Abstract
In mammalian female cells, one X chromosome is inactivated to prevent a dose difference in the expression of X-encoded proteins between males and females. Xist RNA, required for X chromosome inactivation, is transcribed from the future inactivated X chromosome (Xi), where it spreads in cis, to initiate silencing. We have analyzed Xist RNA transcription and localization throughout the cell cycle. It was found that Xist transcription is constant and that the mature RNA remains attached to the Xi throughout mitosis. Diploid and tetraploid cell lines with an MS2-tagged Xist gene were used to investigate spreading of Xist. Most XXXXMS2 tetraploid mouse embryonic stem (ES) cells inactivate the XMS2 chromosome and one other X chromosome. Analysis of cells with two Xi's indicates that Xist RNA is retained by the Xi of its origin and does not spread in trans. Also, in XXMS2 diploid mouse ES cells with an autosomal Xist transgene, there is no trans exchange of Xist RNA from the Xi to the autosome. We propose that Xist RNA does not dissociate from the Xi of its origin, which precludes a model of diffusion-mediated trans spreading of Xist RNA.
During embryonic development of female mammals, one of the two X chromosomes is silenced in all somatic cell lineages. X chromosome inactivation (XCI) is a mechanism to compensate for the difference in gene expression from sex chromosomes between males and females. In mice, XCI is initiated at 5.5 days postcoitus, after which the proliferating cells clonally propagate the inactivated X chromosome (Xi) through many cell cycles. A single locus on the X chromosome, the X inactivation center, is essential for XCI and is home to the Xist gene, one of the main regulators in the process. Xist does not encode a protein but is transcribed into a functional RNA that is sufficient and necessary for silencing. Xist RNA spreads in cis over the X chromosome upon the onset of X inactivation. This RNA contains eight different repeat domains, most of which play a redundant role in the localization of the Xist RNA on the Xi, although the exact mechanism of its association with the Xi is not known. One repeat domain of Xist RNA, the A repeat transcribed from exon 1, is required exclusively for X chromosome silencing. Deletion studies show that loss of this repeat does not interfere with localization of the Xist RNA but completely abolishes the silencing process (31).
After establishment of XCI, the silenced state of the Xi is fixed by DNA methylation and many chromatin modifications, including trimethylation of H3K27 (H3K27me3), ubiquitylation of H2A, hypoacetylation of histones, and accumulation of macroH2A. The Xi also starts DNA replication in late S phase, and its silenced state is propagated after every cell cycle. This phase of XCI is referred to as the maintenance phase (9, 20, 26).
Despite many studies on the function of Xist RNA, it is still not clear how Xist RNA is restricted to the Xi. Thus far, no proteins involved in the recruitment of Xist RNA to DNA have been identified. It has been hypothesized that Xist RNA is targeted to the Xi through LINE1 repeats, which are more abundant on the X chromosome than on autosomes (1, 18, 29). This would be in agreement with the observation that Xist RNA spreading into an autosomal region that has a low density of LINE1 repeats, in cases of X-autosomal translocations, is hampered (5, 27). For silencing of the Xi, sufficient amounts of Xist RNA have to be bound to the Xi only, and after initiation of XCI, the Xist RNA cloud is remarkably stable. Interestingly, when the nucleus of a cell with completed X inactivation is depleted of DNA and chromatin by DNase I treatment and salt extraction, Xist RNA still remains in place, suggesting that Xist RNA localization is facilitated by the nuclear matrix (4). This hypothesis is underlined by the observations that the Xi colocalizes with the well-known nuclear scaffold component SAF-A and that deletion of the RNA binding domain of SAF-A abolishes this colocalization (6, 10). It is unclear, however, whether loss of the RNA binding activity of SAF-A also results in loss of Xist RNA from the Xi. Furthermore, Zhang et al. (32) have shown that, during S phase of the cell cycle, the nuclear position of the Xi shifts from perinuclear to perinucleolar, indicating that Xi interacts with structural components of the nucleus, potentially in the form of interaction with a nuclear matrix and through the action of Xist RNA. Although Xist RNA is confined to the Xi, which is stably propagated to daughter cells, several reports have indicated that Xist RNA dissociates from the Xi around telophase of mitosis. The Xist cloud, as visualized by fluorescent in situ hybridization (FISH), persists into metaphase of mitosis (5) but is eventually lost from the Xi at telophase, after which it can be seen as distinct spots floating in the nucleoplasm (5, 8, 30). This would indicate that Xist RNA is absent during some time from the Xi and diffuses through the cell. We aimed to reinvestigate these dynamic aspects of Xist RNA by using new methods. Transcription and localization of Xist were monitored throughout the cell cycle in differentiated mouse embryonic stem (ES) cells. Using diploid and tetraploid female ES cells with a modified Xist gene encoding tagged Xist RNA and cells carrying an autosomal Xist transgene, we obtained evidence that Xist RNA does not dissociate from its site of origin, where it is confined to the nuclear territory of the silenced X chromosome throughout the cell cycle.
MATERIALS AND METHODS
Generation of the F1 2-1 MS2 cell line.
The MS2 Lox-Neo-Lox targeting vector was created by insertion of a Lox-Neo-Lox resistance cassette into the unique HindIII site in Xist exon 7 of pBglII5k (17), from which Xist exons 3 to 6 were removed by PstI/ClaI digestion. Sixteen MS2 repeats, which were generated by duplication of two tandem repeats of eight MS2 hairpin sequences (28), were inserted in the sense orientation into the unique BamHI site present in the polylinker of the Neo resistance cassette. For targeting, we used the polymorphic Mus musculus/Mus castaneus F1 2-1 ES cell line (23), and the resistance cassette was removed by transient Cre expression.
Cell culture.
ES cells were differentiated into embryoid bodies (EBs) with EB medium (Iscove's modified Dulbecco's medium-GlutaMAX supplemented with 15% [vol/vol] fetal calf serum, 1% [vol/vol] penicillin/streptomycin, 1% [vol/vol] nonessential amino acids, 0.5 mg/ml ascorbic acid, and 0.038 μl/ml monothioglycerol) on nongelatinized bacterial dishes. For FISH analysis, the differentiated cells were trypsinized and plated onto gelatinized glass coverslips 1 day before fixation. For 5-bromo-2′-deoxyuridine (BrdU) incorporation, the cells were grown overnight in the presence of 10 μM BrdU (Sigma).
Generation of tetraploid ES cells.
One million cells of an F1 2-1 cell line with a randomly integrated puromycin resistance gene and 106 cells of the F1 2-1 MS2 cell line containing a randomly integrated neomycin resistance gene were mixed and washed twice with serum-free Dulbecco's modified Eagle's medium. We used polyethylene glycol 1500 (Roche) as a cell fusion agent. Cells were fused as described by the manufacturer. After fusion, the cells were seeded in ES medium (Dulbecco modified Eagle medium [Gibco], 15% fetal calf serum [HyClone], 1% penicillin-streptomycin, 1% nonessential amino acids [Gibco], 0.008 μl/ml β-mercaptoethanol, and 1,000 U of leukemia-inhibiting factor/ml) with 1 μg/ml puromycin and 250 μg/ml G418 and selected for approximately 10 days until clones could be picked. Clones were tested for tetraploidy by measuring the DNA content by fluorescence-activated cell sorter (FACS) analysis and by karyotyping.
Generation of cell lines ectopically expressing Xist RNA.
A kanamycin-neomycin resistance cassette was integrated into bacterial artificial chromosome (BAC) RP24-180B23, which contains the Xist gene and not Tsix, by Lox recombination. Female 30Δ1 XXMS2 ES cells were transfected with the BAC, which was linearized with Sce-I, and were then selected with neomycin. The autosomal integration site of the BAC was verified by DNA FISH, and the copy number was estimated by performing quantitative PCR (Q-PCR) with primers for Xist and Zfp42 on genomic DNA (gDNA) of the clones.
Karyotyping.
Cells were grown in ES medium and blocked in metaphase by addition of 12 μl/ml Karyomax (Gibco) 1 h before harvesting. The cells were trypsinized, resuspended in 0.075 M KCl at 37°C, centrifuged, and fixed in 5 volumes of 0.075 M KCl and 1 volume of fixative (methanol-acetic acid, 3:1). Cells were washed three times in fixative and stored. The cells were spotted onto slides, air dried, stained with 4′,6′-diamidino-2-phenylindole (DAPI), and analyzed.
Cell cycle synchronization.
F1 2-1 cells were synchronized with mimosine according to Krude (14), with slight modifications. Cells were differentiated as described above, trypsinized at day 3 of differentiation, and plated onto gelatin-coated plates for the RNA and FACS samples and on gelatin-coated coverslips for the RNA FISH samples. Cells were blocked for ∼16 h with 1 mM mimosine and released into EB medium after being washed twice with phosphate-buffered saline (PBS). Every 2 h, samples were taken for FACS analysis, RNA isolation, and RNA FISH over a period of 16 h. We analyzed day 4 differentiated ES cells because ES cells become insensitive to mimosine later during EB differentiation.
FACS analysis.
Synchronized cells were trypsinized, washed once with PBS, and fixed for 2 h in 100 μl PBS and 900 μl 70% ethanol (EtOH) at 4°C. After fixation, the cells were washed with PBS and incubated for 3 h in detection buffer (0.1% Triton X-100, 20 mg/liter propidium iodide, 0.2 g/liter RNase). The DNA content was measured on a FACScan (Becton Dickinson).
Allele-specific PCR and Q-PCR.
RNA was isolated with Trizol (Invitrogen), and 2 μg RNA was DNase treated and reverse transcribed with Superscript III (Invitrogen).
Allele-specific reverse transcription (RT)-PCR was performed with primers spanning a length polymorphism identifying the 129 and M. castaneus Xist RNA (forward, 5′-ACTGGGTCTTCAGCGTGA-3′; reverse, 5′-GCAACAACGAATTAGACAACAC-3′).
Q-PCR was performed with primers detecting Xist mRNA spanning an exon-exon junction (forward, 5′-TACTTCAAGATGCACTGCTACCC-3′; reverse, 5′-CTTTGGGGAAGGGTAATATTTGG-3′), Xist primary RNA spanning an intron-exon junction (forward, 5′-GTTCTTACCACCAATTGAAAACG-3′; reverse, 5′-CAAAACAGACTCCAAATTCATCC), and primers detecting beta-actin mRNA as an amplification control (forward, 5′-ACTATTGGCAACGAGCGGTTC-3′; reverse, 5′-AGAGGTCTTTACGGATGTCAACG).
The copy number of the B23 BAC was determined by Q-PCR on gDNA of the targeted cell lines compared to wild-type (WT) gDNA of a diploid XX cell line with the Xist primary RNA primer set and control primer set over the autosomal Zpf42 gene (forward, 5′-GCACCCATATCCGCATCCAC-3′; reverse, 5′-GCATTTCTTCCCGGCCTTTG-3′).
DNA and RNA FISH.
Coverslips with cells obtained during cell synchronization were fixed for 10 min with 4% paraformaldehyde (PFA)-PBS and washed with 70% EtOH. Cells were made permeable by washing twice with PBS and by 5 min of treatment with 25 μg/ml proteinase K-PBS at room temperature or 4 min of treatment with 0.2% pepsin at 37°C. Postfixation was performed for 5 min with 4% PFA-PBS. The coverslips were washed twice with PBS and dehydrated by sequential addition of 70, 90, and 100% EtOH. Nick-translated DNA probes were dissolved in a hybridization mixture containing 50% formamide, 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 50 mM phosphate buffer (pH 7.0), 10% dextran sulfate, and 100 ng/μl mouse Cot DNA to a final concentration of 1 ng/μl. The probe mixture was denatured for 5 min, prehybridized for 45 min at 37°C, and then applied to the slide. Slides were incubated overnight in a humid chamber at 37°C.
After hybridization, coverslips were washed once in 2× SSC and three times in 50% formamide-2× SSC, both at 37°C, and twice in TST (0.1 M Tris, 0.15 M NaCl, 0.05% Tween 20) at room temperature. Then the coverslips were incubated for 30 min in blocking buffer (2 mg/ml bovine serum albumin in 0.1 M Tris and 0.15 M NaCl) in a humidified chamber at room temperature. Detection was done by subsequent steps of incubation with anti-digoxigenin (Boehringer), anti-sheep (fluorescein isothiocyanate [FITC]; Jackson ImmunoResearch Laboratories), and anti-rabbit (FITC; Jackson ImmunoResearch Laboratories) antibodies or anti-biotin (Roche), anti-mouse (rhodamine red; Jackson ImmunoResearch Laboratories), and anti-donkey (rhodamine red; Jackson ImmunoResearch Laboratories) antibodies in blocking buffer for 30 min at room temperature. Slides were washed twice between detection steps with TST. After the last detection step, the coverslips were washed twice with TST and once with TS (0.1 M Tris, 0.15 M NaCl), dehydrated, mounted with Vectashield (Vector Laboratories), and stored at 4°C.
Coverslips with cells differentiated normally as described above were washed twice with PBS and than treated for 30 s with cytoskeletal buffer [100 mM NaCl, 300 mM sucrose, 3 mM MgCl2, 10 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES; pH 6.8)], 2 min with cytoskeletal buffer with detergent (100 mM NaCl, 300 mM sucrose, 3 mM MgCl2, 10 mM PIPES [pH 6.8], 0.5% Triton X-100), and 30 s with cytoskeletal buffer. The cells were fixed for 10 min in 4% PFA-PBS and stored in 70% EtOH. Hybridization and detection were performed as described above.
Coverslips with BrdU-labeled cells were pretreated and fixed with cytoskeletal buffer and detergent and fixed as described above. Then the cells were dehydrated by sequential EtOH steps. To make BrdU accessible to anti-BrdU antibody (Abcam), cells were denatured. For this, 100 μl denaturing buffer (70% formamide, 2× SSC, 10 mM phosphate buffer [pH 7]) was added and cells were incubated on a hot plate for 3 min at 85°C, followed by 5 min of incubation in ice-cold 70% EtOH. Cells were again dehydrated and hybridized overnight, and detection was done as described above. The anti-BrdU (Abcam) and anti-rat (AMCA; Jackson ImmunoResearch Laboratories) antibodies were used for detection.
To determine the integration site of the RP24-180B23 BAC, DNA FISH was performed. Cells were treated with Karyomax (Gibco) and fixed with methanol-acetic acid as described for karyotyping. Permeabilization was done by treating the cells for 4 min with 0.5% pepsin at 37°C. After dehydration, cells were denatured as already described for 3 min at 85°C, followed by 5 min of incubation in ice-cold 70% EtOH. Cells were again dehydrated and hybridized, and detection was done with the antibiotin (Roche) and goat anti-mouse (FITC; Jackson ImmunoResearch Laboratories) antibodies.
Five different probes were used for the various experiments. Xist mRNA was detected with the 5.5-kb cDNA Xist probe described by Gribnau et al. (7). The probe was labeled with digoxigenin by nick translation (Roche). The probe used to identify the MS2-tagged Xist RNA was a 0.5-kb BamHI-HindIII fragment of pBluescript8xMS2 consisting of eight MS2 repeats and was labeled with biotin by nick translation (Roche). The probe used to detect the primary transcript of Xist RNA was a combination of six PCR products from the primer sets intron1P1 (forward, 5′-GTACGCCAAGGGTAGCAAGA-3′; reverse, 5′-CGTACAAAAGGCCAAATGCAA-3′), intron1P2 (forward, 5′-TTGCATTTGCCTTTTGTGACG-3′; reverse, 5′-GCCTCCAGATGGTTTTGTGT-3′), intron1P3 (forward, 5′-TTAGGAGGTGCCATCACACA; reverse, 5′-GGTTCACTGGACTGGGAGAG-3′), intron3 (forward, 5′-GGGGCAAGTCAATAAAGCAC-3′; reverse, 5′-GAGGGGCTTGGAGAGTGAAC-3′), intron5 (forward, 5′-AGCTATTTACGAGTACACTGTTGC-3′; reverse, 5′-CAAAGAACAAAAGAAGCTGTATGAA-3′), and intron6 (forward, 5′-GGATTTGCATTTGCTGGAAG-3′; reverse, 5′-GGACACACCCGTCAACTCTT-3′). The PCR products were labeled with biotin by nick translation (Roche).
The RP24-180B23 BAC was labeled as a whole with biotin by nick translation (Roche). The X chromosome was detected with an X chromosome paint probe directly labeled with Cy3 (Cambio).
Counting and imaging of cells.
In the cell synchronization experiment, only cells with a single Xist RNA cloud were counted. We assumed that these cells had established XCI, whereas cells still containing a pinpoint Tsix/Xist signal together with a cloud had not yet completed XCI. At least 100 cells were counted at each time point for at least two separate experiments.
The tetraploid XXXXMS2 cells were counted similarly. Per separate experiment, the number of Xist clouds in tetraploid XXXXMS2 cells was determined in at least 100 cells per cell line.
The analysis of the fluorescent intensity of the Xist RNA and MS2 repeat RNA FISH signal was done with the ImageJ program.
RESULTS
Xist RNA expression throughout the cell cycle.
One crucial question in understanding how Xist RNA is restricted to the Xi during progression through the cell cycle is whether the Xi of the daughter cells is recoated by Xist RNA still present in the nucleoplasm or by newly synthesized Xist RNA, after loss of Xist RNA during mitosis. For the latter, one would expect that the Xist transcription rate is increased in G1 to compensate for the loss of Xist RNA during each mitotic cell cycle. Moreover, DNA replication itself will reduce the concentration of Xist RNA per Xi during the mid to late S phase. Therefore, we studied if the transcription of Xist is regulated during the cell cycle by analysis of the ratio of primary/mature Xist transcripts. The expression profile of Xist was analyzed by using F1 2-1 ES cells after 3 days of EB differentiation that were synchronized in the cell cycle by treatment with mimosine for 16 h, which blocks the cells at the G1/S transition. The cells were released from the block at day 4 of EB differentiation (14). Samples for FACS analysis, RNA FISH, and Q-PCR were taken every 2 h for the period of approximately one cell cycle.
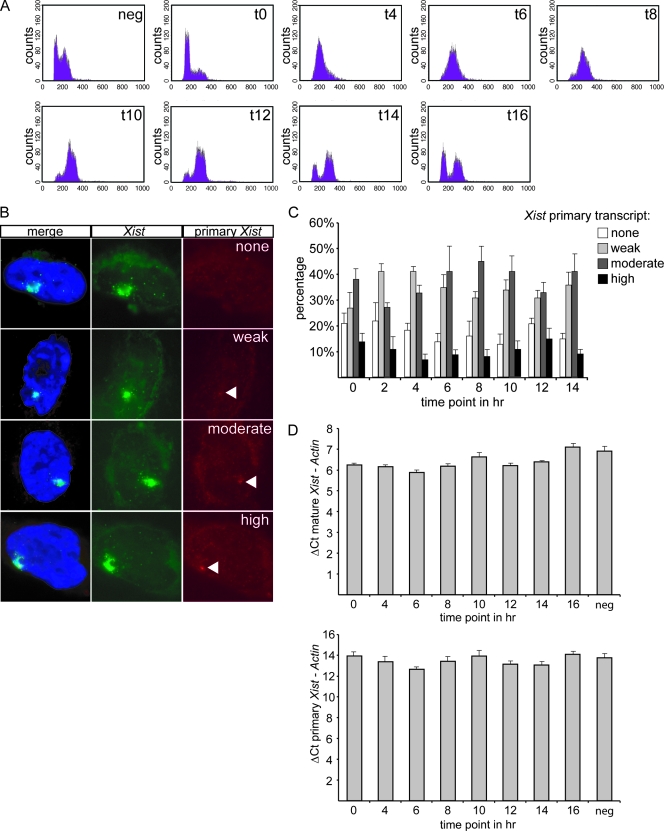
FACS analysis, determining the DNA content, indicated that the vast majority of the cells was synchronized in G1 and went through the cell cycle simultaneously (Fig. 1A). RNA FISH was performed with intronic and exonic Xist probes to visualize primary and mature transcripts. The intensity of the intronic FISH signal was taken as a measurement of Xist expression in cells and was classified as no signal, weak, moderate, or high (Fig. 1B). Only cells with an Xist cloud and without Xist/Tsix pinpoints were counted because these cells have finished the XCI initiation phase and have established an inactive X (15). In four separate experiments we found no significant difference in the abundance of Xist primary transcripts between the different time points of the cell cycle (Fig. 1C).
FIG. 1.
Xist gene transcription during the cell cycle. (A) FACS analysis measuring the DNA content of day 4 differentiated diploid XX ES cells after release from a late G1 phase block. Samples were taken every 2 h. (B) RNA FISH on day 4 differentiated diploid XX ES cells after release from a late G1 phase block. DNA was stained with DAPI (blue), mature Xist RNA was stained with an exon probe (green with FITC), and primary Xist RNA transcripts were stained with an intron probe (rhodamine red). Primary Xist RNA transcript signals were categorized as none, weak, moderate, and high. (C) Percentage of day 4 differentiated diploid XX ES cells having no, weak, moderate, or high primary Xist RNA transcript signals at different time points after release from a late G1 phase block. (D) Q-PCR on day 4 differentiated diploid XX ES cells after release from a late G1 phase block. The threshold cycle (ΔCt) of primary and mature Xist RNA was taken at different time points and normalized with the β-actin control. neg, negative.
This was confirmed by Q-PCR in which the amount of primary Xist RNA and spliced Xist RNA was compared to β-actin mRNA at sequential time points after release from the cell cycle block (Fig. 1D). At all time points, there was no significant change in the ratio of primary to mature Xist RNA amounts. We therefore conclude that the Xist transcription rate is stable during the cell cycle.
Xist RNA associates with the Xi throughout mitosis.
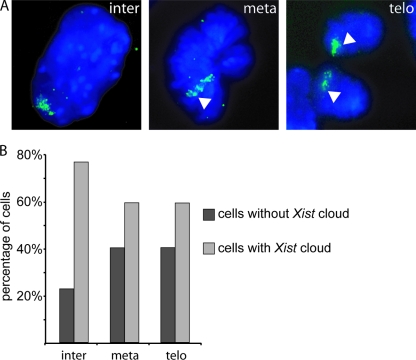
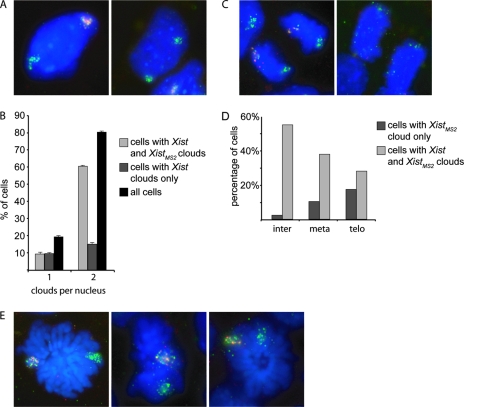
From our finding that Xist transcription is not upregulated at specific time points during the cell cycle and the reported loss of Xist during mitosis (5, 8, 30), one would expect Xist RNA clouds to be absent in early G1 phase of the cell cycle. This prompted us to analyze Xist RNA clouds in female murine embryonic fibroblasts, which were expanded in vitro. After Xist RNA FISH, we detected only a very small number, less than 2% of the cells (5 out of 292 cells), without an Xist cloud and no cells with small pinpoint signals indicative of Xist accumulation just after mitosis (data not shown). These results indicate that Xist may not dissociate from the Xi during mitosis. The previously reported dissociation of Xist (5, 8, 30) might be the result of pretreatment of the cells prior to RNA FISH. We therefore fixed 4-day differentiated female F1 2-1 cells in 4% PFA in the absence of detergent or acetic acid. In this experiment, Xist RNA FISH confirmed the presence of Xist clouds at telophase of mitosis (Fig. 2A). The percentage of cells with an Xist cloud is decreased at metaphase and telophase, but ∼60% of the cells still contained a specific Xist RNA signal (Fig. 2B). We attribute the smaller number of Xist clouds to the pepsin treatment used to permeabilize the cells. Based on these results, we conclude that Xist most likely does not dissociate from the Xi at any phase during progression through the mitotic cell cycle.
FIG. 2.
Xist clouds in day 4 differentiated F1 2-1 cells throughout the cell cycle. (A) Xist cloud in green in an interphase (inter) and a metaphase (meta) cell, followed by Xist clouds in two cells in telophase (telo) (Xist in FITC; DNA is DAPI stained). (B) Percentage of F1 2-1 cells with an Xist cloud in interphase, metaphase, or telophase cells.
Generation of a cell line expressing tagged Xist RNA.
The finding that Xist does not dissociate from the Xi at any phase of the cell cycle would make redundant a diffusion-mediated reassembly of Xist to the Xi after the completion of mitosis. Nonetheless, it is unclear if diffusion has any role in the establishment of the Xist cloud.
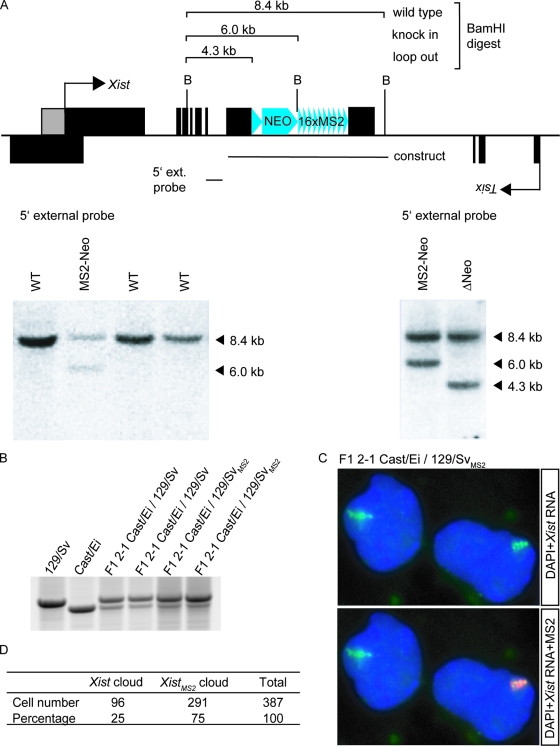
To test whether newly synthesized Xist spreading along the X chromosome is diffusion mediated or if Xist is retained within its own chromosomal territory, we have generated tetraploid XXXX cell lines with one X chromosome harboring a modified Xist gene which is transcribed into a marked Xist RNA, allowing us to determine the origin of the Xist molecules. We started by creating a diploid XX ES line with a marked Xist gene, which was later used to generate tetraploid ES cells. The Xist gene was labeled on one allele by the integration of MS2 repeats in the gene. The phage-specific MS2 sequence was chosen (25) because this sequence is nonfunctional in mammalian cells and therefore not likely to interfere with XCI. Sixteen tandem MS2 repeats were inserted into exon 7 of the Xist gene on the 129/Sv allele of an F1 2-1 M. musculus/M. castaneus (129/Sv/Cast/Ei) ES cell line. After verification of the right insertion, the neomycin resistance cassette was excised by transient Cre recombinase expression (Fig. 3A).
FIG. 3.
Construction of ES cells expressing XistMS2 RNA. (A) A construct containing 16 repeats of the MS2 sequence next to a neomycin selection marker enclosed by Lox sites was integrated into exon 7 of the Xist gene. Positive clones were selected by Southern hybridization with the 5′ external (ext.) probe on BamHI-digested gDNA. Loop out of the neomycin resistance cassette was detected by Southern hybridization with the same digest and probe. (B) Allele-specific RT-PCR on Xist RNA isolated from day 7 differentiated ES cells using length polymorphism to distinguish whether Xist RNA originated from the XCast/Ei or the X129/Sv allele. (C) RNA FISH on day 10 differentiated XXMS2 cells. Xist RNA is in green (FITC), MS2 repeats are in red (rhodamine), and DNA is stained with DAPI. (D) Quantification of the number of cells containing either an Xist cloud or an XistMS2 cloud after 10 days of differentiation.
XCI was analyzed in the mutated XXMS2 cell line by differentiation of the cells into EBs. No increased cell death of the XXMS2 cell line was observed after EB differentiation (data not shown), indicating that XCI was functional. RNA FISH with Xist and MS2 probes on XXMS2 cells that were differentiated for 10 days showed that these cells had either an Xist or an XistMS2 cloud (Fig. 3C).
We tested whether insertion of the MS2 repeat had any effect on XCI by measuring the ratio of tagged and WT Xi's. The XCast/Ei allele and X129/Sv allele of the F1 2-1 cell line are not equally inactivated because both X chromosomes harbor different X chromosome controlling elements (Xce). Since the XCast/Ei allele in the F1 2-1 cell line contains a strong Xcec and the targeted X129/Sv allele contains a weak Xcea, the X129/Sv allele is inactivated in 70% of the cells (3). Allele-specific RT-PCR on Xist using length polymorphism to distinguish the XCast/Ei and X129/Sv alleles indicated that skewing of XCI was not affected by insertion of the repeats (Fig. 3B). Determination of the number and origin of Xi's in day 10 differentiated cells confirmed skewing toward the X129/SvMS2 allele (Fig. 3D).
XCI in tetraploid XXXXMS2 cells.
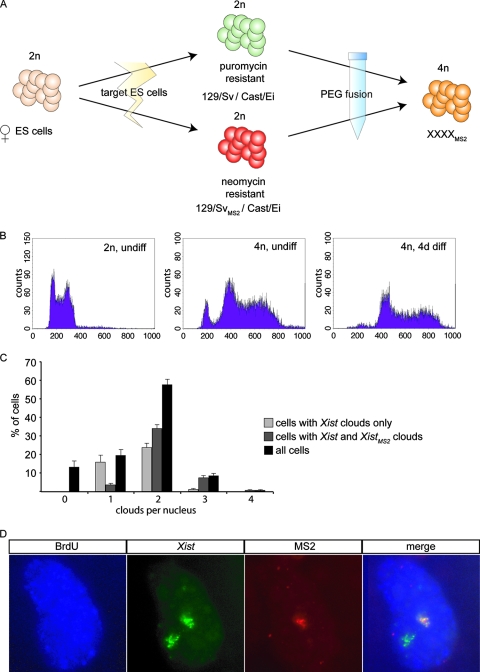
To study the dynamics of the intranuclear localization of Xist RNA throughout the cell cycle, a tetraploid XXXXMS2 cell line was generated. These cells preferentially inactivate two X chromosomes instead of one (19), so Xist RNA is transcribed from two different Xi's. The MS2-tagged allele allows distinction of the origin of Xist RNA in an Xist cloud.
To obtain the tetraploid cell line, a neomycin resistance cassette was randomly integrated into the XXMS2 cell line and fused with an XX ES cell line containing a puromycin resistance cassette. After polyethylene glycol 1500-mediated fusion, XXXXMS2 tetraploid ES cells were selected by puromycin and neomycin double selection (Fig. 4A). The DNA content of the double-resistant XXXXMS2 tetraploid cell lines was verified by FACS analysis before and after differentiation, and this analysis indicated that our cell lines were 4n and stable throughout differentiation (Fig. 4B). This was confirmed by karyotyping, in which we counted the chromosomes in metaphase spreads (data not shown). To test if the tetraploid XXXXMS2 cell lines initiated XCI normally, we differentiated the cells for 7 days and determined the number of Xist and XistMS2 clouds. We found that almost 60% of the cells had two Xist clouds at day 7, which is in agreement with previous data for tetraploid ES cells without MS2 repeats (19). The fact that not all cells have an XaXaXiXi pattern can be attributed to stochastic XCI and loss of X chromosomes (19). There is, however, no indication that the total number of X chromosomes within one nucleus has any effect on the binding and spreading of Xist RNA during mitosis or interphase.
FIG. 4.
Tetraploid XXXXMS2 ES cells. (A) Schematic overview of the formation of tetraploid XXXXMS2 ES cells. (B) FACS analysis of the DNA content of undifferentiated diploid XX ES cells (2n, undiff), undifferentiated tetraploid XXXXMS2 ES cells (4n, undiff), and day 4 differentiated tetraploid XXXXMS2 ES cells (4n, 4d diff). The 2n peak in undifferentiated 4n cells represents male feeders. (C) RNA FISH with Xist and MS2 repeat probes was performed on day 10 differentiated tetraploid XXXXMS2 ES cells. The percentage of cells with a certain number of Xist clouds was determined and is depicted as black bars. The percentages of cells containing WT Xist RNA clouds only are depicted as light gray bars, and the percentages of cells containing no, one, or more WT Xist RNA clouds together with an XistMS2 RNA cloud are depicted as dark gray bars. (D) Xist clouds in tetraploid XXXXMS2 ES cells contain either Xist or XistMS2. RNA FISH on day 7 differentiated tetraploid XXXXMS2 ES cells after overnight incorporation of BrdU. The panels show incorporation of BrdU (blue with Cascade Blue), Xist RNA (green with FITC), and XistMS2 RNA (rhodamine red).
Xist spreading is not diffusion mediated.
Examination of the Xist and XistMS2 clouds of XXXXMS2 tetraploid cells with clearly distinguishable Xi's by RNA FISH with Xist- and MS2-specific probes indicated that Xist clouds from WT Xi's never contain XistMS2 RNA from the targeted Xi (Fig. 5A). After labeling of our cells with BrdU 24 h prior to fixation, we found that the same holds true for XaXaXiXiMS2 cells that went through mitosis, as determined by immuno-RNA FISH detecting Xist, MS2, and BrdU (Fig. 4D). This suggests that Xist RNA is directly retained by the Xi from which it is transcribed and does not diffuse through the nucleus to bind to other Xi's. Thus, Xist RNA is present on an Xi not because it is the only entity in the cell capable of binding Xist RNA, due to epigenetic modifications of the Xi chromatin, but because it does not dissociate from the Xi.
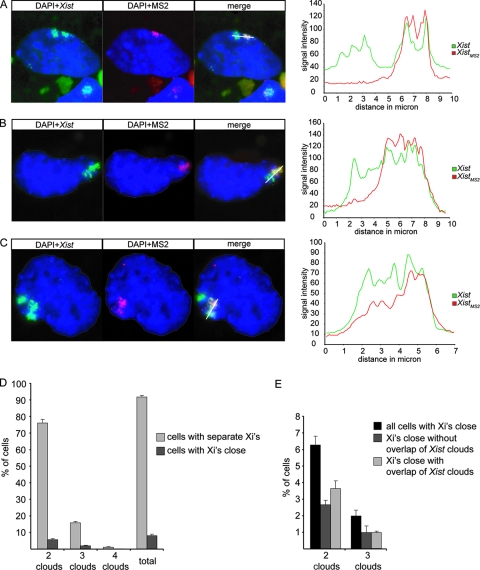
FIG. 5.
Xist and XistMS2 clouds in close proximity. (A, B, and C) The left panels show RNA FISH on day 7 differentiated tetraploid XXXXMS2 ES cells showing DNA (blue with DAPI), Xist RNA (green with FITC), and XistMS2 RNA (rhodamine red). The right panels show the intensities of the Xist and XistMS2 signals measured along the indicated white line across both Xist clouds. (A) A cell with two separate Xist clouds. (B) A cell with two Xist clouds in close proximity to each other but without overlap. (C) A cell with three Xist clouds, two of which are adjacent to each other and have overlapping Xist clouds. (D) Percentage of cells that have separate Xist clouds (light gray bars) or Xist clouds in close proximity to each other (dark gray bars). (E) Percentage of cells with Xist clouds in close proximity to each other that have nonoverlapping or overlapping Xist clouds.
In some cells, Xist clouds were located close together and could only be distinguished as two separate clouds because one was tagged with MS2 repeats (Fig. 5B and C). This was the case in 8% of the cells that had two or more clouds of which one Xist cloud was MS2 tagged (Fig. 5D). To see if this result may point to a targeted and functional association between the two clouds, as opposed to a random nonfunctional association, we performed a simplified mathematical calculation based on literature-derived data showing that the probability of two Xi's being in close proximity to each other is also 8% (see calculation I in the supplemental material). Therefore, our results are feasible and seem to reflect a random positioning of the Xi's in the periphery of the nucleus.
Intriguingly, in about half of the cells that had clouds located in close proximity, the XistMS2 RNA cloud of the targeted Xi seemed to overlap the Xist cloud of the WT Xi, as can be judged by merging the Xist signal and the MS2 signal from RNA FISH experiments (Fig. 5C and E). This result might suggest that Xist RNA is able to spread in trans when nuclear territories of two Xi's are in close proximity to each other. However, two Xi′s and their respective Xist clouds may share the same nuclear territory without any interchromosomal exchange of Xist RNA. Indeed, no cells were found with two MS2-labeled Xist clouds, and only cells with two Xi's in close proximity to each other show an overlap of Xist clouds, which supports the hypothesis that Xist RNA does not leave the Xi of its origin.
Ectopic Xist expression on autosomes.
From the above, we conclude that Xist RNA is directly bound to the Xi from which it is transcribed. To study whether this retention of Xist by the X chromosome requires specific X-chromosomal Xist binding sites which are not abundant on autosomes, we generated transgenic XXMS2 ES cells with an autosomal BAC transgene containing the Xist gene but not Tsix. These cell lines allow us to compare Xist retention on an X chromosome and an autosome within the same cell. A kanamycin-neomycin resistance cassette was integrated into the Xist-containing BAC by Lox recombination, and female XXMS2 ES cells were transfected and were then selected with neomycin. The autosomal integration site of the BAC was verified by DNA FISH (see Fig. S1A in the supplemental material), and the copy number was estimated by performing Q-PCR with primers for Xist and the autosomal Zfp42 gene as a normalization control for diploidy on gDNA of the clones (see Fig. S1B in the supplemental material). High-copy transgenic cell lines (30Δ1 4, 30Δ1 8, 30Δ1 9, and 30Δ1 10) were differentiated for 4 days and subjected to RNA FISH with Xist- and MS2-specific probes which allow discrimination between the transgene (Xist only) and endogenous Xist (Xist only for Xist originating from the Cast/Ei chromosome and Xist and MS2 positive for Xist originating from the 129/Sv chromosome). The number of Xist clouds in cells was determined for each cell line, in which an extra cloud would most likely result from the autosome with the transgenic Xist gene. All of the cell lines showed a significant percentage of cells with double clouds from ∼50% (30Δ1 9) up to ∼80% (30Δ1 8) (see Fig. S1C in the supplemental material). As expected, we found that most cells of 30Δ1 8 line had inactivated the XMS2 chromosome and an autosome (Fig. 6A and B). The WT and transgenic Xist clouds are indistinguishable in nearly all interphase cells. In metaphase cells, the autosomal Xist cloud is of a different size than WT Xist clouds and seems to cover the entire chromosome in most cases (see Fig. S1D and E in the supplemental material; Fig. 6E). Cells with two XistMS2 clouds are never observed. Similar to the present findings with tetraploid XXXXMS2 cells, we found a small but significant proportion of the Xist clouds in close proximity to each other. Again, approximately 50% of the clouds seemed to overlap whereas the rest did not (see Fig. S2A and B in the supplemental material).
FIG. 6.
Transgenic Xist RNA expression in the 30Δ1 8 cell line. (A) Interphase cells with either XistMS2 (in red) and autosomal Xist (green) clouds (left panel) or endogenous WT Xist and autosomal Xist clouds (both in green) (right panel). (B) Percentage of cells with one cloud of Xist or XistMS2, two clouds of either XistMS2 or autosomal Xist, or two clouds of either endogenous WT Xist or autosomal Xist. (C) Cells in telophase with XistMS2 (red) or autosomal Xist (green) still attached (left panel) or with Xist RNA detached and floating in the nucleoplasm (right panel). (D) Percentage of cells with XistMS2 cloud only or both XistMS2 and autosomal Xist clouds at interphase (inter), metaphase (meta), or telophase (telo). (E) Three metaphase cells showing XistMS2 (in red) and autosomal Xist (green) clouds.
Next, we analyzed the binding of the transgenic Xist RNA to its autosome in mitosis of day 4 differentiated 30Δ1 8 ES cells. Similar to the observed localization of endogenous Xist to the Xi, we found that the transgenic Xist RNA is present on the autosome throughout mitosis, including telophase (Fig. 6C). At telophase, we found fewer autosomal Xist clouds relative to Xist clouds on the Xi. This difference is less pronounced in interphase or metaphase cells (Fig. 6D). Increased loss of autosomally located Xist at telophase could represent a real loss of Xist during mitosis but more likely is a consequence of removal of less stringently bound RNA in the RNA FISH procedure (Fig. 6D). Because Xist RNA is less tightly bound to the Xi at telophase, small changes in the affinity of Xist for the autosome compared to the Xi are likely to present themselves at this stage of the cell cycle. Therefore, this finding indicates that the Xi-specific chromatin state or DNA sequence does increase the binding affinity of Xist for the chromosome but is not a necessity.
Taken together, Xist RNA expressed from an autosome can form a tightly bound cloud over the entire chromosome. This cloud is almost indistinguishable in appearance from a WT Xist cloud. Only during telophase, the transgenic Xist RNA is more prone to detach from the autosome, compared to detachment of endogenous Xist RNA from the X chromosome, indicating that an autosome is capable of binding Xist RNA, but with slightly lower affinity than the X chromosome.
DISCUSSION
In this study, the behavior of Xist RNA on the Xi was investigated during different phases of the mitotic cell cycle in mouse ES cells that have established XCI. We found that Xist is retained at the Xi at all phases during mitosis and that Xist transcription is constant throughout the cell cycle. For XXXXMS2 tetraploid cells and Xist transgenic XXMS2 cells, where one of the X chromosomes transcribes a tagged XistMS2 RNA, it was found that XistMS2 RNA is associated with only one Xist cloud, indicating that Xist RNA is retained by the Xi of its origin. In other words, Xist RNA does not leave the territory of the Xi.
Xist RNA is present on the Xi throughout mitosis.
Previous reports have indicated that during mitotic telophase of mouse ES cells, Xist RNA dissociates from the Xi (8, 30) and can be detected as punctate spots floating around in the nucleoplasm. In contrast, we observed mitotic cells in telophase with Xist clouds present on the Xi or autosomes. We attribute this difference to the variation in the procedures used to fix cells prior to RNA FISH analysis. In previous studies (8, 30), cells were pretreated with a hypotonic solution and fixed with a combination of formaldehyde and acetic acid. The Xist interaction with the Xi may be weakened by the chromatin compaction during mitosis, and the absence of a nuclear membrane may have facilitated loss of the Xist cloud during the fixation procedure. In the present study, the cells were fixed with PFA without any pretreatment, which most likely allowed Xist to stay attached to the Xi. Nevertheless, in our analysis we also found a decrease in the number of Xist clouds upon progression through mitosis, which we attribute to weakened interactions of Xist with either DNA or chromatin. The finding that Xist is more readily lost from an autosome than from Xi in the experiments with transgenic female ES cells suggests that this interaction is DNA mediated since the DNA sequence is the most apparent difference between the observed autosomal versus X-chromosomal spreading of Xist RNA, at least at telophase. Nonetheless, we cannot exclude a role for histone modifications present at the X chromosome prior to and after XCI, in the more efficient spreading of Xist RNA over the Xi (22).
Xist RNA retention at the Xi.
The present analysis of tetraploid XXXX ES cells and cells with autosomal Xist transgenes indicates that Xist RNA is retained at its own Xi or at its autosomal origin and spreads only in cis, also when more than one Xi is present in the same nucleus. This result suggests that endogenous Xist RNA is restricted to the nuclear territory of its own Xi, rather than binding the Xi in cis or trans as a result of free diffusion and recognition of specific epigenetic modifications on the Xi. However, in about half of the cases when two Xi's are located close together, we found an overlap between the Xist clouds. A plausible explanation for this finding is that Xist RNA may spread in trans when the nuclear territories of two Xi's are in very close proximity to each other and stay together continuously. Indeed, separate Xist clouds containing XistMS2 RNA were not found. Still, we find it more likely that the chromosome territories of Xi's sometimes intermingle but without any trans exchange of Xist RNA. Intermingling of chromosome territories has previously been described as a common feature of chromosomes in interphase cells (2).
Taken together, there is consensus that Xist RNA can spread in cis on the Xi or autosomal regions. However, as discussed above, we found that Xist RNA does not migrate to an adjacent Xi. In addition, Xist RNA does not spread to autosomal regions in trans, not even when there is very close proximity to the Xi. How can this be explained?
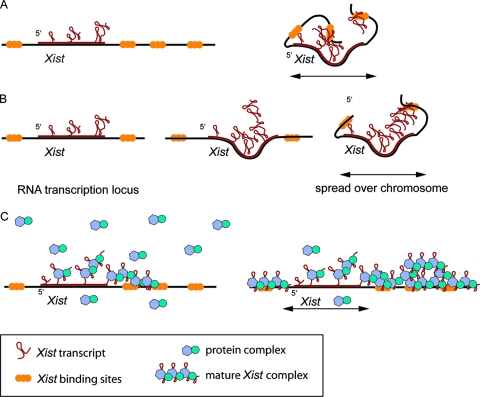
A possible model for this conundrum is that Xist RNA remains on the Xi in cis because of the high local concentration of binding sites, possibly LINE1 repeats, other DNA sequences specific for the X chromosome, or specific X-chromosomal chromatin marks that are present before and after the initiation of XCI (22) (Fig. 7). The Xist binding sites may fold toward the Xist gene, binding the Xist RNA while it is transcribed, preventing diffusion to the (future) active X. Utilization of LINE1 repeats by the inactivation machinery is supported by studies with X:autosomal translocations, which showed that Xist RNA is capable of spreading in cis into the autosomal region, but less efficiently (5, 11, 13, 16, 27). The efficiency with which Xist RNA spreads into the autosomal region seems to be correlated with the density of LINE1 repeats (18), which are good candidates for binding Xist RNA, because they are (i) enriched twice as much on the X chromosome as on autosomes, (ii) less abundant in the pseudoautosomal region of the X chromosome and near X-linked genes that escape XCI, and (iii) increased in abundance around the X inactivation center (1, 29). However, for Xist transgenes inserted at autosomal positions, we and others have shown that Xist RNA spreads in cis (11, 16), showing that even though DNA binding sites might play a role, they are not the only factor in binding Xist to DNA.
FIG. 7.
Schematic models of Xist RNA spreading in M. musculus. (A) Xist RNA binds the chromosome directly, and the DNA folds to capture Xist. (B) Xist RNA binds to itself, creating Xist RNA clouds that are tethered to the chromosome. (C) A protein present in excess captures Xist RNA during transcription, allowing Xist to bind the chromosome.
In a different model, Xist RNA is self-interacting, forming a web of RNA molecules that remain located on the Xi and interact with LINE1 repeats, other specific DNA sequences, or chromatin marks. Xist-Xist interactions could already form during transcription of Xist on the template because roughly eight Xist molecules are simultaneously transcribed from the gene at any time during the cell cycle, thereby preventing diffusion of Xist away from the Xi (see calculation II in the supplemental material).
Finally, lack of trans spreading of Xist RNA could also depend on the amount of Xist RNA transcribed, which might be limited in relation to the abundance of a putative protein that ties Xist RNA to the Xi, acting in synergy with the Xist binding sites. In this model, similar to Drosophila, where association of Rox1 and Rox2 RNAs with the hyperactivated X in male cells requires the MSL complex (12, 21, 24), a putative mammalian protein involved in binding the few hundred copies of Xist RNA to the X chromosome would be present in excess. Such a protein would quickly immobilize Xist RNA, allowing spreading across an X chromosome in cis but not in trans. The reports that Xist remains present on the Xi after removal of DNA from the nucleus (4) and that Xist is more loosely bound to the Xi in mitosis, when a nuclear matrix is no longer present, support the hypothesis that protein-RNA interactions are involved in Xist localization to the Xi. If the amount of Xist RNA per nucleus is quite low, some 300 transcripts per nucleus (30a), it is feasible that the highest-affinity Xist binding sites on the X chromosome will not be saturated. This may result in preferential association of Xist RNA with the binding sites of the X chromosome, usually excluding autosomal regions in X:autosomal translocations in which the autosomal region is not enriched in these or comparable Xist binding sites. However, when Xist is expressed at a higher level, as is the case for most autosomal Xist transgenes, Xist RNA will be forced to spread in cis onto the autosomal region utilizing Xist binding sites with less affinity because the putative Xist binding protein will prevent Xist from going elsewhere.
Supplementary Material
Acknowledgments
We thank Ken Kosik for kindly providing the MS2 repeat sequence and Thasin Stefan Barakat for technical assistance.
This work was supported by HFSP-CDA and NWO-VIDI grants.
Footnotes
Published ahead of print on 14 July 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bailey, J. A., L. Carrel, A. Chakravarti, and E. E. Eichler. 2000. Molecular evidence for a relationship between LINE-1 elements and X chromosome inactivation: the Lyon repeat hypothesis. Proc. Natl. Acad. Sci. USA 976634-6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Branco, M. R., and A. Pombo. 2006. Intermingling of chromosome territories in interphase suggests role in translocations and transcription-dependent associations. PLoS Biol. 4e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cattanach, B. M., and C. Rasberry. 1994. Identification of the Mus castaneus Xce allele. Mouse Genome 922. [Google Scholar]
- 4.Clemson, C. M., J. A. McNeil, H. F. Willard, and J. B. Lawrence. 1996. XIST RNA paints the inactive X chromosome at interphase: evidence for a novel RNA involved in nuclear/chromosome structure. J. Cell Biol. 132259-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duthie, S. M., T. B. Nesterova, E. J. Formstone, A. M. Keohane, B. M. Turner, S. M. Zakian, and N. Brockdorff. 1999. Xist RNA exhibits a banded localization on the inactive X chromosome and is excluded from autosomal material in cis. Hum. Mol. Genet. 8195-204. [DOI] [PubMed] [Google Scholar]
- 6.Fackelmayer, F. O. 2005. A stable proteinaceous structure in the territory of inactive X chromosomes. J. Biol. Chem. 2801720-1723. [DOI] [PubMed] [Google Scholar]
- 7.Gribnau, J., S. Luikenhuis, K. Hochedlinger, K. Monkhorst, and R. Jaenisch. 2005. X chromosome choice occurs independently of asynchronous replication timing. J. Cell Biol. 168365-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall, L. L., and J. B. Lawrence. 2003. The cell biology of a novel chromosomal RNA: chromosome painting by XIST/Xist RNA initiates a remodeling cascade. Semin. Cell Dev. Biol. 14369-378. [DOI] [PubMed] [Google Scholar]
- 9.Heard, E. 2004. Recent advances in X-chromosome inactivation. Curr. Opin. Cell Biol. 16247-255. [DOI] [PubMed] [Google Scholar]
- 10.Helbig, R., and F. O. Fackelmayer. 2003. Scaffold attachment factor A (SAF-A) is concentrated in inactive X chromosome territories through its RGG domain. Chromosoma 112173-182. [DOI] [PubMed] [Google Scholar]
- 11.Herzing, L. B., J. T. Romer, J. M. Horn, and A. Ashworth. 1997. Xist has properties of the X-chromosome inactivation centre. Nature 386272-275. [DOI] [PubMed] [Google Scholar]
- 12.Kelley, R. L., V. H. Meller, P. R. Gordadze, G. Roman, R. L. Davis, and M. I. Kuroda. 1999. Epigenetic spreading of the Drosophila dosage compensation complex from roX RNA genes into flanking chromatin. Cell 98513-522. [DOI] [PubMed] [Google Scholar]
- 13.Keohane, A. M., A. L. Barlow, J. Waters, D. Bourn, and B. M. Turner. 1999. H4 acetylation, XIST RNA and replication timing are coincident and define X;autosome boundaries in two abnormal X chromosomes. Hum. Mol. Genet. 8377-383. [DOI] [PubMed] [Google Scholar]
- 14.Krude, T. 1999. Mimosine arrests proliferating human cells before onset of DNA replication in a dose-dependent manner. Exp. Cell Res. 247148-159. [DOI] [PubMed] [Google Scholar]
- 15.Lee, J. T., L. S. Davidow, and D. Warshawsky. 1999. Tsix, a gene antisense to Xist at the X-inactivation centre. Nat. Genet. 21400-404. [DOI] [PubMed] [Google Scholar]
- 16.Lee, J. T., W. M. Strauss, J. A. Dausman, and R. Jaenisch. 1996. A 450 kb transgene displays properties of the mammalian X-inactivation center. Cell 8683-94. [DOI] [PubMed] [Google Scholar]
- 17.Luikenhuis, S., A. Wutz, and R. Jaenisch. 2001. Antisense transcription through the Xist locus mediates Tsix function in embryonic stem cells. Mol. Cell. Biol. 218512-8520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyon, M. F. 1998. X-chromosome inactivation: a repeat hypothesis. Cytogenet. Cell Genet. 80133-137. [DOI] [PubMed] [Google Scholar]
- 19.Monkhorst, K., I. Jonkers, E. Rentmeester, F. Grosveld, and J. Gribnau. 2008. X inactivation counting and choice is a stochastic process: evidence for involvement of an X-linked activator. Cell 132410-421. [DOI] [PubMed] [Google Scholar]
- 20.Ng, K., D. Pullirsch, M. Leeb, and A. Wutz. 2007. Xist and the order of silencing. EMBO Rep. 834-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh, H., Y. Park, and M. I. Kuroda. 2003. Local spreading of MSL complexes from roX genes on the Drosophila X chromosome. Genes Dev. 171334-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Neill, L. P., T. E. Randall, J. Lavender, H. T. Spotswood, J. T. Lee, and B. M. Turner. 2003. X-linked genes in female embryonic stem cells carry an epigenetic mark prior to the onset of X inactivation. Hum. Mol. Genet. 121783-1790. [DOI] [PubMed] [Google Scholar]
- 23.Panning, B., J. Dausman, and R. Jaenisch. 1997. X chromosome inactivation is mediated by Xist RNA stabilization. Cell 90907-916. [DOI] [PubMed] [Google Scholar]
- 24.Park, Y., R. L. Kelley, H. Oh, M. I. Kuroda, and V. H. Meller. 2002. Extent of chromatin spreading determined by roX RNA recruitment of MSL proteins. Science 2981620-1623. [DOI] [PubMed] [Google Scholar]
- 25.Peabody, D. S. 1993. The RNA binding site of bacteriophage MS2 coat protein. EMBO J. 12595-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plath, K., S. Mlynarczyk-Evans, D. A. Nusinow, and B. Panning. 2002. Xist RNA and the mechanism of X chromosome inactivation. Annu. Rev. Genet. 36233-278. [DOI] [PubMed] [Google Scholar]
- 27.Popova, B. C., T. Tada, N. Takagi, N. Brockdorff, and T. B. Nesterova. 2006. Attenuated spread of X-inactivation in an X;autosome translocation. Proc. Natl. Acad. Sci. USA 1037706-7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rook, M. S., M. Lu, and K. S. Kosik. 2000. CaMKIIα 3′ untranslated region-directed mRNA translocation in living neurons: visualization by GFP linkage. J. Neurosci. 206385-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross, M. T., D. V. Grafham, A. J. Coffey, S. Scherer, K. McLay, D. Muzny, M. Platzer, G. R. Howell, C. Burrows, C. P. Bird, A. Frankish, F. L. Lovell, K. L. Howe, J. L. Ashurst, R. S. Fulton, R. Sudbrak, G. Wen, M. C. Jones, M. E. Hurles, T. D. Andrews, C. E. Scott, S. Searle, J. Ramser, A. Whittaker, R. Deadman, N. P. Carter, S. E. Hunt, R. Chen, A. Cree, P. Gunaratne, P. Havlak, A. Hodgson, M. L. Metzker, S. Richards, G. Scott, D. Steffen, E. Sodergren, D. A. Wheeler, K. C. Worley, R. Ainscough, K. D. Ambrose, M. A. Ansari-Lari, S. Aradhya, R. I. Ashwell, A. K. Babbage, C. L. Bagguley, A. Ballabio, R. Banerjee, G. E. Barker, K. F. Barlow, I. P. Barrett, K. N. Bates, D. M. Beare, H. Beasley, O. Beasley, A. Beck, G. Bethel, K. Blechschmidt, N. Brady, S. Bray-Allen, A. M. Bridgeman, A. J. Brown, M. J. Brown, D. Bonnin, E. A. Bruford, C. Buhay, P. Burch, D. Burford, J. Burgess, W. Burrill, J. Burton, J. M. Bye, C. Carder, L. Carrel, J. Chako, J. C. Chapman, D. Chavez, E. Chen, G. Chen, Y. Chen, Z. Chen, C. Chinault, A. Ciccodicola, S. Y. Clark, G. Clarke, C. M. Clee, S. Clegg, K. Clerc-Blankenburg, K. Clifford, V. Cobley, C. G. Cole, J. S. Conquer, N. Corby, R. E. Connor, R. David, J. Davies, C. Davis, J. Davis, O. Delgado, D. Deshazo, et al. 2005. The DNA sequence of the human X chromosome. Nature 434325-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, K. P., M. Byron, C. M. Clemson, and J. B. Lawrence. 2004. Ubiquitinated proteins including uH2A on the human and mouse inactive X chromosome: enrichment in gene rich bands. Chromosoma 113324-335. [DOI] [PubMed] [Google Scholar]
- 30a.Sun, B. K., A. M. Deaton, and J. T. Lee. 2006. A transient heterochromatic state in Xist preempts X inactivation choice without RNA stabilization. Mol. Cell 21617-628. [DOI] [PubMed] [Google Scholar]
- 31.Wutz, A., T. P. Rasmussen, and R. Jaenisch. 2002. Chromosomal silencing and localization are mediated by different domains of Xist RNA. Nat. Genet. 30167-174. [DOI] [PubMed] [Google Scholar]
- 32.Zhang, L. F., K. D. Huynh, and J. T. Lee. 2007. Perinucleolar targeting of the inactive X during S phase: evidence for a role in the maintenance of silencing. Cell 129693-706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.