Abstract
Eastern equine encephalitis virus (EEEV) produces the most severe human arboviral disease in North America (NA) and is a potential biological weapon. However, genetically and antigenically distinct strains from South America (SA) have seldom been associated with human disease or mortality despite serological evidence of infection. Because mice and other small rodents do not respond differently to the NA versus SA viruses like humans, we tested common marmosets (Callithrix jacchus) by using intranasal infection and monitoring for weight loss, fever, anorexia, depression, and neurologic signs. The NA EEEV-infected animals either died or were euthanized on day 4 or 5 after infection due to anorexia and neurologic signs, but the SA EEEV-infected animals remained healthy and survived. The SA EEEV-infected animals developed peak viremia titers of 2.8 to 3.1 log10 PFU/ml on day 2 or 4 after infection, but there was no detectable viremia in the NA EEEV-infected animals. In contrast, virus was detected in the brain, liver, and muscle of the NA EEEV-infected animals at the time of euthanasia or death. Similar to the brain lesions described for human EEE, the NA EEEV-infected animals developed meningoencephalitis in the cerebral cortex with some perivascular hemorrhages. The findings of this study identify the common marmoset as a useful model of human EEE for testing antiviral drugs and vaccine candidates and highlight their potential for corroborating epidemiological evidence that some, if not all, SA EEEV strains are attenuated for humans.
Eastern equine encephalitis virus (EEEV) is a single-stranded, positive-sense, mosquito-borne RNA virus in the genus Alphavirus (family Togaviridae) that can cause severe encephalitis in humans and horses. EEEV is considered the most deadly of the mosquito-borne alphaviruses due to the high mortality rate associated with apparent infections, reaching as high as 90% in horses. In humans, the estimated case fatality rate approaches 80% and many survivors exhibit crippling sequelae such as mental retardation, convulsions, and paralysis. An increase in the number of equine cases in recent years has raised public health concerns that reflect the continuing importance of EEEV as an emerging arboviral threat. In addition, EEEV is a category B priority agent of the National Institute of Allergy and Infectious Diseases due to its virulence and potential for use as a biological weapon and the lack of a licensed vaccine or effective antiviral drug for human infections.
Previous studies recognized four antigenic subtypes of EEEV. One comprises strains from North America (NA), and the remaining three are found in Central America and South America (SA) (7). These subtypes exhibit important differences in their transmission cycles and virulence. In general, EEEV strains from SA appear to be less virulent for humans than NA strains (29). The former can occasionally cause disease and death in horses, but human infections are rarely recognized and seldom result in neurologic disease (2). In contrast, human infections with NA strains can result in severe disease with neurologic complications. The cause of the apparent difference in human virulence remains unknown; however, a recent study suggests that it may be associated with viral sensitivity to interferons (1).
In experimentally infected laboratory mice, EEEV produces neurologic disease that resembles human and equine infections (27); however, both NA and SA strains of EEEV are highly virulent in mice, causing mortality rates of 70 to 90% following subcutaneous inoculation (1). An exception is SA strain BeAr436087, isolated from a mosquito pool in Brazil, which is a naturally attenuated strain in mice and horses and has been useful for the recent development of an effective live-attenuated vaccine for EEE (28).
Experimental pathogenesis studies of EEEV in primates are few. Nathanson et al. (21) studied intracerebral infection of rhesus macaques with a NA strain and identified a similar distribution of lesions in the central nervous system (CNS) compared to human cases of EEE. The distribution of lesions in the CNS of EEEV-infected macaques was also distinct compared to macaques infected with two virulent flaviviruses, Langat and Japanese encephalitis viruses. More recently, Reed et al. (26) infected cynomolgus macaques via the aerosol route with a NA EEEV strain and reported neurological signs consistent with human cases.
Development of alternative nonhuman primate models to study potential bioterrorism agents and emerging pathogens is warranted due to the current shortage of traditional nonhuman primates (24). Common marmosets (Callithrix jacchus) are small, nonendangered New World primates that have been used extensively in biomedical research and provide an attractive alternative to traditional nonhuman primate species. Compared to larger Old World primates, appreciable advantages of their use in research include small size (320 to 450 g), availability, low purchase price and cost of housing, and ease of breeding in captivity (20). In the present study, common marmosets were evaluated as a disease model for human EEE with both NA and SA strains of EEEV.
MATERIALS AND METHODS
Animals.
Six healthy, adult common marmosets (C. jacchus), 3 to 7 years old and ranging in weight from 364 to 404 g, were obtained from the Southwest National Primate Research Center at the Southwest Foundation for Biomedical Research (SFBR) in San Antonio, TX. The marmosets were used in previous studies of the flavivirus GB virus B that occurred more than 2 years prior to the present study. Liver biopsies were performed prior to EEEV infections to confirm the absence of hepatitis. One week prior to the start of the study, animals were transferred to the biosafety level 4 facilities at SFBR and housed individually in caging specifically developed for marmosets. All experimental animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee and the Institutional Biohazards Committee of the SFBR.
Viruses.
NA strain FL93-939 and SA strain BeAr436087 were provided by the University of Texas Medical Branch World Reference Center for Emerging Viruses and Arboviruses. Strain FL93-939 was isolated from a 1993 Florida pool of Culiseta melanura mosquitoes, and strain BeAr436087 was isolated in 1985 from a mosquito pool collected in Fortaleza, Brazil. To minimize cell culture passages that can attenuate alphaviruses (8, 17), infectious cDNA clones were developed for each virus by reverse transcription-PCR amplification and cloning as described previously (3). A Vero cell plaque assay was used to determine titers of virus stocks, which were shown previously to be indistinguishable from the parent viruses for replication in cell cultures and mice (3).
Virus inoculations and postexposure monitoring.
Three animals received intranasal (i.n.) inoculations of 1 × 106 PFU of strain FL93-393 or BeAr436087 in a 0.1-ml volume. After infection, marmosets were monitored daily for anorexia, depression, and abnormal neurologic signs. On days 0, 1, 2, 4, 7, 9, and 11 after infection, marmosets were sedated, body weights and temperatures were recorded, and blood samples were collected for hematology, biochemical analyses, virus titer determination, and antibody titer measurement. When the marmosets were euthanized, either at day 16 after infection when the experiment was completed or when moribund, tissues were collected for virus titer determination, histopathological analysis, and immunohistochemistry.
Virologic and clinical laboratory determinations.
Virus titers were determined in blood and homogenized tissues by a conventional plaque assay as described previously (25). In accordance with the manufacturer's instructions, complete blood cell counts were performed on the collected blood samples with a VetScan HMT machine (Abaxis, Inc., Union City, CA) and biochemical analyses were performed on the plasma samples with a mammalian liver enzyme profile rotor on a VetScan analyzer (Abaxis, Inc., Union City, CA).
Tissue processing.
When marmosets were euthanized, brain, heart, lung, liver, spleen, mesenteric lymph node, adrenal gland, kidney, and skeletal muscle tissue samples were aseptically removed and either frozen immediately for virus titer determination or fixed in phosphate-buffered (pH 7.2) 4% paraformaldehyde before being paraffin embedded for histology and immunohistochemistry. Cerebrospinal fluid (CSF) was also collected at this time for virus titer determination.
Serological assay.
Neutralizing antibodies were assayed in sera with an 80% plaque reduction neutralization test as previously described (6).
Histopathology and immunohistochemistry.
Paraffin-embedded tissues were cut into 5-μm sections, deparaffinized, and stained with hematoxylin and eosin for histopathological analyses. For immunohistochemical analysis, deparaffinized tissue sections were stained for viral antigen with an indirect immunoperoxidase assay as previously described (23). Briefly, EEEV hyperimmune mouse ascitic fluid was used as the primary antibody (kindly provided by Robert Tesh). Peroxidase-conjugated goat anti-mouse immunoglobulin (KPL, Gaithersburg, MD) was used as the secondary antibody and reacted with aminoethylcarbazole as the substrate (Enzo Life Sciences, Farmingdale, NY). Controls included the use of an irrelevant mouse ascitic fluid as the primary antibody and tissue sections from uninfected marmosets. All sections were counterstained with hematoxylin (Poly Scientific, Bay Shore, NY).
Statistical analysis.
For body weight and temperature measurements, viremia data, and peripheral blood leukocyte values, statistical comparisons were performed with either a Student t test or a one-way analysis of variance, followed by a Tukey multiple-comparison test. Survival data were analyzed with the log rank test (GraphPad Prism, San Diego, CA). P ≤ 0.05 was considered significant.
RESULTS
Clinical observations.
Marmosets were acclimated to their surroundings prior to i.n. exposure to the NA and SA strains of EEEV. There was a significant difference in mortality between NA and SA EEEV-infected marmosets (P < 0.03). SA EEEV-infected marmosets survived infection, whereas NA EEEV-infected marmosets either died or were euthanized on day 4 or 5 after infection. Marmosets exposed to SA EEEV remained active, alert, and in good physical condition throughout the study, with a transient decrease in food consumption during first few days after infection. In contrast, marmosets exposed to NA EEEV developed decreased appetites by day 1 or 2 after infection, with complete anorexia in at least one marmoset by day 4 after infection. By days 2 to 4, these marmosets were inactive, somnolent, either not blinking or repeatedly blinking their eyes, and exhibiting a depressed posture.
Body temperatures and weights of sedated marmosets were measured on days 0, 1, 2, 4, 7, 9, and 11 after infection (Fig. 1). SA EEEV-infected marmosets did not appear to develop a febrile response to infection, although sedation could have affected the accuracy of the body temperature measurements. In contrast, NA EEEV-infected marmosets developed acute fever between days 2 and 4 after infection, which decreased in two of the three animals prior to death or euthanasia on day 4 or 5 (Fig. 1A). There was a significant difference in body temperature in the NA EEEV-infected marmosets between days 0 and 4 after infection.
FIG. 1.
Change in body temperatures and weights of marmosets after i.n. infection with EEEV. Percent changes from the starting (day 0) body temperatures (A; in degrees Celsius) and weights (B; in grams) of marmosets were determined on days 1, 2, 4, 7, 9, and 11 after i.n. infection with either NA EEEV strain FL93-939 (solid lines) or SA EEEV strain 436087 (dashed lines). NA EEEV-1, -2, and -3 and SA EEEV-1, -2, and -3 are individual marmosets within each cohort.
All marmosets experienced body weight losses during the first 4 or 5 days after infection (P > 0.05; Fig. 1B). The SA EEEV-infected marmosets began gaining weight after day 4 postinfection. In contrast, NA EEEV-infected marmosets continued to lose weight until death or euthanasia at day 4 or 5 after infection.
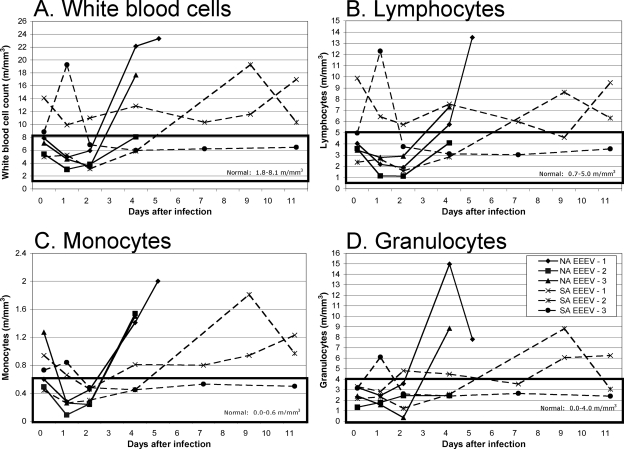
Complete blood cell counts and biochemical analyses were performed on blood samples collected on days 0, 1, 2, 4, 7, 9, and 11 after i.n. exposure to NA and SA EEEVs. Within the first 24 h of infection, all of the NA EEEV-infected animals showed a decrease in white blood cell counts, which were within the normal range for marmosets (Fig. 2A). In contrast, the white blood cell counts of the SA EEEV-infected animals were more variable within the first 24 h of infection. Most likely associated with the excitability of marmosets during handling, the white blood cell counts of two of the three SA EEEV-infected marmosets were above the normal range for marmosets on day 0 after infection, which either increased or decreased by day 1 after infection and remained above the normal range for marmosets. The white blood cell count of the third SA EEEV-infected marmoset was within the normal range on day 0 after infection, and there was a slight increase in the count by day 1 after infection. For both groups, the changes in white blood cell count during the first 24 h after infection nearly paralleled those in the lymphocyte, monocyte, and granulocyte subpopulations of leukocytes (Fig. 2B to D). By day 4 after infection, the NA EEEV-infected marmosets developed marked leukocytosis prior to death or euthanasia at day 4 or 5 after infection. There were no significant differences in white blood cell, lymphocyte, and granulocyte counts between cohorts; however, there was a significant difference in the monocyte counts between NA and SA EEEVs on day 4 after infection.
FIG. 2.
Peripheral blood leukocyte subpopulations after exposure to EEEV. Marmosets were bled on days 0, 1, 2, 4, 7, 9, and 11 after i.n. infection with either NA EEEV strain FL93-939 (solid lines) or SA EEEV strain 436087 (dashed lines) to assess changes in peripheral blood leukocytes. Graphs show cell counts for total white blood cells (A), lymphocytes (B), monocytes (C), and granulocytes (D) for each marmoset. As indicated by the boxes on each graph, normal cell counts of white blood cells, lymphocytes, monocytes, and granulocytes in the peripheral blood of marmosets are 1.8 to 8.1, 0.7 to 5.0, 0.0 to 0.6, and 0.0 to 4.0 m/mm3, respectively. NA EEEV-1, -2, and -3 and SA EEEV-1, -2, and -3 are individual marmosets within each cohort.
For both NA and SA EEEV-infected marmosets, the majority of the biochemistry results were within the normal range for marmosets, including total protein, albumin, glucose, and the liver function enzymes alkaline phosphatase and alanine transferase (data not shown). In marmosets from both groups, there were occasional mild elevations in blood urea nitrogen and creatinine (data not shown).
Viremia and viral burden in tissues.
Previous work has shown that SA strain BeAr436087 is avirulent in mice but induces 10-fold higher viremia than other EEEV strains (1). In marmosets, SA strain BeAr436087 induced higher viremia on day 2 after i.n. infection than NA strain FL93-939 (P < 0.01). SA strain EEEV-infected marmosets developed peak viremia titers of 2.8 to 3.1 log10 PFU/ml at either day 2 or 4 after infection, while there was no detectable viremia in the NA EEEV-infected marmosets (Fig. 3). The limit of detection by the assay was 1.7 log10 PFU/ml.
FIG. 3.
Viremia in marmosets after i.n. infection (106 PFU) with either NA EEEV strain FL93-939 (closed circles; below limit of detection) or SA EEEV strain 436087 (open circles) (three marmosets per cohort). On day 7 after infection, one of two marmosets tested had detectable viremia (a). Error bars indicate the standard deviations, and the limit of detection by the assay was 1.7 log10 PFU/ml.
At the time of euthanasia or death, the brains and other organs of the marmosets were harvested and virus titers were determined by plaque assay. CSF was also collected at this time for virus titer determination. At day 4 or 5 after infection, NA EEEV was detected in the brain, liver, and skeletal muscle (Fig. 4) but SA EEEV was not detected in any tissues at the time of sacrifice, 16 days after infection. The NA EEEV strain replicated to a higher titer in the brain than in the liver or muscle; titers in the brain ranged from 3.6 to 5.8 log10 PFU/100 mg, while viral titers were 2.0 to 2.2 log10 PFU/100 mg in the liver and <1.7 to 2.0 log10 PFU/100 mg in the muscle. There was no detectable virus in the CSF of the infected marmosets.
FIG. 4.
Virus titers in tissues of marmosets on days 4 and 5 after i.n. infection with NA EEEV. Marmosets were infected i.n. (106 PFU) with NA strain FL93-939. At the time of euthanasia or death (day 4 or 5 after infection), brain, lung, heart, liver, adrenal gland, lymph node, and skeletal muscle samples were collected and virus titers were determined by plaque assay. Error bars indicate the standard deviations, and the limit of detection by the assay was 1.7 log10 PFU/ml.
Serology.
SA EEEV-infected marmosets developed neutralizing antibodies to the virus by day 11 or 16 after infection. On these days, the 80% plaque reduction neutralization test titer was 1:20 for SA strain BeAr436087. In contrast, no neutralizing antibodies to virus were detected in the sera of NA EEEV-infected marmosets at day 4 or 5 after infection. The limit of detection by the neutralizing antibody assay was 1:20.
Histological lesions.
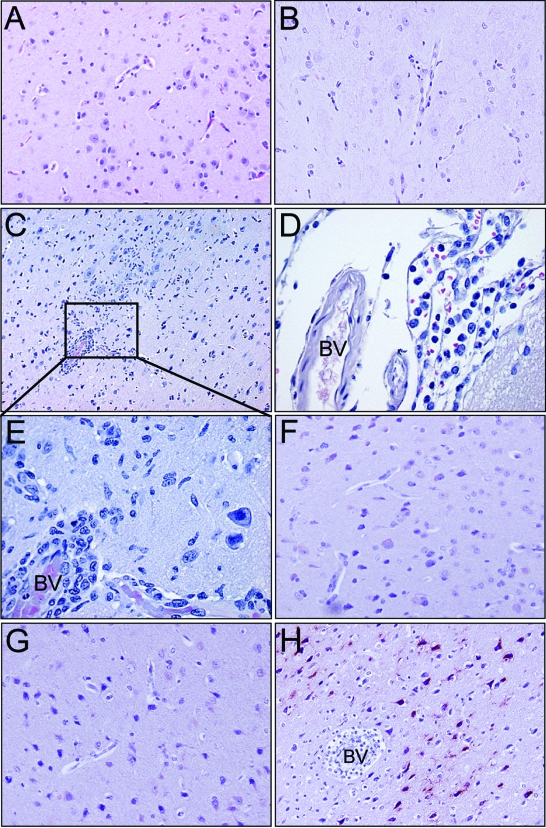
At the time of euthanasia or death, the brains and other organs of the infected marmosets were examined for histopathological lesions. For the histopathological and immunohistochemical analyses, tissue sections from uninfected marmosets were included as negative controls ( Fig. 5A and F). During the necropsies of the SA and NA EEEV-infected marmosets, there were no gross pathological lesions noted. On histopathologic examination of the brains of SA EEEV-infected marmosets, there was no evidence of meningitis or encephalitis associated with infection (Fig. 5B). However, mononuclear cell leptomeningitis and encephalitis of moderate severity were seen in the cerebral cortexes of all NA EEEV-infected marmosets (Fig. 5C to E). Perivascular cuffs and encephalitis were multifocal and primarily composed of mononuclear cells. In two of the three marmosets infected with NA EEEV, there was also a prominent neutrophilic component in the inflammatory infiltrates of the cerebral cortex and hippocampus. Microglial activation and focal neuronal necrosis were also observed within and adjacent to areas of encephalitis, and in one animal, small foci of perivascular hemorrhage were observed.
FIG. 5.
Histopathology and immunohistochemistry of marmoset brains after i.n. infection with EEEV. Similar to uninfected brains (A; magnification, ×20), there were no apparent lesions associated with SA EEEV (B; magnification, ×20); however, NA EEEV-infected marmosets showed multifocal perivascular cuffing and encephalitis in the cerebral cortex (C; magnification, ×10 [inset enlarged in panel E; magnification, ×40]) and a moderate degree of mononuclear cell leptomeningitis (D; magnification, ×40) with some perivascular hemorrhages (not shown). Similar to uninfected brains (F; magnification, ×20), SA EEEV-infected brains (G; magnification, ×20) were negative for viral antigen while NA EEEV-infected brains showed strong cytoplasmic staining (dark red) of neurons in the cerebral cortex (H; magnification, ×20). BV, blood vessel.
Some NA EEEV-infected marmosets showed liver changes suggestive of terminal hypotension or metabolic derangements associated with illness and fever. One animal showed a mild, diffuse microvesicular steatosis, and one animal showed early focal coagulative necrosis around the central veins. In two of the three SA EEEV-infected marmosets, a mild form of acute hepatitis was observed in which there were rare small intralobular and sinusoidal clusters of mononuclear cells and pale, swollen hepatocytes with disorganized trabecular architecture and signs of hepatocyte regeneration. Hemosiderosis and extramedullary hematopoiesis were also noted in the livers of the SA EEEV-infected marmosets, lesions previously identified in common marmosets and other New World primates as incidental findings (13, 19).
In the spleen, two of the three NA EEEV-infected marmosets showed neutrophil accumulations in the red pulp, suggesting either peripheral neutrophilia or local inflammation due to necrosis or complement deposition. The spleens of the SA EEEV-infected animals lacked this lesion.
The adrenal glands and kidneys of both NA and SA EEEV-infected marmosets showed multiple foci of interstitial and/or perivascular mononuclear cell infiltrations (lymphoplasmacytic) that included some neutrophils. These changes have been described previously in uninfected common marmosets (22). Interestingly, the inflammatory cell infiltrates in the kidneys of the SA EEEV-infected marmosets were more abundant than in those of the NA EEEV-infected marmosets. The significance of these findings is unknown.
The mesenteric lymph nodes of all three NA EEEV-infected marmosets showed sinus histiocytosis, whereas only one SA EEEV-infected marmoset showed a mild form of sinus histiocytosis. For both NA and SA EEEV-infected marmosets, no significant pathological changes were noted in the heart, lungs, or skeletal muscle.
Immunohistochemistry.
Immunohistochemical staining for viral antigen was performed on brain tissue sections (Fig. 5F to H). Similar to uninfected marmosets, SA EEEV-infected marmosets showed no apparent infection of the brain on day 16, while NA EEEV-infected marmosets showed strong cytoplasmic staining of infected neurons in the cerebral cortex.
DISCUSSION
The findings of this study identify the common marmoset as a useful model of human EEE for testing antiviral drugs and vaccine candidates and highlight their potential for corroborating epidemiological evidence that some, if not all, SA EEEV strains are attenuated for humans. Despite differences in the ages and weights of the marmosets (and prior exposure to another virus), there was a significant difference in survival between NA and SA EEEV-infected animals. The brain lesions of all three NA EEEV-infected marmosets were also consistent with those described in human cases of EEE.
Although the i.n. route of infection does not account for natural disease progression following a mosquito bite, there were several reasons why the i.n. route was chosen for this study. First, the EEEV marmoset model was developed to ultimately test the efficacy of newly developed vaccines after i.n. or aerosol challenge with a highly virulent strain of EEEV. Second, the epidemiological rate of apparent infection in humans following natural exposure to NA EEEV is relatively low (4%) (15). Therefore, experimental studies of EEEV via subcutaneous inoculation in marmosets would probably require much larger (and more costly) cohort sizes to detect differences between NA and SA EEEVs. Lastly, the i.n. route provides a more direct route of virus to the brain. Thus, the detection of differences between NA and SA EEEVs is more sensitive and reflective of neurovirulence. The results of the present study provide a foundation for conducting more comprehensive experiments on the effect of the route of infection on the early pathogenesis of EEEV in marmosets.
Animal models of EEE.
Insights into human disease caused by EEEV have been gained by studying several different species of animals. In mice, EEEV causes encephalitis but generally fails to induce the vascular manifestations typical of fatal human disease (18). Golden hamsters have been proposed to be a better rodent model of human EEE than mice because they develop vascular lesions after subcutaneous infection that are similar to those described in humans (23). Because alphaviruses are highly infectious by aerosol, additional pathogenesis studies are warranted to determine whether similar lesions occur in hamsters after i.n. or aerosol exposure to EEEV.
Rhesus macaques were the first nonhuman primates used for pathogenesis studies of EEE. When infected with NA EEEV via the intracerebral or i.n. route, juvenile rhesus macaques developed fatal encephalitis with CNS lesions similar to those described in human cases (21, 30). More recently, adult cynomolgus macaques exposed by aerosol to NA EEEV were shown to develop fever and were moribund within 48 to 72 h of fever onset (26). Neurological signs developed rapidly as the fever waned. Gross or histopathologic studies were not reported.
Common marmosets versus other nonhuman primate models of EEE.
We report here that i.n. exposure to a NA strain of EEEV caused lethal encephalitis in marmosets. The onset of fever and neurological signs were similar to those in cynomolgus macaques infected by aerosol exposure (26). Rhesus macaques also develop fever by day 3 after i.n. infection with NA EEEV, with death occurring 2 to 3 days after the onset of fever (30).
In the present study, a decrease in leukocytes was observed in NA EEEV-infected marmosets within 24 to 48 h of infection, followed by marked leukocytosis prior to death or euthanasia. As reported in human cases (14), leukocytosis in the marmosets was primarily attributed to both neutrophilia and lymphocytosis. Cynomolgus macaques also developed leukocytosis prior to death following aerosol exposure to NA EEEV (26); however, the leukocytes were predominantly composed of granulocytes, and a white blood cell count of >20,000/ml was seen in all animals with a poor prognosis.
For both NA and SA EEEV-infected marmosets, the majority of the biochemistry results were within the normal range for marmosets, including liver enzymes. In contrast, EEEV-infected cynomolgus macaques develop elevations in several liver enzymes, including lactate dehydrogenase (LDH), aspartate aminotransferase (AST), and alkaline phosphatase, after aerosol exposure (26). However, liver enzyme elevations have not been reported in human cases of EEE and do not appear to be an important prognostic indicator of disease outcome (11).
The pathological lesions in the CNS of the NA EEEV-infected marmosets were similar to those described for human cases (5, 12, 14, 16, 21), where EEEV causes neuronal loss, neuronophagia, perivascular cuffs, focal and diffuse accumulations of inflammatory cells, and leptomeningitis in the CNS. A neutrophil-rich reaction in brain tissue is characteristic of human EEE meningoencephalitis, which was seen in the NA EEEV-infected marmosets in our study. In human cases of EEE, vascular lesions with breakdown in the structure of the vessel wall and the appearance of thrombi and extravasation of red blood cells have often been noted. We did not observe vasculitis or fibrin thrombi in the cerebral microvasculature, but foci of perivascular hemorrhage were observed in one of the three NA EEEV-infected marmosets. Areas of the CNS most frequently subject to severe lesions in human EEE include the cerebral cortex, basal ganglia, thalamus, hippocampus, and brain stem, with relative sparing of the cerebellum and spinal cord. In the NA EEEV-infected marmosets, the most severe CNS lesions (excluding the spinal cord) were located in the cerebral cortex. Although SA EEEV-infected marmosets showed no overt symptoms of encephalitis and there were no lesions or virus identified in the brain at a later stage of infection, more studies are needed to determine whether lesions and/or virus exist in the brain (or other organs) during early infection and prior to the development of neutralizing antibodies.
The hepatic lesions that were described in the marmosets appear to be associated with EEEV infection. Liver biopsies were performed prior to the EEEV infections to confirm the absence of acute or chronic hepatitis, and based on the biochemistry profiles, there was also no indication of significant liver (or kidney) damage prior to and during EEEV infection. However, future studies should include marmosets that have not been used in prior experiments to corroborate the described hepatic (and other) lesions after EEEV infection.
Common marmoset responses to SA versus NA EEEVs.
Consistent with studies with mice, SA EEEV strain BeAr436087 was attenuated in infected marmosets. Other than transient weight loss during the first few days after infection, the SA EEEV-infected marmosets continued to appear healthy and active throughout the study. This strain also caused no mortality in NIH Swiss mice when inoculated subcutaneously at a dose of 6 log10 PFU, and this attenuation may be correlated to interferon sensitivity that is controlled by both nonstructural and structural protein regions of the virus (3). The availability of cDNA clones for the NA and SA EEEV strains we studied, as well as chimeras with swapped nonstructural and structural protein genome regions, should facilitate further studies with marmosets or macaques to identify viral genes responsible for the dramatic differences in human virulence. Similar studies with other SA strains of EEEV that are virulent in mice should also be performed to determine if the attenuation of strain BeAr436087 for primates can be generalized to the other two SA subtypes. Based on the findings of the present study, cohorts of marmosets of similar ages and body weights will probably be required to detect measurable differences (such as body weight and temperature etc.) between different strains of EEEV.
In addition to disease outcome, there are also host factors that could be measured to identify those that may contribute to the pathogenesis of EEEV. Molecular tools are fairly limited in the marmoset. However, there are several sequences currently available in the GenBank database for marmoset cytokines. In addition, the recent development of a marmoset-specific oligonucleotide microarray and the anticipated release of the complete sequence of the marmoset genome (10) suggest that these animals have the potential to become more relevant and accepted as a nonhuman primate model for various aspects of biomedical research, which include infectious diseases.
Previous work with NIH Swiss mice has shown that SA strain BeAr436087 induces 10-fold higher viremia than other EEEV strains (1). Similarly, in our study, SA EEEV-infected marmosets developed higher peak viremia titers than NA EEEV-infected marmosets, which showed no detectable viremia. Likewise, viremia was either transient or undetectable in cynomolgus macaques that died of encephalitis following aerosol exposure to NA EEEV (26).
There have been only two reported fatal human encephalitis cases of EEE in SA (4, 9), and a recent study hypothesizes that humans in SA are exposed but do not develop apparent infection with EEEV because of poor infectivity and/or avirulence of SA strains (2). The same study showed that only 3% of healthy persons from a region of Peru where EEE is enzootic had EEEV-reactive antibodies (2). In the present study, neutralizing antibodies to EEEV were detected in the SA EEEV-infected marmosets at a titer of 1:20 at day 11 or 16 after infection. However, additional studies are needed to determine peak neutralization antibody titers and duration of immunity compared to other EEEV strains to test this hypothesis.
In summary, the common marmoset appears to be a useful small nonhuman primate model of human EEE for pathogenesis studies, as well as efficacy studies of antiviral therapeutics and vaccine candidates. The focus of future work with this model should include the examination of other SA subtypes of EEEV for attenuation, as well as the identification of viral determinants of human neurovirulence.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases through the Western Regional Center for Excellence for Biodefense and Emerging Infectious Diseases Research (NIH grant U54 AI057156). The laboratories at SFBR were supported by an NIH laboratory construction grant (1C06RR12087). A.P.A. was supported by the James W. McLaughlin fellowship fund.
We thank Slobodan Paessler for help with the experimental design.
Footnotes
Published ahead of print on 9 July 2008.
REFERENCES
- 1.Aguilar, P. V., S. Paessler, A. S. Carrara, S. Baron, J. Poast, E. Wang, A. C. Moncayo, M. Anishchenko, D. Watts, R. B. Tesh, and S. C. Weaver. 2005. Variation in interferon sensitivity and induction among strains of eastern equine encephalitis virus. J. Virol. 7911300-11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilar, P. V., R. M. Robich, M. J. Turell, M. L. O'Guinn, T. A. Klein, A. Huaman, C. Guevara, Z. Rios, R. B. Tesh, D. M. Watts, J. Olson, and S. C. Weaver. 2007. Endemic eastern equine encephalitis in the Amazon region of Peru. Am. J. Trop. Med. Hyg. 76293-298. [PubMed] [Google Scholar]
- 3.Aguilar, P. V., A. P. Adams, E. Wang, A. Carrara, M. Anishchenko, I. Frolov, and S. C. Weaver. 2008. Structural and nonstructural protein genome regions of eastern equine encephalitis virus are determinants of interferon sensitivity and murine virulence. J. Virol. 824920-4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alice, F. J. 1956. Infecção humana pelo virus “leste” da encefalite equina. Bol. Inst. Biol. Bahia (Brazil) 33-9. [Google Scholar]
- 5.Bastian, F. O., R. D. Wende, D. B. Singer, and R. S. Zeller. 1975. Eastern equine encephalomyelitis: histopathologic and ultrastructural changes with isolation of the virus in a human case. Am. J. Clin. Pathol. 6410-13. [DOI] [PubMed] [Google Scholar]
- 6.Beaty, B. J., C. H. Calisher, and R. E. Shope. 1989. Arboviruses, p. 797-855. In N. J. Schmidt and R. W. Emmons (ed.), Diagnostic procedures for viral, rickettsial and chlamydial infections, 6th edition. American Public Health Association, Washington, DC.
- 7.Brault, A. C., A. M. Powers, C. L. V. Chavez, R. N. Lopez, M. F. Cachón, L. F. Gutierrez, W. Kang, R. B. Tesh, R. E. Shope, and S. C. Weaver. 1999. Genetic and antigenic diversity among eastern equine encephalitis viruses from North, Central, and South America. Am. J. Trop. Med. Hyg. 61579-586. [DOI] [PubMed] [Google Scholar]
- 8.Byrnes, A. P., and D. E. Griffin. 2000. Large-plaque mutants of Sindbis virus show reduced binding to heparan sulfate, heightened viremia, and slower clearance from the circulation. J. Virol. 74644-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corniou, B., P. Ardoin, C. Bartholomew, W. Ince, and V. Massiah. 1972. First isolation of a South American strain of eastern equine virus from a case of encephalitis in Trinidad. Trop. Geogr. Med. 24162-167. [PubMed] [Google Scholar]
- 10.Datson, N. A., M. C. Morsink, S. Atanasova, V. W. Armstrong, H. Zischler, C. Schlumbohm, B. E. Dutilh, M. A. Huynen, B. Waegele, A. Ruepp, E. R. de Kloet, and E. Fuchs. 2007. Development of the first marmoset-specific DNA microarray (EUMAMA): a new genetic tool for large-scale expression profiling in a non-human primate. BMC Genomics 8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deresiewicz, R. L., S. J. Thaler, L. Hsu, and A. A. Zamani. 1997. Clinical and neuroradiographic manifestations of eastern equine encephalitis. N. Engl. J. Med. 3361867-1874. [DOI] [PubMed] [Google Scholar]
- 12.Farber, S., A. Hill, M. L. Connerly, and J. H. Dingle. 1940. Encephalitis in infants and children caused by a virus of the eastern variety of equine encephalitis. JAMA 1141725-1731. [Google Scholar]
- 13.Foster, J. R. 2005. Spontaneous and drug-induced hepatic pathology of the laboratory beagle dong, the cynomolgus macaque, and the marmoset. Toxicol. Pathol. 3363-74. [DOI] [PubMed] [Google Scholar]
- 14.Garen, P. D., T. F. Tsai, and J. M. Powers. 1999. Human eastern equine encephalitis: immunohistochemistry and ultrastructure. Mod. Pathol. 12646-652. [PubMed] [Google Scholar]
- 15.Goldfield, M., J. N. Welsh, and B. F. Taylor. 1968. The 1959 outbreak of eastern encephalitis in New Jersey. 5. The apparent infection:disease ratio. Am. J. Epidemiol. 8732-33. [DOI] [PubMed] [Google Scholar]
- 16.Jordan, R. A., J. A. Wagner, and F. R. McCrumb. 1965. Eastern equine encephalitis: report of a case with autopsy. Am. J. Trop. Med. Hyg. 14470-474. [DOI] [PubMed] [Google Scholar]
- 17.Klimstra, W. B., K. D. Ryman, and R. E. Johnston. 1998. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J. Virol. 727357-7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu, C., D. W. Voth, P. Rodina, L. R. Shauf, and G. Gonzalez. 1970. A comparative study of the pathogenesis of western equine and eastern equine encephalomyelitis viral infections in mice by intracerebral and subcutaneous inoculations. J. Infect. Dis. 12253-63. [DOI] [PubMed] [Google Scholar]
- 19.Lowenstine, L. J. 2003. A primer of primate pathology: Lesions and nonlesions. Toxicol. Pathol. 3192-102. [DOI] [PubMed] [Google Scholar]
- 20.Mansfield, K. 2003. Marmoset models commonly used in biomedical research. Comp. Med. 53383-392. [PubMed] [Google Scholar]
- 21.Nathanson, N., P. D. Stolley, and P. J. Boolukos. 1969. Eastern equine encephalitis. Distribution of central nervous system lesions in man and rhesus monkey. J. Comp. Pathol. 79109-115. [DOI] [PubMed] [Google Scholar]
- 22.Okazaki, Y., Y. Kurata, F. Makinodan, F. Kidachi, M. Yokoyama, Y. Wako, Y. Yamagishi, O. Katsuta, M. Takechi, and M. Tsuchitani. 1996. Spontaneous lesions detected in the common cotton-eared marmosets (Callithrix jacchus). J. Vet. Med. Sci. 58181-190. [DOI] [PubMed] [Google Scholar]
- 23.Paessler, S., P. Aguilar, M. Anishchenko, H.-Q. Wang, J. Aronson, G. Campbell, A.-S. Cararra, and S. C. Weaver. 2004. The hamster as an animal model for eastern equine encephalitis and its use in studies of virus entrance into the brain. J. Infect. Dis. 1892072-2076. [DOI] [PubMed] [Google Scholar]
- 24.Patterson, J. L., and R. Carrion, Jr. 2005. Demand for nonhuman primate resources in the age of biodefense. ILAR J. 4615-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powers, A. M., A. C. Brault, R. M. Kinney, and S. C. Weaver. 2000. The use of chimeric Venezuelan equine encephalitis viruses as an approach for the molecular identification of natural virulence determinants. J. Virol. 744258-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed, D. S., M. G. Lackemeyer, N. L. Garza, S. Norris, S. Gamble, L. J. Sullivan, C. M. Lind, and J. L. Raymond. 2007. Severe encephalitis in cynomolgus macaques exposed to aerosolized eastern equine encephalitis virus. J. Infect. Dis. 196441-450. [DOI] [PubMed] [Google Scholar]
- 27.Vogel, P., W. M. Kell, D. L. Fritz, M. D. Parker, and R. J. Schoepp. 2005. Early events in the pathogenesis of eastern equine encephalitis virus in mice. Am. J. Pathol. 166159-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, E., O. Petrakova, A. P. Adams, P. V. Aguilar, W. Kang, S. Paessler, S. M. Volk, I. Frolov, and S. C. Weaver. 2007. Chimeric Sindbis/eastern equine encephalitis vaccine candidates are highly attenuated and immunogenic in mice. Vaccine 257573-7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weaver, S. C., R. B. Tesh, and R. E. Shope. 2006. Alphavirus infections, p. 831-838. In R. I. Guerrant, D. H. Walker, and P. F. Weller (ed.), Tropical infectious diseases. Principles, pathogens, and practice, 2nd edition. Elsevier Churchill Livingstone, Philadelphia, PA.
- 30.Wyckoff, R. W. G., and W. C. Tesar. 1939. Equine encephalomyelitis in monkeys. J. Immunol. 37329-343. [Google Scholar]