Abstract
Secretory leukocyte protease inhibitor (SLPI), an anti-inflammatory mediator of mucosal immunity, inhibits human immunodeficiency virus (HIV) and herpes simplex virus (HSV) in cell culture. Epidemiological studies demonstrate that higher concentrations of SLPI in mucosal secretions are associated with a reduced risk of HIV transmission. The current studies were designed to test the hypothesis that HSV triggers a loss of SLPI to evade innate immunity and that this response may contribute to the increased risk of HIV infection in the setting of HSV infection. Exposure of human cervical epithelial cells to HSV-1 or HSV-2, but not HIV or vesicular stomatitis virus, triggered a significant and sustained reduction in SLPI levels. The reduction persisted when cells were infected in the presence of acyclovir but not following infection with UV-inactivated virus, indicating that viral gene expression, but not replication, is required. Reverse transcriptase PCR studies demonstrated that the loss of SLPI is mediated by downregulation of gene expression. SLPI downregulation was associated with activation of NF-κB signaling pathways and upregulation of proinflammatory cytokines, consistent with the known inhibitor effects of SLPI on NF-κB pathways. The downregulation mapped to viral early-gene expression, as variants impaired in expression of the ICP4 or ICP0 immediate-early gene failed to downregulate SLPI or activate NF-κB. Together, these results identify a novel role for HSV immediate-early-gene expression in regulating mucosal immune responses.
Prevention of genital herpes is a global health priority not only because of the morbidity associated with ulcerative disease itself but also because of the risks of perinatal and sexual transmission as well as the epidemiological link between herpes simplex virus (HSV) infection and human immunodeficiency virus (HIV) acquisition and transmission (9, 10). Women and minorities bear a disproportionate burden of disease (53). Approximately 23% of women of child-bearing age in the United States are HSV-2 seropositive, and the seroprevalence rate among non-Hispanic black women is over 40% (53). In developing countries, 60 to 80% of the population is infected with HSV-2, the serotype most commonly associated with genital herpes (28, 33). Epidemiological studies consistently demonstrate that HSV-2 infection increases the risk of HIV acquisition and transmission (40). An understanding of the molecular mechanisms underlying this link may facilitate the identification of novel preventative strategies for thwarting the overlapping HIV and HSV epidemics.
Cervicovaginal secretions provide intrinsic protection and inhibit HSV infection in vitro by as much as 90% (25, 27). Multiple factors may contribute to this activity, including the acidic pH of the healthy female genital tract and antimicrobial proteins such as mucins, defensins, lactoferrin, lysozyme, and secretory leukocyte protease inhibitor (SLPI) (21, 32, 47). To successfully establish infection, HSV must overcome these mucosal defenses. HSV has evolved several strategies for evading the host immune response targeting components of both innate and acquired immunity, including complement proteins, natural killer cells, major histocompatibility complex class I or class II molecules, and antibody (23). For example, glycoproteins E and C (gE and gC) impair antibody and complement responses. gC inhibits complement activation by binding C3b, whereas gE binds the immunoglobulin G Fc domain, blocking Fc-mediated activities, including complement activation and antibody-dependent cellular cytotoxicity. HSV also expresses several viral genes that are associated with resistance to interferons (IFNs), most notably ICP0 (29).
SLPI is a low-molecular-mass (11.7-kDa) protein found abundantly in mucosal secretions, including saliva, breast milk, seminal fluid, and secretions in the female genital tract. It has pronounced anti-inflammatory, antibacterial, and antifungal activities (7, 12, 22, 44, 46). Importantly, SLPI possesses potent anti-HIV-1 activity at physiological concentrations found in saliva (24, 35, 50), which is presumed to contribute to the endogenous anti-HIV activity of oral secretions. A recent study found that brief exposure of human oral keratinocytes and epithelial cells to HIV-1 stimulated SLPI mRNA and protein production in the absence of direct infection, suggesting that upregulation of SLPI by the virus may protect the oral cavity against HIV infection (24).
High SLPI concentrations are also found in seminal plasma but may not provide protection, because SLPI may be subject to partial proteolytic cleavage by prostate-specific antigen (38). SLPI binds to the membranes of human macrophages through the phospholipid-binding protein annexin II, which acts as a cellular cofactor supporting macrophage HIV-1 infection (31). However, SLPI fails to bind cells under basic conditions, suggesting that the alkaline pH of semen may prevent seminal SLPI from binding to HIV target cells (36).
We recently demonstrated that SLPI also inhibits HSV infection in vitro by binding to epithelial cell surfaces and preventing viral infection, although the precise mechanisms have not yet been elucidated (25). While no epidemiological studies have evaluated the role played by SLPI in protecting against HSV, several studies demonstrate a protective role for SLPI in preventing HIV infection. Higher levels of SLPI in vaginal fluid correlated with reduced rates of perinatal HIV transmission, and higher salivary levels in infants were associated with reduced transmission through breast milk (16, 39). The paradigm being tested in the current studies is that HSV modifies expression of SLPI as an escape mechanism, which may facilitate HIV infection.
MATERIALS AND METHODS
Cells.
The human cervical epithelial cell line CaSki (CRL-1550), immortalized endocervical cells (Endo, CRL-2615) (17), monkey kidney epithelial cells (Vero), and human osteosarcoma cell lines (U-2 OS) were obtained from the American Type Culture Collection (ATCC). Endocervical cells were propagated in keratinocyte serum-free medium (GIBCO-BRL 17005-042) with 0.1 ng/ml human recombinant epidermal growth factor, 0.05 mg/ml bovine pituitary extract, and additional calcium chloride (final concentration, 0.4 mM). U-2 OS cells were propagated in McCoy's 5a medium with 10% fetal bovine serum (FBS). FO6, a derivative Vero cell line expressing ICP4, ICP27, and ICP0 under their own promoters (41), and Vero 2.2, a derivative Vero cell line expressing ICP27 under its own promoter (43), were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 5% FBS. All other cells were grown and maintained in DMEM supplemented with 10% FBS.
Viral strains.
The wild-type strains used in these studies were HSV-1(KOS), HSV-1(F), HSV-1(17+), and HSV-2(G). The ICP0-deficient virus (7134) and its repair (7134R), which were derived from HSV-1(KOS), were a gift from P. Schaffer (Harvard Medical School, Boston, MA) (5). The viral strain dl1403, an ICP0 deletion virus; FXE, which expresses a form of ICP0 lacking the RING finger; and M1, an ICP0 mutant defective in binding to ubiquitin-specific protein USP7, and their respective repairs were derived from HSV-1(17+) and were gifts from R. Everett (MRC Virology Unit, Institute of Virology, Scotland, United Kingdom) (3, 4, 15). The HSV-2(333) vhs deletion mutant (333d41) virus was a gift from D. Leib (Washington University School of Medicine, St. Louis, MO) (42). The HSV-1(KOS) derivative vBSΔ27 (43), in which the α27 gene is replaced by the Escherichia coli lacZ gene, was propagated on Vero 2.2 cells, and its titers were determined. Strain 17 derivative HSV-1(CgalΔ3), which contains the E. coli lacZ gene under the human cytomegalovirus immediate-early (IE) promoter inserted into an intergenic site in the short unique portion of a mutant genome deleted for 3.6 kb of the ICP4 coding region (26), was propagated on FO6 cells, and its titers were determined. Strain F derivative HSV-1(R7802) (obtained from Bernard Roizman, University of Chicago), which lacks the coding domain of ICP22 (26), was grown on Vero cells, and its titers were determined as previously described (41).
Vesicular stomatitis virus (VSV) (Indiana) was a gift from P. Palese, Mount Sinai School of Medicine, New York, NY. The laboratory-adapted HIV-1 strain HIV-1BaL (CCR5-utilizing strain) was grown in PM-1 cells and stored at −180°C after filtration through 0.2-μm filters (Millipore, MA). All herpes viruses were propagated on Vero cells, except for the ICP0 deletion viruses, which were propagated on U2-OS cells. Virus titers on CaSki cells were determined. HSV-2(G) was inactivated by exposure to UV light (at a distance of 10 cm from the light source for 7 min).
Preparation of infected cell lysates, gel electrophoresis, and immunoblot analysis.
Infected cell lysates were prepared at 24 h postinfection (p.i.) and proteins separated in 8% polyacrylamide gel and transferred to nitrocellulose membranes for immunoblotting as previously described (8). The following primary antibodies were used to detect viral proteins: (i) 1113, a mouse anti-ICP27 monoclonal antibody (Goodwin Institute for Cancer Research, Plantation, FL); (ii) 1114, a mouse anti-ICP4 monoclonal antibody (Goodwin); (iii) 1112, a mouse anti-ICP0 monoclonal antibody (Goodwin); (iv) RGST22, a rabbit polyclonal antibody directed against the ICP22 protein (2); and a monoclonal antibody for detection of β-actin (AC-15; Sigma). Secondary goat anti-rabbit (Bio-Rad) and goat anti-mouse antibodies conjugated with alkaline phosphatase were purchased from Calbiochem. The blots were scanned and analyzed using ImageJ (Bethesda, MD).
Impact of viral exposure on SLPI.
Cells were inoculated with different strains of HSV, VSV, or HIV-1BaL at the indicated multiplicities of infection (MOI) (numbers of PFU/cell for HSV and VSV and 50 ng p24 for HIV) or mock infected with phosphate-buffered saline (PBS) in duplicate at 37°C. After a 1- to 2-h adsorption period, the inoculum was removed and the cells were washed and overlaid with serum-free medium (DMEM) in the absence or presence of acyclovir (100 μg/ml) (American Pharmaceutical Partners, Schaumburg, IL). The cell culture supernatants were collected at various times p.i. In some experiments, cell lysates were prepared by incubating the cells with lysis buffer for 10 min (2% Tris base, 0.3% NaCl, 0.5% NP-40, 0.05% deoxycholate). Protease inhibitor cocktail was added to culture supernatants and lysates prior to storage at −20°C. As a positive control, cells were treated with poly(I:C) (25 μg/ml; InvivoGen, San Diego, CA).
SLPI enzyme-linked immunosorbent assay (ELISA).
Frozen samples were thawed and diluted 1:10 in serum-free medium, and SLPI levels were detected by using a Quantikine human SLPI immunoassay (sensitivity, 25 pg/ml; R&D Systems, Minneapolis, MN). The optical density reading (450 nm) was obtained with a multimode detector (Beckman Coulter DTX 880). Standard curves were derived for each individual assay.
Transcription factor assay.
Nuclear extracts were prepared using an Active Motif nuclear extract kit (Carlsbad, CA). Nuclear protein was quantified by using a Quick Start Bradford protein assay (Bio-Rad), and nuclear extracts were stored at −80°C. The samples were analyzed for NF-κB (p65) levels by using an Active Motif TransAM NF-κB kit. Chemiluminescence was determined by using a multimode detector (Beckman Coulter DTX 880).
Total RNA extraction and real-time quantitative RT-PCR (qRT-PCR).
Total RNA was extracted using an Absolutely RNA reverse transcriptase PCR (RT-PCR) mini prep kit (Stratagene, La Jolla, CA). RNA (200 ng) was reverse transcribed with TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA). RT-PCR amplification was performed in duplicate using an ABI PRISM 7000 detection system and analyzed using sequence detector software. Commercially available probes for human SLPI (Hs00268204_m1), interleukin 6 (IL-6) (Hs00985639_m1), and the ribosomal large protein subunit (RPLPO) (4333761F) housekeeping gene were obtained from Applied Biosystems. Quantification was normalized against the number of RPLPO transcripts in the same RNA extracts. Standards for each transcript gave a linear amplification curve over at least 4 logs of template concentrations.
Statistical analysis.
GraphPad Prism (version 4; GraphPad Software) was used for statistical analysis. Results were compared using Student's t test, and differences were considered significant at P values of <0.05.
RESULTS
HSV-1 and HSV-2 trigger a reduction in SLPI independent of viral replication.
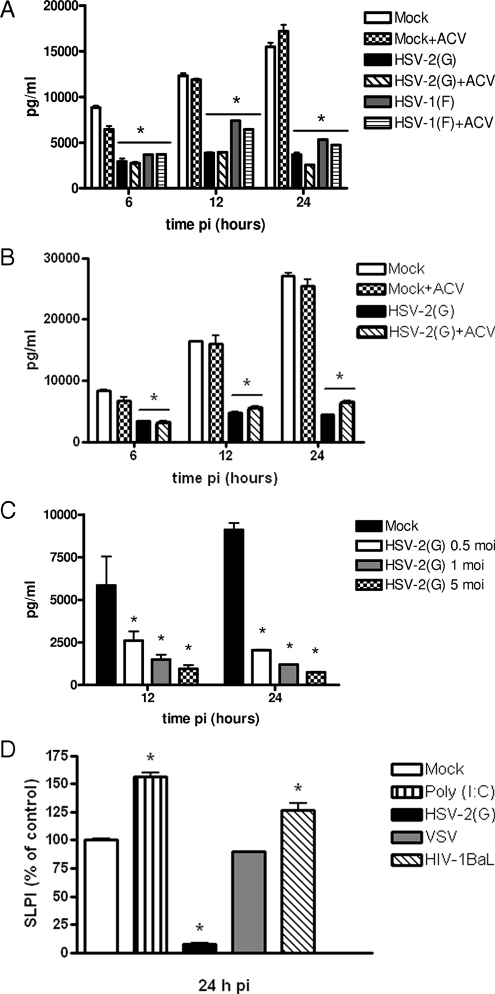
Cells were mock infected or infected with HSV-2(G) or HSV-1(F) in the absence or presence of acyclovir, and the SLPI concentrations in culture supernatants were measured at 6, 12, or 24 h p.i. Mock-infected CaSki and immortalized End1/E6E7 cells released over 10,000 pg/ml of SLPI into the medium within 24 h (Fig. 1A and B). Infection of both cell types with HSV-1 or HSV-2 led to a significant and sustained reduction in SLPI, which was detected as early as 6 h p.i. (P < 0.01). The reduction in SLPI increased following exposure to higher inoculums (Fig. 1C) but was independent of viral replication, as comparable reductions in SLPI levels were observed when cells were infected in the presence of 100 μg/ml of acyclovir (Fig. 1A and B). This concentration of acyclovir consistently inhibits viral replication, as evidenced by significant losses in viral yields and reductions in plaque formation in all of the cell types studied (data not shown). Similar downmodulation of SLPI was observed with primary clinical isolates and following infection of CaCo 2 cells (colonic epithelial cell line) (data not shown).
FIG. 1.
HSV triggers reduction in SLPI. The concentrations of SLPI in culture medium were determined by ELISA following mock infection (PBS) or infection with HSV-2(G) or HSV-1(F) (MOI, 5 PFU/cell) in the absence or presence of acyclovir (ACV). (A) CaSki cells. (B) End1/E6E7 cells. (C) To determine if the effects were dependent on the inoculum, CaSki cells were infected with 0.5, 1, or 5 PFU/cell HSV-2(G). Results are presented as concentrations of SLPI (means ± standard deviations [SD] pg/ml) obtained from at least three independent experiments conducted in duplicate. (D) To examine the specificity of the response, CaSki cells were infected with HSV-2(G), VSV (Indiana) (5 PFU/cell), or HIV-1BaL (50 ng p24) or treated with the TLR3 agonist poly(I:C) (25 μg/ml). Results are presented as percent changes in SLPI concentration relative to levels for PBS-treated cells and are the means ± SD of results from two independent experiments conducted in duplicate. The differences between treated and mock-exposed cells were compared by unpaired t tests; P values of <0.05 were considered to be significant.
To examine whether the loss of SLPI was specific for HSV, CaSki cells were also infected with VSV or exposed to 50 ng p24 of HIV-1BaL, a concentration that binds to CaSki cells (P. M. M. Mesquita and B. C. Herold, unpublished results). Previous studies have demonstrated that exposure of human oral epithelial cells or keratinocytes to HIV-1BaL resulted in an increase in SLPI production, although the effects on genital tract epithelial cells were not examined (24). As an additional control, the cells were treated with the Toll-like receptor 3 (TLR3) agonist poly(I:C), which has been shown to augment SLPI production in the genital tract (1). VSV had little or no effect on SLPI production, whereas both HIV (P = 0.047) and poly(I:C) (P = 0.005) stimulated modest increases in SLPI, indicating that the loss of SLPI following HSV infection is specific (Fig. 1D).
Reduction in SLPI is mediated by transcriptional downregulation.
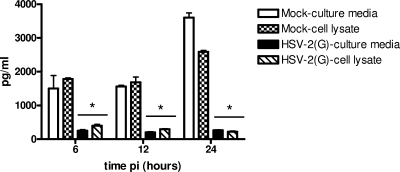
HSV could trigger a reduction in SLPI secretion by either blocking its release, triggering its degradation, or downregulating gene expression. First, to address the possibility that the reduction in SLPI reflects a block in secretion, the concentrations of SLPI observed in cell lysates and culture supernatants following HSV and mock infection were compared. HSV triggered comparable reductions in SLPI in cell lysates and culture supernatants, indicating that HSV does not prevent the protein from being secreted (Fig. 2).
FIG. 2.
HSV triggers reduction in intracellular and secreted SLPI. CaSki cells were mock infected or infected with HSV-2(G) and culture medium and cell lysates harvested at 6, 12, and 24 h p.i. and analyzed for SLPI by ELISA. Results are means ± SD of results from two independent experiments conducted in duplicate. As indicated by the asterisks, P values of <0.05 were considered to be significant.
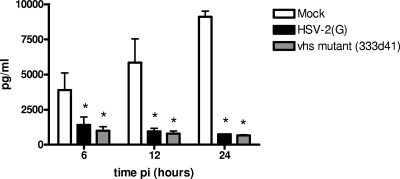
HSV induces rapid destabilization and degradation of host cell mRNA through the activities of the virion host shutoff (vhs) protein (42). To determine whether the reduction in SLPI is mediated by vhs, we took advantage of the HSV-2 vhs deletion mutant 333d41. A comparable reduction in SLPI was observed following infection with the vhs deletion virus, indicating that the loss of SLPI is independent of vhs activity (Fig. 3).
FIG. 3.
HSV retains the ability to reduce SLPI following infection with vhs deletion virus. CaSki cells were mock infected or infected with HSV-2(G) or the vhs-deficient virus (MOI, 5 PFU/cell) and culture medium harvested at the indicated times p.i. and analyzed for SLPI concentration by ELISA. Results are presented as means ± SD obtained from two independent experiments; asterisks indicate P values of <0.05 for comparison with mock-exposed cells.
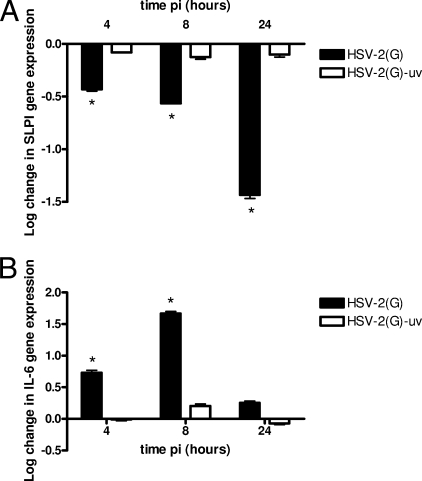
To determine if the reduction in SLPI was transcriptionally regulated, RNA was harvested from cell lysates at 4, 8, and 24 h p.i. and analyzed for SLPI gene expression by qRT-PCR. Infection with HSV-2(G) resulted in at least a 1-log reduction in SLPI gene expression at 24 h p.i. relative to levels for mock-exposed cells. Notably, no significant downregulation was observed following infection with UV-inactivated virus, suggesting that downregulation requires viral gene expression (Fig. 4A). Conversely, infection with HSV-2(G), but not UV-inactivated virus, resulted in rapid and significant upregulation of the proinflammatory cytokine IL-6, which peaked at 8 h p.i. (Fig. 4B).
FIG. 4.
SLPI gene expression is downregulated following infection with HSV-2(G) but not UV-inactivated virus. CaSki cells were infected with either HSV-2(G) or UV-inactivated virus, and the expression of SLPI (A) or IL-6 (B) was analyzed by qRT-PCR. Results are presented as log changes in gene expression relative to the expression of the RPLPO housekeeping gene and are means for duplicate wells obtained from a representative experiment. Similar results were obtained in at least two independent experiments; asterisks indicate P values of <0.01 for comparison with mock-exposed cells.
IE gene expression is required for downregulation of SLPI.
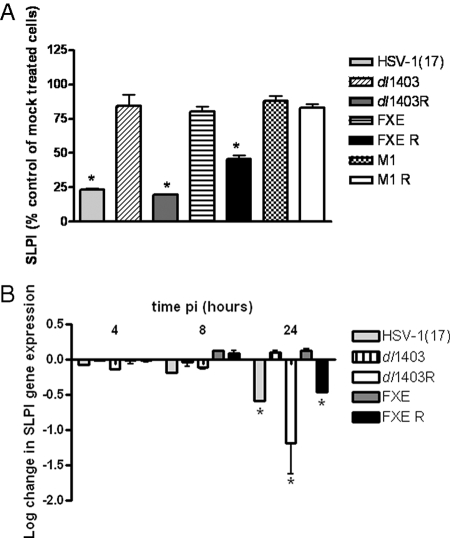
The observation that SLPI downregulation requires viral gene expression but not replication suggests that one or more of the IE genes may play a pivotal role in triggering the loss of SLPI. We initially focused on ICP0 because of its multifunctional roles. ICP0 is a phosphoprotein that transactivates viral and cellular proteins, although no specific promoter sequence has been defined (14). Additionally, ICP0 stimulates the degradation of a number of host proteins, in part because of its E3 ubiquitin ligase activity and its interactions with the cellular ubiquitin-specific protease enzyme USP7 (4, 49). To examine the impact of ICP0 on SLPI expression, we infected cells with dl1403, FXE, and M1 viruses and their respective repairs. These studies were conducted at an MOI of 1 PFU/cell (based on the titer of each virus on CaSki cells) because ICP0 deletion viruses exhibit delayed progression beyond the IE gene expression at low MOI, which is overcome at higher MOI (3, 15). We confirmed that an equivalent number of viral capsids entered the cells, by preparing nuclear extracts and analyzing for VP16 transport to the nuclear pore (data not shown). No reduction in SLPI protein in culture supernatants harvested at 24 h was observed following infection with any of the ICP0 deletion viruses, indicating that ICP0 and both the FXE and the USP7 encoding domains contribute to the SLPI downmodulation (Fig. 5A). The phenotype was restored, at least partially, following infection with the respective repair viruses (Fig. 5). Similar results were obtained by RT-PCR using RNA isolated from the dl1403 and FXE viruses, their respective repairs, and the parental virus HSV-1(17+) (Fig. 5B). Notably, the downregulation of gene expression was delayed relative to results obtained with HSV-2(G) (Fig. 4A).
FIG. 5.
Downregulation of SLPI requires expression of wild-type ICP0. CaSki cells were mock treated or infected with the indicated viruses (MOI, 1 PFU/cell [based on viral titer on CaSki cells]). Culture supernatants (A) were collected at 24 h p.i. and analyzed for SLPI protein levels by ELISA. In parallel studies, cell lysates were harvested at 4, 8, and 24 h p.i. and SLPI gene expression was determined by qRT-PCR. Results are presented as percent changes in SLPI concentration relative to levels for PBS-treated cells (A) and as log changes in SLPI gene expression relative to expression of RPLPO (B). Results are means ± SD obtained from duplicate wells and are representative of at least two independent experiments; asterisks indicate significant changes in SLPI protein (P < 0.01) and gene expression (P < 0.05).
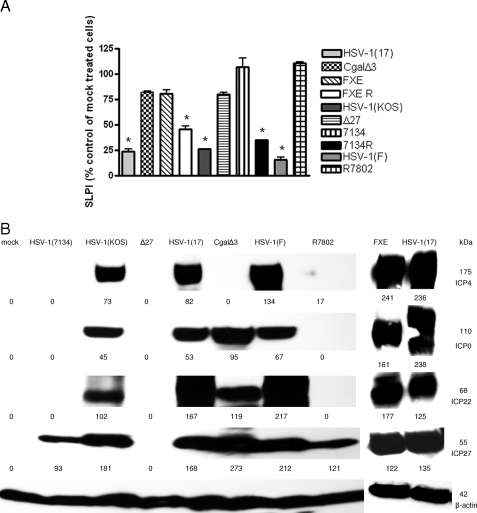
Because deletion of ICP0 affects the expression of other IE genes, we next examined a panel of IE mutant viruses. CaSki cells were mock infected or infected with each virus at an MOI of ∼1 PFU/cell (based on titer on complementing F-06 cells), and at 24 h p.i., the concentrations of SLPI in culture supernatants were determined by ELISA. Whole-cell lysates of infected cells were prepared in parallel, and viral gene expression was evaluated by preparing Western blots and probing for each of the IE proteins, using β-actin as a control. No downmodulation of SLPI was observed following infection with any of the IE mutant variants, whereas each of the parental viruses triggered a significant loss of SLPI (Fig. 6A). Analysis of the Western blots suggests that downmodulation of SLPI requires expression of both full-length ICP0 (with an intact ring domain) and ICP4. This follows from the observation that the ICP4 deletion virus CgalΔ3 expresses each of the other IE genes, including the ICP0 gene, whereas the FXE variant expresses all of the other IE genes, including the ICP4 gene, but expresses a mutated form of ICP0. Thus, neither protein alone is sufficient to trigger SLPI downregulation (Fig. 6B).
FIG. 6.
Neither ICP0 nor ICP4 alone is sufficient to downregulate SLPI. CaSki cells were mock treated or infected with the indicated viruses (MOI, 1 PFU/cell), and at 24 h p.i., the culture medium was collected and analyzed by ELISA for SLPI protein levels (A). Total cell proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the levels of the indicated IE genes were detected by Western blot analysis (B). The quantification was determined using ImageJ software. The numerical values represent the intensity densities of bands relative to β-actin levels.
Downmodulation of SLPI is associated with NF-κB activation.
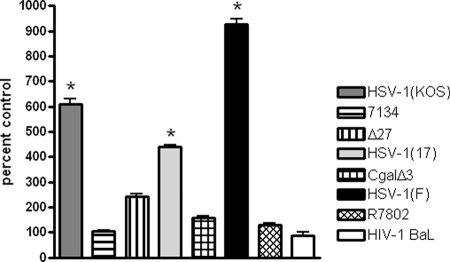
Prior studies have demonstrated that SLPI inhibits LPS-induced NF-κB activation in monocytic cells by blocking the degradation of IκBα and by competitively binding to the NF-κB consensus binding domain (45). This suggests that the virus-induced downregulation of SLPI might be linked to NF-κB activation. Thus, we would predict that infection with HSV viruses that downregulate SLPI would be associated with NF-κB activation and, conversely, that the IE mutant viruses that fail to downregulate SLPI would be impaired in NF-κB activation. To explore this, we compared NF-κB activation in CaSki cells following infection with the IE viral deletion variant viruses at 8 h p.i. HIV-1BaL was also included for comparison. Activated Jurkat cells served as a positive control in the assay. Each of the parental HSV viruses triggered significant activation of NF-κB, whereas little or no NF-κB activation was observed following exposure of the cells to viral variants lacking ICP0, ICP4, ICP27, and ICP22 or to HIV-1BaL (Fig. 7).
FIG. 7.
IE gene expression is required for activation of NF-κB. CaSki cells were exposed to the indicated viruses, and at 8 h p.i., nuclear extracts were prepared and analyzed for NF-κB p65. Activated Jurkat cells are included as a positive control. Results are presented as percent increases in nuclear p65 relative to levels for mock-treated cells and are means for two independent experiments. The asterisks indicate significant increases in nuclear p65 (P < 0.05).
DISCUSSION
These studies demonstrate that HSV-1 and HSV-2 downregulate SLPI, which may serve as a novel immune evasion strategy. Importantly, downmodulation does not require viral replication, as evidenced by the findings that downmodulation occurs within 6 h following infection and persists in the presence of acyclovir. However, downmodulation requires expression of both ICP4 and wild-type ICP0, as evidenced by results obtained with IE viral variants and UV-inactivated virus.
The observation that both of these IE proteins are required to trigger the downmodulation of SLPI is consistent with their known functional interactions. Both IE proteins play critical roles as transcriptional activators of viral gene expression, and the ability of ICP0 to transactivate promoters is increased synergistically in the presence of ICP4 (34). Their vital role in regulating HSV infection and reactivation from latency is highlighted by a recent study, which found that the LAT gene encodes several microRNA precursors (miRNAs) and that one of these miRNAs, miR-H2-3p, is transcribed in an antisense orientation relative to ICP0 and inhibits its expression. Notably, a second miRNA (which derives from a transcript distinct from LAT) inhibits expression of ICP4 (48).
ICP0 has previously been shown to play a major role in immune evasion by overcoming the antiviral IFN response. The ability of ICP0 to transactivate cellular proteins may contribute to its capacity to overcome the IFN-induced block to viral transcription (37). ICP0 blocks IFN regulatory factor 3 (IRF3)- and IRF7-mediated activation of IFN-stimulated genes through the activities of the RING finger domain (30). Additionally, ICP0 stimulates the degradation of a number of host proteins, in part because of its E3 ubiquitin ligase activity as well as its interactions with the cellular ubiquitin-specific protease enzyme USP7 (4, 49). In the current studies, we found that the primary mechanism by which HSV reduces SLPI is downregulation of mRNA, although virus-induced degradation of SLPI protein may also contribute. Further studies are required to determine whether ICP0 and ICP4 directly or indirectly trigger the downregulation and what the precise mechanism underlying this response is. Notably, the loss of SLPI downmodulation was associated with reduced ability to activate NF-κB at early times p.i. (8 h).
There are several potential consequences of downregulating SLPI. First, we previously demonstrated that recombinant SLPI interacts with human epithelial cells to inhibit HSV infection in vitro (25). Downregulation could overcome the antiviral activity of SLPI in mucosal secretions, thus providing a mechanism for immune evasion. A clinical study is currently ongoing to examine the concentrations of SLPI in genital tract secretions in women during an acute HSV outbreak. Second, downmodulation of SLPI could promote the activation of NF-κB by releasing a negative regulator, thus further facilitating HSV infection. The ability of HSV to activate NF-κB has been well documented (2, 19), and interference with NF-κB activation, for example, following expression of a dominant-negative IκBα, resulted in a reduction in virus yield. This has been attributed in part to the role NF-κB may play in preventing virus-induced apoptosis (19). Thus, it has been proposed that persistent NF-κB activation, rather than being a host response to virus infection, may play a positive role in promoting efficient virus replication.
Additionally, the HSV-induced downregulation of SLPI could also contribute to epidemiological observations of an increased risk of HIV acquisition among HSV-2-seropositive individuals (18). SLPI inhibits HIV infection of macrophages, and higher concentrations of SLPI in mucosal fluids are associated with a reduced risk of infection (16, 39). Thus, the reduction in SLPI in the setting of HSV, even in the presence of acyclovir, could promote HIV acquisition. The downregulation of SLPI could also contribute to the increase in HIV viral loads in the genital tract as observed during periods of HSV reactivation in coinfected individuals (20). We found that exposure of the chronically HIV-infected monocytic cell line U1 to wild-type HSV, but not to the ICP4 deletion virus, resulted in enhanced HIV replication, with a significant increase in p24 production (P. M. M. Mesquita and B. C. Herold, unpublished results). These results could be explained by NF-κB-mediated activation of the HIV long-terminal repeat. Whether SLPI downregulation contributes to this response remains to be determined. It is interesting to speculate whether the downmodulation of SLPI or other changes in the mucosal environment triggered by reactivating HSV, even in the absence of viral replication, played a role in the failure of oral acyclovir suppression to reduce the risk of HIV infection in the recently completed clinical trials among high-risk HSV-infected, HIV-negative individuals (6, 51; C. L. Celum, presented at the 15th Conference on Retroviruses and Opportunistic Infections, Boston, MA, 2008).
The observation that HSV downmodulates SLPI is unusual, as most other microbes induce SLPI and other antimicrobial peptides. VSV induced no change in SLPI, and consistent with prior studies, we found that HIV exposure resulted in a modest increase in SLPI levels. Notably, the response induced by the cervical cells was less than that previously reported for oral epithelial cells. In the previous study, the authors found that exposure of oral epithelial cells to HIV resulted in a threefold increase in SLPI production and speculated that this response may contribute to the poor oral transmission of HIV (24). Possibly, the more modest increase in SLPI following exposure of cervical epithelial cells to HIV-1 contributes to the increased risk of genital versus oral mucosal transmission.
Increases in SLPI have also been observed in response to other pathogens. For example, Mycobacterium tuberculosis has also been shown to increase SLPI production by macrophages. Exposure of murine peritoneal macrophages to M. tuberculosis or aerosolized infection of mice with M. tuberculosis triggers an increase in SLPI. Notably, macrophages from TLR2−/− mice are incapable of inducing this response, suggesting a role for TLR2-dependent pathways in triggering the SLPI response (11).
Similarly to results obtained here for HSV, several other pathogens appear to have evolved strategies for escaping the antimicrobial effects of SLPI. Helicobacter pylori triggers a loss of SLPI in cell cultures, and antral biopsies of H. pylori-positive subjects show reduced SLPI expression (52). The H. pylori-induced decrease in SLPI could not be explained by a transcriptional downmodulation and appeared to be regulated posttranslationally. Additionally, cysteine proteases of Trichomonas vaginalis have been demonstrated to degrade SLPI, which has been suggested to contribute to the increased risk of HIV in the setting of trichomonas infection (13).
In conclusion, these studies describe a novel mechanism by which HSV may interfere with innate mucosal immunity and identify yet another role for HSV in immune evasion. Defining the precise mechanisms by which ICP4 and ICP0 trigger downregulation of this mediator of mucosal host defense may promote the development of strategies for prevention of this mucosal immune response, which could foster prevention of both HSV and HIV.
Acknowledgments
We thank Priscilla Shaffer for the generous gift of the ICP0-deficient (7134) and repaired (7134R) viruses, David Leib for the vhs-deficient 333d41 virus, and Roger Everett for the gift of ICP0 viruses dl1403, FXE, and M1 and their respective repairs. We also thank the Quantitative PCR Research Facility at Mount Sinai School of Medicine, New York, NY, and the DNA Core Facility at Albert Einstein College of Medicine, Bronx, NY.
This work was supported by AI065309 (B.C.H.), AI38873 (J.A.B.), and AI48482 (J.A.B.).
Footnotes
Published ahead of print on 30 July 2008.
REFERENCES
- 1.Abrahams, V. M., T. M. Schaefer, J. V. Fahey, I. Visintin, J. A. Wright, P. B. Aldo, R. Romero, C. R. Wira, and G. Mor. 2006. Expression and secretion of antiviral factors by trophoblast cells following stimulation by the TLR-3 agonist, Poly(I : C). Hum. Reprod. 212432-2439. [DOI] [PubMed] [Google Scholar]
- 2.Amici, C., G. Belardo, A. Rossi, and M. G. Santoro. 2001. Activation of I kappa b kinase by herpes simplex virus type 1. A novel target for anti-herpetic therapy. J. Biol. Chem. 27628759-28766. [DOI] [PubMed] [Google Scholar]
- 3.Boutell, C., M. Canning, A. Orr, and R. D. Everett. 2005. Reciprocal activities between herpes simplex virus type 1 regulatory protein ICP0, a ubiquitin E3 ligase, and ubiquitin-specific protease USP7. J. Virol. 7912342-12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boutell, C., S. Sadis, and R. D. Everett. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and is isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai, W., and P. A. Schaffer. 1991. A cellular function can enhance gene expression and plating efficiency of a mutant defective in the gene for ICP0, a transactivating protein of herpes simplex virus type 1. J. Virol. 654078-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celum, C., A. Wald, J. Hughes, J. Sanchez, S. Reid, S. Delany-Moretlwe, F. Cowan, M. Casapia, A. Ortiz, J. Fuchs, S. Buchbinder, B. Koblin, S. Zwerski, S. Rose, J. Wang, and L. Corey. 2008. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet 3712109-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chattopadhyay, A., L. R. Gray, L. L. Patton, D. J. Caplan, G. D. Slade, H. C. Tien, and D. C. Shugars. 2004. Salivary secretory leukocyte protease inhibitor and oral candidiasis in human immunodeficiency virus type 1-infected persons. Infect. Immun. 721956-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheshenko, N., and B. C. Herold. 2002. Glycoprotein B plays a predominant role in mediating herpes simplex virus type 2 attachment and is required for entry and cell-to-cell spread. J. Gen. Virol. 832247-2255. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, M. S. 1998. Sexually transmitted diseases enhance HIV transmission: no longer a hypothesis. Lancet 351(Suppl. 3)5-7. [DOI] [PubMed] [Google Scholar]
- 10.Corey, L., A. Wald, C. L. Celum, and T. C. Quinn. 2004. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J. Acquir. Immune Defic. Syndr. 35435-445. [DOI] [PubMed] [Google Scholar]
- 11.Ding, A., H. Yu, J. Yang, S. Shi, and S. Ehrt. 2005. Induction of macrophage-derived SLPI by Mycobacterium tuberculosis depends on TLR2 but not MyD88. Immunology 116381-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doumas, S., A. Kolokotronis, and P. Stefanopoulos. 2005. Anti-inflammatory and antimicrobial roles of secretory leukocyte protease inhibitor. Infect. Immun. 731271-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Draper, D., W. Donohoe, L. Mortimer, and R. P. Heine. 1998. Cysteine proteases of Trichomonas vaginalis degrade secretory leukocyte protease inhibitor. J. Infect. Dis. 178815-819. [DOI] [PubMed] [Google Scholar]
- 14.Everett, R. D. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 22761-770. [DOI] [PubMed] [Google Scholar]
- 15.Everett, R. D., C. Boutell, and A. Orr. 2004. Phenotype of a herpes simplex virus type 1 mutant that fails to express immediate-early regulatory protein ICP0. J. Virol. 781763-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farquhar, C., T. C. VanCott, D. A. Mbori-Ngacha, L. Horani, R. K. Bosire, J. K. Kreiss, B. A. Richardson, and G. C. John-Stewart. 2002. Salivary secretory leukocyte protease inhibitor is associated with reduced transmission of human immunodeficiency virus type 1 through breast milk. J. Infect. Dis. 1861173-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fichorova, R. N., and D. J. Anderson. 1999. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol. Reprod. 60508-514. [DOI] [PubMed] [Google Scholar]
- 18.Freeman, E. E., H. A. Weiss, J. R. Glynn, P. L. Cross, J. A. Whitworth, and R. J. Hayes. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 2073-83. [DOI] [PubMed] [Google Scholar]
- 19.Goodkin, M. L., A. T. Ting, and J. A. Blaho. 2003. NF-κB is required for apoptosis prevention during herpes simplex virus type 1 infection. J. Virol. 777261-7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray, R. H., X. Li, M. J. Wawer, D. Serwadda, N. K. Sewankambo, F. Wabwire-Mangen, T. Lutalo, N. Kiwanuka, G. Kigozi, F. Nalugoda, M. P. Meehan, M. Robb, and T. C. Quinn. 2004. Determinants of HIV-1 load in subjects with early and later HIV infections, in a general-population cohort of Rakai, Uganda. J. Infect. Dis. 1891209-1215. [DOI] [PubMed] [Google Scholar]
- 21.Hazrati, E., B. Galen, W. Lu, W. Wang, Y. Ouyang, M. J. Keller, R. I. Lehrer, and B. C. Herold. 2006. Human alpha- and beta-defensins block multiple steps in herpes simplex virus infection. J. Immunol. 1778658-8666. [DOI] [PubMed] [Google Scholar]
- 22.Hiemstra, P. S., R. J. Maassen, J. Stolk, R. Heinzel-Wieland, G. J. Steffens, and J. H. Dijkman. 1996. Antibacterial activity of antileukoprotease. Infect. Immun. 644520-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hook, L. M., J. M. Lubinski, M. Jiang, M. K. Pangburn, and H. M. Friedman. 2006. Herpes simplex virus type 1 and 2 glycoprotein C prevents complement-mediated neutralization induced by natural immunoglobulin M antibody. J. Virol. 804038-4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jana, N. K., L. R. Gray, and D. C. Shugars. 2005. Human immunodeficiency virus type 1 stimulates the expression and production of secretory leukocyte protease inhibitor (SLPI) in oral epithelial cells: a role for SLPI in innate mucosal immunity. J. Virol. 796432-6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.John, M., M. J. Keller, E. H. Fam, N. Cheshenko, K. Hogarty, A. Kasowitz, S. Wallenstein, M. J. Carlucci, A. C. Tuyama, W. Lu, M. E. Klotman, R. I. Lehrer, and B. C. Herold. 2005. Cervicovaginal secretions contribute to innate resistance to herpes simplex virus infection. J. Infect. Dis. 1921731-1740. [DOI] [PubMed] [Google Scholar]
- 26.Johnson, P. A., A. Miyanohara, F. Levine, T. Cahill, and T. Friedmann. 1992. Cytotoxicity of a replication-defective mutant of herpes simplex virus type 1. J. Virol. 662952-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keller, M. J., E. Guzman, E. Hazrati, A. Kasowitz, N. Cheshenko, S. Wallenstein, A. L. Cole, A. M. Cole, A. T. Profy, C. R. Wira, K. Hogarty, and B. C. Herold. 2007. PRO 2000 elicits a decline in genital tract immune mediators without compromising intrinsic antimicrobial activity. AIDS 21467-476. [DOI] [PubMed] [Google Scholar]
- 28.Koelle, D. M., and A. Wald. 2000. Herpes simplex virus: the importance of asymptomatic shedding. J. Antimicrob. Chemother. 45(Suppl. T3)1-8. [DOI] [PubMed] [Google Scholar]
- 29.Leib, D. A. 2002. Counteraction of interferon-induced antiviral responses by herpes simplex viruses. Curr. Top. Microbiol. Immunol. 269171-185. [DOI] [PubMed] [Google Scholar]
- 30.Lin, R., R. S. Noyce, S. E. Collins, R. D. Everett, and K. L. Mossman. 2004. The herpes simplex virus ICP0 RING finger domain inhibits IRF3- and IRF7-mediated activation of interferon-stimulated genes. J. Virol. 781675-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma, G., T. Greenwell-Wild, K. Lei, W. Jin, J. Swisher, N. Hardegen, C. T. Wild, and S. M. Wahl. 2004. Secretory leukocyte protease inhibitor binds to annexin II, a cofactor for macrophage HIV-1 infection. J. Exp. Med. 2001337-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MasCasullo, V., E. Fam, M. J. Keller, and B. C. Herold. 2005. Role of mucosal immunity in preventing genital herpes infection. Viral Immunol. 18595-606. [DOI] [PubMed] [Google Scholar]
- 33.Mbopi-Keou, F. X., G. Gresenguet, P. Mayaud, H. A. Weiss, R. Gopal, M. Matta, J. L. Paul, D. W. Brown, R. J. Hayes, D. C. Mabey, and L. Belec. 2000. Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women: opportunities for intervention. J. Infect. Dis. 1821090-1096. [DOI] [PubMed] [Google Scholar]
- 34.McMahan, L., and P. A. Schaffer. 1990. The repressing and enhancing functions of the herpes simplex virus regulatory protein ICP27 map to C-terminal regions and are required to modulate viral gene expression very early in infection. J. Virol. 643471-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNeely, T. B., M. Dealy, D. J. Dripps, J. M. Orenstein, S. P. Eisenberg, and S. M. Wahl. 1995. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J. Clin. Investig. 96456-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNeely, T. B., D. C. Shugars, M. Rosendahl, C. Tucker, S. P. Eisenberg, and S. M. Wahl. 1997. Inhibition of human immunodeficiency virus type 1 infectivity by secretory leukocyte protease inhibitor occurs prior to viral reverse transcription. Blood 901141-1149. [PubMed] [Google Scholar]
- 37.Mossman, K. 2005. Analysis of anti-interferon properties of the herpes simplex virus type I ICP0 protein. Methods Mol. Med. 116195-205. [DOI] [PubMed] [Google Scholar]
- 38.Ohlsson, K., A. Bjartell, and H. Lilja. 1995. Secretory leucocyte protease inhibitor in the male genital tract: PSA-induced proteolytic processing in human semen and tissue localization. J. Androl. 1664-74. [PubMed] [Google Scholar]
- 39.Pillay, K., A. Coutsoudis, A. K. Agadzi-Naqvi, L. Kuhn, H. M. Coovadia, and E. N. Janoff. 2001. Secretory leukocyte protease inhibitor in vaginal fluids and perinatal human immunodeficiency virus type 1 transmission. J. Infect. Dis. 183653-656. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds, S. J., A. R. Risbud, M. E. Shepherd, J. M. Zenilman, R. S. Brookmeyer, R. S. Paranjape, A. D. Divekar, R. R. Gangakhedkar, M. V. Ghate, R. C. Bollinger, and S. M. Mehendale. 2003. Recent herpes simplex virus type 2 infection and the risk of human immunodeficiency virus type 1 acquisition in India. J. Infect. Dis. 1871513-1521. [DOI] [PubMed] [Google Scholar]
- 41.Samaniego, L. A., L. Neiderhiser, and N. A. DeLuca. 1998. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. J. Virol. 723307-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith, T. J., L. A. Morrison, and D. A. Leib. 2002. Pathogenesis of herpes simplex virus type 2 virion host shutoff (vhs) mutants. J. Virol. 762054-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soliman, T. M., R. M. Sandri-Goldin, and S. J. Silverstein. 1997. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J. Virol. 719188-9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song, X., L. Zeng, W. Jin, J. Thompson, D. E. Mizel, K. Lei, R. C. Billinghurst, A. R. Poole, and S. M. Wahl. 1999. Secretory leukocyte protease inhibitor suppresses the inflammation and joint damage of bacterial cell wall-induced arthritis. J. Exp. Med. 190535-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taggart, C. C., S. A. Cryan, S. Weldon, A. Gibbons, C. M. Greene, E. Kelly, T. B. Low, S. J. O'Neill, and N. G. McElvaney. 2005. Secretory leucoprotease inhibitor binds to NF-kappaB binding sites in monocytes and inhibits p65 binding. J. Exp. Med. 2021659-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomee, J. F., P. S. Hiemstra, R. Heinzel-Wieland, and H. F. Kauffman. 1997. Antileukoprotease: an endogenous protein in the innate mucosal defense against fungi. J. Infect. Dis. 176740-747. [DOI] [PubMed] [Google Scholar]
- 47.Tuyama, A. C., N. Cheshenko, M. J. Carlucci, J. H. Li, C. L. Goldberg, D. P. Waller, R. A. Anderson, A. T. Profy, M. E. Klotman, M. J. Keller, and B. C. Herold. 2006. ACIDFORM inactivates herpes simplex virus and prevents genital herpes in a mouse model: optimal candidate for microbicide combinations. J. Infect. Dis. 194795-803. [DOI] [PubMed] [Google Scholar]
- 48.Umbach, J. L., M. F. Kramer, I. Jurak, H. W. Karnowski, D. M. Coen, and B. R. Cullen. 2 July 2008. MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature doi: 10.1038/nature07103. [DOI] [PMC free article] [PubMed]
- 49.Van Sant, C., R. Hagglund, P. Lopez, and B. Roizman. 2001. The infected cell protein 0 of herpes simplex virus 1 dynamically interacts with proteasomes, binds and activates the cdc34 E2 ubiquitin-conjugating enzyme, and possesses in vitro E3 ubiquitin ligase activity. Proc. Natl. Acad. Sci. USA 988815-8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wahl, S. M., T. B. McNeely, E. N. Janoff, D. Shugars, P. Worley, C. Tucker, and J. M. Orenstein. 1997. Secretory leukocyte protease inhibitor (SLPI) in mucosal fluids inhibits HIV-I. Oral Dis. 3(Suppl. 1)S64-S69. [DOI] [PubMed] [Google Scholar]
- 51.Watson-Jones, D., H. A. Weiss, M. Rusizoka, J. Changalucha, K. Baisley, K. Mugeye, C. Tanton, D. Ross, D. Everett, T. Clayton, R. Balira, L. Knight, I. Hambleton, J. Le Goff, L. Belec, and R. Hayes. 2008. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N. Engl. J. Med. 3581560-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wex, T., G. Treiber, M. Venerito, A. Leodolter, U. Peitz, D. Kuester, I. Hritz, S. Krueger, A. Roessner, and P. Malfertheiner. 2006. Helicobacter pylori-induced downregulation of the secretory leukocyte protease inhibitor (SLPI) in gastric epithelial cell lines and its functional relevance for H. pylori-mediated diseases. Biol. Chem. 387893-901. [DOI] [PubMed] [Google Scholar]
- 53.Xu, F., M. R. Sternberg, B. J. Kottiri, G. M. McQuillan, F. K. Lee, A. J. Nahmias, S. M. Berman, and L. E. Markowitz. 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296964-973. [DOI] [PubMed] [Google Scholar]