Abstract
We investigated the mechanism by which the cholesterol-binding compound amphotericin B methyl ester (AME) inhibits human immunodeficiency virus type 1 (HIV-1) particle production. We observed no significant effect of AME on Gag binding to the plasma membrane, Gag association with lipid rafts, or Gag multimerization, indicating that the mechanism of inhibition by AME is distinct from that by cholesterol depletion. Electron microscopy analysis indicated that AME significantly disrupts virion morphology. Interestingly, we found that AME does not inhibit the release of Vpu-defective HIV-1 or Vpu− retroviruses such as murine leukemia virus and simian immunodeficiency virus. We demonstrated that the ability of Vpu to counter the activity of CD317/BST-2/tetherin is markedly reduced by AME. These results indicate that AME interferes with the anti-CD317/BST-2/tetherin function of Vpu.
Several studies have demonstrated that cholesterol-enriched plasma membrane microdomains known as lipid rafts (3, 9, 28) play important roles in the replication of a number of enveloped viruses, including human immunodeficiency virus type 1 (HIV-1) (22). Lipid rafts appear to function in both virus entry and particle egress, and the HIV-1 lipid bilayer itself exhibits a raft-like lipid composition (2, 4). We previously reported that cholesterol depletion interferes with HIV-1 particle production by impairing the association of Gag with membrane (21, 24). Adding further support to the concept that membrane cholesterol plays an important role in HIV-1 biology, we recently demonstrated that the cholesterol-binding compound amphotericin B methyl ester (AME), a water-soluble, relatively noncytotoxic derivative of amphotericin B, potently inhibits HIV-1 replication (34). The antiviral activity of AME is due to profoundly impaired viral infectivity, as well as defective virus particle production (34). Interestingly, the passaging of HIV-1 in the presence of AME leads to viral escape from this compound; mutations that confer resistance map to the cytoplasmic tail of gp41 (34, 35). This analysis revealed a novel mechanism of resistance whereby the gp41 mutations confer resistance to AME by creating PR cleavage sites in the gp41 cytoplasmic tail, leading to the truncation of gp41 after Env incorporation into virions (35). While the resistant mutants overcome the entry defect imposed by AME, they remain sensitive to the AME-imposed disruption of particle assembly and release (34, 35). In the present study, we investigated the mechanism by which AME inhibits HIV-1 assembly and release by evaluating the effect of this compound on the specific steps of the assembly and release pathway and the involvement of viral proteins other than Gag in the AME-imposed inhibition of particle production.
AME inhibits HIV-1 particle production with no significant effect on Gag-membrane binding, raft association, or Gag multimerization.
We previously demonstrated that AME inhibits HIV-1 replication in T-cell lines and primary cell types (34). The inhibitory effect of AME on viral replication appeared to be due predominantly to a 50- to 100-fold reduction in viral infectivity. However, we also noted a significant (∼4-fold) impairment in HIV-1 particle production from infected Jurkat cells. To understand the mechanism by which AME inhibits virus release, in this study we first examined the effect of AME on particle production from HeLa cells following transfection with the full-length, infectious HIV-1 molecular clone pNL4-3. Transfected cells were metabolically radiolabeled, and cell- and virus-associated proteins were immunoprecipitated and quantified. The virus release efficiency was reduced in a concentration-dependent manner; treating virus-producing cells with 5 μM AME reduced virus production by approximately threefold, whereas 10 μM AME reduced virus production over fivefold compared with that in untreated controls (Fig. 1A). Having established that AME treatment impairs HIV-1 particle production in multiple cell types, we next determined whether AME has any effect on Gag binding to membrane or on Gag association with detergent-resistant membrane (DRM), a commonly used biochemical surrogate for raft association. The steady-state distribution of Gag in membrane and DRM was monitored by immunoblotting. Virus-expressing HeLa cells were homogenized, divided into two aliquots, treated with or without a 0.25% final concentration of cold Triton X-100, and subjected to equilibrium flotation centrifugation on sucrose gradients as described previously (18, 19, 21, 25). The distribution of Gag in membrane and DRM fractions was determined by immunoblotting. We observed no significant effect of AME treatment on the distribution of Gag in the total membrane preparation or DRM (data not shown). This finding was confirmed by analyzing Gag association with membrane and DRM following pulse-chase labeling. We detected ∼36 and ∼43% of Pr55Gag in membrane fractions without and with AME treatment, respectively (Fig. 1B). After cold-Triton X-100 treatment, ∼25% of Pr55Gag was associated with DRM without AME treatment and ∼32% of Gag was DRM associated following treatment with 10 μM AME (Fig. 1B). These results indicate that the defect in virus release caused by AME is not due to the disrupted association of Gag with membrane or with DRM. This finding is in contrast to results obtained with cholesterol-depleting agents, which significantly impair Gag-membrane binding (24). We also observed that AME treatment did not affect the distribution of the raft marker caveolin or the nonraft marker transferrin receptor in the membrane or DRM fractions (data not shown). To determine whether AME treatment of virus producer cells affects higher-order Gag multimerization, we used a cell-based assay that measures assembly-induced masking of epitopes recognized by anti-Gag antibodies (25). As reported in our earlier study (25), the effect of sample denaturation on immunoprecipitation efficiency provides a measure of higher-order Gag multimerization. Gag-expressing cells treated or not with AME were metabolically radiolabeled, and cell lysates were immunoprecipitated with or without prior denaturation. We observed that ∼30% of membrane-bound Gag was epitope exposed and that AME treatment did not have a significant effect on this value (Fig. 1C). Similarly, the degree of epitope exposure of DRM-associated Gag was not affected by AME treatment (Fig. 1C). These data indicate that the higher-order multimerization of membrane-bound or DRM-associated Gag measured in this assay was not affected by the treatment of Gag-expressing cells with AME. We previously reported that the propagation of HIV-1 in the presence of AME leads to the emergence of AME-resistant variants (34). The mutations responsible for AME resistance (gp41 mutations P203L and S205L) map to a region of the gp41 cytoplasmic tail close to the membrane-spanning domain. To determine whether AME-resistant mutants overcome the defect in particle production caused by AME, we measured the release of AME-resistant mutants in the presence and absence of AME. We observed that the release of the AME-resistant mutants was inhibited by AME to an extent similar to that of the wild type (WT) (Fig. 1D). Thus, the mutations in gp41 that induce resistance to AME in the context of virus replication and single-cycle infectivity assays do not reverse the effects of AME on virus particle production.
FIG. 1.
AME inhibits HIV-1 particle production with no significant effect on Gag binding to the plasma membrane, Gag association with lipid rafts, or Gag multimerization. (A) HeLa cells were transfected with pNL4-3 (1) and treated 6 h posttransfection with the indicated concentrations of AME for 20 to 24 h. One day posttransfection, cells were metabolically labeled for 2 h with [35S]Met-Cys, and labeled viral proteins in cell and virion lysates were immunoprecipitated with HIV Ig and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by fluorography (6). The virus release efficiency was calculated as the amount of virion-associated p24 relative to total (cell- plus virion-associated) Gag. (B) HeLa cells transfected with pNL4-3/PR− (11) were treated (+) or not treated (−) with 10 μM AME and were pulse-labeled for 5 min and chased in unlabeled medium for 15 min. Postnuclear supernatants were incubated in the absence or presence of 0.25% Triton X-100 and subjected to membrane flotation centrifugation (18, 19, 21). Gradient fractions were treated with radioimmunoprecipitation assay buffer, and the sets of membrane fractions (fractions 1 to 5) and DRM fractions (fractions 1 to 5) were each pooled. Labeled Pr55Gag in each pooled fraction was recovered by immunoprecipitation after denaturation, and the amount of Gag present in membrane and DRM fractions compared to the total amount of Gag in all 10 fractions was determined. (C) HeLa cells transfected with pNL4-3/PR− were treated (+) or not treated (−) with 10 μM AME and subjected to the epitope exposure assay for higher-order Gag multimerization (25). The percentages of Gag epitope exposure in membrane and DRM fractions were determined. (D) HeLa cells were transfected with pNL4-3 or the AME-resistant mutant (P203L and S205L) constructs (34) and treated (+) or not (−) with 10 μM AME, and the virus release efficiency was calculated as described in the legend to panel A. Data are means ± standard deviations (SD; n = 3 to 5).
AME does not affect the subcellular localization of Gag but alters the morphology and density of released HIV-1 particles.
Standard membrane flotation assays do not distinguish between Gag bound to the plasma membrane and Gag associated with intracellular membrane. To determine whether AME treatment affects the trafficking of Gag to the plasma membrane, we compared the localization patterns of Gag in AME-treated and untreated cells by immunostaining. HeLa cells transiently transfected with pNL4-3 were treated overnight with AME, fixed, immunostained with anti-MA antibodies, and analyzed by fluorescence microscopy. Gag in cells either treated or not treated with AME displayed a predominantly punctate, plasma membrane localization pattern (data not shown), indicating that AME did not induce a major change in Gag trafficking.
Because AME treatment does not affect Gag binding to the plasma membrane or Gag multimerization, we examined whether this cholesterol-binding compound might disrupt virion budding from the cell surface. HeLa cells transfected with pNL4-3 were treated with 10 μM AME or were left untreated and were subsequently fixed and examined by transmission electron microscopy. In both AME-treated and untreated cells, numerous released mature particles were observed, with no striking accumulation of immature particles at the plasma membrane detected in treated cells (data not shown). These results indicate that AME does not act by disrupting HIV-1 late domain function.
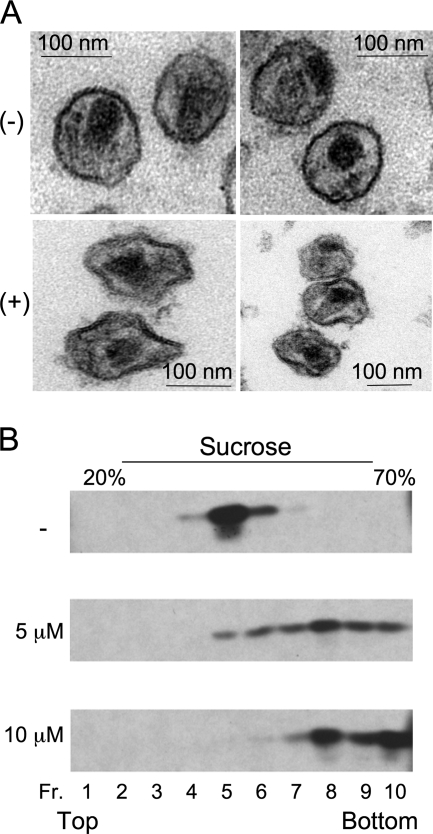
It has been reported previously that the lipid bilayers of HIV-1 virions are enriched with cholesterol relative to the host cell plasma membrane (2, 4), raising the possibility that, as a cholesterol-binding compound, AME may bind preferentially to the viral versus the cellular membrane. To address this issue, we examined the impact on virion morphology of treating purified virions with AME in vitro. Intriguingly, the morphology of AME-treated viral particles differed significantly from that of untreated virions, with severely distorted viral membranes observed among the treated particles (Fig. 2A). The extent of virion distortion was quantified by measuring the deviations from circularity of 100 treated and 100 untreated particles. This analysis was performed by drawing a circle around each virion and measuring the distance between the viral membrane and the periphery of the circle along the radius. Virions whose circularity deviated by more than 20% of the radius were scored as distorted. Approximately 80% of the AME-treated virions were classified as being distorted, whereas only 11% of untreated virions were distorted. To examine the density of virions treated with AME in vitro, we sedimented particles treated or not with AME on linear sucrose gradients. A marked increase in virion density in the AME-treated samples was observed (Fig. 2B). The perturbation of virion morphology and density induced by AME treatment may be a major contributor to the previously reported infectivity defect imposed by this cholesterol-binding compound (34, 35) and is likely to result from direct binding of AME to cholesterol in the viral membrane.
FIG. 2.
AME treatment distorts the morphology and increases the density of purified virions. (A) Virions collected from HeLa cells 24 h posttransfection with pNL4-3 were treated (+) or not (−) with AME for 2 h, pelleted by ultracentrifugation, fixed, and analyzed by electron microscopy. (B) HIV-1 virions purified as described in the legend to panel A and treated with the indicated concentrations of AME were layered onto 20 to 70% (wt/vol) linear sucrose density gradients and subjected to ultracentrifugation (12). Ten fractions (Fr. 1 to 10) were collected from the top of the gradient and analyzed by Western blotting with HIV Ig.
The release of the Fyn(10)fullMA Gag chimera is inhibited by AME.
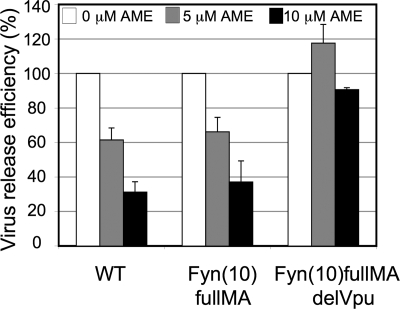
We reported recently that the inhibition of virus release caused by cholesterol depletion is due to disrupted Gag-membrane binding and impaired higher-order Gag multimerization (24). We observed that fusing the Fyn membrane-binding signal to the N terminus of Gag [yielding the chimera Fyn(10)fullMA] reverses the impact of cholesterol depletion on virus production. To determine whether the same effect is seen with the cholesterol-binding compound AME, we performed virus release assays with Fyn(10)fullMA in the presence or absence of AME. Interestingly, the release of Fyn(10)fullMA is reduced by AME to an extent similar to that of the WT (Fig. 3). These results, together with the findings presented above that AME does not inhibit Gag-membrane binding or Gag multimerization, indicate that the mechanism by which AME inhibits HIV-1 particle production is distinct from the mechanism by which cholesterol-depleting agents disrupt virus assembly and release.
FIG. 3.
The insertion of the membrane-targeting signal from c-Fyn does not diminish the ability of AME to disrupt virus particle production. HeLa cells transfected with pNL4-3, pNL4-3/Fyn(10)fullMA (17), or pNL4-3/Fyn(10)fullMA/delVpu [constructed by exchanging the BssHII-SphI fragment (pNL4-3 nucleotides 711 to 1443) of the Fyn(10)fullMA construct (24) with the corresponding fragment from pNL4-3delVpu] were treated with the indicated concentrations of AME and metabolically labeled with [35S]Met-Cys. The virus release efficiency was calculated as described in the legend to Fig. 1. The virus release efficiencies of the WT and the Fyn(10)fullMA Gag constructs were each normalized to 100%. The release efficiency of Fyn(10)fullMA was ∼6-fold higher than that of the WT. The Vpu-defective Fyn(10)fullMA/delVpu mutant displayed an ∼5-fold defect in particle production relative to Fyn(10)fullMA. Data are means ± SD (n = 4).
The inhibition of virus release by AME is Vpu dependent.
The experiments described above were performed in the context of a full-length, infectious HIV-1 molecular clone encoding all HIV-1 proteins. To gain further insights into the mechanism by which AME inhibits HIV-1 particle production, we examined a potential role for viral proteins other than Gag in the ability of AME to disrupt the late stages of the viral replication cycle. We evaluated the effect of AME on the assembly and release of virus particles in the context of clones defective in PR, Env, Nef, or Vpu. As shown in Fig. 4A, the effect of AME on the release of HIV-1 mutants defective in PR, Env, or Nef was comparable to that observed for the WT, indicating that the expression of these proteins is not required for the AME-imposed inhibition of particle production. In contrast, we observed that the mutation of Vpu largely abolished the ability of AME to interfere with HIV-1 assembly and release (Fig. 4B). As reported previously (8, 13, 30, 31), the deletion of Vpu reduces particle production ∼10-fold (9.3% ± 2.4% relative to the WT level). AME treatment at 5 μM had no effect on the release of Vpu-defective HIV-1 and at 10 μM caused a reduction in Vpu− particle release of only ∼30%, compared to the 70% reduction observed for the WT at 10 μM AME (Fig. 4B).
FIG. 4.
The inhibition of virus release is Vpu dependent. (A to C) HeLa cells were transfected with HIV-1 molecular clones defective in PR (pNL4-3/PR−), Env (pNL4-3KFS) (7), or Nef (pNL4-3/delNef) (29) (A) or Vpu (pNL4-3delVpu; delVpu) (13) (B) or with the pNL4-3/PTAP− (PTAP−) mutant (11) or the pNL4-3/MA 29KE/31KE (29KE/31KE) mutant (23) (C). (D) Cells were transfected with pNL4-3 (HIV), an SIVmac239 molecular clone (SIV) (26), or a vector expressing MLV Gag-Pol (MLV) (14). Cells treated with the indicated concentrations of AME were metabolically labeled. Cell and viral lysates were immunoprecipitated with anti-SIVmac239 antiserum or goat anti-MLV Gag p30 antiserum (obtained from ViroMed Biosafety Laboratories, Camden, NJ), and the virus release efficiency was calculated as described in the legend to Fig. 1. In panels A to C, virus release efficiencies for the WT and mutant molecular clones were each normalized to 100%. The release efficiencies of mutants compared to that of the WT (in percentages) were as follows: pNL4-3/PR−, 65 ± 21; pNL4-3KFS, 184 ± 108; pNL4-3/delNef, 120 ± 38; pNL4-3delVpu, 9 ± 2; pNL4-3/PTAP−, 7 ± 1; and pNL4-3/MA 29KE/31KE, 28.0 ± 11.0. In panel D, HIV-1, SIVmac239, and MLV release efficiencies were each normalized to 100%. Data are means ± SD (n = 3 to 5).
To examine the possibility that the level of release of Vpu-defective HIV-1 is so low that further reductions imposed by AME treatment are not detectable, we measured the effect of AME on the release of a late-domain-deficient (PTAP−) p6 mutant (11). The release of the PTAP− mutant was reduced by >10-fold (to 7.2% ± 1.2% of the WT level), yet unlike the Vpu-defective mutant, this mutant showed a further reduction in release of ∼4-fold as a result of AME treatment (Fig. 4C). We also observed that AME treatment significantly impaired the production of a pNL4-3 derivative encoding mutations in the MA domain of Gag (29KE/31KE) (23) (Fig. 4C). The 29KE/31KE mutant exhibits an ∼4-fold defect in virus release efficiency due to the retargeting of Gag to multivesicular bodies (20, 23). Thus, the inability of AME to potently inhibit the release of Vpu-defective HIV-1 is not due simply to the inefficient release of this mutant.
To confirm the requirement for Vpu expression in the ability of AME to inhibit particle production, we examined the effect of AME on the release of two retroviruses that do not encode Vpu: simian immunodeficiency virus SIVmac239 and murine leukemia virus (MLV). We observed that treatment with a 5 μM concentration did not reduce SIVmac239 release and that 10 μM AME reduced particle production by only ∼20% (Fig. 4D). Similarly, MLV release was affected only minimally by AME treatment (Fig. 4D). These results are consistent with a connection between Vpu expression and the AME-imposed defect in virus particle production. To support the finding that Vpu deletion reverses the ability of AME to inhibit HIV-1 particle production, we constructed a Vpu-deficient variant of the Fyn(10)fullMA molecular clone [Fyn(10)fullMAdelVpu]. We observed that the release of the Fyn(10)fullMAdelVpu mutant was not significantly reduced by AME (Fig. 3). These results again highlight that the inhibition of HIV-1 particle production by AME exhibits a clear Vpu dependence.
AME inhibits the ability of Vpu to counter the host factor CD317/BST-2/tetherin.
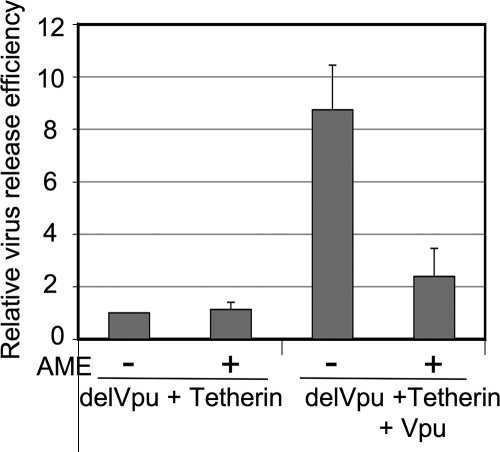
Two recent studies demonstrated that Vpu promotes virus release by counteracting the ability of CD317/BST-2/tetherin to retain HIV-1 virions at the cell surface (15, 32). Because the data presented above provide evidence that the effect of AME on virus release is Vpu dependent, we tested whether AME might prevent Vpu from counteracting the virus-tethering activity of CD317/BST-2/tetherin. To investigate the relationship between AME inhibition and the counteraction of CD317/BST-2/tetherin activity by Vpu, we analyzed virus release in the 293T cell line, which does not express appreciable levels of endogenous tetherin. First, we confirmed that CD317/BST-2/tetherin overexpression in 293T cells induced a strong (∼10-fold) inhibition of Vpu-defective HIV-1 release but had little effect on the release of WT HIV-1 (data not shown). We next tested the effect of AME on virus release in this context. We observed that in the presence of CD317/BST-2/tetherin overexpression, AME had no significant effect on the production of Vpu-defective particles (Fig. 5). This result recapitulates the lack of a major effect of AME on Vpu-defective virus release in HeLa cells, which constitutively express CD317/BST-2/tetherin. The coexpression of Vpu reversed the block in Vpu-defective virus release (Fig. 5), consistent with the findings in previous reports (15, 32). Interestingly, this Vpu-induced rescue of virus release was to a large extent inhibited by AME; in the context of Vpu-defective HIV-1 with exogenous Vpu and CD317/BST-2/tetherin expression, AME inhibited virus release by ∼5-fold (Fig. 5). These results suggest a model whereby AME inhibits HIV-1 particle production at least in part by interfering with the ability of Vpu to counter the virus-retaining function of CD317/BST-2/tetherin. The absence of an accumulation of mature virions tethered to the cell surface in AME-treated cells suggests that AME does not fully block Vpu function. In addition, we observed some reduction (∼2-fold) in particle release from pNL4-3-transfected 293T cells (data not shown), which are not Vpu responsive (15, 33), suggesting that in this cell line AME may impose additional defects in particle production not directly related to CD317/BST-2/tetherin.
FIG. 5.
AME disrupts the ability of Vpu to counter CD317/BST-2/tetherin. 293T cells were cotransfected with a Vpu-defective pNL4-3 derivative (pNL4-3delVpu; delVpu) (13) and a hemagglutinin-tagged CD317/BST-2/tetherin expression vector (tetherin) (15), with or without the Vpu expression plasmid (pCMV-Vphu; Vpu) (16). Transfected cells were treated (+) or not (−) with 10 μM AME and metabolically labeled, and the virus release efficiency was calculated as described in the legend to Fig. 1. Data are means ± SD (n = 3).
In conclusion, in this report we evaluated the mechanism by which the cholesterol-binding compound AME disrupts HIV-1 particle production. Our biochemical analyses indicated that treating virus-producing cells with AME had no significant impact on Gag-membrane binding, DRM association, or higher-order Gag multimerization. Subcellular Gag localization was also not substantially affected by AME. However, AME treatment induced a shift in the density of membrane-associated Gag and caused distortions in the lipid bilayers of treated virions, suggesting that AME binding alters the properties of the viral membrane. Experiments designed to test whether the AME-induced disruption of virus release was linked to the expression of virally encoded proteins other than Gag revealed a requirement for Vpu expression in AME-imposed virus release inhibition. We speculate that AME binding may directly block the ion channel activity of Vpu (5, 27) or may indirectly alter Vpu function via cholesterol/membrane binding. We are currently investigating the cell surface localization and lipid raft association of CD317/BST-2/tetherin and the colocalization of this host factor and HIV-1 Gag in the presence of AME when Vpu is coexpressed. Given that Vpu plays an important role in lentiviral pathogenesis in vivo (10), this accessory protein may represent a viable target for the development of antiretroviral agents.
Acknowledgments
We thank members of the Freed laboratory for helpful discussions and critical review of the manuscript. AME was generously supplied by Karykion Corporation, Princeton, NJ. We thank K. Strebel, K.-T. Jeang, B. Crise, Y. Li, and P. Bieniasz for generously providing plasmids. The following reagents were obtained from the NIH AIDS Research and Reference Reagent Program: HIV immunoglobulin (Ig), anti-SIVmac239 antiserum, and plasmid pSVψ−MLV-env−.
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH, and the NIH Intramural AIDS Targeted Antiviral Program (IATAP) and was funded in part by federal funds from the National Cancer Institute, NIH, under contract N01-CO-12400.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Footnotes
Published ahead of print on 23 July 2008.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aloia, R. C., H. Tian, and F. C. Jensen. 1993. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc. Natl. Acad. Sci. USA 905181-5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, D. A., and E. London. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 27517221-17224. [DOI] [PubMed] [Google Scholar]
- 4.Brugger, B., B. Glass, P. Haberkant, I. Leibrecht, F. T. Wieland, and H. G. Krausslich. 2006. The HIV lipidome: a raft with an unusual composition. Proc. Natl. Acad. Sci. USA 1032641-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ewart, G. D., T. Sutherland, P. W. Gage, and G. B. Cox. 1996. The Vpu protein of human immunodeficiency virus type 1 forms cation-selective ion channels. J. Virol. 707108-7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freed, E. O., and M. A. Martin. 1994. Evidence for a functional interaction between the V1/V2 and C4 domains of human immunodeficiency virus type 1 envelope glycoprotein gp120. J. Virol. 682503-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freed, E. O., and M. A. Martin. 1995. Virion incorporation of envelope glycoproteins with long but not short cytoplasmic tails is blocked by specific, single amino acid substitutions in the human immunodeficiency virus type 1 matrix. J. Virol. 691984-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottlinger, H. G., T. Dorfman, J. G. Sodroski, and W. A. Haseltine. 1991. Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc. Natl. Acad. Sci. USA 883195-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hancock, J. F. 2006. Lipid rafts: contentious only from simplistic standpoints. Nat. Rev. Mol. Cell Biol. 7456-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill, M. S., A. Ruiz, E. Pacyniak, D. M. Pinson, N. Culley, B. Yen, S. W. Wong, and E. B. Stephens. 2008. Modulation of the severe CD4(+) T-cell loss caused by a pathogenic simian-human immunodeficiency virus by replacement of the subtype B vpu with the vpu from a subtype C HIV-1 clinical isolate. Virology 37186-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang, M., J. M. Orenstein, M. A. Martin, and E. O. Freed. 1995. p6Gag is required for particle production from full-length human immunodeficiency virus type 1 molecular clones expressing protease. J. Virol. 696810-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiernan, R. E., A. Ono, G. Englund, and E. O. Freed. 1998. Role of matrix in an early postentry step in the human immunodeficiency virus type 1 life cycle. J. Virol. 724116-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klimkait, T., K. Strebel, M. D. Hoggan, M. A. Martin, and J. M. Orenstein. 1990. The human immunodeficiency virus type 1-specific protein vpu is required for efficient virus maturation and release. J. Virol. 64621-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landau, N. R., and D. R. Littman. 1992. Packaging system for rapid production of murine leukemia virus vectors with variable tropism. J. Virol. 665110-5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neil, S. J., T. Zang, and P. D. Bieniasz. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451425-430. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen, K. L., M. llano, H. Akari, E. Miyagi, E. M. Poeschla, K. Strebel, and S. Bour. 2004. Codon optimization of the HIV-1 vpu and vif genes stabilizes their mRNA and allows for highly efficient Rev-independent expression. Virology 319163-175. [DOI] [PubMed] [Google Scholar]
- 17.Ono, A., S. D. Ablan, S. J. Lockett, K. Nagashima, and E. O. Freed. 2004. Phosphatidylinositol (4,5) bisphosphate regulates HIV-1 Gag targeting to the plasma membrane. Proc. Natl. Acad. Sci. USA 10114889-14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ono, A., D. Demirov, and E. O. Freed. 2000. Relationship between human immunodeficiency virus type 1 Gag multimerization and membrane binding. J. Virol. 745142-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ono, A., and E. O. Freed. 1999. Binding of human immunodeficiency virus type 1 Gag to membrane: role of the matrix amino terminus. J. Virol. 734136-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ono, A., and E. O. Freed. 2004. Cell-type-dependent targeting of human immunodeficiency virus type 1 assembly to the plasma membrane and the multivesicular body. J. Virol. 781552-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ono, A., and E. O. Freed. 2001. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc. Natl. Acad. Sci. USA 9813925-13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ono, A., and E. O. Freed. 2005. Role of lipid rafts in virus replication. Adv. Virus Res. 64311-358. [DOI] [PubMed] [Google Scholar]
- 23.Ono, A., J. M. Orenstein, and E. O. Freed. 2000. Role of the Gag matrix domain in targeting human immunodeficiency virus type 1 assembly. J. Virol. 742855-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ono, A., A. A. Waheed, and E. O. Freed. 2007. Depletion of cellular cholesterol inhibits membrane binding and higher-order multimerization of human immunodeficiency virus type 1 Gag. Virology 36027-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ono, A., A. A. Waheed, A. Joshi, and E. O. Freed. 2005. Association of human immunodeficiency virus type 1 gag with membrane does not require highly basic sequences in the nucleocapsid: use of a novel Gag multimerization assay. J. Virol. 7914131-14140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Regier, D. A., and R. C. Desrosiers. 1990. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res. Hum. Retrovir. 61221-1231. [DOI] [PubMed] [Google Scholar]
- 27.Schubert, U., A. V. Ferrer-Montiel, M. Oblatt-Montal, P. Henklein, K. Strebel, and M. Montal. 1996. Identification of an ion channel activity of the Vpu transmembrane domain and its involvement in the regulation of virus release from HIV-1-infected cells. FEBS Lett. 39812-18. [DOI] [PubMed] [Google Scholar]
- 28.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 131-39. [DOI] [PubMed] [Google Scholar]
- 29.Smith, S. M., R. B. Markham, and K. T. Jeang. 1996. Conditional reduction of human immunodeficiency virus type 1 replication by a gain-of-herpes simplex virus 1 thymidine kinase function. Proc. Natl. Acad. Sci. USA 937955-7960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strebel, K., T. Klimkait, F. Maldarelli, and M. A. Martin. 1989. Molecular and biochemical analyses of human immunodeficiency virus type 1 vpu protein. J. Virol. 633784-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terwilliger, E. F., E. A. Cohen, Y. C. Lu, J. G. Sodroski, and W. A. Haseltine. 1989. Functional role of human immunodeficiency virus type 1 vpu. Proc. Natl. Acad. Sci. USA 865163-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Damme, N., D. Goff, C. Katsura, R. L. Jorgenson, R. Mitchell, M. C. Johnson, E. B. Stephens, and J. Guatelli. 2008. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varthakavi, V., R. M. Smith, S. P. Bour, K. Strebel, and P. Spearman. 2003. Viral protein U counteracts a human host cell restriction that inhibits HIV-1 particle production. Proc. Natl. Acad. Sci. USA 10015154-15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waheed, A. A., S. D. Ablan, M. K. Mankowski, J. E. Cummins, R. G. Ptak, C. P. Schaffner, and E. O. Freed. 2006. Inhibition of HIV-1 replication by amphotericin B methyl ester: selection for resistant variants. J. Biol. Chem. 28128699-28711. [DOI] [PubMed] [Google Scholar]
- 35.Waheed, A. A., S. D. Ablan, J. D. Roser, R. C. Sowder, C. P. Schaffner, E. Chertova, and E. O. Freed. 2007. HIV-1 escape from the entry-inhibiting effects of a cholesterol-binding compound via cleavage of gp41 by the viral protease. Proc. Natl. Acad. Sci. USA 1048467-8471. [DOI] [PMC free article] [PubMed] [Google Scholar]