Abstract
Vascular endothelial cadherin (VE-cadherin) connects neighboring endothelial cells (ECs) via interendothelial junctions and regulates EC proliferation and adhesion during vasculogenesis and angiogenesis. The cytoplasmic domain of VE-cadherin recruits α- and β-catenins and γ-catenin, which interact with the actin cytoskeleton, thus modulating cell morphology. Dysregulation of the adherens junction/cytoskeletal axis is a hallmark of invasive tumors. We now demonstrate that the transmembrane ubiquitin ligase K5/MIR-2 of Kaposi's sarcoma-associated herpesvirus targets VE-cadherin for ubiquitin-mediated destruction, thus disturbing EC adhesion. In contrast, N-cadherin levels in K5-expressing cells were increased compared to those in control cells. Steady-state levels of α- and β-catenins and γ-catenin in K5-expressing ECs were drastically reduced due to proteasomal destruction. Moreover, the actin cytoskeleton was rearranged, resulting in the dysregulation of EC barrier function as measured by electric cell-substrate impedance sensing. Our data represent the first example of a viral protein targeting adherens junction proteins and suggest that K5 contributes to EC proliferation, vascular leakage, and the reprogramming of the EC proteome during Kaposi's sarcoma tumorigenesis.
Cell-cell interactions in tissues are regulated via adherens junctions, cellular structures that mediate tight adhesion between neighboring cells (64). The disruption of adherens junctions changes normal epithelial cells into invasive cells, and defective adherens junctions are regularly observed in malignant tumors (6). Endothelial junctions represent a unique type of cell-cell junction relying on the junction molecule vascular endothelial cadherin (VE-cadherin), which is exclusive to endothelial cells (ECs). The calcium-dependent homotypic interactions of VE-cadherin between neighboring ECs occur both during embryonic vasculogenesis and during angiogenesis. Besides regulating vascular homeostasis, interendothelial junctions also play an important role in contact inhibition of EC growth (31). In addition, endothelial junctions regulate leukocyte extravasation and infiltration into inflamed areas (25). Upon extracellular engagement, junctional proteins such as VE-cadherin and platelet-endothelial cell adhesion molecule 1 (CD31/PECAM-1) transfer intracellular signals to modulate the cytoskeleton and EC growth and apoptosis (37). The cytoplasmic tail of VE-cadherin associates with α- and β-catenins and plakoglobin (or γ-catenin), which dynamically link to the actin cytoskeleton (84). The cytoskeletal architecture is thus regulated by extracellular adhesion processes.
Diseases linked to altered endothelial permeability or vascular morphogenesis are often associated with alterations in the composition of intercellular EC junctions. A particularly striking example of a disease that is based on aberrant EC proliferation, neoangiogenesis, erythrocyte extravasation, and inflammation is Kaposi's sarcoma (KS) (26). KS is the most prevalent AIDS-associated malignancy, accounting for up to 50% of all diagnosed cancers in regions with high AIDS prevalence. Human herpesvirus 8, or KS-associated herpesvirus (KSHV), is consistently identified in all forms of KS (2). Evidence for KSHV's being the etiologic agent of KS comes from the findings that KSHV transforms ECs in vitro, that several open reading frames (ORFs) of KSHV are transforming (54), and that KSHV has close homology to other tumorigenic gammaherpesviruses, such as Epstein-Barr virus (EBV), herpesvirus saimiri, and rhesus rhadinovirus (23). While KSHV is unable to immortalize ECs in vitro, long-term latent infection of dermal microvascular ECs (DMVECs) by KSHV was achieved with DMVECs immortalized with human papillomavirus E6 and E7 proteins (E-DMVECs) and telomerase-immortalized DMVECs (TIME cells) (53).
The KSHV genome comprises over 85 ORFs arranged as seven highly conserved gene blocks separated by regions containing unique or subfamily-specific genes (68). The KSHV-specific “K” genes are involved in the transformation of host cells and immune modulation. Several of these K genes are pirated from the host genome and have now acquired additional functions in immune modulation and/or tumorigenesis (54). The two ORF products K3 and K5 (also known as MIR-1 and MIR-2) are homologous to membrane-associated RING-CH (MARCH) proteins of the host (3, 59). MARCH proteins are type III transmembrane RING-type ubiquitin ligases (3, 51) which transfer ubiquitin to cytoplasmic lysines (3, 9) and, in the absence of lysines, to cysteines or serines and threonines of transmembrane target proteins (13, 79). K3 and K5 (20, 40), as well as the related proteins MK3 of murine herpesvirus 68 (MHV68) (71) and M153R of myxomavirus (32, 51), share the ability to destroy major histocompatibility complex class I (MHC-I) molecules, suggesting a role in viral evasion of the adaptive cellular immune response in immunocompetent hosts. In addition, the members of this family of transmembrane ubiquitin ligases also target other cell surface proteins, mostly immunoreceptors, with partially overlapping, partially unique specificities. For instance, both K3 and K5 additionally target the gamma interferon receptor (47) and the nonpolymorphic MHC-I-like molecule CD1 (69), but only K5 also targets the costimulatory molecules ICAM-1 and B7.2 (22, 40) and activated leukocyte cell adhesion molecule (4).
In addition to these immune modulatory functions, recent evidence suggests that K5 may play a role in regulating the function of ECs by downmodulating the EC-specific cell adhesion molecule CD31/PECAM-1 (52, 75). The efficient removal of both preexisting and newly synthesized CD31/PECAM-1 suggests that inhibiting EC adhesion is an important function of K5 which may thus contribute to KS development in immunosuppressed individuals (52). While it is currently not known which proteins of the EC membrane are targeted by K5, a quantitative membrane proteomics analysis of HeLa cells revealed several novel K5 targets, consistent with K5's selectively eliminating or sequestering a number of yet-to-be-identified host cell proteins (4). It is thus conceivable that K5 contributes on a proteomic level to the well-documented transcriptional reprogramming of ECs by KSHV (78).
We now present evidence that further strengthens the argument that, by expressing K5, KSHV profoundly modulates the function and morphology of ECs. We demonstrate that K5 downregulates VE-cadherin on the EC surface, thus eliminating the major component of endothelial junctions. Importantly, the downregulation of VE-cadherin by K5 also reduces α- and β-catenin and γ-catenin levels, thus dramatically rearranging the actin cytoskeleton. As a consequence, the permeability of the EC monolayer is drastically increased. These data therefore implicate K5 in the dysregulation of ECs during KS development and suggest that K5 may play a role in some of the aberrant features of ECs typical of KS. Moreover, this is the first description of a viral protein targeting the VE-cadherin-catenin complex.
MATERIALS AND METHODS
Viruses and cell culture.
E-DMVECs with long-term KSHV infection were established and maintained as described previously (56). Primary DMVECs (Lonza, Rockland, ME) were infected with KSHV in 1 ml of OptiMEM (Invitrogen, Carlsbad, CA) and spun at 1,000 rpm for 2 h at 20°C, after which the inoculum was removed and replenished with fresh medium. T4 TIME cells were obtained from Dean Kedes (University of Virginia) and infected with KSHV as described previously (75). Adenovirus (Ad) constructs expressing N-terminally (MK3 and K3) or C-terminally (K5) FLAG-tagged protein from sequences inserted under the control of a tetracycline transactivator (tTA)-dependent promoter (36) were generated using a plasmid-based recombinant system (35). The tTA-expressing Ad vector AdTet was obtained from David Johnson (Oregon Health & Science University) and has been described previously (74). pcDNA5/FRT/To expressing the human Notch intracellular domain (NIC) was obtained from Jae Jung (Harvard Medical School) and was used to generate recombinant Ad. An Ad VE-cadherin construct encoding full-length VE-cadherin (AdVE-cadherin) and an Ad VE-cadherin construct lacking the entire cytoplasmic domain (AdECTM) were kindly provided by Andrew Kowalczyk (Emory University, Georgia).
Antibodies and reagents.
The antibodies included anti-transferrin receptor (anti-TfR; U.S. Biological); anti-FLAG and anti-human leukocyte antigen (anti-HLA) antibody W6/32 (Sigma); anti-P4D1, anti-cyclin D1, and antisurvivin (Santa Cruz); anti-α-, -β-, and -γ-catenins (BD Transduction Laboratories); anticalreticulin (Stressgen); anti-VE-cadherin (BV9) and anti-F-actin (Abcam); anti-MHC-I K455 (from P. Peterson); and anti-ORF59 (from Bala Chandran). Phalloidin was purchased from Invitrogen. Concanamycin A (ConA; Sigma) and MG132 (Boston Biochem, Cambridge, MA) were used at final concentrations of 50 nM and 50 μM, respectively. Protein A or G beads were obtained from Santa Cruz Biotechnology. Phorbol 12-myristate 13-acetate was obtained from Sigma and used at 20 ng/ml.
Cell surface protein biotinylation and purification.
Biotinylation with EZ-Link TM Sulfo-NHS-SS-Biotin was performed according to the protocol of the manufacturer (Pierce, Rockford, IL). Briefly, cells were washed three times in ice-cold phosphate-buffered saline (PBS), and proteins at the cell surface were labeled with Sulfo-NHS-SS-Biotin for 30 min at 4°C. The cells were washed and lysed immediately with a nonionic detergent. Labeled proteins were isolated with immobilized NeutrAvidin gel (Pierce, Rockford, IL). The bound proteins were released by incubating the resin with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing 50 mM dithiothreitol.
siRNA transfection.
Cells were transfected with K5 small interfering RNA (siRNA) or scramble siRNA twice at 24-h intervals according to the protocol of the manufacturer (Dharmacon, Lafayette, CO). Four days posttransfection, cells were infected with KSHV as described previously.
Flow cytometry and IFA.
For flow cytometric analysis of surface proteins, cells were removed from tissue culture dishes with 0.05% trypsin-EDTA (Invitrogen), washed with ice-cold PBS, and incubated with primary antibodies for 30 min at 4°C. After several washes in PBS, cells were incubated with phycoerythrin (PE)-conjugated goat anti-mouse secondary antibody (Dako) and washed again before analysis with a FACSCalibur flow cytometer (BD Biosciences, Palo Alto, CA). For intracellular staining, trypsinized and washed cells were fixed in 2% paraformaldehyde for 15 min at room temperature and then washed three times in a permeabilizing solution (1% saponin, 10% NaN3, and 10% fetal calf serum in PBS) prior to incubation with antibodies. Immune fluorescence analysis assays (IFA) were performed as described previously (51). Images were captured with an Axioskop 2 microscope and an AxioCam (Carl Zeiss, Göttingen, Germany). Coverslips were mounted onto slides and covered with Vectashield H-1200 plus DAPI (4′,6-diamidino-2-phenylindole). All pictures were taken in monochrome and were subjected to contrast enhancement and artificially colored by using Openlab 4.0.3 software (Improvision, Lexington, MA). Appropriate specificity controls were included for all experiments.
Immunoprecipitation and immunoblotting.
E-DMVECs grown in 100-mm tissue culture dishes were transduced with Ad constructs at a multiplicity of infection (MOI) of 250 PFU per cell. For tTA-dependent constructs, AdTet was included at a 1:5 ratio. At 20 h after infection, cells were immunoprecipitated as described previously (51). For immunoblotting, the WesternBreeze chemiluminescence detection system (Invitrogen) was used following semidry transfer onto polyvinylidene difluoride membranes (Millipore, Billerica, MA).
Measurement of monolayer formation by the ECIS technique.
Electric cell-substrate impedance sensing (ECIS) measures the attachment and spreading of cells quantitatively in real time (81). ECIS electrode arrays (8W10E), obtained from Applied Biophysics (Troy, NY), resemble a typical eight-chamber tissue culture slide, but the base of each well contains 10 250-μm working electrodes. A low, noninvasive current (20 kHz) is applied to the system, and a larger counter electrode completes the circuit by using standard tissue culture medium as an electrolyte. Arrays are placed in an electrode array holder within a CO2 incubator with leads to the ECIS instrument and a personal computer that controls data acquisition, storage, and analysis (www.appliedbiophysics.com). An increase in resistance is measured as the current is constrained by the insulating membranes of the cells. Small fluctuations in electrode resistance are due to natural micromotion as cells move on the electrodes. Prior to the plating of DMVECs, arrays were cleaned with l-cysteine (10 mM) and coated with 1% gelatin per the manufacturer's instructions. DMVECs cultured in tissue culture dishes under standard conditions were transduced with Ad constructs expressing K5 (AdK5) and K3 (AdK3) and AdTet for 24 h or were untreated. Cells were enzymatically lifted, counted, and seeded in duplicate into 8W10E arrays at a density of 2 × 105 cells/well. Arrays were placed into the array holders immediately after seeding, and resistance was monitored for up to 24 h. Where indicated, the expression of K5 and K3 was repressed by adding tetracycline (Tet; 150 nM).
RESULTS
KSHV K5 downregulates VE-cadherin.
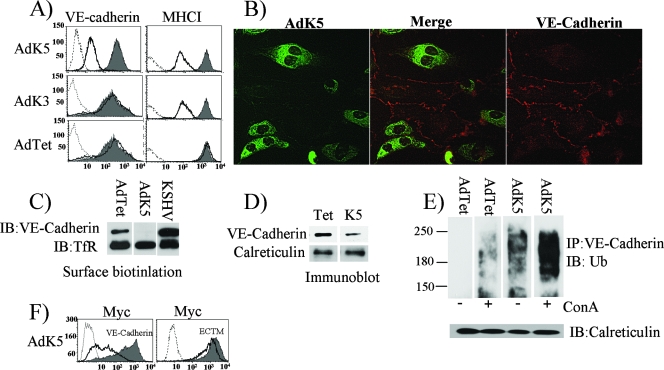
When screening EC-specific surface proteins downregulated by K5, we observed a reduction of VE-cadherin surface levels upon Ad-mediated K5 expression, whereas VE-cadherin expression was not affected by K3 (Fig. 1A). Similarly, IFA revealed strongly reduced expression of VE-cadherin in K5-expressing E-DMVECs, whereas neighboring K5-negative cells showed typical staining of cellular junctions (Fig. 1B). K5 also drastically reduced biotinylated VE-cadherin, consistent with a significant reduction of VE-cadherin levels at the cell surface (Fig. 1C). Interestingly, VE-cadherin levels in latently infected DMVECs that did not express K5 were increased (Fig. 1C). In contrast, residual VE-cadherin was detected in K5-containing cells by immunoblotting (Fig. 1D). This protein could be either residual VE-cadherin at the cell surface or newly synthesized VE-cadherin that had not yet reached the cell surface.
FIG. 1.
Reduced VE-cadherin expression in the presence of K5. (A) Flow cytometry analysis of VE-cadherin expression in E-DMVECs 24 h after transduction with AdTet alone or AdTet together with AdK5 or AdK3 (MOI of 250). Cells were stained with VE-cadherin-specific antibody (BV9) or MHC-I-specific antibody (W6/32) followed by PE-conjugated secondary antibody. Gray shading, untransfected controls; dashed line, background staining with secondary antibody only; solid line, cells transduced with AdTet, AdK5, or AdK3. (B) Immunofluorescence analysis of E-DMVECs transduced with AdK5 and AdTet (MOI of 125). FLAG-tagged K5 was visualized with fluorescein isothiocyanate-conjugated anti-FLAG. VE-cadherin was stained with monoclonal antibody BV9 followed by PE-conjugated secondary antibody. (C) Biotinylation of cell surface proteins of uninfected E-DMVECs, E-DMVECs transduced with AdK5, or E-DMVECs with long-term latent KSHV infection. Biotinylated products, precipitated with streptavidin, were separated by SDS-PAGE and blotted with VE-cadherin- or TfR-specific antibodies. Note the increased expression of VE-cadherin in latently infected cells compared to that in uninfected cells, in contrast to the absence of VE-cadherin expression in K5-expressing cells. IB, immunoblot. (D) Immunoblot of VE-cadherin or calreticulin in total lysates from E-DMVECs transduced with AdTet alone or together with AdK5 as described in the legend to panel A. (E) Immunoblot analysis of anti-VE-cadherin immunoprecipitates (IP) with ubiquitin (Ub)-specific antibody. The expression of K5, cell lysis, and immunoprecipitation were the same as those for panel A except that ConA (50 nM for 3 h) was added where indicated. (F) Intracellular Myc staining of E-DMVECs transduced with AdTet and AdK5 with AdVE-cadherin or AdECTM (ECTM). Dashed line, background staining with secondary antibody only; solid line, cells transduced with AdK5 and AdECTM or AdVE-cadherin; gray shading, cells transduced with AdECTM or AdVE-cadherin only.
Most K5 targets are downregulated by endocytosis, ubiquitin-mediated sorting to multivesicular bodies, and ultimately, lysosomal destruction (21). To determine whether VE-cadherin is ubiquitinated by K5, we immunoprecipitated VE-cadherin from E-DMVECs transduced with AdK5 or AdTet alone as a control and then analyzed the immunoprecipitates by immunoblotting with antiubiquitin antibody. As shown in Fig. 1E, ubiquitinated VE-cadherin was observed only in the presence of K5 and not in cells transduced with the control. To further examine whether ubiquitinated VE-cadherin is degraded in lysosomes, we treated cells with ConA, which inhibits endosomal/lysosomal acidification. Interestingly, this treatment stabilized a ubiquitinated intermediate of VE-cadherin even in the absence of K5 (Fig. 1E), presumably because VE-cadherin is naturally turned over by ubiquitination and lysosomal degradation (24). However, ubiquitinated VE-cadherin was also observed in the absence of ConA in cells transduced with AdK5. In ConA-treated cells, there was a strong increase of ubiquitinated VE-cadherin in the presence of K5, suggesting that both natural and K5-induced turnover had been inhibited. Since K5 ubiquitinates cytoplasmic lysines in its target molecules, we tested whether the cytoplasmic tail of VE-cadherin was required for K5-mediated downregulation. Myc-tagged VE-cadherin or VE-cadherin in which the cytoplasmic tail was replaced by the Myc tag (83) was expressed from an Ad construct, and cells were cotransduced with the construct and AdK5 or control Ad. As shown in Fig. 1F, whereas the full-length tail supported VE-cadherin downregulation by K5, there was a very modest decrease of the tail deletion form of VE-cadherin. (It is possible that this residual decrease was mediated by the single lysine present in the Myc tag very close to the membrane [12].) Taken together, these observations indicate that K5 targets the endothelial junction protein VE-cadherin for ubiquitin-mediated internalization and degradation in the endosomal/lysosomal compartment.
KSHV downregulates VE-cadherin upon de novo infection and reactivation.
To examine whether the downregulation of VE-cadherin also occurs in KSHV-infected cells, we monitored VE-cadherin expression upon the primary infection of T4 TIME cells with KSHV. De novo infection of T4 TIME cells with KSHV results in a temporary downregulation of MHC-I, ICAM-1, and CD31/PECAM-1 due to temporary K5 expression prior to the establishment of latency. We infected T4 TIME cells with KSHV as described previously (75) and evaluated both MHC-I and VE-cadherin expression 24 h postinfection. As shown in Fig. 2A (upper panels), cell surface expression of both molecules was diminished, consistent with K5's inhibiting VE-cadherin expression in KSHV-infected cells. VE-cadherin levels in T4 TIME cells were lower than those in E-DMVECs (Fig. 2A, lower panels), so that the TIME cells became VE-cadherin negative upon KSHV infection.
FIG. 2.
Reduced VE-cadherin expression in KSHV-infected DMVECs upon de novo infection or reactivation. (A, upper panels) T4 TIME cells infected with KSHV for 24 h (solid line) or uninfected (shading) were stained with either MHC-I-specific antibody W6/32 or VE-cadherin-specific antibody as indicated. Dashed line, background staining with secondary antibody only. (Lower panels) E-DMVECs with long-term latent infection were transduced with AdTet (shading) or AdNIC (solid line), and the surface expression of CD31 or VE-cadherin was monitored by flow cytometry. The dashed line represents background staining with secondary antibody only. (B) AdNIC-transduced latently KSHV-infected E-DMVECs were stained for ORF59 (red), FLAG-tagged NIC (green), or nuclei (DAPI; blue). The lower panels show two representative fields of staining for K5 only. (C) T4 TIME cells were transfected with scramble or K5 siRNA. Four days posttransfection, cells were infected with KSHV and stained with VE-cadherin or MHC-I antibody. Gray shading, T4 TIME cells; black line, KSHV-infected T4 TIME cells; blue line, scramble siRNA-transfected KSHV-infected cells; red line, K5 siRNA-transfected KSHV-infected cells.
Except for a small percentage of cells undergoing spontaneous reactivation of the infection, E-DMVECs with long-term latent infection do not express K5 (52). However, K5 is expressed upon the induction of the lytic cycle with phorbol esters (52), although phorbol esters activate ECs and reduce VE-cadherin expression (80), rendering it difficult to interpret the effect of lytic reactivation on VE-cadherin. Recently, it was demonstrated that K5 belongs to a small group of proteins encoded by KSHV lytic genes that can be activated by the Notch-signaling pathway (16, 17). The activation of the Notch receptor by the binding of one of its ligands (Delta, Jagged, or Serrate) leads to proteolytic cleavage of the receptor on the inner side of the membrane, releasing NIC (66). NIC is translocated to the nucleus, where it activates genes by interacting with RBP-Jκ, a transcriptional activator that cooperates with the KSHV replication and transcription activator (RTA) to reactivate KSHV (48, 49). The transfection of cells that harbor the KSHV genome with a construct expressing NIC results in incomplete reactivation, with the expression of a limited number of lytic transcripts, including K5 transcripts (17). To determine whether NIC expressed in latently infected E-DMVECs would induce K5 and thus downregulate VE-cadherin, we inserted NIC into replication-deficient Ad (yielding AdNIC). Consistent with reactivation, the lytic gene product ORF59, as well as K5, was expressed upon transduction with AdNIC (Fig. 2B). In parallel, VE-cadherin and CD31 levels were strongly reduced upon transduction with AdNIC (Fig. 2A).
To further demonstrate that VE-cadherin downregulation by KSHV depends on K5 expression, we monitored VE-cadherin and MHC-I expression upon the de novo infection of T4 TIME cells treated with siRNA for K5 or control siRNA. In pilot experiments, we verified the loss of K5 expression upon siRNA treatment (data not shown). Consistent with the findings in previous reports (1), MHC-I was no longer downregulated when cells were treated with K5 siRNA (Fig. 2C). Remarkably, VE-cadherin levels were also completely restored in the K5 siRNA-treated cells, whereas control siRNA had no effect. Taken together, these data clearly show that K5 mediates VE-cadherin downregulation upon lytic gene expression in KSHV-infected ECs.
KSHV K5 downregulates α-, β-, and γ-catenins.
Cadherins mediate calcium-dependent cell adhesion by interacting with the cadherins on neighboring cells (in homotypic interactions). Thus, cadherins play a major role in the formation of cell-cell junctions. However, cadherins also orchestrate the development of the cytoskeletal architecture (5), with α- and β-catenins and γ-catenin interacting with both the cytosolic faces of cadherins and the actin cytoskeleton.
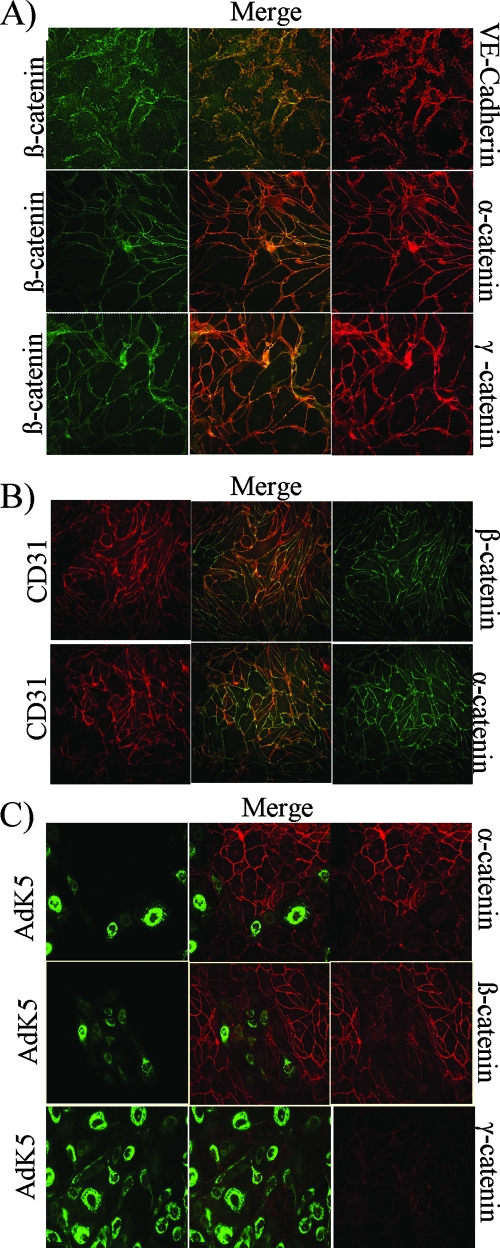
Given the tight association of cadherins with catenins, we monitored the subcellular distribution and expression of catenins in K5-expressing cells. IFA confirmed the colocalization of VE-cadherin with α- and β-catenins, as well as γ-catenin, in E-DMVECs (Fig. 3A). CD31/PECAM-1 is also known to interact with β-catenin (70). However, the costaining of α- and β-catenins and CD31/PECAM-1 revealed only partial colocalization (Fig. 3B), suggesting that VE-cadherin is predominantly responsible for the membrane association of α- and β-catenins in E-DMVECs.
FIG. 3.
K5 downregulates VE-cadherin-associated α-, β-, and γ-catenins. (A) VE-cadherin colocalizes with α-, β-, and γ-catenins in E-DMVECs. Uninfected E-DMVECs were costained for VE-cadherin and β-catenin (upper panel) or for β-catenin and α- or γ-catenin (middle and lower panels). (B) Partial colocalization of CD31/ PECAM-1 with α- and β-catenins. Uninfected E-DMVECs were costained for CD31 and α- or β-catenin as indicated. (C) Reduced expression of α-, β-, and γ-catenins in the presence of K5. E-DMVECs were transduced with AdK5 and AdTet at a low MOI (125) for 24 h prior to fixation and costaining for FLAG and the indicated catenins.
In E-DMVECs transduced with AdK5 at a low MOI, areas positive for K5 showed low levels of α- and β-catenin staining, in contrast to the staining pattern in neighboring untransduced cells (Fig. 3C). Similar results were obtained with γ-catenin (Fig. 3C). Upon transduction with AdK5 at a high MOI, β-catenin levels were uniformly low, whereas expression was unchanged in the presence of K3 (Fig. 4A). Intracellular flow cytometry results were consistent with reduced steady-state levels of α-catenin, β-catenin, and γ-catenin (Fig. 4B). Interestingly, there was a slight, but reproducible, reduction of γ-catenin by K3 as well. The reason for this decrease is currently not known. We hypothesized that K5 liberates catenins from the VE-cadherin scaffold and that free-catenin levels are reduced by constitutive degradation. Free β-catenin is phosphorylated by glycogen synthase kinase-3 (GSK-3), which targets β-catenin for ubiquitination and proteasomal degradation (48). Similarly, α-catenin is degraded by the proteasome, albeit independently of ubiquitin (38). To examine whether β-catenin was reduced by proteasomal degradation, we monitored β-catenin in the presence of the proteasome inhibitor MG132. In addition, we used ConA, which stabilized ubiquitinated VE-cadherin as described above. Interestingly, β-catenin levels in the presence of ConA were still reduced, suggesting that the ubiquitinated VE-cadherin does not interact with β-catenin. However, MG132 restored β-catenin levels, consistent with K5's releasing β-catenin into the cytosol for proteasomal degradation (Fig. 4C). This is the first demonstration of a cytosolic protein's being downregulated as a consequence of viral or cellular MARCH protein expression.
FIG. 4.
The downregulation of β-catenin by K5 is proteasome dependent and does not induce Wnt targets. (A) β-catenin is absent upon transduction with AdK5 but not AdK3. E-DMVECs were transduced for 24 h with a high MOI (250) of AdK5 (with AdTet) or AdK3 (with AdTet). K3 and K5 were visualized with anti-FLAG, and β-catenin was also visualized. (B) Reduction of steady-state levels of α-, β-, and γ-catenins in the presence of K5. E-DMVECs were transduced as described in the legend to panel A and processed for intracellular flow cytometry using the indicated antibodies. Dashed line, background staining with secondary antibody only; gray shading, E-DMVECs transduced with AdTet; solid line, E-DMVECs transduced with AdK5 or AdK3. (C) Increase of β-catenin steady-state levels in the presence of proteasomal inhibitors. E-DMVECs were transduced with AdTet (gray shading) or AdK5 (solid line), treated with ConA (50 nM) or MG132 (50 μM) for 6 h, and subjected to intracellular staining for β-catenin. (D) Immunoblot of the indicated proteins upon the transduction of E-DMVECs with the AdTet control or AdK5 (with AdTet). The bottom two rows are from a different experiment. (E) Costaining of K5 and β-catenin in KSHV-infected E-DMVECs. (Upper panels) Latently infected E-DMVECs were stained for spontaneous K5 expression using K5-specific monoclonal antibody 12G6 (unpublished data), together with anti-β-catenin. (Lower panels) Costaining for LANA-1 and β-catenin.
β-catenin also acts as a transcriptional coactivator in the Wnt pathway, where it induces the transcription of genes involved in the cell cycle and cell differentiation (10). It was previously reported that β-catenin released from the cadherin scaffold induces the transcription of Wnt-regulated genes independently of Wnt signaling (39, 50). Therefore, we examined the expression of the Wnt-/β-catenin-regulated proteins cyclin D1 and survivin. Unexpectedly, the levels of expression of both proteins were lower upon transduction with AdK5 than upon transduction with control Ad (Fig. 4D), suggesting that K5 does not induce β-catenin target proteins but actually lowers the level of steady-state transcription of Wnt-regulated genes.
Previously, it was demonstrated that LANA-1 inhibits GSK-3 in KSHV-infected primary effusion lymphoma cell lines, resulting in markedly increased β-catenin levels in the cytoplasm (34). β-catenin enters the nucleus in LANA-1-expressing cells, thus activating Wnt-responsive genes. However, β-catenin remained associated with the cell surface in LANA-1-expressing latently infected E-DMVECs, whereas β-catenin was absent from K5-positive cells with spontaneously reactivating infection (Fig. 4E). Thus, LANA-1 does not seem to prevent β-catenin degradation upon KSHV reactivation.
K5 upregulates N-cadherin in ECs.
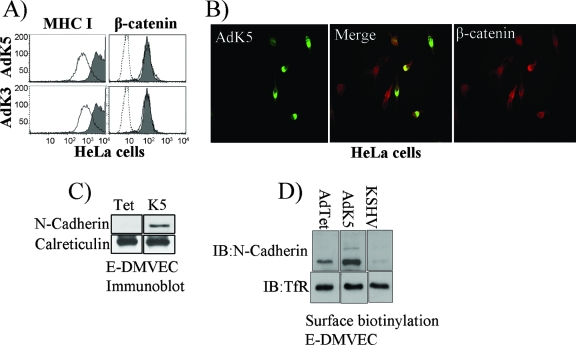
To independently examine whether K5 targets β-catenin directly, we studied β-catenin expression in HeLa cells, a cervical carcinoma line that does not express VE-cadherin. Both K3 and K5 reduced MHC-I levels in HeLa cells but did not reduce steady-state levels of β-catenin as shown by flow cytometry (Fig. 5A) or IFA (Fig. 5B). Thus, K5 does not directly mediate ubiquitin-mediated degradation of β-catenin.
FIG. 5.
Increased N-cadherin expression upon VE-cadherin downregulation by K5. (A) HeLa cells were transduced with AdK5 or AdK3 (each at an MOI of 50, in the presence of AdTet) for 24 h, and surface levels of MHC-I or intracellular levels of β-catenin were monitored by flow cytometry. Gray shading, cells transduced with AdTet alone; solid line, cells transduced with AdK5 or AdK3; dashed line, background staining by the secondary antibody. (B) IFA of β-catenin in HeLa cells transduced with AdK5. Note that cells that expressed high levels of K5 also contained β-catenin. (C) Immunoblot of N-cadherin or calreticulin (as a control) in E-DMVECs transduced with AdTet alone or together with AdK5. (D) The surface proteins of E-DMVECs transduced with AdTet alone or together with AdK5 were biotinylated, and the cells were lysed. Biotinylated proteins were immunoprecipitated with streptavidin beads, separated by SDS-PAGE, and immunoblotted (IB) with VE-cadherin- or TfR-specific antibodies.
Since HeLa cells express N-cadherin (65), this observation also suggested that N-cadherin is K5 resistant. However, N-cadherin is also present in ECs but it does not localize to adherens junctions and is thought to mediate heterotypic adhesion to non-ECs (14). Thus, it was conceivable that K5 ubiquitinates N-cadherin in ECs but not in HeLa cells. Unexpectedly, N-cadherin levels actually increased upon K5 expression in E-DMVECs, as shown by immunoblotting (Fig. 5C) or surface protein biotinylation (Fig. 5D). This observation may be explained by VE-cadherin's normally displacing N-cadherin from adherens junctions, leading to rapid turnover (41, 58). In contrast, K5-mediated removal of VE-cadherin may stabilize N-cadherin at the cell surface. Nevertheless, N-cadherin seems unable to substitute for VE-cadherin with respect to β-catenin association, despite the fact that both cadherins contain conserved cytoplasmic domains associating with catenins (84).
Interestingly, the reverse situation was observed in E-DMVECs latently infected with KSHV. As shown in Fig. 1C, steady-state levels of VE-cadherin were higher in latently infected than in uninfected E-DMVECs. In contrast, N-cadherin was completely absent from latently infected E-DMVECs (Fig. 5C). Thus, latency-associated proteins of KSHV seem to induce VE-cadherin, which may displace N-cadherin from the cell surface. In the presence of K5, VE-cadherin is removed, thus stabilizing N-cadherin expression.
KSHV K5 modulates the actin cytoskeleton.
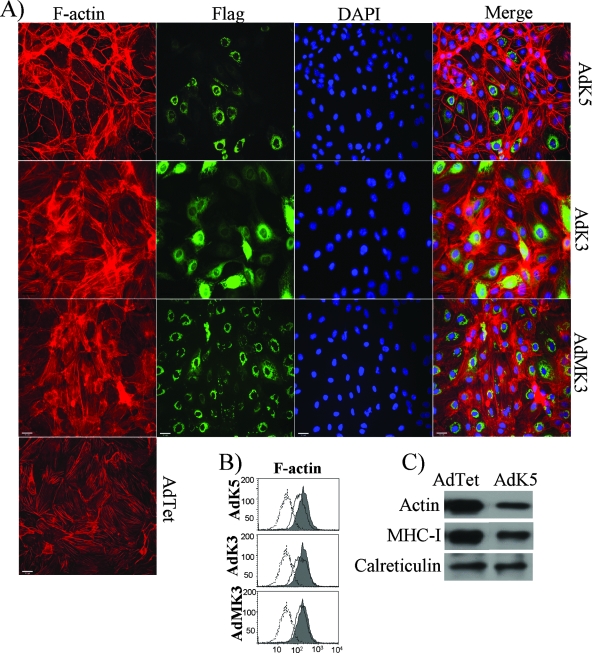
Since catenins link cadherins to actin filaments (F-actin), we investigated the F-actin structure in ECs. In uninfected E-DMVECs, F-actin formed cables that were perpendicular to the membrane, presumably anchoring the adherens junctions (Fig. 6A). The same architecture was observed in DMVECs transduced with Ad expressing MHV68 K3 or KSHV K3 (Fig. 6A). (In some cells with high levels of KSHV K3 expression, actin filaments appeared to form larger actin cables, potentially representing stress fibers [62].) In contrast, F-actin was reorganized to localize along the cell periphery in E-DMVECs expressing K5, both those expressing high levels and those expressing barely detectable levels of K5 (Fig. 6A). Intracellular flow cytometry and immunoblotting also indicated a modest reduction of steady-state levels of F-actin in K5-expressing cells compared to the levels in cells transduced with the control (Fig. 6B and C). These data indicate that K5 drastically dysregulates the actin cytoskeleton, most likely as a consequence of the downregulation of the VE-cadherin-catenin complex.
FIG. 6.
Dysregulation of the actin cytoskeleton by K5. (A) F-actin staining (red) in the presence of K5, KSHV K3, or MHV68 K3 (green). E-DMVECs were transduced with AdTet alone or together AdK5, AdK3, or an Ad vector expressing MHV68 K3 (AdMK3) for 24 h and then subjected to IFA. Viral proteins were visualized with fluorescein isothiocyanate-conjugated anti-FLAG antibody, whereas F-actin was stained with phalloidin. (B) Intracellular flow cytometry analysis for F-actin in E-DMVECs transduced with AdTet alone (shading) or together with the indicated Ad constructs (solid line). Background staining by the secondary antibody is indicated by dashed lines. (C) The total amount of actin in E-DMVECs transduced with AdK5 and that in cells transduced with AdTet were immunoblotted using anti-F-actin antibody from Abcam. MHC-I was detected with a polyclonal antiserum (K455).
Functional consequences of K5 expression for EC function.
To monitor whether K5 affects intercellular junction formation and monolayer permeability, we used ECIS, a noninvasive measurement of cellular behavior in real time (Fig. 7A) (81, 82). A typical resistance curve with an initial increase in resistance was observed when E-DMVECs settled at the bottoms of the ECIS array wells and established firm cell-substrate interactions with the coated electrode surfaces (Fig. 7B). As cells spread and established tight intercellular contacts, the current flow between neighboring cells was further restricted. Cells infected with AdTet alone and cells expressing K3 exhibited similar patterns of attachment, spreading, and junction formation. In the presence of K5, however, resistance remained at a low level throughout (Fig. 7B), despite the fact that the monolayer was visually indistinguishable from controls (data not shown). To examine whether K5 would increase the permeability of a preformed monolayer, we took advantage of the Tet-dependent regulation of K5 expression. When E-DMVECs cotransduced with AdK5 and AdTet were grown in the presence of Tet, monolayer resistance was similar to that of controls. However, upon the induction of K5 by Tet removal, monolayer permeability rapidly decreased (Fig. 7D). In contrast, adding Tet back to the culture restored monolayer resistance (Fig. 7C). Since permeability depends on intact endothelial junctions, we conclude that the downregulation of VE-cadherin by K5 was responsible for the observed increase in E-DMVEC permeability.
FIG. 7.
Increased permeability of K5-expressing E-DMVECs. (A) Schematic drawing portraying ECIS. An 8W10E slide array which was used to monitor real-time attachment and monolayer formation is depicted. The eight culture wells of the slide array are shown, including the eight active electrode areas (circles) and the single counter electrode (rectangle). Front- and side-view enlargements of a single well are shown to highlight the 250-μm-diameter active electrodes at the bottom of each well and the photoresistant layer that insulates the rest of the gold film from the bulk electrolyte. Adapted from Experimental Cell Research (81) with permission of the publisher. (B) ECIS analysis of mock-treated E-DMVECs and E-DMVECs transduced with AdTet alone or together with AdK3 or AdK5 (each treatment or transduction was performed in duplicate, as indicated by the numbers 1 and 2). E-DMVECs were transduced with the indicated Ad constructs for 20 h prior to transfer into an eight-well electrode. (C) At the end of an experiment similar to that described in the legend to panel B, the medium was replaced with fresh medium containing Tet to repress K5 and K3 expression. (D) ECIS upon induction of K5 by Tet removal. The experimental conditions were as described in the legend to panel B, except that the medium contained Tet during plating into the slide array. After 24 h, Tet was removed and resistance was monitored for an additional 65 h.
DISCUSSION
The two RING-CH transmembrane ubiquitin ligases of KSHV eliminate overlapping and nonoverlapping sets of surface proteins. Since most of the proteins targeted by K3 and K5 stimulate recognition by NK, NKT, or T cells, K3 and K5 are modulators of immune responses (MIRs) (19). However, the downregulation of VE-cadherin, together with the elimination of CD31/PECAM by K5 (1, 52), implicates K5 in altering the behavior of KSHV-infected ECs. VE-cadherin was downmodulated in DMVECs transduced with the K5 vector and in latently infected DMVECs upon the reactivation of infection and upon primary infection. Interestingly, the transient infection of human umbilical vein ECs with KSHV reportedly reduced CD31/PECAM and VE-cadherin expression in a previous study (28). Our data suggest that this result was most likely due to K5.
The relevance of this remodeling of endothelial junctions for the establishment and maintenance of chronic infection by KSHV, as well as KS tumor development in immunocompromised individuals, depends on the expression of K5 in vivo. In primary effusion lymphoma-derived cells, KSHV is strictly latent and K5 is expressed only upon lytic reactivation (72). Similarly, in long-term cultures of E-DMVECs, K5 expression requires reactivation (52) (Fig. 2), consistent with the K5 gene's being an immediate-early gene. However, K5 is also expressed in KSHV-infected cells that do not undergo a full-blown lytic cycle. Upon reactivation, K5 expression precedes the expression of the lytic switch gene encoding RTA (60), and in newly infected DMVECs, K5 expression occurs for several days after the shutoff of RTA, suggesting that K5 expression is RTA independent (43). Importantly, K5 targets are downregulated during such primary infection (1, 75) (Fig. 2A). K5 expression can be activated by Notch in the absence of RTA, and Notch is present in KS (17, 34) (Fig. 2A). K5 is also encoded by latent transcripts (73). Previous immunohistochemistry analyses indicated that K5 is widely expressed in KS tumor cells, including the spindle cells typical of KS (33), and K5 was among a small number of lytic gene products upregulated upon the transfer of murine bone marrow-derived ECs transduced with KSHV-bacterial artificial chromosome into nude mice (57). Thus, the expression of K5 in vivo may be more widespread than that of most other lytic gene products. A more detailed study of K5 protein expression in KS tumors has been hampered so far by the fact that very little K5 seems to be required to affect target protein expression. During primary infections, K5 expressed well below IFA detection limits was still able to eliminate cell surface molecules (1, 75). Thus, the presence or absence of K5 target proteins may be a better measure of K5 activity than K5 protein levels.
Both VE-cadherin and CD31 are expressed in KS tumor cells, including the spindle cells typical of KS (27, 77). However, other investigators have reported inconsistent CD31 levels in nodular tumor tissue compared to those in blood vessels or compared to CD34 levels (67). The expression of VE-cadherin mRNA in KS tissue was found previously to be increased compared to that in normal skin (18). However, the same study reported increased message levels for MHC-I. Since K5 downregulates MHC-I or VE-cadherin posttranslationally, mRNA levels may not accurately reflect protein levels. Moreover, KS tumors are composed of both KSHV-infected ECs and uninfected ECs (27), and it has not been established whether VE-cadherin, CD31, and MHC-I expression is more prominent on uninfected cells than on infected cells or in lytic cells than in latent cells. Thus, pending further studies, it is presently not known what percentage of KS cells display reduced expression of K5 target proteins. Using KS-derived cell lines that do not harbor viral genomes, Boccellino et al. detected an upregulation and redistribution of VE-cadherin, α-catenin, and β-catenin during the migration of KS cells in response to platelet-activating factor (8), suggesting that exogenous factors may further affect VE-cadherin expression in KS tumors.
K5-mediated VE-cadherin downregulation during the establishment of latency may accelerate the morphological changes typical of KS, and K5 may render cells more responsive to growth factors since adhesion-dependent contact inhibition renders cells less responsive to vascular endothelial growth factor (31). In latently infected cells undergoing partial lytic reactivation, K5-dependent remodeling of adherens junctions may contribute to proteomic reprogramming of ECs (36, 78). In addition, our ECIS results suggest that K5 increases cell layer permeability, which may contribute to the vascular leakage and the disintegration of vascular architecture typical of KS. Decreasing EC adherence may also contribute to the dissemination of infected ECs, e.g., as circulating ECs are frequently found in KS patients (11, 63).
K5 is the first viral protein shown to destroy endothelial junctions. Since K5 is a homologue of the cellular MARCH family of ubiquitin ligases (3), MARCH-mediated VE-cadherin internalization may also occur during the physiological modulation of adherens junctions. Interestingly, E-cadherin is ubiquitinated and sorted to lysosomes (61), and VE-cadherin can be ubiquitinated and internalized by a cbl-like ubiquitin ligase (24). Moreover, we observed a ubiquitinated intermediate of VE-cadherin in the presence of proton pump inhibitors (Fig. 1E). The viral downregulation of VE-cadherin may thus mimic or accelerate a normal cellular process. It is highly likely that one of the MARCH proteins will modulate VE-cadherin, since all cellular targets of viral proteins are also targeted by one or more cellular MARCH proteins (3).
We observed that the posttranslational downmodulation of VE-cadherin by K5 increased the steady-state levels of N-cadherin but that VE-cadherin levels were higher and N-cadherin levels were lower upon the establishment of latent infection. Since VE-cadherin locates to cell-cell contacts, whereas N-cadherin distributes over the entire membrane, it is thought that VE-cadherin actively excludes N-cadherin from adherens junctions (58). VE-cadherin removal by K5 may thus remove the competitor and stabilize N-cadherin, whereas VE-cadherin upregulation in latently infected cells has the opposite effect. However, N-cadherin cannot functionally replace VE-cadherin with respect to anchoring the adherens junctions to the actin cytoskeleton via α-, β-, and γ-catenins. Interestingly, in a previous study, angiosarcomas lacking VE-cadherin showed higher levels of N-cadherin expression and formed larger tumors in nude mice than the VE-cadherin-expressing controls (85). Similarly, the remodeling of cadherin-dependent adhesion by K5 may contribute to KS tumorigenesis.
K5 is also the first viral protein observed to downregulate α- or β-catenin or γ-catenin. Although β-catenin associates with both CD31 and VE-cadherin (7), β-catenin association with VE-cadherin suggests that β-catenin downregulation is predominantly a consequence of VE-cadherin removal (Fig. 3). In contrast to K5, other viral proteins, mostly from tumor viruses, have been observed previously to stabilize β-catenin, e.g., human T-cell leukemia virus type 1 Tax (76), hepatitis B virus X protein (15), human immunodeficiency virus type 1 vpu (45), latent membrane protein 1 (LMP-1) and LMP-2A of EBV (46, 55), and most interestingly, LANA-1 of KSHV (29, 30). LMP-1 reduces the expression of the β-catenin-degrading ubiquitin ligase SIAH-1 (42), whereas LANA-1 prevents the phosphorylation of β-catenin by sequestering the kinase GSK-3β (30). Stabilization of β-catenin also occurs upon Wnt signaling, in which β-catenin acts as a nuclear cofactor responsible for inducing developmental and proliferative genes. Thus, EBV and KSHV may promote B-cell proliferation by stabilizing β-catenin (34). β-catenin release from cadherins has been implicated in Wnt-dependent and Wnt-independent induction of transcription during cancer (34, 39, 44). While we did not observe increased β-catenin levels in cells coexpressing K5 and LANA and while K5 reduced the expression of Wnt target proteins (Fig. 4), further work is required to examine whether LANA-1 stabilizes nuclear β-catenin levels in ECs since in vitro conditions may not accurately reflect the levels of transcriptionally active versus inactive β-catenin in vivo.
In summary, we have demonstrated that the immune modulator K5 remodels EC adherens junctions, with profound effects on EC adhesion, the cellular cytoskeleton, and potentially, β-catenin signaling. We therefore conclude that K5 not only reprograms the membrane proteome of KS cells but also modulates other cellular proteomes and potentially the transcriptome. We propose that K5 contributes to the reprogramming and dedifferentiation of the ECs during KS tumor development.
Acknowledgments
This work was funded by R01 CA/AI 094011 (to K.F.) and RO1 CA99906 (to A.V.M.). P.P.R. was supported by an N. L. Tartar research fellowship, by a predoctoral research fellowship from the American Heart Association, and by NIAID training grant T32 AI07472.
We thank Dean Kedes and Laura Adang for providing TIME cells and Jae Jung for providing the Notch construct. We also thank Andrew Kowalczyk for providing the VE-cadherin constructs.
M.M. performed most of the experiments in this study and contributed to the design of some of the experiments. P.P.R. performed some of the permeability assays. A.V.M. performed immunofluorecence staining of KSHV-infected E-DMVECs and helped with designing and analyzing the results of permeability assays. K.F. designed the experiments and wrote the paper.
Footnotes
Published ahead of print on 30 July 2008.
REFERENCES
- 1.Adang, L. A., C. Tomescu, W. K. Law, and D. H. Kedes. 2007. Intracellular Kaposi's sarcoma-associated herpesvirus load determines early loss of immune synapse components. J. Virol. 815079-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aluigi, M. G., A. Albini, S. Carlone, L. Repetto, R. De Marchi, A. Icardi, M. Moro, D. Noonan, and R. Benelli. 1996. KSHV sequences in biopsies and cultured spindle cells of epidemic, iatrogenic and Mediterranean forms of Kaposi's sarcoma. Res. Virol. 147267-275. [DOI] [PubMed] [Google Scholar]
- 3.Bartee, E., M. Mansouri, B. T. Hovey Nerenberg, K. Gouveia, and K. Fruh. 2004. Downregulation of major histocompatibility complex class I by human ubiquitin ligases related to viral immune evasion proteins. J. Virol. 781109-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartee, E., A. L. McCormack, and K. Fruh. 2006. Quantitative membrane proteomics reveals new cellular targets of viral immune modulators. PLoS Pathog. 2e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bershadsky, A. 2004. Magic touch: how does cell-cell adhesion trigger actin assembly? Trends Cell Biol. 14589-593. [DOI] [PubMed] [Google Scholar]
- 6.Birchmeier, W., J. Hulsken, and J. Behrens. 1995. Adherens junction proteins in tumour progression. Cancer Surv. 24129-140. [PubMed] [Google Scholar]
- 7.Biswas, P., S. Canosa, D. Schoenfeld, J. Schoenfeld, P. Li, L. C. Cheas, J. Zhang, A. Cordova, B. Sumpio, and J. A. Madri. 2006. PECAM-1 affects GSK-3beta-mediated beta-catenin phosphorylation and degradation. Am. J. Pathol. 169314-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boccellino, M., G. Camussi, A. Giovane, L. Ferro, V. Calderaro, C. Balestrieri, and L. Quagliuolo. 2005. Platelet-activating factor regulates cadherin-catenin adhesion system expression and beta-catenin phosphorylation during Kaposi's sarcoma cell motility. Am. J. Pathol. 1661515-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boname, J. M., and P. G. Stevenson. 2001. MHC class I ubiquitination by a viral PHD/LAP finger protein. Immunity 15627-636. [DOI] [PubMed] [Google Scholar]
- 10.Brembeck, F. H., M. Rosario, and W. Birchmeier. 2006. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr. Opin. Genet. Dev. 1651-59. [DOI] [PubMed] [Google Scholar]
- 11.Browning, P. J., J. M. Sechler, M. Kaplan, R. H. Washington, R. Gendelman, R. Yarchoan, B. Ensoli, and R. C. Gallo. 1994. Identification and culture of Kaposi's sarcoma-like spindle cells from the peripheral blood of human immunodeficiency virus-1-infected individuals and normal controls. Blood 842711-2720. [PubMed] [Google Scholar]
- 12.Cadwell, K., and L. Coscoy. 2008. The specificities of Kaposi's sarcoma-associated herpesvirus-encoded E3 ubiquitin ligases are determined by the positions of lysine or cysteine residues within the intracytoplasmic domains of their targets. J. Virol. 824184-4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cadwell, K., and L. Coscoy. 2005. Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science 309127-130. [DOI] [PubMed] [Google Scholar]
- 14.Cavallaroa, U., S. Liebnerd, and E. Dejana. Endothelial cadherins and tumor angiogenesis. Exp. Cell Res. 312659-667. [DOI] [PubMed]
- 15.Cha, M. Y., C. M. Kim, Y. M. Park, and W. S. Ryu. 2004. Hepatitis B virus X protein is essential for the activation of Wnt/beta-catenin signaling in hepatoma cells. Hepatology 391683-1693. [DOI] [PubMed] [Google Scholar]
- 16.Chang, H. 2006. Notch signal transduction induces a novel profile of Kaposi's sarcoma-associated herpesvirus gene expression. J. Microbiol. 44217-225. [PubMed] [Google Scholar]
- 17.Chang, H., D. P. Dittmer, Y. C. Shin, Y. Hong, and J. U. Jung. 2005. Role of Notch signal transduction in Kaposi's sarcoma-associated herpesvirus gene expression. J. Virol. 7914371-14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornelissen, M., A. C. van der Kuyl, R. van den Burg, F. Zorgdrager, C. J. van Noesel, and J. Goudsmit. 2003. Gene expression profile of AIDS-related Kaposi's sarcoma. BMC Cancer 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coscoy, L. 2007. Immune evasion by Kaposi's sarcoma-associated herpesvirus. Nat. Rev. Immunol. 7391-401. [DOI] [PubMed] [Google Scholar]
- 20.Coscoy, L., and D. Ganem. 2000. Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc. Natl. Acad. Sci. USA 978051-8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coscoy, L., and D. Ganem. 2003. PHD domains and E3 ubiquitin ligases: viruses make the connection. Trends Cell Biol. 137-12. [DOI] [PubMed] [Google Scholar]
- 22.Coscoy, L., and D. Ganem. 2001. A viral protein that selectively downregulates ICAM-1 and B7-2 and modulates T cell costimulation. J. Clin. Investig. 1071599-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damania, B., and J. U. Jung. 2001. Comparative analysis of the transforming mechanisms of Epstein-Barr virus, Kaposi's sarcoma-associated herpesvirus, and herpesvirus saimiri. Adv. Cancer Res. 8051-82. [DOI] [PubMed] [Google Scholar]
- 24.d'Azzo, A., A. Bongiovanni, and T. Nastasi. 2005. E3 ubiquitin ligases as regulators of membrane protein trafficking and degradation. Traffic 6429-441. [DOI] [PubMed] [Google Scholar]
- 25.Dejana, E., R. Spagnuolo, and G. Bazzoni. 2001. Interendothelial junctions and their role in the control of angiogenesis, vascular permeability and leukocyte transmigration. Thromb. Haemost. 86308-315. [PubMed] [Google Scholar]
- 26.Dourmishev, L. A., A. L. Dourmishev, D. Palmeri, R. A. Schwartz, and D. M. Lukac. 2003. Molecular genetics of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) epidemiology and pathogenesis. Microbiol. Mol. Biol. Rev. 67175-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ensoli, B., C. Sgadari, G. Barillari, M. C. Sirianni, M. Sturzl, and P. Monini. 2001. Biology of Kaposi's sarcoma. Eur. J. Cancer 371251-1269. [DOI] [PubMed] [Google Scholar]
- 28.Foglieni, C., S. Scabini, D. Belloni, F. Broccolo, P. Lusso, M. S. Malnati, and E. Ferrero. 2005. Productive infection of HUVEC by HHV-8 is associated with changes compatible with angiogenic transformations. Eur. J. Histochem. 49273-284. [DOI] [PubMed] [Google Scholar]
- 29.Fujimuro, M., and S. D. Hayward. 2003. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus manipulates the activity of glycogen synthase kinase-3β. J. Virol. 778019-8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujimuro, M., F. Y. Wu, C. ApRhys, H. Kajumbula, D. B. Young, G. S. Hayward, and S. D. Hayward. 2003. A novel viral mechanism for dysregulation of beta-catenin in Kaposi's sarcoma-associated herpesvirus latency. Nat. Med. 9300-306. [DOI] [PubMed] [Google Scholar]
- 31.Grazia Lampugnani, M., A. Zanetti, M. Corada, T. Takahashi, G. Balconi, F. Breviario, F. Orsenigo, A. Cattelino, R. Kemler, T. O. Daniel, and E. Dejana. 2003. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J. Cell Biol. 161793-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guerin, J. L., J. Gelfi, S. Boullier, M. Delverdier, F. A. Bellanger, S. Bertagnoli, I. Drexler, G. Sutter, and F. Messud-Petit. 2002. Myxoma virus leukemia-associated protein is responsible for major histocompatibility complex class I and Fas-CD95 down-regulation and defines scrapins, a new group of surface cellular receptor abductor proteins. J. Virol. 762912-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haque, M., K. Ueda, K. Nakano, Y. Hirata, C. Parravicini, M. Corbellino, and K. Yamanishi. 2001. Major histocompatibility complex class I molecules are down-regulated at the cell surface by the K5 protein encoded by Kaposi's sarcoma-associated herpesvirus/human herpesvirus-8. J. Gen. Virol. 821175-1180. [DOI] [PubMed] [Google Scholar]
- 34.Hayward, S. D., J. Liu, and M. Fujimuro. 2006. Notch and Wnt signaling: mimicry and manipulation by gamma herpesviruses. Sci. STKE 2006re4. [DOI] [PubMed] [Google Scholar]
- 35.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 952509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong, Y. K., K. Foreman, J. W. Shin, S. Hirakawa, C. L. Curry, D. R. Sage, T. Libermann, B. J. Dezube, J. D. Fingeroth, and M. Detmar. 2004. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat. Genet. 36683-685. [DOI] [PubMed] [Google Scholar]
- 37.Hordijk, P. 2003. Endothelial signaling in leukocyte transmigration. Cell Biochem. Biophys. 38305-322. [DOI] [PubMed] [Google Scholar]
- 38.Hwang, S. G., S. S. Yu, J. H. Ryu, H. B. Jeon, Y. J. Yoo, S. H. Eom, and J. S. Chun. 2005. Regulation of beta-catenin signaling and maintenance of chondrocyte differentiation by ubiquitin-independent proteasomal degradation of alpha-catenin. J. Biol. Chem. 28012758-12765. [DOI] [PubMed] [Google Scholar]
- 39.Ichikawa, Y., T. Ishikawa, N. Momiyama, M. Kamiyama, H. Sakurada, R. Matsuyama, S. Hasegawa, T. Chishima, Y. Hamaguchi, S. Fujii, S. Saito, K. Kubota, H. Ike, S. Oki, and H. Shimada. 2006. Matrilysin (MMP-7) degrades VE-cadherin and accelerates accumulation of beta-catenin in the nucleus of human umbilical vein endothelial cells. Oncol. Rep. 15311-315. [PubMed] [Google Scholar]
- 40.Ishido, S., C. Wang, B. S. Lee, G. B. Cohen, and J. U. Jung. 2000. Downregulation of major histocompatibility complex class I molecules by Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins. J. Virol. 745300-5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaggi, M., M. Wheelock, and K. R. Johnson. 2002. Differential displacement of classical cadherins by VE-cadherin. Cell Commun. Adhes. 9103-115. [DOI] [PubMed] [Google Scholar]
- 42.Jang, K. L., J. Shackelford, S. Y. Seo, and J. S. Pagano. 2005. Up-regulation of beta-catenin by a viral oncogene correlates with inhibition of the seven in absentia homolog 1 in B lymphoma cells. Proc. Natl. Acad. Sci. USA 10218431-18436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krishnan, H. H., P. P. Naranatt, M. S. Smith, L. Zeng, C. Bloomer, and B. Chandran. 2004. Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi's sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression. J. Virol. 783601-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuphal, F., and J. Behrens. 2006. E-cadherin modulates Wnt-dependent transcription in colorectal cancer cells but does not alter Wnt-independent gene expression in fibroblasts. Exp. Cell Res. 312457-467. [DOI] [PubMed] [Google Scholar]
- 45.Lara-Pezzi, E., S. Roche, O. M. Andrisani, F. Sanchez-Madrid, and M. Lopez-Cabrera. 2001. The hepatitis B virus HBx protein induces adherens junction disruption in a src-dependent manner. Oncogene 203323-3331. [DOI] [PubMed] [Google Scholar]
- 46.Lee, J. O., H. J. Kwun, J. K. Jung, K. H. Choi, D. S. Min, and K. L. Jang. 2005. Hepatitis B virus X protein represses E-cadherin expression via activation of DNA methyltransferase 1. Oncogene 246617-6625. [DOI] [PubMed] [Google Scholar]
- 47.Li, Q., R. Means, S. Lang, and J. U. Jung. 2007. Downregulation of gamma interferon receptor 1 by Kaposi's sarcoma-associated herpesvirus K3 and K5. J. Virol. 812117-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang, Y., J. Chang, S. J. Lynch, D. M. Lukac, and D. Ganem. 2002. The lytic switch protein of KSHV activates gene expression via functional interaction with RBP-Jkappa (CSL), the target of the Notch signaling pathway. Genes Dev. 161977-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang, Y., and D. Ganem. 2003. Lytic but not latent infection by Kaposi's sarcoma-associated herpesvirus requires host CSL protein, the mediator of Notch signaling. Proc. Natl. Acad. Sci. USA 1008490-8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu, Z., and T. Hunter. 2004. Wnt-independent beta-catenin transactivation in tumor development. Cell Cycle 3571-573. [PubMed] [Google Scholar]
- 51.Mansouri, M., E. Bartee, K. Gouveia, B. T. Hovey Nerenberg, J. Barrett, L. Thomas, G. Thomas, G. McFadden, and K. Fruh. 2003. The PHD/LAP-domain protein M153R of myxomavirus is a ubiquitin ligase that induces the rapid internalization and lysosomal destruction of CD4. J. Virol. 771427-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mansouri, M., J. Douglas, P. P. Rose, K. Gouveia, G. Thomas, R. E. Means, A. V. Moses, and K. Fruh. 2006. Kaposi sarcoma herpesvirus K5 removes CD31/PECAM from endothelial cells. Blood 1081932-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McAllister, S. C., and A. V. Moses. 2007. Endothelial cell- and lymphocyte-based in vitro systems for understanding KSHV biology. Curr. Top. Microbiol. Immunol. 312211-244. [DOI] [PubMed] [Google Scholar]
- 54.Moore, P. S., and Y. Chang. 2003. Kaposi's sarcoma-associated herpesvirus immunoevasion and tumorigenesis: two sides of the same coin? Annu. Rev. Microbiol. 57609-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morrison, J. A., A. J. Klingelhutz, and N. Raab-Traub. 2003. Epstein-Barr virus latent membrane protein 2A activates beta-catenin signaling in epithelial cells. J. Virol. 7712276-12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moses, A. V., K. N. Fish, R. Ruhl, P. P. Smith, J. G. Strussenberg, L. Zhu, B. Chandran, and J. A. Nelson. 1999. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J. Virol. 736892-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mutlu, A. D., L. E. Cavallin, L. Vincent, C. Chiozzini, P. Eroles, E. M. Duran, Z. Asgari, A. T. Hooper, K. M. La Perle, C. Hilsher, S. J. Gao, D. P. Dittmer, S. Rafii, and E. A. Mesri. 2007. In vivo-restricted and reversible malignancy induced by human herpesvirus-8 KSHV: a cell and animal model of virally induced Kaposi's sarcoma. Cancer Cell 11245-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Navarro, P., L. Ruco, and E. Dejana. 1998. Differential localization of VE- and N-cadherins in human endothelial cells: VE-cadherin competes with N-cadherin for junctional localization. J. Cell Biol. 1401475-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nicholas, J., V. Ruvolo, J. Zong, D. Ciufo, H. G. Guo, M. S. Reitz, and G. S. Hayward. 1997. A single 13-kilobase divergent locus in the Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genome contains nine open reading frames that are homologous to or related to cellular proteins. J. Virol. 711963-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okuno, T., Y. B. Jiang, K. Ueda, K. Nishimura, T. Tamura, and K. Yamanishi. 2002. Activation of human herpesvirus 8 open reading frame K5 independent of ORF50 expression. Virus Res. 9077-89. [DOI] [PubMed] [Google Scholar]
- 61.Palacios, F., J. S. Tushir, Y. Fujita, and C. D'Souza-Schorey. 2005. Lysosomal targeting of E-cadherin: a unique mechanism for the down-regulation of cell-cell adhesion during epithelial to mesenchymal transitions. Mol. Cell. Biol. 25389-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pellegrino, M., E. Furmaniak-Kazmierczak, J. C. LeBlanc, T. Cho, K. Cao, S. M. Marcovina, M. B. Boffa, G. P. Cote, and M. L. Koschinsky. 2004. The apolipoprotein(a) component of lipoprotein(a) stimulates actin stress fiber formation and loss of cell-cell contact in cultured endothelial cells. J. Biol. Chem. 2796526-6533. [DOI] [PubMed] [Google Scholar]
- 63.Pellet, C., D. Kerob, A. Dupuy, M. V. Carmagnat, S. Mourah, M. P. Podgorniak, C. Toledano, P. Morel, O. Verola, C. Dosquet, Y. Hamel, F. Calvo, C. Rabian, and C. Lebbe. 2006. Kaposi's sarcoma-associated herpesvirus viremia is associated with the progression of classic and endemic Kaposi's sarcoma. J. Investig. Dermatol. 126621-627. [DOI] [PubMed] [Google Scholar]
- 64.Petzelbauer, P., T. Halama, and M. Groger. 2000. Endothelial adherens junctions. J. Investig. Dermatol. Symp. Proc. 510-13. [DOI] [PubMed] [Google Scholar]
- 65.Prozialeck, W. C., M. J. Fay, P. C. Lamar, C. A. Pearson, I. Sigar, and K. H. Ramsey. 2002. Chlamydia trachomatis disrupts N-cadherin-dependent cell-cell junctions and sequesters β-catenin in human cervical epithelial cells. Infect. Immun. 702605-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Radtke, F., and K. Raj. 2003. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat. Rev. Cancer 3756-767. [DOI] [PubMed] [Google Scholar]
- 67.Russell Jones, R., G. Orchard, B. Zelger, and E. Wilson Jones. 1995. Immunostaining for CD31 and CD34 in Kaposi sarcoma. J. Clin. Pathol. 481011-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 9314862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanchez, D. J., J. E. Gumperz, and D. Ganem. 2005. Regulation of CD1d expression and function by a herpesvirus infection. J. Clin. Investig. 1151369-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sciacca, F. L., M. Stuerzl, F. Bussolino, M. Sironi, H. Brandstetter, C. Zietz, D. Zhou, C. Matteucci, G. Peri, et al. 1994. Expression of adhesion molecules, platelet-activating factor, and chemokines by Kaposi's sarcoma cells. J. Immunol. 1534816-4825. [PubMed] [Google Scholar]
- 71.Stevenson, P. G., S. Efstathiou, P. C. Doherty, and P. J. Lehner. 2000. Inhibition of MHC class I-restricted antigen presentation by gamma 2-herpesviruses. Proc. Natl. Acad. Sci. USA 978455-8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun, R., S. F. Lin, K. Staskus, L. Gradoville, E. Grogan, A. Haase, and G. Miller. 1999. Kinetics of Kaposi's sarcoma-associated herpesvirus gene expression. J. Virol. 732232-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taylor, J. L., H. N. Bennett, B. A. Snyder, P. S. Moore, and Y. Chang. 2005. Transcriptional analysis of latent and inducible Kaposi's sarcoma-associated herpesvirus transcripts in the K4 to K7 region. J. Virol. 7915099-15106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tomazin, R., J. Boname, N. R. Hegde, D. M. Lewinsohn, Y. Altschuler, T. R. Jones, P. Cresswell, J. A. Nelson, S. R. Riddell, and D. C. Johnson. 1999. Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cells. Nat. Med. 51039-1043. [DOI] [PubMed] [Google Scholar]
- 75.Tomescu, C., W. K. Law, and D. H. Kedes. 2003. Surface downregulation of major histocompatibility complex class I, PE-CAM, and ICAM-1 following de novo infection of endothelial cells with Kaposi's sarcoma-associated herpesvirus. J. Virol. 779669-9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tomita, M., A. Kikuchi, T. Akiyama, Y. Tanaka, and N. Mori. 2006. Human T-cell leukemia virus type 1 Tax dysregulates β-catenin signaling. J. Virol. 8010497-10505. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Uccini, S., M. C. Sirianni, L. Vincenzi, S. Topino, A. Stoppacciaro, I. Lesnoni La Parola, M. Capuano, C. Masini, D. Cerimele, M. Cella, A. Lanzavecchia, P. Allavena, A. Mantovani, C. D. Baroni, and L. P. Ruco. 1997. Kaposi's sarcoma cells express the macrophage-associated antigen mannose receptor and develop in peripheral blood cultures of Kaposi's sarcoma patients. Am. J. Pathol. 150929-938. [PMC free article] [PubMed] [Google Scholar]
- 78.Wang, H. W., M. W. Trotter, D. Lagos, D. Bourboulia, S. Henderson, T. Makinen, S. Elliman, A. M. Flanagan, K. Alitalo, and C. Boshoff. 2004. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat. Genet. 36687-693. [DOI] [PubMed] [Google Scholar]
- 79.Wang, X., R. A. Herr, W. J. Chua, L. Lybarger, E. J. Wiertz, and T. H. Hansen. 2007. Ubiquitination of serine, threonine, or lysine residues on the cytoplasmic tail can induce ERAD of MHC-I by viral E3 ligase mK3. J. Cell Biol. 177613-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Waschke, J., N. Golenhofen, T. V. Kurzchalia, and D. Drenckhahn. 2006. Protein kinase C-mediated endothelial barrier regulation is caveolin-1-dependent. Histochem. Cell Biol. 12617-26. [DOI] [PubMed] [Google Scholar]
- 81.Wegener, J., C. R. Keese, and I. Giaever. 2000. Electric cell-substrate impedance sensing (ECIS) as a noninvasive means to monitor the kinetics of cell spreading to artificial surfaces. Exp. Cell Res. 259158-166. [DOI] [PubMed] [Google Scholar]
- 82.Xiao, C., B. Lachance, G. Sunahara, and J. H. Luong. 2002. An in-depth analysis of electric cell-substrate impedance sensing to study the attachment and spreading of mammalian cells. Anal. Chem. 741333-1339. [DOI] [PubMed] [Google Scholar]
- 83.Xiao, K., D. F. Allison, M. D. Kottke, S. Summers, G. P. Sorescu, V. Faundez, and A. P. Kowalczyk. 2003. Mechanisms of VE-cadherin processing and degradation in microvascular endothelial cells. J. Biol. Chem. 27819199-19208. [DOI] [PubMed] [Google Scholar]
- 84.Yamada, S., S. Pokutta, F. Drees, W. I. Weis, and W. J. Nelson. 2005. Deconstructing the cadherin-catenin-actin complex. Cell 123889-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zanetta, L., M. Corada, M. Grazia Lampugnani, A. Zanetti, F. Breviario, L. Moons, P. Carmeliet, M. S. Pepper, and E. Dejana. 2005. Downregulation of vascular endothelial-cadherin expression is associated with an increase in vascular tumor growth and hemorrhagic complications. Thromb. Haemost. 931041-1046. [DOI] [PubMed] [Google Scholar]