Abstract
Nef-induced podocyte proliferation and dedifferentiation via mitogen-activated protein kinase 1,2 (MAPK1,2) activation plays a role in human immunodeficiency virus (HIV) nephropathy pathogenesis. All-trans retinoic acid (atRA) reverses the HIV-induced podocyte phenotype by activating cyclic AMP (cAMP)/protein kinase A (PKA) and inhibiting MAPK1,2. Here we show that atRA, through cAMP and PKA, triggers a feed-forward loop involving CREB and USF1 to induce biphasic stimulation of MKP1. atRA stimulated CREB and USF1 binding to the MKP1 gene promoter, as shown by gel shifting and chromatin immunoprecipitation assays. CREB directly mediated the early phase of atRA-induced MKP1 stimulation; whereas the later phase was mediated by CREB indirectly through induction of USF1. These findings were confirmed by a reporter gene assay using the MKP1 promoter with mutation of CRE or Ebox binding sites. Consistent with these findings, the biological effects of atRA on podocytes were inhibited by silencing either MKP1, CREB, or USF1 with small interfering RNA. atRA also induced CREB phosphorylation and MKP1 expression and reduced MAPK1,2 phosphorylation in kidneys of HIV type 1-infected transgenic mice. We conclude that atRA induces sustained activation of MKP1 to suppress Nef-induced activation of the Src-MAPK1,2 pathway, thus returning the podocyte to a more differentiated state. The mechanism involves a feed-forward loop where activation of one transcription factor (TF) (CREB) leads to induction of a second TF (USF1).
Human immunodeficiency virus (HIV)-associated nephropathy (HIVAN) is an important cause of end stage renal disease in patients infected with HIV (40). Unlike most other glomerulopathies, HIVAN is characterized by proliferation and dedifferentiation of podocytes, which are glomerular visceral epithelial cells (3, 4, 17). Recently, we showed that the HIV-1 nef gene is the primary determinant of this unusual podocyte phenotype (17, 36) and that Nef stimulates podocyte proliferation and dedifferentiation through the Src-dependent mitogen- activated protein kinase 1,2 (MAPK1,2) pathways (15). Increased MAPK1,2 phosphorylation is also observed in mouse and human kidney sections of HIVAN (15).
Retinoids are derivatives of vitamin A that have multiple cellular functions including inhibition of proliferation, induction of differentiation, regulation of apoptosis, and inhibition of inflammation (11). In addition to their established benefits in treating a variety of cancers, retinoids reduce proteinuria and glomerulosclerosis in several experimental models of kidney disease (14, 23, 28, 39). Retinoids exert their effects by binding to two families of nuclear receptors, the retinoic acid receptors and the retinoid X receptors, which in turn bind to the retinoic acid response elements of gene promoters (2, 13). The activation of cytoplasmic signaling molecules by retinoids has also been reported as an important pathway for induction of leukemia cell differentiation (25). A recent study reported that all-trans retinoic acid (atRA) induces rapid cyclic AMP (cAMP) production and increased protein kinase A (PKA) activity in acute promyeloblastic leukemia cells, leading to cell differentiation (42). The antiproliferative and prodifferentiation effects of atRA suggested a rationale for studying its effect on podocytes infected with HIV type 1 (HIV-1). We found that atRA inhibits HIV-induced proliferation and dedifferentiation in podocytes (14). The mechanism involves activation of the cAMP/PKA pathway through retinoic acid receptor α, resulting in Nef-induced MAPK1,2 activation in podocytes (14).
The MAPK and PKA pathways are known to interact at the level of MAPK phosphatase (MKP). Expression of MKP1 is induced by activation of PKA, protein kinase C, or MAPKs (MAPK1,2, p38, and Jun N-terminal protein kinase [JNK]) in response to different stimulants (5, 16, 38). atRA has also been shown to increase MKP1 expression (30). While transcriptional induction of MKP1 by atRA has been proposed (41), the mechanism by which atRA regulates MKP1 and the identity of the involved transcription factors remain unknown. MKP1 has been shown to act as an anti-inflammatory and antiapoptotic agent, mostly through inhibition of p38 phosphorylation (1, 34). A recent study suggests that MKP1 mediates the anti-inflammatory effects of dexamethasone in vitro and in vivo (1). The role of MKP1 in kidney disease, but not specifically in podocytes, has been studied. One report suggests that MKP1 is suppressed in kidneys with diabetic nephropathy, leading to increased MAPK phosphorylation (19).
Here, we have studied the mechanisms by which atRA stimulates MKP1 expression. We found that atRA induced a biphasic stimulation of MKP1 in HIV-infected podocytes through cAMP-PKA pathways. CREB (cAMP response element binding protein) mediates the early phase of MKP1 transcription, whereas USF1 (upstream stimulatory factor 1) mediates the later phase. CREB activation is required for atRA-induced USF1 activation. Thus, we describe a novel feed-forward mechanism for temporal regulation of the MKP1 gene through sequential activation of one transcription factor by a second. We also examined the biological relevance of this feed-forward activation of the MKP1 gene for HIV-induced podocyte proliferation and dedifferentiation.
MATERIALS AND METHODS
Infection of conditionally immortalized murine podocytes with HIV-1 or control vector.
Conditionally immortalized murine podocytes were isolated as previously described (33). These cells proliferate under permissive conditions (gamma interferon at 33°C) but differentiate under nonpermissive conditions (37°C). The HIV-1 constructs have been described previously (17). Expression of HIV-1 genes was confirmed by immunoblot analysis. The HIV-1 gag/pol genes and vesicular stomatitis virus G envelope glycoprotein were provided in trans using pCMV R8.91 and pMD.G plasmids, respectively (gifts from Didier Trono, Salk Institute, La Jolla, CA). For this study, podocytes were infected or transfected at passage 15 and then multiple vials of cells were frozen. Prior to experiments, cells were grown at 37°C on type 1 collagen-coated dishes for 10 days to inactivate the temperature-sensitive T antigen and to allow for differentiation. Immunoblotting was used to confirm that T antigen was absent in these cells.
Transfection of podocytes.
Podocytes were transfected with a K-CREB (a gift from R. H. Goodman, Portland, OR) or MKP1 (6) vector or a control vector using a nucleofection kit according to manufacturer's protocols (Amaxa Biosystems, Gaithersburg, MD). We were able to achieve 80 to 90% efficiency based on green fluorescent protein expression. We use a technique combining Dharmacon On TargetPlus SMARTpool small interfering RNA (siRNA) reagents and an Amaxa RNA interference (RNAi) nucleofection kit to introduce siRNA into podocytes. We were able to achieve 60 to 70% knockdown of specific genes (MKP1, USF1, and CREB1 genes). miRIDIAN microRNA mimic negative control sequences are based on Caenorhabditis elegans microRNA for use as negative experimental controls in mammalian cells. Efficiency of siRNA was confirmed by real-time PCR for mRNA levels and by Western blotting for protein levels.
Real-time PCR.
Real-time PCR was performed with a Roche Lightcycler and the QuantiTect one-step real-time PCR Sybr green kit (Qiagen) according to the manufacturer's instructions. Primers were designed using OMIGA design software and selected for having the same melting temperatures. Sequences were as follows: MKP1, 5′AGATCCTGTCCTTCCTGTACC3′ and 5′AGTCAATAGCCTCGTTGAACC3′; synaptopodin, 5′GCCAGGGACCAGCCAGATA 3′ and 5′AGGAGCCCAGGCCTTCTCT3′; luciferase, 5′GTCTGAATTCCAGTCGATGTACACGTTCG3′ and 5′CACGAAGCTTGCATGCGAGAACTCCACGC3′; tubulin, 5′TGCCTTTGTGCACTGGTATG3′ and 5′CTGGAGCAGTTTGACGACAC3′. Lightcycler analysis software was used for determining crossing points using the second derivative method. Data were normalized to those for housekeeping genes and presented as increases compared to control using the 2−ΔΔCT method (26).
Immunoblot analysis.
Podocytes were lysed with buffer containing 1% NP-40, a protease inhibitor cocktail, and tyrosine and serine-threonine phosphorylation inhibitors. Cell lysates were subjected to immunoblot analysis using the following antibodies: anti-phospho-MAPK1,2, anti-MAPK1,2, anti-phospho-CREB, and anti-CREB (all Cell Signaling Laboratory, Beverly, MA) and anti-MKP1, anti-MKP2, anti-USF1, and anti-USF2 (Santa Cruz Biotechnology, Santa Cruz, CA). For immunoprecipitation, total cell lysates were first incubated with anti-CBP (Santa Cruz) and, after precipitation, immunoblotted with anti-CREB1 (Santa Cruz).
Panomics TranSignal protein/DNA arrays.
For Panomics TranSignal protein/DNA arrays, the protocol provided by the manufacturer was followed. In contrast to gel shift assays, which characterize the activation of one transcription factor at a time, the TranSignal transcription factor arrays profile multiple transcription factor-DNA interactions simultaneously. Use of the Panomics array entails incubating transcription factor-specific biotinylated DNA oligonucleotides with nuclear extracts. The protein-DNA complexes are purified and separated from free probes by spin column separation. After hybridization of the purified oligonucleotides to the array, positive spots are visualized by enhanced chemiluminescence. This allows simultaneous examination of over 350 transcription factors. Experiments were performed in triplicate with podocytes incubated with or without atRA for 60 min.
Electromobility shift assay (EMSA).
The following DNA fragments containing the binding sequences of specific transcription factors were obtained from published information and synthesized: USF1, 5′-CACCCGGTCACGTGGCCTACACC-3′ and 5′-GGTGTAGGCCACGTGACCGGGTG-3′; CREB, 5′-TCAAATTGACGTCATGGTAA-3′ and 5′-TTACCATGACGTCAATTTGA-3′). DNA probes were prepared by annealing complementary single-stranded oligonucleotides with 5′-GATC overhangs (Genosys Biotechnologies, Inc.) and were labeled by filling in with [α-32P]dGTP and [α-32P]dCTP using Klenow enzyme. DNA binding complexes were separated by electrophoresis on a 5% polyacrylamide-Tris-glycine-EDTA gel, which was dried and exposed to X-ray film. The specificity of DNA-protein binding was verified by incubation with a 100-fold excess of cold probe and by supershifting with specific antibodies for CREB (Cell Signaling Technology) and USF1 (Santa Cruz). All experiments were repeated at least three times, and representative experiments are shown.
ChIP assay.
Chromatin immunoprecipitation (ChIP) assays were performed as described previously (22). Briefly, 3 × 107 cultured murine podocytes per experimental condition were serum starved for 16 h and then treated with either atRA (1 μM) or control vehicle for 30 min for CREB and 90 min for USF1. Cells were crossed-linked with formaldehyde for 10 min, followed by the addition of 1/20 volume of 2.5 M glycine to quench unreacted formaldehyde. Cells were lysed using a series of non-sodium dodecyl sulfate-containing buffers as described previously (22). Chromatin extracted from the lysed cells was sonicated using a Misonix 3000 sonicator with microtip to generate chromatin fragments of between 300 and 1,000 bp. Immunoprecipitation of USF1- or CREB-cross-linked chromatin was carried out using M-280 Dynabeads (Invitrogen) with sheep anti-rabbit immunoglobulin G (IgG) preincubated with either rabbit anti-USF1 (sc-229; Santa Cruz Biotechnology) antibody or a rabbit anti-CREB (sc-186; Santa Cruz Biotechnology) antibody, respectively. To control for nonspecific IgG binding, rabbit IgG (Sigma) was used. After overnight incubation of chromatin with antibody-coupled Dynabeads, the beads were washed several times and immunoprecipitated chromatin complexes were eluted from the beads. DNA-protein cross-links were reversed by incubation at 65°C for 6 h, and then RNase A and proteinase K were added sequentially to remove RNA and proteins. DNA was purified using the QIAquick PCR purification kit (Qiagen) by following the manufacturer's instructions. Purified DNA was used for the analysis of the MKP1 proximal promoter by semiquantitative PCR with the following primer set: 5′-GTCTTTGCTTTTGGCTTTGG-3′ and 5′-CGCGGTTTTATGTAGCCTCT-3′. PCR products were electrophoresed on 8% agarose gel using a Tris-borate-EDTA buffer. Electrophoresed gel was stained with ethidium bromide, and bands were visualized under UV light.
Mutagenesis and reporter gene assay for MKP1.
For construction of mutant constructs, the MKP1 luciferase expression vector (CL100) (21) containing a 5′ regulatory element of the MKP1 gene (2,900 bp) was obtained from Yusen Liu. Mutations of CRE1, CRE2 (CREB binding sites), and Ebox (USF1 binding site) were created by using the QuikChange II-E site-directed mutagenesis kit (Stratagene Agilent Technology) according to the manufacturer's instructions. The following primers contained mutation sites: sense Cre mut1 (GGA CCC AGC TCC GAG GCT GAT GGA TCC TCC CCC TCT GGC TCG GCG GCG CC), antisense Cre mut1 (CGC CGA GCC AGA GGG GGA GAC GTC ATC AGC CTC GGA GCT GGG TCC GCT), sense Cre mut2 (CTG GCC TGG CAG GGC GGG TGG ATC CAC CGC CCC GTC ACG TGA TCA CCA), antisense Cre mut2 (GTG ATC ACG TGA CGG GGC GGT GGA TCC ACC CGC CCT GCC AGG CCA GGC GC), sense Ebox mut (GGT GAC GTC ACC GCC CCG TCA ATC GAT CAC CAT TCA AAC AAA CAC CC), and antisense Ebox mut (TGT TTG TTT GAA TGG TGA TCG ATT GAC GGG GCG GTG ACG TCA CC).
Reporter gene assay.
Podocytes were transfected with either wild-type (WT) or mutant reporter genes using Amaxa nucleofection. At day 3, cells were stimulated with atRA as indicated in Fig. 3D and cells were harvested for total RNA isolation. Real-time PCR was performed using primers described previously (35). Cells were also cotransfected with both the firefly luciferase reporter gene and Renilla luciferase gene as an internal control. Cells were then treated with atRA or the control for 6 h. The luciferase activity was determined using a dual-luciferase assay system (Promega). The firefly luciferase activity of the MKP1 reporter gene was normalized with the Renilla luciferase activity of the control construct.
FIG. 3.
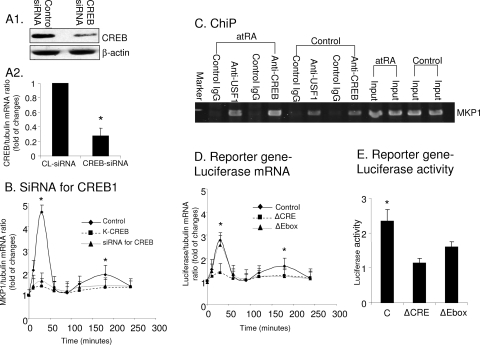
The role of CREB and USF1 in mediating the stimulatory effects of atRA on MKP1 was further confirmed. (A) Podocytes were transfected with either control (CL) siRNA or siRNA specific for CREB for 3 days. CREB expression was determined in these cells by immunoblotting (A1) and real-time PCR (A2) (n = 3; *, P < 0.01). (B) Podocytes were transfected with either K-CREB or siRNA for CREB or control vector for 3 days and then stimulated with atRA for the indicated times. Real-time PCR was performed in these cells to determine the ratio of MKP1/tubulin mRNA levels (n = 3; *, P < 0.01). (C) ChIP analysis. Podocytes were stimulated with atRA or control vehicle for 30 min for CREB and 90 min for USF1. The MKP1 promoter-specific sequence was immunoprecipitated by anti-CREB and anti-USF1 antibodies but not by control IgG. The representative blots of three independent experiments are shown. (D) Podocytes were transfected with WT or mutant MKP1 reporter genes for 3 days. Cells were then stimulated with atRA (1 μM) for the indicated times. Cells were harvested for total RNA isolation and real-time PCR for luciferase mRNA levels (n = 3; *, P < 0.01). (E) Podocytes were transfected with WT or mutant MKP1 reporter genes for 3 days. Cells were stimulated by atRA for 6 h, and then luciferase activity was determined as described in Materials and Methods (n = 3; *, P < 0.05). C, control.
Cell proliferation assays.
Podocytes were plated on collagen-coated 24-well plates at a density of 20,000 cells/well. At day 1, cells were further cultured for 3 to 5 days with atRA (1 μM) (Sigma, St. Louis, MO) or dimethyl sulfoxide as the control in RPMI medium containing 10% fetal bovine serum. At days 3 and 5, cells were trypsinized and counted in triplicate.
Animal studies.
HIV-1-infected transgenic mice were injected with atRA (16 mg/kg body weight) or vehicle alone (corn oil) (three mice in each group), as described previously (14). Mice were sacrificed 2 h after injection. Glomeruli were isolated from cortices of kidneys for immunoblotting after homogenization and lysed in a buffer containing protease and phosphatase inhibitors.
Data analysis.
For cell proliferation assays and real-time PCRs, the data were provided as means ± standard deviations of the means. For real-time PCR, all data were expressed as changes compared to control cells. Immunoblot experiments were repeated at least three times, and representative experiments are shown. Statistically significant differences between the means were determined by unpaired t test. Significance was defined as a P of <0.05.
RESULTS
atRA through cAMP/PKA induces a biphasic stimulation of MKP1.
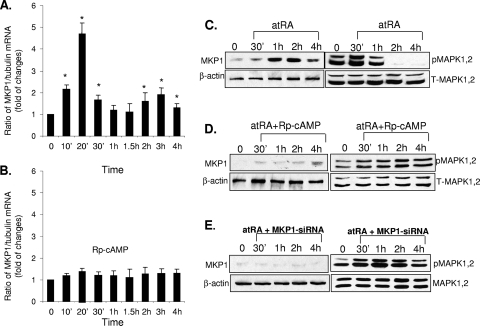
Previous reports have shown that atRA can increase expression of MKP1 at the transcriptional level in neuronal cells (30, 41). By real-time PCR, we found that atRA induced biphasic stimulation of MKP1 mRNA in podocytes (Fig. 1A). Significant increases in mRNA were observed between 10 min and 30 min. This was followed by a second peak, which occurred between 2 and 3 h (Fig. 1A). We found that the expression of MKP2 and MKP3 was not affected by atRA (data not shown). To determine the role of the cAMP/PKA pathway in regulation of MKP1 expression, we treated cells with Rp-cAMP, an antagonist of PKA. We found that Rp-cAMP significantly blocked atRA-induced MKP1 expression, as shown by real-time PCR (Fig. 1B). Elevated MKP1 expression was observed from 1 h to 4 h after stimulation by atRA (Fig. 1C). atRA induced a slight increase in MAPK1,2 phosphorylation at 30 min, followed by significant inhibition at 1 h and complete suppression at 2 h (Fig. 1C). Rp-cAMP also blocked the atRA-induced increase in MKP protein levels, which resulted in more-sustained MAPK1,2 phosphorylation (Fig. 1D). To further confirm that MKP1 plays a major role in the regulation of HIV-induced MAPK1,2 phosphorylation, we silenced MKP1 in podocytes using siRNA and then treated these cells with atRA. We found that suppression of MKP1 caused more-sustained MAPK1,2 phosphorylation in podocytes treated by atRA (Fig. 1E).
FIG. 1.
atRA-induced MKP1 gene expression is biphasic and requires cAMP/PKA activation. (A) Podocytes were stimulated with 1 μM atRA for the indicated time intervals. Total RNA was isolated for real-time PCR of MKP1. The ratios of MKP1/tubulin mRNA levels were compared with those for control (untreated) cells. *, P < 0.01 compared to unstimulated cells (n = 3). (B) HIV-infected podocytes were pretreated with Rp-cAMP (100 μM) for 2 h and then stimulated with atRA (1 μM) for the time intervals indicated. Total RNA was isolated for real-time PCR of MKP1 as described above (n = 3). (C) Podocytes were stimulated with 1 μM atRA for the indicated time intervals. Cell lysates were prepared for immunoblot analysis using anti-phospho-MAPK1,2 (pMAPK1,2), total MAPK1,2 (T-MAPK1,2), MKP1, and β-actin antibodies. (D) HIV-infected podocytes were pretreated with Rp-cAMP at 100 μM for 2 h and then stimulated with atRA (1 μM) at the indicated time points. Immunoblotting was performed for phosphor-MAPK1,2, total MAPK1,2, MKP1, and β-actin. (E) HIV-infected podocytes were transfected with siRNA for MKP1 and then stimulated with atRA (1 μM) at the indicated time points. Immunoblotting was performed for phosphor- and total MAPK1,2, MKP1, and β-actin. All experiments were repeated at least three times, and representative blots are shown.
CREB and USF1 mediate the temporal regulation of MKP1 expression by atRA.
To identify transcription factors that mediate the effects of atRA on podocytes, we used Panomics TranSignal protein/DNA arrays for podocytes stimulated with atRA or the control for 1 h. Of the transcription factors identified, CREB and USF1 were found to have binding motifs within the MKP1 gene promoter.
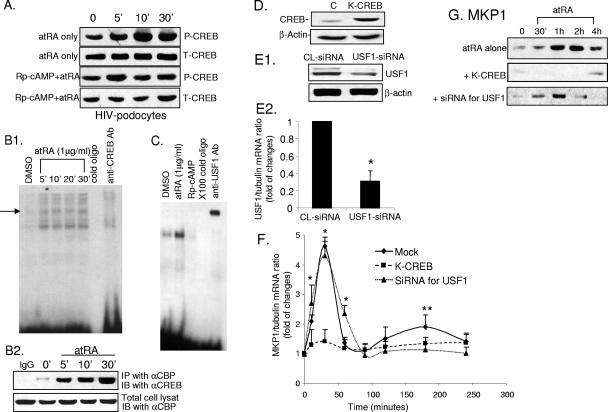
Since atRA induces cAMP production (14), we examined whether treatment with atRA leads to CREB phosphorylation through activation of PKA. We found that atRA induced CREB phosphorylation, which was inhibited at least partially by Rp-cAMP (Fig. 2A). We also confirmed that atRA induced CREB activation by EMSA and by coimmunoprecipitation of CREB with CBP/p300 (Fig. 2B1 and B2). USF1 activation was also confirmed by EMSA (Fig. 2C). Furthermore, atRA-induced USF1 activation was inhibited by Rp-cAMP (Fig. 2C).
FIG. 2.
CREB and USF1 mediate the effects of atRA on MKP1 stimulation. (A) Control and HIV-infected podocytes were pretreated with either Rp-cAMP (100 μM) or control vehicle for 60 min and then incubated with atRA (1 μM) for the times indicated. Cell lysates were obtained for immunoblot analysis using phosphor- (P-CREB) and total CREB (T-CREB) antibodies. (B1) EMSA was performed for CREB as described in Materials and Methods. Anti-CREB antibody (Ab) (Cell Signaling) was used for the supershift experiment. DMSO, dimethyl sulfoxide. (B2) Cells were stimulated with atRA (1 μM) for the indicated times. Cell lysates were used for immunoprecipitation (IP) with monoclonal anti (α)-CBP antibody (Santa Cruz). The immunoprecipitates and total cell lysates were used for immunoblots (IB) using anti-CREB antibody (Santa Cruz). (C) EMSA was performed for USF1 as described in Materials and Methods. Anti-USF1 antibody (Santa Cruz) was used for the supershift experiment. (D) Podocytes were transfected with either K-CREB or control vector as described in Materials and Methods. Cell lysates were prepared for immunoblot analysis of total CREB in order to verify overexpression of K-CREB. Representative blots of three independent experiments are shown. (E) Podocytes were transfected with siRNA for USF1 or control (CL) siRNA. Immunoblotting (E1) and real-time PCR (E2) were performed to confirm suppression of USF1 expression, *, P < 0.001 (n = 3). (F) Podocytes were transfected with K-CREB, siRNA for USF1, or control siRNA for 3 days. Cells were stimulated with atRA (1 μM) for different time intervals as indicated. Total RNA was isolated for real-time PCR for MKP1 as described in Materials and Methods. The ratios of MKP1/tubulin mRNA levels in atRA-stimulated cells were compared with those for control cells. The means of three independent experiments are shown. The stimulation by atRA relative to that for the control is shown, *, P < 0.01 for K-CREB-transfected cells compared to mock-transfected cells; **, P < 0.01 for siRNA for USF1 siRNA-transfected cells compared to mock-transfected cells. (G) Podocytes were transfected with K-CREB, siRNA for USF1, or control siRNA for 3 days. Cells were stimulated with atRA (1 μM) for different time intervals as indicated. Cell lysates were prepared for immunoblot analysis with MKP1 antibody. All experiments were repeated at least three times, and the representative blots are shown.
We examined the role of CREB and USF1 in regulation of MKP1 expression. Podocytes were transfected with a dominant negative mutant of CREB (K-CREB) that competitively inhibits endogenous CREB activity (Fig. 2D) or with siRNA against USF1 that suppressed 70% of USF1 expression (Fig. 2E1 and E2). K-CREB inhibited both the early and late peaks of atRA-induced MKP1 gene expression at the mRNA and protein levels (Fig. 2F and G). In contrast, siRNA against USF1 abolished only the late phase (2 to 3 h) of MKP1 expression induced by atRA at both the mRNA and protein levels (Fig. 2F and G). These data demonstrate for the first time that both CREB and USF1 can regulate the MKP1 gene in a sequential manner.
To confirm that the inhibitory effects of K-CREB (dominant negative mutant) are specific, we performed additional experiments using siRNA to silence CREB. As shown in Fig. 3A, we were able to knock down CREB expression at both the mRNA and protein levels. We found that siRNA for CREB exhibited inhibitory effects on atRA-induced MKP1 expression similar to that for K-CREB (Fig. 3B). To further confirm the interaction of CREB and USF1 with the MKP1 promoter, we performed a ChIP assay. We found that both CREB and USF1 are immunoprecipitated with specific MKP1 gene promoter sequences in resting cells and more prominently in atRA-treated cells (Fig. 3C). To further confirm that CREB and USF1 are key trans-regulators for MKP1 gene expression, we performed a reporter gene assay using mutant constructs with deletion of either CREB (CRE) or USF1 binding sites (Ebox). Consistent with our findings in Fig. 2F, we found that deletion of CRE abolished both early and late phases of MKP1 reporter expression, as determined by real-time PCR of luciferase mRNA, whereas deletion of Ebox suppressed only the late phase of activation (Fig. 3D). Overall luciferase expression was lower than that of MKP1 (Fig. 3B and D); however, this phenomenon has been previously described (32). When we determined the luciferase activity after 6 h of stimulation by atRA, we found that deletion of CRE abolished the luciferase activity whereas deletion of EBox only partially reduced luciferase activity in podocytes (Fig. 3E).
New protein synthesis is required for USF1-mediated MKP1 expression.
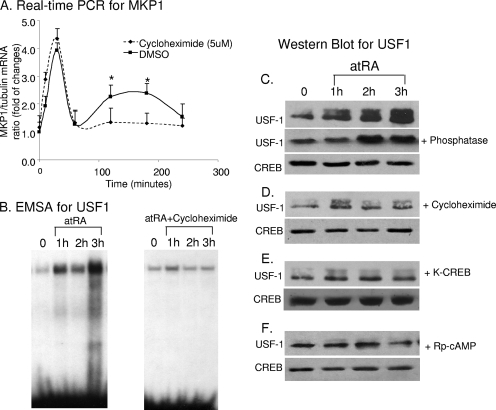
We hypothesized that protein synthesis is required for the USF1-mediated late phase of MKP1 gene transcription but not for the early phase of CREB-mediated stimulation. To address this question, we pretreated cells with cycloheximide (CHX) to block protein synthesis prior to stimulation by atRA. We found that CHX inhibited late USF1-mediated increases in MKP1 mRNA levels whereas it did not affect CREB-mediated effects (Fig. 4A). Furthermore, we found that CHX inhibited DNA-protein interactions for USF1 by EMSA (Fig. 4B).
FIG. 4.
atRA activates CREB, which in turn activates USF1 through stimulation of its expression. (A) HIV-infected podocytes were pretreated with CHX (5 mM) for 30 min and then stimulated with atRA (1 μM) or dimethyl sulfoxide (DMSO) for the indicated time intervals. Total RNA was isolated for real-time PCR for MKP1 (P < 0.01; n = 3). (B) HIV-infected podocytes were treated with CHX or atRA as for panel A. Nuclear proteins were isolated for EMSA. (C) Podocytes were treated with atRA (1 μM) for the indicated time intervals. Cell lysates with or without treatment with calf intestine alkaline phosphatase (Invitrogen) were used for immunoblotting for USF1 after running on a gradient gel. Total CREB was used as loading control for nuclear proteins. (D) Podocytes were pretreated with CHX (5 mM) for 30 min and then treated with atRA as for panel A. Immunoblotting was performed for USF1 and CREB as for panel C. (E) Podocytes were transfected with K-CREB for 3 days and then treated with atRA as for panel A. Immunoblotting was performed for USF1 and CREB as for panel C. (F) Podocytes were treated with Rp-cAMP (100 μM) for 1 h and then treated with atRA as for panel A. Immunoblotting were performed for USF1 and CREB as for panel C. The representative blots of at least three independent experiments are shown here.
Next, we determined whether the reduced DNA-protein interaction was due to reduced USF1 phosphorylation or to a fall in total USF1 protein levels. By immunoblotting, we found that atRA stimulated both USF1 phosphorylation (represented by the top band) and total protein levels in podocytes after 1 to 3 h of stimulation (Fig. 4C). The higher-molecular-weight band was confirmed to be phosphorylated USF1 because treatment of the samples with a phosphatase caused these bands to disappear (Fig. 4C). CHX significantly inhibited atRA-induced USF1 protein levels, but it did not affect the ratio of phosphor-USF1 to total USF1 (Fig. 4D). Dominant negative K-CREB also blocked atRA-induced USF1 protein levels, indicating that CREB mediates the effects of atRA on USF1 expression (Fig. 4E). Furthermore, Rp-cAMP inhibited both the phosphorylation and expression of USF1 protein stimulated by atRA (Fig. 4F), suggesting that USF1 is phosphorylated by PKA. Treatment of cells with MG132, an inhibitor for proteasomal degradation, did not affect the levels of USF1 in atRA-treated cells (data not shown), suggesting that the increased levels of USF1 are not due to an alteration of the degradation process. These studies suggest a new mechanism involving a feed-forward loop of gene regulation for MKP1 whereby activation of one transcription factor leads to induction of MKP1 as well as a second transcription factor, which further induces MKP1.
MKP1 plays a key role in HIV-infected podocytes.
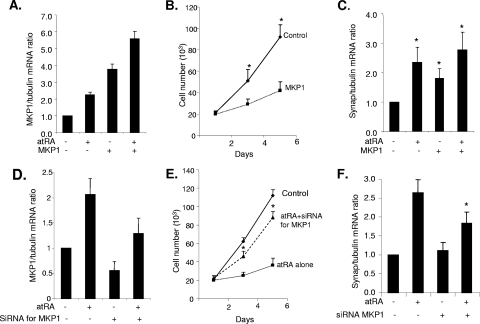
Next, we determined the biological relevance of this signaling network. First, we examined whether MKP1 mediates the effects of atRA on podocytes. As we have shown previously, atRA can restore the normal phenotype of HIV-infected podocytes by inhibition of proliferation and stimulation of differentiation markers including synaptopodin (14). Here, we examined the effects of MKP1 overexpression alone on podocyte proliferation and synaptopodin expression. Overexpression of MKP1 was confirmed by real-time PCR as shown in Fig. 5A. MKP1 expression was highest in cells both transfected with MKP1 and treated with atRA due to an additive effect. Overexpression of MKP1 in HIV-infected podocytes significantly reduced proliferation and increased synaptopodin mRNA levels, similar to treatment with atRA (Fig. 5B and C). Next, we used siRNA to knock down MKP1 expression in HIV-1 podocytes. As shown in Fig. 5D, siRNA against MKP1 reduced MKP1 expression at both basal and atRA-stimulated conditions. The antiproliferative effect of atRA was inhibited in podocytes treated with siRNA for MKP1 (Fig. 5E). The effect of atRA on synaptopodin expression was also partially inhibited (Fig. 5F). These data indicate that MKP1 at least partially mediates the effects of atRA on podocytes.
FIG. 5.
MKP1 mediates the effects of atRA on podocytes. HIV-infected podocytes were transfected with either control vector or MKP1 (A to C) or transfected with a control oligonucleotide or siRNA against MKP1 (D to F) for 3 days. Cells were then stimulated with atRA at 1 μM for 1 to 5 days. Real-time PCR was used to assess MKP1 expression levels (A and D). Cell number was assessed at days 1, 3, and 5 (B and D), and real-time PCR was performed using primers for synaptopodin (C and F). All experiments were repeated at least three times, *, P < 0.01.
Activation of both CREB and USF1 is required for atRA to switch the podocyte from proliferation to a differentiated state.
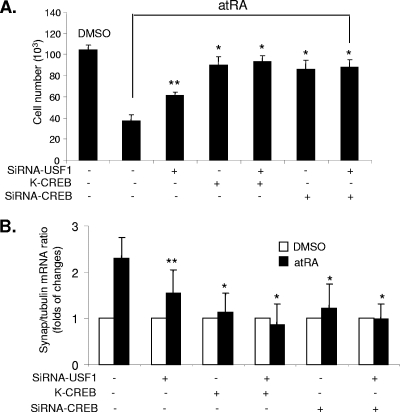
We next investigated whether activation of CREB and USF1 is required for atRA-induced MKP1 expression and therefore mediation of atRA's effects on the podocyte phenotype. Transfection with K-CREB or siRNA for CREB with or without siRNA for USF1 inhibited completely the antiproliferative effects of atRA on HIV-infected podocytes (Fig. 6A). Transfection with siRNA for USF1 alone inhibited partially the antiproliferative effects of atRA (Fig. 6A). Furthermore, transfection of podocytes with either K-CREB or siRNA for CREB with or without siRNA for USF1 inhibited completely the stimulatory effects of atRA on synaptopodin expression, whereas transfection with siRNA against USF1 alone had partial effects (Fig. 6B). These data indicate a role for CREB and USF1 in mediating atRA-induced MKP1 expression and changes in cell phenotype. Inhibition of CREB completely abolished the effects of atRA, whereas inhibition of USF1 partially abolished the effects of atRA.
FIG. 6.
Role of CREB and USF1 in mediating the effects of atRA on podocytes in vitro. HIV-1-infected podocytes were transfected with control vector, K-CREB, siRNA for CREB1, siRNA for USF1, or control siRNA for 3 days and then were incubated with or without atRA (1 μM) for 1 to 3 days. (A) Cell number was assessed in these conditions at day 3. The means ± standard deviations of three independent experiments performed in triplicate are shown, *, P < 0.001; **, P < 0.05. DMSO, dimethyl sulfoxide. (B) Real-time PCR of synaptopodin was performed in these cells with or without atRA stimulation for 1 day. *, P < 0.01.
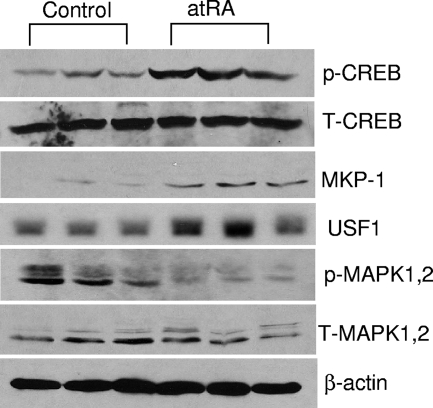
atRA increases CREB phosphorylation and MKP1 expression in vivo.
To verify our findings in vivo, HIV-infected transgenic mice were injected with atRA and the control vehicle as described previously (14). Glomeruli were isolated from kidney cortex for immunoblot analysis with phosphor-CREB, USF1, MKP1, and phosphor-MAPK1,2. As shown in Fig. 7, injection of atRA in mice induced CREB phosphorylation and USF1 and MKP1 expression in kidneys while MAPK1,2 phosphorylation was reduced. These data suggest that CREB phosphorylation and MKP1 stimulation also mediate the effects of atRA in vivo.
FIG. 7.
atRA induces CREB phosphorylation and MKP1 expression in vivo. HIV-1-infected transgenic mice were injected with atRA or vehicle alone (three mice in each group) as described in Materials and Methods. After perfusion with phosphate-buffered saline containing phosphatase inhibitors, the cortex of the kidney was removed and isolated glomeruli were used for immunoblotting using specific antibodies for phosphor (p)- and total (T) CREB, MKP1, USF1, phosphor- and total MAPK1,2, and β-actin.
DISCUSSION
HIVAN is the most common kidney disease in HIV-seropositive patients, and specific treatment for this disease is unavailable. While antiretroviral therapy ameliorates the disease, adjunctive treatment targeted to specific pathogenic pathways induced by HIV-1 awaits further development. Recently, we found that atRA induces HIV-infected podocytes to differentiate and inhibits HIV-induced podocyte proliferation in vitro (14). atRA also reduces proteinuria and glomerulosclerosis in vivo in HIV-1 transgenic mice (14). Inhibition of MAPK1,2 phosphorylation is a critical part of the mechanism (14). In the current study, we have explored the molecular and cellular mechanisms responsible for MAPK1,2 inhibition as well as the impact of this pathway on disease phenotype. We conclude that atRA inhibits MAPK1,2 phosphorylation through stimulation of MKP1 based on the following findings: (i) the time course of MKP1 stimulation by atRA at protein levels correlates with the time course of MAPK1,2 phosphorylation levels, (ii) silencing MKP1 expression by siRNA causes sustained MAPK1,2 phosphorylation, and (iii) atRA did not induce MKP2 expression.
atRA has been shown to cause increased MKP1 expression in other cell types (30). The mechanism, however, has remained unclear. Expression of MKP1 can be induced by activation of PKA, protein kinase C, or MAPKs (MAPK1,2, p38, and JNK) in response to multiple stimuli (8, 24). While upstream events are fairly well known, the identity of downstream, target transcription factors has been largely unknown. We show that atRA induces MKP1 gene expression in podocytes through PKA-mediated CREB and USF1 activation. The early phase of MKP1 stimulation is regulated by CREB directly; the late phase of stimulation is controlled by CREB indirectly through induction of USF1 expression and activation. These findings were confirmed by our reporter gene assay using a mutant MKP1 promoter with deletion of either CRE or Ebox. Furthermore, both CREB and USF1 can bind to cis elements of the MKP1 gene promoter, as shown by our ChIP experiments as well as by others (32).
Our findings suggest that a feed-forward motif is required for sustained MKP induction. Since CREB acts upstream of this feed-forward loop, inhibition of CREB by K-CREB or siRNA completely inhibited MKP1 stimulation and cell proliferation. USF1 mediated only the late phase of MKP1 stimulation and produced less-sustained stimulation of MKP1 expression. Therefore, inhibition of USF1 only partially reduced the antiproliferative effects of atRA. Our data suggest that this feed-forward loop (CREB-USF1) is required for sustained MKP1 expression and for the full effects of atRA on podocytes. Of note, while atRA induced a clear biphasic stimulation of MKP1 at the mRNA level, immunoblotting demonstrated monophasic and sustained expression of MKP1 at the protein level. Inhibition of USF1 reduced the time frame of MKP1 activation by atRA.
CREB is phosphorylated by several protein kinases, including PKA and MAPK1,2 (27). We have already demonstrated the beneficial effects of atRA in HIV-1-infected transgenic mice (14). We found here that atRA induced CREB phosphorylation through activation of PKA. It was recently reported that atRA induces neurite outgrowth in PC12 cells through CREB phosphorylation and that this is independent of the retinoic acid response elements (9). While the importance of CREB in neuronal differentiation is well documented, the role of CREB in podocytes and in kidney disease has never been studied. We found here that inhibition of CREB activity diminished the effects of atRA on podocytes, indicating that CREB acts downstream of atRA in maintaining the podocyte in a differentiated state. Our data indicate a new pathway in which CREB stimulates the expression of the MKP1 gene, leading to podocyte differentiation. atRA also induced CREB phosphorylation in vivo, which is associated with increased MKP1 expression and reduced MAKP1,2 phosphorylation. This is the first study to show that atRA induces a CREB signaling pathway in vivo.
USF1 is a ubiquitously expressed transcription factor controlling several critical genes in lipid and glucose metabolism (20). Of some 40 genes regulated by USF1, several are involved in the molecular pathogenesis of cardiovascular disease. Polymorphisms of this gene are associated with kidney disease as well (10, 29). The USF1 gene promoter in the podocin (NPHS2) gene promoter has been shown to affect gene expression. Podocin (NPHS2) expression in podocytes is associated with variable degrees of proteinuria and progression to renal failure in different glomerular diseases (7). We found here that USF1 mediates the protective effects of atRA on podocytes by stimulation of the MKP1 gene along with CREB. Dual regulation of genes with USF1 and other transcription factors is well known. It has been reported that USF1 and AP-2b cooperatively regulate the human lipocalin-type prostaglandin D synthase gene in TE671 cells (12). USF1/2 and CREB together mediate Helicobacter pylori-dependent COX-2 gene transcription via a MEK/extracellular signal-regulated kinase-dependent pathway (18). Furthermore, both CREB and USF1 contribute to Ca2+ signal-mediated activation of brain-derived neurotrophic factor gene promoter 1 expression in rat cortical neurons in culture (37). However the role of network topology in these other cases is not known. Furthermore, the temporal regulation of genes by different transcription factors is not well studied. Only one study has reported that UTP stimulates osteopontin expression via coordinated regulation of the NF-κB, USF, and AP-1 signaling pathways (31). While NF-κB mediates early activation (15 min), USF1/2 and AP-1 mediate late-phase activation (1 h). The authors speculate that NF-κB mediates the early phase because phosphorylation is sufficient for its activation whereas activation of USF1 and AP1 requires de novo synthesis of cofactors. In contrast, our study provides a mechanistic understanding of the relationship between CREB and USF1. atRA-induced CREB activation mediated increases in USF1 protein levels that were required for enhancement of USF1 activity in podocytes. Therefore, the effect of USF1 is delayed compared to that of CREB, which is activated by direct phosphorylation. Thus our data provide evidence that a feed-forward loop is required for sustained increases in MKP levels.
Our data also support a role for this increase in MKP1 in mediating the protective effects of atRA on podocytes. MKP1 has been shown to be important in the innate immune responses and plays a critical role in suppressing endotoxin-induced shock (43). A recent study suggests that MKP1 contributes to the anti-inflammatory effects of dexamethasone both in vitro and in vivo (1). MKP1 has been reported to mediate the inhibitory effects of atRA on the hypertrophic growth of cardiomyocytes (30). The role of MKP1 in kidney disease has also been determined. It has been shown that connective tissue growth factor promoters activate mesangial cell survival via upregulation of MKP1 (39a). In kidneys of diabetic rats, MKP1 expression increases in parallel with p38 at the early phase (19, 19a). However, other studies suggest that MKP1 is suppressed in kidneys with diabetic nephropathy, leading to increased MAPK phosphorylation (2a). These controversial findings require further studies to clarify. MKP1 expression may change at different stages of kidney disease. It is very interesting that MAPK (extracellular signal-regulated kinase, p38, and JNK) and MKP1 are highly expressed in proliferative cells in renal development (2b, 29a). An increase of MKP1, possibly induced by MAPK, may not be strong enough to suppress MAPK activation. The temporal relationship between MAPK activation and MKP1 activation in vivo remains to be determined. Our study indicates that MKP1 plays a key role in mediating the effects of atRA to inhibit proliferation and induce differentiation of podocytes infected by HIV. We also show that these effects are mediated through the inhibition of MAPK activation.
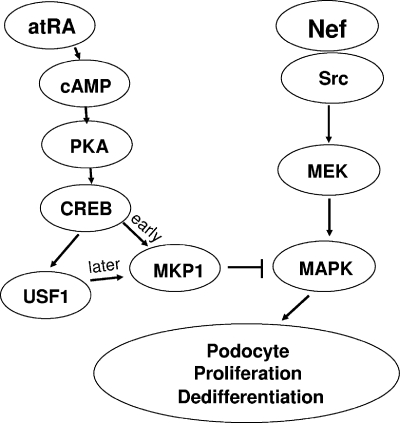
In conclusion, the current study shows that atRA, by triggering a feed-forward gene regulatory motif between CREB and UFS1, induced sustained upregulation of MKP1 to suppress HIV Nef-induced activation of the Src-MAPK1,2 pathway and returned the podocyte to a more differentiation state (Fig. 8).
FIG. 8.
Schematic of cross talk between cAMP/PKA and MAPK1,2 downstream of atRA signaling in HIV-infected podocytes. Nef, an HIV accessory protein, interacts with Src, leading to Ras/C-Raf/MAPK1,2 phosphorylation in podocytes. This sustained MAPK1,2 phosphorylation contributes to podocyte proliferation and dedifferentiation. atRA stimulates intracellular cAMP production, which activates PKA, leading to CREB phosphorylation. CREB directly mediated the early phase of atRA-induced MKP1 stimulation, whereas the later phase was mediated indirectly by CREB through induction of USF1 expression. Through this feed-forward gene-regulatory motif, atRA induced sustained upregulation of MKP1 to suppress HIV-induced activation of the MAPK1,2 pathway, leading to podocyte differentiation and growth arrest.
Acknowledgments
Ting-Chi Lu is a recipient of an NIH career development award (K08 DK079781). John Cijiang He is a recipient of an NIH career development award (K08 DK065495) and is supported by NIH R01 DK078897. This work was also supported by NIH grants P01 DK056492 to Paul E. Klotman and R01 DK38761 to Ravi Iyengar.
Footnotes
Published ahead of print on 14 July 2008.
REFERENCES
- 1.Abraham, S. M., T. Lawrence, A. Kleiman, P. Warden, M. Medghalchi, J. Tuckermann, J. Saklatvala, and A. R. Clark. 2006. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J. Exp. Med. 2031883-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allenby, G., M. T. Bocquel, M. Saunders, S. Kazmer, J. Speck, M. Rosenberger, A. Lovey, P. Kastner, J. F. Grippo, P. Chambon, et al. 1993. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc. Natl. Acad. Sci. USA 9030-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Awazu, M., K. Ishikura, et al. 1999. Mechanisms of mitogen-activated protein kinase activation in experimental diabetes. J. Am. Soc. Nephrol. 10738-745. [DOI] [PubMed] [Google Scholar]
- 2b.Awazu, M., S. Omori, et al. 2002. MAP kinase in renal development. Nephrol. Dial. Transplant. 17(Suppl. 9)5-7. [DOI] [PubMed] [Google Scholar]
- 3.Barisoni, L., L. A. Bruggeman, P. Mundel, V. D. D'Agati, and P. E. Klotman. 2000. HIV-1 induces renal epithelial dedifferentiation in a transgenic model of HIV-associated nephropathy. Kidney Int. 58173-181. [DOI] [PubMed] [Google Scholar]
- 4.Barisoni, L., W. Kriz, P. Mundel, and V. D'Agati. 1999. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J. Am. Soc. Nephrol. 1051-61. [DOI] [PubMed] [Google Scholar]
- 5.Bey, P., A. B. Gorostizaga, P. M. Maloberti, R. C. Lozano, C. Poderoso, F. C. Maciel, E. J. Podesta, and C. Paz. 2003. Adrenocorticotropin induces mitogen-activated protein kinase phosphatase 1 in Y1 mouse adrenocortical tumor cells. Endocrinology 1441399-1406. [DOI] [PubMed] [Google Scholar]
- 6.Bhalla, U. S., P. T. Ram, and R. Iyengar. 2002. MAP kinase phosphatase as a locus of flexibility in a mitogen-activated protein kinase signaling network. Science 2971018-1023. [DOI] [PubMed] [Google Scholar]
- 7.Boute, N., O. Gribouval, S. Roselli, F. Benessy, H. Lee, A. Fuchshuber, K. Dahan, M. C. Gubler, P. Niaudet, and C. Antignac. 2000. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat. Genet. 24349-354. [DOI] [PubMed] [Google Scholar]
- 8.Brondello, J. M., A. Brunet, J. Pouyssegur, and F. R. McKenzie. 1997. The dual specificity mitogen-activated protein kinase phosphatase-1 and -2 are induced by the p42/p44MAPK cascade. J. Biol. Chem. 2721368-1376. [DOI] [PubMed] [Google Scholar]
- 9.Canon, E., J. M. Cosgaya, S. Scsucova, and A. Aranda. 2004. Rapid effects of retinoic acid on CREB and ERK phosphorylation in neuronal cells. Mol. Biol. Cell 155583-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Duca, M., R. Oleggini, S. Sanna-Cherchi, L. Pasquali, A. Di Donato, S. Parodi, R. Bertelli, G. Caridi, G. Frasca, G. Cerullo, A. Amoroso, F. P. Schena, F. Scolari, and G. M. Ghiggeri. 2006. Cis and trans regulatory elements in NPHS2 promoter: implications in proteinuria and progression of renal diseases. Kidney Int. 701332-1341. [DOI] [PubMed] [Google Scholar]
- 11.Evans, T. R., and S. B. Kaye. 1999. Retinoids: present role and future potential. Br. J. Cancer 801-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujimori, K., and Y. Urade. 2007. Cooperative activation of lipocalin-type prostaglandin D synthase gene expression by activator protein-2β in proximal promoter and upstream stimulatory factor 1 within intron 4 in human brain-derived TE671 cells. Gene 397143-152. [DOI] [PubMed] [Google Scholar]
- 13.Gronemeyer, H., and R. Miturski. 2001. Molecular mechanisms of retinoid action. Cell. Mol. Biol. Lett. 63-52. [PubMed] [Google Scholar]
- 14.He, J. C., T.-C. Lu, M. Fleet, M. Sunamoto, M. Husain, W. Fang, S. Neves, Y. Chen, S. J. Shankland, R. Iyengar, and P. E. Klotman. 2007. Retinoic acid inhibits HIV-1 induced podocyte proliferation through the cAMP pathway. J. Am. Soc. Nephrol. 1893-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He, J. C., M. Husain, M. Sunamoto, V. D. D'Agati, M. E. Klotman, R. Iyengar, and P. E. Klotman. 2004. Nef stimulates proliferation of glomerular podocytes through activation of Src-dependent Stat3 and MAPK1,2 pathways. J. Clin. Investig. 114643-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu, J. H., T. Chen, Z. H. Zhuang, L. Kong, M. C. Yu, Y. Liu, J. W. Zang, and B. X. Ge. 2007. Feedback control of MKP-1 expression by p38. Cell. Signal. 19393-400. [DOI] [PubMed] [Google Scholar]
- 17.Husain, M., G. L. Gusella, M. E. Klotman, I. H. Gelman, M. D. Ross, E. J. Schwartz, A. Cara, and P. E. Klotman. 2002. HIV-1 Nef induces proliferation and anchorage-independent growth in podocytes. J. Am. Soc. Nephrol. 131806-1815. [DOI] [PubMed] [Google Scholar]
- 18.Juttner, S., T. Cramer, S. Wessler, A. Walduck, F. Gao, F. Schmitz, C. Wunder, M. Weber, S. M. Fischer, W. E. Schmidt, B. Wiedenmann, T. F. Meyer, M. Naumann, and M. Hocker. 2003. Helicobacter pylori stimulates host cyclooxygenase-2 gene transcription: critical importance of MEK/ERK-dependent activation of USF1/-2 and CREB transcription factors. Cell. Microbiol. 5821-834. [DOI] [PubMed] [Google Scholar]
- 19.Kang, S. W., S. G. Adler, J. Lapage, and R. Natarajan. 2001. p38 MAPK and MAPK kinase 3/6 mRNA and activities are increased in early diabetic glomeruli. Kidney Int. 60543-552. [DOI] [PubMed] [Google Scholar]
- 19a.Komers, R., J. N. Lindsley, et al. 2007. Renal p38 MAP kinase activity in experimental diabetes. Lab. Investig. 87548-558. [DOI] [PubMed] [Google Scholar]
- 20.Komulainen, K., M. Alanne, K. Auro, R. Kilpikari, P. Pajukanta, J. Saarela, P. Ellonen, K. Salminen, S. Kulathinal, K. Kuulasmaa, K. Silander, V. Salomaa, M. Perola, and L. Peltonen. 2006. Risk alleles of USF1 gene predict cardiovascular disease of women in two prospective studies. PLoS Genet. 2e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwak, S. P., D. J. Hakes, K. J. Martell, and J. E. Dixon. 1994. Isolation and characterization of a human dual specificity protein-tyrosine phosphatase gene. J. Biol. Chem. 2693596-3604. [PubMed] [Google Scholar]
- 22.Lee, T. I., S. E. Johnstone, and R. A. Young. 2006. Chromatin immunoprecipitation and microarray-based analysis of protein location. Nat. Protoc. 1729-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehrke, I., M. Schaier, K. Schade, C. Morath, R. Waldherr, E. Ritz, and J. Wagner. 2002. Retinoid receptor-specific agonists alleviate experimental glomerulonephritis. Am. J. Physiol. (Renal Physiol.) 282F741-F751. [DOI] [PubMed] [Google Scholar]
- 24.Li, J., M. Gorospe, D. Hutter, J. Barnes, S. M. Keyse, and Y. Liu. 2001. Transcriptional induction of MKP-1 in response to stress is associated with histone H3 phosphorylation-acetylation. Mol. Cell. Biol. 218213-8224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Licht, J. D., and A. Zelent. 2005. Retinoid and growth factor receptor signaling in APL. Blood 1051381-1382. [Google Scholar]
- 26.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 27.Lonze, B. E., and D. D. Ginty. 2002. Function and regulation of CREB family transcription factors in the nervous system. Neuron 35605-623. [DOI] [PubMed] [Google Scholar]
- 28.Moreno-Manzano, V., F. Mampaso, J. C. Sepulveda-Munoz, M. Alique, S. Chen, F. N. Ziyadeh, M. C. Iglesias-de la Cruz, J. Rodriguez, E. Nieto, J. M. Orellana, P. Reyes, I. Arribas, Q. Xu, M. Kitamura, and F. J. Lucio Cazana. 2003. Retinoids as a potential treatment for experimental puromycin-induced nephrosis. Br. J. Pharmacol. 139823-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oleggini, R., R. Bertelli, A. Di Donato, M. Di Duca, G. Caridi, S. Sanna-Cherchi, F. Scolari, L. Murer, L. Allegri, R. Coppo, F. Emma, G. Camussi, F. Perfumo, and G. M. Ghiggeri. 2006. Rare functional variants of podocin (NPHS2) promoter in patients with nephrotic syndrome. Gene Expr. 1359-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.Omori, S., M. Hida, et al. 2002. Expression of mitogen-activated protein kinase family in rat renal development. Kidney Int. 5827-37. [DOI] [PubMed] [Google Scholar]
- 30.Palm-Leis, A., U. S. Singh, B. S. Herbelin, G. D. Olsovsky, K. M. Baker, and J. Pan. 2004. Mitogen-activated protein kinases and mitogen-activated protein kinase phosphatases mediate the inhibitory effects of all-trans retinoic acid on the hypertrophic growth of cardiomyocytes. J. Biol. Chem. 27954905-54917. [DOI] [PubMed] [Google Scholar]
- 31.Renault, M. A., S. Jalvy, M. Potier, I. Belloc, E. Genot, L. V. Dekker, C. Desgranges, and A. P. Gadeau. 2005. UTP induces osteopontin expression through a coordinate action of NFκB, activator protein-1, and upstream stimulatory factor in arterial smooth muscle cells. J. Biol. Chem. 2802708-2713. [DOI] [PubMed] [Google Scholar]
- 32.Ryser, S., A. Massiha, I. Piuz, and W. Schlegel. 2004. Stimulated initiation of mitogen-activated protein kinase phosphatase-1 (MKP-1) gene transcription involves the synergistic action of multiple cis-acting elements in the proximal promoter. Biochem. J. 378473-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saleem, M. A., M. J. O'Hare, J. Reiser, R. J. Coward, C. D. Inward, T. Farren, C. Y. Xing, L. Ni, P. W. Mathieson, and P. Mundel. 2002. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 13630-638. [DOI] [PubMed] [Google Scholar]
- 34.Simonson, M. S. 1994. Anti-AP-1 activity of all-trans retinoic acid in glomerular mesangial cells. Am. J. Physiol. 267F805-F815. [DOI] [PubMed] [Google Scholar]
- 35.Skaggs, H. S., H. Xing, D. C. Wilkerson, L. A. Murphy, Y. Hong, C. N. Mayhew, and K. D. Sarge. 2007. HSF1-TPR interaction facilitates export of stress-induced HSP70 mRNA. J. Biol. Chem. 28233902-33907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sunamoto, M., M. Husain, J. C. He, E. J. Schwartz, and P. E. Klotman. 2003. Critical role for Nef in HIV-1-induced podocyte dedifferentiation. Kidney Int. 641695-1701. [DOI] [PubMed] [Google Scholar]
- 37.Tabuchi, A., H. Sakaya, T. Kisukeda, H. Fushiki, and M. Tsuda. 2002. Involvement of an upstream stimulatory factor as well as cAMP-responsive element-binding protein in the activation of brain-derived neurotrophic factor gene promoter I. J. Biol. Chem. 27735920-35931. [DOI] [PubMed] [Google Scholar]
- 38.Valledor, A. F., J. Xaus, M. Comalada, C. Soler, and A. Celada. 2000. Protein kinase C epsilon is required for the induction of mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J. Immunol. 16429-37. [DOI] [PubMed] [Google Scholar]
- 39.Wagner, J., C. Dechow, C. Morath, I. Lehrke, K. Amann, R. Waldherr, J. Floege, and E. Ritz. 2000. Retinoic acid reduces glomerular injury in a rat model of glomerular damage. J. Am. Soc Nephrol. 111479-1487. [DOI] [PubMed] [Google Scholar]
- 39a.Wahab, N., D. Cox, et al. 2007. Connective tissue growth factor (CTGF) promotes activated mesangial cell survival via up-regulation of mitogen-activated protein kinase phosphatase-1 (MKP-1). Biochem. J. 406131-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winston, J. A., G. C. Burns, and P. E. Klotman. 1998. The human immunodeficiency virus (HIV) epidemic and HIV-associated nephropathy. Semin. Nephrol. 18373-377. [PubMed] [Google Scholar]
- 41.Xu, Q., T. Konta, A. Furusu, K. Nakayama, J. Lucio-Cazana, L. G. Fine, and M. Kitamura. 2002. Transcriptional induction of mitogen-activated protein kinase phosphatase 1 by retinoids. Selective roles of nuclear receptors and contribution to the antiapoptotic effect. J. Biol. Chem. 27741693-41700. [DOI] [PubMed] [Google Scholar]
- 42.Zhao, Q., J. Tao, Q. Zhu, P. M. Jia, A. X. Dou, X. Li, F. Cheng, S. Waxman, G. Q. Chen, S. J. Chen, M. Lanotte, Z. Chen, and J. H. Tong. 2004. Rapid induction of cAMP/PKA pathway during retinoic acid-induced acute promyelocytic leukemia cell differentiation. Leukemia 18285-292. [DOI] [PubMed] [Google Scholar]
- 43.Zhao, Q., X. Wang, L. D. Nelin, Y. Yao, R. Matta, M. E. Manson, R. S. Baliga, X. Meng, C. V. Smith, J. A. Bauer, C. H. Chang, and Y. Liu. 2006. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J. Exp. Med. 203131-140. [DOI] [PMC free article] [PubMed] [Google Scholar]