Abstract
Hepatitis delta virus (HDV) contains a viroid-like, 1.7-kb circular RNA genome, which replicates via a double-rolling-circle model. However, the exact mechanism involved in HDV genome RNA replication and subgenomic mRNA transcription is still unclear. Our previous studies have shown that the replications of genomic and antigenomic HDV RNA strands have different sensitivities to α-amanitin and are associated with different nuclear bodies, suggesting that these two strands are synthesized in different transcription machineries in the cells. In this study, we developed a unique quantitative reverse transcription-PCR (qRT-PCR) procedure for detection of various HDV RNA species from an RNA transfection system. Using this qRT-PCR procedure and a series of HDV mutants, we demonstrated that Arg-13 methylation, Lys-72 acetylation, and Ser-177 phosphorylation of small hepatitis delta antigen (S-HDAg) are important for HDV mRNA transcription. In addition, these three S-HDAg modifications are dispensable for antigenomic RNA synthesis but are required for genomic RNA synthesis. Furthermore, the three RNA species had different sensitivities to acetylation and deacetylation inhibitors, showing that the metabolic requirements for the synthesis of HDV antigenomic RNA are different from those for the synthesis of genomic RNA and mRNA. In sum, our data support the hypothesis that the cellular machinery involved in the synthesis of HDV antigenomic RNA is different from that of genomic RNA synthesis and mRNA transcription, even though the antigenomic RNA and the mRNA are made from the same RNA template. We propose that acetylation and deacetylation of HDAg may provide a molecular switch for the synthesis of the different HDV RNA species.
Hepatitis delta virus (HDV) is a satellite virus that requires hepatitis B virus to supply envelope proteins for virus assembly and production (38). It contains a single-stranded, circular RNA genome of 1.7 kb, which replicates through a double-rolling-circle mechanism in the nucleus (29). The genomic RNA (G-RNA) strand is first replicated into the full-length antigenomic RNA (AG-RNA) and is also transcribed into a 0.8-kb mRNA, which encodes the only HDV protein, hepatitis delta antigen (HDAg). The AG-RNA, in turn, is replicated into G-RNA by another round of rolling-circle replication. The production of HDAg, which is intimately involved in HDV RNA replication, is a unique feature distinguishing HDV from plant viroids, the latter of which do not encode any protein. HDAg consists of two species, the small form (S-HDAg; 195 amino acids, 24 kDa) and the large form (L-HDAg; 214 amino acids, 27 kDa), which play different roles in HDV replication. S-HDAg is an essential activator for HDV RNA replication (19). In contrast, L-HDAg inhibits a certain stage of HDV RNA replication but is required for virion assembly (4, 7, 21, 28). HDAg is modified posttranslationally by phosphorylation (6, 8, 35, 37), acetylation (36), methylation (24), and in the case of L-HDAg, isoprenylation (11). Arg-13 methylation, Lys-72 acetylation, and Ser-177 phosphorylation are three major modifications of S-HDAg and are important for the functions of S-HDAg in HDV RNA replication (24, 35, 36). Isoprenylation on Cys-211 of L-HDAg is required for virus assembly (11).
Cellular DNA-dependent RNA polymerase II (Pol II) has been implicated as the key enzyme for HDV RNA replication (5, 9, 10, 22, 23, 25, 32-34, 42). However, relevant studies have found that the metabolic requirements for the replication of HDV genome and antigenome are significantly different, supporting the hypothesis that the synthesis of HDV G-RNA and the synthesis of AG-RNA are carried out by independent mechanisms, probably by different polymerases (16, 23, 24, 26, 29, 30, 32, 35, 36). On the basis of the sensitivity to α-amanitin and the site of synthesis, Pol II is likely responsible for the synthesis of G-RNA. However, the enzyme responsible for the synthesis of AG-RNA is still controversial; both Pol I and Pol II have been suggested (5, 20, 23, 27, 29, 40).
What are the metabolic requirements for the transcription of the HDAg-encoding mRNA? Similar to the synthesis of G-RNA, transcription of this mRNA is also sensitive to low concentrations of α-amanitin (1 to 5 μg/ml) (32). In addition, this mRNA has all of the hallmarks of the conventional mRNA transcripts in the cells (e.g., 5′ capped and 3′ polyadenylated) (13, 14). Accordingly, transcription of the HDV mRNA was thought to occur via the Pol II transcription machinery, similar to G-RNA synthesis (20, 27). Previously, it was first suggested that HDV mRNA transcription is the extension of AG-RNA synthesis and occurs only at the beginning of the HDV RNA replication cycle (14). However, recent evidence indicated that mRNA transcription is independent of either G-RNA or AG-RNA synthesis and occurs throughout the HDV replication cycle (31). Previous studies of HDV mRNA transcription were hampered by the relatively low quantity of the mRNA species. In this study, we developed a quantitative reverse transcription-PCR (qRT-PCR) procedure which can specifically and sensitively detect various HDV RNA species synthesized from an HDV cDNA-free RNA transfection system (31). Using this qRT-PCR procedure, we showed that, similar to G-RNA synthesis but opposite to AG-RNA synthesis, transcription of mRNA requires an S-HDAg that is methylated, acetylated, and phosphorylated. This result adds to the growing list of differences in metabolic requirements between G-RNA/mRNA synthesis and AG-RNA synthesis. These differences also support the hypothesis that the cellular machinery involved in the synthesis of HDV AG-RNA is different from that of G-RNA synthesis and mRNA transcription.
MATERIALS AND METHODS
Plasmid construction and in vitro transcription.
Genomic and antigenomic HDV RNA (1.9-kb G-RNA and AG-RNA, respectively), which contain the entire HDV genome plus approximately 200 additional nucleotides of HDV sequence, were transcribed from pKS/HDV1.9 (18) with T7 MEGAscript (Ambion) after linearization by NotI and SnaBI digestion, respectively. Plasmid PX9-p(A), which was used for in vitro transcription of S-HDAg-encoding mRNA-I [containing a poly(A) stretch to mimic HDAg-encoding mRNA (genotype I) in the cells in which HDV was replicating], was derived from plasmid PX9 (31) by inserting 70 adenosines immediately after the polyadenylation site of S-HDAg-encoding sequence by using the jumping-PCR method. Capped and poly(A)-tailed S-HDAg-encoding mRNA-I/II and mRNA-I were transcribed from plasmids PX9-I/II (31) and PX9-p(A), respectively, with T7 mMESSAGE mMACHINE Ultra (Ambion) after linearization by HindIII digestion. Plasmids used for in vitro transcription of HDV G-RNA, AG-RNA, and S-HDAg-encoding mRNA with the R13A, K72A, or S177A substitution were derived from plasmid pKS/HDV1.9 or PX9-I/II by PCR-based site-directed mutagenesis. Plasmid PX9-I/II-d1nt, which was used for in vitro transcription of an S-HDAg-encoding defective mutant (d1nt), in which the adenosine of the ATG start codon was deleted to abolish S-HDAg translation, was also derived from plasmid PX9-I/II by PCR-based site-directed mutagenesis.
Cell culture, RNA transfection, and drug treatment.
Human hepatoma cell line Huh7 was cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and incubated at 37°C in 5% CO2. HDV RNA transfection experiments were performed by use of the DMRIE-C transfection reagent (Invitrogen) according to the manufacturer's directions. To study the effects of curcumin and trichostatin A (TSA) on HDV genome replication and mRNA transcription, curcumin or TSA (Sigma) was added to culture medium at 4 h or 48 h after HDV RNA transfection and incubated for 24 h.
qRT-PCR.
Total RNA was extracted from cells by using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. To detect the HDAg-encoding mRNA, 200 ng of total RNA was reverse transcribed in a 20-μl final reaction mixture volume containing 1× reverse transcriptase buffer, 2.5 μM of oligo(dT)20, 0.2 mM (each) of dATP, dGTP, dCTP, and dTTP (Roche), 5 mM of dithiothreitol, 40 U of RNaseOUT recombinant RNase inhibitor, and 200 U of SuperScript III reverse transcriptase (Invitrogen). The RT reaction was performed at 55°C for 50 min and then inactivated by heating at 95°C for 45 min. For the detection of HDV G-RNA or AG-RNA, 200 ng of total RNA was reverse transcribed in a 20-μl final reaction mixture volume containing 1× rTth reverse transcriptase buffer, 0.1 μM of tagged strand-specific primer (Table 1), 40 U of RNaseOUT recombinant RNase inhibitor (Invitrogen), 1 mM of MnCl2, 0.2 mM (each) of dATP, dGTP, dCTP, and dTTP, and 5 U of rTth (Applied Biosystems). The RT reaction was performed at 70°C for 15 min and then terminated by adding 2 μl of 10× chelating buffer. Real-time PCR was performed by using the LightCycler FastStart DNA master Sybr green I kit (Roche). Briefly, 5 μl of 10-fold serially diluted cDNA was mixed with 15 μl of master mix. The reaction consisted of an initiating step of 10 min at 95°C, followed by 45 cycles of amplification, with each cycle consisting of 10 s at 95°C, 3 s at 60°C, and 16 s at 72°C for HDV G-RNA and AG-RNA detection and of 10 s at 95°C, 3 s at 55°C, and 26 s at 72°C for HDV mRNA detection. The reactions, data acquisition, and analyses were performed using the LightCycler 2.0 system (Roche).
TABLE 1.
Primers used for the HDV RNA species-specific RT-PCR procedurea
| Primer | Positions (5′-3′)b | Sequence (5′-3′)c |
|---|---|---|
| Tagged GS | 416-394 | GCTGTAGCCTACCAGCAGTCAGTCATCACTTTCCTCCTCGCTTCGGTCTC |
| Tagged AGS | 1510-1535 | GTAGGCTACGACTGAAGCCTAGTGCAGTCGAGTTCCTCTAACTTCTTTCTTCC |
| Tagm-T20-F | 938-945 | GCCGCCCTGCAGTTTTTTTTTTTTTTTTTTTTGAGTGGAA |
| mI-R | 1536-1511 | CGGAAGAAAGAAGTTAGAGGAACTCG |
| mII-R | 1376-1361 | GGTCCCGGACGGATCA |
| mI-F | 994-1014 | TCGGCTGGGAAGAGTATATCC |
| TagG-F | GCTGTAGCCTACCAGCAGTCAGTCATC | |
| GS-R | 61-84 | GTCGGTAAAGAGCATTGGAACGTC |
| TagAG-F | GTAGGCTACGACTGAAGCCTAGTGCAG | |
| AGS-R | 166-139 | CCTTCCTAGAGTTTCACTAGCGTTATGG |
All of the primers are designed according to the sequence of HDV genotype I, except for primer mII-R, which is designed according to the sequence of HDV genotype II.
The positions are presented using the HDV genome numbering of Wang et al. (41). The numbers go “up” and “down” in the 5′-to-3′ direction of genomic and antigenomic HDV RNA, respectively.
Underlined sequences are tag sequences that are not present in the HDV genome or antigenome.
RESULTS
Establishment of qRT-PCRs specific for HDV G-RNA, AG-RNA, and HDAg-encoding mRNA.
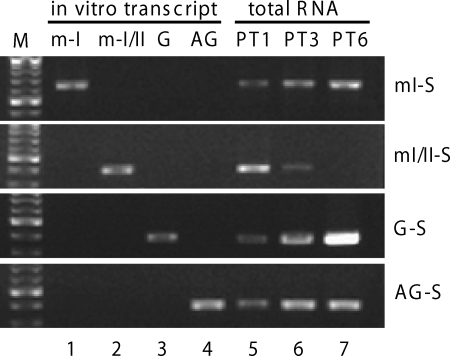
To study the importance of posttranslational modifications of S-HDAg in HDV replication, we set out to develop a qRT-PCR procedure that can specifically detect different HDV RNA species using a previously established HDV cDNA-free RNA transfection system (31). This transfection system avoids the artifact of DNA-dependent RNA transcription step inherent in the DNA transfection systems. To establish HDV RNA replication, cells were transfected with either 1.9-kb genomic or antigenomic RNA (genotype I) and mRNA encoding a hybrid genotype I-II S-HDAg. To study nascent mRNA synthesis in this system, several HDV RNA species need to be discriminated, namely, the transfected and nascent G-RNA and AG-RNA of genotype I, the transfected HDAg-encoding mRNA (mRNA-I/II), and the nascent HDAg-encoding mRNA (mRNA-I) (Fig. 1). Since the transfected mRNA-I/II is a chimeric HDAg-encoding RNA that contains sequences from genotype II at the 5′ half and genotype I at its 3′ half, whereas the G-RNA used for transfection is genotype I, only the nascent mRNA produced from the G-RNA (genotype I), not the transfected mRNA-I/II, will be detected if the genotype I sequence-specific PCR primers are used. The most challenging issues for developing an HDV RNA species-specific qRT-PCR procedure are (i) to discriminate between the nascent mRNA-I and AG-RNA because the mRNA-I sequence is included entirely in the AG-RNA except for its 3′-poly(A) tail and (ii) to discriminate between the G- and AG-RNA, since half of the G- and AG-RNA are self-complementary. The RT-PCR protocols eventually established, mI-S, mI/II-S, G-S, and AG-S, are specific for detection of mRNA-I, mRNA-I/II, G-RNA, and AG-RNA, respectively (Tables 1 and 2), as demonstrated by using in vitro-transcribed RNAs as templates (Fig. 2, lanes 1 to 4). In addition, using this RT-PCR procedure, we were able to detect the dynamic changes of these four RNA species in the HDV RNA-transfected cells. As expected, the transfected mRNA-I/II decayed over time, whereas the newly synthesized mRNA-I, G-RNA, and AG-RNA accumulated over time (Fig. 2, lanes 5 to 7). The AG-RNA did not increase appreciably at later time points, indicating that it reached the plateau of synthesis early.
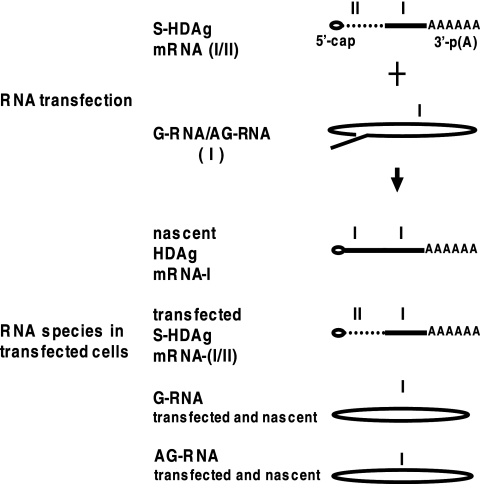
FIG. 1.
Schematic representation of the strategy for detection of different HDV RNA species. For detection of nascent antigenomic RNA (AG-RNA, genotype I) and HDAg-encoding mRNA (mRNA-I, genotype I), the in vitro-transcribed genomic RNA (G-RNA) of genotype I was cotransfected with a genotype I/II chimeric HDAg-encoding mRNA (mRNA-I/II) into Huh7 cells. In contrast, for detection of G-RNA produced from cells in which HDV RNA replicated, in vitro-transcribed AG-RNA was used for cotransfection with mRNA-I/II. The origins of each HDV RNA species are indicated.
TABLE 2.
Characteristics of the HDV RNA species-specific RT-PCR procedures
| Protocol | RNA detected | RNAs transfected | RT primera | Reverse transcriptase enzyme | Temp of RT reaction (°C) | PCR primera (F/R)b | Sensitivity | Specificityc (discrimination) |
|---|---|---|---|---|---|---|---|---|
| mI-S | mRNA-I | G-RNA and mRNA-I/II | Oligo(dT)20 | SuperScript III | 55 | Tagm-T20-F/mI-R | 103 | 6 log10 |
| mI/II-S | mRNA-I/II | G-RNA and mRNA-I/II | Oligo(dT)20 | SuperScript III | 55 | mII-R/mI-F | —d | — |
| G-S | G-RNA | AG-RNA and mRNA-I/II | Tagged GS | rTth | 70 | TagG-F/GS-R | 103 | 5 log10 |
| AG-S | AG-RNA | G-RNA and mRNA-I/II | Tagged AGS | rTth | 70 | TagAG-F/AGS-R | 103 | 5 log10 |
The sequences of the RT-PCR primers are listed in Table 1.
F, forward primer; R, reverse primer.
The specificity for the mI-S protocol is defined as the log difference between the titer at which the mRNA-I was detectable and the titer at which the G-RNA or AG-RNA generated the first positive signal. The specificity for the G-S protocol is defined as the log difference between the titer at which the G-RNA was detectable and the titer at which the AG-RNA generated the first positive signal. The specificity for AG-S protocol is defined as the log difference between the titer at which the AG-RNA was detectable and the titer at which the G-RNA generated the first positive signal.
—, not determined.
FIG. 2.
Verification of the RT-PCR products using HDV RNA species-specific protocols. In vitro-transcribed mRNA-I, mRNA-I/II, G-RNA, and AG-RNA (lanes 1 to 4) (104 copies each) and 200 ng of total cellular RNAs harvested at day 1, day 3, and day 6 posttransfection (PT1, PT3, and PT6, respectively) (lanes 5 to 7) of the HDV RNA-transfected cells were amplified by RT-PCR using the mI-S, mI/II-S, G-S, or AG-S protocol (Table 2). The products of resultant PCR were analyzed by gel electrophoresis and stained with ethidium bromide. Lane M contains molecular size markers.
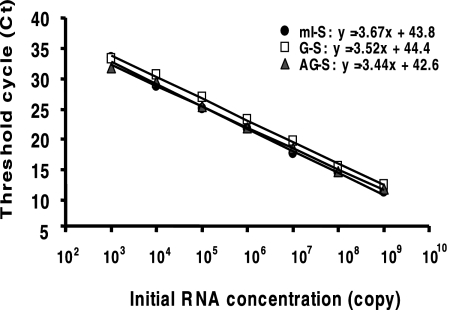
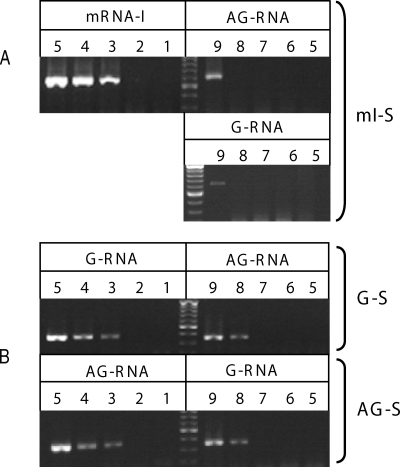
For the purpose of quantifying the amount of each RNA species, the real-time PCR step of this qRT-PCR procedure was conducted by use of Roche LightCycler 2.0 system. To determine the sensitivity of this qRT-PCR procedure, 10-fold dilutions of in vitro-transcribed RNAs were spiked into total RNA extracted from Huh7 cells and used to produce standard curves by plotting threshold cycle value against copy number. These representative standard curves demonstrate a high degree of linearity (R2 = 0.99) over 6 orders of magnitude (103 to 109 copies), with a detection limit of 103 copies for all the three protocols (Fig. 3 and Table 2). The specificity of this real-time qRT-PCR procedure was also determined using in vitro-transcribed RNAs as templates. The mI-S protocol allowed a 106-fold discrimination between mRNA-I and AG-RNA as well as between mRNA-I and G-RNA. The G-S and AG-S protocols allowed a 105-fold discrimination between their target RNA and nontarget cRNA (Fig. 4 and Table 2). Therefore, this qRT-PCR procedure can specifically and sensitively detect various HDV RNA species from the HDV cDNA-free RNA transfection system.
FIG. 3.
Amplification standard curves for the HDV RNA species-specific real-time qRT-PCR assay. RT-PCRs with 103 to 109 copies of in vitro-transcribed mRNA-I, G-RNA, and AG-RNA, which were mixed with total RNA extracted from Huh7 cells, were carried out using mI-S, G-S, and AG-S protocols, respectively. The threshold cycle (Ct) was plotted against the log10 of the corresponding initial template concentration.
FIG. 4.
Sensitivity and specificity of the qRT-PCR assay for the detection of HDV genomic RNA, antigenomic RNA, and HDAg-encoding mRNA. RT-PCRs with 103 to 109 copies of in vitro-transcribed mRNA-I, G-RNA, or AG-RNA, which were mixed with total RNA extracted from Huh7 cells, were carried out by using the mI-S protocol (A) or the G-S or AG-S protocol (B) as indicated. The products of resultant PCR were analyzed by gel electrophoresis. The numbers above each lane in the gel represent the log10 number of copies of in vitro-transcribed mRNA-I, G-RNA, or AG-RNA added to the RT reaction mixture.
Posttranslational modifications at R13, K72, and S177 are important for HDV mRNA transcription.
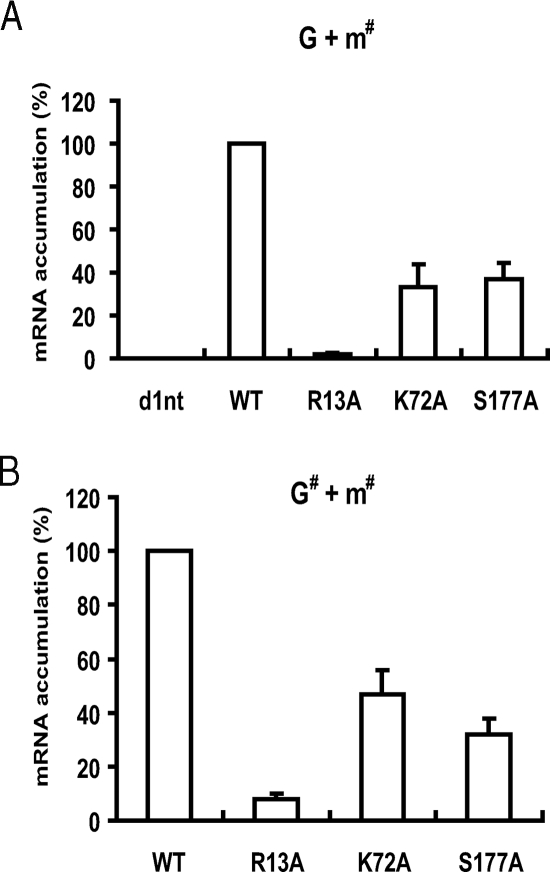
Previous studies (24, 35, 36) showed that Arg-13 methylation, Lys-72 acetylation, and Ser-177 phosphorylation are three major modifications of S-HDAg and are critical for HDV replication. It is not clear, however, whether these three modifications are involved in HDV mRNA transcription. Therefore, we examined the ability of the S-HDAg methylation, acetylation, and phosphorylation mutants to promote HDV mRNA transcription by using the established HDV cDNA-free RNA transfection method, in which HDV G-RNA was cotransfected with a wild-type or mutant S-HDAg-encoding mRNA (31). The first event in the HDV replication cycle is probably the transcription of HDAg-encoding mRNA species, since this mRNA is necessary for the production of S-HDAg, which, in turn, is required for the initiation of HDV RNA replication. Utilizing the mI-S protocol (Table 2), we were able to detect HDV mRNA species on day 1 posttransfection, which could not detected by Northern blotting previously (31). We found that, compared to the wild-type RNA, cotransfection of mRNA encoding S-HDAg with R13A, K72A, or S177A substitution led to significantly lower levels of HDV mRNA transcription (Fig. 5A). In particular, the R13A mutation almost completely suppressed mRNA transcription. No nascent HDV mRNA species was detected when the S-HDAg-encoding defective mutant (d1nt), in which the adenosine of the ATG start codon was deleted to abolish S-HDAg translation, was used for cotransfection, indicating the specificity of this assay. Similar results were obtained when the same mutations (R13A, K72A, or S177A) were introduced into the HDV genomic RNA and when these mutant RNAs were cotransfected with their corresponding mutant S-HDAg-encoding mRNAs (Fig. 5B). This protocol ensures that no residual wild-type mRNA is made during the entire RNA replication cycle. These results strongly suggest that S-HDAg methylation, acetylation, and phosphorylation are important for HDV mRNA transcription.
FIG. 5.
Effects of arginine-13, lysine-72, and serine-177 mutations of S-HDAg on HDV mRNA transcription. (A) The wild-type HDV genomic RNA was transfected together with the S-HDAg-encoding defective mutant (d1nt) or with mRNAs encoding wild-type (WT) S-HDAg or S-HDAg with the R13A, K72A, or S177A mutation into Huh7 cells. (B) Alternatively, the HDV genomic RNA encoding wild-type, R13A, K72A, or S177A HDAg was transfected together with the corresponding S-HDAg mRNA encoding the same mutation. At day 1 posttransfection, total RNAs were extracted, and HDV mRNA was detected by qRT-PCR using the mI-S protocol (Table 2). The data were normalized relative to the values detected in the wild-type HDV RNA transfection. Error bars indicate standard deviations. G + m#, wild-type genomic RNA cotransfected with mutant mRNA; G# + m#, mutant genomic RNA cotransfected with the corresponding mutant mRNA.
S-HDAg methylation, acetylation, and phosphorylation are dispensable for AG-RNA synthesis but are required for G-RNA synthesis.
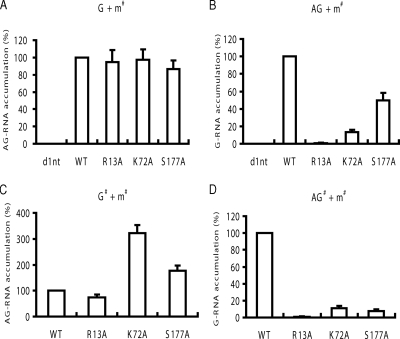
We next studied the requirement of S-HDAg modifications in the synthesis of HDV G-RNA and AG-RNA. To study the synthesis of AG-RNA from the genomic template, G-RNA was cotransfected with the S-HDAg-encoding mRNA. On the other hand, to study the synthesis of G-RNA from the antigenomic template, AG-RNA was used for cotransfection. The nascent AG-RNA and G-RNA strands were then detected by the AG-S and G-S protocols (Table 2), respectively, on day 6 posttransfection. The results showed that AG-RNA synthesis was not significantly affected when any one of the mutant S-HDAg-encoding mRNAs was used (Fig. 6A). In contrast, G-RNA synthesis was drastically reduced when mRNA encoding S-HDAg with R13A, K72A, or S177A substitution was used (Fig. 6B). These results are in agreement with the previous studies, in which Northern blotting was used to evaluate HDV RNA production (24, 35, 36), and indicated that S-HDAg methylation, acetylation, and phosphorylation are all required for HDV G-RNA synthesis but not for AG-RNA synthesis.
FIG. 6.
Effects of arginine-13, lysine-72, and serine-177 mutations of S-HDAg on HDV genomic and antigenomic RNA synthesis. (A and B) The wild-type HDV genomic RNA (A) or antigenomic RNA (B) was transfected together with the S-HDAg-encoding defective mutant (d1nt) or with S-HDAg mRNAs encoding the wild-type (WT) S-HDAg or R13A, K72A, or S177A S-HDAg mutant into Huh7 cells. (C and D) Alternatively, the HDV genomic RNA (C) or antigenomic RNA (D) encoding the wild-type (WT) or R13A, K72A, or S177A HDAg was transfected together with the corresponding S-HDAg mRNA encoding the same mutation. At day 6 posttransfection, total RNAs were extracted, and HDV antigenomic RNA (A and C) and genomic RNA (B and D) were detected by qRT-PCR using the AG-S and G-S protocols, respectively (Table 2). The data were normalized relative to the values detected in the wild-type HDV RNA transfection. Error bars indicate standard deviations. G + m#, wild-type genomic RNA cotransfected with mutant mRNA; AG + m#, wild-type antigenomic RNA cotransfected with mutant mRNA; G# + m#, mutant genomic RNA cotransfected with mutant mRNA; AG# + m#, mutant antigenomic RNA cotransfected with mutant mRNA.
To further verify the importance of posttranslational modifications of S-HDAg in HDV RNA synthesis throughout the replication cycle of HDV, we introduced the same mutations (R13A, K7A, or S177A) into the HDV G-RNA and AG-RNA and cotransfected these mutant RNAs with their corresponding mutant S-HDAg-encoding mRNAs. Under this condition, the mutant mRNA was present throughout the replication cycle. We found that all of these mutations drastically reduced G-RNA synthesis, indicating that the posttranslational modifications are required for G-RNA synthesis (Fig. 6D). This is consistent with the findings above (Fig. 6A and B). In contrast, these mutations did not reduce AG-RNA synthesis. Not only that, K72A and S177A mutant mRNAs supported a significantly higher level of AG-RNA synthesis than the wild-type RNA did (Fig. 6C). These results suggested that acetylation and phosphorylation of S-HDAg are not required for AG-RNA synthesis but may interfere with AG-RNA synthesis. These results further confirmed that S-HDAg methylation, acetylation, and phosphorylation are required for G-RNA synthesis. In contrast, AG-RNA synthesis requires an unmodified form of HDAg.
Differential metabolic requirements between HDV mRNA transcription/G-RNA synthesis and AG-RNA synthesis.
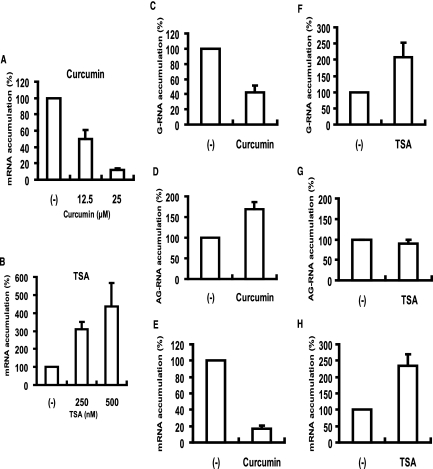
The above experiments suggested that the modified S-HDAg favors HDV mRNA transcription/G-RNA synthesis, whereas the unmodified S-HDAg supports HDV AG-RNA synthesis. To further verify this conclusion, we examined the effects of the compounds that affect protein modification/demodification. S-HDAg has been shown to be acetylated by p300, which is an A-type histone acetyltransferase (36). We used curcumin, a specific inhibitor of p300/CBP histone acetyltransferase activity (1), and trichostatin A, a broad-spectrum protein deacetylation inhibitor (2), to verify the importance of S-HDAg acetylation in HDV mRNA transcription. Huh7 cells were cotransfected with wild-type HDV genomic RNA and S-HDAg-encoding mRNA and then treated with curcumin or TSA after transfection. The newly transcribed HDV mRNA was detected by qRT-PCR 24 h after drug treatment. We found that HDV mRNA transcription was reduced in a dose-dependent manner in the cells treated with curcumin (Fig. 7A). In contrast, HDV mRNA transcription was upregulated by TSA, also in a dose-dependent manner (Fig. 7B). These results further confirmed the importance of protein acetylation in HDV mRNA transcription.
FIG. 7.
Effects of curcumin and TSA treatments on HDV genome replication and mRNA transcription. HDV genomic RNA was transfected together with the S-HDAg-encoding mRNA. Four hours after transfection, curcumin (A) or TSA (B) was added at the indicated concentrations and incubated for 24 h. Total RNAs were extracted, and HDV mRNA was detected by qRT-PCR using the mI-S protocol. (C to E) Curcumin (100 μM) was added at 48 h posttransfection for 24 h. (F to H) TSA (25 μM) was added at 4 h posttransfection for 24 h. HDV G-RNA, AG-RNA, and mRNA were detected by qRT-PCR using the G-S, AG-S, and mI-S protocols, respectively. The data were normalized relative to the values without the drug treatment. Error bars indicate standard deviations. (−), negative control (no curcumin or TSA added).
At an even higher concentration (100 μM), curcumin also caused significant reduction of G-RNA synthesis, similar to mRNA transcription, but caused a significant increase of AG-RNA synthesis (Fig. 7C to E). In contrast, TSA treatment (25 μM) increased HDV mRNA transcription and G-RNA synthesis, but not AG-RNA synthesis (Fig. 7F to H). These results together confirmed the results obtained by using S-HDAg modification-defective mutants and suggested that the modified S-HDAg is involved in G-RNA synthesis and mRNA transcription but that the unmodified form of HDAg is involved in AG-RNA synthesis.
DISCUSSION
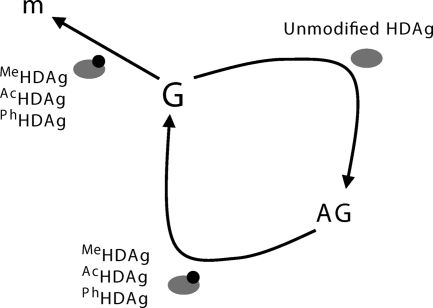
We have described here a unique qRT-PCR procedure with high sensitivity and specificity for the detection of HDV genomic RNA, antigenomic RNA, and HDAg-encoding mRNA in an HDV cDNA-free RNA transfection system. By using this qRT-PCR procedure and a series of site-directed mutagenic mutants, we demonstrated that the three major posttranslational modification sites of S-HDAg, methylation (R13), acetylation (K72), and phosphorylation (S177), are important for HDV mRNA transcription. In addition, we confirmed that these three S-HDAg modifications are dispensable for AG-RNA synthesis but are required for G-RNA synthesis. Furthermore, the results of inhibitory drug treatments (TSA and curcumin) also suggested that HDV mRNA transcription and G-RNA synthesis require an S-HDAg that is acetylated, whereas AG-RNA synthesis is mediated by an unacetylated S-HDAg. The importance of methylation and phosphorylation of S-HDAg have also been corroborated by inhibitor studies (24, 35). In sum, our data presented here indicated that, in contrast to AG-RNA synthesis, which is mediated by an unmodified S-HDAg, posttranslational modifications of S-HDAg are involved in both HDV mRNA transcription and G-RNA synthesis (Fig. 8).
FIG. 8.
Model of the HDAg requirement for the synthesis of the three HDV RNA species. MeHDAg, AcHDAg, and PhHDAg represent methylated, acetylated, and phosphorylated HDAg, respectively. M, HDAg-encoding mRNA; G, HDV genomic RNA; AG, HDV antigenomic RNA.
The above finding adds to the growing list of similar metabolic requirements between HDV mRNA transcription and G-RNA synthesis. Previous studies have shown that the syntheses of these two RNA species are sensitive to a low concentration of α-amanitin (26, 29, 32). Besides, both of these two RNA species are synthesized in the nucleoplasm (23) and exported to the cytoplasm immediately after synthesis (26). Combined with the fact that HDV mRNA has all of the hallmarks of the conventional Pol II-mediated mRNA transcripts and the recent report that Pol II possesses RNA-dependent RNA polymerase activity (22) and is able to use the partial HDV antigenomic-sense RNA hairpin fragments as templates for elongation reactions in vitro (9, 22), these data support the proposition that HDV mRNA transcription and G-RNA synthesis are carried out by the Pol II machinery. In contrast, AG-RNA synthesis has drastically different metabolic requirements. However, the enzyme involved in AG-RNA synthesis is still debatable. It is significant to note that both mRNA transcription and AG-RNA synthesis take place on the same template, and yet they have different HDAg requirements. This finding gives further support to our previous conclusion that mRNA transcription is independent of AG-RNA synthesis (31) but is not an extension of AG-RNA synthesis as previously proposed (14).
Other than the fact that AG-RNA synthesis is mediated by an unmodified S-HDAg, which was shown in the present and previous studies (24, 35, 36), some other metabolic requirements for its synthesis are significantly different from that for the synthesis of G-RNA or the transcription of mRNA. For instance, AG-RNA synthesis is resistant to a very high concentration of α-aminitin (100 μg/ml) (40, 43, 47), AG-RNA is not exported to the cytoplasm after its synthesis (26), AG-RNA synthesis is not inhibited by L-HDAg when the latter is expressed at the beginning of the replication cycle (28), and the newly synthesized AG-RNA accumulates in the nucleolus (16, 23). Collectively, these prominent differences in metabolic requirements between G-RNA synthesis and AG-RNA synthesis support the hypothesis that the cellular transcription machinery involved in the synthesis of HDV AG-RNA is different from that of G-RNA synthesis and mRNA transcription.
The detailed mechanism concerning how the cellular RNA polymerases are redirected for HDV genome replication and mRNA transcription is still unclear. However, HDAg has many features reminiscent of a transcription factor and is indispensable for HDV replication (as reviewed in references 15, 20, and 27). Besides, HDAg binds to HDV RNA and serves as an RNA chaperone to alter RNA conformation (17). Accordingly, HDAg must play a very important role in the redirection of the cellular RNA polymerases for HDV replication. In this study, we found that the S-HDAg methylation-defective mutants lost the ability to carry out G-RNA synthesis and mRNA transcription. However, the acetylation- and phosphorylation-defective mutants still allow some degree of G-RNA synthesis and mRNA transcription (Fig. 5 and 6). These results may imply that S-HDAg can be posttranslationally modified sequentially and/or synergistically for its optimum function in HDV replication in a way similar to the modifications of transcription factors and core histones, which are subjected to multisite protein modifications (44). In this respect, R-13 methylation of S-HDAg may play a critical role in regulating HDV replication at a higher hierarchical level than that of K-72 acetylation and S-177 phosphorylation.
Lysine acetylation has been shown to occur in over 80 proteins of different origins, including core histones, about 40 transcription factors, and over 30 other proteins (3, 12, 39, 43). This modification is reversible and dynamically regulated by acetyltransferases and deacetylases for exerting its effect through “loss of function” and “gain of function.” In our experiments using the S-HDAg acetylation-defective mutants, we found that K72A mutants mediated a drastically decreased level of HDV mRNA transcription and G-RNA synthesis (Fig. 5, 6B, and 6D). In contrast, it strongly enhanced AG-RNA synthesis (Fig. 6C). In the experiments with curcumin (acetyltransferase inhibitor) and TSA (deacetylase inhibitor) treatments, we found that inhibition of deacetylase activity preferentially increased HDV mRNA transcription and G-RNA synthesis (Fig. 7F to H). By contrast, inhibition of acetyltransferase activity significantly decreased HDV mRNA transcription and G-RNA synthesis, yet it enhanced AG-RNA synthesis (Fig. 7C to E). These results combined suggested that S-HDAg K72 acetylation/deacetylation plays a critical role in regulating the synthesis of different HDV RNA species and that acetylation may enable S-HDAg to recruit the Pol II transcription complex for G-RNA transcription and mRNA transcription, whereas the S-HDAg with unmodified K72 helps its interaction with other transcription machineries to carry out AG-RNA synthesis.
Multisite protein modification/demodification events have been suggested to play regulatory functions in cells. Since HDAg is the only protein encoded by HDV, the modification status of HDAg must play a critical role in the redirection of the cellular RNA polymerases for HDV genome replication and mRNA transcription. Our data presented here indicated that the requirements for S-HDAg modifications are significantly different between G-RNA synthesis/mRNA transcription and AG-RNA synthesis. What is the detailed mechanism involved and are there other kinds of S-HDAg modifications that are required for HDV RNA replication? The HDV RNA species-specific qRT-PCR procedure that we described here could be a useful tool for these studies.
Footnotes
Published ahead of print on 23 July 2008.
REFERENCES
- 1.Balasubramanyam, K., R. A. Varier, M. Altaf, V. Swaminathan, N. B. Siddappa, U. Ranga, and T. K. Kundu. 2004. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J. Biol. Chem. 27951163-51171. [DOI] [PubMed] [Google Scholar]
- 2.Bolden, J. E., M. J. Peart, and R. W. Johnstone. 2006. Anticancer activities of histone deacetylase inhibitors. Nat. Rev. Drug Discov. 5769-784. [DOI] [PubMed] [Google Scholar]
- 3.Caron, C., E. Col, and S. Khochbin. 2003. The viral control of cellular acetylation signaling. Bioessays 2558-65. [DOI] [PubMed] [Google Scholar]
- 4.Chang, F. L., P. J. Chen, S. J. Tu, C. J. Wang, and D. S. Chen. 1991. The large form of hepatitis delta antigen is crucial for assembly of hepatitis delta virus. Proc. Natl. Acad. Sci. USA 888490-8494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, J., X. Nie, H. E. Chang, Z. Han, and J. Taylor. 2008. Transcription of hepatitis delta virus RNA by RNA polymerase II. J. Virol. 821118-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, M.-F., S. C. Baker, L. H. Soe, T. Kamahora, J. G. Keck, S. Makino, S. Govindarajan, and M. M. C. Lai. 1988. Human hepatitis delta antigen is a nuclear phosphoprotein with RNA-binding activity. J. Virol. 622403-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao, M., S. Y. Hsieh, and J. Taylor. 1990. Role of two forms of hepatitis delta virus antigen: evidence for a mechanism of self-limiting genome replication. J. Virol. 645066-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi, S. H., K. J. Park, and S. B. Hwang. 2002. Large hepatitis delta antigen is phosphorylated at multiple sites and phosphorylation is associated with protein conformational change. Intervirology 45142-149. [DOI] [PubMed] [Google Scholar]
- 9.Filipovska, J., and M. M. Konarska. 2000. Specific HDV RNA-templated transcription by pol II in vitro. RNA 641-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu, T. B., and J. Taylor. 1993. The RNAs of hepatitis delta virus are copied by RNA polymerase II in nuclear homogenates. J. Virol. 676965-6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glenn, J. S., J. A. Watson, C. M. Havel, and J. M. White. 1992. Identification of a prenylation site in delta virus large antigen. Science 2561331-1333. [DOI] [PubMed] [Google Scholar]
- 12.Glozak, M. A., N. Sengupta, X. Zhang, and E. Seto. 2005. Acetylation and deacetylation of non-histone proteins. Gene 36315-23. [DOI] [PubMed] [Google Scholar]
- 13.Gudima, S., S. Y. Wu, C. M. Chiang, G. Moraleda, and J. Taylor. 2000. Origin of hepatitis delta virus mRNA. J. Virol. 747204-7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh, S. Y., and J. Taylor. 1991. Regulation of polyadenylation of hepatitis delta virus antigenomic RNA. J. Virol. 656438-6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang, W. H., C. W. Chen, H. L. Wu, and P. J. Chen. 2006. Post-translational modification of delta antigen of hepatitis D virus. Curr. Top. Microbiol. Immunol. 30791-112. [DOI] [PubMed] [Google Scholar]
- 16.Huang, W. H., Y. S. Chen, and P. J. Chen. 2008. Nucleolar targeting of hepatitis delta antigen abolishes its ability to initiate viral antigenomic RNA replication. J. Virol. 82692-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, Z. S., and H. N. Wu. 1998. Identification and characterization of the RNA chaperone activity of hepatitis delta antigen peptides. J. Biol. Chem. 27326455-26461. [DOI] [PubMed] [Google Scholar]
- 18.Jeng, K.-S., A. Daniel, and M. M. C. Lai. 1996. A pseudoknot ribozyme structure is active in vivo and required for hepatitis delta virus RNA replication. J. Virol. 702403-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuo, M. Y., M. Chao, and J. Taylor. 1989. Initiation of replication of the human hepatitis delta virus genome from cloned DNA: role of delta antigen. J. Virol. 631945-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai, M. M. C. 2005. RNA replication without RNA-dependent RNA polymerase: surprises from hepatitis delta virus. J. Virol. 797951-7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, C. Z., P. J. Chen, and D. S. Chen. 1995. Large hepatitis delta antigen in packaging and replication inhibition: role of the carboxyl-terminal 19 amino acids and amino-terminal sequences. J. Virol. 695332-5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehmann, E., F. Brueckner, and P. Cramer. 2007. Molecular basis of RNA-dependent RNA polymerase II activity. Nature 450445-449. [DOI] [PubMed] [Google Scholar]
- 23.Li, Y.-J., T. Macnaughton, L. Gao, and M. M. C. Lai. 2006. RNA-templated replication of hepatitis delta virus: genomic and antigenomic RNAs associate with different nuclear bodies. J. Virol. 806478-6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, Y.-J., M. R. Stallcup, and M. M. C. Lai. 2004. Hepatitis delta virus antigen is methylated at arginine residues, and methylation regulates subcellular localization and RNA replication. J. Virol. 7813325-13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macnaughton, T. B., E. J. Gowans, S. P. McNamara, and C. J. Burrell. 1991. Hepatitis delta antigen is necessary for access of hepatitis delta virus RNA to the cell transcriptional machinery but is not part of the transcriptional complex. Virology 184387-390. [DOI] [PubMed] [Google Scholar]
- 26.Macnaughton, T. B., and M. M. C. Lai. 2002. Genomic but not antigenomic hepatitis delta virus RNA is preferentially exported from the nucleus immediately after synthesis and processing. J. Virol. 763928-3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macnaughton, T. B., and M. M. Lai. 2006. HDV RNA replication: ancient relic or primer? Curr. Top. Microbiol. Immunol. 30725-45. [DOI] [PubMed] [Google Scholar]
- 28.Macnaughton, T. B., and M. M. C. Lai. 2002. Large hepatitis delta antigen is not a suppressor of hepatitis delta virus RNA synthesis once RNA replication is established. J. Virol. 769910-9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macnaughton, T. B., S. T. Shi, L. E. Modahl, and M. M. C. Lai. 2002. Rolling circle replication of hepatitis delta virus RNA is carried out by two different cellular RNA polymerases. J. Virol. 763920-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Modahl, L. E., and M. M. C. Lai. 2000. The large delta antigen of hepatitis delta virus potently inhibits genomic but not antigenomic RNA synthesis: a mechanism enabling initiation of viral replication. J. Virol. 747375-7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Modahl, L. E., and M. M. C. Lai. 1998. Transcription of hepatitis delta antigen mRNA continues throughout hepatitis delta virus (HDV) replication: a new model of HDV RNA transcription and replication. J. Virol. 725449-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Modahl, L. E., T. B. Macnaughton, N. Zhu, D. L. Johnson, and M. M. C. Lai. 2000. RNA-dependent replication and transcription of hepatitis delta virus RNA involve distinct cellular RNA polymerases. Mol. Cell. Biol. 206030-6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monjardino, J. 1996. Replication of hepatitis delta virus. J. Viral Hepat. 3163-166. [DOI] [PubMed] [Google Scholar]
- 34.Moraleda, G., and J. Taylor. 2001. Host RNA polymerase requirements for transcription of the human hepatitis delta virus genome. J. Virol. 7510161-10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mu, J. J., D. S. Chen, and P. J. Chen. 2001. The conserved serine 177 in the delta antigen of hepatitis delta virus is one putative phosphorylation site and is required for efficient viral RNA replication. J. Virol. 759087-9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mu, J. J., Y. G. Tsay, L. J. Juan, T. F. Fu, W. H. Huang, D. S. Chen, and P. J. Chen. 2004. The small delta antigen of hepatitis delta virus is an acetylated protein and acetylation of lysine 72 may influence its cellular localization and viral RNA synthesis. Virology 31960-70. [DOI] [PubMed] [Google Scholar]
- 37.Mu, J. J., H. L. Wu, B. L. Chiang, R. P. Chang, D. S. Chen, and P. J. Chen. 1999. Characterization of the phosphorylated forms and the phosphorylated residues of hepatitis delta virus delta antigens. J. Virol. 7310540-10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rizzetto, M., B. Hoyer, M. G. Canese, J. W. Shih, R. H. Purcell, and J. L. Gerin. 1980. δ Agent: association of δ antigen with hepatitis B surface antigen and RNA in serum of δ-infected chimpanzees. Proc. Natl. Acad. Sci. USA 776124-6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sterner, D. E., and S. L. Berger. 2000. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 64435-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor, J. M. 2006. Structure and replication of hepatitis delta virus RNA. Curr. Top. Microbiol. Immunol. 3071-23. [DOI] [PubMed] [Google Scholar]
- 41.Wang, K.-S., Q.-L. Choo, A. J. Weiner, J.-H. Ou, R. C. Najarian, R. M. Thayer, G. T. Mullenbach, K. J. Denniston, J. L. Gerin, and M. Houghton. 1986. Structure, sequence and expression of the hepatitis delta (δ) viral genome. Nature 323508-514. [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi, Y., J. Filipovska, K. Yano, A. Furuya, N. Inukai, T. Narita, T. Wada, S. Sugimoto, M. M. Konarska, and H. Handa. 2001. Stimulation of RNA polymerase II elongation by hepatitis delta antigen. Science 293124-127. [DOI] [PubMed] [Google Scholar]
- 43.Yang, X. J. 2004. Lysine acetylation and the bromodomain: a new partnership for signaling. Bioessays 261076-1087. [DOI] [PubMed] [Google Scholar]
- 44.Yang, X. J. 2005. Multisite protein modification and intramolecular signaling. Oncogene 241653-1662. [DOI] [PubMed] [Google Scholar]