Abstract
Two major routes of preprotein targeting into mitochondria are known. Preproteins carrying amino-terminal signals mainly use Tom20, the general import pore (GIP) complex and the Tim23–Tim17 complex. Preproteins with internal signals such as inner membrane carriers use Tom70, the GIP complex, and the special Tim pathway, involving small Tims of the intermembrane space and Tim22–Tim54 of the inner membrane. Little is known about the biogenesis and assembly of the Tim proteins of this carrier pathway. We report that import of the preprotein of Tim22 requires Tom20, although it uses the carrier Tim route. In contrast, the preprotein of Tim54 mainly uses Tom70, yet it follows the Tim23–Tim17 pathway. The positively charged amino-terminal region of Tim54 is required for membrane translocation but not for targeting to Tom70. In addition, we identify two novel homologues of the small Tim proteins and show that targeting of the small Tims follows a third new route where surface receptors are dispensable, yet Tom5 of the GIP complex is crucial. We conclude that the biogenesis of Tim proteins of the carrier pathway cannot be described by either one of the two major import routes, but involves new types of import pathways composed of various features of the hitherto known routes, including crossing over at the level of the GIP.
INTRODUCTION
Many mitochondrial precursor proteins are synthesized with amino-terminal targeting sequences, termed presequences, that direct the proteins to the organelle and across the outer and inner membranes (Ryan and Jensen, 1995; Schatz and Dobberstein, 1996; Neupert, 1997; Pfanner et al., 1997; Ryan and Pfanner, 1998). In the matrix, the presequences are typically cleaved off by the mitochondrial processing peptidase. A number of mitochondrial preproteins are not synthesized with cleavable targeting signals. A few preproteins were shown to contain the targeting information at the amino-terminal portion of the protein that is to carry a “noncleaved presequence” (Hurt et al., 1985; Arakawa et al., 1990; Rospert et al., 1993; Hahne et al., 1994; Jarvis et al., 1995); however, most noncleavable preproteins, notably those that are membrane proteins, seem to contain internal targeting information distributed over various regions of the preprotein (Pfanner et al., 1987a; Smagula and Douglas, 1988; Davis et al., 1998; Káldi et al., 1998). Typical representatives are the members of the large family of inner membrane metabolite carriers, such as the ADP/ATP carrier (AAC).
The mitochondrial machinery for the import of presequence-containing preproteins has been studied in detail. The presequences are typically recognized by the surface receptor Tom20, the 20-kDa subunit of the translocase of the outer membrane (Söllner et al., 1989; Ramage et al., 1993; Brix et al., 1997). Subsequently, the preproteins are transferred to the general import pore (GIP) complex where they interact with Tom22 and Tom5 and are translocated through the import channel formed by Tom40 (Vestweber et al., 1989; Kiebler et al., 1993; Dietmeier et al., 1997; Hill et al., 1998). The presequences bind to Tim23 of the translocase of the inner membrane and in a membrane potential (Δψ)-dependent reaction move through the inner membrane channel, that is, the Tim core complex formed by Tim23 and Tim17 (Dekker et al., 1993, 1997; Emtage and Jensen, 1993; Ryan et al., 1994; Bauer et al., 1996). Matrix-located heat shock protein 70 (mtHsp70) cooperates with Tim44 to drive the completion of preprotein translocation (Kronidou et al., 1994; Rassow et al., 1994; Schneider et al., 1994; Voos et al., 1996; Bömer et al., 1998).
The inner membrane carriers follow a different import route that converges with the presequence pathway only at the level of the GIP complex. The carrier preproteins such as AAC are preferentially recognized by Tom70 before their transfer to the GIP (Hines et al., 1990; Söllner et al., 1990). At the trans side of the outer membrane, the presequence and carrier pathways diverge (Moczko et al., 1997; Kübrich et al., 1998). Recent studies led to the identification of a number of new Tim proteins that mediate the translocation through the intermembrane space and insertion into the inner membrane. Three homologous small Tim proteins of the intermembrane space, Tim9, Tim10, and Tim12, bind the carrier preproteins (Koehler et al., 1998a,b; Sirrenberg et al., 1998) and transfer them to a translocase of the inner membrane that contains Tim22 and Tim54 (Sirrenberg et al., 1996; Kerscher et al., 1997). The carrier proteins contain six membrane-spanning segments each and are inserted into the inner membrane in a Δψ-dependent manner via Tim22–Tim54. Tim23, Tim17, and Tim22 contain a homologous membrane domain with four predicted membrane-spanning segments, and recent evidence indicates that Tim23 and most likely Tim17 and Tim22 are also imported via the carrier Tim pathway (Kerscher et al., 1997; Káldi et al., 1998).
Little is known about the actual biogenesis of the Tim proteins that are involved in the import of carrier preproteins. For this study, we have analyzed targeting and translocation of the precursors of these Tim proteins. We report that the targeting pathways of Tim22 and Tim54 reveal a new principle of combination of different portions of the main (presequence) pathway and the special (carrier) pathway. The crossing over occurs at the level of the GIP complex. Moreover, import of the small Tims provides the first example for a preferential targeting via Tom5 and not via the trypsin-accessible domains of the larger receptors.
MATERIALS AND METHODS
Construction of Plasmids for in Vitro Transcription
The open reading frames of yeast TIM54 (Kerscher et al., 1997), TIM22 (Sirrenberg et al., 1996), TIM13 (Accession No. P53299), TIM12 (Jarosch et al., 1996), TIM10 (Jarosch et al., 1997), TIM9 (Koehler et al., 1998b), and TIM8 (Accession No. Y13136) were amplified by PCR and individually cloned into pGEM-4Z (Promega, Madison, WI). Tim54ΔN was obtained by PCR using a downstream vector primer and a primer containing the SP6 polymerase binding site and an 18-nucleotide stretch encoding residues 39–44 of Tim54 (5′-GGA TTA GGT GAC ACT ATA GAA ATG ATC TTT TGG TCT GTG-3′).
Import of Preproteins Into Isolated Mitochondria
The yeast strains used in this study are shown in Table 1. Mitochondria were isolated from yeast cells grown in YPG media (1% yeast extract, 2% bacto-peptone, and 3% glycerol) according to Daum et al. (1982) and Hartl et al. (1987). Radiolabeled preproteins were obtained by in vitro transcription and translation reactions using rabbit reticulocyte lysate (Amersham, Arlington Heights, IL) in the presence of [35S]methionine/cysteine (Söllner et al., 1991).
Table 1.
Saccharomyces cerevisiae strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| YPH499 | ade2-101 his3-Δ200 leu2-Δ1 ura3-52 trp1-Δ63 lys2-801 | Sikorski and Hieter, 1989 |
| MM208 | ade2-101 his3-Δ200 leu2-Δ1 ura3-52 trp1-Δ63 lys2-801 tom70∷HIS3 | Moczko et al., 1994 |
| MM112-C |
ade2-101 his3-Δ200 leu2-Δ1 ura3-52 trp1-Δ63 lys2-801 tom20∷URA3 + pG1 (TRP1)-TOM22 |
Hönlinger et al., 1995b |
| KD56 | ade2-101 his3-Δ200 leu2-Δ1 ura3-52 trp1-Δ63 lys2-801 tom5∷HIS3 | Dietmeier et al., 1997 |
| MB3 | ade2-101 his3-Δ200 leu2-Δ1lys2-801 ura3∷LYS2 | Maarse et al., 1992 |
| MB3-46 | ade2-101 his3-Δ200 leu2-Δ1lys2-801 ura3∷LYS2 tim23-2 | Dekker et al., 1993 |
| PK82 | his4-713 lys2 ura3-52 Δtrp1 leu2-3,112 | Gambill et al., 1993 |
| PK83 | ade2-101 lys2 ura3-52 Δtrp1 leu2-3,112 ssc1-3(LEU2) | Gambill et al., 1993 |
Mitochondrial in vitro import reactions were performed in BSA-containing buffer (3% [wt/vol] fatty acid-free BSA, 80 mM KCl, 5 mM MgCl2, 10 mM MOPS/KOH, pH 7.2) in the presence of 2 mM ATP and 2 mM NADH. To dissipate the membrane potential, 8 μM antimycin A, 20 μM oligomycin, and 1 μM valinomycin (Sigma, St. Louis, MO) were added to the import reaction. Reticulocyte lysate containing radiolabeled preproteins (2.5–10% [vol/vol] of import reaction) was incubated with mitochondria (25–50 μg protein) at 25°C for varying times. Valinomycin (1 μM) was added to stop import, and samples were subsequently treated with or without proteinase K (50 μg/ml) on ice for 15 min. The protease was inactivated by the addition of 1 mM PMSF, and samples were incubated for a further 10 min at 4°C.
For trypsin treatment of accessible Tom receptor domains, mitochondrial samples in SEM buffer (250 mM sucrose, 1 mM EDTA, 10 mM MOPS/KOH, pH 7.2) were incubated with trypsin (20 μg/ml) for 20 min on ice. Trypsin was inactivated on the addition of a 30-fold excess of soybean trypsin inhibitor (Type II-S, Sigma) and samples were incubated for an additional 10 min on ice before further manipulations. For control samples, a 30-fold excess of soybean trypsin inhibitor was added to mitochondria before trypsin addition. Preproteins were imported for 15 min at 25°C before proteinase K digestion.
For import of preproteins into ssc1–3 mitochondria (Gambill et al., 1993), a 15 min incubation at 37°C was performed with both wild-type and ssc1–3 mitochondria before import studies were performed at 25°C.
Swelling of mitochondrial samples was prepared by resuspending the mitochondrial pellets in EM buffer (1 mM EDTA, 10 mM MOPS/KOH, pH 7.2) and incubating the samples on ice for 15 min. ATP was depleted from mitochondrial samples and reticulocyte lysates according to Glick (1995). Preproteins were imported for 15 min at 25°C before proteinase K digestion.
After treatments, mitochondrial pellets were lysed in the appropriate detergent-containing buffer and applied to SDS or blue native polyacrylamide gels.
Accumulation of b2(167)Δ-dihydrofolate Reductase Across Mitochondrial Membranes
Mitochondria (50 μg) were incubated with 1 μg purified b2(167)Δ-dihydrofolate reductase (DHFR) (Dekker et al., 1997) for 15 min at 25°C in the presence of 2 μM methotrexate. After accumulation, mitochondria were reisolated, washed with SEM buffer containing 2 μM methotrexate, and finally resuspended in BSA-containing buffer containing 2 μM methotrexate before use in further import assays.
Blue Native Gel Electrophoresis
Blue native PAGE was performed essentially as described previously (Schägger and von Jagow, 1991; Schägger et al., 1994; Dekker et al., 1997). Briefly, mitochondrial pellets (25–100 μg protein) were lysed in 50 μl ice-cold digitonin buffer (1% [wt/vol] digitonin, 20 mM Tris-HCl, pH 7.4, 0.1 mM EDTA, 50 mM NaCl, 10% [vol/vol] glycerol, 1 mM PMSF) (Blom et al., 1995). After a clarifying spin, 5 μl of sample buffer (5% [wt/vol] Coomassie brilliant blue G-250, 100 mM Bis-Tris, pH 7.0, 500 mM 6-aminocaproic acid) were added, and the samples were electrophoresed at 4°C through a 6–16% polyacrylamide gradient gel.
For immunoblotting, the native gel was soaked in blot buffer (20 mM Tris-base, 150 mM glycine, 20% [vol/vol] methanol, 0.08% [wt/vol] SDS) before transfer onto PVDF membranes (Millipore, Bedford, MA) using the semidry blotting technique (Harlow and Lane, 1988). Immunodecoration was performed according to standard procedures (Harlow and Lane, 1988), and detection was achieved using the ECL method (Amersham). For detection of radiolabeled proteins, the dried gel or PVDF membrane was exposed to phosphorimage storage cassettes before phosphorimage analysis (Molecular Dynamics, Sunnyvale, CA).
Miscellaneous
Sequence alignments were generated with MegAlign (DNA Star Inc., Madison, WI) using the Clustal method and the PAM250 weight table.
SDS-PAGE of larger proteins (e.g., Tim54 and Tim22) was performed according to Laemmli (1970), and urea SDS-PAGE (Ito et al., 1980) was used for the analysis of the small Tim proteins.
RESULTS
The Preproteins of Tim22 and Tim54 Require Different Surface Receptors for Import
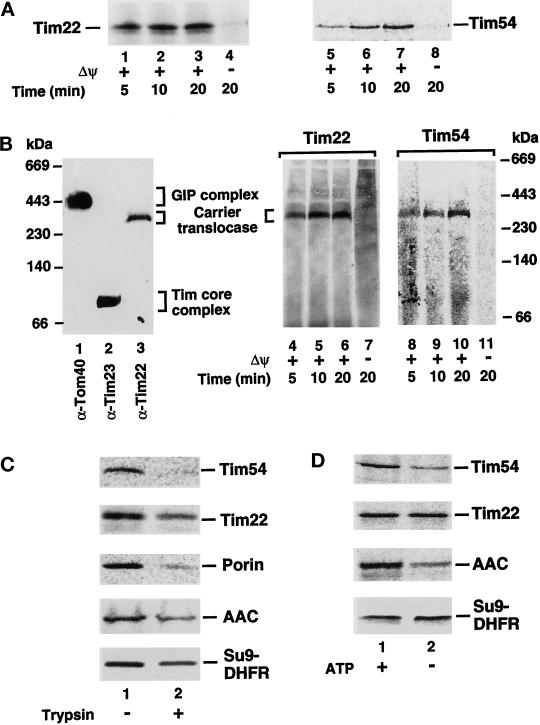
The preproteins of Tim22 and Tim54 were synthesized in vitro in rabbit reticulocyte lysate in the presence of [35S]methionine/cysteine and incubated with isolated yeast wild-type mitochondria. In the presence of a membrane potential across the inner membrane, the preproteins were transported to a protease-protected location (Figure 1A, lanes 1–3 and 5–7). On dissipation of the Δψ, import was blocked (Figure 1A, lanes 4 and 8).
Figure 1.
Biogenesis of Tim22 and Tim54. (A) Import kinetics of Tim22 and Tim54. 35S-labeled Tim22 and Tim54 preproteins were imported into mitochondria for different times in the presence (lanes 1–3, 5–7) or absence (lanes 4 and 8) of a membrane potential (Δψ) as described in MATERIALS AND METHODS. After import, mitochondria were treated with 50 μg/ml proteinase K and subjected to SDS-PAGE. (B) Assembly of Tim22 and Tim54 into the carrier translocase. Mitochondria were solubilized in digitonin-containing buffer and subjected to blue native gel electrophoresis. The gel was blotted, and lanes were immunodecorated with antibodies specific for Tom40 (lane 1), Tim23 (lane 2), or Tim22 (lane 3). For lanes 4–11, Tim22 and Tim54 preproteins were imported into mitochondria as in A, and mitochondria were electrophoresed on blue native PAGE as above. (C) The import of Tim54 and Tim22 requires surface receptors. Mitochondria were pretreated with 20 μg/ml trypsin for 20 min, before inactivation with a 30-fold excess of trypsin inhibitor (lane 2). For control samples (lane 1), trypsin-inhibitor was added to mitochondria before the addition of trypsin. 35S-labeled Tim22 and Tim54 preproteins were subjected to mitochondrial import as well as 35S-labeled porin, AAC, and Su9-DHFR preproteins, which served as controls. Samples were treated with proteinase K before SDS-PAGE analysis. (D) The import of Tim22 and Tim54 show differential requirements for ATP. 35S-labeled Tim22, Tim54, AAC, and Su9-DHFR in reticulocyte lysates were depleted of ATP. Preproteins were imported into mitochondria in the presence (lane 1) or absence of added ATP. In lane 2, mitochondria were additionally blocked in the export of ATP from the matrix according to Glick (1995). Samples were treated with proteinase K before SDS-PAGE analysis. Radiolabeled proteins were detected by storage phosphorimage cassette technology.
To determine whether the proteins were correctly imported and assembled, we used blue native electrophoresis of digitonin-lysed mitochondria, which allows a separation of the mitochondrial translocase complexes (Dekker et al., 1996–1998): the large Tom complex, termed the GIP complex (Figure 1B, lane 1); the Tim core complex containing Tim23 and Tim17 (Figure 1B, lane 2); and a ∼300-kDa complex containing Tim22, termed the carrier translocase (Figure 1B, lane 3). We found that both imported Tim22 and Tim54 were efficiently assembled into the 300-kDa translocase complex in a Δψ-dependent manner (Figure 1B, lanes 4–6 and 8–10).
By a pretreatment of the mitochondria with trypsin, the cytosolic domains of the import receptors Tom20, Tom22, and Tom70 are removed (Alconada et al., 1995; Dietmeier et al., 1997). The import of both Tim22 and Tim54 into trypsin-treated mitochondria was inhibited, indicating a dependence on one or more of these surface receptors (Figure 1C, lane 2). Such pretreatment also inhibited the import of the outer membrane protein porin and a matrix-targeted fusion protein between the presequence of Fo-ATPase subunit 9 and the entire dihydrofolate reductase (Su9-DHFR), which mainly uses Tom20 (Hines et al., 1990; Moczko et al., 1994; Pfanner et al., 1997). The import of AAC, which mainly uses Tom70 as its receptor (Hines et al., 1990; Söllner et al., 1990), was also inhibited (Figure 1C). It has been shown previously that there is a differential dependence on cytosolic ATP and cofactors for the targeting of preproteins to either Tom20 or Tom70 (Hachiya et al., 1995; Komiya et al., 1996, 1997). We asked how the depletion of cytosolic ATP affected the import of Tim22 and Tim54 and found a strong difference between both preproteins. Although the import of Tim54 was inhibited by the ATP depletion, the import of Tim22 was unchanged (Figure 1D, lane 2). As a control, we show that depletion of cytosolic ATP also inhibited the import of the preprotein AAC but not Su9-DHFR (Figure 1D) (Wachter et al., 1994).
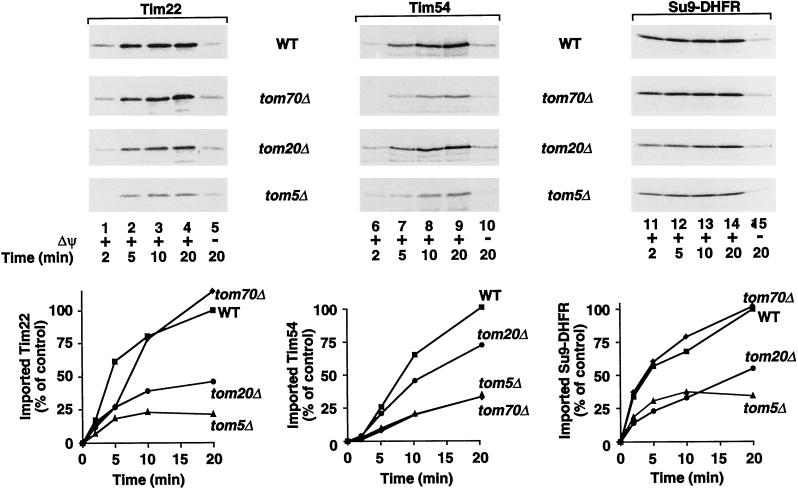
We thus examined the possibility that Tim22 and Tim54 interacted with different receptors by using mitochondria isolated from yeast strains lacking TOM20 or TOM70 genes, respectively. Because a lack of Tom20 causes a reduction in the mitochondrial levels of Tom22 and thus indirectly a reduction of the mitochondrial membrane potential (Lithgow et al., 1994; Gärtner et al., 1995b; Hönlinger et al., 1995b), we used a tom20Δ strain where TOM22 was put on a high-copy number plasmid to restore the mitochondrial levels of Tom22 and the membrane potential (Hönlinger et al., 1995b). Import of the preprotein of Tim22 was strongly inhibited in tom20Δ mitochondria but practically unchanged in tom70Δ mitochondria in comparison to wild-type mitochondria (Figure 2, left panel). In contrast, the import of Tim54 was strongly inhibited in tom70Δ mitochondria, but only mildly affected in tom20Δ mitochondria (Figure 2, middle panel). Furthermore, the presequence-containing preprotein Su9-DHFR displayed import characteristics similar to those of Tim22 in its receptor requirements (Figure 2, right panel). In the absence of a membrane potential across the inner membrane, import of the preproteins was inhibited in all cases (Figure 2, lanes 5, 10, and 15), confirming the specificity of the import processes into the mutant mitochondria.
Figure 2.
Tim22 and Tim54 require different outer membrane receptors for their import into mitochondria. In vitro synthesized 35S-labeled Tim22 (left panel), Tim54 (middle panel), and Su9-DHFR (right panel) were imported into mitochondria isolated from wild-type (WT), tom70Δ, tom20Δ, or tom5Δ yeast cells for the indicated times in the presence (lanes 1–4, 6–9, and 11–14) or absence (lanes 5, 10, and 15) of a membrane potential (Δψ). Samples were treated with proteinase K before SDS-PAGE analysis. Radiolabeled proteins were detected by storage phosphorimage cassette technology and quantitated using ImageQuant software (Molecular Dynamics). The import observed in wild-type mitochondria after 20 min was set at 100% (control).
In addition, we used mitochondria from a yeast strain lacking the small subunit Tom5 of the GIP complex. Tom5 is resistant to a treatment with trypsin and functions after the surface receptors at the entry site of the import pore where the presequence and carrier routes converge (Dietmeier et al., 1997). The import of both Tim22 and Tim54 along with Su9-DHFR were inhibited in tom5Δ mitochondria (Figure 2, bottom panels). We conclude that the preprotein of Tim54 is directed into mitochondria preferentially by Tom70 in an ATP-dependent reaction, whereas the preprotein of Tim22, like the presequence-containing preprotein Su9-DHFR, uses Tom20 as receptor in a manner independent of cytosolic ATP. Both import pathways join at the entry site of the GIP that includes Tom5.
Different Tim Pathways for the Preproteins of Tim22 and Tim54
Koehler et al. (1998a) and Sirrenberg et al. (1998) showed that mutations in Tim components of the carrier pathway, including Tim10, caused a strong reduction in the mitochondrial level of Tim22, suggesting that the preprotein of Tim22 itself was imported via the carrier Tim pathway, as shown previously for the homologous proteins Tim23 and Tim17 (Dekker et al., 1997; Kerscher et al., 1997; Káldi et al., 1998). We used two assays to determine which Tim pathway was used by the preprotein of Tim54 in comparison with the import of Tim22.
Accumulation of Chemical Amounts of a Presequence-Containing Preprotein in Translocation Contact Sites
A matrix-targeted preprotein, consisting of a portion of precytochrome b2 and the entire dihydrofolate reductase [b2(167)Δ-DHFR], can be prepared as a soluble species in large amounts. After stabilization of the DHFR moiety with methotrexate, the preprotein can be accumulated in the mitochondrial import sites, spanning both the Tom machinery and the Tim23–Tim17 machinery (Dekker et al., 1997). Thereby the subsequent import of preproteins using the Tim23–Tim17 pathway is impaired in mitochondria with accumulated b2(167)Δ-DHFR. The import of carrier proteins is only slightly affected because the Tom complexes are approximately four times more abundant than the Tim23–Tim17 complexes, and thus most Tom complexes are not occupied by the b2(167)Δ-DHFR preprotein (Dekker et al., 1997). We accumulated methotrexate-bound b2(167)Δ-DHFR in mitochondria and tested the import kinetics of different preproteins. Although the import of Tim22 was only slightly inhibited (Figure 3A), a significant reduction in import was observed for the preprotein of Tim54 (Figure 3B). For comparison, the import of AAC was only slightly affected (Figure 3C), but the import of the Rieske Fe/S protein, a typical presequence-containing preprotein, was inhibited (Figure 3D). Furthermore, the analysis of inner-membrane insertion and assembly of the imported preproteins by blue native electrophoresis revealed that Tim54 assembly was reduced in mitochondria containing accumulated preprotein (Figure 3E).
Figure 3.
The preproteins of Tim22 and Tim54 use different Tim pathways for their import. Mitochondria were incubated in the presence or absence of b2(167)Δ-DHFR and methotrexate for 15 min at 25°C. Mitochondria were reisolated, washed, and resuspended in import buffer containing methotrexate before the addition of 35S-labeled (A) Tim22, (B) Tim54, (C) the ADP/ATP carrier (AAC), or (D) the Fe/S protein (Fe/Sp). Import proceeded for the indicated times, and mitochondria were subsequently treated with 50 μg/ml proteinase K. Where indicated, the membrane potential (Δψ) was dissipated before the addition of preprotein. Mitochondrial proteins were separated by SDS-PAGE and radiolabeled proteins were detected by storage phosphorimage cassette technology. Processing of the presequence of the Fe/S protein by matrix-located proteases generates intermediate (i) and mature (m) forms as indicated. The amount of imported preprotein was quantitated using ImageQuant software. The import in the absence of b2(167)Δ-DHFR after the longest import time was set to 100% for each preprotein. (E) Tim22, Tim54, and AAC preproteins were imported into mitochondria for 30 min in the presence or absence of accumulated b2(167)Δ-DHFR and subjected to blue native PAGE. Assembled preprotein was quantitated using ImageQuant software where 100% was set to preprotein assembled in the absence of b2(167)Δ-DHFR.
A Point Mutation in TIM23
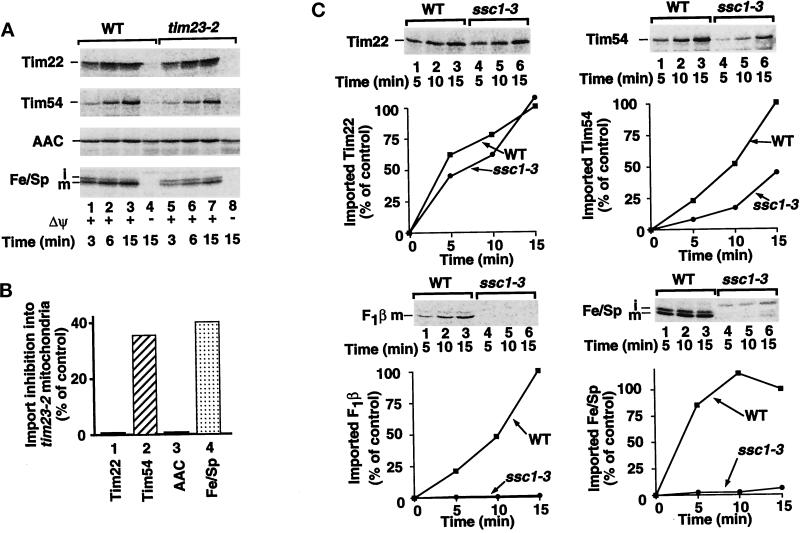
Mitochondria from the yeast mutant tim23–2 carry an amino acid substitution in Tim23 that causes a labilization of the Tim23–Tim17 complex and thus a reduction in preproteins imported via Tim23–Tim17 (Bömer et al., 1997a; Dekker et al., 1997). The import of Tim22 into tim23–2 mitochondria occurred with wild-type rates, like the import of the ADP/ATP carrier (Figure 4A, first and third panels). The import of Tim54 into tim23–2 mitochondria was partly reduced, comparable to the import of the Fe/S protein (Figure 4A, compare second and fourth panels, lanes 5–7 with lanes 1–3; Figure 4B, compare columns 2 and 4 [showing the degree of inhibition]).
Figure 4.
Dependence of Tim54 import on Tim23 and matrix Hsp70. (A) The import of the Fe/S protein and Tim54 is impaired in tim23–2 mitochondria. 35S-labeled preproteins of Tim22, Tim54, the ADP/ATP carrier (AAC), or the Fe/S protein (Fe/Sp) were imported into mitochondria isolated from wild-type (WT) cells or tim23–2 cells in the presence (lanes 1–3, 5–7) or absence (lanes 4 and 8) of a membrane potential (Δψ) for the indicated times. After import, samples were treated with 50 μg/ml proteinase K and subjected to SDS-PAGE. (B) The inhibition of preprotein import in tim23–2 mitochondria observed after 15 min import was quantified in comparison to the import yield into wild-type mitochondria (control). (C) Inactivation of matrix Hsp70 has a partial effect on the import of Tim54 but no effect on Tim22. 35S-labeled preproteins corresponding to Tim22, Tim54, the β-subunit of the F1-ATPase (F1β), or the Fe/S protein (Fe/Sp) were imported into wild-type mitochondria and mitochondria containing a temperature-sensitive mutation in SSC1, encoding the matrix-located Hsp70 (ssc1–3). Processing of F1β generates the mature (m) product as shown. After import, samples were treated with 50 μg/ml proteinase K and subjected to SDS-PAGE. The amount of protein imported into wild-type mitochondria after 15 min was set to 100% (control).
These results demonstrate the independence of the import of Tim22 from the Tim23–Tim17 pathway, yet a dependence of the import of Tim54 on this pathway. Most preproteins using the Tim23–Tim17 pathway are transported into the matrix and thereby depend strictly on the mtHsp70 system (Gambill et al., 1993; Glick et al., 1993; Voos et al., 1993, 1996; Stuart et al., 1994). Some preproteins with hydrophobic sorting signals/membrane anchors branch from this pathway at the level of Tim23 (Bömer et al., 1997a). These preproteins apparently leave the Tim23–Tim17 machinery laterally into the membrane without a strict need for mtHsp70 and are thus not at all, or only partially, inhibited by an inactivation of mtHsp70 (Glick et al., 1993; Voos et al., 1993; Gärtner et al., 1995a,b; Gruhler et al., 1995). To test whether Tim54 belonged to this special class of preproteins, we used ssc1–3 mitochondria that have an inactivated mtHsp70 (Gambill et al., 1993; Voos et al., 1993). Indeed, the import of Tim54 was only partially impaired by the inactivation of mtHsp70 (Figure 4C, top right panel), whereas the import of the matrix-targeted preproteins F1-ATPase subunit β and Fe/S protein was severely inhibited (Figure 4C, bottom panels). The import of Tim22, which is imported via the carrier Tim route, was not affected by the ssc1–3 mutation (Figure 4C, top left panel), as observed with the precursors of Tim17 and Tim23 (Bömer et al., 1997b). These results suggest that Tim54 uses the Tim23–Tim17 pathway for its import, but leaves the matrix route before the Fe/S protein, at about the level where the action of mtHsp70 is needed.
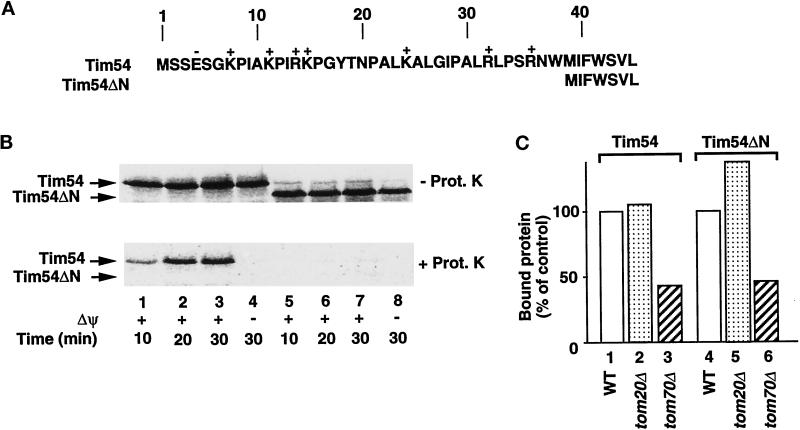
Most preproteins using Tim23–Tim17 that we studied so far contain an amino-terminal targeting signal that is a positively charged presequence. Although Tim54 is not proteolytically processed during import (Kerscher et al., 1997), an inspection of the primary structure revealed that the amino-terminal region of Tim54 contains an abundance of positively charged residues (Figure 5A). We deleted the first 38 residues of Tim54, leading to the construct Tim54ΔN, which is synthesized from the methionine at residue 39 (Figure 5A). The import of Tim54ΔN into a protease-protected location of mitochondria was completely blocked (Figure 5B, bottom panel, lanes 5–7). The binding of the mutant preprotein to mitochondria, however, was still possible (Figure 5B, top panel, lanes 5–8). The binding of preproteins to isolated mitochondria typically includes two fractions: a specific binding to receptors and a nonspecific binding to the organelle surface (Pfanner et al., 1987b; Söllner et al., 1991; Alconada et al., 1995). To test whether Tim54ΔN bound to mitochondria included specific binding to receptors, we compared its association with both wild-type mitochondria and mitochondria lacking Tom20 or Tom70. The binding of Tim54ΔN to mitochondria was reduced by ∼60% tom70Δ mitochondria (Figure 5C, column 6), comparable to the binding of full-length Tim54 (Figure 5C, column 3), whereas for tom20Δ mitochondria, an increase in the binding of Tim54ΔN was observed (Figure 5C, column 2 vs. 5). We conclude that a significant fraction of Tim54ΔN bound to mitochondria specifically interacts via Tom70, indicating that the positively charged amino-terminal segment is dispensable for this receptor interaction. The amino-terminal segment, however, is required for translocation of Tim54 into mitochondria, suggesting that both the amino-terminal portion of Tim54 and a more C-terminal region are involved in the import of this preprotein.
Figure 5.
Different portions of Tim54 are needed for receptor interaction and membrane translocation. (A) Generation of a construct lacking the first 38 amino acids of Tim54 (Tim54ΔN) removes a number of basic residues. Positively and negatively charged residues are indicated. Tim54ΔN initiates from the internal methionine shown at position 39. (B) Tim54ΔN is not imported into mitochondria. 35S-labeled preproteins corresponding to Tim54 (lanes 1–4) and Tim54ΔN (lanes 5–8) were incubated with mitochondria for the indicated times in the presence (lanes 1–3, 5–7) or absence (lanes 4 and 8) of a membrane potential (Δψ). After import, samples were halved and treated with (bottom panel) or without (top panel) proteinase K (Prot. K). (C) Binding of Tim54ΔN to mitochondria is inhibited by a lack of Tom70. 35S-labeled Tim54 and Tim54ΔN preproteins were arrested on the outer membrane by depleting ATP from mitochondria and reticulocyte lysates. Samples were analyzed by SDS-PAGE, and radiolabeled proteins were quantified using ImageQuant software. Binding to isolated mitochondria from wild-type cells (WT; control) was compared with binding to mitochondria lacking Tom20 (tom20Δ) or Tom70 (tom70Δ).
A Role for Tom5 in Targeting of the Small Tims to Mitochondria
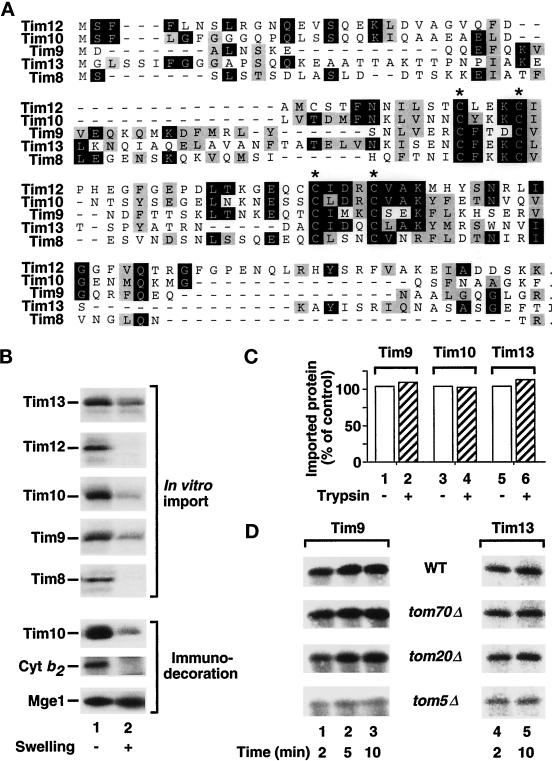
Tim9, Tim10, and Tim12 are the three currently known small Tim proteins that are homologous to each other. A search in the yeast genome revealed the presence of two additional small Tim proteins, termed Tim8 and Tim13, with significant homology to the other three Tims, including a complete conservation of four cysteine residues (Figure 6A). The preproteins of the small Tims were synthesized in reticulocyte lysates and labeled with [35S]methionine/cysteine. Like the known small Tims, Tim8 and Tim13 were transported to a protease-protected location in mitochondria (Figure 6B, lane 1). After swelling of the mitochondria that led to an opening of the intermembrane space with an efficiency of ∼80–90% (Bömer et al., 1997a,b), each small Tim became accessible to protease (Figure 6B, lane 2), demonstrating that Tim8 and Tim13 also were located in the intermembrane space. As control, immunodecoration showed that endogenous Tim10 along with the intermembrane space protein cytochrome b2 were accessible to protease after swelling but not the matrix-located protein Mge1 (Figure 6B, Immunodecoration).
Figure 6.
A family of small Tim proteins with a preference for import via Tom5. (A) Identification of two new small Tim homologues. Sequence alignment of the five small Tim proteins. Amino acid identities are shown in white lettering against black; conserved residues are shown in black lettering against gray. The putative Zn-finger motif cysteines are indicated by an asterisk above. (B) The small Tim proteins are imported into the intermembrane space. 35S-labeled Tim13, Tim12, Tim10, Tim9, and Tim8 preproteins were imported into mitochondria for 10 min at 25°C. After import, mitochondria were isolated and treated with proteinase K (lane 1) or first swollen before proteinase K treatment (lane 2). As controls for swelling and proteinase K treatment, mitochondria were immunodecorated with antibodies against the intermembrane space proteins Tim10 and cytochrome b2 (Cyt b2) and the matrix protein Mge1. (C) Mitochondrial import of the small Tim proteins can occur in the absence of trypsin-accessible receptor domains. Before import of 35S-labeled Tim9, Tim10, and Tim13, mitochondria were pretreated with active (lanes 2, 4, 6) or inactive trypsin (lanes 1, 3, 5; control). All import reactions were treated with proteinase K before SDS-PAGE and quantification. (D) The import of the small Tim proteins preferentially depends on Tom5. 35S-labeled Tim9 and Tim13 were imported into wild-type (WT) mitochondria or mitochondria lacking Tom70 (tom70Δ), Tom20 (tom20Δ), or Tom5 (tom5Δ) for the indicated time points. Samples were treated with proteinase K and analyzed by SDS-PAGE and digital autoradiography.
We then investigated the targeting principles of the small Tims. No obvious mitochondrial targeting signals are observed in any of the small Tim sequences (Figure 6A), and our unpublished results along with those of Koehler et al. (1998b) showed that the small Tim proteins do not require a membrane potential for their insertion into the intermembrane space. Surprisingly, a pretreatment of the mitochondria with trypsin did not inhibit the import of the small Tims, as shown here with the preproteins of Tim9, Tim10, and Tim13 (Figure 6C, columns 2, 4, and 6), indicating that they did not strictly require mitochondrial surface receptors. Indeed, import of the small Tims was not affected by a deletion of TOM70 (Figure 6D, tom70Δ) and was only slightly impaired, in the case of Tim9, by a deletion of TOM20 (Figure 6D, tom20Δ). We wondered whether the small Tim proteins showed a requirement for Tom5 as receptor because Tom5 is resistant to trypsin treatment and can suffice as a receptor under bypass import conditions (Dietmeier et al., 1997). Indeed, the import of the small Tims was strongly inhibited in tom5Δ mitochondria (Figure 6D, tom5Δ). Previous work showed that the tom5Δ mitochondria are selectively deficient in Tom5, whereas the other Tom proteins are present in normal amounts and the GIP complex is not altered (Dietmeier et al., 1997; Dekker et al., 1998). Although the import of preproteins in tom5Δ mitochondria is generally reduced compared with wild-type mitochondria (Dietmeier et al., 1997), the small Tim proteins showed an even stronger reliance on Tom5 for their import compared with Tim22 and Tim54 (Figure 2). We conclude that Tom5, but not the trypsin-accessible surface receptors, plays an important role in the targeting of the small Tims into mitochondria.
DISCUSSION
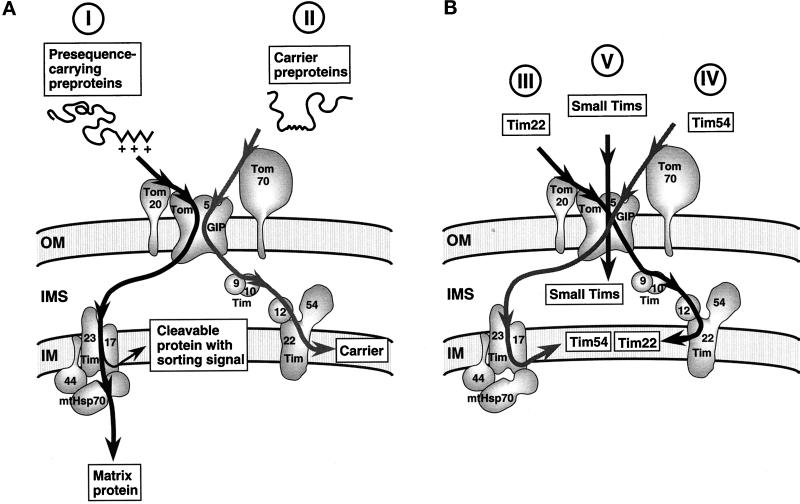
The biogenesis of the Tim proteins of the carrier import route neither follows one of the known major import pathways for mitochondrial preproteins (Figure 7A, routes I and II) nor fits into a common new mechanism. Three different targeting principles seem to be necessary to import the Tim components of the carrier route (Figure 7B).
Figure 7.
Multiple targeting routes of preproteins into mitochondria. (A) Established pathways for presequence-containing preproteins and carrier preproteins. Presequence-containing preproteins (I) follow an import route that preferentially involves binding to Tom20, whereas carrier preproteins (II) preferentially bind to Tom70. The pathways converge at the general import pore (GIP) consisting of, among other components, Tom5 and Tom40. The pathways diverge after translocation across the outer membrane and use different Tim complexes: the Tim23–Tim17 core complex along with mt-Hsp70 and Tim44 for presequence-carrying preproteins and the carrier translocase for carrier preproteins. The requirement of mtHsp70 for the import of preproteins using pathway I, but sorted to the inner membrane, seems to be sequence dependent. (B) Three distinct routes for the biogenesis of Tim proteins of the carrier pathway. Shown are the main import routes that are used by the Tim proteins of the carrier pathway. The import of Tim54 and Tim22 uses different features of the presequence-carrying preprotein and carrier preprotein pathways to reach the same destination. The small Tims seem to preferentially require Tom5, but not the surface receptors, for their import.
The preprotein of Tim22 is the first preprotein found that preferentially uses the receptor Tom20 for targeting to the outer membrane but follows the Tim route for carrier proteins (Figure 7B, route III). Tim22 is a quite hydrophobic protein and therefore would have been a typical candidate for binding to Tom70 like the carrier preproteins, but import signals in Tim22 that are not yet defined direct the preprotein to Tom20, which usually functions as a receptor for presequence-containing preproteins.
In contrast, Tim54 carries an amino-terminal noncleaved translocation sequence that is positively charged like mitochondrial presequences, yet Tim54 preferentially depends on Tom70 for its targeting to the outer membrane (Figure 7B, route IV). After translocation through the GIP, Tim54 then follows the typical route for presequence-containing preproteins until it reaches the Tim23–Tim17 core complex. The amino-terminal positively charged sequence of Tim54 is required for translocation of the preprotein into the mitochondria, whereas interaction with the outer membrane can occur without this sequence. This indicates that the remainder of Tim54, which includes two predicted hydrophobic segments (Kerscher et al., 1997), contains a signal for its interaction with Tom70. Although carrying an abundance of positive residues, the amino-terminal sequence of Tim54 is not predicted to form an amphipathic α-helix because of the presence of several helix-breaking prolyl residues, distinguishing this sequence from typical mitochondrial presequences (Roise et al., 1986; Von Heijne, 1986) and providing an explanation for the only weak dependence on Tom20. Tim54 branches from the main import route at the level of Tim23 before a strict requirement for matrix Hsp70 becomes crucial. It has been observed that a hydrophobic signal anchor following the positively charged amino-terminal region of a preprotein minimizes its requirement for mtHsp70. This is apparently due to the sorting/membrane insertion activity of the hydrophobic segment at an early stage of translocation of an unfolded preprotein (Glick et al., 1993; Voos et al., 1993; Stuart et al., 1994; Gärtner et al., 1995a, 1995b). In agreement with this model, the first predicted hydrophobic segment of Tim54 is located immediately after the positively charged sequence (Kerscher et al., 1997).
In an elegant series of experiments, Mihara and colleagues (Hachiya et al., 1995; Komiya et al., 1996, 1997) demonstrated that cytosolic cofactors from rabbit reticulocyte lysate are important determinants for the selection of import receptors by preproteins. By using purified preproteins and cytosolic chaperones, they showed that preproteins bound to the mitochondrial import stimulation factor are imported via Tom70 in an ATP-dependent reaction, whereas preproteins interacting with cytosolic Hsp70 preferentially use Tom20 in an ATP-independent manner. In agreement with these observations we found that the import of Tim22 via Tom20 did not require cytosolic ATP, whereas the import of Tim54 via Tom70 was ATP-dependent. Because we used complete rabbit reticulocyte lysate, the full set of cytosolic chaperones was present during the import reaction, suggesting that the preproteins of Tim22 and Tim54 were bound to different chaperones before their delivery to the mitochondria. Some preproteins bound to Tom70 seem to be transferred to Tom20 before their insertion into the GIP, i.e., they require both Tom70 and Tom20 for import (Keil and Pfanner, 1993; Keil et al., 1993; Hachiya et al., 1995; Hönlinger et al., 1995a; Komiya et al., 1997), whereas other preproteins can be directly transferred from Tom70 to the GIP complex (Söllner et al., 1990; Steger et al., 1990). The preprotein of Tim54 mainly behaves like the latter preproteins. Tim54 bound to Tom70 does not strictly need to be transferred to Tom20 but can be directly transferred to the GIP complex in the absence of Tom20.
A third and quite short import pathway is followed by the small Tim proteins (Figure 7B, route V). The small Tims are directly translocated into the intermembrane space without an insertion into the inner membrane because their import does not require an inner membrane potential. Other intermembrane space proteins that only use the Tom machinery are the cytochrome c heme lyase and the cytochrome c1 heme lyase. These preproteins strongly require Tom20 for their transfer to the GIP complex (Lill et al., 1992; Steiner et al., 1995). For the import of the small Tims, however, the trypsin-accessible surface domains of import receptors were dispensable, including Tom20, Tom22, and Tom70. Here, the trypsin-resistant Tom5 that typically functions as the link between the receptor and the general import pore (Dietmeier et al., 1997) plays the crucial role. Tom5 seems to represent the first Tom protein that interacts with the majority of preproteins of the small Tims. Besides the import of apocytochrome c (Stuart et al., 1990a,b), the import of the small Tims is therefore one of the simplest mitochondrial membrane translocation mechanisms known to date.
In addition to the various targeting pathways, this study led to two additional pieces of information. 1) Two new homologues of the small Tims, termed Tim13 and Tim8, were identified and found to be located in the intermembrane space. The four cysteines that were suggested to be of functional importance for the small Tims by formation of Zn-finger–like motifs are fully conserved in both Tim8 and Tim13. These small Tims were also identified independently as mitochondrial intermembrane space proteins interacting with Tim9 and Tim10 (Koehler et al., 1999). 2) Blue native electrophoresis provides a simple and efficient method to assess the correct assembly of in vitro imported Tim22 and Tim54 into a ∼300-kDa complex. This complex also contains peripherally associated Tim12 (Koehler et al., 1998a,b; Sirrenberg et al., 1998; our unpublished results) and is termed the carrier translocase.
In summary, we conclude that multiple mechanisms exist for targeting and membrane translocation of mitochondrial preproteins. Depending on the preprotein, distinct pieces of the known major import routes are combined to yield novel pathways. This includes crossing over of pathways at the level of the GIP complex of the outer membrane. For special preproteins like the small Tims, Tom5, which typically functions at the second or third stage of import, can become the first level import component and may thus have receptor-like functions.
ACKNOWLEDGMENTS
We thank Drs. Elizabeth Craig, Bernard Guiard, Michiel Meijer, and Falk Martin for yeast strains and preproteins, and Dr. Wolfgang Voos for helpful discussion. We are grateful to Hanne Müller for expert technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 388 Freiburg, the Fonds der Chemischen Industrie, and a long-term fellowship from the Alexander-von-Humboldt Stiftung to M.T.R.
REFERENCES
- Alconada A, Gärtner F, Hönlinger A, Kübrich M, Pfanner N. Mitochondrial receptor complex from Neurospora crassa and Saccharomyces cerevisiae. Methods Enzymol. 1995;260:263–286. doi: 10.1016/0076-6879(95)60144-9. [DOI] [PubMed] [Google Scholar]
- Arakawa H, Amaya YZ, Mori M. The NH2-terminal 14–16 amino acids of mitochondrial and bacterial thiolases can direct mature ornithine carbamoyltransferases into mitochondria. J Biochem. 1990;107:160–164. doi: 10.1093/oxfordjournals.jbchem.a123001. [DOI] [PubMed] [Google Scholar]
- Bauer MF, Sirrenberg C, Neupert W, Brunner M. Role of Tim23 as voltage sensor and presequence receptor in protein import into mitochondria. Cell. 1996;87:33–41. doi: 10.1016/s0092-8674(00)81320-3. [DOI] [PubMed] [Google Scholar]
- Blom J, Dekker PJT, Meijer M. Functional and physical interactions of components of the yeast mitochondrial inner-membrane import machinery (MIM) Eur J Biochem. 1995;232:309–314. doi: 10.1111/j.1432-1033.1995.tb20813.x. [DOI] [PubMed] [Google Scholar]
- Bömer U, Maarse AC, Martin F, Geissler A, Merlin A, Schönfisch B, Meijer M, Pfanner N, Rassow J. Separation of structural and dynamic functions of the mitochondrial translocase: Tim44 is crucial for the inner membrane import sites in translocation of tightly folded domains, but not of loosely folded preproteins. EMBO J. 1998;17:4226–4237. doi: 10.1093/emboj/17.15.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bömer U, Meijer M, Guiard B, Dietmeier K, Pfanner N, Rassow J. The sorting route of cytochrome b2 branches from the general mitochondrial import pathway at the preprotein translocase of the inner membrane. J Biol Chem. 1997a;272:30439–30446. doi: 10.1074/jbc.272.48.30439. [DOI] [PubMed] [Google Scholar]
- Bömer U, Meijer M, Maarse AC, Dekker PJT, Pfanner N, Rassow J. Multiple interactions of components mediating preprotein translocation across the inner mitochondrial membrane. EMBO J. 1997b;16:2205–2216. doi: 10.1093/emboj/16.9.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brix J, Dietmeier K, Pfanner N. Differential recognition of preproteins by the purified cytosolic domains of the mitochondrial import receptors Tom20, Tom22, and Tom70. J Biol Chem. 1997;272:20730–20735. doi: 10.1074/jbc.272.33.20730. [DOI] [PubMed] [Google Scholar]
- Daum G, Böhni PC, Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982;257:13028–13033. [PubMed] [Google Scholar]
- Davis AJ, Ryan KR, Jensen RE. Tim23p contains separate and distinct signals for targeting to mitochondria and insertion into the inner membrane. Mol Biol Cell. 1998;9:2577–2593. doi: 10.1091/mbc.9.9.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker PJT, Keil P, Rassow J, Maarse AC, Pfanner N, Meijer M. Identification of MIM23, a putative component of the protein import machinery of the mitochondrial inner membrane. FEBS Lett. 1993;330:66–70. doi: 10.1016/0014-5793(93)80921-g. [DOI] [PubMed] [Google Scholar]
- Dekker PJT, Martin F, Maarse AC, Bömer U, Müller H, Guiard B, Meijer M, Rassow J, Pfanner N. The Tim core complex defines the number of mitochondrial translocation contact sites and can hold arrested preproteins in the absence of matrix Hsp70-Tim44. EMBO J. 1997;16:5408–5419. doi: 10.1093/emboj/16.17.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker PJT, Müller H, Rassow J, Pfanner N. Characterization of the preprotein translocase of the outer mitochondrial membrane by blue native electrophoresis. Biol Chem. 1996;377:535–538. [PubMed] [Google Scholar]
- Dekker PJT, Ryan MT, Brix J, Müller H, Hönlinger A, Pfanner N. The preprotein translocase of the outer mitochondrial membrane: molecular dissection and assembly of the general import pore complex. Mol Cell Biol. 1998;18:6515–6524. doi: 10.1128/mcb.18.11.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietmeier K, Hönlinger A, Bömer U, Dekker PJT, Eckerskorn C, Lottspeich F, Kübrich M, Pfanner N. Tom5 functionally links mitochondrial preprotein receptors to the general import pore. Nature. 1997;388:195–200. doi: 10.1038/40663. [DOI] [PubMed] [Google Scholar]
- Emtage JLT, Jensen RE. MAS6 encodes an essential inner membrane component of the yeast mitochondrial protein import pathway. J Cell Biol. 1993;122:1003–1012. doi: 10.1083/jcb.122.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambill D, Voos W, Kang PJ, Miao B, Langer T, Craig EA, Pfanner N. A dual role for mitochondrial heat shock protein 70 in membrane translocation of preproteins. J Cell Biol. 1993;123:109–117. doi: 10.1083/jcb.123.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gärtner F, Bömer U, Guiard B, Pfanner N. The sorting signal of cytochrome b2 promotes early divergence from the general mitochondrial import pathway and restricts the unfoldase activity of matrix Hsp70. EMBO J. 1995a;14:6043–6057. doi: 10.1002/j.1460-2075.1995.tb00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gärtner F, Voos W, Querol A, Miller B, Craig EA, Cumsky MG, Pfanner N. Mitochondrial import of subunit Va of cytochrome c oxidase characterized with yeast mutants: independence from receptors, but requirement for matrix hsp70 translocase function. J Biol Chem. 1995b;270:3788–3795. doi: 10.1074/jbc.270.8.3788. [DOI] [PubMed] [Google Scholar]
- Glick BS. Pathways and energetics of mitochondrial import in Saccharomyces cerevisiae. Methods Enzymol. 1995;260:224–231. doi: 10.1016/0076-6879(95)60140-6. [DOI] [PubMed] [Google Scholar]
- Glick BS, Wachter C, Reid GA, Schatz G. Import of cytochrome b2 to the mitochondrial intermembrane space: the tightly folded heme-binding domain makes import dependent upon matrix ATP. Protein Sci. 1993;2:1901–1917. doi: 10.1002/pro.5560021112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruhler A, Ono H, Guiard B, Neupert W, Stuart RA. A novel intermediate on the import pathway of cytochrome b2 into mitochondria: evidence for conservative sorting. EMBO J. 1995;3:1349–1359. doi: 10.1002/j.1460-2075.1995.tb07121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachiya N, Mihara K, Suda K, Horst M, Schatz G, Lithgow T. Reconstitution of the initial steps of mitochondrial protein import. Nature. 1995;376:705–709. doi: 10.1038/376705a0. [DOI] [PubMed] [Google Scholar]
- Hahne K, Haucke V, Ramage L, Schatz G. Incomplete arrest in the outer membrane sorts NADH-cytochrome b5 reductase to two different submitochondrial compartments. Cell. 1994;79:829–839. doi: 10.1016/0092-8674(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: a laboratory manual. Plainview, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- Hartl FU, Ostermann J, Guiard B, Neupert W. Successive translocation into and out of the mitochondrial matrix: targeting of proteins to the intermembrane space by a bipartite signal peptide. Cell. 1987;51:1027–1037. doi: 10.1016/0092-8674(87)90589-7. [DOI] [PubMed] [Google Scholar]
- Hill K, Model K, Ryan MT, Dietmeier K, Martin F, Wagner R, Pfanner N. Tom40 forms the hydrophilic channel of the mitochondrial import pore for preproteins. Nature. 1998;395:516–521. doi: 10.1038/26780. [DOI] [PubMed] [Google Scholar]
- Hines V, Brandt A, Griffiths G, Horstmann H, Brutsch H, Schatz G. Protein import into yeast mitochondria is accelerated by the outer membrane protein MAS 70. EMBO J. 1990;9:3191–3200. doi: 10.1002/j.1460-2075.1990.tb07517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hönlinger A, Keil P, Nelson RJ, Craig EA, Pfanner N. Posttranslational mitochondrial protein import in a homologous yeast in vitro system. Biol Chem. 1995a;376:515–519. [PubMed] [Google Scholar]
- Hönlinger A, et al. The mitochondrial receptor complex: Mom22 is essential for cell viability and directly interacts with preproteins. Mol Cell Biol. 1995b;15:3382–3389. doi: 10.1128/mcb.15.6.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt EC, Müller U, Schatz G. The first twelve amino acids of a yeast mitochondrial outer membrane protein can direct a nuclear-encoded cytochrome oxidase subunit to the mitochondrial inner membrane. EMBO J. 1985;4:3509–3518. doi: 10.1002/j.1460-2075.1985.tb04110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Date T, Wickner W. Synthesis, assembly into the cytoplasmic membrane, and proteolytic processing of the precursor of coliphage M13 coat protein. J Biol Chem. 1980;255:2123–2130. [PubMed] [Google Scholar]
- Jarosch E, Rödel G, Schweyen RJ. A soluble 12-kDa protein of the mitochondrial intermembrane space affects protein import into yeast mitochondria. Mol Gen Genet. 1997;255:157–165. doi: 10.1007/s004380050484. [DOI] [PubMed] [Google Scholar]
- Jarosch E, Tuller G, Daum G, Waldherr M, Voskova A, Schweyen RJ. Mrs5p, an essential protein of the mitochondrial intermembrane space, affects protein import into yeast mitochondria. J Biol Chem. 1996;271:17219–17225. doi: 10.1074/jbc.271.29.17219. [DOI] [PubMed] [Google Scholar]
- Jarvis JA, Ryan MT, Hoogenraad NJ, Craik DJ, Hoj PB. Solution structure of the acetylated and noncleavable mitochondrial targeting signal of rat chaperonin 10. J Biol Chem. 1995;270:1323–1331. doi: 10.1074/jbc.270.3.1323. [DOI] [PubMed] [Google Scholar]
- Káldi K, Bauer MF, Sirrenberg C, Neupert W, Brunner M. Biogenesis of Tim23 and Tim17, integral components of the TIM machinery for matrix-targeted preproteins. EMBO J. 1998;17:1569–1576. doi: 10.1093/emboj/17.6.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil P, Pfanner N. Insertion of MOM22 into the mitochondrial outer membrane strictly depends on surface receptors. FEBS Lett. 1993;321:197–200. doi: 10.1016/0014-5793(93)80107-6. [DOI] [PubMed] [Google Scholar]
- Keil P, Weinzierl A, Kiebler M, Dietmeier K, Söllner T, Pfanner N. Biogenesis of the mitochondrial receptor complex: two receptors are required for binding of MOM38 to the outer membrane surface. J Biol Chem. 1993;268:19177–19180. [PubMed] [Google Scholar]
- Kerscher O, Holder J, Srinivasan M, Leung SS, Jensen RE. The Tim54p-Tim22p complex mediates insertion of proteins into the mitochondrial inner membrane. J Cell Biol. 1997;7:1663–1675. doi: 10.1083/jcb.139.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebler M, Keil P, Schneider H, van der Klei IJ, Pfanner N, Neupert W. The mitochondrial receptor complex: a central role of MOM22 in mediating preprotein transfer from receptors to the general insertion pore. Cell. 1993;74:483–492. doi: 10.1016/0092-8674(93)80050-o. [DOI] [PubMed] [Google Scholar]
- Koehler CM, Jarosch E, Tokatlidis K, Schmid K, Schweyen RJ, Schatz G. Import of mitochondrial carriers mediated by essential proteins of the intermembrane space. Science. 1998a;279:369–373. doi: 10.1126/science.279.5349.369. [DOI] [PubMed] [Google Scholar]
- Koehler CM, Leuenberger D, Merchant S, Renold A, Junne T, Schatz G. Human deafness dystonia syndrome is a mitochondrial disease. Proc Natl Acad Sci USA. 1999;96:2141–2146. doi: 10.1073/pnas.96.5.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler CM, Merchant S, Oppliger W, Schmid K, Jarosch E, Dolfini L, Junne T, Schatz G, Tokatlidis K. Tim9p, an essential partner subunit of Tim10p for the import of mitochondrial carrier proteins. EMBO J. 1998b;17:6477–6486. doi: 10.1093/emboj/17.22.6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya T, Rospert S, Schatz G, Mihara K. Binding of mitochondrial precursor proteins to the cytoplasmic domains of the import receptors Tom70 and Tom20 is determined by cytoplasmic chaperones. EMBO J. 1997;16:4267–4275. doi: 10.1093/emboj/16.14.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya T, Sakaguchi M, Mihara K. Cytoplasmic chaperones determine the targeting pathway of precursor proteins to mitochondria. EMBO J. 1996;15:399–407. [PMC free article] [PubMed] [Google Scholar]
- Kronidou NG, Oppliger W, Bolliger L, Hannavy K, Glick BS, Schatz G, Horst M. Dynamic interaction between Isp45 and mitochondrial hsp70 in the protein import system of the yeast mitochondrial inner membrane. Proc Natl Acad Sci USA. 1994;91:12818–12822. doi: 10.1073/pnas.91.26.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kübrich M, Rassow J, Voos W, Pfanner N, Hönlinger A. The import route of ADP/ATP carrier into mitochondria separates from the general import pathway of cleavable preproteins at the trans side of the outer membrane. J Biol Chem. 1998;273:16374–16381. doi: 10.1074/jbc.273.26.16374. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lill R, Stuart RA, Drygas ME, Nargang FE, Neupert W. Import of cytochrome c heme lyase into mitochondria: a novel pathway into the intermembrane space. EMBO J. 1992;11:449–456. doi: 10.1002/j.1460-2075.1992.tb05074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithgow T, Junne T, Suda K, Gratzer S, Schatz G. The mitochondrial outer membrane protein Mas22p is essential for protein import and viability of yeast. Proc Natl Acad Sci USA. 1994;91:11973–11977. doi: 10.1073/pnas.91.25.11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maarse AC, Blom J, Grivell LA, Meijer M. MPI1, an essential gene encoding a mitochondrial membrane protein, is possibly involved in protein import into yeast mitochondria. EMBO J. 1992;11:3619–3628. doi: 10.1002/j.1460-2075.1992.tb05446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczko M, Bömer U, Kübrich M, Zufall N, Hönlinger A, Pfanner N. The intermembrane space domain of mitochondrial Tom22 functions as a trans binding site for preproteins with N-terminal targeting sequences. Mol Cell Biol. 1997;17:6574–6584. doi: 10.1128/mcb.17.11.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczko M, Ehmann B, Gärtner F, Hönlinger A, Schäfer E, Pfanner N. Deletion of the receptor MOM19 strongly impairs import of cleavable preproteins into Sacharomyces cerevisiae mitochondria. J Biol Chem. 1994;269:9045–9051. [PubMed] [Google Scholar]
- Neupert W. Protein import into mitochondria. Annu Rev Biochem. 1997;66:863–917. doi: 10.1146/annurev.biochem.66.1.863. [DOI] [PubMed] [Google Scholar]
- Pfanner N, Craig EA, Hönlinger A. Mitochondrial preprotein translocase. Annu Rev Cell Dev Biol. 1997;13:25–51. doi: 10.1146/annurev.cellbio.13.1.25. [DOI] [PubMed] [Google Scholar]
- Pfanner N, Hoeben P, Tropschug M, Neupert W. The carboxy-terminal two-thirds of the ADP/ATP carrier polypeptide contains sufficient information to direct translocation into mitochondria. J Biol Chem. 1987a;262:14851–14854. [PubMed] [Google Scholar]
- Pfanner N, Müller HK, Harmey MA, Neupert W. Mitochondrial protein import: involvement of the mature part of a cleavable precursor protein in the binding to receptor sites. EMBO J. 1987b;6:3449–3454. doi: 10.1002/j.1460-2075.1987.tb02668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage L, Junne T, Hahne K, Lithgow T, Schatz G. Functional cooperation of mitochondrial protein import receptors in yeast. EMBO J. 1993;12:4115–4123. doi: 10.1002/j.1460-2075.1993.tb06095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassow J, Maarse AC, Krainer E, Kübrich M, Müller H, Meijer M, Craig EA, Pfanner N. Mitochondrial protein import: biochemical and genetic evidence for interaction of matrix hsp70 and the inner membrane protein MIM44. J Cell Biol. 1994;127:1547–1556. doi: 10.1083/jcb.127.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roise D, Horvath SJ, Tomich TM, Richards JH, Schatz G. A chemically synthesized presequence of an imported mitochondrial protein can form an amphiphilic helix and perturb natural and artificial phospholipid bilayers. EMBO J. 1986;5:1327–1334. doi: 10.1002/j.1460-2075.1986.tb04363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rospert S, Junne T, Glick BS, Schatz G. Cloning and disruption of the gene encoding yeast mitochondrial chaperonin 10, the homolog of E. coli groES. FEBS Lett. 1993;335:358–360. doi: 10.1016/0014-5793(93)80419-u. [DOI] [PubMed] [Google Scholar]
- Ryan KR, Jensen RE. Protein translocation across mitochondrial membranes: what a long, strange trip it is. Cell. 1995;83:517–519. doi: 10.1016/0092-8674(95)90089-6. [DOI] [PubMed] [Google Scholar]
- Ryan KR, Menold MM, Garret S, Jensen RE. SMS1, a high copy suppressor of the yeast mas6 mutant, encodes an essential inner membrane protein required for mitochondrial protein import. Mol Biol Cell. 1994;5:529–538. doi: 10.1091/mbc.5.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MT, Pfanner N. The preprotein translocase of the mitochondrial outer membrane. Biol Chem. 1998;379:289–294. [PubMed] [Google Scholar]
- Schägger H, Cramer WA, von Jagow G. Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal Biochem. 1994;217:220–230. doi: 10.1006/abio.1994.1112. [DOI] [PubMed] [Google Scholar]
- Schägger H, von Jagow G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- Schatz G, Dobberstein B. Common principles of protein translocation across membranes. Science. 1996;271:1519–1526. doi: 10.1126/science.271.5255.1519. [DOI] [PubMed] [Google Scholar]
- Schneider H-C, Berthold J, Bauer MF, Dietmeier K, Guiard B, Brunner M, Neupert W. Mitochondrial Hsp70/MIM44 complex facilitates protein import. Nature. 1994;371:768–774. doi: 10.1038/371768a0. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirrenberg C, Bauer MF, Guiard B, Neupert W, Brunner M. Import of carrier proteins into the mitochondrial inner membrane mediated by Tim22. Nature. 1996;384:582–585. doi: 10.1038/384582a0. [DOI] [PubMed] [Google Scholar]
- Sirrenberg C, Endres M, Fölsch H, Stuart RA, Neupert W, Brunner M. Carrier protein import into mitochondria mediated by the intermembrane proteins Tim10/Mrs11 and Tim12/Mrs5. Nature. 1998;391:912–915. doi: 10.1038/36136. [DOI] [PubMed] [Google Scholar]
- Smagula C, Douglas MG. Mitochondrial import of the ADP/ATP carrier protein in Saccharomyces cerevisiae. Sequences required for receptor binding and membrane translocation. J Biol Chem. 1988;263:6783–6790. [PubMed] [Google Scholar]
- Söllner T, Griffiths G, Pfaller R, Pfanner N, Neupert W. MOM19, an import receptor for mitochondrial precursor proteins. Cell. 1989;59:1061–1070. doi: 10.1016/0092-8674(89)90762-9. [DOI] [PubMed] [Google Scholar]
- Söllner T, Pfaller R, Griffiths G, Pfanner N, Neupert W. A mitochondrial import receptor for the ADP/ATP carrier. Cell. 1990;62:107–115. doi: 10.1016/0092-8674(90)90244-9. [DOI] [PubMed] [Google Scholar]
- Söllner T, Rassow J, Pfanner N. Analysis of mitochondrial protein import using translocation intermediates and specific antibodies. Methods Cell Biol. 1991;3:345–358. doi: 10.1016/s0091-679x(08)61689-1. [DOI] [PubMed] [Google Scholar]
- Steger HF, Söllner T, Kiebler M, Dietmeier K, Pfaller R, Trülzsch KS, Tropschug M, Neupert W, Pfanner N. Import of ADP/ATP carrier into mitochondria: two receptors act in parallel. J Cell Biol. 1990;111:2353–2363. doi: 10.1083/jcb.111.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Zollner A, Haid A, Neupert W, Lill R. Biogenesis of mitochondrial heme lyases in yeast. Import and folding in the intermembrane space. J Biol Chem. 1995;270:22842–22849. doi: 10.1074/jbc.270.39.22842. [DOI] [PubMed] [Google Scholar]
- Stuart RA, Gruhler A, van der Klei I, Guiard B, Koll H, Neupert W. The requirement of matrix ATP for the import of precursor proteins into the mitochondrial matrix and intermembrane space. Eur J Biochem. 1994;220:9–18. doi: 10.1111/j.1432-1033.1994.tb18593.x. [DOI] [PubMed] [Google Scholar]
- Stuart RA, Nicholson DW, Neupert W. Early steps in mitochondrial protein import: receptor functions can be substituted by the membrane insertion activity of apocytochrome c. Cell. 1990a;60:31–43. doi: 10.1016/0092-8674(90)90713-o. [DOI] [PubMed] [Google Scholar]
- Stuart RA, Nicholson DW, Wienhues U, Neupert W. Import of apocytochrome c into the mitochondrial intermembrane space along a cytochrome c1 sorting pathway. J Biol Chem. 1990b;265:20210–20219. [PubMed] [Google Scholar]
- Vestweber D, Brunner J, Baker A, Schatz G. A 42K outer-membrane protein is a component of the yeast mitochondrial protein import site. Nature. 1989;341:205–209. doi: 10.1038/341205a0. [DOI] [PubMed] [Google Scholar]
- Von Heijne G. Mitochondrial targeting signals form amphiphilic helices. EMBO J. 1986;5:1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voos W, Gambill D, Guiard B, Pfanner N, Craig EA. Presequence and mature part of preproteins strongly influence the dependence of mitochondrial protein import in heat shock protein 70 in the matrix. J Cell Biol. 1993;123:119–126. doi: 10.1083/jcb.123.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voos W, von Ahsen O, Müller H, Guiard B, Rassow J, Pfanner N. Differential requirement for the mitochondrial Hsp70-Tim44 complex in unfolding and translocation of preproteins. EMBO J. 1996;15:2668–2677. [PMC free article] [PubMed] [Google Scholar]
- Wachter C, Schatz G, Glick BS. Protein import into mitochondria: the requirement for external ATP is precursor-specific whereas intramitochondrial ATP is universally needed for translocation into the matrix. Mol Biol Cell. 1994;5:465–474. doi: 10.1091/mbc.5.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]