Abstract
Chaperone-mediated autophagy (CMA) is a selective type of autophagy by which specific cytosolic proteins are sent to lysosomes for degradation. Substrate proteins bind to the lysosomal membrane through the lysosome-associated membrane protein type 2A (LAMP-2A), one of the three splice variants of the lamp2 gene, and this binding is limiting for their degradation via CMA. However, the mechanisms of substrate binding and uptake remain unknown. We report here that LAMP-2A organizes at the lysosomal membrane into protein complexes of different sizes. The assembly and disassembly of these complexes are a very dynamic process directly related to CMA activity. Substrate proteins only bind to monomeric LAMP-2A, while the efficient translocation of substrates requires the formation of a particular high-molecular-weight LAMP-2A complex. The two major chaperones related to CMA, hsc70 and hsp90, play critical roles in the functional dynamics of the LAMP-2A complexes at the lysosomal membrane. Thus, we have identified a novel function for hsc70 in the disassembly of LAMP-2A from these complexes, whereas the presence of lysosome-associated hsp90 is essential to preserve the stability of LAMP-2A at the lysosomal membrane.
Chaperone-mediated autophagy (CMA) is a selective form of autophagy by which cytosolic proteins bearing in their amino acid sequences a common targeting motif are recognized by a chaperone complex which targets them to lysosomes for degradation (13, 26). The lysosomal uptake of these proteins requires their binding to the lysosome-associated membrane protein type 2A (LAMP-2A), a CMA receptor at the lysosomal membrane. Substrate proteins are unfolded and then translocated into the lysosomal lumen across the membrane with the assistance of a luminal chaperone (lys-hsc70).
LAMP-2A is a type I integral membrane protein with a heavily glycosylated luminal region, a single transmembrane region, and a short cytosolic tail (17). LAMP-2A is one of the three splice variants of the lamp2 gene. LAMP-2 proteins protect the lysosomal membrane from degradation by lysosomal hydrolases and participate in intracellular cholesterol trafficking (16), lysosomal biogenesis (16), and lysosomal motility along microtubules (19). In addition to these common functions, the different splice variants of LAMP-2 also have specialized functions. LAMP-2A serves as a receptor for the cytosolic proteins that undergo degradation via CMA (7) and for the cytoplasmic antigens presented on major histocompatibility complex class II (37). The impaired lysosome/autophagosome fusion in patients with mutations in the LAMP-2B exon supports a role for this isoform in macroautophagy (32), and LAMP-2C seems to be involved in lysosomal biogenesis (17).
Specific chaperones and cochaperones play a major role in CMA during substrate recognition, targeting, unfolding, and transport (1, 2). The cytosolic form of the heat shock cognate protein of 70 kDa (hsc70) recognizes the CMA-targeting motif in the substrate proteins (6). hsc70 associates to the lysosomal membrane (1, 2), but its interaction with the substrate proteins and the CMA receptor once at this compartment remains poorly understood. A form of hsc70 also exists with the lysosomal lumen (1, 2, 10). The blockage of luminal hsc70 with antibodies internalized via endocytosis results in the inhibition of CMA (2). Supporting the essential role of lys-hsc70 in CMA, lysosomes that contain in their membrane all the components involved in substrate translocation but lack luminal hsc70 are unable to degrade proteins via CMA (10). hsp90 has also been identified as part of the chaperone complex associated with the lysosomal membrane (2). However, its function at this location is currently unknown.
CMA is maximally activated under stress conditions such as oxidative stress, prolonged starvation, or exposure to toxic compounds that induce protein damage (5, 13, 26). Lysosomal levels of both hsc70 and LAMP-2A increase when CMA is maximally activated (2, 8, 11). The binding of substrates to LAMP-2A is the limiting step for their degradation via CMA (7). Lysosomal levels of LAMP-2A are tightly controlled by at least three different mechanisms: regulated cleavage at the lysosomal membrane in discrete lipid microdomains, dynamic distribution of LAMP-2A between the lysosomal membrane and lumen, and transcriptional regulation of the lamp2 gene (8, 22, 23). LAMP-2A has been shown to organize into high-molecular-weight multimeric complexes at the lysosomal membrane (9), but the composition and function of these complexes, as well as their dynamics of assembly/disassembly and the effect of possible changes in this organization on CMA activity, are unknown.
In this work, we have used different approaches to characterize the LAMP-2A-containing complexes at the lysosomal membrane. We have found that LAMP-2A organizes into dynamic multimeric complexes that assemble in a stepwise manner directly related to the different steps involved in CMA. Both hsc70 and hsp90 play critical roles in the dynamics of these multimeric complexes. We have identified a novel role for hsc70 in the disassembly of the LAMP-2A complexes which is essential for the maintenance of CMA activity, whereas hsp90 at the lysosomal membrane is critical for guaranteeing LAMP-2A stability while transitioning between complexes.
MATERIALS AND METHODS
Animals and cells.
Adult male Wistar rats (200 to 250 g) were used. Mouse fibroblasts (NIH 3T3) were from the American Type Culture Collection (Manassas, VA). H460 cells (lung carcinoma), LS cells (mouse fibroblasts), and HCT-116 cells (colon carcinoma cells) were from the NCI repository. Clones of the NIH 3T3 cells subjected to stable LAMP-2A RNA interference (RNAi) were generated as described previously (27).
Chemicals.
Reagents and chemicals were as previously described (8, 23). The polyclonal antibodies against the cytosolic tail of LAMP-2A and LAMP-2B were developed in our laboratory (7; C. Zhang and A. M. Cuervo, unpublished data). The antibodies against the luminal region of LAMP-2 and LAMP-1 were from the Hybridoma Bank (University of Iowa), against hsc70 from Maine Biotechnology Services, Inc. (Portland, ME), and against hsp90 from Stressgen (Canada). Geldanamycin was from Sigma (St. Louis, MO) and the geldanamycin derivative 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG) was from the National Cancer Institute, Bethesda, MD. The high-molecular-weight calibration kit was from Amersham Biosciences.
Isolation of subcellular fractions.
Rat liver lysosomes were isolated from a light mitochondrial-lysosomal fraction by centrifugation through a discontinuous metrizamide density gradient (3). In some experiments, two separate lysosomal fractions with different CMA activity were isolated as described before (10). Lysosomes from cultured mouse fibroblasts were isolated by centrifugation of a postnuclear supernatant fraction through metrizamide/percoll discontinuous density gradients (31). The integrity of the lysosomal membrane was measured by β-hexosaminidase latency assays, as described previously (33). Only preparations with lysosomes that were more than 95% intact were used. Lysosomal matrices and membranes were separated by centrifugation after hypotonic shock as described previously (28). A cytosolic fraction was prepared by centrifugation of the light mitochondrial-lysosomal fraction supernatant at 100,000 × g for 1 h at 4°C.
Isolation of LAMP-2A multimeric complexes.
Lysosomal membranes were solubilized by resuspending them in 20 mM MOPS [3-(N-morpholine)propanesulfonic acid], 150 mM NaCl, and 0.5% NP-40 buffer (except where indicated) and incubating them for 15 min on ice. After centrifugation for 15 min at 16,000 × g, the soluble proteins were recovered in the supernatant. The different protein multimeric complexes present in this solubilized fraction were separated by the following four methods.
(i) Native gel electrophoresis.
Lysosomal membrane complexes were solubilized in solubilization buffer and subjected to native electrophoresis (non-sodium dodecyl sulfate-polyacrylamide gel electrophoresis [non-SDS-PAGE]) after boiling with nonreducing sample buffer (non-β-mercaptoethanol, non-SDS Laemmli buffer) as previously described (35). Briefly, 10% acrylamide gels were polymerized in the absence of SDS, and the detergent was also omitted in the standard running buffer. The samples were resolved by electrophoresis at a constant voltage of 150 mV, and the LAMP-2A-containing complexes were detected by immunoblotting using anti-LAMP-2A antibody and quantified by densitometry.
(ii) BNE.
Lysosomal membranes were solubilized in 1% octyl glycoside (or other detergents, as indicated) by a 15-min incubation on ice followed by centrifugation at 16,000 × g for 15 min. The supernatant was supplemented with nonreducing sample buffer and subjected to blue native electrophoresis (BNE) using 3 to 12% NativePAGE Novex bis-Tris precast gels (Invitrogen, Carlsbad, CA).
(iii) Centrifugation in continuous density sucrose gradients.
The solubilized lysosomal membranes were loaded on a 4.5-ml continuous sucrose gradient (10 to 60%) in the above-mentioned solubilization buffer. The samples were ultracentrifuged at 210,000 × g for 20 h at 4°C in a swinging-bucket rotor (SW60 rotor; Beckman). Aliquots of 350 to 500 μl collected from the top of the gradients were precipitated with 10% trichloroacetic acid (TCA), washed with acetone, and resuspended in 100 μl of 5% β-mercaptoethanol in phosphate-buffered saline (1.37 M NaCl, 0.003 M KCl, 0.07 M Na2HPO4, 0.11 M K2HPO4 [pH 7.4]). Each aliquot was subjected to SDS-PAGE and immunoblotting or silver staining (24, 34). The level of LAMP-2A in each complex was expressed as a percentage of the total amount of LAMP-2A in each lane of the gel.
(iv) Gel filtration chromatography.
Solubilized lysosomal membranes were subjected to gel filtration chromatography through a Sephacryl S-400HR matrix (void volume of column, 25 ml), and 30-μl fractions were collected. Each fraction was subjected to 10% TCA precipitation and washed with acetone, and after resuspension in phosphate-buffered saline, all samples were boiled with reducing Laemmli buffer. The presence of LAMP-2A in the fractions was detected by SDS-PAGE and immunoblotting. Partially deglycosylated forms of LAMP-2A, resulting from sample manipulation, were considered part of the LAMP-2A presented in each fraction for densitometry purposes.
Site-directed mutagenesis.
Point mutations were performed with the QuikChange site-directed mutagenesis kit (Stratagene) and were verified by DNA sequencing. The cells were transfected with the cDNAs for native and mutated human LAMP-2A subcloned in the pCR3 mammalian expression vector (Invitrogen) using the calcium phosphate method as described before (7, 9).
Uptake and degradation of substrate proteins by isolated lysosomes.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was radiolabeled with [14C]formaldehyde by reductive methylation as described before (21). Cytosolic proteins from mouse fibroblasts in culture were metabolically radiolabeled by incubation with [14C]leucine (2 μCi/ml) at 37°C for 2 days (10). [14C]GAPDH or a pool of radiolabeled cytosolic proteins (2) were incubated in MOPS buffer (10 mM MOPS [pH 7.3], 0.3 M sucrose, 1 mM dithiothreitol) with intact lysosomes for 30 min (7, 10, 11). The degradation of the radiolabeled substrates was measured after precipitation with TCA as described previously (7, 10, 11). Proteolysis was expressed as the percentage of the initial acid-insoluble radioactivity (protein) transformed into acid-soluble radioactivity (amino acids and small peptides) at the end of the incubation. Where indicated, lysosomes were disrupted by a hypotonic shock before incubation with the substrates to assess changes in enzymatic proteolytic activity. In other experiments, the lysosomal uptake of GAPDH was calculated separately from binding by comparing the association of GAPDH with lysosomes pretreated or not with protease inhibitors as described before (22, 23).
Intracellular protein turnover.
To measure the degradation of long-lived proteins, confluent cells were labeled with leucine (2 μCi/ml) for 48 h at 37°C and then extensively washed and maintained in complete (10% NCS) or serum-deprived medium containing an excess of unlabeled leucine (2.8 mM) to prevent the reutilization of radiolabeled leucine (4). Aliquots of the medium taken at different times were precipitated with TCA, and proteolysis was measured as above. The total radioactivity incorporated into the cellular proteins was determined as the amount of acid-precipitable radioactivity in the labeled cells immediately after washing.
General methods.
The protein concentration was determined by the Lowry method using bovine serum albumin as a standard (25). After SDS-PAGE and immunoblotting (24), the proteins recognized by the specific antibodies were visualized using secondary antibodies conjugated to horseradish peroxidase by chemiluminescence methods (Renaissacence; NEN-Life Science Products, Boston, MA). The densitometric quantification of the immunoblotted membranes was performed on Kodak scientific imaging film using an image analyzer system (S-100; Inotech, Sunnyvale, CA).
RESULTS
Characterization of different LAMP-2A multimeric complexes at the lysosomal membrane.
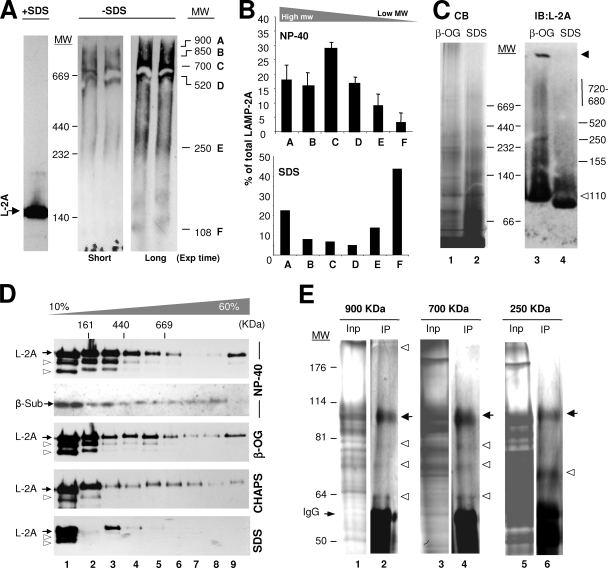
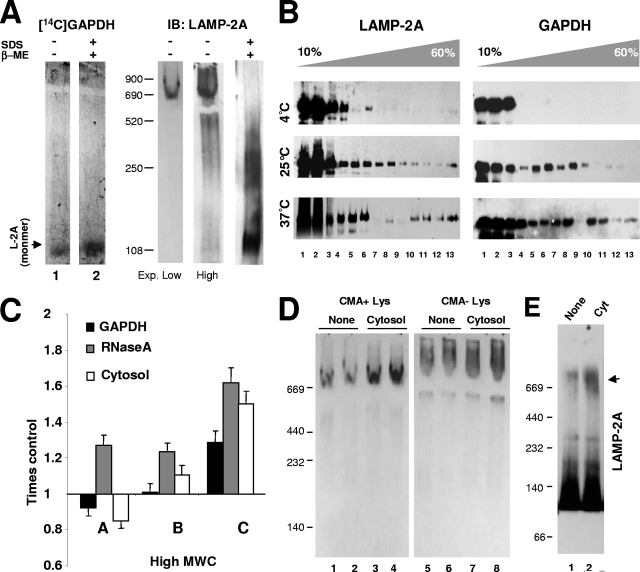
We have previously described, using density gradient centrifugation, that LAMP-2A forms high-molecular-mass complexes at the lysosomal membrane (9). However the nature, stability, and functional consequences of the formation of these complexes have not been analyzed before. To this purpose, we have used in this work native gel electrophoresis, BNE, molecular exclusion chromatography, and sucrose density gradient centrifugation of lysosomes isolated from the livers of rats starved for 48 h, a condition known to activate CMA (13, 26). While only LAMP-2A monomers (90 to 110 kDa) are detected by immunoblotting after resolving rat liver lysosomes in reducing (with β-mercaptoethanol) SDS electrophoresis (Fig. 1A, left), we found five well-resolved higher-molecular-mass complexes containing LAMP-2A (in a range from 150 kDa up to >850 kDa) when samples were subjected to nonreducing native electrophoresis (Fig. 1A, right; complexes are labeled from A to E [F being the monomer] with their estimated molecular weights indicated on the right). Low concentrations of an anionic detergent (SDS) disrupted most of the complexes into monomeric LAMP-2A (Fig. 1B). The reproducibility of the size of these complexes, along with the fact that LAMP-2A was never detected in the stacking of the gel or in the filter when the samples were subjected to filter retardation assays (data not shown), makes it unlikely that these complexes correspond to random protein aggregates. In addition, despite LAMP-2A being a heavily glycosylated protein, it is unlikely that the different complexes result from the partial deglycosylation of the protein, because when 5-mm slices (11 slices from top to bottom) of the native gel were subjected to reducing SDS electrophoresis and immunoblotting for LAMP-2A, only the full-size LAMP-2A was detected in each of the fractions positive for the protein in the native gel (see Fig. S1 in the supplemental material).
FIG. 1.
LAMP-2A organizes into defined protein complexes at the lysosomal membrane. (A) Lysosomes isolated from the livers of 48-h-starved rats solubilized in 0.5% NP-40 were subjected to electrophoresis in the presence or absence of SDS and without β-mercaptoethanol and immunoblotted for LAMP-2A (L-2A). The LAMP-2A complexes are named alphabetically from A to F, and their estimated molecular weights (MW) are indicated in thousands. Exp, exposure. (B) Effect of the solubilization of lysosomes with NP-40 (top) or SDS (bottom) on the distribution of LAMP-2A among different molecular complexes resolved by native gel electrophoresis. Values are expressed as percentages of the total LAMP-2A in the membrane present in each complex and are the means ± SE of the densitometric quantification of two to six immunoblots as the one shown in panel A. (C) Isolated lysosomal membranes solubilized with the indicated detergents were subjected to BNE and stained with Coomassie blue (CB) (left) or subjected to immunoblotting (IB) for LAMP-2A (L-2A) (right). The black arrowhead indicates protein retained in the well, and the white arrowhead indicates full-size monomeric LAMP-2A. β-OG, β-octyl-glycoside. (D) Lysosomal membranes isolated and solubilized with the indicated detergents were subjected to continuous sucrose density gradient centrifugation. Immunoblots for LAMP-2A (L-2A) of aliquots collected from the top to the bottom of the gradients are shown. The estimated molecular masses were calculated with molecular markers run through the gradients. The distribution of the 20S proteasome β subunits (β-Sub) after subjecting cytosol to the same gradients is shown as a control of the >700-kDa complexes. The arrowheads point to partially deglycosylated forms of LAMP-2A. (E) Protein composition of three of the LAMP-2A-containing complexes. Solubilized lysosomal membranes were resolved by gel filtration, and the fractions in which the 900-, 700-, and 250-kDa LAMP-2A-containing complexes were detected were subjected to immunoprecipitation with an antibody specific against LAMP-2A. The inputs (Inp; 1/10 of total) and immunoprecipitates (IP) were subjected to SDS-PAGE, and the gels were stained with a periodic acid-modified silver staining procedure to stain heavily glycosylated proteins. The bands corresponding to IgG are indicated. Black arrows indicate LAMP-2A, and white arrowheads indicate proteins reproducibly coimmunoprecipitated with LAMP-2A in each fraction.
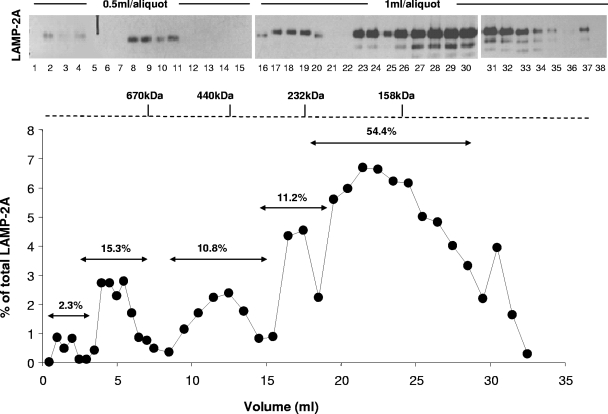
A similar distribution of LAMP-2A in different molecular weight complexes was observed when the solubilized lysosomes were subjected to molecular exclusion chromatography (Fig. 2), BNE (Fig. 1C), or centrifugation through a continuous sucrose density (10 to 60%) gradient (Fig. 1D; the distribution of the β subunits of the 20S proteasome [complexes up to 700 kDa] in a cytosolic fraction is shown as a reference). These complexes were also disrupted by the exposure of lysosomes to low concentrations of anionic detergent (Fig. 1C and D). The lower-molecular-weight bands observed in the sucrose gradient fractions (arrowheads) correspond to deglycosylated LAMP-2A generated during the precipitating procedures rather than to the proteolytic fragments, as those bands were only observed in the lysosomal preparation after precipitation and, in addition, LAMP-2A was still detected as a single 40-kDa band when the precipitated fraction was completely deglycosylated by enzymatic treatment (see Fig. S2 in the supplemental material). The apparently different amount of monomers of LAMP-2A at the lysosomal membrane observed when using density gradient centrifugation (Fig. 1D) or molecular-exclusion chromatography (Fig. 2) compared to native electrophoresis is due, for the most part, to the different affinity of the antibody for native and denatured LAMP-2A. As shown before (see Fig. S1 in the supplemental material), when native gels were subjected to denaturing and reducing electrophoresis, the initially weak signal for LAMP-2A in the monomeric region becomes considerably more intense (40% of the total LAMP-2A in the membrane, which is close to the 54% observed after molecular-exclusion chromatography [Fig. 2]). The negative charge provided to all proteins by Coomassie blue seems to facilitate the recognition of LAMP-2A monomers by the antibody after BNE (Fig. 1C). In this respect, the two types of native electrophoresis provided complementary advantages, as the standard native electrophoresis favored the recognition of LAMP-2A in the very-high-molecular-weight complexes without the high background of the monomeric form, whereas BNE allowed better resolution of the monomers and multimeric complexes and more accurate information about the size and relative abundance of each complex. Using this procedure, we calculated the relative distribution (±5% standard error [SE]) of LAMP-2A in the complexes as follows: 3% in the >800-kDa complexes, 21.5% in the 720- to 680-kDa complexes, 4% in the 520-kDa complexes, 14.5% in the 250-kDa complexes, 12% in the 155-kDa complexes, and 45% in 110-kDa (monomer) complexes. In summary, the four methods used in this study revealed the presence of LAMP-2A at the lysosomal membrane at any given time as part of different well-resolved protein multimeric complexes.
FIG. 2.
Resolution of different LAMP-2A-containing protein complexes at the lysosomal membrane by gel filtration. Distribution of LAMP-2A through the different fractions collected after subjecting lysosomes isolated from 48-h-starved rats and solubilized in 0.5% NP-40 to gel filtration chromatography. The graph (bottom) shows the percentages of the total LAMP-2A at the lysosomal membrane present in each of the fractions monitored by immunoblotting (top). The relative percentage of LAMP-2A in the resolved peaks was calculated after the addition of the LAMP-2A present in the different aliquots included in each peak.
Previous immunoprecipitation studies from our group of cells expressing hemagglutinin-tagged LAMP-2A have revealed that at least three molecules of LAMP-2A are able to associate together in protein complexes at the lysosomal membrane (9). To determine whether or not the high-molecular-mass complexes containing LAMP-2A detected at the lysosomal membrane corresponded to different orders of LAMP-2A homomultimers, we subjected three of these complexes (>800 kDa, 700 kDa, and 250 kDa) to immunoprecipitation for LAMP-2A and analyzed the electrophoretic pattern of the precipitated fraction after silver staining. As shown in Fig. 1E, although LAMP-2A was the most abundant protein in each of these complexes, other proteins were also detected in association with LAMP-2A. Although the presence of contaminant immunoglobulin G (IgG) in the precipitate prevented us from discriminating any protein below 50 kDa, we were able to determine in the molecular-mass range that could be resolved that the protein composition of the two high-molecular-mass complexes was more similar than that observed in the 250-kDa complex. Note that because the silver-staining protocol was modified to allow staining of heavily glycosylated proteins such as LAMP-2A, it is not possible to determine the stoichiometry of the components of these complexes. Our results thus support that LAMP-2A associates with other proteins in lysosomes to form well-resolved protein complexes.
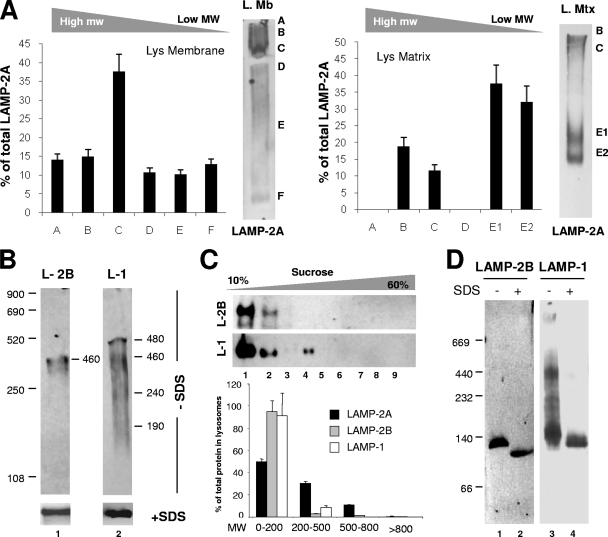
Although LAMP-2A is predominantly located at the lysosomal membrane, a percentage of the full-size protein can also be detected in the lysosomal lumen (9, 20), from where it can be mobilized into the lysosomal membrane in conditions requiring the maximal activation of CMA (such as prolonged starvation) (8). Native gel electrophoresis of the two lysosomal compartments revealed that while LAMP-2A organized into high- and medium-molecular-mass complexes at the lysosomal membrane, the predominant forms of LAMP-2A in the lysosomal matrix were protein complexes of about 200 to 400 kDa, composed mainly of LAMP-2A and luminal hsc70 (9; Fig. 3A). Native electrophoresis (Fig. 3B), sucrose density gradient centrifugation (Fig. 3C), and BNE (Fig. 3D) revealed that the organization of LAMP-2A at the lysosomal membrane differs from that of other major lysosomal membrane proteins of very similar molecular weight and structural characteristics, such as LAMP-1 and even LAMP-2B, a splice variant of LAMP-2, which only differs from LAMP-2A in the amino acid composition of its transmembrane and cytosolic regions (about 15% of the total protein) (17) and which we have recently shown is not required for CMA (C. Zhang and A. M. Cuervo, unpublished data).
FIG. 3.
Organization into protein complexes of different membrane proteins in lysosomes. (A) Lysosomal membranes (L. Mb) and matrices (L. Mtx) solubilized with 0.5% NP-40 were subjected to native gel electrophoresis and immunoblotting for LAMP-2A. Graphs show the means + SE of the densitometric quantification of three different experiments as the ones shown. Values are expressed as percentages of the total LAMP-2A present in each complex. Two bands (E1 and E2) with different molecular weights (MW) were detected in the region that corresponds to complex E in total lysosomes (Fig. 1A). (B) Lysosomal membranes solubilized as described for panel A were subjected to native gel electrophoresis (−SDS; top) or SDS-PAGE (+SDS; bottom) and immunoblotted for LAMP-2B (L-2B) and LAMP-1 (L-1). The estimated molecular weights of the immunoreactive bands are indicated. (C) Lysosomal membranes solubilized as described for panel A were subjected to continuous sucrose density gradient centrifugation. The content of LAMP-2A, LAMP-2B (L-2B), and LAMP-1 (L-1) in each of the aliquots collected from the gradients was determined by immunoblotting with specific antibodies. Values are expressed as percentages of the total protein in lysosomes and correspond to the densitometric quantification of five immunoblots as the ones shown here. Aliquots were grouped by MW (in thousands) regions as shown in panel D. (D) Lysosomal membranes solubilized as described for panel C were subjected to BNE and immunoblotting for LAMP-2B or LAMP-1.
Our results support the organization of LAMP-2A at the lysosomal membrane into defined high-molecular-weight protein complexes distinct from those in the lumen and from the ones formed by other lysosomal membrane proteins.
The organization of LAMP-2A at the lysosomal membrane changes with changes in CMA activity.
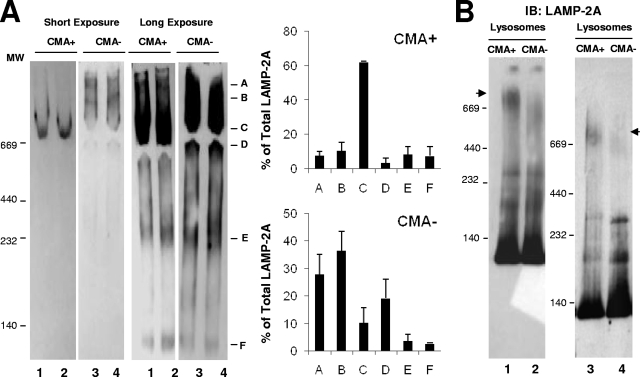
To gain insight into the functional relevance of the different protein complexes containing LAMP-2A at the lysosomal membrane and into the relationship of these complexes to CMA, we compared the lysosomal organization of LAMP-2A in two groups of lysosomes known to have very different ability for CMA. We have previously identified two populations of lysosomes with very similar morphologies and enzymatic contents but with very different abilities to degrade proteins via CMA due to the different levels of hsc70 in their lumen (10). Thus, hsc70-containing lysosomes (CMA+ lysosomes) are active for CMA, while CMA activity is barely detectable in the group of lysosomes lacking this chaperone (CMA− lysosomes) (10). An analysis of these two groups of lysosomes by native gel electrophoresis (Fig. 4A) or BNE (Fig. 4B) revealed distinctive patterns of organization of LAMP-2A in their membranes, particularly in the high-molecular-weight complexes (Fig. 4). Some of the LAMP-2A-containing protein complexes particularly enriched in one lysosomal population were not detectable in the other. Thus, whereas the 700-kDa complex (band C) was highly enriched in CMA+ lysosomes, the abundance of this complex was markedly lower in CMA− lysosomes, where only a less abundant, lower-molecular-mass form (approximately 520 kDa) was observed. Native electrophoresis revealed an enrichment of the very-high-molecular-mass forms of LAMP-2A (>700 kDa; bands A and B) in CMA− lysosomes, although these complexes were not detected by BNE. The higher exposure of the native gels and the BNE revealed no changes in the composition of the low-molecular-weight complexes between both groups of lysosomes (Fig. 4). The two major differences between both groups of lysosomes identified to date are the lower content of luminal hsc70 and higher pH (5.6) of CMA− lysosomes compared to those of CMA+ lysosomes. However, we demonstrate that neither of these two factors was essential for the formation/maintenance of the 700-kDa complex. Thus, the addition of hsc70 into the lumen of CMA− lysosomes as described before (10) was not sufficient to promote the formation of the >700-kDa complex in their membrane, and an increase of the luminal pH of CMA+ lysosomes, to resemble that found in CMA− lysosomes, did not disrupt the 700-kDa complex (see Fig. S3 in the supplemental material). Nevertheless, the differences in the organization of LAMP-2A in the membranes of both groups of lysosomes support a possible functional relevance for this organization and point toward the 700-kDa complex detected in lysosomes active for CMA as the putative functional complex, since that is the one mainly absent in the CMA-inactive group of lysosomes.
FIG. 4.
The organization of LAMP-2A into protein complexes is different in lysosomes with different CMA activity. (A) Lysosomes with high (CMA+) and low (CMA−) CMA activity isolated from the livers of 48-h-starved rats were subjected to native gel electrophoresis and immunoblotted for LAMP-2A. Left, two different exposures of a representative immunoblot. Right, mean values + SE of the distribution of LAMP-2A in the different complexes were calculated by the densitometric quantification of three to four immunoblots as the ones shown. Values are expressed as percentages of the total amount of LAMP-2A at the lysosomal membrane. (B) The same groups of lysosomes were subjected to BNE and immunoblotting (IB) of LAMP-2A. The immunoblot on the right shows a gel run for a longer time to increase the resolution of the intermediate complexes. Arrowheads indicate the approximately 700-kDa complex.
Different steps in CMA depend on the formation of different LAMP-2A-containing protein complexes at the lysosomal membrane.
We first analyzed which LAMP-2A-containing protein complexes are required for the binding of CMA substrates. Because the antibody specific for LAMP-2A recognizes only the cytosolic tail of the protein, and this is where CMA substrates bind (9); classical pull-down experiments to determine the association of substrate proteins with particular LAMP-2A complexes cannot be used. Instead, we first analyzed the binding of CMA substrate proteins to each of these complexes using far-Western blotting/panning after native gel electrophoresis. We have previously used this approach to identify the binding of substrates to LAMP-2A immobilized in nitrocellulose after SDS-PAGE (7). Using the same binding conditions, we incubated the nitrocellulose membranes containing the lysosomal samples electrotransferred from native gels with radiolabeled [14C]GAPDH, a well-characterized CMA substrate (7, 10, 12). As shown in Fig. 5A, the radiolabeled protein was only detected as being associated with LAMP-2A monomers, and we did not detect the direct binding of GAPDH to any of the high-molecular-weight complexes of LAMP-2A (immunoblots for LAMP-2A of the lysosomal membranes subjected to native or SDS electrophoresis are shown as a reference on the right). The binding of GAPDH to LAMP-2A during the panning process was specific to the cytosolic tail of this protein because it could be competed by a synthetic peptide with the amino acid composition of the carboxy terminus region of LAMP-2A (data not shown). These results support that CMA substrates bind preferentially to monomers of LAMP-2A and that once LAMP-2A is organized into high-molecular-weight complexes, substrates can no longer bind to the LAMP-2A cytosolic tail.
FIG. 5.
Functional differences of the LAMP-2A complexes at the lysosomal membrane. (A) Lysosomal membranes isolated from the livers of 48-h-starved rats were subjected to native and reducing electrophoresis as labeled and electrotransferred to nitrocellulose membranes which were then incubated in renaturalization buffer with [14C]GAPDH and exposed to a phosphorimager screen (left) or subjected to immunoblotting (IB) for LAMP-2A (L-2A) (right). Two different time exposures (Exp.) are shown for the immunoblot. β-ME, β-mercaptoethanol. (B) Lysosomes isolated as described for panel A were incubated with GAPDH at the indicated temperatures, cross-linked, and then subjected to centrifugation through sucrose density gradients. Aliquots from the top to the bottom of the gradient were subjected to SDS-PAGE and immunoblotting for LAMP-2A (left) or GAPDH (right). (C) Lysosomes incubated with the indicated CMA substrates were solubilized and subjected to native gel electrophoresis and immunoblotted for LAMP-2A. Changes in the percentage of LAMP-2A in the indicated complexes (as defined in Fig. 1A) relative to the amount in lysosomes incubated without additions (control) are shown and are the means + SE of the results from four to five different experiments. (D) The effect of the incubation of lysosomes (Lys) with high (+) and low (−) CMA activity with cytosolic substrates on the organization of LAMP-2A at their membranes was analyzed by native gel electrophoresis and immunoblotting for LAMP-2A. Samples from two different animals are shown. (E) Lysosomes incubated alone or with a pool of cytosolic proteins (Cyt) were solubilized and subjected to BNE and immunoblotting for LAMP-2A. Incubation with substrates increased the intensity of the LAMP-2A-containing complex of approximately 700 kDa (indicated by the black arrowhead) by 4.2- ± 0.8- fold.
To further support this preferential binding of CMA substrates to LAMP-2A monomers, we incubated lysosomes with GAPDH under conditions favoring either substrate binding or translocation. We have previously shown that the selective binding of the substrate to lysosomes still occurs at low temperatures but that substrate uptake can only be detected at temperatures ≥23°C (2, 12, 33). Because translocation across the lysosomal membrane via CMA is a very rapid event, we stabilized these multimeric complexes with reversible cross-linkers to be able to capture a detectable amount of substrate proteins across the membrane. At low temperatures, only monomers and low-molecular-weight complexes of LAMP-2A were detectable in lysosomal membranes subjected to sucrose density gradient centrifugation (Fig. 5B, left). Accordingly, lysosome-associated GAPDH did not migrate beyond the 250-kDa fraction (Fig. 5B, right) (in the absence of lysosomes, GAPDH was only detected in the two first fractions, corresponding to the range between GAPDH monomers [36 kDa] to tetramers [144 kDa] [data not shown]). In contrast, the incubation of GAPDH with lysosomes at 25°C or 37°C resulted in the migration of both LAMP-2A and GAPDH toward higher-density regions of the gradient (Fig. 5B, middle and bottom panels). Incubation with different CMA substrates or with a cytosolic fraction containing a variety of CMA substrates also increased the amount of LAMP-2A detectable in the 700-kDa complex (complex C) by native gel electrophoresis (Fig. 5C) and by BNE (Fig. 5E), whereas the multimeric distribution of LAMP-2A in the membrane of CMA− lysosomes remained unchanged (Fig. 5D).
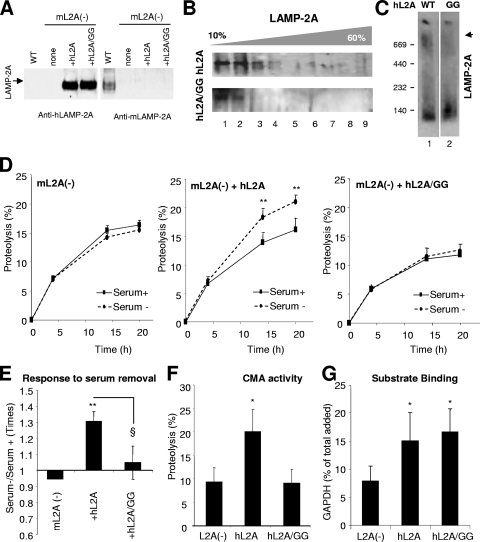
To directly confirm that the formation of this high-molecular-weight protein complex containing LAMP-2A at the lysosomal membrane is required for substrate uptake, we carried out site-directed mutagenesis in LAMP-2A in search of a mutation that altered its ability to form this complex. We targeted our mutations to the transmembrane region because, along with the cytosolic tail, it is the only region different among the three LAMP-2 splice variants and because transmembrane regions have been shown to be critical for the multimerization of other membrane proteins. We expressed wild-type or mutated human LAMP-2A in mouse fibroblasts in culture in which we have previously knocked down the expression of the endogenous LAMP-2A (27) (Fig. 6A). We have previously shown that human LAMP-2A transfected in mouse cells is functional for CMA (9). Of the different mutations analyzed, we found that the replacement of two glycine residues (G) with alanines (A) in the transmembrane region (GG/AA), which resembles a dimerization motif (GXXXG) described for other proteins (29), did not affect the targeting of LAMP-2A into the lysosomal compartment, insertion, orientation, or stability of the protein in the lysosomal membrane (data not shown), but it modified the multimeric pattern of LAMP-2A (Fig. 6B and C). In fact, upon the centrifugation of the lysosomes isolated from these cells in continuous density sucrose gradients, we did not detect the mutant protein in the 700-kDa complex as it did not migrate beyond the 250-kDa region of the gradient (Fig. 6B). BNE of the isolated lysosomes also revealed a marked decrease in the amount of LAMP-2A in the 700-kDa complex (Fig. 6C).
FIG. 6.
Identification of a LAMP-2A-containing protein complex required for substrate uptake by lysosomes via CMA. NIH 3T3 mouse fibroblasts subjected to stable LAMP-2A RNAi [mL2A(−)] (27) were transfected with cDNA encoding for the wild type (+hL2A) or a GG/AA mutant (+hL2A/GG) of human LAMP-2A. (A) Immunoblot with an antibody against human LAMP-2A (left) or against mouse LAMP-2A (right) of total cellular extracts of untransfected mouse fibroblasts subjected to LAMP-2A RNAi (none) or the same fibroblasts transfected with the indicated constructs. A cellular extract of mouse fibroblasts not subjected to RNAi (WT) is shown in lane 1. (B and C) Lysosomes from the LAMP-2A RNAi-treated cells transfected with wild-type (hL2A) or mutant (hL2A/GG) human LAMP-2A were subjected to centrifugation through sucrose density gradients (B) or BNE (C). The SDS-PAGE of the aliquots from the top to the bottom of the gradient and the BNE were subjected to immunoblotting for human LAMP-2A. (D) Rates of long-lived-protein degradation via CMA (sensitive to ammonium chloride and insensitive to 3-methyl adenine) were calculated in the cells maintained in the presence (Serum+) or absence (Serum−) of serum, as described in Materials and Methods. Values are expressed as percentages of the total acid-precipitable radioactivity (proteins) transformed into acid-soluble radioactivity (amino acids and small peptides) at the indicated time points and are means + SE of the results from three different experiments with triplicate wells. *, P < 0.01; **, P < 0.001. (E) Changes in protein degradation in response to serum removal were analyzed in the cells described for panel D. Values are expressed as the fold increases in protein degradation in untransfected wild-type cells and are the means + SE of the results from two different experiments with triplicate wells. * (P < 0.001) and § (P < 0.01) are differences with respect to the control and the cells transfected with the wild-type protein, respectively. (F) Proteolysis of a pool of labeled cytosolic proteins by intact lysosomes isolated as described for panel B. Values are shown as percentages of proteolysis and are the means + SE of the results from two different experiments with triplicate samples. *, P < 0.01. (G) Binding of GAPDH to intact lysosomes isolated as described for panel B. Lysosomes were incubated at 4°C to eliminate differences due to impaired uptake. Values are shown as percentages of the total GAPDH associated with lysosomes collected by centrifugation after correcting for the total amount of lysosomal protein in each sample and are the means + SE of the results from three individual experiments. *, P < 0.05.
We have previously shown that in cells in which the expression of LAMP-2A is blocked by RNAi, the ability to increase the rates of long-lived-protein degradation via CMA (sensitive to ammonium chloride and insensitive to 3-methyladenine) in response to serum removal is practically abolished but that the normal response can be restored by expressing an exogenous copy of LAMP-2A (7, 27) (Fig. 6D and E). We found that the expression of the mutant form of LAMP-2A with altered multimerization failed to restore the cellular ability to increase protein degradation in the absence of serum (Fig. 6D and E). To directly assess the CMA activity of lysosomes containing the GG/AA mutant form of LAMP-2A, we isolated lysosomes from untransfected LAMP-2A knockdown cells and from the same set of cells transfected with the wild-type or mutant form of LAMP-2A and incubated them with a pool of radiolabeled CMA substrate proteins. In agreement with the metabolic studies, lysosomes from the cells expressing the GG/AA LAMP-2A mutant displayed significantly lower ability to translocate and degrade the radiolabeled substrates via CMA (Fig. 6F). Interestingly, an analysis of the binding of CMA substrates at lower temperatures, which allows its dissociation from uptake, revealed comparable binding abilities between lysosomes from cells expressing the wild type and the GG/AA LAMP-2A mutant (Fig. 6G). The expression of both proteins resulted in a significant increase in substrate binding compared to that of lysosomes from untransfected cells, supporting our previous conclusion (Fig. 5) that the recruitment of LAMP-2A into high-molecular-weight multimeric complexes at the lysosomal membrane is not required for substrate binding. In summary, the facts that (i) the amount of LAMP-2A associated with the 700-kDa complex was higher in lysosomes with higher CMA activity (Fig. 4A and B) and when CMA was activated in lysosomes by incubation with substrates (Fig. 5C to E), (ii) the 700-kDa complex is prevalent under conditions favoring substrate translocation and not substrate binding (Fig. 5B), and (iii) a decrease in CMA rate is observed when the formation of this complex is eliminated through LAMP-2A mutagenesis (Fig. 6) strongly support the role of the 700-kDa complex in the translocation of substrates across the lysosomal membrane via CMA.
Lysosomal chaperones regulate the organization of LAMP-2A into multimeric complexes at the lysosomal membrane.
A particular subset of chaperones has been shown to associate with the lysosomal membrane and to participate in CMA, but their specific roles in this process are unknown (1, 2, 10). Furthermore, although the interaction of both lys-hsc70 and hsp90 with LAMP-2A has been previously shown by coimmunoprecipitation and colocalization by confocal immunofluorescence (1, 2, 10), there is no information on the form of LAMP-2A (monomer, multimer) that they bind to.
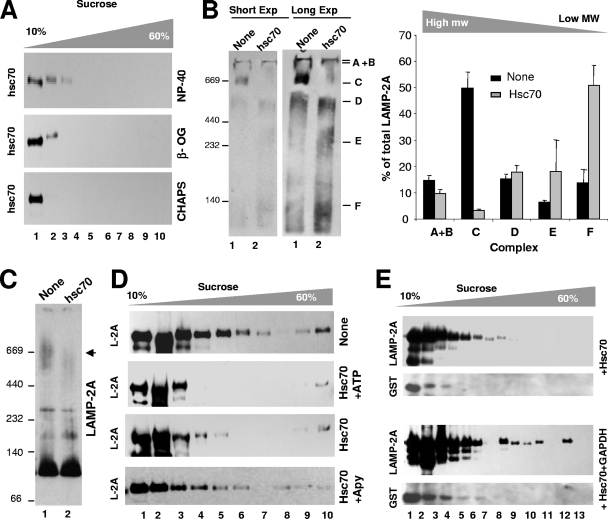
We first analyzed the contribution of hsc70 to the different LAMP-2A complexes at the lysosomal membrane using continuous sucrose density gradient centrifugation (Fig. 7A). We found that membrane-associated hsc70 did not migrate with LAMP-2A to the 700-kDa LAMP-2A complex required for substrate translocation. Instead, hsc70 was preferentially located in the lower density region of the gradient (≤250 kDa). A similar distribution was found when the lysosomal membranes were subjected to gel filtration (data not shown). The failure to see hsc70 in the high-molecular-mass region of the gradients was not a problem of sensitivity or dilution, because immunoblotting for hsc70 of the proteins coimmunoprecipitated with LAMP-2A (as shown in Fig. 1E) revealed the presence of the chaperone only in the 250-kDa complex (compatible with the association of one molecule of LAMP-2A and two of hsc70, or two molecules of LAMP-2A and one of hsc70).
FIG. 7.
Hsc70 induces disassembly of LAMP-2A from the high-molecular-weight protein complexes at the lysosomal membrane. (A) Lysosomes isolated from the livers of 48-h-starved rats were solubilized with the indicated detergents and subjected to sucrose density gradient centrifugation and immunoblotting for hsc70. (B to D) Lysosomes isolated as described for panel A were incubated with GST-hsc70 and/or ATP and apyrase (Apy), as labeled, solubilized, and subjected to native gel electrophoresis (two different exposures [Exp] are shown) (B), BNE (C), or sucrose density gradient centrifugation (D) and immunoblotted for LAMP-2A (L-2A). Changes in the distribution of LAMP-2A throughout the native gel were calculated by the densitometric quantification of the immunoblots from three different experiments. Values are expressed as times the values in lysosomes incubated without additions. The black arrowhead in panel C indicates the approximately 700-kDa complex. (E) Lysosomes incubated with the indicated additions were subjected to sucrose density gradient centrifugation and immunoblotted for LAMP-2A or GST.
We then compared the distribution of the endogenous chaperone to that of exogenously added hsc70. We have previously shown that the purified glutathione S-transferase (GST)-hsc70 recombinant fusion protein binds to the lysosomal membrane and when added in conjunction with CMA substrates stimulates their binding/uptake by lysosomes via CMA (12, 33). The incubation of lysosomes isolated from 48-h-starved rats (high CMA activity) with GST-hsc70 in the absence of CMA substrates decreased the amount of LAMP-2A present in the high-molecular-weight complexes. In fact, native gel electrophoresis (Fig. 7B), BNE (Fig. 7C), and sucrose density gradient centrifugation (Fig. 7D) all revealed a marked reduction in the amount of LAMP-2A in the 700-kDa complex and a shift of LAMP-2A toward the low-molecular-mass complex (≤250 kDa) and monomers (the amount of LAMP-2A in the 700-kDa complex changed from 18.2% ± 1.3% to 3.2% ± 0.5% of the total LAMP-2A in the membrane, as quantified by BNE). Although the addition of ATP to the reaction mixture did not increase hsc70-mediated disassembly (Fig. 7D), the complete depletion of ATP by apyrase treatment prevented this disassembly (Fig. 7D). We thereby conclude that the amount of ATP usually present in our lysosomal preparation was enough to facilitate hsc70 activity and that hsc70 disassembles the multimeric LAMP-2A complexes in an ATP-dependent manner. Similarly to the endogenous hsc70, the exogenously added hsc70 bound to the lysosomal membrane (tracked with an antibody against GST) was preferentially located in the lower density of the sucrose gradient (≤250 kDa) (Fig. 7E).
The ability of GST-hsc70 to disassemble LAMP-2A complexes was, however, abolished when the chaperone was added in conjunction with CMA substrates (GAPDH shown in Fig. 7E, bottom panels). Under these conditions, the high-molecular-mass complexes of LAMP-2A persisted as such, and as shown before (Fig. 5B), a higher amount of LAMP-2A migrated to the 700-kDa region of the sucrose density gradient (Fig. 7E, bottom panel), where it was also possible to detect part of the added substrate (Fig. 5B). The incubation of hsc70 with GAPDH in the absence of lysosomes did not induce the migration of GAPDH toward the high-density part of the gradient (data not shown). The binding of GAPDH-hsc70 to monomeric LAMP-2A could explain the slight shift observed in the migration of hsc70 bound to the lysosomal membrane when the substrate was added (Fig. 7E, bottom panel). Altogether, these results support a very dynamic organization of LAMP-2A at the lysosomal membrane and a novel role for hsc70 in the mobilization of LAMP-2A from the high-molecular-weight complexes, which are required for substrate translocation, toward lower-molecular-weight complexes and monomers—where substrate binding occurs. However, once substrate proteins interact with LAMP-2A, the formation of the translocating complex (700 kDa) is favored over disassembly.
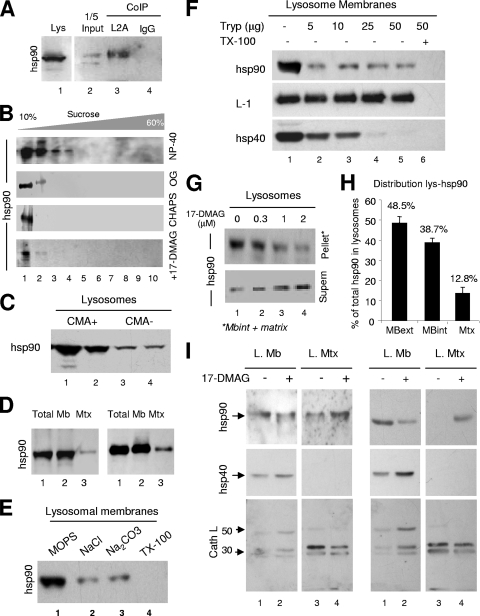
The function of hsp90, the other lysosome-associated chaperone that has been shown to colocalize with LAMP-2A at the lysosomal membrane (1), remains unknown. We confirmed that hsp90 interacts with LAMP-2A at the lysosomal membrane as it can be coimmunoprecipitated from solubilized rat liver lysosomal membranes (Fig. 8A). To gain insight into the possible functional role of the association of hsp90 to LAMP-2A, we first analyzed the nature of this complex. As in the case of hsc70, after density gradient centrifugation of the solubilized lysosomal membranes, most of the hsp90 associated with the lysosomal membrane remained in the lower-density region of the gradient (Fig. 8B). This result indicates that neither of the two CMA-related chaperones, hsc70 or hsp90, is part of the LAMP-2A-containing high-molecular-mass protein complex (700 kDa) required for substrate translocation.
FIG. 8.
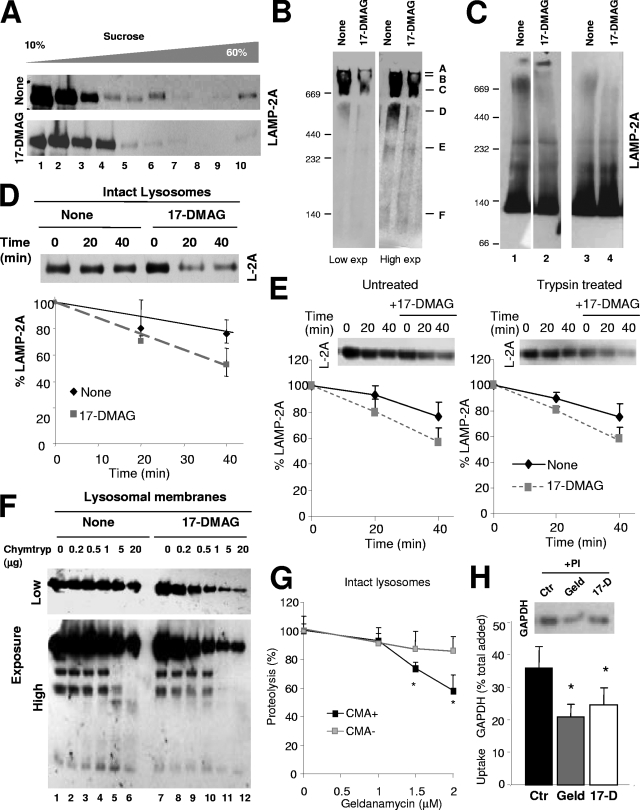
Characterization of lysosome-associated hsp90. (A) Lysosomes from the livers of 48-h-starved rats were solubilized and subjected to immunoprecipitation (CoIP) with an antibody against LAMP-2A (L2A) or an irrelevant immunoglobulin (IgG). Precipitates were subjected to SDS-PAGE and immunoblotting for hsp90. Lane 1 shows total lysosomes (Lys), and lane 2 contains 1/5 of the starting solubilized membranes. (B) Lysosomes isolated from the livers of 48-h-starved rats were solubilized with the indicated detergents and subjected to sucrose density gradient centrifugation and immunoblotting for hsp90. Bottom panel, lysosomes were incubated with the geldanamycin derivative 17-DMAG before solubilization with NP-40. (C) Intact lysosomes with high (CMA+) and low (CMA−) CMA activity isolated from the livers of 48-h-starved rats were subjected to SDS-PAGE and immunoblotted for hsp90 (duplicate samples for each type of lysosome are shown). (D) Intact lysosomes (total) with high CMA activity were subjected to hypotonic shock and centrifugation, and membranes (Mb) and matrices (Mtx) collected by centrifugation were processed as described for panel C. Duplicate samples are shown. (E) Intact lysosomes were subjected to washes with MOPS buffer, 1 M NaCl, 0.1 M Na2CO3, or 1% Triton X-100 as labeled. The amount of hsp90 associated with the membrane was detected after isolation of the membranes as above and immunoblotting for hsp90. (F) Intact lysosomes were incubated with increasing concentrations of trypsin (Tryp) supplemented or not with 1% Triton X-100, as labeled. Samples were subjected to SDS-PAGE and immunoblotting for the indicated proteins. (G) Intact lysosomes with high CMA activity were incubated with increasing concentrations of 17-DMAG, collected by centrifugation and supernatant (Supern) and pellet were subjected to SDS-PAGE and immunoblotting for hsp90. Mbint, luminal part of the membrane. (H) The percentage of lysosomal hsp90 present on the cytosolic side of the lysosomal membrane (MBext), the luminal part of the membrane (MBint), and the lysosomal lumen (Mtx) was calculated by combining the results obtained after membrane/matrix separation (D), membrane washing (E) and trypsinization (F), and treatment with hsp90 inhibitors (G). Values are the means ± SE of the results from five to eight different experiments. (I) Membranes (L. Mb) and matrices (L. Mtx) from lysosomes incubated alone (−) or with (+) 17-DMAG were subjected to immunoblotting for hsp90, hsp40, or cathepsin L (Cath L). Two representative immunoblots are shown. Arrows indicate the precursor (50 kDa) and active subunits (20 and 30 kDa) of cathepsin L.
To further characterize the involvement of hsp90 in the organization of LAMP-2A at the lysosomal membrane, we first analyzed the distribution and topology of this chaperone in lysosomes. We found that, as for hsc70, hsp90 is more abundant in the subpopulation of lysosomes with higher CMA activity (Fig. 8C). In this subgroup of lysosomes, the separation of lysosomal membranes and matrices after hypotonic shock and centrifugation revealed that hsp90 is preferentially located in the membrane (87.2% ± 2.3% in the membrane and 13.5% ± 3.8% in the matrix) (Fig. 8D). A portion of the hsp90 at the lysosomal membrane (30 to 40%, depending on the method) (Fig. 8H) remains associated with this fraction even in lysosomal membranes isolated from intact lysosomes subjected to washes of increasing astringency (Fig. 8E) or to treatment with an exogenous protease (Fig. 8F), supporting its association with the luminal side of the membrane. The resistance of the luminal region of LAMP-1 to the protease treatment and the susceptibility of hsp40, a chaperone located at the cytosolic side of the lysosomal membrane, were used as controls to show that the protease treatment was efficient in removing proteins located on the cytosolic side of the membrane but that it was not gaining access into the lysosomal lumen (Fig. 8F). These results thus confirm that, as previously described for hsc70, lysosomal hsp90 associates with both sides of the lysosomal membrane.
We then used 17-DMAG, a potent derivative of the irreversible hsp90 inhibitor geldanamycin (30), to modify the levels/activity of lysosomal hsp90. Treatment with this inhibitor released both the hsp90 associated with the lysosomal membrane, which could be recovered in the supernatant after centrifugation of the lysosomes (Fig. 8G-H), as well as that located on the luminal side of the membrane, which fractionated now with the lysosomal matrix (Fig. 8I). Notice that in the experiments in Fig. 8I, after treatment with the inhibitor, lysosomes were first collected by centrifugation to remove the hsp90 released from the cytosolic side of the membrane, and then lysosomal membranes and matrices were isolated by hypotonic shock and centrifugation. The release of hsp90 from both sides of the lysosomal membrane after treatment with the hsp90 inhibitor seems specific for this protein, as the same treatment did not decrease the association of hsp40 with the cytosolic side of the membrane or of the portion of cathepsin L normally detected on the luminal side of the lysosomal membrane (Fig. 8I).
The chemical inhibition of lysosomal hsp90 resulted in changes in the normal distribution of LAMP-2A at the lysosomal membrane. Thus, sucrose gradient centrifugation (Fig. 9A), native gel electrophoresis (Fig. 9B), and BNE (Fig. 9C) revealed that the release of hsp90 from the lysosomal membrane after incubation with 17-DMAG markedly decreased the amount of LAMP-2A detected in multimeric complexes and, in particular, at the 700-kDa complex, supporting a possible role for hsp90 in LAMP-2A multimerization. However, in contrast to the effect observed for hsc70, where the decrease in the amount of LAMP-2A in the 700-kDa complex associated with an increase in monomeric LAMP-2A (Fig. 7B to D), we did not observe an increase in the amount of LAMP-2A monomers upon treatment with the hsp90 inhibitors. Instead, the total levels of LAMP-2A as a monomer and in most of the multimeric complexes were reduced after exposure to the inhibitor, pointing toward a possible decreased stability of this protein in lysosomes. We have previously shown that LAMP-2A is normally turned over in lysosomes following a regulated sequential cleavage from the lysosomal membrane (15). To directly analyze the effect of hsp90 inhibition on LAMP-2A stability we incubated freshly isolated rat liver lysosomes with the hsp90 inhibitor and followed LAMP-2A degradation as the loss of the cytosolic tail. As shown in Fig. 9D, the degradation of LAMP-2A was faster in lysosomes previously treated with 17-DMAG. To determine which of the membrane-associated hsp90s (cytosolic or luminal) was responsible for this stabilizing effect on LAMP-2A, we removed the cytosolic hsp90 by high-concentration salt washes (see Fig. S4A in the supplemental material) or by trypsin cleavage (protecting the cytosolic tail of LAMP-2A with the excess of substrate) (Fig. 9E). The fact that the removal of the cytosolic hsp90 did not have an effect on LAMP-2A stability (Fig. 9E) but that the addition of 17-DMAG still increased the degradation rate of LAMP-2A supports the fact that most of the stabilizing effect of hsp90 over LAMP-2A was due to the protein associated with the luminal side of the lysosomal membrane.
FIG. 9.
Novel role for hsp90 as a stabilizer of LAMP-2A at the lysosomal membrane. (A) Intact rat liver lysosomes with high CMA activity were incubated alone or with the geldanamycin derivative 17-DMAG. At the end of the incubation, lysosomes were subjected to sucrose density gradient centrifugation (A), native gel electrophoresis (B), or BNE (C) and immunoblotted for LAMP-2A. Two exposures (Exp) of the same immunoblot are shown in panel B to better appreciate the region corresponding to the monomeric form of LAMP-2A. Two independent experiments are shown in C (lanes on the left were run in the same gel but were cut as they were separated by irrelevant samples). (D) Resealed membranes from lysosomes preincubated or not with 17-DMAG were incubated at 37°C in an isotonic buffer, and the remaining levels of LAMP-2A at the indicated times were detected by immunoblotting. Values are expressed as percentages of the LAMP-2A at time zero and are the means ± SE of the densitometric quantification of the immunoblots from three different experiments. A representative immunoblot is shown. (E) Intact lysosomes untreated or previously preincubated with 25 μg of trypsin and with 17-DMAG as indicated were incubated (after neutralization of the protease with a trypsin inhibitor) at 37°C in an isotonic buffer, and the remaining levels of LAMP-2A at the indicated times were detected by immunoblotting. Values are expressed as described for panel D and are the means ± SE of the densitometric quantification of the immunoblots from two different experiments. (F) Proteolytic cleavage pattern of LAMP-2A in lysosomal membranes isolated from lysosomes incubated as described for panel B and treated with increasing concentrations of chymotrypsin (Chymtryp). Images of a low (top) and a high (bottom) exposure time of the immunoblot for LAMP-2A are shown. (G to H) Effect of geldanamycin (Geld) and 17-DMAG (17-D) on CMA of GAPDH by isolated lysosomes. Panel G shows the effect of increasing concentrations of geldanamycin on the degradation of GAPDH by intact lysosomes with high (+) or low (−) CMA activity. Values are expressed as percentages of the degradation in untreated lysosomes to be able to compare the samples despite the low rates of degradation observed in the CMA− lysosomes and are the means + SE of the results from two different experiments with triplicate samples. In panel H, untreated lysosomes (control; Ctr) or lysosomes treated with the indicated hsp90 inhibitors and preincubated or not with protease inhibitors (PI) were incubated with GAPDH. Lysosomes were collected by centrifugation, and the amount of GAPDH associated with each sample was determined by SDS-PAGE and immunoblotting. Uptake was calculated after densitometric quantification as the difference between association and binding. Values are expressed as percentages of total GAPDH added and are the means + SE of six different experiments. *, P < 0.01.
We have previously shown that LAMP-2A associates with cathepsin A at the lysosomal membrane and that the formation of this complex is essential for the normal turnover of LAMP-2A at the membrane (15). However, the incubation of freshly isolated rat liver lysosomes with the hsp90 inhibitor did not enhance cathepsin A activity associated with the lysosomal membrane, neither did it promote the mobilization of LAMP-2A to discrete lipid membrane microdomains, previously shown to be the site for regulated LAMP-2A degradation at the lysosomal membrane (22) (see Fig. S4B and C in the supplemental material). Based on these results, we hypothesized that the degradation of LAMP-2A in the absence of hsp90 in the membrane did not follow the usual regulated cleavage. Instead, a conformational change of LAMP-2A rendered this receptor unstable in the absence of hsp90. In fact, we found that treatment with the hsp90 inhibitor increased the susceptibility of LAMP-2A to proteolytic cleavage by different proteases (chymotrypsin is shown in Fig. 9F). The degradation of LAMP-2A in lysosomes lacking hsp90 could be blocked by ammonium chloride (data not shown), supporting the participation of acidic luminal hydrolases in this degradation. Therefore, we propose that the association of hsp90 with LAMP-2A stabilizes the receptor at the lysosomal membrane likely in the transition stages between the monomeric and high-molecular-weight protein complexes. The instability of LAMP-2A in the absence of hsp90 could also explain why the treatment of lysosomes with hsp90 inhibitors (17-DMAG or geldanamycin) reduces the uptake of CMA substrates by isolated lysosomes (Fig. 9G; see also Fig. S5B in the supplemental material). In support that the effect of the hsp90 inhibitors is mostly on uptake, and not on other lysosomal properties, treatment with the inhibitors did not have any effect on the residual degradation of substrates observed in lysosomes inactive for CMA (Fig. 9G; see also the supplemental material) or on the degradation of CMA substrates by both groups of lysosomes once the membrane was disrupted (see Fig. S5C in the supplemental material).
In summary, our results support that the two chaperones related to CMA, hsc70 and hsp90, modulate the dynamic organization of LAMP-2A into different molecular weight complexes at the lysosomal membrane.
DISCUSSION
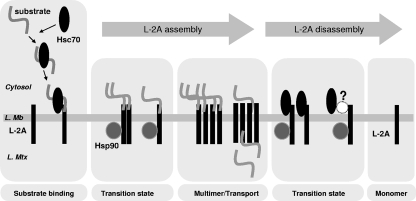
We have shown here that LAMP-2A organizes in dynamic, defined protein complexes at the lysosomal membrane (Fig. 1 to 3) and that the contribution of LAMP-2A to each complex changes with changes in CMA activity (Fig. 4 and 5). CMA substrates bind preferentially to LAMP-2A monomers (Fig. 5), while the efficient translocation of substrate proteins across the lysosomal membrane requires the formation of a particular 700-kDa LAMP-2A-containing complex (Fig. 6). The two chaperones related to CMA, hsc70 and hsp90, play distinctive roles in the stepwise assembly of LAMP-2A into protein complexes at the lysosomal membrane. Whereas hsc70 promotes the organization of LAMP-2A into monomers or smaller complexes (Fig. 7), the interaction of hsp90 with LAMP-2A is required to maintain the stability of the receptor likely while transitioning through different levels of multimerization (Fig. 9; see model in Fig. 10).
FIG. 10.
Hypothetical model for the dynamic assembly and disassembly of LAMP-2A (L-2A) into multimeric complexes at the lysosomal membrane (L. Mb). Based on the results presented in this work, we hypothesize that CMA substrates delivered by cytosolic hsc70 bind preferentially to monomers of LAMP-2A at the lysosomal membrane and that the binding of substrates promotes the multistep organization of LAMP-2A into higher-order multimeric complexes. The translocation of substrates requires the formation of an approximately 700-kDa LAMP-2A complex that is disassembled into smaller complexes in an hsc70-dependent manner when substrates are no longer present. The interaction of hsp90 with LAMP-2A at the luminal side of the lysosomal membrane stabilizes this receptor while transitioning between the multimeric membrane complexes. L. Mtx, lysosomal matrix.
Our results explain the failure of our previous attempts to coimmunoprecipitate with LAMP-2A other components of the CMA translocation complex using the antibody against the cytosolic tail of LAMP-2A, as this tail would be occupied by the substrates once they bind to the lysosomal membrane, making it inaccessible to the antibody. In this work, we have used instead four antibody-independent approaches—native gel electrophoresis, BNE, continuous sucrose density gradient centrifugation, and gel filtration—to characterize the organization of LAMP-2A complexes in the membrane. Although the specific size and number of LAMP-2A-containing complexes vary slightly depending on the resolution and harshness of each approach, we have been able to identify particularly stable complexes common to all four procedures. Other LAMP-2A complexes detected with some of these procedures probably represent assembly/disassembly intermediates. Their varied molecular weights could result from the different sizes of CMA substrates interacting with LAMP-2A at the lysosomal membrane at any given time.
The nature of the translocation complex at the lysosomal membrane responsible for substrate internalization via CMA has remained elusive. Different pieces of evidence compiled in this work support the 700-kDa complex containing LAMP-2A as a candidate for the CMA translocation complex: (i) the amount of LAMP-2A present in this complex increases in lysosomes active for CMA (Fig. 4), (ii) the 700-kDa complex is prevalent under conditions promoting substrate uptake (Fig. 5B to E), (iii) a portion of the CMA substrates transiently associates with the 700-kDa complex (Fig. 5B, right), and (iv) the inability to form the 700-kDa complex results in decreased protein translocation via CMA (Fig. 6D to F). Although LAMP-2A seems to be one of the major components of this complex, three other proteins of estimated molecular weights of 62, 70, and 78 were also recovered in this protein complex (Fig. 1E). In addition, the presence in this fraction of a highly hydrophobic protein difficult to resolve by electrophoresis cannot be discarded. The identity and role of these proteins in the transport complex are currently an object of study in our laboratory.
At this point, the nature and function of the >800-kDa LAMP-2A-containing complex at the lysosomal membrane remain unknown. However, our panning experiments and studies under conditions of different CMA activity do not support an active role for the formation of these very-high-molecular-weight complexes in CMA.
A surprising finding was the preferential binding of substrate proteins to LAMP-2A monomers, rather than to the large LAMP-2A-containing complexes. We cannot discard that the failure of substrates to interact with the high-molecular-weight forms of LAMP-2A in the panning experiments (Fig. 5A) might result from a failure of the LAMP-2A present in those complexes to renaturalize. However, the facts that when uptake is prevented, the substrate bound to the lysosomal membrane and does not migrate farther than the 300-kDa region, even in the presence of cross-linkers, in the sucrose density gradients (Fig. 5B) and in the gel filtration (7) and that the binding of CMA substrates to the lysosomal membrane is preserved in cells expressing a multimerization-impaired mutant of LAMP-2A (Fig. 6G) strongly support the binding of the substrate to small-size LAMP-2A complexes and/or monomers.
We have identified here a novel role for the membrane-associated hsc70 in the disassembly of the LAMP-2A multimeric complexes. Cytosolic chaperones have been previously shown to participate in the assembly and disassembly of multimeric complexes (36). In fact, one of the best-characterized intracellular functions of hsc70 is in the disassembly of the clathrin coat surrounding endocytic vesicles. However, to the best of our knowledge, this is the first time that such a role has been described for a chaperone at the lysosomal membrane. Based on this new function, it is possible that the increased binding of CMA substrates to lysosomes observed when exogenous hsc70 is added may be not only due to the previously described targeting of the substrate through the selective interaction of the chaperone with its CMA-targeting motif (6) but also to the hsc70-mediated disassembly of LAMP-2A into monomers (the form of LAMP-2A preferentially bound by the substrates). The fact that we could only detect net LAMP-2A disassembly when hsc70 was added alone but not when added in the presence of substrate proteins (Fig. 7E) suggests that the assembly and disassembly of translocation complexes are in a continuous balance at the lysosomal membrane. The advantages of a transitory over a stable translocation complex at the lysosomal membrane could be related to the peculiar characteristics of the lysosomal lumen (low pH, high content of hydrolases) which would make persistent continuity between the cytosol and the lysosomal matrix undesirable.
The dynamic character of LAMP-2A at the lysosomal membrane forces this protein to adopt transitory conformations until the proper assembly or complete disassembly is attained. Hsp90 has been shown to stabilize transient conformations of many proteins (i.e., steroid receptors, regulatory serine/threonine kinases, pathogen-resistance proteins, etc.) (36). We describe here a similar stabilizing effect of this chaperone on LAMP-2A at the lysosomal membrane (Fig. 9). This stabilizing effect, and the consequent decrease in CMA activity observed upon treatment with hsp90 inhibitors, contrasts with the stimulatory effect of geldanamycin on CMA previously described in human fibroblasts in culture (18). The discrepant results between that study and our work cannot be attributed to (i) differences between geldanamycin (used in their study) and 17-DMAG, as, other than the efficient dose range (Fig. S5B), we found a similar effect for both compounds on CMA substrate uptake, protein degradation, and LAMP-2A stability (Fig. 9; see also Fig. S5 in the supplemental material), or (ii) different effects of the drugs in vitro and in cultured cells, as in contrast with the previously described stimulatory effect (18), both drugs decreased the rates of protein degradation induced by serum removal when added to our cultured cells (Fig. S5A). The only condition in which we observed increased lysosomal protein degradation was upon the addition of very high concentrations of 17-DMAG or geldanamycin to intact lysosomes (see Fig. S5B in the supplemental material), which, we verified, induced lysosomal membrane breakage (data not shown). Although the previous report was done using doses of geldanamycin below those producing breakage, cell type-dependent differences in the sensitivity of lysosomes to the drugs are plausible. In addition, due to the pleiotropic function of hsp90 inside cells, we cannot discard that the changes in CMA in cultured cells treated with the inhibitors could be indirectly due to the inhibition of any of these other functions. Lastly, a cell type-dependent response to the loss of LAMP-2A is also possible. In this respect, we have found that the treatment of different cell types with 17-DMAG, although to a different extent for each, always resulted in decreased levels of LAMP-2A (see Fig. S5D and E in the supplemental material). However, whereas in some cells we did not find changes in other components of the CMA system, in other cell types we observed a marked increase in the levels of hsc70 (Fig. S5D and E). Higher levels of hsc70 in response to decreased levels of the CMA receptor have been previously described in other conditions such as aging (14). Although further studies are needed, this compensatory effect of hsc70 could be behind the previously observed stimulatory effect of geldanamycin and explain the different effects of the hsp90 inhibitors, depending on the cell type. Although hsp90 and hsc70 often work together and are considered part of a single multichaperone machinery (36), their possible functional interaction at the lysosomal membrane also requires future characterization.
The dynamic organization of LAMP-2A at the lysosomal membrane and the novel role of hsc70 and hsp90 in this organization offer a new level for the modulation of CMA activity. Although hsc70 is a very abundant cytosolic protein, changes in the ability of this chaperone to interact with the lysosomal membrane should have consequences for LAMP-2A multimerization and therefore on CMA activity. Changes in the organization of LAMP-2A at the lysosomal membrane could be behind the malfunctioning of CMA in different pathological conditions (26). Consequently, the modulation of the lysosomal membrane chaperones could help to restore normal CMA activity in those conditions.
Supplementary Material
Acknowledgments
This work was supported by NIH/NIA grants AG021904, AG25355, and DK041918 and by an Ellison Medical Foundation award.
We are grateful to Roberta Kiffin for her technical help and to Ashish Massey and Fernando Macian for suggestions and comments.
Footnotes
Published ahead of print on 21 July 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Agarraberes, F., and J. F. Dice. 2001. A molecular chaperone complex at the lysosomal membrane is required for protein translocation. J. Cell Sci. 1142491-2499. [DOI] [PubMed] [Google Scholar]
- 2.Agarraberes, F., S. Terlecky, and J. Dice. 1997. An intralysosomal hsp70 is required for a selective pathway of lysosomal protein degradation. J. Cell Biol. 137825-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aniento, F., E. Roche, A. M. Cuervo, and E. Knecht. 1993. Uptake and degradation of glyceraldehyde-3-phosphate dehydrogenase by rat liver lysosomes. J. Biol. Chem. 26810463-10470. [PubMed] [Google Scholar]
- 4.Auteri, J. S., A. Okada, V. Bochaki, and J. F. Dice. 1983. Regulation of intracellular protein degradation in IMR-90 human diploid fibroblasts. J. Cell. Physiol. 115167-174. [DOI] [PubMed] [Google Scholar]
- 5.Bandhyopadhyay, U., and A. M. Cuervo. 2006. Chaperone-mediated autophagy in aging and neurodegeneration: lessons from alpha-synuclein. Exp. Gerontol. 42120-128. [DOI] [PubMed] [Google Scholar]
- 6.Chiang, H., S. Terlecky, C. Plant, and J. Dice. 1989. A role for a 70 kDa heat shock protein in lysosomal degradation of intracellular protein. Science 246382-385. [DOI] [PubMed] [Google Scholar]
- 7.Cuervo, A., and J. Dice. 1996. A receptor for the selective uptake and degradation of proteins by lysosomes. Science 273501-503. [DOI] [PubMed] [Google Scholar]
- 8.Cuervo, A., and J. Dice. 2000. Regulation of lamp2a levels in the lysosomal membrane. Traffic 1570-583. [DOI] [PubMed] [Google Scholar]
- 9.Cuervo, A., and J. Dice. 2000. Unique properties of lamp2a compared to other lamp2 isoforms. J. Cell Sci. 1134441-4450. [DOI] [PubMed] [Google Scholar]
- 10.Cuervo, A., J. Dice, and E. Knecht. 1997. A lysosomal population responsible for the hsc73-mediated degradation of cytosolic proteins in lysosomes. J. Biol. Chem. 2725606-5615. [DOI] [PubMed] [Google Scholar]
- 11.Cuervo, A., E. Knecht, S. Terlecky, and J. Dice. 1995. Activation of a selective pathway of lysosomal proteolysis in rat liver by prolonged starvation. Am. J. Physiol. 269C1200-C1208. [DOI] [PubMed] [Google Scholar]
- 12.Cuervo, A., S. Terlecky, J. Dice, and E. Knecht. 1994. Selective binding and uptake of ribonuclease A and glyceraldehyde-3-phosphate dehydrogenase by isolated rat liver lysosomes. J. Biol. Chem. 26926374-26380. [PubMed] [Google Scholar]
- 13.Cuervo, A. M. 2004. Autophagy: many pathways to the same end. Mol. Cell. Biochem. 26355-72. [DOI] [PubMed] [Google Scholar]
- 14.Cuervo, A. M., and J. F. Dice. 2000. Age-related decline in chaperone-mediated autophagy. J. Biol. Chem. 27531505-31513. [DOI] [PubMed] [Google Scholar]
- 15.Cuervo, A. M., L. Mann, E. Bonten, A. d'Azzo, and J. Dice. 2003. Cathepsin A regulates chaperone-mediated autophagy through cleavage of the lysosomal receptor. EMBO J. 2212-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eskelinen, E., C. Schmidt, S. Neu, M. Willenborg, G. Fuertes, N. Salvador, Y. Tanaka, R. Lullmann-Rauch, D. Hartmann, J. Heeren, K. von Figura, E. Knecht, and P. Saftig. 2004. Disturbed cholesterol traffic but normal proteolytic function in LAMP-1/LAMP-2 double-deficient fibroblasts. Mol. Biol. Cell 153132-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eskelinen, E. L., Y. Tanaka, and P. Saftig. 2003. At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol. 13137-145. [DOI] [PubMed] [Google Scholar]
- 18.Finn, P., N. Mesires, M. Vine, and J. F. Dice. 2005. Effects of small molecules on chaperone-mediated autophagy. Autophagy 1141-145. [DOI] [PubMed] [Google Scholar]
- 19.Huynh, K., E. Eskelinen, C. Scott, A. Malevanets, P. Saftig, and S. Grinstein. 2007. LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J. 26313-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jadot, M., R. Wattiaux, F. Mainferme, F. Dubois, A. Claessens, and S. Wattiaux-De Coninck. 1996. Soluble form of Lamp II in purified rat liver lysosomes. Biochem. Biophys. Res. Commun. 223353-359. [DOI] [PubMed] [Google Scholar]
- 21.Jentoft, N., and D. Dearborn. 1983. Protein labeling by reductive alkylation. Methods Enzymol. 91570-579. [DOI] [PubMed] [Google Scholar]
- 22.Kaushik, S., A. C. Massey, and A. M. Cuervo. 2006. Lysosome membrane lipid microdomains: novel regulators of chaperone-mediated autophagy. EMBO J. 253921-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiffin, R., C. Christian, E. Knecht, and A. Cuervo. 2004. Activation of chaperone-mediated autophagy during oxidative stress. Mol. Biol. Cell 154829-4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laemmli, U. 1970. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 25.Lowry, O., N. Rosebrough, A. Farr, and R. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193265-275. [PubMed] [Google Scholar]
- 26.Massey, A., C. Zhang, and A. Cuervo. 2006. Chaperone-mediated autophagy in aging and disease. Curr. Top. Dev. Biol. 73205-235. [DOI] [PubMed] [Google Scholar]
- 27.Massey, A. C., S. Kaushik, G. Sovak, R. Kiffin, and A. M. Cuervo. 2006. Consequences of the selective blockage of chaperone-mediated autophagy. Proc. Natl. Acad. Sci. USA 1035805-5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohsumi, Y., T. Ishikawa, and K. Kato. 1983. A rapid and simplified method for the preparation of lysosomal membranes from rat liver. J. Biochem. 93547-556. [PubMed] [Google Scholar]
- 29.Russ, W. P., and D. M. Engelman. 2000. The GxxxG motif: a framework for transmembrane helix-helix association. J. Mol. Biol. 296911-919. [DOI] [PubMed] [Google Scholar]
- 30.Sharp, S., and P. Workman. 2006. Inhibitors of the HSP90 molecular chaperones: current status. Adv. Cancer Res. 95323-348. [DOI] [PubMed] [Google Scholar]
- 31.Storrie, B., and E. Madden. 1990. Isolation of subcellular organelles. Methods Enzymol. 182203-225. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka, Y., G. Guhde, A. Suter, E.-L. Eskelinen, D. Hartmann, R. Lullmann-Rauch, P. Janssen, J. Blanz, K. von Figura, and P. Saftig. 2000. Accumulation of autophagic vacuoles and cardiomyopathy in Lamp-2-deficient mice. Nature 406902-906. [DOI] [PubMed] [Google Scholar]
- 33.Terlecky, S., and J. Dice. 1993. Polypeptide import and degradation by isolated lysosomes. J. Biol. Chem. 26823490-23495. [PubMed] [Google Scholar]
- 34.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. 764350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waki, H., T. Yamauchi, J. Kamon, Y. Ito, S. Uchida, S. Kita, K. Hara, Y. Hada, F. Vasseur, P. Froguel, S. Kimura, R. Nagai, and T. Kadowaki. 2003. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J. Biol. Chem. 27840352-40363. [DOI] [PubMed] [Google Scholar]
- 36.Young, J. C., V. R. Agashe, K. Siegers, and F. U. Hartl. 2004. Pathways of chaperone-mediated protein folding in the cytosol. Nat. Rev. Mol. Cell Biol. 5781-791. [DOI] [PubMed] [Google Scholar]
- 37.Zhou, D., P. Li, Y. Lin, J. M. Lott, A. D. Hislop, D. H. Canaday, R. R. Brutkiewicz, and J. S. Blum. 2005. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity 22571-581. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.