Abstract
Foot-and-mouth disease virus (FMDV) utilizes different cell surface macromolecules to facilitate infection of cultured cells. Virus, which is virulent for susceptible animals, infects cells via four members of the αV subclass of cellular integrins. In contrast, tissue culture adaptation of some FMDV serotypes results in the loss of viral virulence in the animal, accompanied by the loss of virus' ability to use integrins as receptors. These avirulent viral variants acquire positively charged amino acids on surface-exposed structural proteins, resulting in the utilization of cell surface heparan sulfate (HS) molecules as receptors. We have recently shown that FMDV serotypes utilizing integrin receptors enter cells via a clathrin-mediated mechanism into early endosomes. Acidification within the endosome results in a breakdown of the viral capsid, releasing the RNA, which enters the cytoplasm by a still undefined mechanism. Since there is evidence that HS internalizes bound ligands via a caveola-mediated mechanism, it was of interest to analyze the entry of FMDV by cell-surface HS. Using a genetically engineered variant of type O1Campos (O1C3056R) which can utilize both integrins and HS as receptors and a second variant (O1C3056R-KGE) which can utilize only HS as a receptor, we followed viral entry using confocal microscopy. After virus bound to cells at 4°C, followed by a temperature shift to 37°C, type O1C3056R-KGE colocalized with caveolin-1, while O1C3056R colocalized with both clathrin and caveolin-1. Compounds which either disrupt or inhibit the formation of lipid rafts inhibited the replication of O1C3056R-KGE. Furthermore, a caveolin-1 knockdown by RNA interference also considerably reduced the efficiency of O1C3056R-KGE infection. These results indicate that HS-binding FMDV enters the cells via the caveola-mediated endocytosis pathway and that caveolae can associate and traffic with endosomes. In addition, these results further suggest that the route of FMDV entry into cells is a function solely of the viral receptor.
Foot-and-mouth disease virus (FMDV) is the type species of the genus Aphthovirus of the family Picornaviridae. The virus is the cause of a highly contagious disease of cloven-hoofed livestock, which is characterized by vesicular lesions in the mouth and on the feet, teats, and nares (see reference 31 and references therein). It has been well established that, via a conserved Arg-Gly-Asp (RGD) sequence in the βG-to-βH (G-H) loop of VP1 (6, 26, 41, 49), the virus utilizes four members of the αV subgroup of cellular integrins (αVβ1, αVβ3, αVβ6, and αVβ8) as receptors in tissue culture (12, 21, 34, 37, 38, 54).
In addition to cellular integrins, FMDV is also able to utilize alternative membrane molecules as receptors. The first evidence of this was presented in reports that found antibody-complexed virus was able to bind to Fc receptors and that this binding led to internalization and viral replication (7, 47, 49). This work was expanded to develop an artificial receptor by fusing a single-chain monoclonal antibody against FMDV (46) to the intercellular adhesion molecule 1 (ICAM-1), engineering a receptor designed to allow virus with an altered receptor binding site with which to attach and replicate in cells (67). Finally, it was demonstrated that some FMDV serotypes, when adapted to tissue culture, gain the ability to utilize cell surface heparan sulfate (HS) as a viral receptor (2, 35, 45, 54), resulting in the attenuation of virulence in host species (70). The interactions between these virions and HS have recently been delineated by crystallographic analyses (19, 27-29). These data, along with recent evidence that FMDV interaction with soluble αVβ6 integrin does not result in structural alterations to the virion (22), suggest that the viral receptor plays a role only in docking and internalizing the virion. However, in spite of the ability of the virion to utilize alternative receptors, the available evidence suggests that the integrin receptor is the only one involved in viral pathogenesis in the susceptible host (52, 54, 68; V. O'Donnell, A. Koser, D. Gregg, and B. Baxt. Abstr. XIIIth Meeting of the European Study Group for the Molecular Biology of Picornaviruses. Lunteren, The Netherlands, 23 to 29 May 2005).
Recently, studies of the entry of FMDV into cultured cells have been initiated. Results from both our laboratory and that of Jackson and colleagues have demonstrated that integrin-binding FMDV types A and O utilize a clathrin-dependent mechanism to infect cells, trafficking through early endosomes into recycling endosomes (13, 58). It is within these endosomes that the virion breaks down (3-5, 16, 51), releasing the viral genome to the cytosol. More recently, Martin-Acebes et al. (44) also demonstrated the involvement of a clathrin-dependent mechanism in productive FMDV type C endocytosis. It was of interest to us to determine the mechanism of entry for FMDV when the virus uses a receptor other than an integrin, for example, HS. There is strong evidence that ligands that bind to HS on HS proteoglycans, including those of bacteria and viruses, are internalized via caveola-mediated endocytosis, although this route is dependent on the protein moiety of the HS proteoglycan (11, 23, 25, 65). A recent report, however, suggests that human papillomavirus binds to HS on dendritic cells and is internalized by a clathrin-dependent pathway (14).
In this study, we followed the internalization, via confocal microscopy, of an FMDV variant which can utilize either HS or integrins as the receptor. In addition, we utilized a genetically engineered variant of this virus which can utilize only HS as a receptor and compared variant entry events to those of the integrin-binding parent virus. Our results demonstrate that the entry mechanism of FMDV into cultured cells is dependent on the receptor to which the virus binds.
MATERIALS AND METHODS
Cell lines and viruses.
Human mammary gland epithelial cells (MCF-10A) were obtained from ATCC (catalogue no. CRL-10317) and were maintained in a mixture of Dulbecco's minimum essential medium (DMEM) and Ham's F-12 medium (1:1; Invitrogen, CA) containing 5% heat-inactivated fetal bovine serum (FBS), 20 ng/ml epidermal growth factor, 100 ng/ml cholera toxin, 10 μg/ml insulin, and 500 ng/ml hydrocortisone. COS-1 cells were maintained in DMEM containing 10% FBS, an additional 2 mM l-glutamine, and 1 mM sodium pyruvate.
The FMDV type O1 strain Campos (O1Campos) was derived from the vesicular fluid of an experimentally infected steer. The HS and integrin-binding variant, O1C3056R, was derived from an O1Campos vaccine seed stock by the insertion of the capsid-coding region of a small-plaque variant virus, selected on CHO cells, into an FMDV type A12 infectious cDNA clone (in reference 70, the virus was referred to as vCRM4). A variant of this virus, in which the RGD integrin recognition site in VP1 was mutated to a Lys-Gly-Glu (KGE) sequence by site-directed mutagenesis (O1C3056R-KGE), was derived from the infectious cDNA clone pCRM48 (70).
Antibodies and reagents.
Monoclonal antibody (MAb) 12FB directed against type O1 was diluted 1/5 for use and has been previously described (77). The MAbs LM609 (catalogue no. MAB1976), which recognizes the αVβ3 heterodimer (17), and CSβ6 (catalogue no. MAB2076), which recognizes the β6 integrin subunit (86) and the αVβ6 heterodimer (22), were purchased from Chemicon (Billerica, MA) and diluted 1/100 for use. To study virus entry, antibodies against the following human cellular structures were used. A rabbit polyclonal antibody against early endosomal antigen-1 (EEA-1; Affinity Bioreagents, Golden, CO) was used at a 1/100 dilution to identify early endosomes; early and recycling endosomes also were identified by using rabbit polyclonal antibodies against Rab4 (72, 76, 83) (StressGen, Ann Arbor, MI) and Rab11 (75, 76, 87) (Affinity BioReagents) at a 1/100 dilution; a rabbit polyclonal antibody against caveolin-1 and a MAb against clathrin heavy chain (Affinity Bioreagents), each at a 1/200 dilution, were used as markers for caveolae and clathrin endocytosis pathways, respectively; MAbs directed against the endoplasmic reticulum marker protein disulfide isomerase (PDI; clone RL77; 1/100 dilution; Affinity Bioreagents), the Golgi apparatus markers, the Golgi zone area (clone 371-4; 1/50 dilution; Chemicon), and the coatomer protein I (COPI; clone maD; 1/100 dilution; Sigma, St. Louis, MO), and transferrin receptor (TfnR; clone RVS-10; 1/50 dilution; Chemicon) were used.
The following chemicals and inhibitors were used. Chlorpromazine (Sigma), which causes the loss of coated pits from the surface of the cell and the appearance of clathrin coats, composed of the same subunits on endosomal membranes (39, 85), was used at a 12.5 μM concentration; cytochalasin D (Sigma), which disrupts the actin cytoskeleton (18) and inhibits lipid raft-mediated internalization (59), was used at a 2 μM concentration; nystatin (Invitrogen), which sequesters cholesterol and disrupts lipid rafts (1, 74), and progesterone (Sigma), which inhibits synthesis of cholesterol (50), were used at concentrations of 25 μg/ml and 10 μg/ml, respectively; amiloride (Sigma), which inhibits macropinocytosis, was used at a 10 μM concentration; brefeldin A (Sigma), which disrupts the Golgi apparatus and affects the budding of COPI-coated membranes (30), was used at a concentration of 5 μg/ml; nocodazole (Sigma), which causes the depolymerization of microtubules and blocks endosomal traffic between peripheral early and late endosomes (10, 32), was used at a concentration of 20 μM; monensin ionophore (Sigma) and ammonium chloride (Sigma), lysosomotropic agents which raise the pH in endocytic vesicles (43), were used at concentrations of 50 μM and 50 mM, respectively.
Viral growth curve.
Monolayers of MCF-10A cells were infected with the type virus O1Campos, O1C3056R, or O1C3056R-KGE at a multiplicity of infection (MOI) of 10 PFU/cell for 1 h at 37°C. At the end of the adsorption period, the inoculum was removed, and the cells were rinsed with ice-cold 2-morpholinoethanesulfonic acid (MES)-buffered saline (25 mM MES [pH 5.5], 145 mM NaCl) to inactivate residual virus particles. The monolayers were then rinsed with MEM (Invitrogen) containing 1% FBS and 25 mM HEPES (pH 7.4) and incubated at 37°C. At the appropriate times postinfection (p.i.), the cells were frozen at −70°C, and the thawed lysates were used to determine titers by plaque assay on BHK-21 cell monolayers.
Infection of cells and confocal microscopy.
Subconfluent monolayers of MCF-10A cells grown on 12-mm glass coverslips in 24-well tissue culture dishes were infected with FMDV at an MOI ranging from 50 to 100 PFU/cell for 1 h at 4°C in MEM containing 0.5% FBS and 25 mM HEPES (pH 7.4). After the adsorption period, the inoculum was removed, the monolayers were washed with MEM (Invitrogen), fresh medium was added, and the cells were incubated at 37°C. To analyze the role of lipid rafts in the internalization, MCF-10A cells were infected with virus as described above in the presence of 1 μg/ml of cholera toxin subunit B conjugated with Alexa Fluor 488 (Molecular Probes, Invitrogen, CA). To study the role of early endosomes and recycling endosomes, we infected MCF-10A cells with virus, as described before, in the presence of 200 μg/ml of transferrin conjugated with Alexa Fluor 488 (Molecular Probes). At the appropriate times, cells were fixed with 4% paraformaldehyde (EMS, Hatfield, PA) and processed for immunofluorescence and confocal microscopy as previously described (58). Briefly, after fixation, the paraformaldehyde was removed, and the cells were permeabilized with 0.5% Triton X-100 for 5 min at room temperature (RT) and incubated in blocking buffer (phosphate-buffered saline [PBS], 5% normal goat serum, 2% bovine serum albumin, 10 mM glycine) for 1 h at RT. The fixed cells were then incubated with the primary antibodies overnight at 4°C. After cells were washed with PBS, they were incubated with the appropriate secondary antibody, goat anti-rabbit immunoglobulin G (IgG) (1/400 dilution; Alexa Fluor 594; Molecular Probes) or goat anti-mouse isotype-specific IgG (1/400 dilution; Alexa Fluor 488 or Alexa Fluor 594; Molecular Probes), for 1 h at RT. Following this incubation, the cells were washed with PBS, counterstained with the nuclear stain TOPRO-iodide 642/661 (Molecular Probes) for 5 min at RT, washed, mounted, and examined with a Leica scanning confocal microscope (model TCS2). Data were collected utilizing the appropriate prepared controls lacking the primary antibodies, as well as the anti-FMDV antibodies with uninfected cells to give the negative background levels and to determine channel crossover settings. The captured images were adjusted for contrast and brightness, using Adobe Photoshop software.
Viral replication in the presence of internalization inhibitors.
MCF-10A cells were incubated with the compounds at the concentrations listed above for 30 min to 2 h at 37°C prior to infection. The cells were then infected with FMDV O1Campos or O1C3056R-KGE at an MOI of 10 PFU/cell in the presence of the compounds. At the end of the adsorption period, the virus was removed, and the cells were rinsed once with ice-cold MES-buffered saline to inactivate unadsorbed virus. The cells were washed once with medium, and then medium containing the inhibitors was added. One set of cultures was immediately frozen at −70°C, and a second set was incubated for an additional 4 h at 37°C and then removed to −70°C. The plates were thawed, cell debris was removed by centrifugation, and viral titer was determined by plaque assay with BHK-21 cell monolayers. The results are represented as the ratio between the titers at 5 h postinfection (p.i.) and those at 1 h p.i.
Knockdown of caveolin-1 by RNA interference.
The short interfering RNA (siRNA) SMARTpool, consisting of four RNA duplexes designed to target the gene of interest, human caveolin-1 (catalog no. D-003467), and a control siRNA, siGlo RISC-free (catalog no. D-001600-01), were purchased from Dharmacon (Lafayette, CO). Pools consisted of equal amounts of each duplex. The siRNA sequences are given as sense/antisense pairs. The four siRNA sequences for caveolin-1 were CUAAACACCUCAACGUGAUU/5′-PUCAUCGUUGAGGUGUUUAGUU, GCAGUUGUACCAUGCAUUAUU/5′-PUAAUGCAUGGUACAACUGCUU, AUUAAGAGCUUCCUGAUUGUU/5′-PCAAUCAGGAAGCUCUUAAUUU, and GCAAAUACGUAGACUCGGAUU/5′-PUCCGAGUCUACGUAUUUGCUU.
MCF-10A cells were grown to densities of 1.1 × 105 cells per well in 24-well tissue culture dishes in 1 ml of medium without antibiotics and transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. For each 2-cm dish, 100 total pmol of pooled siRNA was diluted in 50 μl of serum-free OptiMEM (Invitrogen), and, separately, 5 μl of Lipofectamine 2000 was diluted in 50 μl of OptiMEM. After an incubation of 5 min at RT, the diluted RNA and the Lipofectamine 2000 were combined and incubated for 20 min at RT. MCF-10A medium was replaced with 400 μl of serum-free OptiMEM, and the 100-μl mixture was then added to each dish, which was then rocked gently to spread the lipid-RNA complexes. Forty-eight hours after cells were transfected, they were infected with the O1Campos or O1C3056R-KGE virus at an MOI of 1 for 1 h at 37°C in MEM containing 0.5% FBS and 25 mM HEPES (pH 7.4). After the adsorption period, the inoculum was removed, and the cells were rinsed with ice-cold MES-buffered saline to inactivate residual virus particles. The monolayers were then rinsed with MEM containing 1% FBS and 25 mM HEPES (pH 7.4) and incubated at 37°C. One set of cultures was immediately frozen at −70°C, and a second set was incubated for an additional 4 h at 37°C and then removed to −70°C. The plates were thawed, cell debris was removed by centrifugation, and the viral titer was determined by plaque assay with BHK-21 cell monolayers. For analysis of caveolin-1 protein expression, Western blotting was performed 48 h after transfection. Total cell extracts were made using NET-1% Triton extraction buffer (150 mM NaCl, 1 mM EDTA,10 mM Tris [pH 7.5], 1% Triton X-100) supplemented with protease inhibitors (Roche, Mannheim, Germany). Extract was separated on a 15% Laemmli gel and transferred to a polyvinylidene difluoride membrane for Western blotting. Anti-caveolin-1 immunoblotting was performed using a rabbit anti-caveolin-1 antibody (Affinity Bioreagents), and anti-GAPDH immunoblotting was performed using a mouse anti-GAPDH antibody (Research Diagnostics). Antibodies were diluted 1/500 in a PBS solution containing 0.1% Tween-20 and 5% milk and detected with alkaline-phosphatase-conjugated goat anti-rabbit or anti-mouse antibody at a 1/1,000 dilution and 5-bromo-4-chloro-3-indolyl phosphate disodium salt (BCIP)/Nitro Blue Tetrazolium (NBT) (KPL, Baltimore, MD) as the substrate.
RESULTS
Growth kinetics of FMDV type O1Campos variants in MCF-10A cells.
We had previously shown that type O1Campos replicates in MCF-10A cells via its interaction with the αVβ6 integrin (58) and that O1C3056R was able to infect cells lacking the integrin receptor expression via its interaction with HS (54). To compare the growth of these two variants with that of type O1C3056R-KGE, which is unable to bind to αV integrin receptors, we analyzed the growth kinetics of these viruses in MCF-10A cells. The results shown in Fig. 1 demonstrate that O1Campos and O1C3056R both grew with similar kinetics and reached comparable titers in this cell line. Type O1C3056R-KGE also grew in this cell line, although at a slower rate and to about 10-fold lower titers. These results indicated that MCF-10A cells are suitable for the study of the internalization events in vitro.
FIG. 1.
Growth curve of FMDV type O1 variants in MCF-10A cells. Cells were infected with FMDV type O1Campos, O1C3056R, or O1C3056R-KGE at an MOI of 10 PFU/cell, as described in Materials and Methods. Combined intracellular and extracellular plaque titers were determined at different times p.i. in BHK-21 cells.
Heparin-binding type O1Campos variants can bind and enter cells lacking integrin receptors.
The αVβ6 integrin is present only on a subset of MCF-10A cells (58), so it was of interest to examine the early interactions between the O1Campos variants and this cell type, which allowed us to analyze different entry mechanisms in the same cell line. Figure 2 shows the expression of the αVβ6 integrin and the binding and entry of each of the variants to MCF-10A cells. As we reported previously (58), upon adsorption, type O1Campos bound only to cells expressing the αVβ6 integrin and colocalized with the integrin on the cell membrane at 4°C (Fig. 2a), and after the temperature was shifted to 37°C, the virus internalized in association with the integrin (Fig. 2b to d). In contrast, type O1C3056R-KGE bound to cells regardless of whether the integrin was expressed or not, but in cells in which it was expressed, the virus and integrin did not colocalize (Fig. 2). This could be seen more dramatically when the temperature was shifted to 37°C and the entry of the virus followed (Fig. 2f to h). The binding of virus to the cell surface was abolished when the cells were treated with heparinase III (more than 70% inhibition; not shown). Type O1C3056R, which contains the RGD sequence in VP1 and can utilize both integrins and HS as the receptors (54), also bound to cells regardless of αVβ6 expression (Fig. 2i). Unlike the variant with the KGE sequence in VP1, the type O1C3056R virus colocalized with the integrin in cells expressing the receptor (Fig. 2j to l).
FIG. 2.
Analysis of FMDV interaction with the αVβ6 integrin in MCF-10A cells. Cells were infected with type O1Campos (a to d), type O1C3056R-KGE (e to h), and type O1C3056R (i to l) or were mock infected (m) at 4°C as described in Materials and Methods. Cells were fixed and probed for viral proteins (red) and the αVβ6 integrin (green), as described in Materials and Methods, immediately p.a. (a, e, and i) or at various times after cells were shifted to 37°C (b to d, f to h, and j to l). Only the merged photomicrographs are shown.
Integrin-binding and HS-binding FMDVs enter cells via different mechanisms.
Since integrin-binding FMDVs enter cells via clathrin-mediated endocytosis (13, 58), it was of interest to examine HS-binding viruses to determine whether they utilized the same route of entry. Virus was allowed to adsorb to cells at 4°C for 1 h, the temperature shifted to 37°C, and the cells were fixed at different times after the temperature shift. Cells were probed for virus and either clathrin or caveolin-1 as described in Materials and Methods (Fig. 3), 15 min after the temperature shift. Type O1Campos colocalized with clathrin (Fig. 3c); however, type O1C3056R-KGE did not colocalize with clathrin (Fig. 3i), and type O1C3056R colocalized with clathrin in some cells (Fig. 3o). When we probed with an antibody to caveolin-1, we observed that type O1Campos did not colocalize with this protein (Fig. 3f), while type O1C3056R-KGE did (Fig. 3l), and type O1C3056R colocalized in some cells (Fig. 3r). Thus, virus which utilized HS as the receptor appeared to enter the cells via a different route than virus which bound to the αVβ6 receptor.
FIG. 3.
Analysis of FMDV interaction with clathrin or caveolin-1 in MCF-10A cells. Cells were infected with type O1Campos (a to f), O1C3056R-KGE (g to l), and O1C3056R (m to r) or were mock infected (s to x) at 4°C as described in Materials and Methods. Cells were fixed and probed, as described in Materials and Methods, for viral proteins (red), clathrin (green), or caveolin-1 (green) at 15 min after the temperature was shifted to 37°C.
HS-binding FMDV moves through the endocytic pathway at a rate that is slower than integrin-binding virus.
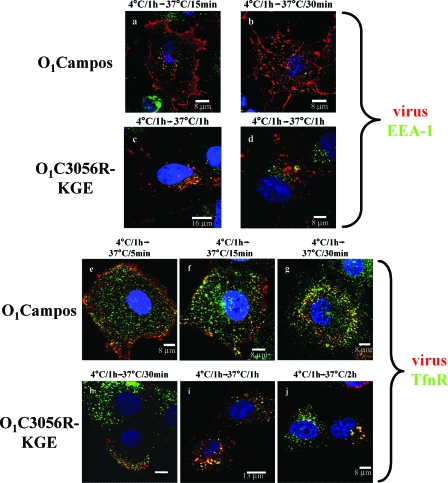
We, and others, had previously reported that integrin-binding FMDVs move rapidly through early endosomes into recycling endosomes (13, 58). Our results were based on the rapid colocalization of the incoming virus with the EEA-1 protein, as well as its colocalization with TfnR, which also utilizes the same entry pathway (66, 82). We compared the movement of integrin-binding type O1Campos with that of the HS-binding variant O1C3056R-KGE. To perform these experiments, we utilized COS-1 cells, since the anti-EEA-1 antibody did not react with this protein in MCF-10A cells. To be able to use COS-1 cells for this experiment, we demonstrated that the HS-binding virus utilized the same entry route into this cell line as it did into MCF-10A cells by looking at the colocalization of the virus with caveolae and cholera toxin (data not shown). Prior to infecting cells with type O1Campos, we transfected these cells with plasmids encoding the αV and β6 integrin subunits as previously described (21). As we have shown previously (58), type O1Campos colocalized with the early endosomal marker EEA-1 within 15 min after the temperature was shifted to 37°C (Fig. 4a). At 30 min postadsorption (p.a.), the virus did not colocalize with this protein, indicating that the virus had already been translocated from the early endosomes (Fig. 4b). In contrast, type O1C3056R-KGE showed only partial colocalization with EEA-1 by 1 h after the temperature shift (Fig. 4c), and there were many cells in the culture that showed no colocalization of virus with EEA-1 (Fig. 4d). To try and resolve the temporal differences of viral movement through the endocytic system, we followed the internalization of both viruses along with that of TfnR, which traffics through early and recycling endosomes before returning to the cell surface (66, 82). Type O1Campos rapidly began to colocalize with TfnR by 5 min p.a., and by 30 min p.a., there was almost complete colocalization of virus with the TfnR (Fig. 4e to g). In contrast, type O1C3056R-KGE was only slightly colocalized with TfnR by 30 min p.a., but there was extensive colocalization by 2 h p.a. (Fig. 4h to j). These results suggested that integrin-binding and HS-binding viruses may traffic through different endocytic compartments. To analyze this further, we examined the association of the incoming viruses with two members of the Rab protein family, Rab4, a marker for early and recycling endosomes (72, 76, 83), and Rab11, a marker for recycling endosomes and the Golgi apparatus (75, 76, 87). The type O1C3056R-KGE virus began to colocalized with Rab4 at between 15 min (not shown) and 30 min p.a. (Fig. 5a) and with Rab11 at 30 min p.a. (Fig. 5d), showing a complete colocalization with both markers by 1 h p.a. (Fig. 5b and e). In contrast, the integrin-binding type O1Campos virus colocalized with Rab4 (not shown) but not with Rab11 (Fig. 5g to i).
FIG. 4.
Analysis of FMDV movement through early endosomes. COS-1 cells were infected with either type O1Campos (a to b) or type O1C3056R-KGE (c to d) at 4°C as described in Materials and Methods. MCF-10A cells were infected with either type O1Campos (e to g) or type O1C3056R-KGE (h to j) at 4°C as described in Materials and Methods. Cells were shifted to 37°C and, at the times indicated over each panel, were fixed and probed, as described in Materials and Methods, for viral proteins (red), EEA-1 (green), or TfnR (green). Only the merged photomicrographs are shown.
FIG. 5.
Analysis of FMDV interactions with the Rab proteins in MCF-10A cells. Cells were infected with either type O13056R-KGE (a to f) or type O1Campos (g to i) at 4°C as described in Materials and Methods. Cells were shifted to 37°C and, at the times indicated over each panel, were fixed and probed, as described in Materials and Methods, for viral proteins (red), Rab4 (green, a to c), or Rab11 (green, d to i). Only the merged photomicrographs are shown.
In addition, we compared the entry of both viruses with that of fluorescent transferrin (Tfn-AF488), a well-established marker for the clathrin pathway, in the presence or absence of nocodazole, which blocks endosomal traffic from early to late endosomes by depolymerization of microtubules (32, 39). When the entry of the O1C3056R-KGE virus and that of the integrin-binding virus were examined, colocalization with Tfn-AF488 by 30 min p.a. was observed for both, without showing any differences for the nocodazole-treated cells (not shown).
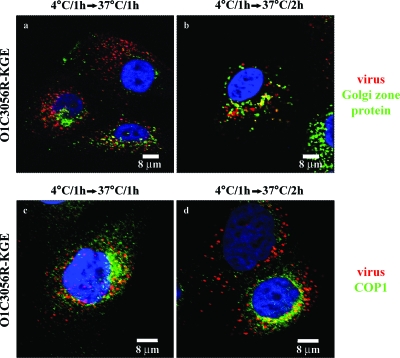
Type O1C3056R-KGE also partially colocalized with the Golgi zone area protein (Fig. 6a and b) and COPI (Fig. 6c and d). We have previously shown that O1Campos does not colocalize with proteins found in the Golgi apparatus (58). As we had previously shown for type O1Campos (58), neither type O1C3056R nor type O1C3056R-KGE was delivered to the endoplasmic reticulum during internalization (not shown).
FIG. 6.
Analysis of HS-binding FMDV with the Golgi apparatus in MCF-10A cells. Cells were infected with type O13056R-KGE at 4°C as described in Materials and Methods. Cells were shifted to 37°C and, at the times indicated over each panel, were fixed and probed, as described in Materials and Methods, for viral proteins (red), Golgi zone protein (green, a to b), or COPI (green, c to d). Only the merged photomicrographs are shown.
Taken together, these results indicate that integrin-binding viruses traffic only from the clathrin-coated vesicle to the early endosome, where virus uncoating presumably occurs. Viruses which utilize HS as a receptor traffic from the caveola through the early endosome and into the recycling endosome and/or the Golgi apparatus.
Effects of inhibitors on the endocytic pathways on replication of integrin- and HS-binding FMDV.
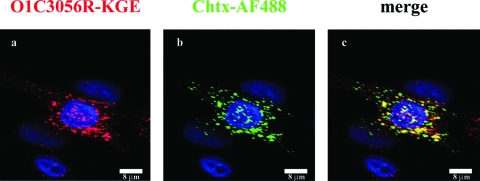
To confirm the microscopy analyses just presented, we analyzed viral replication in the presence of a number of inhibitors of endocytosis (Fig. 7). We had previously reported that chlorpromazine, an inhibitor of clathrin-mediated endocytosis (39, 85), inhibited type O1Campos replication (58). In addition to inhibiting the integrin-binding virus, chlorpromazine also inhibited type O1C3056R replication (not shown), but it did not inhibit the replication of type O1C3056R-KGE, which utilizes only HS as the receptor and seems to be internalized via a caveola-mediated endocytosis pathway. Since the caveola-mediated entry of a number of picornaviruses is dependent on lipid rafts (20, 39, 42, 63, 79), we utilized three inhibitors of lipid raft formation, cytochalasin D (59), nystatin (1, 74), and nystatin plus progesterone. Not surprisingly, these inhibitors had no effect on the replication of O1Campos (Fig. 7). MCF-10A cells treated for 2 h with nystatin or a combination of nystatin and progesterone resulted in an inhibition of O1C3056R-KGE replication, which was also observed with confocal microscopy (not shown). To further analyze this result, we followed the entry of type O1C3056R-KGE along with cholera toxin, which enters cells via caveolae and lipid raft-dependent endocytosis (33). Figure 8 show a colocalization of the HS-binding virus and cholera toxin AF488 starting at 15 min p.a. after the temperature was shifted to 37°C.
FIG. 7.
Effect of inhibitors of internalization on FMDV replication in MCF-10A cells. Cells were treated with each of the inhibitors and infected with the O1Campos or O13056R-KGE variant, as described in Materials and Methods. Immediately p.a., one set of plates was frozen at −70°C and a second set of plates was frozen at −70°C at 5 h after infection. Plaque titers were determined in BHK-21 cells and are represented as the ratio of plaque titers at 1 to those at 5 h p.i.
FIG. 8.
Internalization of type O13056R-KGE and fluorescent cholera toxin in MCF-10A cells. Cells were infected with virus in the presence of Alexa Fluor 488-labeled cholera toxin as described in Materials and Methods. Cells were fixed 15 min after infection, and after they were shifted to 37°C, they were probed for virus (red). Cholera toxin was detected as Alex Fluor 488 (green).
We had previously shown that late endosomes were not involved in the entry of type O1Campos (58) and that the lack of effect of nocodazole, which causes the depolymerization of microtubules and inhibits translocation between peripheral early and late endosomes (10), on the replication of these viruses indicate that these viruses do not traffic through late endosomes. Amiloride, a compound which inhibits macropinocytosis, had no effect on viral replication of either of the viruses. In addition, brefeldin A, which disrupts the Golgi apparatus (30), had no effect on the replication of either the integrin- or the HS-binding virus (not shown), suggesting that the movement of type O1C3056R-KGE to the Golgi apparatus (Fig. 6) might represent a dead-end pathway.
Finally, we examined viral replication in the presence of two compounds which raise the pH of endosomal vesicles, monensin and ammonium chloride. We previously showed that these compounds inhibited the replication of a number of different FMDV serotypes and subtypes by preventing the acid-induced breakdown of the viral capsid to pentamers, resulting in the release of the viral RNA (3). As observed for previous studies, monensin and ammonium chloride inhibited the replication of all three viral variants, O1Campos, O1C3056R-KGE (Fig. 7), and O1C3056R (not shown).
Inhibition of FMDV replication by caveolin-1 knockdown.
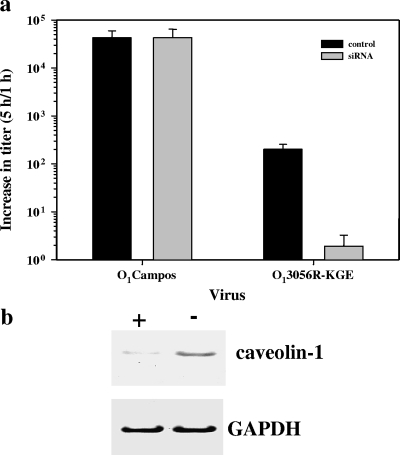
To verify whether caveolin-1 plays a critical role in HS-binding virus entry, we tested the effect of a caveolin-1 knockdown on O1Campos and O1C3056R-KGE infection. Western blot analysis showed that the amount of caveolin-1 in MCF-10A cells could be reduced by 90% after cells were transfected with the caveolin-1 siRNA but did not change after they were transfected with a control siRNA (Fig. 9b). This reduction of caveolin-1 resulted in no inhibition of viral replication when the cells were incubated with O1Campos (Fig. 9a). In contrast, when the cells were transfected with caveolin-1 siRNA and infected with the HS-binding virus O1C3056R-KGE, an approximately 100-fold inhibition of replication was observed compared to that for the control (Fig. 9a). These results indicated that the expression of caveolin-1 is important and necessary for the infection by O1C3056R-KGE.
FIG. 9.
Viral yields from FMDV infections in MCF-10A cells treated with siRNA to reduce the intracellular concentration of caveolin-1. (a) MCF-10A cells transfected with four RNA duplexes targeted to caveolin-1 or with a control siRNA, siGlo, for 48 h at 37°C, as described in Materials and Methods. Triplicate plates were infected with FMDV at an MOI of 1 PFU/cell for the indicated times. Plaque titers were determined in BHK-21 cells and are represented as the ratios of plaque titers at 1 to those at 5 h p.i. (b) The relative abundance of caveolin-1 protein in the cells treated with the control or that with cells with the caveolin-1-targeted siRNA duplexes was determined by immunoblotting using polyclonal antibodies to caveolin-1 and GAPDH.
DISCUSSION
FMDV is able to utilize a number of different cell surface molecules as viral receptors in cell culture, including at least four different αV integrins and HS (8, 9, 36, 48). However, the viruses that use integrin-mediated binding are those responsible for disease in susceptible animals (52, 54, 70). It has recently been reported that these viruses enter cells via clathrin-mediated endocytosis (13, 58). The results we have presented in the present report indicate that FMDV O1C3056R-KGE, which utilizes the alternative receptor HS, enters the cell via a caveola-mediated endocytic pathway. This conclusion is supported by the colocalization of HS-binding virus with caveolin-1 but not with the markers of the clathrin-mediated endocytosis, the inhibition of replication of the variant O1C3056R-KGE but not the O1Campos by caveolin-1 knockdown, and the sensitivity of HS-binding virus internalization to drugs that impair lipid raft components, such as nystatin and progesterone, or disturb anchorage of caveolae to the actin cytoskeleton, such as cytochalasin D. In addition, our results further suggest that the entry pathway of FMDV into cells is dependent on the receptor to which the virus binds.
To understand the HS-mediated entry of FMDV, we analyzed the internalization of the variant O1C3056R-KGE, where the RGD sequence of the G-H loop responsible for targeting the virus to integrins has been mutated to KGE and can utilize only HS as a receptor, and a second variant of type O1Campos, O1C3056R, which can bind to both HS and integrin receptors (8, 54). For most of our studies, we utilized the human breast epithelial cell line MCF-10A, which is a heterogeneous population of cells, some of which express the αVβ3 or αVβ6 integrin receptors (58).
All three of the viruses replicated in the MCF-10A cells (Fig. 1), and the analysis of viral binding shows that O1Campos bound only to cells expressing the αVβ6 integrin (Fig. 2), as reported previously (58). In contrast, type O1C3056R was bound to cells which either did or did not express the integrin and colocalized with the integrin in expressing cells (Fig. 2). Type O1C3056R-KGE bound to cells irrespective of their integrin expression and never colocalized with the integrin (Fig. 2). Treatment of cells with heparinase abolished binding of the O1C3056R-KGE virus (not shown). Thus, this virus bound only to the HS receptor, while the O1C3056R variant bound to either HS or the integrin. We followed the entry of these viruses along with markers for clathrin and caveola-mediated entry. As has been shown previously (13, 58), integrin-binding virus colocalized with clathrin upon entering the cell (Fig. 3), while the HS-binding virus appeared to colocalize with caveolin-1 (Fig. 3), indicating that the HS-virus complex was entering via the caveolae. The viral variant which bound to both integrin and HS appeared to colocalize with both clathrin and caveolin-1 (Fig. 3). We confirmed these results by using the inhibitor of clathrin-mediated entry, chlorpromazine. This compound inhibited the replication of both type O1Campos (Fig. 7) and type O1C3056R (not shown); however, the latter virus was inhibited to a lesser extent. In contrast, chlorpromazine had no effect on the replication of type O1C3056R-KGE (Fig. 7).
Caveolae are a type of lipid raft present on the cell surface which contain the caveolin protein (69). Caveolin is a cholesterol-binding protein (53, 80), and caveolae are membrane domains that are cholesterol dependent (80). The replication of two other nonenveloped viruses, simian virus 40 (SV40) and echovirus type 1 (EV1), which enter cells via caveolae (62, 63), are inhibited by compounds that deplete cholesterol and disrupt lipid rafts (1, 63). In the presence of nystatin and progesterone, compounds that are cholesterol-sequestering and lipid-raft disrupting (1, 40, 74), O1C3056R-KGE viral infection was inhibited (Fig. 7). We also found that the replication of the HS-binding virus was inhibit to a lesser extent by cytochalasin D, which disrupts the actin cytoskeleton and inhibits lipid raft-mediated internalization (18, 59). For EV1, it has been demonstrated that cytochalasin D does not inhibit viral replication (63), suggesting that EV1, may enter in an actin-independent manner. The authors of that study, however, speculated that this result may depend on the cell type used (63). Disruption of the microtubules with the drug nocodazole had no effect on the replication of either of the viruses, O1Campos or O1C3056R-KGE, which indicates that these viruses do not traffic from early to late endosomes.
To verify that caveolae play a critical role, we tested the effect of a caveolin-1 knockdown on O1Campos and O1C3056R-KGE infection. When the abundance of the caveolin-1 protein was reduced by more than 90% by siRNA, an inhibitory effect on viral replication was observed for the O1C3056R-KGE HS-binding virus, indicating that the expression of caveolin-1 is important and necessary for infection by this virus, while the caveolin-1 knockdown had no effect on the replication of O1Campos.
To further define the role of lipid rafts in the internalization of type O1C3056R-KGE, we analyzed virus entry along with that of cholera toxin. Cholera toxin has been shown to be associated with lipid rafts (56, 57, 73) and enters cells partially through caveolae (55); therefore, cholera toxin can be used as a marker for lipid rafts. By using fluorescence-labeled cholera toxin, we were able to follow the internalization of the virus. We observed that the O1C3056R-KGE virus colocalizes with cholera toxin on entering the cell (Fig. 8), indicating again that HS-binding virus enters the cell in a structure containing caveolin-1 which is dependent on cholesterol. It has been shown that cholera toxin binds to GM1 ganglioside and partially passes through caveosomes during transport to the Golgi complex (55), suggesting that the HS-binding virus may be entering caveosomes at late stages of infection.
It has been demonstrated that the dynamics of the caveolar endocytosis pathway are slow compared with those of the clathrin-endocytosis pathway (24, 81, 84). This is in agreement with our results, since we found that the HS-binding FMDV moves through the endocytic pathway at a slower rate than that of the integrin-binding virus that uses the clathrin-endocytosis pathway.
We had previously suggested that type O1Campos enters the cell in an early endosome, followed by movement into a recycling endosome, where the virus breaks down due to the acidic environment, releasing the RNA (58). The results presented in this work indicate that the integrin-binding virus enters into the early endosomes, where breakdown presumably occurs. These results are based on following the entry of virus in association with Rab4 and Rab11. The Rab proteins are small GTPases, members of the Ras superfamily, which are distributed and regulate transport through intracellular organelles (see 75, 78, and 88 and references therein). Rab4 regulates endocytic recycling to the plasma membrane and is found on both early and recycling endosomes (72, 76, 83), while Rab11 is found on recycling endosomes and the Golgi apparatus and regulates recycling through the recycling endosomes and Golgi-plasma membrane traffic (75, 76, 87). We found that both variants, O1Campos and O1C3056R-KGE, colocalized with the early endosomal marker EEA-1, TfnR, and Rab4 (Fig. 4 and 5), indicating that both variants trafficked into the early endosomes. Interestingly, only the HS-binding virus colocalized with Rab11 (Fig. 5) and colocalized partially with the Golgi zone area protein and the COPI protein (Fig. 6), which is part of a complex of proteins involved in intra-Golgi apparatus transport (64). Thus, we believe that the HS-binding viruses enter the cell through caveolae and traffic through early endosomes and into recycling endosomes, where the breakdown and release of the RNA may occur. Our results are consistent with several other studies demonstrating that cargo internalized by caveolae and markers of the clathrin-endocytosis pathway merge at early endosomes, with the detection of caveolin-1 on early endosomes. These results suggest that cargo and membranes internalized through caveolae are predominantly transported to the early endosomes for further sorting and delivery to cellular locations, although exceptions may occur, depending on the cell type (60, 61, 71).
We cannot rule out the possibility that this virus also traffics through the Golgi apparatus; however, brefeldin A, which disrupts the Golgi apparatus (30), did not inhibit the replication of either the integrin-binding or the HS-binding virus (not shown). Moreover, a colocalization of the HS-binding virus with cholera toxin, which is known to traffic to the Golgi complex, was observed at late stages of infection. Taken together, these results suggest that traffic through the Golgi apparatus, if it occurs, may be a dead-end pathway.
We, and others have previously reported that monensin, a compound which raises the pH of endocytic vesicles, inhibited the replication of a wide variety of FMDV serotypes and subtypes by preventing the acid-induced breakdown of virus particles, resulting in genome release (3, 15). We also recently reported that in the presence of monensin, integrin-binding viruses were not released from the early endosomal compartment (58). In this study, pretreatment of cells with monensin inhibited the replication of both viruses, O1Campos and the variant O1C3056R-KGE. Monensin probably raises the pH of both the early and the recycling endosomes enough to prevent viral uncoating and therefore inhibits the replication of the viruses. Similar results were observed when cells were pretreated with ammonium chloride, although there was a greater effect on the replication of the O1C3056R-KGE variant (Fig. 7).
Our studies were undertaken to identify targets for antiviral intervention to control FMD. Even though it has been shown that the HS-binding viruses are relatively avirulent (70), it is important to determine if FMDV can enter cells via alternative mechanisms. FMDV has been shown to be remarkably resilient and variable in the field, and the emergence of new viral variants, which might manifest different modes of viral entry, needs to be addressed.
Acknowledgments
This work was supported by the U.S. Department of Agriculture, Agricultural Research Service, through Current Research Information System Project (CRIS) no. 1940-32000-052-00D and Specific Cooperative Agreement no. 58-1940-2-245 with the Department of Pathobiology and Veterinary Sciences, University of Connecticut, Storrs, CT.
We thank Marvin Grubman for reading the manuscript and providing valuable suggestions.
Footnotes
Published ahead of print on 9 July 2008.
REFERENCES
- 1.Anderson, H. A., Y. Chen, and L. C. Norkin. 1996. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell 71825-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baranowski, E., N. Sevilla, N. Verdaguer, C. M. Ruiz-Jarabo, E. Beck, and E. Domingo. 1998. Multiple virulence determinants of foot-and-mouth disease virus in cell culture. J. Virol. 726362-6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxt, B. 1987. Effect of lysosomotropic compounds on early events in foot-and-mouth disease virus replication. Virus Res. 7257-271. [DOI] [PubMed] [Google Scholar]
- 4.Baxt, B., and H. L. Bachrach. 1982. The adsorption and degradation of foot-and-mouth disease virus by isolated BHK-21 cell plasma membranes. Virology 116391-405. [DOI] [PubMed] [Google Scholar]
- 5.Baxt, B., and H. L. Bachrach. 1980. Early interactions of foot-and-mouth disease virus with cultured cells. Virology 10442-55. [DOI] [PubMed] [Google Scholar]
- 6.Baxt, B., and Y. Becker. 1990. The effect of peptides containing the arginine-glycine-aspartic acid sequence on the adsorption of foot-and-mouth disease virus to tissue culture cells. Virus Genes 473-83. [DOI] [PubMed] [Google Scholar]
- 7.Baxt, B., and P. W. Mason. 1995. Foot-and-mouth disease virus undergoes restricted replication in macrophage cell cultures following Fc receptor-mediated adsorption. Virology 207503-509. [DOI] [PubMed] [Google Scholar]
- 8.Baxt, B., S. Neff, E. Rieder, and P. Mason. 2002. Foot-and-mouth disease virus-receptor interactions: role in pathogenesis and tissue culture adaptation, p. 115-123. In B. L. Semler and E. Wimmer (ed.), Molecular biology of picornaviruses. ASM Press, Washington, DC.
- 9.Baxt, B., and E. Rieder. 2004. Molecular aspects of foot-and-mouth disease virus virulence and host range: role of host cell receptors and viral factors, p. 145-172. In F. Sobrino and E. Domingo (ed.), Foot and mouth disease: current perspectives. Horizon Bioscience, Norfolk, England.
- 10.Bayer, N., D. Schober, E. Prchla, R. F. Murphy, D. Blaas, and R. Fuchs. 1998. Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection. J. Virol. 729645-9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belting, M. 2003. Heparan sulfate proteoglycan as a plasma membrane carrier. Trends Biochem. Sci. 28145-151. [DOI] [PubMed] [Google Scholar]
- 12.Berinstein, A., M. Roivainen, T. Hovi, P. W. Mason, and B. Baxt. 1995. Antibodies to the vitronectin receptor (integrin αVβ3) inhibit binding and infection of foot-and-mouth disease virus to cultured cells. J. Virol. 692664-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berryman, S., S. Clark, P. Monaghan, and T. Jackson. 2005. Early events in integrin αVβ6-mediated cell entry of foot-and-mouth disease virus. J. Virol. 798519-8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bousarghin, L., P. Hubert, E. Franzen, N. Jacobs, J. Boniver, and P. Delvenne. 2005. Human papillomavirus 16 virus-like particles use heparan sulfates to bind dendritic cells and colocalize with langerin in Langerhans cells. J. Gen. Virol. 861297-1305. [DOI] [PubMed] [Google Scholar]
- 15.Carrillo, E. C., C. Giachetti, and R. H. Campos. 1984. Effect of lysosomotropic agents on the foot-and-mouth disease virus replication. Virology 135542-545. [DOI] [PubMed] [Google Scholar]
- 16.Cavanagh, D., D. J. Rowlands, and F. Brown. 1978. Early events in the interaction between foot-and mouth disease virus and primary pig kidney cells. J. Gen. Virol. 41255-264. [DOI] [PubMed] [Google Scholar]
- 17.Cheresh, D. A., and R. C. Spiro. 1987. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J. Biol. Chem. 26217703-17711. [PubMed] [Google Scholar]
- 18.Cooper, J. A. 1987. Effects of cytochalasin and phalloidin on actin. J. Cell Biol. 1051473-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curry, S., E. Fry, W. Blakemore, R. Abu-Ghazaleh, T. Jackson, A. King, S. Lea, J. Newman, D. Rowlands, and D. Stuart. 1996. Perturbations in the surface structure of A22 Iraq foot-and-mouth disease virus accompanying coupled changes in host cell specificity and antigenicity. Structure 4135-145. [DOI] [PubMed] [Google Scholar]
- 20.DeTulleo, L., and T. Kirchhausen. 1998. The clathrin endocytic pathway in viral infection. EMBO J. 174585-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duque, H., and B. Baxt. 2003. Foot-and-mouth disease virus receptors: comparison of bovine αV integrin utilization by type A and O viruses. J. Virol. 772500-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duque, H., M. LaRocco, W. T. Golde, and B. Baxt. 2004. Interactions of foot-and-mouth disease virus with soluble bovine αVβ3 and αVβ6 integrins. J. Virol. 789773-9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eguchi, A., T. Akuta, H. Okuyama, T. Senda, H. Yokoi, H. Inokuchi, S. Fujita, T. Hayakawa, K. Takeda, M. Hasegawa, and M. Nakanishi. 2001. Protein transduction domain of HIV-1 Tat protein promotes efficient delivery of DNA into mammalian cells. J. Biol. Chem. 27626204-26210. [DOI] [PubMed] [Google Scholar]
- 24.Fittipaldi, A., A. Ferrari, M. Zoppe, C. Arcangeli, V. Pellegrini, F. Beltram, and M. Giacca. 2003. Cell membrane lipid rafts mediate caveolar endocytosis of HIV-1 Tat fusion proteins. J. Biol. Chem. 27834141-34149. [DOI] [PubMed] [Google Scholar]
- 25.Fittipaldi, A., and M. Giacca. 2005. Transcellular protein transduction using the Tat protein of HIV-1. Adv. Drug Deliv. Rev. 57597-608. [DOI] [PubMed] [Google Scholar]
- 26.Fox, G., N. R. Parry, P. V. Barnett, B. McGinn, D. J. Rowlands, and F. Brown. 1989. The cell attachment site on foot-and-mouth disease virus includes the amino acid sequence RGD (arginine-glycine-aspartic acid). J. Gen. Virol. 70625-637. [DOI] [PubMed] [Google Scholar]
- 27.Fry, E. E., S. M. Lea, T. Jackson, J. W. Newman, F. M. Ellard, W. E. Blakemore, R. Abu-Ghazaleh, A. Samuel, A. M. King, and D. I. Stuart. 1999. The structure and function of a foot-and-mouth disease virus-oligosaccharide receptor complex. EMBO J. 18543-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fry, E. E., J. W. Newman, S. Curry, S. Najjam, T. Jackson, W. Blakemore, S. M. Lea, L. Miller, A. Burman, A. M. King, and D. I. Stuart. 2005. Structure of foot-and-mouth disease virus serotype A1061 alone and complexed with oligosaccharide receptor: receptor conservation in the face of antigenic variation. J. Gen. Virol. 861909-1920. [DOI] [PubMed] [Google Scholar]
- 29.Fry, E. E., D. I. Stuart, and D. J. Rowlands. 2005. The structure of foot-and-mouth disease virus. Curr. Top. Microbiol. Immunol. 28871-101. [DOI] [PubMed] [Google Scholar]
- 30.Fujiwara, T., K. Oda, S. Yokota, A. Takatsuki, and Y. Ikehara. 1988. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J. Biol. Chem. 26318545-18552. [PubMed] [Google Scholar]
- 31.Grubman, M. J., and B. Baxt. 2004. Foot-and-mouth disease. Clin. Microbiol. Rev. 17465-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gruenberg, J., G. Griffiths, and K. E. Howell. 1989. Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J. Cell Biol. 1081301-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henley, J. R., E. W. Krueger, B. J. Oswald, and M. A. McNiven. 1998. Dynamin-mediated internalization of caveolae. J. Cell Biol. 14185-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson, T., S. Clark, S. Berryman, A. Burman, S. Cambier, D. Mu, S. Nishimura, and A. M. King. 2004. Integrin αVβ8 functions as a receptor for foot-and-mouth disease virus: role of the β-chain cytodomain in integrin-mediated infection. J. Virol. 784533-4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson, T., F. M. Ellard, R. A. Ghazaleh, S. M. Brookes, W. E. Blakemore, A. H. Corteyn, D. I. Stuart, J. W. Newman, and A. M. King. 1996. Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J. Virol. 705282-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson, T., A. M. King, D. I. Stuart, and E. Fry. 2003. Structure and receptor binding. Virus Res. 9133-46. [DOI] [PubMed] [Google Scholar]
- 37.Jackson, T., A. P. Mould, D. Sheppard, and A. M. King. 2002. Integrin αVβ1 is a receptor for foot-and-mouth disease virus. J. Virol. 76935-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson, T., D. Sheppard, M. Denyer, W. Blakemore, and A. M. King. 2000. The epithelial integrin αVβ6 is a receptor for foot-and-mouth disease virus. J. Virol. 744949-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joki-Korpela, P., V. Marjomaki, C. Krogerus, J. Heino, and T. Hyypia. 2001. Entry of human parechovirus 1. J. Virol. 751958-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kilsdonk, E. P., P. G. Yancey, G. W. Stoudt, F. W. Bangerter, W. J. Johnson, M. C. Phillips, and G. H. Rothblat. 1995. Cellular cholesterol efflux mediated by cyclodextrins. J. Biol. Chem. 27017250-17256. [DOI] [PubMed] [Google Scholar]
- 41.Leippert, M., E. Beck, F. Weiland, and E. Pfaff. 1997. Point mutations within the βG-βH loop of foot-and-mouth disease virus O1K affect virus attachment to target cells. J. Virol. 711046-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marjomaki, V., V. Pietiainen, H. Matilainen, P. Upla, J. Ivaska, L. Nissinen, H. Reunanen, P. Huttunen, T. Hyypia, and J. Heino. 2002. Internalization of echovirus 1 in caveolae. J. Virol. 761856-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marsh, M., J. Wellsteed, H. Kern, E. Harms, and A. Helenius. 1982. Monensin inhibits Semliki Forest virus penetration into culture cells. Proc. Natl. Acad. Sci. USA 795297-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin-Acebes, M. A., M. Gonzalez-Magaldi, K. Sandvig, F. Sobrino, and R. Armas-Portela. 2007. Productive entry of type C foot-and-mouth disease virus into susceptible cultured cells requires clathrin and is dependent on the presence of plasma membrane cholesterol. Virology 369105-118. [DOI] [PubMed] [Google Scholar]
- 45.Martinez, M. A., N. Verdaguer, M. G. Mateu, and E. Domingo. 1997. Evolution subverting essentiality: dispensability of the cell attachment Arg-Gly-Asp motif in multiply passaged foot-and-mouth disease virus. Proc. Natl. Acad. Sci. USA 946798-6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mason, P., A. Berinstein, B. Baxt, R. Parsells, A. Kang, and E. Rieder. 1996. Cloning and expression of a single-chain antibody fragment specific for foot-and-mouth disease virus. Virology 224548-554. [DOI] [PubMed] [Google Scholar]
- 47.Mason, P. W., B. Baxt, F. Brown, J. Harber, A. Murdin, and E. Wimmer. 1993. Antibody-complexed foot-and-mouth disease virus, but not poliovirus, can infect normally insusceptible cells via the Fc receptor. Virology 192568-577. [DOI] [PubMed] [Google Scholar]
- 48.Mason, P. W., M. J. Grubman, and B. Baxt. 2003. Molecular basis of pathogenesis of FMDV. Virus Res. 919-32. [DOI] [PubMed] [Google Scholar]
- 49.Mason, P. W., E. Rieder, and B. Baxt. 1994. RGD sequence of foot-and-mouth disease virus is essential for infecting cells via the natural receptor but can be bypassed by an antibody-dependent enhancement pathway. Proc. Natl. Acad. Sci. USA 911932-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Metherall, J. E., K. Waugh, and H. Li. 1996. Progesterone inhibits cholesterol biosynthesis in cultured cells. Accumulation of cholesterol precursors. J. Biol. Chem. 2712627-2633. [DOI] [PubMed] [Google Scholar]
- 51.Miller, L. C., W. Blakemore, D. Sheppard, A. Atakilit, A. M. King, and T. Jackson. 2001. Role of the cytoplasmic domain of the β-subunit of integrin αVβ6 in infection by foot-and-mouth disease virus. J. Virol. 754158-4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monaghan, P., S. Gold, J. Simpson, Z. Zhang, P. H. Weinreb, S. M. Violette, S. Alexandersen, and T. Jackson. 2005. The αVβ6 integrin receptor for foot-and-mouth disease virus is constitutively expressed on the epithelial cells targeted in cattle. J. Gen. Virol. 862769-2780. [DOI] [PubMed] [Google Scholar]
- 53.Murata, M., J. Peranen, R. Schreiner, F. Wieland, T. V. Kurzchalia, and K. Simons. 1995. VIP21/caveolin is a cholesterol-binding protein. Proc. Natl. Acad. Sci. USA 9210339-10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neff, S., D. Sa-Carvalho, E. Rieder, P. W. Mason, S. D. Blystone, E. J. Brown, and B. Baxt. 1998. Foot-and-mouth disease virus virulent for cattle utilizes the integrin αVβ3 as its receptor. J. Virol. 723587-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nichols, B. J. 2002. A distinct class of endosome mediates clathrin-independent endocytosis to the Golgi complex. Nat. Cell Biol. 4374-378. [DOI] [PubMed] [Google Scholar]
- 56.Nichols, B. J., A. K. Kenworthy, R. S. Polishchuk, R. Lodge, T. H. Roberts, K. Hirschberg, R. D. Phair, and J. Lippincott-Schwartz. 2001. Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 153529-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nichols, B. J., and J. Lippincott-Schwartz. 2001. Endocytosis without clathrin coats. Trends Cell Biol. 11406-412. [DOI] [PubMed] [Google Scholar]
- 58.O'Donnell, V., M. LaRocco, H. Duque, and B. Baxt. 2005. Analysis of foot-and-mouth disease virus internalization events in cultured cells. J. Virol. 798506-8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parton, R. G., B. Joggerst, and K. Simons. 1994. Regulated internalization of caveolae. J. Cell Biol. 1271199-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pelkmans, L. 2005. Secrets of caveolae- and lipid raft-mediated endocytosis revealed by mammalian viruses. Biochim. Biophys. Acta 1746295-304. [DOI] [PubMed] [Google Scholar]
- 61.Pelkmans, L., T. Burli, M. Zerial, and A. Helenius. 2004. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell 118767-780. [DOI] [PubMed] [Google Scholar]
- 62.Pelkmans, L., J. Kartenbeck, and A. Helenius. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3473-483. [DOI] [PubMed] [Google Scholar]
- 63.Pietiainen, V., V. Marjomaki, P. Upla, L. Pelkmans, A. Helenius, and T. Hyypia. 2004. Echovirus 1 endocytosis into caveosomes requires lipid rafts, dynamin II, and signaling events. Mol. Biol. Cell 154911-4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rabouille, C., and J. Klumperman. 2005. Opinion: The maturing role of COPI vesicles in intra-Golgi transport. Nat. Rev. Mol. Cell Biol. 6812-817. [DOI] [PubMed] [Google Scholar]
- 65.Reilly, J. F., E. Mizukoshi, and P. A. Maher. 2004. Ligand dependent and independent internalization and nuclear translocation of fibroblast growth factor (FGF) receptor 1. DNA Cell Biol. 23538-548. [DOI] [PubMed] [Google Scholar]
- 66.Richardson, D. R., and P. Ponka. 1997. The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochim. Biophys. Acta 13311-40. [DOI] [PubMed] [Google Scholar]
- 67.Rieder, E., A. Berinstein, B. Baxt, A. Kang, and P. W. Mason. 1996. Propagation of an attenuated virus by design: engineering a novel receptor for a noninfectious foot-and-mouth disease virus. Proc. Natl. Acad. Sci. USA 9310428-10433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rieder, E., T. Henry, H. Duque, and B. Baxt. 2005. Analysis of foot-and-mouth disease virus type A24 isolate containing an SGD receptor recognition site in vitro and its pathogenesis in cattle. J. Virol. 7912989-12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rothberg, K. G., J. E. Heuser, W. C. Donzell, Y. S. Ying, J. R. Glenney, and R. G. Anderson. 1992. Caveolin, a protein component of caveolae membrane coats. Cell 68673-682. [DOI] [PubMed] [Google Scholar]
- 70.Sa-Carvalho, D., E. Rieder, B. Baxt, R. Rodarte, A. Tanuri, and P. W. Mason. 1997. Tissue culture adaptation of foot-and-mouth disease virus selects viruses that bind to heparin and are attenuated in cattle. J. Virol. 715115-5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharma, D. K., A. Choudhury, R. D. Singh, C. L. Wheatley, D. L. Marks, and R. E. Pagano. 2003. Glycosphingolipids internalized via caveolar-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. J. Biol. Chem. 2787564-7572. [DOI] [PubMed] [Google Scholar]
- 72.Sheff, D. R., E. A. Daro, M. Hull, and I. Mellman. 1999. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 145123-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387569-572. [DOI] [PubMed] [Google Scholar]
- 74.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 131-39. [DOI] [PubMed] [Google Scholar]
- 75.Somsel Rodman, J., and A. Wandinger-Ness. 2000. Rab GTPases coordinate endocytosis. J. Cell Sci. 113 Pt. 2183-192. [DOI] [PubMed] [Google Scholar]
- 76.Sonnichsen, B., S. De Renzis, E. Nielsen, J. Rietdorf, and M. Zerial. 2000. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 149901-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stave, J. W., J. L. Card, and D. O. Morgan. 1986. Analysis of foot-and-mouth disease virus type O1 Brugge neutralization epitopes using monoclonal antibodies. J. Gen. Virol. 672083-2092. [DOI] [PubMed] [Google Scholar]
- 78.Stenmark, H., and V. M. Olkkonen. 2001. The Rab GTPase family. Genome Biol. 2REVIEWS3007. http://genomebiology.com/2001/2/5/reviews/3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stuart, A. D., H. E. Eustace, T. A. McKee, and T. D. Brown. 2002. A novel cell entry pathway for a DAF-using human enterovirus is dependent on lipid rafts. J. Virol. 769307-9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tagawa, A., A. Mezzacasa, A. Hayer, A. Longatti, L. Pelkmans, and A. Helenius. 2005. Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters. J. Cell Biol. 170769-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thomsen, P., K. Roepstorff, M. Stahlhut, and B. van Deurs. 2002. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol. Biol. Cell 13238-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Dam, E. M., and W. Stoorvogel. 2002. Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles. Mol. Biol. Cell 13169-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van der Sluijs, P., M. Hull, P. Webster, P. Male, B. Goud, and I. Mellman. 1992. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell 70729-740. [DOI] [PubMed] [Google Scholar]
- 84.Wacker, I., C. Kaether, A. Kromer, A. Migala, W. Almers, and H. H. Gerdes. 1997. Microtubule-dependent transport of secretory vesicles visualized in real time with a GFP-tagged secretory protein. J. Cell Sci. 1101453-1463. [DOI] [PubMed] [Google Scholar]
- 85.Wang, L. H., K. G. Rothberg, and R. G. Anderson. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 1231107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weinacker, A., A. Chen, M. Agrez, R. I. Cone, S. Nishimura, E. Wayner, R. Pytela, and D. Sheppard. 1994. Role of the integrin αVβ6 in cell attachment to fibronectin. Heterologous expression of intact and secreted forms of the receptor. J. Biol. Chem. 2696940-6948. [PubMed] [Google Scholar]
- 87.Wilcke, M., L. Johannes, T. Galli, V. Mayau, B. Goud, and J. Salamero. 2000. Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-golgi network. J. Cell Biol. 1511207-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2107-117. [DOI] [PubMed] [Google Scholar]