Abstract
Viral fusogenic membrane proteins have been proposed as tools to increase the potency of oncolytic viruses, but there is a need for mechanisms to control the spread of fusogenic viruses in normal versus tumor cells. We have previously shown that a mutant of the paramyxovirus simian virus 5 (SV5) that harbors mutations in the P/V gene from the canine parainfluenza virus (P/V-CPI−) is a potent inducer of type I interferon (IFN) and apoptosis and is restricted for spread through normal but not tumor cells in vitro. Here, we have used the cytopathic P/V-CPI− as a backbone vector to test the hypothesis that a virus expressing a hyperfusogenic glycoprotein will be a more effective oncolytic vector but will retain sensitivity to IFN. A P/V mutant virus expressing an F protein with a glycine-to-alanine substitution in the fusion peptide (P/V-CPI−-G3A) was more fusogenic than the parental P/V-CPI− mutant. In two model prostate tumor cell lines which are defective in IFN production (LNCaP and DU145), the hyperfusogenic P/V-CPI−-G3A mutant had normal growth properties at low multiplicities of infection and was more effective than the parental P/V-CPI− mutant at cell killing in vitro. However, in PC3 cells which produce and respond to IFN, the hyperfusogenic P/V-CPI−-G3A mutant was attenuated for growth and spread. Killing of PC3 cells was equivalent between the parental P/V-CPI− mutant and the hyperfusogenic P/V-CPI−-G3A mutant. In a nude mouse model using LNCaP cells, the hyperfusogenic P/V-CPI−-G3A mutant was more effective than P/V-CPI− at reducing tumor burden. In the case of DU145 tumors, the two vectors based on P/V-CPI− were equally effective at limiting tumor growth. Together, our results provide proof of principle that a cytopathic SV5 P/V mutant can serve as an oncolytic virus and that the oncolytic effectiveness of P/V mutants can be enhanced by a fusogenic membrane protein without compromising sensitivity to IFN. The potential advantages of SV5-based oncolytic vectors are discussed.
A number of paramyxoviruses have shown promise as oncolytic vectors for tumor therapy, including measles virus, mumps virus, Sendai virus, and Newcastle disease virus (15, 30, 34, 36, 42, 51). The goal of the work described here was to define the relative oncolytic potential of cytopathic and hyperfusogenic mutants of the paramyxovirus simian virus 5 (SV5).
Many paramyxoviruses that are being developed as oncolytic vectors have the inherent property of causing extensive cytopathic effects (CPE) and inducing apoptotic cell death (15, 28, 30, 37). By comparison, SV5 has the unusual property among paramyxoviruses of being largely noncytopathic in most epithelial and fibroblast cell types (39, 49) and thus would not be expected to have inherent oncolytic properties. Noncytopathic wild-type (WT) SV5 can be converted into a virus that induces apoptosis by engineered substitutions in the viral P/V gene (11, 54). The SV5 P/V gene encodes the phosphoprotein P and the accessory protein V (31), which share an identical 164-residue amino-terminal domain (the shared P/V region) but have unique C-terminal domains. The P protein is an essential subunit of the viral RNA-dependent RNA polymerase (31). The V protein contains a highly conserved cysteine-rich zinc-binding domain that is required for many V-associated functions. V protein is thought to function in the regulation of viral RNA synthesis (33) but also has additional roles in counteracting host cell antiviral responses (reviewed in reference 18). These functions include blocking type I interferon (IFN) signaling by targeting STAT1 for degradation (10) and inhibiting IFN-β gene expression through binding to mda-5 (7). An SV5 mutant with substitutions in the shared region of the canine parainfluenza virus P/V genes (P/V-CPI−) (Fig. 1A) overexpresses viral RNA and protein, is a potent inducer of IFN-β and proinflammatory cytokines, cannot block IFN signaling, and induces cell death (11, 54). Here, we have tested the oncolytic potential of this P/V-CPI− mutant in human prostate cancer cells.
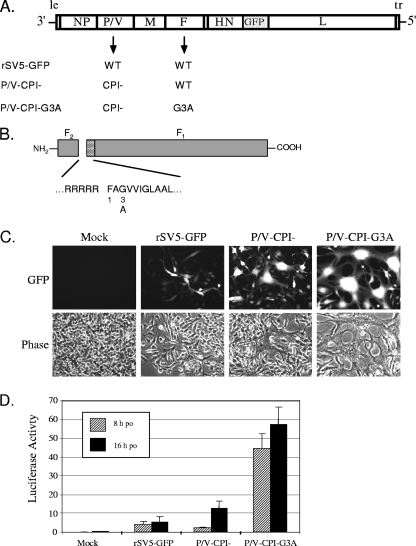
FIG. 1.
Incorporation of the G3A mutation into the F protein of P/V-CPI− results in a virus with a hyperfusogenic phenotype. (A) Schematic of viruses used in this study. The genome structure of SV5 is shown with addition of the GFP gene between HN and L as described previously (20). Arrows indicate WT and mutant P/V and F genes occurring in rSV5-GFP, P/V-CPI−, and P/V-CPI−-G3A recombinant viruses. (B) Location of G3A mutation. The SV5 F protein is shown schematically as F1 and F2 subunits with the amino acid sequence at the proteolytic cleavage site. The location of the G3A substitution in the fusion peptide (hatched box) is indicated (adapted from Lamb et al. [32]). (C) P/V-CPI−-G3A is hyperfusogenic. BSR-T7 cells were mock infected or infected at an MOI of 0.05 with rSV5-GFP, P/V-CPI−, or P/V-CPI−-G3A, and microscopy pictures were taken at 48 h p.i. (D) Quantitative fusion assay. BSR-T7 cells were mock infected or infected at an MOI of 5 with rSV5-GFP, P/V-CPI−, or P/V-CPI−-G3A. At 21 h p.i., infected cells were overlaid with Vero cells that had been previously transfected with the pEMCLucβbAn plasmid, which encodes luciferase under the control of a T7 promoter. At 8 and 16 h postoverlay (po) luciferase activity was measured as the relative increase over mock-infected cells. Results are the average of three transfections, with error bars representing standard deviations.
Viral fusogenic membrane glycoproteins (FMGs) have been investigated as new tools to promote killing of tumor cells through cell-cell fusion (2). For example, oncolytic strains of adenovirus (17, 24, 25, 26), vesicular stomatitis virus (VSV) (14, 46), and herpes simplex 1 virus (24, 25) that have been engineered to express paramyxovirus FMGs show increased oncolytic efficacy in tissue culture and rodent model systems. Additionally, FMG expression by viral vectors in combination with chemotherapy treatments can have synergistic oncolytic effects in models of colon and pancreatic cancer (24, 26). Importantly, these fused cells can ultimately die through pathways that have properties of necrosis (3). This type of cell death has advantages for tumor therapy, since necrotic cells are known to induce potent inflammatory responses that could aid in immune-mediated tumor clearance (16, 35). Similarly, tumor cells that may have lower potential for apoptotic cell death should still be sensitive to killing through cell-cell fusion.
The paramyxovirus fusion protein F is an excellent candidate as a viral FMG since efficient cell-cell fusion occurs at neutral pH, fusion is catalyzed by a single protein with a relatively simple structure, and F fusion activity is controlled by proteolytic cleavage (31, 32). During the entry process, the paramyxovirus F protein fuses the virion lipid bilayer with the plasma membrane (31) to release the nucleocapsid structure into the cytoplasm. The F protein also promotes fusion of an infected cell that is expressing cell surface F protein with a neighboring cell, resulting in multinucleated syncytia. F protein is synthesized as a fusion-inactive precursor Fo which is cleaved during transport to the cell surface to yield F1 and F2 (Fig. 1B). For the SV5 F protein, cleavage occurs by a furin-like cellular protease at a single site composed of a stretch of Arg residues (40), and this exposes the fusogenic peptide responsible for membrane fusion (reviewed in references 31 and 32). This cleavage requirement for fusion activity has been exploited to generate recombinant viruses where F protein is engineered with sites for activation by tumor-selective proteases (30, 47).
SV5 F protein mutants have been described that contain single Gly-to-Ala substitutions in the fusion peptide (27), resulting in hyperfusogenic F mutants that induce massive cell-cell fusion when they are expressed in cells by heterologous vectors. Positions 3, 7, and 12 within the fusion peptide appear to be particularly sensitive to the Gly substitutions (Fig. 1B). Here, we have exploited the properties of a previously described SV5 F protein with a Gly-to-Ala substitution in the third position of the fusion peptide (G3A) (27) to test the hypothesis that a hyperfusogenic glycoprotein will enhance tumor cell killing by an oncolytic SV5 vector.
Many viruses naturally show preferential replication in tumor versus normal cell types (reviewed in references 4 and 45). During the transformation process, tumor cells can accumulate specific defects in IFN pathways that contribute to resistance to the antiproliferative effects of IFN (13, 55, 56), and it is proposed that this also confers increased susceptibility to viral infection (4). Thus, it was hypothesized that oncolytic viruses could be engineered for greater selectivity for replication in tumor cells by engineering viruses to induce a strong IFN response and/or be defective in blocking IFN signaling (48). Thus, virus replication would be crippled in normal cells while replication would be unhindered in tumor cells that contain defects in these pathways (48).
Given the ability of paramyxoviruses to spread and kill through cell-cell fusion and the emphasis on IFN as a natural mediator of selectivity for tumor versus normal cells, an important question to address is whether viral vectors with FMGs would still be restricted for spread by IFN. From a safety point of view, engineering viruses for enhanced killing through cell-cell fusion would be an advantage only if the vector had properties that restricted replication to tumor cells and not normal cells. Here, we have addressed this question by testing the hypotheses that the IFN-inducing SV5 mutant P/V-CPI− can serve as an oncolytic vector and that increasing the fusogenic activity of this virus does not compromise IFN sensitivity. Our results have general implications for the design of more effective and safer paramyxovirus-based vectors, as well as nonparamyxovirus vectors that are engineered to kill tumor cells through FMGs with enhanced fusion activity.
MATERIALS AND METHODS
Cells, viruses, and growth analysis.
Cultures of LNCaP, DU145, and PC3 cells were grown in RPMI medium containing 10% fetal bovine serum (FBS). Vero cells were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS. BSRT7/5 (5) cells were kindly provided by K. Conzelmann (University of Munich) and Robert A. Lamb (Northwestern University) and were cultured in DMEM containing 10% FBS supplemented with 1 mg/ml G418 every other passage. A recombinant SV5 (rSV5) expressing green fluorescent protein (GFP) was recovered as described previously (20) from a cDNA plasmid provided by Robert Lamb (Northwestern University) and Bio He (Pennsylvania State University). The recovery and growth properties of rSV5 expressing herpes simplex virus thymidine kinase (rSV5-TK) and P/V-CPI− have been previously described (39, 54). The P/V-CPI−-G3A F protein mutation was engineered into the backbone of the rSV5 P/V-CPI− using standard molecular biology techniques and was recovered as described previously (39). Details are available upon request. For tumor studies, virus was purified as described previously (6) by centrifugation through a glycerol cushion (5 h at 25,000 rpm; SW28 rotor), and virus pellets were resuspended in a small volume of DMEM containing 0.75% bovine serum albumin (BSA).
Fluorescence microscopy and MTS viability assays.
Fluorescence microscopy was performed using a Nikon Eclipse fluorescence microscope and a 20×lens as described previously (54). MTS [3,4-(5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy phenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt] assays were performed in 96-well dishes using Cell Titer 96 Aqueous One solution reagent (Promega) according to the manufacturer's instructions. Data are expressed as a percentage of mock-infected cells.
Modified BSR-T7 fusion assay.
Cell-cell fusion was quantified by a modification of an assay described previously by Nussbaum et al. (38). Briefly, 50% confluent, six-well dishes of BHK cells that constitutively express the T7 RNA polymerase (BSR-T7 cells) (5) were mock infected or infected with the WT rSV5-GFP, P/V-CPI−, or P/V-CPI−-G3A viruses at a multiplicity of infection (MOI) of 5. At 21 h postinfection (p.i.), cells were washed with phosphate-buffered saline (PBS) and overlaid with 105 Vero cells that had been transfected 12 h previously (6 μg of DNA per 5 × 106 cells) using FuGene 6 reagent with pEMCLucβbAn, a plasmid encoding an internal ribosome entry site-luciferase gene under the control of the T7 promoter (9). At 8 and 16 h after overlay, cells were washed with PBS and lysed in reporter lysis buffer (Promega), and luciferase activity was determined on a TD-20/20 luminometer.
IFN-β ELISA and IFN antibody neutralization.
Medium from mock-infected or infected cells was collected, clarified for 3 min at 13,000 rpm, and then assayed using an IFN-β enzyme-linked immunosorbent assay (ELISA) kit according to manufacturer's instructions (PBL Biomedical Laboratories). In experiments involving neutralization of IFN, neutralizing antibodies against IFN-α (Millipore MAB411) and IFN-β (Chemicon International AB1431) were included in medium during infections at final concentrations of 5,000 neutralizing units/ml for each antibody. Control neutralizing tumor necrosis factor alpha antibody (Biosource AHC3812) was used at a final concentration of 10,000 neutralizing units/ml.
LNCaP and DU145 xenograft implantation and virus injection.
Male, nude mice 8 to 10 weeks of age were implanted subcutaneously with 4 × 106 tumor cells in 100 μl of Matrigel (for LNCaP cells; BD Biosciences) or RPMI medium (for DU145 cells). Palpable tumors were allowed to form, and then mice were randomly separated into three groups for treatment with PBS, P/V-CPI−, or P/V-CPI−-G3A. At days 0 and 8 of the study, mice were intratumorally injected with PBS containing 10% BSA or 108 PFU of P/V-CPI− or P/V-CPI−-G3A diluted in PBS-10% BSA (100-μl total volume for LNCaP tumors; 50-μl total volume for DU145 tumors). Tumors were measured every other day with calipers [mm3 = 1/2(length × width2)], and mice were weighed. At day 18 of the study, mice bearing LNCaP tumors were euthanized, and tumors were excised, weighed and fixed with 4% paraformaldehyde, and representative tumors were photographed.
RESULTS
An rSV5 with a G3A substitution in the fusion peptide shows enhanced cell-cell fusion.
It has previously been shown that a glycine-to-alanine substitution in position 3 of the SV5 F protein fusion peptide (G3A) increased the ability of this protein to mediate cell-cell fusion when expressed from non-SV5-based vectors (27, 44). We hypothesized that expression of the G3A F protein in the context of an rSV5 would also result in a virus with hyperfusogenic properties. To test this, the G3A F protein mutant was engineered into the P/V-CPI− genome (Fig. 1A and B), and virus (P/V-CPI−-G3A) was recovered from cDNA (20). As shown in Fig. 1A, P/V-CPI−-G3A also contains a GFP gene inserted between the HN and L genes. WT rSV5 harboring the G3A mutation was not analyzed as WT virus is largely noncytopathic, and the goal of this study was to evaluate the cytopathic P/V-CPI− mutant in tumor reduction.
Infection with the P/V-CPI−-G3A mutant virus resulted in larger pockets of cell-cell fusion than with either WT rSV5-GFP or the parental P/V-CPI− virus. This is evident in the microscopy pictures in Fig. 1C taken at 48 h after infection of BHK-derived cells with rSV5-GFP, P/V-CPI−, or P/V-CPI−-G3A at low MOIs. A luciferase-based assay was used to quantitate cell-cell fusion by a modification of the approach described by Nussbaum et al. (38). BSR-T7 cells that constitutively express the bacteriophage T7 polymerase (5) were mock infected or infected at an MOI of 5 with rSV5-GFP, P/V-CPI−, or P/V-CPI−-G3A. At 21 h p.i., cells were overlaid with Vero cells that had been transfected 12 h previously with a plasmid encoding a luciferase gene under the control of the T7 promoter. At 8 and 16 h after overlay, lysates were harvested, and luciferase activity was measured as a readout for F-mediated cell-cell fusion of the transfected Vero cells with infected BSR-T7 cells (Fig. 1D). Mock-infected cells and cells infected with rSV5-GFP and P/V-CPI− showed very low levels of luciferase activity at 8 h postoverlay (po), with luciferase activity at 16 h po. being slightly increased for P/V-CPI− samples. In contrast, samples from cells infected with the G3A mutant virus showed luciferase activity that was much higher than that of P/V-CPI− at both 8 and 16 h po. Taken together, these data indicate that introduction of the G3A F protein mutation into the P/V-CPI− backbone results in a virus with hyperfusogenic properties.
Growth and cell-killing properties of the hyperfusogenic P/V-CPI−-G3A mutant in cells that do not produce IFN.
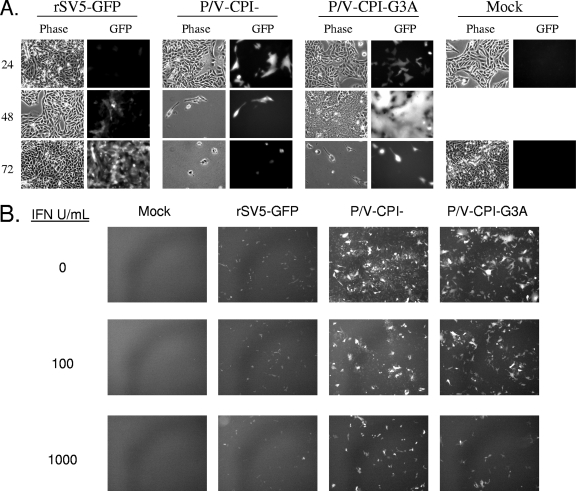
To determine the spread of the G3A mutant through a cell population, Vero cells were mock infected or infected at an MOI of 0.05 with rSV5-GFP, P/V-CPI−, or P/V-CPI−-G3A, and microscopy pictures were examined at 24, 48, and 72 h p.i. As shown in Fig. 2A, rSV5-GFP virus spread through the cell population slowly, and most cells were infected by 72 h p.i. The P/V-CPI− virus spread through Vero cells faster than WT virus, and nearly all cells were dead by 48 h p.i., as described previously (53). For the hyperfusogenic mutant virus, large syncytia were evident by 48 h p.i., and the fused monolayer of P/V-CPI−-G3A-infected cells remained intact longer than cells infected with P/V-CPI− before eventually lysing. Thus, both of the P/V mutant viruses spread through Vero cells faster than WT virus and effectively kill cells.
FIG. 2.
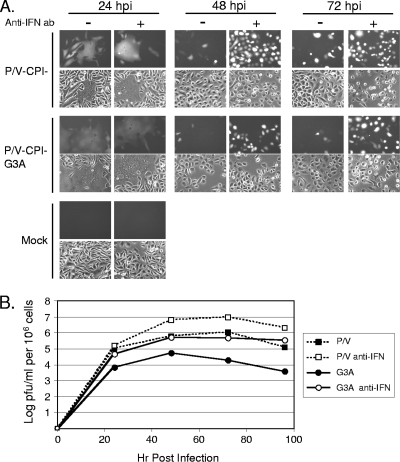
Spread at low MOIs and IFN sensitivity of the hyperfusogenic P/V-CPI−-G3A mutant. (A) Virus spread at low MOIs. Vero cells were mock infected or infected at an MOI of 0.05 with the indicated viruses, and microscopy pictures were taken at 24, 48 and 72 h p.i. (B) IFN sensitivity. Vero cells were mock treated or treated for 24 h with 100 or 1,000 units of IFN before infection with the indicated viruses at an MOI of 0.05. At 30 h p.i., cells were examined by microscopy for GFP expression.
The above data with Vero cells which do not produce IFN raised the question of whether spread of the hyperfusogenic virus would be limited by IFN treatment. To test this, Vero cells were mock treated or treated with 100 or 1,000 units of universal type I IFN for 24 h prior to mock infection or infection at low MOIs. At 30 h p.i, GFP expression was monitored by microscopy as an assay for virus spread through the population. In the absence of IFN treatment, more cells were infected with the P/V-CPI− and P/V-CPI−-G3A mutants than with rSV5-GFP (0 IFN samples) (Fig. 2B), and P/V mutant-infected cells showed higher levels of GFP fluorescence, as described previously (54). For all viruses, there was a dose-dependent decrease in the number of GFP-positive cells with increasing levels of IFN pretreatment. Most importantly, the increased levels of fusion seen with the G3A mutant were greatly decreased by IFN pretreatment, and there was no substantial difference in IFN sensitivity compared to the parental P/V-CPI− virus.
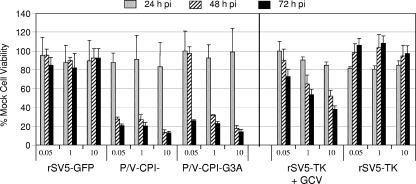
To quantitate the relative ability of the fusogenic G3A mutant to kill cells, Vero cells were mock infected or infected at increasing MOIs of 0.05, 1, and 10 with rSV5-GFP, P/V-CPI−, or P/V-CPI−-G3A, and cell viability was measured at 24-h intervals by an MTS assay. As seen in Fig. 3, cells infected with WT rSV5-GFP did not show a major loss of viability at any MOI or time tested, and viability was always between ∼80 to 110% of mock-infected cells, as described previously (12). Cells infected with P/V-CPI− virus showed a very rapid loss of viability down to ∼20% of mock-infected cells, and this was largely independent of the MOI. The kinetics of cell killing by the fusogenic G3A virus was similar to that of P/V-CPI−, with the exception of the results at 48 h p.i at an MOI of 0.05. In this sample, cell viability remained high for cells infected with the G3A mutant, and this was consistent with the microscopy pictures of Fig. 2C above showing giant syncytia that had not yet lysed.
FIG. 3.
Cell killing by the hyperfusogenic P/V-CPI−-G3A mutant. Vero cells were mock infected or infected with the indicated viruses at an MOI of 0.05, 1, or 10. For cells infected with rSV5-TK, medium was used with or without 10 μg/ml GCA as described previously (39). Cell viability was determined at 24, 48, and 72 h p.i. using an MTS viability assay as described in Materials and Methods. Data are from quadruplicate samples and are expressed as a percentage of the value obtained with mock-infected cells, with error bars representing the standard deviation.
We have previously described the properties of an rSV5 vector that expresses herpes simplex TK (39), an enzyme that converts the nontoxic prodrug ganciclovir (GCV) to a drug that kills cells. Cells infected with rSV5-TK are efficiently killed but only when exposed to GCV. To define the relative ability of our SV5 vectors to kill cells, the viability of Vero cells infected with P/V-CPI− and P/V-CPI−-G3A was compared to cells infected with the rSV5-TK virus with or without GCV. As shown in Fig. 3, loss of viability for cells infected with the rSV5-TK virus was time and MOI dependent only in the presence of GCV, as described previously (39). Both the kinetics and the extent of cell killing by rSV5-TK were reduced compared to results with either of the P/V mutant vectors. Together, these data indicate that cell killing by the hyperfusogenic G3A mutant is similar to that of the parental P/V-CPI− virus in IFN-deficient Vero cells and that the cytopathic vectors based on P/V gene mutants are more effective at cell killing than the previously described rSV5-TK virus.
Growth properties of P/V-CPI−-G3A in human prostate cancer cell lines that differ in production of IFN.
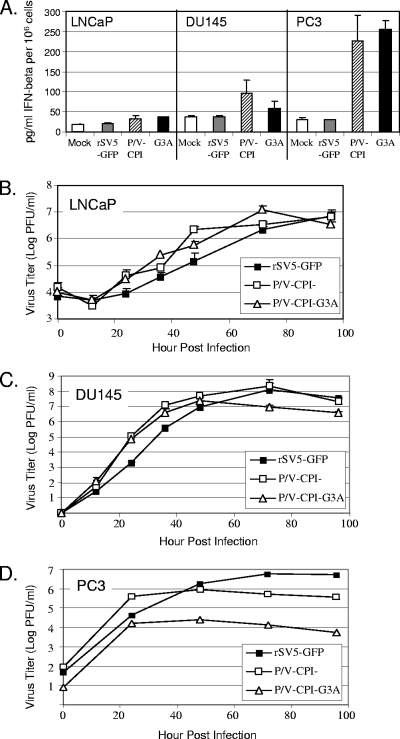
Since the above data in Vero cells indicated that both the hyperfusogenic and parental P/V-CPI− viruses were sensitive to IFN, we examined the role of IFN in limiting virus growth in LNCaP, DU145, and PC3 cells, three well-established prostate tumor cell lines. To quantitate the amount of IFN produced following infection of these three cell lines, LNCaP, DU145, and PC3 cells were mock infected or infected at an MOI of 10 with rSV5-GFP, P/V-CPI−, or P/V-CPI−-G3A, and IFN-β levels were measured at 24 h p.i. by ELISA (Fig. 4A). In LNCaP cells, there was no significant IFN-β released following infection with any virus tested, and only moderate amounts (96 and 59 pg/ml) were released from DU145 cells infected with the P/V-CPI− and P/V-CPI−-G3A mutants, respectively. PC3 cells produced 228 and 254 pg/ml of IFN-β in response to infection with P/V-CPI− and P/V-CPI−-G3A, respectively.
FIG. 4.
IFN induction and growth at low MOIs of the P/V-CPI−-G3A mutant in representative prostate cancer cell lines. (A) IFN production in human prostate cancer cell lines. LNCaP, DU145, or PC3 cells were mock infected or infected at an MOI of 10 with the indicated viruses. At 24 h p.i., medium was collected, and the amount of IFN-β was determined by an ELISA. Data are the average of three measurements, with error bars representing standard deviations. (B to D) Growth at low MOIs. LNCaP, DU145, or PC3 cells were infected at an MOI of 0.05 with rSV5-GFP, P/V-CPI−, or P/V-CPI−-G3A. Medium was harvested at the indicated times p.i., and the amount of infectious virus was determined by plaque assay. LNCaP and DU145 time courses represent one experiment done in triplicate. The PC3 time course represents the average of two independent experiments.
To determine multistep growth properties in these cell lines, LNCaP, DU145, and PC3 cell lines were infected at an MOI of 0.05, and medium was harvested to assay infectious virus by plaque assay. As shown in Fig. 4B and C, the hyperfusogenic P/V-CPI−-G3A mutant showed growth kinetics and titers similar to those of P/V-CPI− in both LNCaP and DU145 cell lines, and both viruses grew to titers higher than WT at early time points. Virus growth in PC3 cells differed from the other two prostate cell lines. As shown in Fig. 4D, PC3 cells infected with rSV5-GFP showed a steady increase in virus production out to 72 h p.i. In contrast, cells infected with the P/V-CPI− virus showed an initial burst of progeny virus at 24 h p.i., but virus production did not increase further, as shown previously for A549 cells (54). The hyperfusogenic P/V-CPI−-G3A virus also displayed an initial burst of virus growth but differed by reaching a plateau ∼2 logs below the level of P/V-CPI− (Fig. 4D) in PC3 cells. Thus, of the three prostate tumor cell lines tested here, only PC3 cells showed a striking difference in growth at low MOIs between the hyperfusogenic and parental P/V-CPI− mutants.
The above results on the differential ability of infected prostate tumor cells to produce IFN-β (Fig. 4A) suggested the hypothesis that IFN contributed to growth restriction of P/V-CPI− and P/V-CPI−-G3A in PC3 cells. To test this, PC3 cells were mock infected or infected at an MOI of 0.05 with the parental P/V-CPI− or P/V-CPI−-G3A viruses, and replacement medium was supplemented with an excess of neutralizing antibodies against IFN-α and IFN-β. Microscopy pictures were taken at 24-h intervals to monitor viral spread and CPE, and medium was collected to monitor virus growth. As shown in Fig. 5A, both P/V-CPI− and P/V-CPI−-G3A induced syncytia formation by 24 h p.i., with P/V-CPI−-G3A syncytia being much larger than those caused by P/V-CPI−. The addition of IFN-neutralizing antibodies had no observable effect at 24 h p.i. By 48 to 72 h p.i. in untreated samples, cells that were incorporated into syncytia had died or detached from the dish, and only a small percentage of remaining cells expressed GFP. However, in samples that had been treated with neutralizing IFN antibodies, a much higher percentage of cells infected with P/V-CPI− and P/V-CPI−-G3A expressed GFP than in untreated samples. Increased CPE caused by P/V-CPI− and P/V-CPI−-G3A was also evident in cells treated with the neutralizing antibodies. Addition of a control neutralizing tumor necrosis factor alpha antibody to infected cells had no effect on viral spread (data not shown).
FIG. 5.
Growth at low MOIs and spread of P/V-CPI− and P/V-CPI−-G3A are restricted by IFN in the PC3 prostate cancer cell line. (A) Effect of IFN on viral spread. PC3 cells were mock infected or infected at an MOI of 0.05 with P/V-CPI− or P/V-CPI−-G3A. Cells were incubated in medium with (+) or without (−) neutralizing IFN-α and IFN-β antibodies as described in Materials and Methods. At 24, 48, and 72 h p.i., microscopy pictures were taken. (B) Effect of IFN on viral growth. The medium from PC3 cells treated as described in panel A was harvested to assay infectious virus by plaque assay. Results are representative of two experiments. Ab, antibody.
IFN-neutralizing antibodies in the medium also had a substantial effect on the growth of both mutant viruses at low MOIs in PC3 cells. This is evident in Fig. 5B, where the growth of P/V-CPI− and the G3A mutant is shown to be increased by ∼1 log or greater at late times p.i. in the presence of IFN-neutralizing antibodies compared to control samples. Interestingly, despite the increase in growth seen with the addition of IFN-neutralizing antibodies, titers for the hyperfusogenic mutant G3A were still lower than the titer of the P/V-CPI− virus plus neutralizing IFN antibodies. Taken together, these data indicate that even though P/V-CPI−-G3A demonstrated an increased fusion capability, its spread, growth, and CPE were still limited by IFN in the PC3 cell line. Furthermore, the replication of P/V-CPI−-G3A was defective in the PC3 cell line compared to P/V-CPI−, and this defect was at least in part independent of extracellular IFN.
The hyperfusogenic P/V-CPI−-G3A is more cytopathic than P/V-CPI− in two out of three prostate cancer cell lines tested.
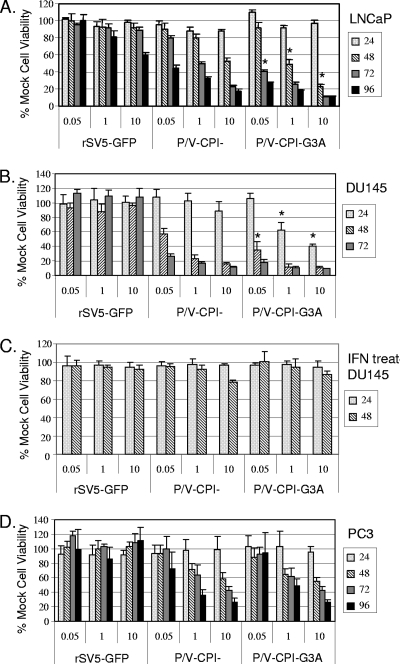
We hypothesized that the G3A F protein mutation that increases the fusogenic activity of P/V-CPI− would also increase the ability of the mutant virus to kill prostate tumor cells. To test this, LNCaP, DU145, or PC3 cells were mock infected or infected at increasing MOIs of 0.05, 1, and 10 with rSV5-GFP, P/V-CPI−, or P/V-CPI−-G3A, and cell viability was measured in 24-h increments by an MTS assay. In the LNCaP cell line (Fig. 6A), both P/V-CPI− and P/V-CPI−-G3A caused a time- and MOI-dependent decrease in cell viability. At some time points and MOIs tested (Fig. 6A, P/V-CPI−-G3A), there was a statistically significant decrease in cell viability for cells infected with the fusogenic G3A mutant compared to the parental P/V-CPI− virus. This difference was most apparent at 72 h at the MOI of 0.05, and 48 h at the MOIs of 1 and 10. With the DU145 cell line (Fig. 6B), both P/V-CPI− and P/V-CPI−-G3A showed a drastic reduction in cell viability, with a reduction of greater than 75% in cell viability at all MOIs tested by 72 h p.i. Similar to results with LNCaP cells, P/V-CPI−-G3A-infected DU145 cells showed a decreased viability compared to P/V-CPI− at all MOIs tested. This difference was most readily apparent at 48 h p.i. at an MOI of 0.05 and at 24 h for the MOIs of 1 and 10 (Fig. 6B, P/V-CPI−-G3A). As shown in Fig. 6C, IFN treatment of DU145 cells prevented loss of viability following infection with the two P/V mutants (compare with Fig. 6B). This is consistent with DU145 cells responding to IFN (23) and with the conclusion that the hyperfusogenic P/V mutant retains sensitivity to IFN.
FIG. 6.
P/V-CPI−-G3A is more cytopathic than P/V-CPI− in two out of three prostate cancer cell lines tested. Results of cell viability assays are shown. LNCaP, DU145, or PC3 cells were mock infected or infected at an MOI of 0.05, 1, or 10 with the indicated viruses, and cell viability was measured by MTS assay at 24-h increments. In panel C, DU145 cells were treated overnight with 1,000 units/ml of IFN, washed, and infected as described for the other panels. Data are expressed as a percentage of the value obtained with mock-infected cells and are representative of two or more independent experiments done in quadruplicate. An asterisk denotes values from the P/V-CPI−-G3A samples that are statistically significant (P < 0.05; Students t test) compared to the corresponding P/V-CPI− samples.
PC3 cells differed from the other cell lines, as shown in Fig. 6D, since neither P/V-CPI− nor P/V-CPI−-G3A virus was able to reduce cell viability below 50% at an MOI of 0.05. Both P/V-CPI− and P/V-CPI−-G3A reduced cell viability by at least 50% at MOIs of 1 and 10. Most importantly, in contrast to what was observed in LNCaP and DU145 cell lines, the fusogenic G3A mutant did not reduce cell viability to a greater extent than P/V-CPI−. Pretreatment of PC3 cells with 1,000 units of IFN prior to infection protected cells from loss of viability (data not shown).
The hyperfusogenic G3A mutant is more effective than the parental P/V mutant in reducing in vivo tumor burden in an LNCaP xenograft model system.
Based on the above finding that the hyperfusogenic G3A mutant was more cytopathic than P/V-CPI− during in vitro infections of LNCaP cells, we hypothesized that P/V-CPI−-G3A would also be more effective at reducing LNCaP tumor burden in an in vivo xenograft model system. To test this, groups of male nude mice were implanted with LNCaP cells, and after tumors grew to a palpable size, they were injected intratumorally on day 0 and day 8 of the study with either PBS (n = 8) or 108 PFU of purified P/V-CPI− (n = 10) or P/V-CPI−-G3A (n = 10). Mouse weight was measured every other day for 18 days. On day 18, mice were sacrificed, and tumors were removed and weighed.
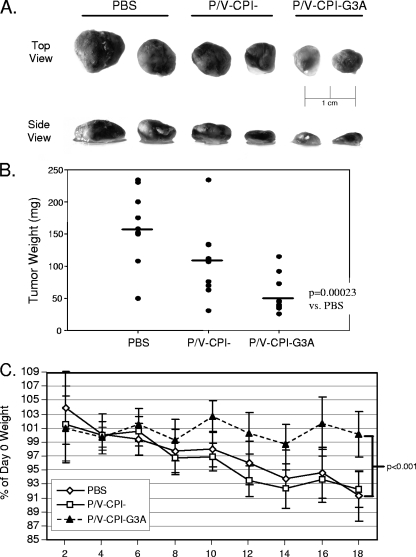
Figure 7A shows representative pictures of excised tumors from each experimental group. Nearly all tumors treated with the hyperfusogenic G3A mutant were smaller and lighter in color than the control PBS-treated tumors. Likewise, tumors treated with the parental P/V-CPI− mutant were smaller than control PBS-treated tumors, but the effect was less dramatic than that seen with the G3A mutant virus. This conclusion is supported by results shown in Fig. 7B, where the average final tumor weight for the group treated with the hyperfusogenic P/V-CPI−-G3A mutant was significantly lower than the PBS (P = 0.00023) and P/V-CPI− groups. During this study, it was observed that mice bearing LNCaP tumors lost weight over time, presumably due to negative effects of tumor burden. However, mice whose tumors were treated with the hyperfusogenic P/V-CPI−-G3A mutant, on average, did not lose weight during this study, and this average weight at day 18 was statistically significant (P < 0.001) compared to mice in the PBS and P/V-CPI− treatment groups that lost ∼10% of their original body weight (Fig. 7C). The weight loss observed in mice in the P/V-CPI− group was likely due to remaining tumor burden and was not a side effect of virus injection since intravenous injection of 108 PFU of P/V-CPI− or P/V-CPI−-G3A into nude mice lacking tumors did not cause any weight decline over a 10-day time period (data not shown). Together, these data indicate that the hyperfusogenic G3A mutant is more effective than P/V-CPI− in reducing LNCaP tumor size and in preventing mouse weight loss associated with tumor burden.
FIG. 7.
P/V-CPI−-G3A is more effective at reducing LNCaP tumor burden than P/V-CPI−. (A) LNCaP tumor pictures. Groups of 10 male nude mice were implanted with LNCaP tumor cells. Mice that formed palpable tumors were injected intratumorally with PBS or 108 PFU of purified P/V-CPI− or P/V-CPI−-G3A at day 0 and day 8 of the study. On day 18 after the initial injection, tumors were harvested and fixed, and representative photographs were taken. (B) LNCaP tumor weight. The tumors harvested as described in panel A were weighed. Note that some symbols overlap. The bars indicate average tumor weights. vs, versus. (C) Mouse weight loss. Mice bearing LNCaP tumors and treated as described in panel A were monitored every other day for 18 days. Error bars represent ±95% confidence intervals.
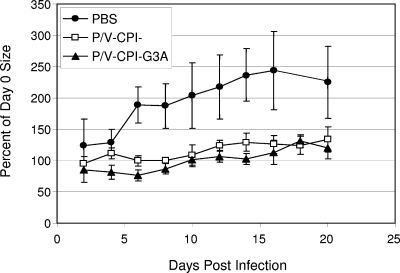
To define the relative effectiveness of the two CPI−-based vectors in a second model system, male nude mice were implanted with DU145 prostate tumor cells, and tumors were allowed to grow to a palpable size. Mice were then injected intratumorally on days 0 and 8 with either PBS or 108 PFU of purified P/V-CPI− or P/V-CPI−-G3A (n = 3 or 4). Tumor size and mouse weight were measured every other day for 20 days. As shown in Fig. 8, PBS-treated tumors continued to rapidly increase in size out to day 16, when tumor volume had increased to ∼250% that of day 0. In contrast, the sizes of tumors treated with either P/V-CPI− or P/V-CPI−-G3A appeared to plateau after initial treatment but did not further decrease after a second injection at day 8. By day 10, tumors treated with P/V-CPI− and P/V-CPI−-G3A appeared to increase slightly but then plateaued at ∼120% of day 0 tumor volume. Two virus injections were required to obtain the results shown in Fig. 8. These results support the conclusion that both of the P/V mutant vectors can limit increases in tumor burden in two model tumor systems. However, the increased effectiveness of the G3A mutant with LNCaP but not DU145 tumors suggests that the contribution of a hyperfusogenic F protein to tumor reduction may depend on the particular type of tumor being treated.
FIG. 8.
Both P/V-CPI− and P/V-CPI−-G3A delay DU145 tumor growth. Groups of 3 or 4 male nude mice were implanted with DU145 tumor cells. Mice with palpable tumors were injected intratumorally with PBS or 108 PFU of P/V-CPI− or P/V-CPI−-G3A on days 0 and 8 of the study. Tumor size was measured by calipers every other day for 20 days. Error bars represent standard deviations from the average.
DISCUSSION
The goal of the work described here was to provide proof of principle that a cytopathic and hyperfusogenic P/V mutant of the paramyxovirus SV5 could serve as an oncolytic virus. In two tumor model systems, the cytopathic P/V mutants were effective at limiting tumor growth in vivo. In one system involving LNCaP tumor cells, the P/V mutant encoding a hyperfusogenic mutant F protein was more effective than the parental P/V mutant at reducing tumor burden. Importantly, in vitro experiments demonstrated that despite the enhanced ability to cause cell-cell fusion, the P/V-CPI−-G3A mutant was still sensitive to IFN. Together, the work described here establishes the potential for SV5-based oncolytic vectors and SV5 P/V mutants as backbones for the design of more effective therapeutic vectors.
We have shown that the noncytopathic SV5 can be engineered for controlled cell killing through the expression of the herpes virus TK gene (39). Previous work compared the cell-killing potential of the rSV5-TK virus to WT rSV5, which is largely noncytopathic, and, thus, the effectiveness of rSV5-TK compared to inherently cytopathic viruses was not previously known. Here, we show using MTS-based viability assays that infection of Vero cells with the rSV5-TK virus did not reduce cell viability unless the culture was supplemented with GCV, as described previously (39). However, the loss of viability in the presence of GCV was slower and less extensive than that seen with the cytopathic vectors based on CPI− substitutions in the P/V gene. While we have not tested the rSV5-TK virus in an animal model system, the results with Vero cells, which do not restrict virus spread, suggest that the P/V mutants would be more effective for tumor therapy than WT rSV5-TK in tumors containing IFN-defective cells.
In addition to inherent features of negative-strand RNA viruses as vectors (52), our SV5 P/V-CPI− mutants have a number of properties that could be exploited for further development as an oncolytic vector. First, SV5 P/V gene mutants have been shown to induce apoptosis in a wide range of human tumor cell lines (11, 54), but virus spread in primary normal human prostate epithelial cells is highly restricted (53). While we have not determined virus titers in tumors infected with the P/V mutants, we were able to detect viral antigen by immunohistochemistry in LNCaP tumors at the time of harvest (data not shown). The P/V-CPI− cytopathic properties appear to be a stable phenotype since the ability of P/V-CPI− to kill cells was not lost even after sequential passages at low MOIs in A549 cells (data not shown). Our recent work has shown that the cell-killing phenotype of P/V-CPI− can be suppressed by expression of either WT P or WT V protein (12). While expression of the measles virus V protein is sufficient to block apoptosis induced by exogenous activators of p53-related pathways (8), it remains to be seen if the SV5 P and V proteins are also direct inhibitors of apoptosis. A second desirable oncolytic property is that the P/V-CPI− mutant is defective in limiting at least two phases of IFN responses: P/V-CPI− is a potent inducer of IFN but also cannot block IFN signaling (54). Thus, P/V-CPI− is restricted by IFN for growth in normal and immortalized human epithelial cells but not in a range of tumor cell lines (53). This has been proposed to be a major factor in the efficacy of oncolytic viruses and in prevention of virus spread to normal cells (4). Likewise, many RNA viruses that are defective in counteracting IFN responses are also defective for growth. By contrast, our P/V-CPI− mutant grows more effectively than WT SV5. Finally, our results in both mouse and ferret model systems indicate that intranasal infection with P/V-CPI− does not cause overt clinical symptoms, and infected animals have a low viral load that is cleared from the respiratory tract by 7 to 8 days p.i. with no obvious pathological effects (6). When high doses of the P/V-CPI− mutant (108 PFU) were delivered intravenously, nude mice showed no adverse effect and did not lose weight (data not shown). While we have not evaluated systemic spread of the P/V mutants, these results suggest that the P/V-CPI− virus establishes acute infections with minimal side effects in these animals.
There is interest in exploiting properties of the paramyxovirus glycoproteins to increase cell-killing potential and the specificity of these viruses for tumor versus normal cells (19, 41). Paramyxovirus F proteins have been used previously to enhance the efficacy of tumor cell killing (17, 24, 25) and can be engineered with cleavage sites for tumor-selective proteases (30, 47). Here, we have extended this concept to test the hypothesis that expression of a mutant SV5 F protein with enhanced fusogenic properties would be an effective means to increase tumor cell killing. In the context of the cytopathic P/V-CPI− mutant, the G3A mutation resulted in a recombinant virus with increased capacity to generate cell-cell fusion, as predicted by previous work expressing SV5 F protein mutants from heterologous vectors (27). The hyperfusogenic P/V-CPI−-G3A mutant grew to titers ∼1 log below the titer of the parental P/V-CPI− in the majority of cell lines tested (data not shown); however, growth appeared unaffected in other cell lines. Why would a glycine-to-alanine mutation in the fusion peptide of the SV5 F protein lead to reduced virus yield? Using recombinant SV40 vectors, Horvath and Lamb (27) showed that processing, transport, and cell surface expression of a newly synthesized G3A mutant were similar but not identical to WT F protein. This suggests that the biosynthetic steps that lead to cell surface expression of the G3A F mutant are not likely to be a major factor in lower virus yields. The G3A mutation reduces the energy of activation needed for the SV5 F protein to be triggered to cause membrane fusion (44). Thus, it is possible that there is premature triggering of F proteins on the surface of viral particles, which, in turn, reduces the infectivity of progeny virions. It is known that membrane lipid composition can affect the ability of SV5 to mediate fusion (43), suggesting that alterations in the ability of the G3A mutant F to mediate entry may account for growth defects at low MOIs in some but not all cell lines tested. Most importantly for the purposes of evaluating SV5 as a safe oncolytic vector, the addition of a hyperfusogenic F protein did not increase SV5 growth properties and in most cases led to attenuated growth.
A potential danger of engineering a hyperfusogenic virus for oncolytic therapies is that virus spread may no longer show IFN-mediated restriction since spread via cell-cell fusion could, a priori, be less sensitive to IFN-induced antiviral responses than spread through the production of progeny virions. Here, we show that despite the increased ability of P/V-CPI−-G3A to cause CPE in a large number of cell lines, at low MOIs spread of P/V-CPI−-G3A and cell killing are still restricted by type I IFN. Unlike LNCaP cells, which are defective in IFN synthesis and signaling (Fig. 4) (13), PC3 tumor cells have been shown to mount a strong IFN-induced antiviral response (1), and this is capable of restricting the growth and spread of both the parental P/V-CPI− and hyperfusogenic G3A mutant at low MOIs. While neutralization of IFN increased viral titers during P/V-CPI−-G3A and P/V-CPI− infection by ∼1 log at most time points examined, P/V-CPI−-G3A virus yields never reached the same level as P/V-CPI−. This result supports the hypothesis that the fusogenic P/V-CPI−-G3A will have an inherent defect in its viral life cycle in some cell types not observed with the parental P/V-CPI−. Our P/V-CPI−-based vectors induce IRF-3 activation (12), and it has been proposed that there are enhanced innate responses when new cells are recruited into growing syncytia (21). Thus, spread of the G3A mutant could be restricted by mechanisms that are independent of IFN signaling but dependent on IRF-3 or other preexisting factors that are activated during fusion between infected and uninfected cells. Our results suggest that hyperfusogenic vectors should be constructed in the context of a virus that is defective in counteracting IFN responses in order to maintain a level of safety.
This work is the first report of the use of SV5 as an anticancer therapeutic agent. As part of our focus on a panel of human prostate cancer cell lines, we found that P/V-CPI−-G3A was more cytopathic than P/V-CPI− in vitro in LNCaP cells. Interestingly, there were also differences in the therapeutic efficacy of P/V-CPI−-G3A and P/V-CPI− in an in vivo LNCaP xenograft model system since mice treated with the fusogenic G3A mutant had consistently smaller tumors than those treated with PBS or P/V-CPI−. Additionally, while mice treated with PBS or P/V-CPI− lost ∼10% of their initial body weight, those treated with P/V-CPI−-G3A maintained their original body weight and appeared much healthier throughout the time course of treatment, presumably due to the reduced tumor burden following treatment with the hyperfusogenic G3A mutant.
P/V-CPI−-G3A exhibited a greater CPE than P/V-CPI− in vitro in the DU145 cell line. However, in an in vivo DU145 xenograft model, P/V-CPI− and P/V-CPI−-G3A showed similar abilities to delay tumor growth. Taken together, these data indicate that both P/V-CPI− and P/V-CPI−-G3A show promise as potential prostate cancer therapeutic agents but that P/V-CPI−-G3A may have an increased therapeutic effect compared to P/V-CPI− in some, but not all, tumor systems. It is currently unclear why P/V-CPI−-G3A was more effective at reducing LNCaP tumor burden than P/V-CPI−, but these two viruses showed similar efficacy at delaying DU145 tumor growth. Differences in lipid composition of DU145 cell membranes or the type of extracellular matrix formed around these cells in vivo could influence the ability of P/V-CPI−-G3A infection to cause syncytia formation in these cells (43). An alternative explanation is that IFN signaling plays a role in the effectiveness of these two viruses in vivo. LNCaP cells are unresponsive to IFN due lack of Jak1 expression (13). Within the LNCaP tumors, virus replication would not be inhibited, and this could lead to greater tumor killing due to enhanced cell fusion by the P/V-CPI− mutant. In contrast, DU145 cells respond to IFN (23), and as shown in Fig. 6, IFN limited spread of the two P/V mutants. Thus, the similarities between these two viruses in limiting DU145 tumor growth could be due to their inability to block IFN signaling from infected cells or from cells recruited to the site of infection.
The immune response to tumor cells infected with SV5 could influence the oncolytic potential of P/V-CPI− and P/V-CPI−-G3A (50). In transfected tumor cells, fusogenic membrane glycoproteins are reported to cause necrotic cell death, which can be a highly immunostimulatory event (3, 22, 35), and this could lead to recruitment of immune cells to the site of infected tumors. Thus, the SV5 G3A mutant could serve to promote fusion using other virus systems where immune recruitment could be an additional safety feature. We have recently shown that within a small population of human donors, very few individuals had high levels of antibodies that neutralized SV5 infectivity (29). While we have not evaluated cellular immunity to SV5, it is possible that immunity prior to or following SV5 treatment could limit the effectiveness of our vectors. Alternatively, innate or adaptive anti-SV5 responses could provide a mechanism to stimulate the immune system to aid in clearance (35).
Acknowledgments
We are grateful to Nancy Kock for help with histological experiments, Steve Kridel for LNCaP cells, K. Conzelmann and R. A. Lamb for BSR-T7 cells, D. Ornelles for the pLuc plasmid, R. A. Lamb and B. He for rSV5 cDNA clones, and Maryam Ahmed for guidance with the tumor studies.
This work was supported in part by NIH grant AI42023, Susan G. Komen Breast Cancer Foundation grant BCTR0402596, and the Merle and Larry Andrew Fund for Breast Cancer Research.
Footnotes
Published ahead of print on 30 July 2008.
REFERENCES
- 1.Ahmed, M., S. D. Cramer, and D. S. Lyles. 2004. Sensitivity of prostate tumors to wild type and M protein mutant vesicular stomatitis viruses. Virology 33034-49. [DOI] [PubMed] [Google Scholar]
- 2.Bateman, A., F. Bullough, S. Murphy, L. Emiliusen, D. Lavillette, F. L. Cosset, R. Cattaneo, S. J. Russell, and R. G. Vile. 2000. Fusogenic membrane glycoproteins as a novel class of genes for the local and immune-mediated control of tumor growth. Cancer Res. 601492-1497. [PubMed] [Google Scholar]
- 3.Bateman, A. R., K. J. Harrington, T. Kottke, A. Ahmed, A. A. Melcher, M. J. Gough, E. Linardakis, D. Riddle, A. Dietz, C. M. Lohse, S. Strome, T. Peterson, R. Simari, and R. G. Vile. 2002. Viral fusogenic membrane glycoproteins kill solid tumor cells by nonapoptotic mechanisms that promote cross presentation of tumor antigens by dendritic cells. Cancer Res. 626566-6578. [PubMed] [Google Scholar]
- 4.Bell, J. C., B. Lichty, and D. Stojdl. 2003. Getting oncolytic virus therapies off the ground. Cancer Cell 47-11. [DOI] [PubMed] [Google Scholar]
- 5.Buchholz, U. J., S. Finke, and K. K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capraro, G. A., J. B. Johnson, N. Kock, and G. D. Parks. 2008. Growth and antibody responses to respiratory tract infection of ferrets and mice with WT and P/V mutants of the paramyxovirus simian virus 5. Virology 376416-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Childs, K. S., N. Stock, C. Ross, J. Andrejeva, L. Hilton, M. Skinner, R. E. Randall, and S. Goodbourn. 2007. mda-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology 359190-200. [DOI] [PubMed] [Google Scholar]
- 8.Cruz, C. D., H. Palosaari, J. P. Parisien, P. Devaux, R. Cattaneo, T. Ouchi, and C. M. Horvath. 2006. Measles virus V protein inhibits p53 family member p73. J. Virol. 805644-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng, H., C. Wang, G. Acsadi, and J. A. Wolff. 1991. High-efficiency protein synthesis from T7 RNA polymerase transcripts in 3T3 fibroblasts. Gene 109193-201. [DOI] [PubMed] [Google Scholar]
- 10.Didcock, L., D. F. Young, S. Goodbourn, and R. E. Randall. 1999. The V protein of simian virus 5 inhibits interferon signaling by targeting STAT1 for proteasome-mediated degradation. J. Virol. 739928-9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dillon, P. J., E. K. Wansley, V. A. Young, M. A. Alexander-Miller, and G. D. Parks. 2006. Exchanges of P/V genes between two noncytopathic SV5 variants results in a recombinant virus that kills cells through death pathways that are sensitive to caspase inhibitors. J. Gen. Virol. 873643-3648. [DOI] [PubMed] [Google Scholar]
- 12.Dillon, P. J., and G. D. Parks. 2007. Role for the phosphoprotein P subunit of the paramyxovirus polymerase in limiting induction of host cell antiviral responses. J. Virol. 8111116-11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn, G. P., K. C. Sheehan, L. J. Old, and R. D. Schreiber. 2005. IFN unresponsiveness in LNCaP cells due to the lack of JAK1 gene expression. Cancer Res. 653447-3453. [DOI] [PubMed] [Google Scholar]
- 14.Ebert, O., K. Shinozaki, C. Kournioti, M. S. Park, A. Garcia-Sastre, and S. L. Woo. 2004. Syncytia induction enhances the oncolytic potential of vesicular stomatitis virus in virotherapy for cancer. Cancer Res. 643265-3270. [DOI] [PubMed] [Google Scholar]
- 15.Elankumaran, S., D. Rockemann, and S. K. Samal. 2006. Newcastle disease virus exerts oncolysis by both intrinsic and extrinsic caspase-dependent pathways of cell death. J. Virol. 807522-7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Errington, F., J. Jones, A. Merrick, A. Bateman, K. harrington, M. Gough, D. O'Donnell, P. Selby, R. Vile, and A. Melcher. 2006. Fusogenic membrane glycoprotein-mediated tumour cell fusion activates human dendritic cells for enhanced IL-12 production and T-cell priming. Gene Ther. 13138-149. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Trevino, A., S. Castel, C. Lopez-Iglesias, N. Cortadellas, J. Comas-Riu, and E. Mercade. 2003. Effects of adenovirus-mediated SV5 fusogenic glycoprotein expression on tumor cells. J. Gene Med. 5483-492. [DOI] [PubMed] [Google Scholar]
- 18.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signaling, immune modulation, antiviral responses and virus countermeasures. J. Gen. Virol. 812341-2364. [DOI] [PubMed] [Google Scholar]
- 19.Hammond, A. L., R. K. Plemper, J. Zhang, U. Schneider, S. J. Russell, and R. Cattaneo. 2001. Single-chain antibody displayed on recombinant measles virus confers entry through the tumor-associated carcinoembryonic antigen. J. Virol. 752087-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He, B., R. G. Paterson, C. D. Ward, and R. A. Lamb. 1997. Recovery of infectious SV5 from cloned DNA and expression of a foreign gene. Virology 237249-260. [DOI] [PubMed] [Google Scholar]
- 21.Herschke, F., S. Plumet, T. Duhen, O. Azocar, J. Druelle, D. Laine, T. F. Wild, C. Rabourdin-Combe, D. Gerlier, and H. Valentin. 2007. Cell-cell fusion induced by measles virus amplifies the type I interferon response. J. Virol. 8112859-12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higuchi, H., S. F. Bronk, A. Bateman, K. Harrington, R. G. Vile, and G. J. Gores. 2000. Viral fusogenic membrane glycoprotein expression causes syncytia formation with bioenergetic cell death: implications for gene therapy. Cancer Res. 606396-6402. [PubMed] [Google Scholar]
- 23.Hobeika, A. C., P. S. Subramaniam, and H. M. Johnson. 1997. IFN-alpha induces the expression of the cyclin-dependent kinase inhibitor p21 in human prostate cancer cells. Oncogene 141165-1170. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann, D., J. M. Bangen, W. Bayer, and O. Wildner. 2006. Synergy between expression of fusogenic membrane proteins, chemotherapy and facultative virotherapy in colorectal cancer. Gene Ther. 131534-1544. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann, D., W. Bayer, and O. Wildner. 2007. Local and distant immune-mediated control of colon cancer growth with fusogenic membrane glycoproteins in combination with viral oncolysis. Hum. Gene Ther. 18435-450. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann, D., and O. Wildner. 2006. Enhanced killing of pancreatic cancer cells by expression of fusogenic membrane glycoproteins in combination with chemotherapy. Mol. Cancer Ther. 52013-2022. [DOI] [PubMed] [Google Scholar]
- 27.Horvath, C. M., and R. A. Lamb. 1992. Studies on the fusion peptide of a paramyxovirus fusion glycoprotein: roles of conserved residues in cell fusion. J. Virol. 662443-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iseni, F., D. Garcin, M. Nishio, N. Kedersha, P. Anderson, and D. Kolakofsky. 2002. Sendai virus trailer RNA binds TIAR, a cellular protein involved in virus-induced apoptosis. EMBO J. 215141-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson, B., G. A. Capraro, and G. D. Parks. 2008. Differential mechanisms of complement-mediated neutralization of the closely related paramyxoviruses SV5 and mumps virus. Virology 376112-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinoh, H., M. Inoue, K. Washizawa, T. Yamamoto, S. Fujikawa, Y. Tokusumi, A. Iida, Y. Nagai, and M. Hasegawa. 2004. Generation of a recombinant Sendai virus that is selectively activated and lyses human tumor cells expressing matrix metalloproteinases. Gene Ther. 111137-1145. [DOI] [PubMed] [Google Scholar]
- 31.Lamb, R. A., and G. D. Parks. 2007. Paramyxoviridae: the viruses and their replication, p.1449-1496. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 32.Lamb, R. A., R. G. Paterson, and T. S. Jardetzky. 2006. Paramyxovirus membrane fusion: lessons from the F and HN atomic structures. Virology 34430-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin, Y., F. Horvath, J. A. Aligo, R. Wilson, and B. He. 2005. The role of simian virus 5 V protein on viral RNA synthesis. Virology 338270-280. [DOI] [PubMed] [Google Scholar]
- 34.Lorence, R. M., K. W. Reichard, B. B. Katubig, H. M. Reyes, A. Phuangsab, B. R. Mitchell, C. J. Cascino, R. J. Walter, and M. E. Peeples. 1994. Complete regression of human neuroblastoma zenografts in athymic mice after local Newcastle disease virus therapy. J. Natl. Cancer Inst. 861228-1233. [DOI] [PubMed] [Google Scholar]
- 35.Melcher, A., M. Gough, S. Todryk, and R. Vile. 1999. Apoptosis or necrosis for tumor immunotherapy: what's in a name? J. Mol. Med. 77824-833. [DOI] [PubMed] [Google Scholar]
- 36.Myers, R., S. Greiner, M. Harvey, D. Soeffker, M. Frenzke, K. Abraham, A. Shaw, S. Rozenblatt, M. J. Federspiel, S. J. Russell, and K. W. Peng. 2005. Oncolytic activities of approved mumps and measles vaccines for therapy of ovarian cancer. Cancer Gene Ther. 12593-599. [DOI] [PubMed] [Google Scholar]
- 37.Myers, R. M., S. M. Greiner, M. E. Harvey, G. Griesmann, M. J. Kuffel, S. A. Buhrow, J. M. Reid, M. Federspiel, M. M. Ames, D. Dingli, K. Schweikart, A. Welch, A. Dispenzieri, K. W. Peng, and S. J. Russell. 2007. Preclinical pharmacology and toxicology of intravenous MV-NIS, an oncolytic measles virus administered with or without cyclophosphamide. Clin. Pharmacol. Ther. 82700-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nussbaum, O., C. C. Broder, and E. A. Berger. 1994. Fusogenic mechanisms of enveloped-virus glycoproteins analyzed by a novel recombinant vaccinia virus-based assay quantitating cell fusion-dependent reporter gene activation. J. Virol. 685411-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parks, G. D., V. A. Young, C. Koumenis, E. K. Wansley, J. L. Layer, and K. M. Cooke. 2002. Controlled cell killing by a recombinant nonsegmented negative-strand RNA virus. Virology 293192-203. [DOI] [PubMed] [Google Scholar]
- 40.Paterson, R. G., M. A. Shaughnessy, and R. A. Lamb. 1989. Analysis of relationship between cleavability of paramyxovirus fusion protein and length of connecting peptide. J. Virol. 631293-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng, K. W., K. A. Donovan, U. Schneider, R. Cattaneo, J. A. Just, and S. J. Russell. 2003. Oncolytic measles viruses displaying a single-chain antibody against CD38, a myeloma cell marker. Blood 1012557-2562. [DOI] [PubMed] [Google Scholar]
- 42.Reichard, K. W., R. M. Lorence, C. J. Cascino, M. E. Peeples, R. J. Walter, M. B. Fernando, H. M. Reyes, and J. A. Greager. 1992. Newcastle disease virus selectively kills human tumor cells. J. Surg. Res. 52448-453. [DOI] [PubMed] [Google Scholar]
- 43.Roos, D. S., C. S. Duchala, C. B. Stephensen, K. V. Holmes, and P. W. Choppin. 1990. Control of virus-induced cell fusion by host cell lipid composition. Virology 175345-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russell, C. J., T. S. Jardetzky, and R. A. Lamb. 2004. Conserved glycine residues in the fusion peptide of the paramyxovirus fusion protein regulate activation of the native state. J. Virol. 7813727-13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russell, S. J. 2002. RNA viruses as virotherapy agents. Cancer Gene Ther. 9961-966. [DOI] [PubMed] [Google Scholar]
- 46.Shin, E. J., J. I. Chang, B. Choi, G. Wanna, O. Ebert, E. M. Genden, and S. L. Woo. 2007. Fusogenic vesicular stomatitis virus for the treatment of head and neck squamous carcinomas. Otolaryngol. Head Neck Surg. 136811-817. [DOI] [PubMed] [Google Scholar]
- 47.Springfeld, C., V. von Messling, M. Frenzke, G. Ungerechts, C. J. Buchholz, and R. Cattaneo. 2006. Oncolytic efficacy and enhanced safety of measles virus activated by tumor-secreted matrix metalloproteinases. Cancer Res. 667694-7700. [DOI] [PubMed] [Google Scholar]
- 48.Stojdl, D. F., B. D. Lichty, B. R. tenOver, J. M. Paterson, A. T. Power, S. Knowles, R. Marius, J. Rynard, L. Poiquin, H. Atkins, E. G. Brown, R. K. Durbin, J. E. Durbin, J. Hiscott, and J. C. Bell. 2003. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4263-275. [DOI] [PubMed] [Google Scholar]
- 49.Sun, M., T. A. Rothermel, L. Shuman, J. A. Aligo, S. Xu, Y. Lin, R. A. Lamb, and B. He. 2004. Conserved cysteine-rich domain of paramyxovirus simian virus 5 V protein plays an important role in blocking apoptosis. J. Virol. 785068-5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ungerechts, G., C. Springfeld, M. E. Frenzke, J. Lampe, W. B. Parker, E. J. Sorscher, and R. Cattaneo. 2007. An immunocompetent murine model for oncolysis with an armed and targeted measles virus. Mol. Ther. 151991-1997. [DOI] [PubMed] [Google Scholar]
- 51.Vigil, A., M. S. Park, O. Martinez, M. A. Chua, S. Xiao, J. F. Cros, L. Martinez-Sobrido, S. L. Woo, and A. Garcia-Sastre. 2007. Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus. Cancer Res. 678285-8292. [DOI] [PubMed] [Google Scholar]
- 52.von Messling, V., and R. Cattaneo. 2004. Toward novel vaccines and therapies based on negative-strand RNA viruses. Curr. Top. Microbiol. Immunol. 283281-312. [DOI] [PubMed] [Google Scholar]
- 53.Wansley, E. K., P. J. Dillon, M. D. Gainey, J. Tam, S. D. Cramer, and G. D. Parks. 2005. Growth sensitivity of a recombinant simian virus 5 P/V mutant to type I interferon differs between tumor cell lines and normal primary cells. Virology 335131-144. [DOI] [PubMed] [Google Scholar]
- 54.Wansley, E. K., and G. D. Parks. 2002. Naturally occurring substitutions in the P/V gene convert the noncytopathic paramyxovirus simian virus 5 into a virus that induces alpha/beta interferon synthesis and cell death. J. Virol. 7610109-10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong, L. H., K. G. Krauer, I. Hatzinisiriou, M. J. Estcourt, P. Hersey, N. D. Tam, S. Edmondson, R. J. Devenish, and S. J. Ralph. 1997. Interferon-resistant human melanoma cells are deficient in ISGF3 components, STAT1, STAT2, and p48-ISGF3γ. J. Biol. Chem. 27228779-28785. [DOI] [PubMed] [Google Scholar]
- 56.Xu, B., D. Grander, O. Sangfelt, and S. Einhorn. 1994. Primary leukemia cells resistant to alpha-interferon in vitro are defective in the activation of the DNA-binding factor interferon-stimulated gene factor 3. Blood 841942-1949. [PubMed] [Google Scholar]