Abstract
Angiogenesis, the formation of new blood vessels from existing vasculature, is regulated primarily by endothelial cell activity. We show herein that the Ras family GTPase Rap1 has a key role in the regulation of angiogenesis by modulating endothelial cell functions. Blood vessel growth into fibroblast growth factor 2 (FGF2)-containing Matrigel plugs was absent from rap1a−/− mice, and aortic rings derived from rap1a−/− mice failed to sprout primitive tubes in response to FGF2, when the tissue was embedded in Matrigel. Knocking down either rap1a or rap1b, two closely related rap1 family members, in human microvascular endothelial cells (HMVECs) by utilizing siRNA confirmed that Rap1 plays key roles in endothelial cell function. The rap1a or rap1b knockdown resulted in decreased adhesion to extracellular matrices and impaired cell migration. HMVEC monolayers lacking Rap1 had increased permeability, and Rap1-deficient endothelial cells failed to form three-dimensional tubular structures when they were plated on Matrigel in vitro. Finally, the activation levels of extracellular signal-regulated kinase (ERK), p38, and Rac, which are important signaling molecules in angiogenesis, were all reduced in response to FGF2 when either of the Rap1 proteins was depleted. These observations place Rap1 centrally in the human angiogenic process and suggest that both the Rap1a and Rap1b proteins are required for angiogenesis and that Rap1 is a critical mediator of FGF-induced ERK activation.
Angiogenesis, the formation of new capillaries from preexisting vasculature, is an essential process, both during development (46) and throughout life (5). Maladaptive angiogenesis contributes to numerous cardiovascular diseases such as atherosclerosis, brain ischemia, hypertension, and stroke (5). The process of angiogenesis is usually initiated by increased endothelial cell permeability and proliferation, followed by the proteolysis of basement membrane components. These cells then sprout and migrate toward a site of needed blood supply where they reestablish junctions, form tube structures, and subsequently stabilize into mature capillaries (37). However, the mechanisms underlying this process are only poorly understood.
Rap1 is a Ras family small GTPase that serves as a molecular switch. It couples extracellular stimuli to intracellular effectors and their resulting biological responses by cycling between inactive GDP- and active GTP-bound states. In response to ligand binding to multiple cell surface receptors, Rap1 is activated by guanine nucleotide exchange factors (GEFs) and is subsequently converted back to its inactive GDP-bound state by GTPase-activating proteins (GAPs) (3, 4, 44). There exist two closely related Rap1 family members, Rap1a and Rap1b, which are encoded by separate genes but share 95% amino acid identity and may have complementary as well as distinct biological functions.
Rap1 was first reported to antagonize Ras by binding to but not activating the c-Raf-1 kinase (11, 27). However, in cell types that express B-Raf, Rap1 can trigger a B-Raf→MEK→extracellular signal-regulated kinase (ERK) kinase cascade (54). Rap1 can also activate Akt upon stimulating certain cells with 8-(4-chlorophenylthio)-2-O methyladenosine-3,5-cyclic monophosphate (8CPT-cyclic AMP [cAMP]), a cAMP analog that activates the Rap GEFs Epac1 and Epac2 (36, 51).
Another important function of Rap1 is the modulation of integrin-dependent cell adhesion and motility via inside-out signaling to β1, β2, and β3 family integrins (16). Mouse embryonic fibroblasts lacking the Rap GEF C3G showed decreased integrin-dependent cell adhesion and increased random cell motility (40). Activation of Rap1 in T cells induced the αLβ2 integrin (LFA-1)-mediated adhesion to intracellular adhesion molecules (26). These events are mediated by Rap effector proteins, RAPL and/or RIAM (21, 26, 30).
Genetic ablation of rap1a in mice impaired integrin activation and reduced the adhesion and migration of leukocytes (15, 32), recapitulating the pivotal role Rap1 plays in mediating cell adhesive and migratory functions. Loss of Rap1b in mice also resulted in the inactivation of the αIIbβ3 integrin in platelets, and consequently, the Rap1b null mice had a hemorrhagic phenotype not seen with rap1a−/− mice (9). These findings suggest both redundant and distinctive roles for Rap1a and Rap1b in cells.
Recently, Rap1 has also been implicated in the regulation of vascular endothelial-cadherin (VE-cadherin)-mediated cell-cell adhesion. Activation of Rap1 by 8CPT-cAMP/Epac1 enhances endothelial barrier formation (18, 55). 8CPT-cAMP also reverts thrombin-induced hyperpermeability (13). These findings strongly suggest that Rap1 is involved in the modulation of vascular permeability and function and thus may play a key role in angiogenesis. Unexpectedly, the loss of Rap1 blocked rather than potentiated angiogenesis, and this appeared to be due to the previously uncharacterized role of Rap1 in mediating FGF2-induced cell signaling events.
MATERIALS AND METHODS
Animals.
rap1a−/− mice were generated as previously described (32) and backcrossed into a C57BL/6 background for 11 generations. Experiments were carried out in accordance with protocols approved by the Indiana University institutional animal care and use committee.
In vivo Matrigel plug assay.
Matrigel (BD Biosciences, San Jose, CA) was kept on ice and mixed with phosphate-buffered saline (PBS) and 60 U/ml heparin (Sigma-Aldrich, St. Louis, MO), with or without 600 ng/ml fibroblast growth factor 2 (FGF2; Peprotech, Rocky Hill, NJ). Matrigel (400 μl) was subcutaneously injected into the mouse groin area, using a 27-guage needle. After 7 days, plugs were harvested, photographed, and assayed for total hemoglobin, using a kit (Pointe Scientific, Lincoln Park, MI) according to the manufacturer's instructions. Additionally, plugs were fixed in immunohistochemistry zinc fixative (BD Biosciences) for 36 h at room temperature and embedded in paraffin. Serial 5-μm cross-sections were made at 100-μm intervals across the length of the Matrigel plug. For immunostaining, sections were blocked for endogenous peroxidase activity with 3% hydrogen peroxide in methanol following antigen retrieval in antigen-unmasking solution (Vector Laboratories, Burlingame, CA) at 95°C. Sections were blocked in 3% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO) for 1 h and then stained for CD31 (anti-CD31, 1:50; BD Pharmingen, San Jose, CA). Purified class- and species-matched immunoglobulins (BD Pharmingen) were used for isotype controls. Sections were incubated with biotinylated anti-rat immunoglobulin G (Vector Laboratories, Burlingame, CA), and then incubated with streptavidin-Cy3 (Molecular Probes). The slides were mounted in 90% glycerol-10% PBS (pH 8.0) containing 6-diamidino-2-phenylindole dihydrochloride (Sigma) to permit nuclear identification. Sections were examined, and images of sections were collected using a Zeiss Axioskop microscope (Carl Zeiss, Chester, VA) with a 20× CP-achromat/0.12-numerical aperture objective and a SPOT RT color camera (Diagnostic Instruments, Sterling Heights, MI).
Ex vivo mouse aortic ring assay.
Freshly dissected aortas were placed in ice-cold α-minimal essential medium (α-MEM), cleaned of fatty tissue under a dissecting microscope, and rinsed in cold α-MEM three times to remove residual blood before they were sliced into 1-mm-thick rings, using a surgical scalpel. The rings were washed again before they were embedded between two layers of 50-μl growth factor-reduced Matrigel (BD Biosciences) supplemented with 20 U/ml heparin. Matrigel was overlaid with 100 μl of endothelial cell basal medium type 2 (EBM-2; Lonza, Walkersville, MD) with 2% FBS (Atlanta Biologicals, Lawrenceville, GA) plus 25 ng/ml FGF2 or PBS and cultured at 37°C in a 5% CO2 humidified incubator for 7 days, with a medium change every 3 days. The outgrowth and branching activity of endothelial tubes were counted using a Nikon Diaphot 300 inverted microscope at a magnification of ×100 and photographed using an attached Nikon Coolpix 995 camera.
Generation of Rap1b antiserum.
Rabbit antiserum against human Rap1b protein was raised against peptide sequence TPVPGKARKKSS conjugated to keyhole limpet hemocyanin (QCB, Hopkinton, MA). The specificity of the antiserum was tested with Rap1b null mouse embryonic fibroblasts or with 293T cells expressing either hemagglutinin-Rap1a (HA-Rap1a) or -1b (J. Yan and L. A. Quilliam, unpublished data).
Cell culture.
Human microvascular endothelial cells (HMVECs) (Lonza) were maintained in complete microvascular endothelial cell growth medium (mvEGM-2; Lonza) in a humidified 5% CO2 incubator at 37°C.
Transfection of siRNA.
Short interfering RNAs (siRNAs) (set I) against the human rap1a or rap1b gene were described previously (19) and synthesized by Ambion (Austin, TX). Nontargeting control siRNAs were purchased from Dharmacon (siControl no. 2 and no. 3; Dharmacon, Lafayette, CO). siRNAs were transfected into HMVECs by using Lipofectamine2000 (Invitrogen, Carlsbad, CA) per the manufacturer's protocol. Additional Stealth rap1a siRNA pool (Invitrogen) and rap1b SMARTpool (Dharmacon) siRNAs were used for the confirmation of findings (set II). Sequences within the set II pools were distinct from those described in reference 19.
Western blotting.
To detect Rap1 levels, HMVEC lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by immunoblotting with anti-total-Rap1 (7) and anti-GAPDH (Biodesign, Saco, ME) antiantibodies. For detecting phospho-ERK1/2 and phospho-p38 levels, HMVECs were lysed with phospho-Tyr protecting lysis buffer (1% Triton X-100, 10% glycerol, 50 mM NaCl, 50 mM HEPES, 2 mM EDTA, 1 mM Na3VO4, 10 mM NaF, 10 mM NaPO4, 10 mM p-nitrophenyl phosphate, 10 mM β-glycerol phosphate, and protease inhibitor cocktail). Samples were subjected to SDS-PAGE and immunoblotted with anti-phospho-ERK1/2 and anti-total-ERK1/2 or anti-phospho-p38 and anti-total-p38 (Cell Signaling) antibodies.
Rap1 activation assay.
HMVECs were deprived of serum and growth factors for 18 h and stimulated with 25 ng/ml FGF2. GTP-Rap1 was pulled down using RalGDS-RBD-glutathione S-transferase (GST) immobilized to glutathione agarose beads (Sigma) and detected using anti-Rap1 antibody after SDS-PAGE (7).
Rac activation assay.
HMVECs transfected with different siRNAs were deprived of serum and growth factors for 18 h and stimulated with 25 ng/ml FGF2 and 10 μg/ml heparin. GTP-Rac was pulled down using GST-PAK1-RBD immobilized to glutathione agarose beads and detected using anti-Rac antibody (Upstate Biotechnology, Lake Placid, NY) after SDS-PAGE.
Wound healing assay.
HMVECs transfected with different siRNAs were seeded at 8 × 105 cells/plate on 60-mm dishes and cultured for 1 day to reach confluence. The cell monolayer was scraped with a sterile razor blade to remove cells in one direction as well as to create a small incision on the plate to mark the start line of cell migration. The plate was washed twice with PBS to remove floating cells and incubated with EBM-2 supplemented with 25 ng/ml FGF2 to stimulate migration. Photographs were taken after 24 h, and the distance of cell migration was measured using ImageJ software (NIH).
Adhesion assay.
A 96-well plate was coated with 10 μg/ml rat tail collagen I (BD Biosciences) or 5 μg/ml fibronectin (BD Biosciences) at 4°C overnight. The wells were washed twice with PBS and blocked in 1% BSA at 37°C for 1 h. HMVECs were transfected with different siRNAs at 1 × 104 cells/well in 50 μl EBM-2. The cells were allowed to adhere for 30 min before they were washed twice with PBS and fixed in 10% methanol-10% acetic acid. The fixed cells were stained for protein with 0.5% crystal violet (Sigma)-10% methanol for 10 min. The wells were then washed extensively with PBS, the dye was dissolved in 10% acetic acid, and absorbance at a 600-nm wavelength was measured using a Spectra Max 250 model plate reader (Molecular Devices, Sunnyvale, CA).
Permeability assay.
Transwell (BD Biosciences) filters (24 mm) with a 0.4-μm pore size were coated with 10 μg/ml rat tail collagen I at 4°C overnight and washed twice with PBS. HMVECs transfected with various siRNAs were plated onto the filters at 2 × 105 cells/well in mvEGM-2. Cells were cultured for 48 h to reach confluence. Transendothelial resistance was measured using an Evom volt-ohm meter (World Precision Instruments, Sarasota, FL).
In vitro tube formation assay.
HMVECs transfected with siRNAs were seeded on growth factor-reduced Matrigel at 1 × 104 cells/well in a 96-well plate in EBM-2 supplemented with 25 ng/ml FGF2 or PBS. Cells were cultured for 12 h, and photographs were taken at 4, 8, and 12 h. Photos were processed using Photoshop software (Adobe Software).
Proliferation assay.
HMVECs transfected with siRNAs were seeded at 1 × 104 cells/well in a 96-well plate and cultured overnight in mvEGM-2 medium. The cells were then washed twice with PBS and cultured in 100 μl of EBM-2 supplemented with different concentrations of FGF2 for 72 h. Cell proliferation was measured using a CellTiter 96 proliferation assay kit (Promega, Madison, WI) per the manufacturer's protocol.
Statistical analysis.
Data from mouse Matrigel and aortic ring assays were analyzed with one-way analysis of variance. Data from all other experiments were compared and analyzed using Student's t test. A P value of <0.05 was considered significant.
RESULTS
Loss of Rap1a in mice abolished the angiogenic response to FGF2.
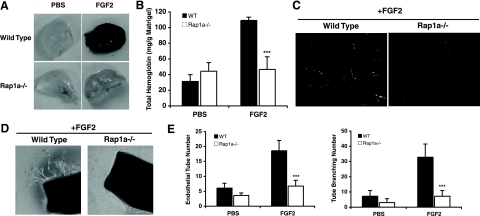
Increased vascular permeability is a key early step during angiogenesis. Since Rap1 activation enhances endothelial cell junction formation and inhibition of Rap1 promotes endothelial permeability (13, 18, 29, 55), we determined whether the loss of Rap1a would potentiate the angiogenic response to FGF2, a potent stimulator of blood vessel formation (37). The wild-type and rap1a−/− mice were injected with Matrigel impregnated with heparin in the presence or absence of FGF2. New blood vessel formation was then examined after 6 days. Surprisingly, despite a robust angiogenic response to FGF2 in wild-type mice (Fig. 1A) that resulted in a threefold increase in the hemoglobin content of Matrigel plugs (Fig. 1B), little or no blood vessel formation was induced by the growth factor in the rap1a−/− mice (Fig. 1A and B). This result was also reflected in the reduced migration of CD31-positive endothelial cells into the Matrigel plugs in the rap1a−/− mice, as determined by immunofluorescence staining (Fig. 1C).
FIG. 1.
rap1a knockout mice had an impaired angiogenic response to FGF2. (A) Matrigel plugs containing 20 U/ml heparin plus either PBS or 600 ng/ml FGF2 were injected subcutaneously into wild-type and rap1a−/− mice. The Matrigel plugs were recovered at day 7, and images were taken. (B) Hemoglobin content of recovered Matrigel plugs, reflecting new blood vessel formation. Bars show means ± standard errors. ***, P < 0.001 (n = 8). (C) Endothelial cells migrated into Matrigel plugs were revealed by CD31 staining. Images are representative of experiments from five mice of each genotype. (D) Aortic rings of 1-mm thickness from either the wild-type or the rap1a−/− mice were embedded in Matrigel supplemented with 20 U/ml heparin and 2% FBS, with or without 25 ng/ml FGF2. The outgrowth of aortic tubes was observed at day 7, and representative images are shown. (E) Quantification of aortic tube outgrowth and branching. Bars show means ± standard deviations. ***, P < 0.001 (n = 5).
Because endothelial cells, pericytes, and inflammatory cells all contribute to angiogenesis (49, 52) and we have previously established numerous defects in leukocyte function in the rap1a−/− mice (32), we next wished to determine if the reduction in angiogenesis observed for Rap1a null mice was attributable to defects in endothelial cell function. Therefore, the ability of FGF2 to induce endothelial tube outgrowth from small slices of aorta embedded in Matrigel was measured. Aortic rings from wild-type mice had profound sprouting and branching of endothelial cell-derived tubes. In contrast, rings derived from the rap1a−/− mice exhibited only minimum tube outgrowth or branching even following FGF2 stimulation (Fig. 1D and E). This finding suggested that an endothelial cell defect in Rap1a null mice might be responsible for the observed attenuation of angiogenesis.
siRNA successfully knocked down rap1a and rap1b in HMVECs.
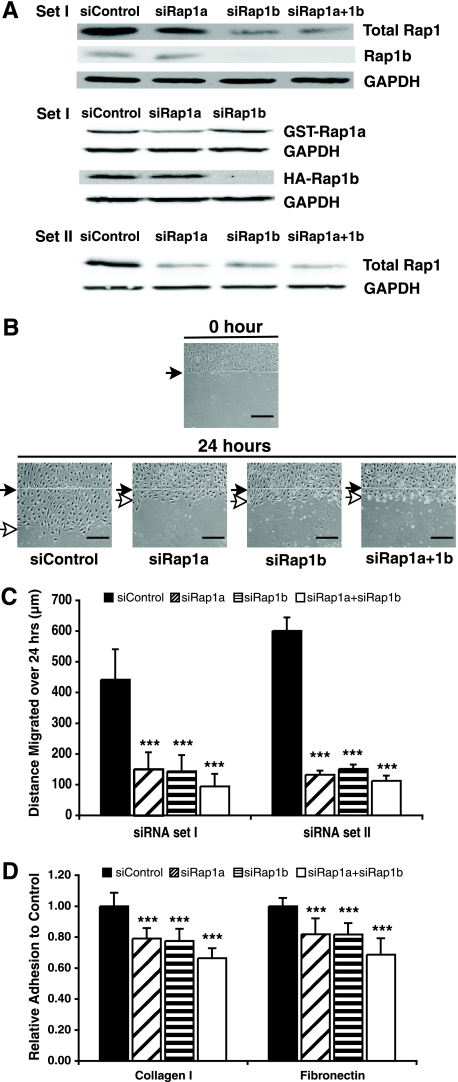
Intrigued by the striking phenotypes manifested by the rap1a−/− mice and given the high similarity of the two Rap1 family members, Rap1a and Rap1b, we began to investigate those endothelial cell activities that were blocked by the loss of Rap1 and to determine whether these effects were Rap1a or Rap1b specific. We also wished to determine if Rap1 mediated similar events in human cells. Therefore, siRNAs against rap1a or rap1b were used to reduce Rap1a or Rap1b expression in HMVECs. These siRNAs specifically and effectively reduced Rap1 levels (Fig. 2A).
FIG. 2.
Suppression of Rap1 expression decreased HMVEC migration, as shown in a wound healing assay, and adhesion to extracellular matrix proteins. (A) siRNAs selectively knocked down endogenous Rap1a or Rap1b expression in HMVECs and exogenous Rap1a or Rap1b in 293T cells. (Upper panel) Endogenous total Rap1, Rap1b, and GAPDH levels in HMVECs; (middle panel) GST-Rap1a and HA-Rap1b levels in 293T cells; (bottom panel) total Rap1 level and GAPDH from an additional set of siRNAs. (B) HMVECs transfected with two distinct sets of siRNAs were cultured until cells were confluent. Representative data are shown for set I siRNAs. Cells were removed in one direction by a razor blade, and a small incision was made in the plastic to mark the starting point of migration. The distance that cells migrated was recorded after 24 h. Filled arrows indicate the starting point of migration; open arrows indicate the end point of migration. Scale bar = 100 μm. (C) Quantification of cell migration. Bars show means ± standard deviations (SD). ***, P < 0.001 (n = 3). (D) HMVECs transfected with different siRNAs (set I) were plated on type I collagen or fibronectin matrices in 96-well plates for 30 min. Adherent cells were quantitated by staining with crystal violet and measuring absorbance at 600 nm. Bars show means ± SD. ***, P < 0.001 (n = 4).
Rap1a- or Rap1b-depleted endothelial cells had reduced migration and adhesion.
Migration of endothelial cells is essential during angiogenesis, and the inactivation of all Rap family members (Rap1a, -1b, -2a, -2b, and -2c) by Rap1GAP in HUVECs impaired the cells' directional migration as shown in a wound-healing assay (17). To address the individual impacts Rap1a and Rap1b have on cell migration, HMVEC monolayers treated with different siRNAs were wounded, and cell migration into the cleared area was monitored. HMVECs depleted of either Rap1a or Rap1b displayed dramatically delayed directional migration compared to that of cells transfected with control siRNA (more than 60% reduction over the area of migration over 24 h [Fig. 2B and C]).
Cell migration is achieved by regulating integrin-mediated adhesion at the leading and trailing edges of a cell (22). Since Rap1 plays a role in inside-out signaling to regulate integrin activation (16, 21, 26), we suspected that endothelial cell adhesion might also be reduced upon the loss of Rap1. Indeed, HMVEC adhesion to both collagen and fibronectin was significantly decreased in Rap1a null or Rap1b null cells, and an additional 10% decrease in adhesion was observed for cells depleted of both GTPases (Fig. 2D). Similar findings were obtained with additional (set II) rap1a and rap1b siRNAs (J. Yan and L. A. Quilliam, unpublished data).
Cell-cell contact was compromised when rap1a or rap1b was knocked down in endothelial cells.
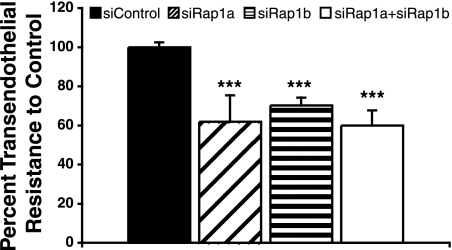
VE-cadherin mediates endothelial cell junction formation, and Rap1 has been shown to regulate VE-cadherin activity (13, 18, 29, 55). We therefore determined the ability of Rap1 null endothelial cells to form an intact monolayer. HMVECs transfected with various siRNAs were plated in Transwells, and transendothelial resistance was measured. Endothelial cells lacking either isoform exhibited decreased transendothelial resistance (Fig. 3), indicating inefficiency in junction formation and increased permeability.
FIG. 3.
Knockdown of Rap1 expression increased HMVEC junction permeability. HMVECs transfected with control, rap1a, rap1b, or rap1a plus rap1b siRNAs were plated on 0.4-μm filters and cultured until confluent. Transendothelial resistance was measured as an indicator of endothelial monolayer integrity. Bars show means ± standard deviations. ***, P < 0.001 (n = 8).
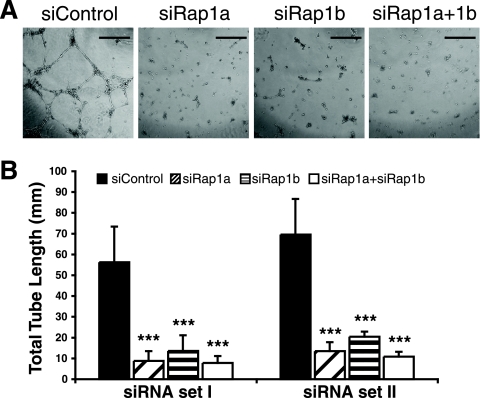
Depletion of Rap1 in endothelial cells inhibited tube formation in vitro.
To further verify that endothelial cell Rap1 expression is critical for FGF2-induced angiogenesis, we performed an in vitro endothelial cell tube formation assay with Matrigel that mimics capillary formation in vivo. Suppressing the expression of Rap1a or -1b in endothelial cells completely blocked tube formation, whereas control cells developed an intricate tubular structure upon induction by FGF2 (Fig. 4). When control endothelial cells were plated on Matrigel in the absence of growth factors, they transiently formed tubules that rapidly regressed, but this too was lost upon depletion of Rap1 (J. Yan, and L. A. Quilliam, unpublished data).
FIG. 4.
Reducing Rap1 expression abolished HMVEC tube formation on Matrigel. HMVECs transfected with two distinct sets of control, rap1a, rap1b, or rap1a plus rap1b siRNAs were plated on Matrigel in vitro in the presence of 25 ng/ml FGF2, and tubular structures were allowed to develop for 12 h. (A) Representative images of HMVEC tube formation. Scale bar = 500 μm. (B) Quantification of total tube length. Bars show means ± standard deviations. ***, P < 0.001 (n = 3).
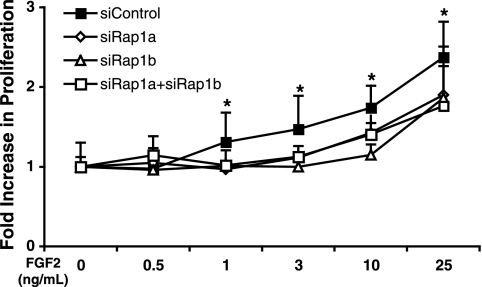
Rap1-deficient HMVECs have a slower proliferation rate.
Angiogenesis is usually accompanied by endothelial cell proliferation (37). We, thus, sought to determine whether the loss of Rap1 affected this process. HMVECs transfected with different rap1 siRNAs were treated with various concentrations of FGF2 to stimulate proliferation. Modest yet statistically significant decreases in the proliferation rate at concentrations of 1, 3, 10, and 25 ng/ml FGF2 were observed (Fig. 5).
FIG. 5.
Loss of Rap1 decreased HMVEC proliferation. HMVECs transfected with control, rap1a, rap1b, or rap1a plus rap1b siRNAs were cultured for 72 h in EBM-2 with different concentrations of FGF2 as indicated. Cell proliferation was measured and expressed as the increase in absorbance versus the control. *, P < 0.05 on siRNA control versus that of rap1 siRNA-treated cells (n = 3).
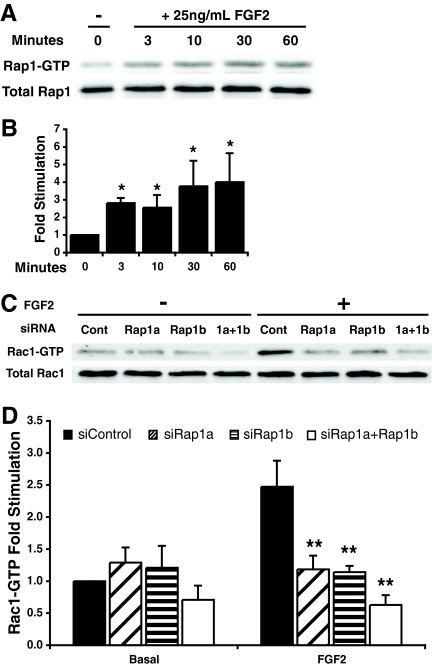
Rac activation was abolished in endothelial cells depleted of Rap1.
The fact that FGF2-induced blood vessel formation was blocked despite increased vascular permeability suggested that Rap1 might be required to mediate the FGF2-induced signaling events responsible for angiogenesis. Consistent with this notion, Rap1 was activated by FGF2 within 3 min of HMVEC stimulation, and this activation was sustained for at least 60 min (Fig. 6A and B). Since the Rho family GTPase Rac is a downstream effector of Rap1 (1) and is required for endothelial cell tube formation on Matrigel (10), we next determined if Rap1-mediated FGF2-induced Rac activation. HMVECs transfected with rap1a or rap1b siRNAs failed to activate Rac1/2 in response to FGF2 (Fig. 6C and D), suggesting that Rap1 also regulates actin cytoskeleton rearrangement in endothelial cells via Rac.
FIG. 6.
Rap1 depletion abolished Rac activation in HMVECs. (A) HMVECs were starved overnight and stimulated with 25 ng/ml FGF2 for 3 to 60 min. Immunoblotting for Rap1-GTP and total Rap1 levels was performed. (B) Densitometric quantification of protein levels. Bars show means ± standard deviations (SD). *, P < 0.05 (n = 4). (C) HMVECs transfected with control, rap1a, rap1b, or rap1a plus rap1b siRNAs were starved overnight and stimulated with 25 ng/ml FGF2 and 10 μg/ml heparin for 10 min. Immunoblot assays for Rac-GTP and total Rac level were performed. (D) Densitometric quantification of protein levels. Bars show means ± SD; **, P < 0.01; n = 4.
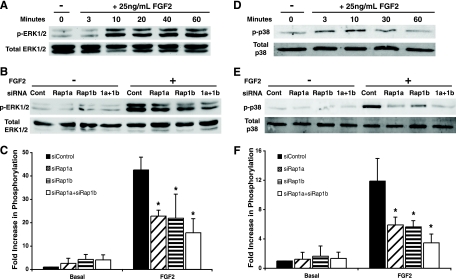
ERK1/2 and p38 phosphorylation was reduced in Rap1-depleted endothelial cells.
FGF2 signaling through ERK has been shown to play vital roles in endothelial cell functions, including proliferation and vascularization (14), and Rap1 can activate ERK in cell types that express B-Raf (54). ERK phosphorylation in response to FGF2 in HMVECs correlated with Rap1 activation, which occurred within 10 min and was sustained for at least 1 h (Fig. 6C). To test whether endothelial defects might be attributable to reduced ERK phosphorylation, HMVECs treated with rap1 siRNAs were stimulated with FGF2, and ERK phosphorylation was measured. Suppression of either rap1a or rap1b expression significantly reduced ERK1/2 phosphorylation in response to FGF2 (Fig. 6D and E). The use of additional rap1a and rap1b siRNAs (set II) resulted in a similar reduction in ERK phosphorylation (J. Yan and L. A. Quilliam, unpublished data). These findings suggest that Rap1 is responsible for coupling FGFR1 to ERK phosphorylation to regulate angiogenesis.
In an attempt to identify other downstream targets of Rap1 in endothelial cells, we examined the phosphorylation of p38, another mitogen-activated protein kinase (MAPK) that is important for endothelial cell migration and angiogenesis (14, 24). FGF2 induced a rapid but transient phosphorylation of HMVEC p38 that peaked at 10 min (Fig. 7D). This activation was reduced by ∼50% following the transfection of rap1 siRNAs (Fig. 7E and F).
FIG. 7.
Rap1 mediated ERK1/2 and p38 activation by FGF2 in HMVECs. (A) HMVECs were starved overnight and stimulated with 25 ng/ml FGF2 for 3 to 60 min. Immunoblot assays for phosphorylated ERK1/2 and total ERK1/2 are shown. (B) HMVECs transfected with control, rap1a, rap1b, or rap1a plus rap1b siRNAs were starved overnight and incubated with or without 25 ng/ml FGF2 for 10 min. Immunoblot assays for phosphorylated and total ERK1/2 are shown. (C) Densitometric quantification of protein levels. (D) HMVECs were starved overnight and stimulated with 25 ng/ml FGF2 for 3 to 60 min. Immunoblot assays for phosphorylated p38 and total p38 are shown. (E) HMVECs were treated as described for panel B. Immunoblot assays for phosphorylated and total p38 are shown. (F) Densitometric quantification of protein levels. Bars in panels C and F show means ± standard deviations. *, P < 0.05 (n = 3).
DISCUSSION
Multiple studies have implicated Rap1 in endothelial cell functions that include migration and junction formation (13, 17, 18, 29, 39, 41, 55), suggesting that this GTPase might also participate in angiogenesis. However, a direct role for Rap1 in this process had not been established. Here, we have identified Rap1a as an indispensable factor for FGF2-induced angiogenesis in mice, and we determined that both Rap1a and Rap1b are required for normal human endothelial cell function. Suppressing the expression of either of the Rap1 proteins severely impaired human endothelial cell adhesion, migration, cell junction formation, and vascular tube formation. Rac, ERK, and p38 have all been implicated in FGF2-induced angiogenesis. Suppressing Rap1a or -1b expression attenuated the ability of FGF2 to activate each of these signaling molecules, suggesting that Rap1 is responsible for coupling the FGF receptor 1 to multiple downstream pathways to mediate blood vessel formation.
The failure of new blood vessels to develop within the Matrigel plugs introduced into Rap1a null mice is supportive of an endothelial cell defect in these animals. While Rap1a could also contribute to angiogenesis via regulation of pericytes/vascular smooth muscle cells (25, 42, 43), macrophages (32, 49), and/or endothelial progenitor cells (6, 20, 37), data from the ex vivo aortic ring assay are supportive of a key role for Rap1 in endothelial cells. Increased vascular permeability is usually considered the first step in angiogenesis (37). Since this and previous studies have implicated Rap1 in the regulation of endothelial cell junction formation (13, 18, 29, 55), we anticipated that the loss of Rap1a would lead to enhanced angiogenesis. However, the robust vessel formation observed for wild-type mice in response to FGF2 was completely absent from the knockouts. Therefore, the lack of vessel leakiness was not a major determinant for the defective angiogenic response of Rap1a null mice to FGF2.
A key step during angiogenesis is the migration of endothelial cells to the source of oxygen insufficiency and cytokine release. Findings that support a role for Rap in this process include the fact that reduced expression of the Rap GEF C3G is embryonic lethal due to vascular defects (53) and that Rap has been implicated in endothelial cell migration (17, 23, 39). However, previous studies employed Rap GAPs and GEFs that do not discriminate between Rap family members (Rap1a, -1b, -2a, -2b, and/or -2c), making it impossible to determine the specific role(s) that each GTPase plays. We have shown here that Rap1a and Rap1b are equally important in the regulation of endothelial cell function. The fact that suppressing the expression of either of the Rap1 proteins had similar effects on endothelial cell adhesion/migration and that knocking them both down had additional impacts suggest that these two closely related proteins could serve nonredundant functions. However, despite the fact that Rap1a and Rap1b have divergent C termini and a unique codon 48 adjacent to the effector-binding loop, no protein has been identified that interacts specifically with Rap1a and not Rap1b. It is possible, therefore, that just as Rap1 and Ras can be independently activated to regulate B-Raf, Rap1a and Rap1b utilize the same downstream effectors and that knocking down the expression of either GTPase simply reduced the intracellular pool of total Rap1, causing a similar cellular outcome. After the completion of this study, Chrzanowska-Wodnicka et al. reported that rap1b−/− mice also exhibited defective angiogenesis in response to vascular endothelial growth factor (VEGF) or a combination of FGF2 plus VEGF and that lung endothelial cells derived from these mice exhibited similarly reduced migration (8). These findings support our observation for human cells that the loss of Rap1a or -1b achieves the same biological outcome.
The formation of delicate tubular structures by HMVECs with Matrigel requires extensive cytoskeletal rearrangements. Since Rap1 can activate Rac, a Rho family GTPase required for endothelial cell tube formation (10, 33), and can control cell morphology by localizing the Rac GEFs Vav2 and Tiam1 (1), it was not surprising that the loss of Rap1 not only reduced HMVEC tubule formation following siRNA knockdown of rap1 but this was associated with decreased Rac activation. Both Tiam1 and Vav2 have been implicated downstream of Rap1 in regulating endothelial cell permeability/monolayer integrity (2).
Our data also suggest that a novel FGF2-Rap1-ERK signaling pathway in endothelial cells is important for angiogenesis. ERK1 and ERK2 play a pivotal role during both developmental neovascularization and pathological angiogenesis (14). It is well established that FGF2 signals via Ras to activate ERK and to induce endothelial cell proliferation and differentiation (12, 28, 56). Here, FGF2 was also found to promote both rapid and sustained Rap1 activation, and knocking down either rap1a or rap1b substantially reduced FGF2-induced ERK activation. While this is the first demonstration of Rap1-mediated ERK activation by FGF2, it is consistent with a previous finding in brain endothelial cells in which dominant-negative H-Ras abrogated FGF2-induced Ras activation but only partially reduced ERK activation and did not block FGF2-induced differentiation (45). The phosphorylation of ERK in the absence of Ras activation was likely mediated by Rap1-GTP. Since ERK activation is important for proliferation, the reduced phospho-ERK levels may have been responsible for the slower growth rate of HMVECs following the suppression of Rap1a or -1b expression in the current study.
The ERK and p38 MAPKs work in a coordinated fashion downstream of FGF2 to regulate endothelial cell functions, and p38 has been reported to induce an antiproliferative, promigratory phenotype (34, 35). In other cell types, Rap1 has been described both upstream and downstream of p38 activation (48, 50). In our hands, p38 was rapidly but transiently activated by FGF2 in HMVECs in a Rap1-dependent manner, suggesting that this GTPase utilizes p38 during the early events of FGF2-induced endothelial tubulogenesis. These studies indicate that Rap1 regulates MAPK pathways previously associated with FGF2-induced Ras activation in endothelial cells. It is not surprising, therefore, that the loss of Rap1 directly impacts FGF2-induced angiogenesis.
FGF2 promotes autophosphorylation of its receptor, FGFR-1, providing binding sites for several signaling molecules. The adaptor protein Crk binds to phospho-Y463 in the juxtamembrane region of FGFR-1 (31) and recruits RapGEF1/C3G. Crk1, Rap1-GTP, and the Rap1 effector RAPL all localize to the leading edge of human aortic endothelial cells (17, 39). It is possible, therefore, that FGF2 activates Rap1 via Crk-C3G. FGFR-1 also activates ERK via phospholipase C-γ (28, 38) in a protein kinase C-independent manner to regulate tubule formation (12, 28). Since RasGRP3, a Rap1 GEF that is activated by diacylglycerol and/or Ca2+ downstream of phospholipase C, has been identified in angiogenic vessels (47), it is possible that FGF2 utilizes both Crk/C3G and/or phospholipase C-γ/RasGRP3 pathways to activate Rap1 in endothelial cells.
In summary, the data presented here identify Rap1 as a downstream target of FGFR1 and demonstrate that Rap1a is involved in FGF2-induced angiogenesis, both in mouse cells and in human cells. Both Rap1a and Rap1b play a role in human endothelial cell adhesion, migration, monolayer permeability, and tubule formation induced by FGF receptors, coupling them to ERK, p38, and Rac activation. These data suggest that Rap1 is a key mediator of new blood vessel formation and that the rap1a−/− mouse may be a useful model for understanding cell signaling events associated with human angiogenesis.
Acknowledgments
This work was supported by National Institutes of Health grants HL080166 and CA108645 (L.A.Q.) and American Heart Association predoctoral fellowship 0810081Z (J.Y.).
Footnotes
Published ahead of print on 14 July 2008.
REFERENCES
- 1.Arthur, W. T., L. A. Quilliam, and J. A. Cooper. 2004. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J. Cell Biol. 167111-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birukova, A. A., T. Zagranichnaya, E. Alekseeva, G. M. Bokoch, and K. G. Birukov. 2008. Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J. Cell. Physiol. 215715-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bos, J. L. 1998. All in the family? New insights and questions regarding interconnectivity of Ras, Rap1 and Ral. EMBO J. 176776-6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bos, J. L., H. Rehmann, and A. Wittinghofer. 2007. GEFs and GAPs: critical elements in the control of small G proteins. Cell 129865-877. [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet, P. 2003. Angiogenesis in health and disease. Nat. Med. 9653-660. [DOI] [PubMed] [Google Scholar]
- 6.Carmona, G., E. Chavakis, U. Koehl, A. M. Zeiher, and S. Dimmeler. 2007. Activation of Epac stimulates integrin-dependent homing of progenitor cells. Blood 1112640-2646. [DOI] [PubMed] [Google Scholar]
- 7.Castro, A. F., J. F. Rebhun, and L. A. Quilliam. 2005. Measuring Ras-family GTP levels in vivo—running hot and cold. Methods 37190-196. [DOI] [PubMed] [Google Scholar]
- 8.Chrzanowska-Wodnicka, M., A. E. Kraus, D. Gale, G. C. White II, and J. Vansluys. 2007. Defective angiogenesis, endothelial migration, proliferation and MAPK signaling in Rap1b-deficient mice. Blood 1112647-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chrzanowska-Wodnicka, M., S. S. Smyth, S. M. Schoenwaelder, T. H. Fischer, and G. C. White II. 2005. Rap1b is required for normal platelet function and hemostasis in mice. J. Clin. Investig. 115680-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connolly, J. O., N. Simpson, L. Hewlett, and A. Hall. 2002. Rac regulates endothelial morphogenesis and capillary assembly. Mol. Biol. Cell 132474-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook, S. J., B. Rubinfeld, I. Albert, and F. McCormick. 1993. RapV12 antagonizes Ras-dependent activation of ERK1 and ERK2 by LPA and EGF in Rat-1 fibroblasts. EMBO J. 123475-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cross, M. J., L. Lu, P. Magnusson, D. Nyqvist, K. Holmqvist, M. Welsh, and L. Claesson-Welsh. 2002. The Shb adaptor protein binds to tyrosine 766 in the FGFR-1 and regulates the Ras/MEK/MAPK pathway via FRS2 phosphorylation in endothelial cells. Mol. Biol. Cell 132881-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cullere, X., S. K. Shaw, L. Andersson, J. Hirahashi, F. W. Luscinskas, and T. N. Mayadas. 2005. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 1051950-1955. [DOI] [PubMed] [Google Scholar]
- 14.Depeille, P. E., Y. Ding, J. L. Bromberg-White, and N. S. Duesbery. 2007. MKK signaling and vascularization. Oncogene 261290-1296. [DOI] [PubMed] [Google Scholar]
- 15.Duchniewicz, M., T. Zemojtel, M. Kolanczyk, S. Grossmann, J. S. Scheele, and F. J. Zwartkruis. 2006. Rap1A-deficient T and B cells show impaired integrin-mediated cell adhesion. Mol. Cell. Biol. 26643-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enserink, J. M., L. S. Price, T. Methi, M. Mahic, A. Sonnenberg, J. L. Bos, and K. Tasken. 2004. The cAMP-Epac-Rap1 pathway regulates cell spreading and cell adhesion to laminin-5 through the alpha3beta1 integrin but not the alpha6beta4 integrin. J. Biol. Chem. 27944889-44896. [DOI] [PubMed] [Google Scholar]
- 17.Fujita, H., S. Fukuhara, A. Sakurai, A. Yamagishi, Y. Kamioka, Y. Nakaoka, M. Masuda, and N. Mochizuki. 2005. Local activation of Rap1 contributes to directional vascular endothelial cell migration accompanied by extension of microtubules on which RAPL, a Rap1-associating molecule, localizes. J. Biol. Chem. 2805022-5031. [DOI] [PubMed] [Google Scholar]
- 18.Fukuhara, S., A. Sakurai, H. Sano, A. Yamagishi, S. Somekawa, N. Takakura, Y. Saito, K. Kangawa, and N. Mochizuki. 2005. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol. Cell. Biol. 25136-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao, L., Y. Feng, R. Bowers, M. Becker-Hapak, J. Gardner, L. Council, G. Linette, H. Zhao, and L. A. Cornelius. 2006. Ras-associated protein-1 regulates extracellular signal-regulated kinase activation and migration in melanoma cells: two processes important to melanoma tumorigenesis and metastasis. Cancer Res. 667880-7888. [DOI] [PubMed] [Google Scholar]
- 20.Goichberg, P., A. Kalinkovich, N. Borodovsky, M. Tesio, I. Petit, A. Nagler, I. Hardan, and T. Lapidot. 2006. cAMP-induced PKCzeta activation increases functional CXCR4 expression on human CD34+ hematopoietic progenitors. Blood 107870-879. [DOI] [PubMed] [Google Scholar]
- 21.Han, J., C. J. Lim, N. Watanabe, A. Soriani, B. Ratnikov, D. A. Calderwood, W. Puzon-McLaughlin, E. M. Lafuente, V. A. Boussiotis, S. J. Shattil, and M. H. Ginsberg. 2006. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr. Biol. 161796-1806. [DOI] [PubMed] [Google Scholar]
- 22.Hogg, N., M. Laschinger, K. Giles, and A. McDowall. 2003. T-cell integrins: more than just sticking points. J. Cell Sci. 1164695-4705. [DOI] [PubMed] [Google Scholar]
- 23.Hong, J., R. C. Doebele, M. W. Lingen, L. A. Quilliam, W. J. Tang, and M. R. Rosner. 2007. Anthrax edema toxin inhibits endothelial cell chemotaxis via Epac and Rap1. J. Biol. Chem. 28219781-19787. [DOI] [PubMed] [Google Scholar]
- 24.Huang, C., K. Jacobson, and M. D. Schaller. 2004. MAP kinases and cell migration. J. Cell Sci. 1174619-4628. [DOI] [PubMed] [Google Scholar]
- 25.Kanda, Y., and Y. Watanabe. 2007. Adrenaline increases glucose transport via a Rap1-p38MAPK pathway in rat vascular smooth muscle cells. Br. J. Pharmacol. 151476-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katagiri, K., A. Maeda, M. Shimonaka, and T. Kinashi. 2003. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat. Immunol. 4741-748. [DOI] [PubMed] [Google Scholar]
- 27.Kitayama, H., Y. Sugimoto, T. Matsuzaki, Y. Ikawa, and M. Noda. 1989. A ras-related gene with transformation suppressor activity. Cell 5677-84. [DOI] [PubMed] [Google Scholar]
- 28.Klint, P., S. Kanda, Y. Kloog, and L. Claesson-Welsh. 1999. Contribution of Src and Ras pathways in FGF-2 induced endothelial cell differentiation. Oncogene 183354-3364. [DOI] [PubMed] [Google Scholar]
- 29.Kooistra, M. R., M. Corada, E. Dejana, and J. L. Bos. 2005. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 5794966-4972. [DOI] [PubMed] [Google Scholar]
- 30.Lafuente, E. M., A. A. van Puijenbroek, M. Krause, C. V. Carman, G. J. Freeman, A. Berezovskaya, E. Constantine, T. A. Springer, F. B. Gertler, and V. A. Boussiotis. 2004. RIAM, an Ena/VASP and Profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev. Cell 7585-595. [DOI] [PubMed] [Google Scholar]
- 31.Larsson, H., P. Klint, E. Landgren, and L. Claesson-Welsh. 1999. Fibroblast growth factor receptor-1-mediated endothelial cell proliferation is dependent on the Src homology (SH) 2/SH3 domain-containing adaptor protein Crk. J. Biol. Chem. 27425726-25734. [DOI] [PubMed] [Google Scholar]
- 32.Li, Y., J. Yan, P. De, H.-C. Chang, A. Yamauchi, K. W. Christopherson II, N. C. Paranavitana, X. Peng, C. Kim, V. Munugalavadla, R. Kapur, J. C. Stone, M. H. Kaplan, M. C. Dinauer, D. L. Durden, and L. A. Quilliam. 2007. Rap1a null mice have altered myeloid cell functions suggesting distinct roles for the closely related Rap1a and 1b proteins. J. Immunol. 1798322-8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maillet, M., S. J. Robert, M. Cacquevel, M. Gastineau, D. Vivien, J. Bertoglio, J. L. Zugaza, R. Fischmeister, and F. Lezoualc'h. 2003. Crosstalk between Rap1 and Rac regulates secretion of sAPPalpha. Nat. Cell Biol. 5633-639. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto, T., I. Turesson, M. Book, P. Gerwins, and L. Claesson-Welsh. 2002. p38 MAP kinase negatively regulates endothelial cell survival, proliferation, and differentiation in FGF-2-stimulated angiogenesis. J. Cell Biol. 156149-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMullen, M. E., P. W. Bryant, C. C. Glembotski, P. A. Vincent, and K. M. Pumiglia. 2005. Activation of p38 has opposing effects on the proliferation and migration of endothelial cells. J. Biol. Chem. 28020995-21003. [DOI] [PubMed] [Google Scholar]
- 36.Mei, F. C., J. Qiao, O. M. Tsygankova, J. L. Meinkoth, L. A. Quilliam, and X. Cheng. 2002. Differential signaling of cyclic AMP: opposing effects of exchange protein directly activated by cyclic AMP and cAMP-dependent protein kinase on protein kinase B activation. J. Biol. Chem. 27711497-11504. [DOI] [PubMed] [Google Scholar]
- 37.Milkiewicz, M., E. Ispanovic, J. L. Doyle, and T. L. Haas. 2006. Regulators of angiogenesis and strategies for their therapeutic manipulation. Int. J. Biochem. Cell Biol. 38333-357. [DOI] [PubMed] [Google Scholar]
- 38.Mohammadi, M., A. M. Honegger, D. Rotin, R. Fischer, F. Bellot, W. Li, C. A. Dionne, M. Jaye, M. Rubinstein, and J. Schlessinger. 1991. A tyrosine-phosphorylated carboxy-terminal peptide of the fibroblast growth factor receptor (Flg) is a binding site for the SH2 domain of phospholipase C-γ1. Mol. Cell. Biol. 115068-5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagashima, K., A. Endo, H. Ogita, A. Kawana, A. Yamagishi, A. Kitabatake, M. Matsuda, and N. Mochizuki. 2002. Adaptor protein Crk is required for ephrin-B1-induced membrane ruffling and focal complex assembly of human aortic endothelial cells. Mol. Biol. Cell 134231-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohba, Y., K. Ikuta, A. Ogura, J. Matsuda, N. Mochizuki, K. Nagashima, K. Kurokawa, B. J. Mayer, K. Maki, J. Miyazaki, and M. Matsuda. 2001. Requirement for C3G-dependent Rap1 activation for cell adhesion and embryogenesis. EMBO J. 203333-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orlova, V. V., M. Economopoulou, F. Lupu, S. Santoso, and T. Chavakis. 2006. Junctional adhesion molecule-C regulates vascular endothelial permeability by modulating VE-cadherin-mediated cell-cell contacts. J. Exp. Med. 2032703-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quarck, R., E. Berrou, C. Magnier, R. Bobe, R. Bredoux, G. Tobelem, J. Enouf, and M. Bryckaert. 1996. Differential up-regulation of Rap1a and Rap1b proteins during smooth muscle cell cycle. Eur. J. Cell Biol. 70269-277. [PubMed] [Google Scholar]
- 43.Quarck, R., M. Bryckaert, C. Magnier, E. Corvazier, R. Bredoux, J. de Gunzburg, M. Fontenay, G. Tobelem, and J. Enouf. 1994. Evidence for Rap1 in vascular smooth muscle cells. Regulation of their expression by platelet-derived growth factor BB. FEBS Lett. 342159-164. [DOI] [PubMed] [Google Scholar]
- 44.Quilliam, L. A., J. F. Rebhun, and A. F. Castro. 2002. A growing family of guanine nucleotide exchange factors is responsible for activation of Ras-family GTPases. Prog. Nucleic Acid Res. Mol. Biol. 71391-444. [DOI] [PubMed] [Google Scholar]
- 45.Rennel, E., M. J. Cross, P. Klint, X. Bai, J. L. Arbiser, and P. Gerwins. 2003. Regulation of endothelial cell differentiation and transformation by H-Ras. Exp. Cell Res. 291189-200. [DOI] [PubMed] [Google Scholar]
- 46.Risau, W. 1997. Mechanisms of angiogenesis. Nature 386671-674. [DOI] [PubMed] [Google Scholar]
- 47.Roberts, D. M., A. L. Anderson, M. Hidaka, R. L. Swetenburg, C. Patterson, W. L. Stanford, and V. L. Bautch. 2004. A vascular gene trap screen defines RasGRP3 as an angiogenesis-regulated gene required for the endothelial response to phorbol esters. Mol. Cell. Biol. 2410515-10528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawada, Y., K. Nakamura, K. Doi, K. Takeda, K. Tobiume, M. Saitoh, K. Morita, I. Komuro, K. De Vos, M. Sheetz, and H. Ichijo. 2001. Rap1 is involved in cell stretching modulation of p38 but not ERK or JNK MAP kinase. J. Cell Sci. 1141221-1227. [DOI] [PubMed] [Google Scholar]
- 49.Schmid, M. C., and J. A. Varner. 2007. Myeloid cell trafficking and tumor angiogenesis. Cancer Lett. 2501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt, A., E. Caron, and A. Hall. 2001. Lipopolysaccharide-induced activation of β2-integrin function in macrophages requires Irak kinase activity, p38 mitogen-activated protein kinase, and the Rap1 GTPase. Mol. Cell. Biol. 21438-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsygankova, O. M., A. Saavedra, J. F. Rebhun, L. A. Quilliam, and J. L. Meinkoth. 2001. Coordinated regulation of Rap1 and thyroid differentiation by cyclic AMP and protein kinase A. Mol. Cell. Biol. 211921-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.von Tell, D., A. Armulik, and C. Betsholtz. 2006. Pericytes and vascular stability. Exp. Cell Res. 312623-629. [DOI] [PubMed] [Google Scholar]
- 53.Voss, A. K., P. Gruss, and T. Thomas. 2003. The guanine nucleotide exchange factor C3G is necessary for the formation of focal adhesions and vascular maturation. Development 130355-367. [DOI] [PubMed] [Google Scholar]
- 54.Vossler, M. R., H. Yao, R. D. York, M. G. Pan, C. S. Rim, and P. J. Stork. 1997. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell 8973-82. [DOI] [PubMed] [Google Scholar]
- 55.Wittchen, E. S., R. A. Worthylake, P. Kelly, P. J. Casey, L. A. Quilliam, and K. Burridge. 2005. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J. Biol. Chem. 28011675-11682. [DOI] [PubMed] [Google Scholar]
- 56.Zubilewicz, A., C. Hecquet, J. C. Jeanny, G. Soubrane, Y. Courtois, and F. Mascarelli. 2001. Two distinct signalling pathways are involved in FGF2-stimulated proliferation of choriocapillary endothelial cells: a comparative study with VEGF. Oncogene 201403-1413. [DOI] [PubMed] [Google Scholar]