Abstract
Virus-specific CD8+ T cells play a central role in the control of viral infections, including human immunodeficiency virus type 1 (HIV-1) infection. However, despite the presence of strong and broad HIV-specific CD8+ T-cell responses in chronic HIV-1 infection, these cells progressively lose critical effector functions and fail to clear the infection. Mounting evidence suggests that the upregulation of several inhibitory regulatory receptors on the surface of CD8+ T cells during HIV-1 infection may contribute directly to the impairment of T-cell function. Here, we investigated the role of killer immunoglobulin receptors (KIR), which are expressed on NK cells and on CD8+ T cells, in regulating CD8+ T-cell function in HIV-1 infection. KIR expression was progressively upregulated on CD8+ T cells during HIV-1 infection and correlated with the level of viral replication. Expression of KIR was associated with a profound inhibition of cytokine secretion, degranulation, proliferation, and activation by CD8+ T cells following stimulation with T-cell receptor (TCR)-dependent stimuli. In contrast, KIR+ CD8+ T cells responded potently to TCR-independent stimulation, demonstrating that these cells are functionally competent. KIR-associated suppression of CD8+ T-cell function was independent of ligand engagement, suggesting that these regulatory receptors may constitutively repress TCR activation. This ligand-independent repression of TCR activation of KIR+ CD8+ T cells may represent a significant barrier to therapeutic interventions aimed at improving the quality of the HIV-specific CD8+ T-cell response in infected individuals.
The immune response to infection relies on a highly coordinated set of events involving a variety of cell subsets. The cellular immune response to viruses relies heavily on cytotoxic CD8+ T lymphocytes (CTLs). Despite the efficacy of CD8+ T-cell responses in reducing viral loads in acute human immunodeficiency virus type 1 (HIV-1) infection (21), viral replication persists at a viral set point throughout chronic HIV-1 infection (14). In contrast to the setting of cleared viral infections, CD8+ T cells are not able to eliminate HIV-1 completely (24).
Accumulating evidence suggests that several aspects of a normal CTL response are altered during chronic HIV infection (12, 22, 24) due to exposure to persistent viral replication. During chronic infection, changes within the T-cell compartment include a significant skewing of the hierarchy of the immune response (1, 38), improper maturation of effector T-cell populations (10), and changes in both the phenotypic and the functional quality of the HIV-specific CTL (7). Subjected to the protracted exposure to high doses of antigen, HIV-specific T cells have evolved elaborate homeostatic mechanisms that allow them to resist elimination while, simultaneously, they attempt to dampen immunopathogenesis (36).
While the activation of T cells is dependent on the recognition of specific major histocompatibility complex (MHC)/peptide complexes by the T-cell receptor (TCR), the outcome of this interaction is modulated by a vast array of additional receptor-ligand interactions such as those involved in the adhesion and costimulation of T cells by the target cell (32). Recent studies have shown that a significant subset of CD8+ T cells appears to upregulate inhibitory receptors typically expressed on NK cells following an encounter with antigen (4, 5, 8, 11, 13, 27, 28, 36). These receptors include members of the killer immunoglobulin-like receptors (KIR) that can be both inhibiting and activating (4, 8, 31). In humans, peripheral blood KIR+ CD8+ T cells represent nearly 5% of T cells in a normal healthy adult and can reach up to 30% in elderly subjects (3, 36); these cells become enriched in the setting of infections with influenza virus (19), lymphocytic choriomeningitis virus (28), Listeria monocytogenes (28), hepatitis C virus (8), and HIV (4, 13, 33).
A small number of studies have demonstrated that KIR expression is elevated on CD8+ T cells in asymptomatic HIV-1 infection, predominantly on memory CD8+ T cells, associated with a reduced ability to kill target cells (4, 13, 33). However, the extent of KIR-induced dysregulation at different stages of HIV-1 infection is poorly understood. Here, we demonstrate that KIR expression is dramatically enriched on CD8+ T cells from subjects with ongoing HIV-1 replication. KIR expression was associated with reduced cytokine secretion, proliferation, activation, and killing by antigen-specific CD8+ T cells following stimulation via the TCR. However, stimulation of T cells that did not depend on signals delivered via the TCR (phorbol-12-myristate-13-acetate [PMA]) resulted in potent stimulation of the T cells, suggesting a specific defect in TCR-mediated stimulation of KIR+ CD8+ T-cell function and not a generalized T-cell defect. Furthermore, KIR-associated repression of CD8+ T-cell function occurred in subjects that did or did not possess the respective KIR ligands, demonstrating a ligand-independent repression of CD8+ T-cell function. Thus, these studies demonstrate a generalized suppression of TCR-mediated activity of KIR+ CD8+ T cells during HIV-1 infection via a ligand-independent blockade of TCR activation.
MATERIALS AND METHODS
Subjects.
A total of 50 subjects were recruited for this study, including 10 healthy HIV-1-negative control subjects; 10 untreated viremic HIV-1-infected subjects with an average viral load of 148,270 copies HIV-1 RNA per ml of plasma (range, 8,010 to >750,000 copies per ml) and an average CD4 count of 273 cells per mm3 (range, 42 to 483 cells per mm3); 10 HIV-1-infected subjects receiving highly active antiretroviral therapy (HAART), with undetectable viral loads (<50 copies) and an average CD4 count of 611 cells per mm3 (range, 278 to 803 cells per mm3); 10 subjects that spontaneously control HIV-1 infection without antiretroviral therapy, with an average viral load of 137 copies of RNA per ml plasma (range, <50 to 1,800 copies) and an average CD4 count of 704 cells per mm3 (range, 375 to 1,305 cells per mm3); and 10 acutely HIV-1-infected individuals with an average viral load of 1,526,125 copies per ml (range, 94,000 to >750,000 copies) and an average CD4 count of 715 cells per mm3 (range, 398 to 985 cells per mm3) (Table 1). The MGH institutional review board approved the study, and each subject gave written informed consent for participation in the study.
TABLE 1.
Patient characteristics
| Patients | Mean CD4 count (cells/μl ± SD)a | Mean viral load (RNA copies/ml ± SD) |
|---|---|---|
| Healthy controls | N/A | N/A |
| Untreated chronic | 273 ± 204 | 148,270 ± 294,908 |
| Treated chronic | 611 ± 277 | <50 |
| Controllers | 704 ± 204 | 137 ± 199 |
| Acute | 715 ± 310 | 1,526,125 ± 1,459,987 |
N/A, not applicable. SD, standard deviation.
Phenotyping.
The frequency and phenotype of KIR-expressing CD8+ T cells were assessed by flow cytometry. Peripheral blood mononuclear cells (PBMC) were stained with KIR (CD158a, CD158b, and NKB1)-fluorescein isothiocyanate (FITC), CD3-Pacific Blue, CCR7 PECy7 (Becton Dickinson, San Jose, CA), CD45RA PECy5.5 (Caltag, Burlingame, CA), CD4 Qdot655, and CD8 Qdot605 (Custom Conjugates). For the coexpression of inhibitory molecules, PBMC were stained with PD-1-allophycocyanin (APC), CD57-biotin, KIR (CD158a, CD158b, and NKB1)-phycoerythrin (PE), CD3-Pacific Blue, and CD8-APCCy7 (BD Biosciences); washed and stained with streptavidin Cascade Yellow (Invitrogen); washed, fixed, and permeabilized; and then stained with CTLA-4-PE (BD Biosciences). A minimum of 2 × 105 cells was acquired on an LSRII cytometry system, and data were analyzed with FlowJo.
TCR staining.
To determine whether KIR+ CD8+ T cells expressed TCR αβ or γδ, PBMC were stained with KIR (CD158a, CD158b, and NKB1)-PE, TCRαβ-APC, TCRγδ-FITC, CD3-Pacific Blue (Becton Dickinson, San Jose, CA), CD4 Qdot655, and CD8 Qdot605 (Custom Conjugates). A minimum of 2 × 105 cells was acquired and then analyzed with FlowJo software.
Intracellular cytokine staining.
The proportion of cytokines secreting and degranulating CD8+ T cells was quantified by multiparameter intracellular cytokine staining. One million cells were stimulated with peptides corresponding to HIV-1 epitopes (2 μg/ml), the CEF peptide pool (cytomegalovirus [CMV]-, Epstein-Barr virus [EBV]-, and influenza virus-optimal epitopes) (NIH AIDS Reagent Bank) (2 μg/ml), or staphylococcal enterotoxin B (SEB; Sigma Aldrich, St. Louis, MO) (2 μg/ml); medium alone served as the negative control, and PMA (2.5 μg/ml) and ionomycin (0.5 μg/ml) served as a positive control. Brefeldin A (Sigma, St. Louis, MO) (5 μg/ml), Golgi Stop (3 μg/ml), anti-CD28 (1 μg/ml), anti-CD49d (1 μg/ml), and anti-CD107a-PECy5 (10 μg/ml) (BD Biosciences, San Jose, CA) were added to cells, which were then incubated for 6 h at 37°C in 5% CO2. PBMC were stained for surface markers with KIR (CD158a, CD158b, and NKB1)-PE, CD3-Pacific Blue, (Becton Dickinson, San Jose, CA), CD4 Qdot655, and CD8 Qdot605 (Custom Conjugates) (Caltag, Burlingame, CA) for 30 min. Samples were then fixed and permeabilized according to the manufacturer's directions (Caltag, Burlingame, CA) and stained for intracellular gamma interferon (IFN-γ)-PECy7, interleukin-2 (IL-2)-APC, and tumor necrosis factor (TNF)-Alexa 700 (Becton Dickinson, San Jose, CA) for an additional 30 min. After cells were washed, they were resuspended in 1% paraformaldehyde (Sigma, St. Louis, MO) until acquisition was performed on an LSRII (BD Biosciences, San Jose, CA) system.
T-cell activation assay.
Differences between the activation of KIR+ CD8+ T cells and that of KIR− CD8+ T cells were assessed by the quantification of CD69 expression on the surface of CD8+ T cells following stimulation. One million cells were stimulated with SEB (2 μg/ml; Sigma Aldrich, St. Louis, MO), anti-CD3 (OKT3; Biolegend), medium alone (as a negative control), and PMA (2.5 μg/ml) and ionomycin (0.5 μg/ml) (as a positive control). Anti-CD28 (1 μg/ml) and anti-CD49d (1 μg/ml; BD Biosciences, San Jose, CA) were added, and the samples were incubated for 6 h at 37°C in 5% CO2. The cells were then stained with KIR (CD158a, CD158b, NKB1)-FITC, CD3-Pacific Blue, CD8-APCCy7, and CD69-PECy7 (BD Biosciences San Jose, CA) for 30 min. Samples were then fixed in 1% paraformaldehyde (Sigma, St. Louis, MO) until four-color flow cytometry analysis was performed on an LSRII instrument (BD Biosciences, San Jose, CA). Two hundred thousand to 106 events were acquired and analyzed using FlowJo software.
Proliferation assay.
PBMC were resuspended to 106 cells/ml in phosphate-buffered saline (PBS) and incubated at 37°C for 7 min with 0.25 μM carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes). After the cells received serum and were washed with PBS, they were resuspended at 106 cells/ml in RPMI medium supplemented with glutamine, 10% fetal calf serum, penicillin, and streptomycin. No exogenous cytokines were added to the medium. Pools of overlapping HIV-1-specific peptides representing the entire amino acid sequence of either Gag, Nef, or Pol were then added at a concentration of 20 ng/ml per peptide. Phytohemagglutinin (PHA; Sigma, St. Louis, MO) and SEB (Sigma, St. Louis, MO) were used as positive controls, and medium alone served as the negative control. On day 6, cells were harvested, washed with PBS, and stained with purified KIR (CD158a, CD158b, NKB1)-PE, CD3-Pacific Blue, CD4-Qdot655, and CD8-Qdot605. Cells were then fixed in 1% paraformaldehyde and acquired on an LSRII machine. The mean background proliferation was calculated based on the proliferation fraction of cells in the negative wells containing medium alone. Thus, the antigen-specific proportion of proliferating cells was calculated by subtracting the proportion of proliferating cells in unstimulated samples from the proliferating fraction in the stimulated wells.
Chromium release cytotoxicity assay.
The ability of CD8+ T cells to kill HLA-matched B cell lines was examined using a standard chromium release cytotoxicity assay. A CD8+ T-cell clone specific for the HLA-B51-restricted epitope (TAFTIPSI [TI8]) in HIV-1 HXB2 that expressed CD158b (specific for KIR2DL2/L3/S3 but not the other KIR2DL2/L3/S3 ligand CD158a or NKB1) was sorted into CD158b+ and CD158b− CD8+ T cells as the cells were labeled with the CD158b-PE antibody and then sorted into the two populations using Miltenyi magnetic beads directed at the PE stain (Miltenyi Biotech, Auburn, CA). The resulting separation resulted in an average purity range of 93.2 to 97.4% in the CD158b+ cells and only 0.1 to 0.3% contamination of these cells in the CD158b− cells remaining in the eluted sample. The abilities of these two populations of sorted CD8+ T cells to kill peptide-loaded HLA-matched B cell lines were compared to that of the bulk B51-TI8-specific CD8+ T-cell clone. Target cells included 2 × 106 HLA-C1/C1, -C2/C2, or -C1/C2 HLA-B51-matched B cells which were labeled with 50 μCi of 51CrO4-Na2 (1 Ci = 37 GBq; New England Nuclear) for 1 h at 37°C in 5% CO2. B51-TI8-specific T cells were added as effectors at effector-to-target (E:T) ratios of 10:1 and 25:1. Supernatant was harvested after a 4-h incubation at 37°C in 5% CO2. The percentage of lysis was calculated as (sample count − spontaneous release)/(maximal release − spontaneous release) × 100.
HIV inhibition assay.
The antiviral effects of KIR+ and KIR− CD8+ T cells were assessed using the viral inhibition assay. Two CD8+ T-cell clones were employed. The first was an HLA-B51-restricted T-cell clone directed at TI8, and the second was a CD8+ T-cell clone that recognizes the B27 epitope KRWIILGLNK (KK10) in HIV-1 HXB2. A small population of both clones expressed CD158b (specific for KIR2DL2/L3/S3) but not CD158a or NKB1. PBMC from the same donor from which the clones were generated were thawed and resuspended in 10 ml of R10 medium supplemented with 0.5 μg/ml of a bispecific antibody directed at CD3 and CD8 in the presence of 50 units/ml of IL-2. After 4 days, the resulting suspension was composed largely of activated, pure CD4+ T cells. The cells were counted and infected at a multiplicity of infection of 0.1 with the R5 virus JRCSF or the X4 virus IIIB for 3 h. In parallel, the clones were sorted into KIR+ and KIR− populations, using a FACSAria cell sorting system. The resulting purity was >98% (range, 97.5 to 99.1%) for both clones. HIV-infected CD4+ T cells were then cocultured for 14 days in the presence or the absence of autologous KIR+ or KIR− CD8+ T cells at an E:T ration of 1:1. Supernatant was then collected every 3 to 4 days for 14 days. The level of viral replication was then quantified by p24 enzyme-linked immunosorbent assay. The overall capacity of T cells to inhibit viral replication was calculated as the difference between the p24 production in wells containing CD8+ T cells and that in wells where HIV-infected CD4+ T cells were cultured alone.
Statistical analysis.
Analysis of variance was employed to analyze differences among groups. A post hoc Tukey's test was then employed for comparisons that were significant. P values of less then 0.05 were considered significant.
RESULTS
Increased KIR− expression on CD8+ T cells in viremic HIV-1 infection.
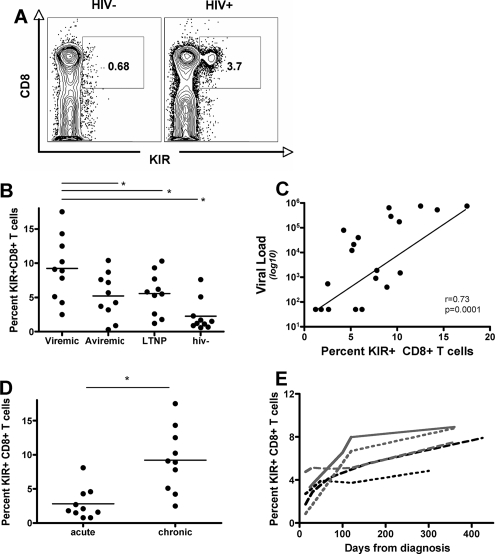
Profound changes in the phenotype of CD8+ T cells occur during HIV-1 infection (10). These changes appear to differ among subjects who are treated with suppressed viral replication and those who exhibit persistent viral replication. Previous work has demonstrated a modulation of KIR expression on CD8+ T cells; however, these studies were performed with small groups of subjects with quite different clinical characteristics (4, 13, 33), making it difficult to ascertain the precise level of NK cell receptor accumulation on CD8+ T cells during different stages of HIV-1 infection. Thus, we compared the levels of KIR− expression, using a pool of KIR antibodies (CD158a for KIR2DL1/2DS1, CD158b for KIR2DL2/L3/S3, and NKB1 for KIR3DL1), on the surface of CD8+ T cells from subjects with treated aviremic, viremic, and controlled HIV-1 infection, as well as those from HIV-1-negative subjects (Table 1). An average of 12.2% (range, 4.23 to 19.1%), 8.2% (range, 3.7 to 13.2%), 6.4 (range, 1.11 to 15.8%), and 2.1% (range, 0.58 to 7.6%) of CD8+ T cells expressed KIR in subjects with chronic viremic infection, treated aviremic infection, and spontaneous controlled infection compared to HIV-1-negative control subjects, respectively (P < 0.05 for all comparisons) (Fig. 1B). Furthermore, the proportion of KIR+ CD8+ T cells correlated with the level of HIV-1 replication, suggesting that the level of viral replication drives the expansion of larger proportions of KIR+ CD8+ T cells (r = 0.73; P = 0.0001) (Fig. 1C). Interestingly, despite the reduction of viral loads to undetectable levels with antiretroviral therapy, the proportion of KIR+ CD8+ T cells in HIV-positive subjects never reached the levels of those seen in HIV-1-uninfected controls (Fig. 1B). Similarly, despite low levels of detectable HIV-1 replication in controllers, the proportion of KIR+ CD8+ T cells was significantly expanded compared to that of HIV-1-uninfected controls (P < 0.05, KIR) (Fig. 1B). These data suggest that populations of KIR+ CD8+ T cells are induced in the setting of active viral replication and may persist in the settings of HAART and spontaneous control due to the continued low-level replication of the virus in tissue compartments, resulting in a significant continuous expansion of KIR+ CD8+ T cells in chronic HIV infection.
FIG. 1.
Increased expression of KIR on CD8+ T cells occurs with persistent viral replication. (A) The flow plots demonstrate the dramatic increase of KIR+ CD8+ T cells in a representative HIV-positive subject (right panel) compared to that in a negative control (left panel). (B) The dot plot represents the overall size of the KIR+ CD8+ T-cell population in subjects at different stages of HIV-1 infection, demonstrating significantly higher proportions of KIR+ CD8+ T cells in subjects with untreated HIV-1 infection and reduced but not normal levels of these cells in treated chronic infection subjects or controllers than in HIV-negative controls. (C) The proportion of KIR+ CD8+ T cells correlates with the level of viral replication. (D) The dot plot shows the size of the KIR+ CD8+ T-cell population in subjects in the acute and chronic untreated infection groups, demonstrating that these cells accumulate with progressive infection. (E) The line graph shows the delayed accumulation of KIR+ CD8+ T cells with disease progression in a total of five subjects in the acute HIV infection group (less than three bands in the Western blot analysis). *, P < 0.05.
Longitudinal accumulation of KIR+ CD8+ T cells.
The above-reported data demonstrated the accumulation of KIR+ CD8+ T cells in chronic HIV-1 infection and are in line with previous data (4, 13, 33). However, it is uncertain at what point during the infection these KIR+ T cells begin to expand. To determine whether these cells are present from the time of infection or whether they expand due to exposure to viral replication, we quantified the proportion of KIR+ CD8+ T cells in a group of 10 acutely HIV-1-infected individuals who all had less than three bands by Western blotting at diagnosis. KIR+ CD8+ T cells were observed to be at significantly lower proportions during acute HIV-1 infection than that in individuals with chronic, untreated HIV-1 infection (P = 0.008) (Fig. 1D). Furthermore, longitudinal quantification of the accumulation of KIR+ CD8+ T cells starting in acute HIV-1 infection was performed with five untreated individuals (Fig. 1E). The proportion of KIR+ CD8+ T cells accumulated steadily over the first year of infection, suggesting that persistent viral replication may have contributed to the steady increase of this subset of CD8+ T cells (Fig. 1E).
KIR+ CD8+ T cells are enriched in HIV-specific memory CD8+ T cells.
As described previously, KIR expression is restricted to memory CD8+ T cells (4, 29). These observations were confirmed in the context of HIV infection (Fig. 2A). Additionally, some but not all KIR+ CD8+ T cells expressed several additional markers of anergy/exhaustion, including programmed death receptor 1 (PD-1), CD57, and CTLA-4 (Fig. 2D) (9, 12, 20). Furthermore, given that KIR expression was restricted primarily to memory CD8+ T cells, we were interested in determining whether these receptors were preferentially expressed on HIV-specific CD8+ T cells. Thus, KIR expression was assessed on the surface of tetramer-positive populations from a total of four HIV-positive individuals. Chronically infected individuals who exhibited strong CD8+ T-cell responses to the HLA-B8-restricted Nef epitope FLKEKGGL (FL8) were identified by use of an IFN-γ enzyme-linked immunospot (ELISpot) assay (34). The expression of KIR on the surface of B8-FL8-specific CD8+ T cells was heterogeneous among the four individuals. KIR was expressed at an average of 38.3% (range, 18 to 64.1%) of the tetramer-positive antigen-specific cells. Only tetramer-positive KIR− HIV-specific CD8+ T cells were able to produce IFN-γ following stimulation compared to that of tetramer-positive KIR+ cells (P < 0.001) (Fig. 2C). Similar results were obtained with the B27-KK10-specific CD8+ T cells and the B57-KF11-specific CD8+ T cells (data not shown). Overall, KIR was expressed on a subset of HIV-specific CD8+ T cells that respond poorly to antigenic stimulation.
FIG. 2.
KIR is expressed on memory HIV-specific CD8+ T cells. (A) The flow cytometric plots show the effector memory phenotype of KIR+ CD8+ T cells from a representative subject. (B) The plots depict the gating strategy employed to gate on tetramer-positive CD8+ T cells (top line), which show the reduced potential of B8-FL8-specific CD8+ T cells expressing KIR to secrete cytokines following stimulation (lower line). (C) The bar graph demonstrates the overall reduced capacity of tetramer-positive CD8+ T cells that express KIR to secrete cytokines. (D) The flow plots depict the distribution of the additional inhibitory receptors PD-1, CD57, and CTLA-4 on KIR− and KIR+ CD8+ T cells from a representative individual. (E) The overall expression proportions of the receptors PD-1, CD57, and CTLA-4 are shown in the bar graph for a group of HIV-negative individuals (n = 6), showing a trend toward elevated levels of CD57 on KIR+ CD8+ T cells.
KIR+ CD8+ T cells are functionally compromised.
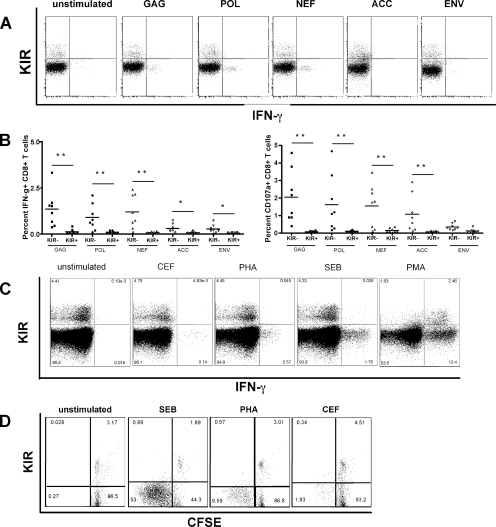
Antigen-specific CD8+ T-cell function becomes progressively compromised in chronic viral infections (22, 23). Given that KIR+ CD8+ T cells appear to accumulate with progressive infection and are antigen specific but respond poorly to stimulation with peptide, we were intrigued to determine whether the expression of KIR on CD8+ T cells was associated with different levels of CD8+ T-cell dysfunction in response to a variety of different stimuli. Thus, multiparameter flow cytometry was performed with PBMC stimulated with various antigens. PBMC were stimulated with pools of overlapping HIV-Gag, -Nef, -Env, -Pol, accessory proteins (Tat, Rev, Vpu, Vif, Vpr), and envelope peptide pools. Expression of KIR on the surface of CD8+ T cells was associated with a significant reduction in CD107a and IFN-γ (Fig. 3A and B) (P < 0.05 for all comparisons) and in MIP-1β and a complete loss of TNF-α and IL-2 (data not shown) following stimulation with peptide pools and PHA. Thus, overall, the expression of KIR on CD8+ T cells was associated with a significant decline in cytokine secretion and degranulation following stimulation with peptides or PHA.
FIG. 3.
KIR expression results in reduced TCR-mediated CD8+ T-cell activation. (A) The flow plots demonstrate the reduced capacity of KIR+ CD8+ T cells from a representative HIV-positive subject to secrete cytokines following stimulation with HIV peptide pools. (B) The dot plots show the level of IFN-γ secretion (left panel) and CD107a upregulation (right panel) on KIR+ and KIR− CD8+ T cells following stimulation with peptide pools spanning individual HIV gene products. (C) Flow plots depict the ability of KIR+ and KIR− CD8+ T cells to secrete IFN-γ following stimulation via the TCR (CEF, PHA, and SEB) and PMA, which activates T cells outside the TCR in a single representative subject. (D) The diagram shows the level of CFSE dilution in KIR+ and KIR− CD8+ T cells following stimulation and demonstrates that proliferation is also impaired in KIR+ CD8+ T cells.
Given the remarkably low level of effector activity of KIR+ CD8+ T cells, we were interested in determining whether CD8+ T cells that expressed these NK cell receptors were unable to respond to more potent stimuli, such as the immunodominant pool of CEF, PHA, SEB, or PMA. To eliminate the confounding factor of HIV-1 infection-induced T-cell dysfunction and look at the pure effect of KIR expression on CD8+ T-cell function, we examined the ability of KIR+ CD8+ T cells from HIV-uninfected controls to respond to these stimuli. Stimulation with either PHA or SEB induced potent degranulation and secretion of cytokines by KIR− CD8+ T cells (Fig. 3C). Interestingly, all the cells that responded to CEF also belonged to the KIR− CD8+ T-cell population, implying that virus-specific cells that are not chronically exposed to antigen express lower levels of KIR. In contrast, CD8+ T cells expressing KIR were unable to respond to stimulation with either of these stimuli that are delivered via the TCR (Fig. 3C). Surprisingly, both KIR+ and KIR− CD8+ T cells secreted cytokines and degranulated in response to stimulation with PMA (Fig. 3C), which occurs independently of TCR engagement. Furthermore, the population of KIR+ CD8+ T cells responded more potently than the negative CD8+ T cells did to stimulation with PMA, likely due to the fact that these cells belong to an effector memory phenotype. These data demonstrate that the defect in KIR+ CD8+ T-cell activity is specific to stimulation through the TCR and is not an intrinsic defect in cell function.
Antigen-specific proliferation is an additional critical functional readout of T-cell function that is lost with progressive infection (22). To determine whether KIR expression affects the ability of T cells from healthy controls to proliferate following stimulation with the pool of CEF peptides, SEB, or PHA, we measured CFSE dilution following 6 days of in vitro stimulation in six HIV-1-negative controls. Similar to the defect observed with respect to cytokine secretion and degranulation, KIR+ CD8+ T cells exhibited a markedly reduced proliferative capacity following stimulation, while KIR− CD8+ T cells from the same individuals proliferated readily (Fig. 3D). These data demonstrate that additional critical effector CD8+ T-cell functions are also suppressed in KIR+ CD8+ T-cell subpopulations.
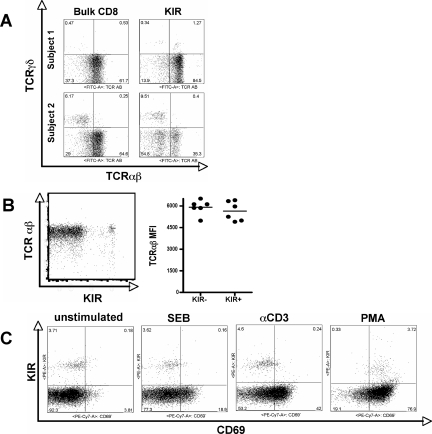
KIR+ CD8+ T cells express normal levels of αβ-positive TCR.
KIR can be expressed on both αβ and γδ T cells (15). Given that γδ T cells have been shown to accumulate in chronic HIV-1 infection (16, 35), we sought to determine whether the accumulation of KIR was due simply to the accumulation of γδ T cells in chronic HIV-1 infection. Thus, PBMC were stained with antibodies against both αβ and γδ in a group of six HIV-positive individuals. As described previously, KIR− expression was observed on the surface of both αβ and γδ CD8+ T cells. The distribution of KIR on the surface of both T-cell subsets varied greatly among subjects, but the larger populations of KIR+ T cells always appeared to belong to the αβ+ T-cell populations (Fig. 4A). Thus, the accumulation of KIR in chronic HIV-1 infection is due to the upregulation of these molecules on αβ+ T cells rather than to the accumulation of γδ T cells.
FIG. 4.
Normal expression of αβ TCR on KIR+ CD8+ T cells. To evaluate whether KIR+ CD8+ T cells were unable to respond to stimulation due to reduced TCR levels or to the expression of the γδ TCR, we evaluated TCR expression on KIR+ T cells. (A) The proportion of αβ and γδ TCR+ CD8+ T cells was assessed on bulk CD8+ T cells (left panel) and KIR+ CD8+ T cells (right panel) from two subjects. (B) The mean fluorescence intensity (MFI) of αβ TCR was evaluated on the surfaces of KIR+ and KIR− CD8+ T cells from a single representative subject (left panel) and from six separate individuals (right panel), demonstrating that reduced T-cell activation is not attributable to lower TCR expression. (C) The potential of KIR+ and KIR− CD8+ T cells to become activated following stimulation via the TCR (SEB and anti-CD3 antibody) or outside of the TCR with PMA/ionomycin was evaluated by the upregulation of CD69 in a representative subject.
Given that KIR+ T cells were especially impaired in their ability to respond to stimuli elicited through the TCR, we speculated that KIR+ T cells might express reduced levels of TCR on their surfaces. Thus, we compared levels of expression of αβ TCR on the surfaces of KIR+ and KIR− T cells. There was no difference in the level of αβ TCR mean fluorescence intensity on the surface of KIR+ T cells compared to that on the surface of KIR− T cells from a total of six subjects (Fig. 4B). These data demonstrate that reduced activation in KIR+ T cells is due to an active suppression of TCR signaling rather than to a constitutive downregulation of TCR expression in the presence of these innate immune receptors.
KIR expression is associated with reduced TCR-mediated activation of CD8+ T cells.
Inhibitory molecules can block T-cell activation at different levels of the signaling cascade, affecting the effector functions differentially. To determine whether KIR expression blocked only downstream activity (i.e., cytokine secretion and degranulation) while sparing T-cell activation (CD69 expression), we compared the ability of KIR+ T cells to upregulate CD69 with that of KIR− T cells following stimulation. Stimuli elicited via the TCR did not activate KIR+ CD8+ T cells to upregulate CD69 (Fig. 4C), while KIR− CD8+ T cells were potently activated. In contrast, nearly all KIR+ CD8+ T cells upregulated CD69 following stimulation with PMA/ionomycin, which drives T-cell activation in a TCR-independent manner. These data demonstrate that KIR+ CD8+ T-cell dysfunction occurs early in the T-cell activation cascade, prior to the generation of signals to induce cytokine secretion or degranulation.
KIR+ CD8+ T cells exhibit poor antiviral activity.
Ultimately, to date the most critical measure of an antiviral CD8+ T-cell response hinges on its capacity to suppress HIV-1 replication in vitro. Thus, to determine whether any differences existed between the antiviral activity of KIR+ and that of KIR− CD8+ T cells derived from the same clone (Fig. 5A), we performed a viral inhibition assay with these two separate cell groups. Thus, KIR+ and KIR− CD8+ T cells from two different CD8+ T-cell clones, B51-TI8 and B27-KK10, were sorted and cocultured with HIV-1-infected autologous purified CD4+ T cells. The levels of viral replication of the KIR+ CD8+ T cells were compared with those of the KIR− CD8+ T cells from each clone and with the levels of viral replication observed for the wells containing CD4+ T cells alone. KIR− and not KIR+ CD8+ T cells suppressed viral replication in autologous CD4+ T cells (Fig. 5B). These data suggest that the expression of KIR is associated with a loss of antiviral activity that is exhibited by the KIR− CD8+ T-cell clones.
FIG. 5.
KIR+ CD8+ T-cell clones suppress viral replication poorly. To determine whether KIR expression affects the quality of CD8+ T-cell clone-mediated control of viral replication in autologous HIV-infected CD4+ T cells, we performed a viral inhibition assay using KIR+ and KIR− T cells derived from two clones, a B51-TI8-specific clone (left panel) and a B27-KK10-specific clone (right panel). (A) The plots show the gating strategy used to sort KIR+ and KIR− CD8+ T cells derived from two separate clones (at left, B51-TI8; at right, B27-KK10). (B) The level of viral replication was measured by p24 enzyme-linked immunosorbent assay and was monitored over the course of 2 weeks in wells containing CD4+ T cells alone (solid gray), cocultures of KIR− CD8+ T cells with CD4+ T cells (filled), and KIR+ CD8+ T cells with CD4+ T cells (dashed line). Reduced levels of viral replication were observed in cocultures containing KIR− but not KIR+ clones, demonstrating that the expression of KIR on CD8+ T-cell clones is associated with a loss of antiviral HIV-specific control in vitro.
KIR-associated functional blockade is independent of ligand expression.
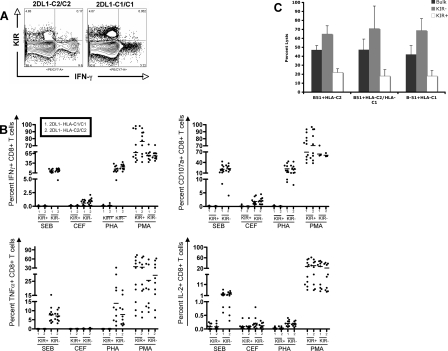
Previous studies suggested that the inhibition of CD8+ T-cell activity by KIR is mediated in a ligand-dependent manner (17, 18). Thus, in these studies KIR+ CD8+ T cells were able to lyse B cell targets that did not express the KIR− ligand, whereas targets expressing the ligand were not lysed, suggesting that engagement of KIR to its ligand was necessary to suppress TCR activation. However, in our larger ex vivo intracellular staining data set, we observed a generalized suppression of KIR+ T-cell activity following stimulation via TCR, despite the fact that not all individuals possessed ligands for all their KIR. Thus, to try to understand whether KIR− ligand interaction was critical, we selected a subgroup of six HIV-negative individuals based on their genotype that expressed KIR2DL1 in the presence of a group HLA-C2 allele (n = 3) or that expressed KIR2DL1 in the absence of any group C2 alleles (n = 3) (the ligand for KIR2DL1 is HLA-C2 alleles). PBMC from these individuals were then stimulated with CEF, SEB, PHA, and PMA/ionomycin and monitored for cytokine production and degranulation of CD158a (KIR2DL1/2DS1)+, CD158b (KIR2DL2/3/2DS2)+, and NKB1 (KIR3DL1)+ subpopulations. There were no differences between the levels of degranulation or cytokine secretion among individuals that expressed the KIR ligand and those who did not, for all stimuli tested (Fig. 6A and B). These data suggest that the presence of the ligand was not required to block T-cell activation.
FIG. 6.
KIR-associated inhibition of CD8+ T-cell activity is independent of the presence of its ligand. To understand whether KIR ligation was required for T-cell inactivation, we evaluated the level of KIR+ CD8+ T-cell activation in subjects that did or did not express the ligand, HLA-C2 alleles, for KIR2DL1. (A) The flow plot shows the ability of KIR+ CD8+ T cells to secrete IFN-γ following stimulation with SEB in an individual that is homozygous for the KIR2DL1 ligand HLA-C2 (left panel) compared with that of an individual that does not express the ligand (right panel). (B) The dot plot shows the levels of IFN-γ secretion (top left), CD107a upregulation (top right), TNF-α secretion (bottom left), and IL-2 secretion (bottom right) by KIR+ and KIR− CD8+ T cells (n = 6), three of which were homozygous for HLA-C2, and three of which did not express the ligand. (C) The bar graph shows the percent of lysis of HLA-B51+ B cells loaded with the HIV-B51-restricted epitope TI8 that either coexpressed or did not express HLA-C2, by autologous KIR+ and KIR− CD8+ T-cell clones specific to the B51-TI8 peptide. These data demonstrate the ligand-independent impairment of KIR+ CD8+ T cells.
To further determine whether KIR engagement was required for the inhibition of T-cell killing of target cells, an HIV-specific HLA-B51-restricted CD8+ T-cell clone specific for the TI8 epitope in HIV-1 HXB2 that expressed CD158b (KIR2DL2, KIR2DL2/KIR2DS2) in the absence of CD158a (KIR2DL1/2DL2/2DS1) and NKB1 (KIR3DL1) was sorted into two populations, one that expressed CD158b and one that did not express CD158b. Three different B cell lines were used as targets: an HLA-B51 + HLA-C2 homozygous, an HLA-B51 + HLA-C2/C1 heterozygous, and an HLA-B51 + HLA-C1 homozygous B cell line. Peptide-loaded target cells were labeled with chromium and cocultured for a total of 6 h in the presence of the bulk, CD158b+ and CD158b− HLA-B51-restricted CD8+ T cells specific for TI8. CD158b+ T-cell clones killed all target cells poorly compared to both the bulk and the KIR− CD8+ T-cell clones, irrespective of the HLA-C alleles on the target cell (Fig. 6C). Although we observed a slight reduction in the ability of CD158b+ T cells to kill target cells that expressed the C1 ligand, the difference was not statistically significant compared to that of the C2 homozygous and the heterozygous cell lines. These data suggest that ligand interaction is not necessary for the inhibition of KIR+ T-cell killing.
DISCUSSION
Despite the presence of significant numbers of HIV-specific CD8+ T cells during active chronic HIV-1 infection, mounting evidence has demonstrated that these cells become progressively impaired (22, 23). Recently, it has been suggested that the upregulation of inhibitory receptors on the surface of CD8+ T cells may contribute to the gradual dysregulation of cellular function (12). In the absence of infection, these inhibitory receptors are upregulated on CD8+ T cells progressively with age and have also been observed to expand in the setting of other viral infections (4). Here we demonstrate that a family of receptors that are normally expressed on NK cells, killer immunoglobulin-like receptors, are significantly expanded with progressive HIV-1 infection on the surfaces of CD8+ T cells and that the level of KIR expression appears to be driven by the level of viral replication in vivo. KIR expression was observed to be upregulated preferentially on the surface of memory antigen-specific CD8+ T cells and was associated with significantly reduced secretion of MIP-1β, TNF-α and IFN-γ, impaired degranulation, obstructed cell proliferation, and reduced T-cell activation in response to TCR-mediated stimulation. In contrast, PMA/ionomycin stimulation resulted in potent KIR+ CD8+ T-cell activation. Furthermore, the expression of KIR was associated with a loss of the potential to suppress viral replication in vitro. Interestingly, impaired T-cell function was observed for the absence of KIR ligand engagement, suggesting that ligation of the receptor is not required for the inhibition of T-cell function. Overall, these data demonstrate that KIR is upregulated over time on CD8+ T cells in the presence of protracted exposure to HIV-1 replication and is associated with a generalized blockade of TCR signaling.
Unlike cells of the innate immune system that proliferate in response to infection and then perish upon resolution, antigen-specific cells of the adaptive immune system must persist to maintain a memory response. Thus, T cells have developed a number of different homeostatic mechanisms to prevent their elimination following antigenic stimulation. These include downmodulation of TCR (25), dephosphorylation of signaling molecules (30), and upregulation of inhibitory receptors that prevent chronic activation-induced cell death (12, 36). In the case of the last mechanism, several inhibitory receptors have been identified recently on the surface of virus-specific T cells in the setting of persistent viral replication (37), while these molecules are normally observed only transiently following peak viral replication in the setting of a resolved acute infection (6). These molecules are therefore likely to play a critical role in the homeostatic control of virus-specific memory and are involved in protecting virus-specific cells from activation-induced cell death, which would result in clonal deletion of antigen-specific T cells. These molecules include PD-1, CTLA-4, CD85J, NKG2A, and KIR (4, 12, 20, 36).
Inhibitory NK cell receptor expression has been described on the surface of CD8+ T cells in HIV-1-negative individuals and accumulates significantly with age (3, 36). Previous studies have demonstrated an increase of KIR expression on CD8+ T cells in HIV-1-infected individuals (4, 13, 33); however, these studies were performed with small groups of poorly clinically defined patient populations. We quantified the expression of KIR (including KIR2DL1/2DS1/2DL2/2DS2/2DL3/3DL1) on the surface of CD8+ T cells from a group of 50 well-characterized subjects. Here we demonstrate a significant upregulation of KIR on CD8+ T cells of subjects with ongoing viral replication and show that the expression of KIR correlates with the level of HIV-1 replication, suggesting that the virus itself or the associated immune activation directly drives the expansion of these cells. Interestingly, KIR expression declined in aviremic subjects receiving HAART; however, the levels of this receptor never reached those levels seen in HIV-1-negative controls. Similarly, the level of KIR was elevated in individuals who spontaneously controlled viral replication at very low levels, suggesting that some persisting low-level viral replication in tissue compartments such as the gut or lymph nodes may continue to drive the persistence of these markers on the surface of antigen-specific CD8+ T cells.
KIR expression on CD8+ T cells was associated with a loss of multiple effector functions, including cytokine secretion, degranulation, proliferation, activation, killing of target cells, and inhibition of viral replication in vitro. Remarkably, functional blockade by these markers was observed only for stimuli that were mediated via the TCR. Stimulation of PBMC with PMA/ionomycin elicited potent stimulation of KIR+ CD8+ T cells at levels significantly higher than those observed for KIR− CD8+ T cells, suggesting that these cells are not exhausted but rather blocked. It is likely that increased responsiveness to PMA/ionomycin is due to the fact that the KIR+ CD8+ T cells display an effector memory phenotype and are therefore primed to respond potently upon stimulation. Furthermore, inhibition of TCR activation was independent of whether the signal was administered in a conventional MHC/peptide-dependent manner or whether they were administered on the outside of the binding groove by PHA cross-linking or superantigen ligation, demonstrating an antigen-independent repression of TCR signaling. Thus, KIR+ T cells are not inherently exhausted, as they respond potently to TCR-independent stimuli but are unable to respond to stimuli administered via the TCR.
Previous studies that examined the role of the KIR blockade on TCR function utilized a limited number of T-cell clones to address whether KIR ligand engagement was necessary for inhibitory KIR activity (17). However, KIR ligand expression is variable within a population. Thus, according to these previous studies, KIR+ CD8+ T cells should be repressed only in the context of ligand expression. However, CD158a+ (which recognizes KIR2DL1/S1/S2) CD8+ T cells were unresponsive to stimulation with peptides, PHA, and SEB in both subjects that were homozygous for the ligand (HLA-C2), as well as in subjects that did not carry genes to code for the ligand. We therefore hypothesized that in contrast to previous data suggesting ligand-induced repression of KIR+ CD8+ T cells, KIR expression induced a blockade of T-cell function in a ligand-independent manner. To further confirm this phenomenon, we sorted an HIV-1-specific HLA-B51-restricted CD8 T-cell clone that expressed only HLA-C binding KIR recognized by the CD158b antibody (KIR2DL2/L3/S3) and sorted these cells into a population that expressed KIR and a population that did not express this KIR. To further eliminate the confounding factor of HLA-E binding inhibitory receptors, we ensured that the sorted cell populations did not express NKG2A. HLA-matched peptide-loaded B cell lines expressing C1 or C2 HLA-C alleles were lysed effectively by both the bulk CD8+ T-cell clones and the KIR− CD8+ T-cell clones, but the KIR+ CD8+ T-cell clones lysed all peptide-loaded HLA-matched B cell clones ineffectively, irrespective of the HLA-C expressed on their surface. It is possible that differences reported in this study and the previous studies may reflect differences in techniques employed to cultivated CD8+ T-cell clones. However, our data demonstrate that fresh ex vivo KIR+ CD8+ T-cell function is blocked even in the absence of KIR ligands and that sorted KIR+ CD8+ T-cell clones are unable to kill peptide-loaded target cells irrespective of ligand expression on the target cells. These data suggest that KIR represses TCR signaling in a constitutive manner without the need for ligand interaction.
Two potential models that could explain the interference of KIR with TCR activation are conceivable. The first model would involve the exclusion of TCR from engagement with MHC-class I alleles, as KIR also binds to these alleles and could therefore mask MHC from engaging and activating TCR. However, this scenario is unlikely as SEB and PHA engagement occur on the outside of the MHC-class I binding groove and because KIR affinity for MHC is significantly lower than that of TCR (26). The second and more likely scenario is that KIR may be recruited into the TCR synapse upon engagement with MHC-class I by TCR. Due to the constitutive association of KIR with the phosphatases SHP-1 and -2, KIR may draw these phosphatases in sufficient proximity to the TCR and thus block downstream signaling events. The latter scenario may also account for the ligand-independent effect of KIR, as ligand engagement is not always required for the association of KIR with SHP1/2 (2) and can therefore block the activation of T cells at the synapse without the requirement of ligand interaction.
Thus, overall, HIV-1 infection results in a significant accumulation of KIR on the surface of HIV-1-specific CD8+ T cells. This homeostatic mechanism that may be aimed at reducing unabated CD8+ T-cell activity might contribute to the overall generalized dysfunction observed for HIV-specific CD8+ T cells during chronic HIV infection. The fact that these receptors have the potential to repress T-cell activation in the absence of ligand interaction poses a difficult obstacle for immunotherapeutic interventions designed to overcome T-cell dysfunction and suggests that interventions that target inhibitory receptor signaling might be needed to reconstitute T-cell function in chronic persistent viral infections.
Acknowledgments
These studies were supported by NIH grant K99AI072973 and the Harvard University Center for AIDS Research (HU CFAR).
Footnotes
Published ahead of print on 25 June 2008.
REFERENCES
- 1.Alter, G., G. Hatzakis, C. M. Tsoukas, K. Pelley, D. Rouleau, R. LeBlanc, J. G. Baril, H. Dion, E. Lefebvre, R. Thomas, P. Cote, N. Lapointe, J. P. Routy, R. P. Sekaly, B. Conway, and N. F. Bernard. 2003. Longitudinal assessment of changes in HIV-specific effector activity in HIV-infected patients starting highly active antiretroviral therapy in primary infection. J. Immunol. 171477-488. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Arias, D. A., and K. S. Campbell. 2007. Protein kinase C regulates expression and function of inhibitory killer cell Ig-like receptors in NK cells. J. Immunol. 1795281-5290. [DOI] [PubMed] [Google Scholar]
- 3.Andre, P., C. Brunet, S. Guia, H. Gallais, J. Sampol, E. Vivier, and F. Dignat-George. 1999. Differential regulation of killer cell Ig-like receptors and CD94 lectin-like dimers on NK and T lymphocytes from HIV-1-infected individuals. Eur. J. Immunol. 291076-1085. [DOI] [PubMed] [Google Scholar]
- 4.Anfossi, N., J. M. Doisne, M. A. Peyrat, S. Ugolini, O. Bonnaud, D. Bossy, V. Pitard, P. Merville, J. F. Moreau, J. F. Delfraissy, J. Dechanet-Merville, M. Bonneville, A. Venet, and E. Vivier. 2004. Coordinated expression of Ig-like inhibitory MHC class I receptors and acquisition of cytotoxic function in human CD8+ T cells. J. Immunol. 1737223-7229. [DOI] [PubMed] [Google Scholar]
- 5.Arlettaz, L., S. Degermann, C. De Rham, E. Roosnek, and B. Huard. 2004. Expression of inhibitory KIR is confined to CD8+ effector T cells and limits their proliferative capacity. Eur. J. Immunol. 343413-3422. [DOI] [PubMed] [Google Scholar]
- 6.Barber, D. L., E. J. Wherry, D. Masopust, B. Zhu, J. P. Allison, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439682-687. [DOI] [PubMed] [Google Scholar]
- 7.Betts, M. R., B. Exley, D. A. Price, A. Bansal, Z. T. Camacho, V. Teaberry, S. M. West, D. R. Ambrozak, G. Tomaras, M. Roederer, J. M. Kilby, J. Tartaglia, R. Belshe, F. Gao, D. C. Douek, K. J. Weinhold, R. A. Koup, P. Goepfert, and G. Ferrari. 2005. Characterization of functional and phenotypic changes in anti-Gag vaccine-induced T cell responses and their role in protection after HIV-1 infection. Proc. Natl. Acad. Sci. USA 1024512-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonorino, P., V. Leroy, T. Dufeu-Duchesne, S. Tongiani-Dashan, N. Sturm, M. Pernollet, E. Vivier, J. P. Zarski, P. N. Marche, and E. Jouvin-Marche. 2007. Features and distribution of CD8 T cells with human leukocyte antigen class I-specific receptor expression in chronic hepatitis C. Hepatology 461375-1386. [DOI] [PubMed] [Google Scholar]
- 9.Brenchley, J. M., N. J. Karandikar, M. R. Betts, D. R. Ambrozak, B. J. Hill, L. E. Crotty, J. P. Casazza, J. Kuruppu, S. A. Migueles, M. Connors, M. Roederer, D. C. Douek, and R. A. Koup. 2003. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 1012711-2720. [DOI] [PubMed] [Google Scholar]
- 10.Champagne, P., G. S. Ogg, A. S. King, C. Knabenhans, K. Ellefsen, M. Nobile, V. Appay, G. P. Rizzardi, S. Fleury, M. Lipp, R. Forster, S. Rowland-Jones, R. P. Sekaly, A. J. McMichael, and G. Pantaleo. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410106-111. [DOI] [PubMed] [Google Scholar]
- 11.D'Andrea, A., C. Chang, J. H. Phillips, and L. L. Lanier. 1996. Regulation of T cell lymphokine production by killer cell inhibitory receptor recognition of self HLA class I alleles. J. Exp. Med. 184789-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443350-354. [DOI] [PubMed] [Google Scholar]
- 13.Galiani, M. D., E. Aguado, R. Tarazona, P. Romero, I. Molina, M. Santamaria, R. Solana, and J. Pena. 1999. Expression of killer inhibitory receptors on cytotoxic cells from HIV-1-infected individuals. Clin. Exp. Immunol. 115472-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi, R. T., and B. D. Walker. 2002. Immunologic control of HIV-1. Annu. Rev. Med. 53149-172. [DOI] [PubMed] [Google Scholar]
- 15.Gougeon, M. L., S. Boullier, V. Colizzi, and F. Poccia. 1999. NKR-mediated control of gammadelta T-cell immunity to viruses. Microbes Infect. 1219-226. [DOI] [PubMed] [Google Scholar]
- 16.Gougeon, M. L., F. Poccia, and S. Boullier. 2000. Human gamma delta T lymphocytes in HIV disease: effector functions and control by natural killer cell receptors. Springer Semin. Immunopathol. 22251-263. [DOI] [PubMed] [Google Scholar]
- 17.Guerra, N., F. Michel, A. Gati, C. Gaudin, Z. Mishal, B. Escudier, O. Acuto, S. Chouaib, and A. Caignard. 2002. Engagement of the inhibitory receptor CD158a interrupts TCR signaling, preventing dynamic membrane reorganization in CTL/tumor cell interaction. Blood 1002874-2881. [DOI] [PubMed] [Google Scholar]
- 18.Henel, G., K. Singh, D. Cui, S. Pryshchep, W. W. Lee, C. M. Weyand, and J. J. Goronzy. 2006. Uncoupling of T cell effector functions by inhibitory killer immunoglobulin-like receptors. Blood. 1074449-4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kambayashi, T., E. Assarsson, J. Michaelsson, P. Berglund, A. D. Diehl, B. J. Chambers, and H. G. Ljunggren. 2000. Emergence of CD8+ T cells expressing NK cell receptors in influenza A virus-infected mice. J. Immunol. 1654964-4969. [DOI] [PubMed] [Google Scholar]
- 20.Kaufmann, D. E., D. G. Kavanagh, F. Pereyra, J. J. Zaunders, E. W. Mackey, T. Miura, S. Palmer, M. Brockman, A. Rathod, A. Piechocka-Trocha, B. Baker, B. Zhu, S. Le Gall, M. T. Waring, R. Ahern, K. Moss, A. D. Kelleher, J. M. Coffin, G. J. Freeman, E. S. Rosenberg, and B. D. Walker. 2007. Upregulation of CTLA-4 by HIV-specific CD4(+) T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 81246-1254. [DOI] [PubMed] [Google Scholar]
- 21.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 684650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichterfeld, M., D. E. Kaufmann, X. G. Yu, S. K. Mui, M. M. Addo, M. N. Johnston, D. Cohen, G. K. Robbins, E. Pae, G. Alter, A. Wurcel, D. Stone, E. S. Rosenberg, B. D. Walker, and M. Altfeld. 2004. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J. Exp. Med. 200701-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lichterfeld, M., X. G. Yu, M. T. Waring, S. K. Mui, M. N. Johnston, D. Cohen, M. M. Addo, J. Zaunders, G. Alter, E. Pae, D. Strick, T. M. Allen, E. S. Rosenberg, B. D. Walker, and M. Altfeld. 2004. HIV-1-specific cytotoxicity is preferentially mediated by a subset of CD8(+) T cells producing both interferon-gamma and tumor necrosis factor-alpha. Blood 104487-494. [DOI] [PubMed] [Google Scholar]
- 24.Lieberman, J., P. Shankar, N. Manjunath, and J. Andersson. 2001. Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection. Blood 981667-1677. [DOI] [PubMed] [Google Scholar]
- 25.Liu, H., M. Rhodes, D. L. Wiest, and D. A. Vignali. 2000. On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity 13665-675. [DOI] [PubMed] [Google Scholar]
- 26.Maenaka, K., T. Juji, T. Nakayama, J. R. Wyer, G. F. Gao, T. Maenaka, N. R. Zaccai, A. Kikuchi, T. Yabe, K. Tokunaga, K. Tadokoro, D. I. Stuart, E. Y. Jones, and P. A. van der Merwe. 1999. Killer cell immunoglobulin receptors and T cell receptors bind peptide-major histocompatibility complex class I with distinct thermodynamic and kinetic properties. J. Biol. Chem. 27428329-28334. [DOI] [PubMed] [Google Scholar]
- 27.McMahon, C. W., and D. H. Raulet. 2001. Expression and function of NK cell receptors in CD8+ T cells. Curr. Opin. Immunol. 13465-470. [DOI] [PubMed] [Google Scholar]
- 28.McMahon, C. W., A. J. Zajac, A. M. Jamieson, L. Corral, G. E. Hammer, R. Ahmed, and D. H. Raulet. 2002. Viral and bacterial infections induce expression of multiple NK cell receptors in responding CD8(+) T cells. J. Immunol. 1691444-1452. [DOI] [PubMed] [Google Scholar]
- 29.Moser, J. M., J. Gibbs, P. E. Jensen, and A. E. Lukacher. 2002. CD94-NKG2A receptors regulate antiviral CD8(+) T cell responses. Nat. Immunol. 3189-195. [DOI] [PubMed] [Google Scholar]
- 30.Mustelin, T., and K. Tasken. 2003. Positive and negative regulation of T-cell activation through kinases and phosphatases. Biochem. J. 37115-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poon, K., D. Montamat-Sicotte, N. Cumberbatch, A. J. McMichael, and M. F. Callan. 2005. Expression of leukocyte immunoglobulin-like receptors and natural killer receptors on virus-specific CD8+ T cells during the evolution of Epstein-Barr virus-specific immune responses in vivo. Viral Immunol. 18513-522. [DOI] [PubMed] [Google Scholar]
- 32.Reichardt, P., B. Dornbach, and M. Gunzer. 2007. The molecular makeup and function of regulatory and effector synapses. Immunol. Rev. 218165-177. [DOI] [PubMed] [Google Scholar]
- 33.Sirianni, M. C., F. Ensoli, C. Alario, V. Fiorelli, G. Sacco, S. Topino, F. Iebba, I. Mezzaroma, and F. Aiuti. 2001. Distribution of the natural killer-related receptor for HLA-C during highly active antiretroviral therapy for human immunodeficiency virus infection. Hum. Immunol. 621328-1334. [DOI] [PubMed] [Google Scholar]
- 34.Streeck, H., M. Lichterfeld, G. Alter, A. Meier, N. Teigen, B. Yassine-Diab, H. K. Sidhu, S. Little, A. Kelleher, J. P. Routy, E. S. Rosenberg, R. P. Sekaly, B. D. Walker, and M. Altfeld. 2007. Recognition of a defined region within p24 Gag by CD8+ T cells during primary human immunodeficiency virus type 1 infection in individuals expressing protective HLA class I alleles. J. Virol. 817725-7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taupin, J. L., F. Halary, J. Dechanet, M. A. Peyrat, J. M. Ragnaud, M. Bonneville, and J. F. Moreau. 1999. An enlarged subpopulation of T lymphocytes bearing two distinct gammadelta TCR in an HIV-positive patient. Int. Immunol. 11545-552. [DOI] [PubMed] [Google Scholar]
- 36.Ugolini, S., C. Arpin, N. Anfossi, T. Walzer, A. Cambiaggi, R. Forster, M. Lipp, R. E. Toes, C. J. Melief, J. Marvel, and E. Vivier. 2001. Involvement of inhibitory NKRs in the survival of a subset of memory-phenotype CD8+ T cells. Nat. Immunol. 2430-435. [DOI] [PubMed] [Google Scholar]
- 37.Wherry, E. J., S. J. Ha, S. M. Kaech, W. N. Haining, S. Sarkar, V. Kalia, S. Subramaniam, J. N. Blattman, D. L. Barber, and R. Ahmed. 2007. Molecular signature of CD8(+) T cell exhaustion during chronic viral infection. Immunity 27670-684. [DOI] [PubMed] [Google Scholar]
- 38.Yu, X. G., M. M. Addo, E. S. Rosenberg, W. R. Rodriguez, P. K. Lee, C. A. Fitzpatrick, M. N. Johnston, D. Strick, P. J. Goulder, B. D. Walker, and M. Altfeld. 2002. Consistent patterns in the development and immunodominance of human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T-cell responses following acute HIV-1 infection. J. Virol. 768690-8701. [DOI] [PMC free article] [PubMed] [Google Scholar]