Abstract
Adaptive CD4+ and CD8+ T-cell responses have been associated with control of human immunodeficiency virus/simian immunodeficiency virus (HIV/SIV) replication. Here, we have designed a study with Indian rhesus macaques to more directly assess the role of CD8 SIV-specific responses in control of viral replication. Macaques were immunized with a DNA prime-modified vaccinia virus Ankara (MVA)-SIV boost regimen under normal conditions or under conditions of antibody-induced CD4+ T-cell deficiency. Depletion of CD4+ cells was performed in the immunized macaques at the peak of SIV-specific CD4+ T-cell responses following the DNA prime dose. A group of naïve macaques was also treated with the anti-CD4 depleting antibody as a control, and an additional group of macaques immunized under normal conditions was depleted of CD8+ T cells prior to challenge exposure to SIVmac251. Analysis of the quality and quantity of vaccine-induced CD8+ T cells demonstrated that SIV-specific CD8+ T cells generated under conditions of CD4+ T-cell deficiency expressed low levels of Bcl-2 and interleukin-2 (IL-2), and plasma virus levels increased over time. Depletion of CD8+ T cells prior to challenge exposure abrogated vaccine-induced protection as previously shown. These data support the notion that adaptive CD4+ T cells are critical for the generation of effective CD8+ T-cell responses to SIV that, in turn, contribute to protection from AIDS. Importantly, they also suggest that long-term protection from disease will be afforded only by T-cell vaccines for HIV that provide a balanced induction of CD4+ and CD8+ T-cell responses and protect against early depletion of CD4+ T cells postinfection.
In mice, a brief encounter with antigen can initiate programmed differentiation in CD8+ T cells that leads to T-cell clonal expansion and acquisition of effector function (2, 3, 21). Effector T-cell populations at the peak of CD8+ T-cell responses are programmed to undergo antigen-independent contraction, with a small portion of these cells persisting to constitute the long-term memory T-cell population (22, 41, 49). Thus, during an immune response to an infection, intrinsic external signals regulate the quality and frequency of long-term memory CD8+ T-cell responses.
In nonhuman primates, the currently available vaccine candidates for human immunodeficiency virus (HIV) do not protect from infection, and their ability to confer long-term protection from disease likely will depend on the elicitation of durable CD8+ and CD4+ T-cell responses. Vaccines based on the combination of DNA and poxvirus elicit broad simian immunodeficiency virus (SIV)-specific CD4+ and CD8+ T-cell responses, fail to prevent infection, but decrease viral replication and delay disease (15-17). In previous studies, the reduction of virus levels correlated with the levels of both SIV-specific CD4+ and CD8+ T-cell responses induced by vaccination, and the level of virus-specific CD8+ T cells correlated with the level of CD4+ T cells (15), suggesting the importance of CD4+ help in the induction of long-term memory CD8+ T cells. Importantly, the frequency of vaccine-induced Gag-specific central memory, but not effector memory, CD8+ T cells inversely correlated with virus levels (47).
In mice, CD4+ T-cell help is not required for priming of CD8+ T-cell responses against pathogens but is required for optimal maintenance of protective CD8+ memory (43). Indeed, upon reencounter with antigen, “helpless” CD8+ T cells undergo apoptosis mediated by tumor necrosis factor-related apoptosis-inducing ligand (44, 20, 34). Memory CD8+ T cells and secondary responses to bacterial or viral challenge are decreased over time in CD4+ T-cell-deficient animals (13, 29, 45).
These issues are particularly relevant in the development of vaccines for SIV/HIV type 1 (HIV-1) (50) since both of these pathogens induce significant depletion of CD4+ T cells early in infection (10, 24, 29, 32, 33, 48), recapitulating, in principle, the priming of virus-specific CD8+ T-cell responses under conditions of CD4+ T-cell deficiency.
Here, we have used a combination of DNA and recombinant modified vaccinia virus Ankara-SIV (MVA-SIV) vaccine as a model to address more directly how SIV-specific CD4+ T cells induced by the DNA prime dose influence the maintenance of long-lasting memory CD8+ T cells and protection from high levels of viral replication. We depleted CD4+ T cells at the time of the DNA-induced maximal expansion of SIV-specific CD4+ T cells and evaluated the quantity and quality of SIV-specific CD8+ T-cell responses induced by MVA-SIV boost. In addition, we evaluated the kinetics of CD8+ T-cell responses and the ability of SIV-specific CD8+ T cells to produce cytokines following challenge exposure to SIVmac251. CD4 depletion during immunization resulted in CD8+ T cells that express lower levels of Bcl-2 and interleukin-2 (IL-2) and a progressive loss of control of viral replication. In a parallel experiment, we controlled for the importance of total CD8+ T cells, including SIV-specific CD8+ T cells, by performing depletion of CD8+ cells in immunized macaques prior to challenge exposure to SIVmac251. CD8+ T-cell depletion in immunized macaques resulted in the loss of vaccine-induced protection and accelerated death. Together, these data suggest the importance of adaptive CD4+ and CD8+ T-cell responses in the control of SIV/HIV replication and indicate that SIV/HIV, by destroying CD4+ T cells at an early stage of infection, poses a formidable challenge for the long-term protection from disease afforded by T-cell vaccines.
MATERIALS AND METHODS
Animals, treatments, and SIVmac251 challenge.
All animals used in this study were colony-bred rhesus macaques (Macaca mulatta) obtained from Covance Research Products (Alice, TX). The animals were housed and handled in accordance with the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care International. The care and use of the animals were in compliance with all relevant institutional (NIH) guidelines.
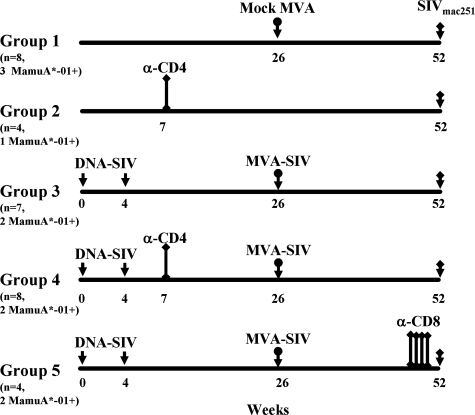
Thirty-one macaques were enrolled and divided into five groups, as indicated in Fig. 1. Macaques in groups 3, 4, and 5 were immunized at weeks 0 and 4 with 3 mg and 1 mg, by the intramuscular and intradermal routes, respectively, of SIV DNA containing Retanef (a vaccine encoding the SIV Rev, Tat, and Nef genes) and SIV DNA containing gag, pol, and env. At week 26 these animals were boosted with MVA-SIV-Retanef and MVA-SIV-gag-pol-env given intramuscularly at a dose of 1 × 108 PFU. Groups 2 and 4 received a single intravenous inoculation of 50 mg/kg of body weight of OKT4A-immunoglobulin G1 ([IgG1] 12F11) 3 weeks after the second immunization with DNA (week 7). Group 5 received one inoculation of 10 mg/kg of the mouse-human chimeric monoclonal antibody directed to CD8+ T cells (cM-T807) 3 days before the challenge by the subcutaneous route and two intravenous inoculations at a dose of 5 mg/kg, one at the time of the challenge and the other 5 days following challenge exposure to SIVmac251.
FIG. 1.
Study design. Immunization regimens. The total numbers of animals per group and of MamuA*-01+ macaques included in each group are indicated in parentheses. A single inoculation of the anti-CD4 antibody was given 45 weeks before challenge exposure to macaques in groups 2 and 4. Four inoculations of the anti-CD8 antibody were given before challenge exposure to animals in group 5. α, anti.
CD4+ and CD8+ T-cell counts.
CD4+ and CD8+ T-cell counts were periodically determined from whole blood and by fluorescence-activated cell sorter (FACS) analysis, according to a FACS/Lyse kit (BD Immunocytometry Systems, San Jose, CA), and analyses were performed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). The antibodies used were anti-CD3 (SP34; BD Biosciences), anti-CD4 (L200; BD Biosciences), anti-CD8 (DK25; DakoCytomation, Carpinteria, CA), and anti-CD20 (B9E9; Beckman Coulter, Fullerton, CA).
Viral RNAs.
SIVmac251 in plasma was quantified by nucleic acid sequence-based amplification (40). Briefly, RNA was extracted from plasma and tissues, as previously described (35), and isothermally amplified using SIVmac251-specific primers. Quantification was performed by using an electro-chemiluminescence chemistry-based probe hybridization system with a coextracted internal standard. The copy number was expressed per 100 μl of plasma or per microgram of RNA, and the detection limit of the assay was 2 × 103 RNA copies.
Cell-associated viral loads in sorted subsets of naïve and memory CD4+ T cells (discriminated on the basis of CD45RA and CD95 expression) were determined by a quantitative PCR assay for SIV gag using a Perkin-Elmer ABI 7700 instrument (PerkinElmer, Waltham, MA). The SIV gag primers and probe used were previously described (29, 38).
Lymphocyte proliferation assay.
Peripheral blood mononuclear cells were cultured at 105 cells per well in triplicates for 3 days in the absence or presence of native high-performance liquid chromatography-purified SIV p27 Gag or gp120 Env proteins (Advanced BioScience Laboratories, Kensington, MD) or concavalin-A, as positive control. The cells were then pulsed overnight with 1 μCi of [3H]thymidine before harvest. The relative rate of lymphoproliferation was calculated as the increase of thymidine incorporation into cellular DNA relative to the medium control (stimulation index).
ELISPOT assay.
Monkey gamma interferon (IFN-γ)-specific enzyme-linked immunospot (ELISPOT) assay kits manufactured by U-Cytech (Utrecht, The Netherlands) were used. Ninety-six-well, flat-bottom plates were coated with anti-IFN-γ monoclonal antibody MD-1 overnight at 4°C and blocked with 2% bovine serum albumin in phosphate-buffered saline for 1 h at 37°C. A total of 105 cells/well were loaded in triplicate in RPMI 1640 medium containing 5% human serum and 10 μg/ml of a specific peptide pool (15-mer overlapping by 11 amino acids encompassing the full SIV Gag protein). Analyses were performed by counting the spots with a microscope and using the software KS ELISPOT (Carl Zeiss Vision GmbH, Hallbergmoos, Germany).
Detection of anti-SIVmac251-binding and -neutralizing antibodies.
To detect anti-SIVmac251-binding antibodies, serial dilutions of plasma were incubated with the lysate of SIVmac251 spiked with native purified gp120 Env protein of SIVmac251 bound to microtiter enzyme-linked immunosorbent assay plates, as described elsewhere (16). Endpoint titers were defined as the reciprocal of the highest serum dilution that gave an optical absorbency at 450 nm at least 2 standard deviations greater than average values obtained with negative control serum.
Antibodies and flow cytometry.
For the physical detection of SIV-specific CD8+ T cells, staining was performed with anti-human CD8β antibody (clone 2ST8.5H7; Beckman Coulter), anti-human CD28 antibody (clone CD28.2; Pharmingen, San Diego, CA), anti-human CD95 antibody (clone DX2, BD Pharmingen), anti-human Bcl-2 antibody (clone 100; Caltag), and with pretitered, allophycocyanin (APC)-conjugated (Molecular Probes) Gag181-189 CM9 (p11C; CTPYDINQM)-Mamu-A*01 tetrameric complexes (Beckman Coulter) for 30 min at room temperature (23). For the characterization of CD4+ T cells, anti-human Ki67 (clone B56; BD Pharmingen) and CCR5 (clone 3A9; BD Pharmingen) antibodies were used. A total of 100,000 events were collected in the lymphocyte region and analyzed with CellQuest software (BD Biosciences).
For phenotypic analysis, freshly isolated cells were labeled simultaneously with the following combinations of antibodies: CD3Cy7-APC, CD8-Cy5.5-phycoerythrin (PE), CD4-cascade blue, CD45RA-Texas Red PE, CD95-APC, CCR5-PE, CD11a-Cy7-PE. SIV Gag-specific responses were determined using overlapping peptides as previously described (39, 4, 25). All antibodies were purchased from BD Pharmingen and validated and titrated using rhesus macaque peripheral blood mononuclear cells. Control cultures were set up for each sample without SIV peptides. Following stimulation, cells were labeled with cell surface markers (CD3, CD4, CD8, CD28, and CD95) and Vivid (amine-reactive dye to discriminate live/dead cells) (36). After cells were fixed (Fix/Perm kit; Pharmingen), they were permeabilized and labeled with IL-2-PE, IFN-γ-fluorescein isothiocyanate, and tumor necrosis factor alpha (TNF-α)-Cy7-PE (Pharmingen). Labeled cells were fixed with 0.5% paraformaldehyde and analyzed using either an LSR II or a modified Becton Dickinson Digital Vantage. Data were analyzed using FlowJo, version 6.1 (Tree Star, Inc., Ashland, OR) and SPICE, version 4.1.5 (M. Roederer, Vaccine Research Center, NIH, Bethesda, MD).
RESULTS
In vivo CD4+ and CD8+ T-cell depletion.
CD8+ T-cell depletion of SIV-infected animals causes an increase in plasma virus levels suggesting that CD8+ T cells participate in the control of viral replication. However, this effect cannot be directly ascribed to adaptive CD8+ cytotoxic T-lymphocyte (CTL) responses because of the presence in rhesus macaques of a sizeable number of NK CD8+ cells (31).
We therefore designed a study in the SIVmac251 model to investigate more directly the importance of SIV-specific CD8+ T cells in the control of SIV replication. Thirty-one Indian rhesus macaques were enrolled and divided into five groups (Fig. 1). The control groups 1 and 2 were mock immunized with the MVA vector (group 1) or left naïve (group 2). Macaques in group 2 were depleted of CD4+ T cells at the time indicated in Fig. 1, using the OKT4A-IgG1 antibody. Animals in groups 3, 4, and 5 were all immunized with two inoculations of SIV-DNA containing gag, pol, env, and Retanef, a chimeric vaccine encoding SIV regulatory genes (14), followed by a boost with a highly attenuated recombinant MVA-based poxvirus expressing the same antigens. Group 3 did not receive any depleting antibody treatment; group 4 was treated with the OKT4A antibody at 3 weeks after the second DNA immunization, the time at which maximal expansion of CD4+ helper cells occurs (15, 16). The importance of CD8+ cells (natural killer, NK, and adaptive CD8+ T cells) was investigated by treating macaques in group 5 with the cM-T807 antibody prior to challenge exposure. All groups were challenged with SIVmac251 by the intrarectal route, as indicated in Fig. 1.
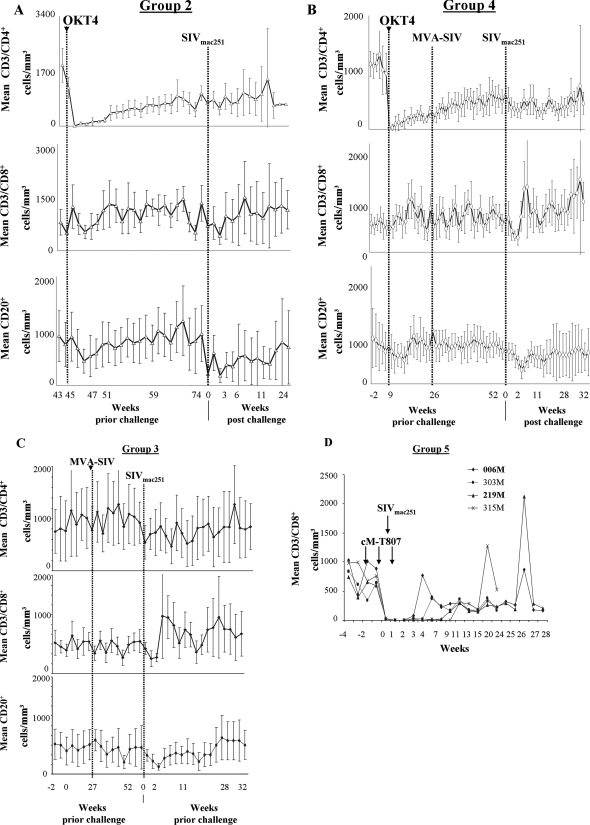
Treatment with the OKT4A-IgG1 antibody resulted in depletion of CD4+ T cells in the blood of macaques from groups 2 and 4, and the number of CD8+ T cells or CD20+ B cells was not affected by this treatment (Fig. 2A and B). The number of CD4+ T cells in the blood of these animals was reconstituted slowly over time, as also observed in transplant patients (18).
FIG. 2.
In vivo depletion of T-cell subsets. Absolute CD3+ CD4+, CD3+ CD8+, and CD20+ T-cell counts per mm3 of blood in macaques treated with the anti-CD4 antibody (OKT4A-IgG1) (A and B), left untreated (C), and treated with the anti-CD8 antibody (cM-T807) (D) are shown. Arrows represent times of inoculation of CM-T807 and challenge exposure to SIV. Results for MamuA*-01+ macaques in group 5 are shown in bold.
As expected, the number of CD8+ T cells, CD4+ T cells, or B cells did not change in the vaccinated macaques in group 3 that were not treated with antibodies (Fig. 2C), and treatment with the anti CD8 cM-T807 antibody resulted in a transient depletion of CD8+ T cells in blood of all macaques in group 5 (Fig. 2D), consistent with results by other investigators using this antibody (27, 42).
Ki67+ T cells in CD4+- and CD8+-depleted macaques.
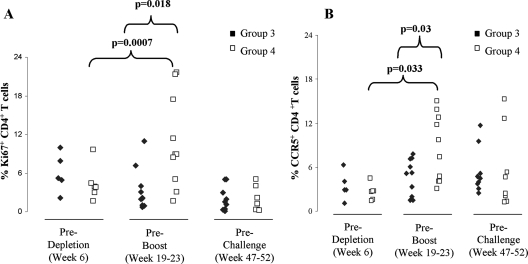
As depletion of T-cell subsets could result in a homeostatic proliferation of T cells that, in turn, could influence viral replication following challenge exposure to SIV, we monitored Ki67 expression on CD4+ T cells over time in macaques that underwent either CD4+ or CD8+ T-cell depletion. CD4+ T-cell depletion was followed by a significant increase in T-cell proliferation in macaques from group 4, as expected (Fig. 3A); however, at time of challenge, there was no difference in the level of Ki67 expression on CD4+ T cells between macaques from groups 3 and 4. Similar results were obtained when the number of activated CCR5+ CD4+ T cells was measured (Fig. 3B). In macaques that underwent CD8+ T-cell depletion, we found no significant difference in the numbers of Ki67+ T cells, but we observed a decrease in CCR5+ CD4+ T cells, likely because of the increase in viral replication observed in this group during primary infection (data not shown).
FIG. 3.
Frequency of Ki67+ and CCR5+ CD4+ T cells in blood of macaques either untreated or treated with the OKT4A-IgG1 depleting antibody. (A) Percentage of Ki67+ CD4+ T cells at weeks 6, 19 to 23, and 47 to 52 in blood of macaques from groups 3 (untreated) and 4 (treated). (B) Percentage of CCR5+ CD4+ T cells at weeks 6, 19 to 23, and 47 to 52 in blood of macaques from groups 3 (untreated) and 4 (treated).
Depletion of CD8+ T cells prior to challenge exposure in immunized macaques or depletion of CD4+ T cells during immunization results in decreased protection from SIVmac251.
Challenge exposure of mock-vaccinated (group 1) and unvaccinated CD4-depleted macaques (group 2) resulted in infection of all macaques, and no significant difference was observed in plasma virus levels between the two groups (Fig. 4A). This result was expected since the level of CD4+ T cells in the animals in group 2 was up to 1,000/mm3 prior to challenge exposure, and normal levels of T-cell activation were observed at that time (Fig. 2A and data not shown). Therefore, these two control groups were combined in further analyses. The vaccinated macaques in group 3 demonstrated a significant decrease in plasma virus levels compared to the mock-vaccinated groups 1 and 2 (weeks 1 to 4; P = 0.003) (Fig. 4B and E). In contrast, macaques that were depleted of CD8+ T cells failed to control viral replication during primary infection, and their plasma levels were equivalent to those of the control animals in groups 1 and 2 (Fig. 4D and E). Interestingly, the macaques in this group progressed to disease faster than the mock-vaccinated group, and three of four macaques succumbed to disease within 27 weeks from SIVmac251 infection. These data confirm that CD8+ T-cell-mediated responses, either native or adaptive, contribute to the control of viral replication.
FIG. 4.
Levels of viral RNA in plasma following challenge exposure to SIVmac251. Plasma virus levels in each macaque from groups 1 (mock infected) and 2 (mock infected and T-cell depleted) (A), group 3 (vaccinated) (B), group 4 (vaccinated and CD4+ T-cell depleted) (C), group 5 (vaccinated and CD8+ T-cell depleted) (D) are shown. The asterisk refers to the MamuA*-01+ macaques. (E) Comparative analysis of the geometrical mean plasma virus levels in macaques from groups 1 and 2 versus groups 3, 4, and 5. Comparative analysis of the geometrical mean plasma virus levels in Mamu-A*01+ (F) and Mamu-A*01− (G) macaques within groups 3 and group 4. Depl, depleted; vacc, vaccinated.
Challenge exposure of macaques in group 4 resulted in significantly lower plasma virus levels than those of macaques in group 1 and 2 during primary viremia (Fig. 4C and E). Analysis of virus plasma levels in macaques from groups 3 and 4 did not differ significantly during primary infection, demonstrating that vaccination also protected the macaques that were subjected to CD4+ T-cell depletion from high virus levels following the DNA prime dose (Fig. 4E). However, with time, we noticed a progressive increase in plasma virus in macaques in group 4, suggesting a progressive loss of control of SIV replication. This result was also confirmed when Mamu-A*01-negative (Mamu-A*01−) and Mamu-A*01-positive (Mamu-A*01+) macaques were analyzed independently (Fig. 4F and G). The difference in virus load was more evident among the MamuA*-01+ macaques, as expected, given their ability to control SIVmac251 replication through a major histocompatibility complex class I-mediated mechanism.
To validate the difference in the virus levels observed in the plasma of macaques in groups 3 and 4, we obtained blood and lymph nodes at weeks 6 and 24 following challenge exposure. We sorted total or SIV-specific memory CD4+ T cells and quantified the amount of SIV gag DNA in these cells. At 6 weeks, following challenge exposure, no differences were observed in the amount of SIV gag in either total CD4+ T cells (Fig. 5A) or SIV-specific naïve or memory CD4+ T cells (Fig. 5B) in lymph nodes from macaques from groups 1, 3, and 4. In contrast, at week 24, a significantly higher level of SIV gag DNA was found in SIV-specific CD4+ T cells from both blood and lymph nodes of macaques in group 4 (Fig. 5C). Analysis of the sorted subsets of naïve or memory CD4+ T cells also revealed a significant increase of viral DNA in the macaques from group 4 compared to those of group 3 (Fig. 5D).
FIG. 5.
Virus level in tissues of macaques mock vaccinated, vaccinated, and vaccinated under conditions of CD4 deficiency. Mean SIV Gag copies in total CD4+ T cells (A) or CD4+ T-cell subsets (B) from lymph nodes (LN) at week 6 are shown. The standard deviation is derived from a total of five animals in group 3, four animals in group 4, and two animals in group 1. SIV Gag copies in total CD4+ T cells (C) or CD4+ T-cell subsets (D) in lymph nodes or blood at week 24 are shown. The standard deviation is derived from a total of three animals in group 3, and three animals in group 4. One animal in group 1 was analyzed at this time point. CM, central memory; TM, total memory CD4 T cells.
Altogether, these data support the notion that the difference in viral replication observed in these animals was not due to different levels of T-cell activation per se; rather, macaques that amplified their CD8+ T cells under conditions of CD4+ T-cell help deficiency failed to control virus replication over time.
Qualitative and quantitative differences in the SIV-specific immune response in macaques immunized in the absence of CD4+ T cells prior to challenge exposure to SIVmac251.
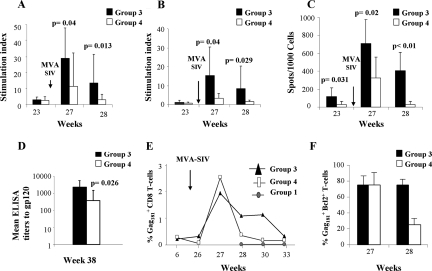
The data presented above provided a unique opportunity to assess how the quantity and quality of immune responses relate to viral replication within the same animals over time. Thus, we investigated at first how immunization in the absence of CD4+ T cells influenced the development and maintenance of a SIV-specific adaptive memory CD8+ T-cell response, particularly of the central memory type (37, 47). Proliferative responses to both the SIV-p27 Gag and the SIV-gp120 Env proteins were measured in all animals from group 4 at 3 weeks before (week 23), and at 1 or 2 weeks after (weeks 27 and 28) the boost with MVA-SIV (Fig. 1) and compared to animals in group 3. As expected, the proliferative response to p27 Gag was significantly lower at weeks 27 and 28 in macaques that were boosted in the absence of CD4+ T cells (group 4) than in group 3 macaques that were vaccinated under normal conditions (P = 0.04 by the exact Wilcoxon rank test) (Fig. 6A). Similarly, proliferative response to SIV Env was also significantly lower (P = 0.04 at week 27 and P = 0.02 at week 28) in group 4 than in group 3 (Fig. 6B). Proliferation to subunit antigens has been used as a surrogate for IL-2 production, and their reduction in macaques immunized in the absence of CD4+ T cells goes hand in hand with a reduced CD4+ T-helper response.
FIG. 6.
Immune responses to SIV following vaccination. Proliferative responses induced by the MVA boost to p24 Gag (A) and the gp120 Env (B). (C) ELISPOT assay of Gag in blood of the immunized macaques (D) Titers of serum antibodies to gp120 following vaccination with MVA-SIV. (E) Frequency of Gag181-189 (Gag181) tetramer-positive CD8+ T cells in blood of macaques from groups 1, 3, and 4 before and after booster immunization with MVA-SIV. (F) Percentage of Gag181-189 tetramer-positive T cells expressing Bcl-2 at 1 or 2 weeks (weeks 27 and 28) following booster immunization with MVA-SIV. ELISA, enzyme-linked immunosorbent assay.
ELISPOT for IFN-γ responses to SIV gag also differed between the macaques from groups 3 and 4, and the difference was significant at week 23 (Fig. 6C), suggesting that the maintenance of DNA-induced immune responses was affected by CD4 depletion during immunization. Differences between the two groups continued to be significant after the MVA boost (week 27, P = 0.031; week 28, P < 0.01) (Fig. 6C). Consistent with a decrease in CD4 help, the titers of antibody to SIV gp120 were also significantly decreased (P = 0.026) in the macaques from group 4 (Fig. 6D).
The extent and quality of CD8+ T cells specific for SIV gag was quantified by tetramer staining in Mamu-A*01+ animals from groups 3 and 4. At week 27, which was 1 week following the MVA-SIV boost, the frequency of SIV Gag181-189-specific CD8+ T cells was found to expand similarly in both groups. However, following the MVA-SIV boost, the contraction of this response was faster in macaques boosted in the absence of CD4+ T cells (group 4) than in macaques vaccinated under normal conditions (group 3) (Fig. 6E); a qualitative analysis of the two subpopulations showed that in contrast to group 4, a larger fraction of tetramer+ CD8+ T cells in group 3 expressed Bcl-2 at week 28 (Fig. 6F) (45). Bcl-2 is an antiapoptotic gene whose expression has been described to be higher in central memory T (TCM) cells than effector memory T cells. Accordingly, when the absolute number of CD28+ CD95+ or CD28− CD95+ tetramer+ T cells was measured at week 33 in both groups, TCM cells were predominant only in the macaques from group 3 (data not shown), but this difference did not reach statistical significance because of the small number of Mamu-A*01+ macaques included in these studies.
Qualitative and quantitative differences in SIV-specific CD8+ T cells in macaques immunized in the absence of CD4+ T cells following exposure to SIVmac251.
Following challenge exposure to SIVmac251, we found a difference in the kinetics of expansion of SIV gag tetramer responses in blood of all animals, and this response contracted faster in the macaques in group 4 that were boosted by MVA under conditions of CD4+ T-cell deficiency (Fig. 7A). However, once more, because of the small number of Mamu-A*01+ macaques included in the studies, we could not demonstrate a significant qualitative difference in the frequency of TCM and effector memory T cells (data not shown).
FIG. 7.
Immune responses to SIV following challenge exposure. (A) Expansion of Gag181-189 (Gag181) tetramer-positive CD8+ T cells in blood of macaques from groups 1, 3, and 4 following challenge exposure to SIVmac251. (B) SIV Gag-specific IFN-γ, TNF-α, and IL-2 production in CD8+ and CD4+ T-cell responses in blood and lymph nodes from macaques 898L, 899L, 905L, 215M, 881L, 317M, 319M, 222M, 550M, 556M, 564M, 217M, 220M, 901L, 223M, 225M, 298M, and 299M, obtained at week 6 following SIVmac251 challenge exposure. (C) Quantification of immune responses in purified mononuclear cells collected from blood and lymph nodes of macaques 889L, 898L, 215M, 217M, 220M, 223M, and 22H obtained at week 24 (left). SIV Gag-specific IFN-γ, TNF-α, and IL-2 production in CD8+ and CD4+ T-cell responses at week 24 in blood of the same macaques.
IL-2 production is a hallmark of TCM cells (26), and polyfunctional CD8+ T-cell responses have been correlated with a better response to HIV (38). We evaluated the qualitative and quantitative differences in SIV-specific immune responses by multicolor flow cytometry in lymph nodes and blood that were collected at weeks 6 and 24 after challenge exposure.
At week 6, following challenge exposure to SIVmac251, neither the quantity (data not shown) nor the quality (Fig. 7B) of SIV-specific CD4+ or CD8+ T-cell responses differed among macaques immunized under normal conditions (group 3) or under conditions of CD4+ T-cell deficiency (group 4); and even though production of IFN-γ prevailed, IL-2 responses were readily measurable in both groups. In contrast, no IL-2 response was detected in the mock-vaccinated macaques (group 1). Interestingly, at week 24 we observed both quantitative and qualitative differences in macaques that were immunized in the absence of CD4+ T cells compared to animals that were immunized under normal conditions. Animals in group 4 had exclusively IFN-γ and TNF-α functional responses in both CD4+ and CD8+ T-cell subsets (Fig. 7C), whereas macaques in group 3 maintained significantly higher polyfunctional responses in both CD4+ and CD8+ T cells (IFN-γ, TNF-α, and IL-2). Because at this time point (week 24) there was also a significantly higher level of virus in tissues of macaques from group 4 (Fig. 4D and E), these data provide further support to the notion that polyfunctional rather than monofunctional CD4+ and CD8+ T-cell responses better correlate with control of SIV/HIV replication. However, an alternative explanation could also be that the higher virus level in the tissues of macaques in group 4 induced monofunctional responses.
DISCUSSION
Evolution has armed pathogens with strategies to evade immune recognition. Several viruses, including HIV, encode proteins that affect both innate and adaptive T-cell responses (1). HIV is able to spread from cell to cell, integrate into the host chromosomes, and become latent. Thus, evading the CTL response is crucial for persistence in the host and successful transmission. HIV infects activated CD4+ CCR5+ T cells that populate the gastrointestinal tract and causes their massive depletion by both direct and indirect mechanisms (12, 30). Thus, HIV-specific CD8+ T cells largely develop under conditions of CD4+ T-cell deficiency, as observed in CD4-depleted mice; the CTL responses are unable to clear the virus, and persistent infection is established (28). We have validated the impact of reduced CD4+ T-cell help on the generation of adaptive CD8+ T-cell responses in nonhuman primates. By depleting total CD4+ T cells during immunization, we also decreased SIV-specific CD4+ T cells induced by DNA immunization and changed the quality and persistence of the SIV-specific CD8+ T-cell-adaptive response. The impact of this treatment was also evaluated over the short and long term following challenge exposure of rhesus macaques to SIVmac251, a pathogenic virus that causes AIDS, in this nonhuman primate species (5). Our data clearly demonstrate that even though the expansion and efficacy of SIV-specific CD8+ T cells during primary viremia was not affected, the ability of these cells to persist and contain viral replication was greatly diminished over time in macaques immunized in the absence of CD4+ T cells. Importantly, the early contraction of CD8+ T-cell responses was associated with less control of viral replication several months later. This suggests that the initial containment of viremia may be due to the expansion of primary SIV-specific CD8+ T cells and that this may mask the inability of memory CD8+ T cells to expand and survive under similar circumstances. This clearly establishes the important role of SIV-specific CD4+ T cells in regulating the protective CD8+ T-cell responses in vivo after vaccination and suggests that the generation of HIV-specific CD4+ T cells will be a critical component of a successful HIV vaccine (15).
The data presented here further our understanding of SIV/HIV pathogenesis as they demonstrate the impact of early CD4+ T-cell depletion in AIDS pathogenesis. In the absence of antiretroviral therapy, the progressive destruction of helper CD4+ T cells for other pathogens is likely responsible for AIDS-defining opportunistic infections. Even if some degree of CD4+ T-cell repopulation occurs, there is a threshold below which AIDS occurs, and the ability to respond to vaccines is lost (6-8).
The data presented here stress the need for early treatment with antiretroviral therapy to curtail the loss of CD4+ T cells in tissues and explain the failure of therapeutic vaccination in SIV/HIV (11, 17, 19, 46) as adequate CD4+ T-cell counts need to be reconstituted. Thus, immune modulatory regimens able to repopulate the tissues of CD4+ T cells may be key to the success of therapeutic vaccines and to reversing the immunological damage caused by SIV/HIV.
Furthermore, our data underscore at least two difficult problems for HIV vaccines that cannot prevent infection. The first is that vaccines based on nonreplicating live vectors may be unable to maintain sufficient levels of CD8+ T cells at the portal of entry in order to prevent CD4+ T-cell loss during acute infection. The second is that because HIV replicates very fast, its spread from the gut or the genital tract to the systemic lymph nodes occurs long before the expansion of an HIV-specific CD8+ T-cell response occurs (39). Indeed, several lines of evidence indicate that while T-cell vaccines induce T-cell responses able to limit viral replication during primary viremia, their protective effect is not sustained (9). Thus, vaccines for HIV that are unable to elicit neutralizing antibodies should elicit CD8+ T-cell responses of sufficient magnitude and durability at the portal of entry of HIV/SIV to curtail CD4+ T-cell depletion. Perhaps this goal can be achieved only with safe attenuated vaccines that replicate in the gut; certainly, it will require the generation of a sufficient number of HIV-specific CD4+ T cells so as to provide the help necessary for long-term maintenance of the expanded CD8+ T-cell population. Hence, strategies aimed at eliciting effective T-cell responses to HIV by vaccination must broaden their scope beyond generation of antigen-specific CD8+ T cells to include optimization of the generation of CD4+ T cells of the right quality and magnitude.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, and the National Institute of Allergy and Infectious Diseases.
We thank V. S. Kalyanaraman for the detection of anti-SIVmac251 antibodies and P. D. Markham, S. Orndorff, D. Weiss, and J. Treece of Advanced BioScience Laboratories, Inc., Kensington, MD. We thank also Barbara Felber and George Pavlakis for providing the Gag, Pol, and Env DNA plasmids and Steven Snodgrass and Ryan Kelly for editorial assistance.
Author contributions were as follows: M.V. and G.F. designed the study, performed experiments, and wrote the paper; J.M., K.S., W.-P.T., and A.H. performed research; D.V., M.R., J.J.M., and K.S. performed analysis; and K.A.R. and M.R. contributed vital reagents and software for analysis, respectively. M.Z. helped with the study design and editing.
We declare that we have no competing financial interests.
Footnotes
Published ahead of print on 30 July 2008.
REFERENCES
- 1.Ambagala, A. P., J. C. Solheim, and S. Srikumaran. 2005. Viral interference with MHC class I antigen presentation pathway: the battle continues. Vet. Immunol. Immunopathol. 1071-15. [DOI] [PubMed] [Google Scholar]
- 2.Badovinac, V. P., K. A. Messingham, S. E. Hamilton, and J. T. Harty. 2003. Regulation of CD8+ T cells undergoing primary and secondary responses to infection in the same host. J. Immunol. 1704933-4942. [DOI] [PubMed] [Google Scholar]
- 3.Badovinac, V. P., B. B. Porter, and J. T. Harty. 2002. Programmed contraction of CD8(+) T cells after infection. Nat. Immunol. 3619-626. [DOI] [PubMed] [Google Scholar]
- 4.Betts, M. R., D. R. Ambrozak, and D. C. Douek. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 7511983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desrosiers, R. C. 1990. The simian immunodeficiency viruses. Annu. Rev. Immunol. 8557-578. [DOI] [PubMed] [Google Scholar]
- 6.Edghill-Smith, Y., H. Golding, J. Manischewitz, L. R. King, D. Scott, M. Bray, A. Nalca, J. W. Hooper, C. A. Whitehouse, J. E. Schmitz, K. A. Reimann, and G. Franchini. 2005. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 11740-747. [DOI] [PubMed] [Google Scholar]
- 7.Edghill-Smith, Y., M. Bray, C. A. Whitehouse, D. Miller, E. Mucker, J. Manischewitz, L. R. King, M. Robert-Guroff, A. Hryniewicz, D. Venzon, C. Meseda, J. Weir, A. Nalca, V. Livingston, J. Wells, M. G. Lewis, J. Huggins, S. H. Zwiers, H. Golding, and G. Franchini. 2005. Smallpox vaccine does not protect macaques with AIDS from a lethal monkeypox virus challenge. J. Infect. Dis. 191372-381. [DOI] [PubMed] [Google Scholar]
- 8.Edghill-Smith, Y., D. Venzon, T. Karpova, J. McNally, J. Nacsa, W. P. Tsai, E. Tryniszewska, M. Moniuszko, J. Manischewitz, L. R. King, S. J. Snodgrass, J. Parrish, P. Markham, M. Sowers, D. Martin, M. G. Lewis, J. A. Berzofsky, I. M. Belyakov, B. Moss, J. Tartaglia, M. Bray, V. Hirsch, H. Golding, and G. Franchini. 2003. Modeling a safer smallpox vaccination regimen, for human immunodeficiency virus type 1-infected patients, in immunocompromised macaques. J. Infect. Dis. 1881181-1191. [DOI] [PubMed] [Google Scholar]
- 9.Franchini, G., S. Gurunathan, L. Baglyos, S. Plotkin, and J. Tartaglia. 2004. Poxvirus-based vaccine candidates for HIV: two decades of experience with special emphasis on canarypox vectors. Exp. Rev. Vaccines 3(Suppl. 1)S75-S88. [DOI] [PubMed] [Google Scholar]
- 10.Gordon, S. N., N. R. Klatt, S. E. Bosinger, J. M. Brenchley, J. M. Milush, J. C. Engram, R. M. Dunham, M. Paiardini, S. Klucking, A. Danesh, E. A. Strobert, C. Apetrei, I. V. Pandrea, D. Kelvin, D. C. Douek, S. I. Staprans, D. L. Sodora, and G. Silvestri. 2007. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J. Immunol. 1793026-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorochov, G., A. U. Neumann, A. Kereveur, C. Parizot, T. Li, C. Katlama, M. Karmochkine, G. Raguin, B. Autran, and P. A. Debré. 1998. Perturbation of CD4+ and CD8+ T-cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nat. Med. 4215-221. [DOI] [PubMed] [Google Scholar]
- 12.Guadalupe, M., E. Reay, S. Sankaran, T. Prindiville, J. Flamm, A. McNeil, and S. Dandekar. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 7711708-11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton, S. E., M. C. Wolkers, S. P. Schoenberger, and S. C. Jameson. 2006. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat. Immunol. 7475-481. [DOI] [PubMed] [Google Scholar]
- 14.Hel, Z., J. M. Johnson, E. Tryniszewska, W. P. Tsai, R. Harrod, J. Fullen, J. Tartaglia, and G. Franchini. 2002. A novel chimeric Rev, Tat, and Nef (Retanef) antigen as a component of an SIV/HIV vaccine. Vaccine 203171-3186. [DOI] [PubMed] [Google Scholar]
- 15.Hel, Z., J. Nacsa, E. Tryniszewska, W. P. Tsai, R. W. Parks, D. C. Montefiori, B. K. Felber, J. Tartaglia, G. N. Pavlakis, and G. Franchini. 2002. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T cell responses. J. Immunol. 1694778-4787. [DOI] [PubMed] [Google Scholar]
- 16.Hel, Z., W. P. Tsai, E. Tryniszewska, J. Nacsa, P. D. Markham, M. G. Lewis, G. N. Pavlakis, B. K. Felber, J. Tartaglia, and G. Franchini. 2006. Improved vaccine protection from simian AIDS by the addition of nonstructural simian immunodeficiency virus genes. J. Immunol. 17685-96. [DOI] [PubMed] [Google Scholar]
- 17.Hel, Z., D. Venzon, M. Poudyal, W. P. Tsai, L. Giuliani, R. Woodward, C. Chougnet, G. Shearer, J. D. Altman, D. Watkins, N. Bischofberger, A. Abimiku, P. Markham, J. Tartaglia, and G. Franchini. 2000. Viremia control following antiretroviral treatment and therapeutic immunization during primary SIV251 infection of macaques. Nat. Med. 61140-1146. [DOI] [PubMed] [Google Scholar]
- 18.Ho, V. T., and R. J. Soiffer. 2001. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood 983192-3204. [DOI] [PubMed] [Google Scholar]
- 19.Hryniewicz, A., D. A. Price, M. Moniuszko, A. Boasso, Y. Edghill-Spano, S. M. West, D. Venzon, M. Vaccari, W. P. Tsai, E. Tryniszewska, J. Nacsa, F. Villinger, A. A. Ansari, C. J. Trindade, M. Morre, D. Brooks, P. Arlen, H. J. Brown, C. M. Kitchen, J. A. Zack, D. C. Douek, G. M. Shearer, M. G. Lewis, R. A. Koup, and G. Franchini. 2007. Interleukin-15 but not interleukin-7 abrogates vaccine-induced decrease in virus level in simian immunodeficiency virus mac251-infected macaques. J. Immunol. 1783492-3504. [DOI] [PubMed] [Google Scholar]
- 20.Janssen, E. M., E. E. Lemmens, T. Wolfe, U. Christen, M. G. von Herrath, and S. P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421852-856. [DOI] [PubMed] [Google Scholar]
- 21.Kaech, S. M., and R. Ahmed. 2001. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaech, S. M., J. T. Tan, E. J. Wherry, B. T. Konieczny, C. D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 41191-1198. [DOI] [PubMed] [Google Scholar]
- 23.Kuroda, M. J., J. E. Schmitz, D. H. Barouch, A. Craiu, T. M. Allen, A. Sette, D. I. Watkins, M. A. Forman, and N. L. Letvin. 1998. Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus-infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I-peptide complex. J. Exp. Med. 1871373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 4341148-1152. [DOI] [PubMed] [Google Scholar]
- 25.Maecker, H. T., H. S. Dunn, M. A. Suni, E. Khatamzas, C. J. Pitcher, T. Bunde, N. Persaud, W. Trigona, T. M. Fu, E. Sinclair, B. M. Bredt, J. M. McCune, V. C. Maino, F. Kern, and L. J. Picker. 2001. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J. Immunol. Methods 25527-40. [DOI] [PubMed] [Google Scholar]
- 26.Malek, T. R. 2002. T helper cells, IL-2 and the generation of cytotoxic T-cell responses. 2002. Trends Immunol. 23465-467. [DOI] [PubMed] [Google Scholar]
- 27.Matano, T., R. Shibata, C. Siemon, M. Connors, H. C. Lane, and M. Martin. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matloubian, M., R. J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 688056-8065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattapallil, J. J., D. C. Douek, A. Buckler-White, D. Montefiori, N. L. Letvin, G. J. Nabel, and M. Roederer. 2006. Vaccination preserves CD4 memory T cells during acute simian immunodeficiency virus challenge. J. Exp. Med. 2031533-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 4341093-1097. [DOI] [PubMed] [Google Scholar]
- 31.Mavilio, D., G. Lombardo, A. Kinter, M. Fogli, A. La Sala, S. Ortolano, A. Farschi, D. Follmann, R. Gregg, C. Kovacs, E. Marcenaro, D. Pende, A. Moretta, and A. S. Fauci. 2005. Characterization of CD56−/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc. Natl. Acad. Sci. USA 1022886-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehandru, S., M. A. Poles, K. Tenner-Racz, V. Manuelli, P. Jean-Pierre, P. Lopez, A. Shet, A. Low, H. Mohri, D. Boden, P. Racz, and M. Markowitz. 2007. Mechanisms of gastrointestinal CD4+ T-cell depletion during acute and early human immunodeficiency virus type 1 infection. J. Virol. 81599-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehandru, S., M. A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D. Boden, P. Racz, and M. Markowitz. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Northrop, J. K., R. M. Thomas, A. D. Wells, and H. Shen. 2006. Epigenetic remodeling of the IL-2 and IFN-gamma loci in memory CD8 T cells is influenced by CD4 T cells. J. Immunol. 1771062-1069. [DOI] [PubMed] [Google Scholar]
- 35.Pal, R., D. Venzon, S. Santra, V. S. Kalyanaraman, D. C. Montefiori, L. Hocker, L. Hudacik, N. Rose, J. Nacsa, Y. Edghill-Smith, M. Moniuszko, Z. Hel, I. M. Belyakov, J. A. Berzofsky, R. W. Parks, P. D. Markham, N. L. Letvin, J. Tartaglia, and G. Franchini. 2006. Systemic immunization with an ALVAC-HIV-1/protein boost vaccine strategy protects rhesus macaques from CD4+ T-cell loss and reduces both systemic and mucosal simian-human immunodeficiency virus SHIVKU2 RNA levels. J. Virol. 803732-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perfetto, S. P., P. K. Chattopadhyay, L. Lamoreaux, R. Nguyen, D. Ambrozak, R. A. Koup, and M. Roederer. 2006. Amine reactive dyes: an effective tool to discriminate live and dead cells in polychromatic flow cytometry. J. Immunol. Methods 313199-208. [DOI] [PubMed] [Google Scholar]
- 37.Pitcher, C. J., S. I. Hagen, J. M. Walker, R. Lum, B. L. Mitchell, V. C. Maino, M. K. Axthelm, and L. J. Picker. 2002. Development and homeostasis of T cell memory in rhesus macaque. J. Immunol. 16829-43. [DOI] [PubMed] [Google Scholar]
- 38.Precopio, M. L., M. R. Betts, J. Parrino, D. A. Price, E. Gostick, D. R. Ambrozak, T. E. Asher, D. C. Douek, A. Harari, G. Pantaleo, R. Bailer, B. S. Graham, M. Roederer, and R. A. Koup. 2007. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J. Exp. Med. 2041405-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reynolds, M. R., E. Rakasz, P. J. Skinner, C. White, K. Abel, Z. M. Ma, L. Compton, G. Napoé, N. Wilson, C. J. Miller, A. Haase, and D. I. Watkins. 2005. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. J. Virol. 799228-9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romano, J. W., K. G. Williams, R. N. Shurtliff, C. Ginocchio, and M. Kaplan. 1997. NASBA technology: isothermal RNA amplification in qualitative and quantitative diagnostics. Immunol. Investig. 2615-28. [DOI] [PubMed] [Google Scholar]
- 41.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 40708-712. [DOI] [PubMed] [Google Scholar]
- 42.Schmitz, J. E., R. P. Johnson, H. M. McClure, K. H. Manson, M. S. Wyand, M. J. Kuroda, M. A. Lifton, R. S. Khunkhun, K. J. McEvers, J. Gillis, M. Piatak, J. D. Lifson, G. Grosschupff, P. Racz, K. Tenner-Racz, E. P. Rieber, K. Kuus-Reichel, R. S. Gelman, N. L. Letvin, D. C. Montefiori, R. M. Ruprecht, R. C. Desrosiers, and K. A. Reimann. 2005. Effect of CD8+ lymphocyte depletion on virus containment after simian immunodeficiency virus SIVmac251 challenge of live attenuated SIVmac239Δ3-vaccinated rhesus macaques. J. Virol. 798131-8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shedlock, D. J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300337-339. [DOI] [PubMed] [Google Scholar]
- 44.Sun, J. C., M. A. Williams, and M. J. Bevan. 2004. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 5927-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun, Y., E. Iglesias, A. Samri, G. Kamkamidze, T. Decoville, G. Carcelain, and B. Autran. 2003. A systematic comparison of methods to measure HIV-1 specific CD8 T cells. J. Immunol. Methods 27223-34. [DOI] [PubMed] [Google Scholar]
- 46.Tryniszewska, E., J. Nacsa, M. G. Lewis, P. Silvera, D. Montefiori, D. Venzon, Z. Hel, R. W. Parks, M. Moniuszko, J. Tartaglia, K. A. Smith, and G. Franchini. 2002. Vaccination of macaques with long-standing SIVmac251 infection lowers the viral set point after cessation of antiretroviral therapy. J. Immunol. 1695347-5357. [DOI] [PubMed] [Google Scholar]
- 47.Vaccari, M., C. J. Trindade, D. Venzon, M. Zanetti, and G. Franchini. 2005. Vaccine-induced CD8+ central memory T cells in protection from simian AIDS. J. Immunol. 1753502-3507. [DOI] [PubMed] [Google Scholar]
- 48.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280427-431. [DOI] [PubMed] [Google Scholar]
- 49.Wherry, E. J., V. Teichgräber, T. C. Becker, D. Masopust, S. M. Kaech, R. Antia, U. H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4225-234. [DOI] [PubMed] [Google Scholar]
- 50.Zanetti, M., and G. Franchini. 2006. T cell memory and protective immunity by vaccination: is more better? Trends Immunol. 27511-517. [DOI] [PubMed] [Google Scholar]