Abstract
Group A rotavirus is one of the most common causes of severe diarrhea in human infants and newborn animals. Rotavirus virions are triple-layered particles. The outer capsid proteins VP4 and VP7 are highly variable and represent the major neutralizing antigens. The inner capsid protein VP6 is conserved among group A rotaviruses, is highly immunogenic, and is the target antigen of most immunodiagnosis tests. Llama-derived single-chain antibody fragments (VHH) are the smallest molecules with antigen-binding capacity and can therefore be expected to have properties different from conventional antibodies. In this study a library containing the VHH genes of a llama immunized with recombinant inner capsid protein VP6 was generated. Binders directed to VP6, in its native conformation within the viral particle, were selected and characterized. Four selected VHH directed to conformational epitopes of VP6 recognized all human and animal rotavirus strains tested and could be engineered for their use in immunodiagnostic tests for group A rotavirus detection. Three of the four VHH neutralized rotavirus in vivo independently of the strain serotype. Furthermore, this result was confirmed by in vivo partial protection against rotavirus challenge in a neonatal mouse model. The present study demonstrates for the first time a broad neutralization activity of VP6 specific VHH in vitro and in vivo. Neutralizing VHH directed to VP6 promise to become an essential tool for the prevention and treatment of rotavirus diarrhea.
Group A rotavirus (RV) is the leading cause of acute gastroenteritis in human infants less than 5 years old, causing 611,000 deaths per year (41). It is also the main cause of severe diarrhea in the neonates of many animal species of economic interest (43, 47). RV virions are triple-layered particles composed by a core (protein VP2), an inner capsid (protein VP6), and an outer capsid (proteins VP7 and VP4) (16, 29). The inner capsid protein, VP6, is a trimer representing 51% of the virion mass. According to the antigenic variation of VP6, RVs are classified into seven groups (A to G) (16). Depending on the presence or absence of two different epitopes in the VP6 protein, group A RV strains are further divided into subgroups (Sb) I, II, I+II, and no I no II. Despite the different subgroups mentioned, VP6 is a strongly conserved protein among all group A RVs (>90% amino acid homology). It is highly immunogenic and constitutes the target antigen of most immunodiagnosis tests for group A RV detection. In contrast, the outer capsid proteins VP7 (glycoprotein) and VP4 (protease sensitive) are highly variable and constitute the major neutralizing antigens. Based on the variation of VP7 and VP4, group A RVs are further classified into G and P types, respectively. RVs with different G- and P-type combinations induce low or no cross neutralization in vitro. The neutralizing antibodies directed to VP7 and VP4 correlate with protection in vivo against subsequent homologous RV infection (16, 29).
Since VP6 is a highly conserved protein, several attempts to investigate its use as a broadly protective antigen were carried out. Contradictory results were obtained. Some studies showed that anti-VP6 maternal antibodies did not induce passive protection against RV-induced diarrhea in neonatal mice (7, 8), and active vaccination with VP2/6 virus-like particles failed to protect against RV infection and diarrhea in gnotobiotic pigs (27), while other studies of vaccination with VP6 protein or DNA induced protection in vivo in a mouse model (10-13). Anti-VP6 secretory immunoglobulin A (IgA) binds to RV and mediates protection by intracellular neutralization during transcytosis in mice (3, 6, 52). VP6 could, therefore, be considered as a potential broadly reactive vaccine. Regarding in vitro neutralization, most studies showed that antibodies to VP6 lack neutralizing activity (20, 21, 44, 56). However, it has been reported that a monospecific polyclonal antiserum to VP6 of C486 RV has low neutralizing activity in vitro (46).
It is well known that the continuous presence of high titers of passive RV antibodies in the gut lumen (naturally produced or artificially added to the milk) fully protects against diarrhea and significantly reduces virus shedding (20, 48, 49). Passive immunity strategies such as oral administration of specific antibodies from different sources (bovine colostrum or chicken egg yolk) have been explored and were shown to be effective immunotherapies to prevent RV infections in both humans and animals (15, 23, 31-33, 51). However, there are some practical limitations related to the high cost of scaling up immunoglobulin purification and sterilization and the fact that they are animal-derived products. To develop a pharmaceutical product complying with the rigorous requirements for human application (i.e., high purity, high batch reproducibility, absence of adventitious viruses derived from an animal host or mammalian cell cultures, etc.), a more reliable technology based on recombinant antibodies might be an ideal choice. Especially, fragments of heavy-chain antibodies (VHH) of Camelidae come into the focus of research and are expected to have superior properties compared to conventional recombinant antibodies (scFv) (28). The VHH domain (15 kDa) is the smallest known natural domain with full antigen-binding capacity. It is ideal for the generation of libraries of single-chain antibodies (24, 25, 38). Due to their small size, VHH molecules have been shown to act as strong enzyme inhibitors, reaching enzyme pockets not accessible to common antibodies (4). Recombinant VHH antibody fragments are emerging as new versatile reagents for the diagnosis and also for the therapy of infectious diseases, including RV-induced diarrhea (40, 57). We describe here the selection and characterization of VHH antibodies directed to the inner capsid protein VP6 of group A RV. We show that these VHH are broadly reactive reagents that can be engineered for their use in immunodiagnostic tests for group A RV detection. Furthermore, we demonstrate a broad neutralization activity of VP6 specific VHH in vitro and confirmed this result by in vivo protection against RV challenge in a neonatal mouse model.
MATERIALS AND METHODS
RVs.
The bovine RV (BRV) reference strain IND (SbI, P[5]G6) was used as an antigen in enzyme-linked immunosorbent assay (ELISA) and virus neutralization (VN) tests to monitor the immune response of the llama and the biopanning process for binder selection.
In order to have a panel of RVs representing different subgroup reactivities and G- and P-type combinations from different species, the following strains were included in the different assays (ELISA and VN) for binder characterization: BRV strain C486 (SbI, P[1]G6), homologous to the VP6 used as immunizing antigen; BRV B223 (SbI, P[11]G10); human RV Wa (SbII, P1A[8]G1); and equine H2 (Sb no I no II, P[12]G3).
Reference RVs were propagated in monkey kidney cells (MA-104) for use in Western blot, enzyme-linked immunospot (ELISPOT), ELISA, and VN assays. The tissue culture-adapted BRV C486 (Sb1, P[1]G6) was used for mouse challenge. Fecal samples from newborn colostrum-deprived calves experimentally infected with BRV IND, taken at preinoculation and at the peak of virus shedding, were also included (42).
Wild-type murine RV strain ECw (Sb no I no II, P[17]G3) was kindly supplied by A. Castello, Quilmes University. Stock EC strain was prepared as 10% intestinal homogenate derived from infected suckling mice as previously described (17).
Llama immunization.
A 1-year-old male llama received five doses of crude Sf9 cell extract containing 500 μg of recombinant VP6 protein derived from the BRV C486 strain (SbI, P[1]G6; kindly supplied by L. Babiuk, VIDO, Canada), emulsified in INTA oil adjuvant (42.5% Marcol 52, 6.5% Arlacel C, 1% Tween 80), at days 0, 21, 28, 35, and 246. Serum and blood samples were taken at days 0, 4, and 7 after each inoculation (Fig. 1). The antibody response was monitored by ELISA and VN assay (see below). To evaluate the effector B-cell response, an ELISPOT assay determining the number of RV-specific antibody-secreting cells (ASC) in the peripheral blood of the inoculated llama was adapted from previous ELISPOT assays conducted in pigs and calves (42, 58). Briefly, IND BRV-infected MA-104 cells (with >80% infection as detected by immunofluorescence) grown in 96-well plates were fixed with 70% acetone, air dried, and stored at −20°C until used. Suspensions of mononuclear cells derived from peripheral blood of the inoculated llama were added to quadruplicate wells (1 × 106, 5 × 105, 2.5 × 105, and 1.25 × 105 cells/well). After centrifugation at 500 × g for 5 min, plates were incubated for 12 to 14 h at 37°C in 5% CO2. The plates were washed with phosphate-buffered saline (PBS)-0.05% Tween 20 to remove adherent cells, and the spots were developed by adding a peroxidase-labeled goat anti-llama immunoglobulin G (IgG; H+L; Bethyl Labs, Inc., Montgomery, CA) at a 1/1,500 dilution for 2 h at 37°C, followed by 50 μl of a tetramethylbenzidine membrane peroxidase substrate system (KLP, Maryland).
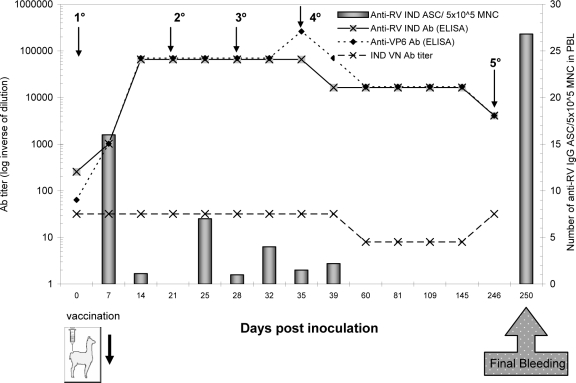
FIG. 1.
Llama immunization. The schedule for immunization, sample collection, and final bleeding is shown. The evaluation of RV antibody response in serum during the time course of immunization is depicted. Antibody titers were measured by (i) ELISA using recombinant VP6, (ii) ELISA using RV (IND, Sb I, P[5]G6), (iii) VN assay, and (iv) ELISPOT assay using the same RV (IND, Sb I, P[5]G6). Vaccination time points are represented by arrows.
Llama management, inoculation, and sample collection were conducted by trained personnel under the supervision of a veterinarian and in accordance with protocols approved by the INTA's ethical committee of animal welfare.
VHH library production and enrichment of VP6 binders.
From a total of 900 ml of blood collected 4 days after the last injection, 6 × 108 mononuclear cells were extracted by Ficoll-Paque gradient centrifugation, pelleted, frozen in liquid nitrogen, and then kept at −80°C. The total RNA was extracted by using an RNA extraction kit (Nucleospin RNA II; Macherey Nagel), yielding 256 μg of RNA. Subsequently, first-strand cDNA was synthesized from 210 μg of RNA by using Superscript III reverse transcriptase (Invitrogen), with oligo(dT)12-18 primers (Invitrogen) or random primers (Invitrogen). In a 20-μl reaction mixture 0.2, 1, or 5 μg of total RNA was used. The cDNA encoding VHH and VH was specifically amplified by PCR using the primers CALL01 and CALL02 (GTCCTGGCTGCTCTTCTACAAGG and GGTACGTGCTGTTGAACTGTTCC, respectively) annealing at the leader and at the CH2 sequences. The 600-bp fragment (VHH-CH2 without the CH1 exon) was eluted from an 1.6% agarose gel after separation from the 900-bp fragment (VH-CH1-CH2 exons). VHH fragments were then amplified with one additional nested PCR with primers annealing at the framework 1 and framework 4 regions (AACATGCCATGACTCGCGGCTCAACCGGCCATGGCTGAKGTBCAGCTGCAGGCGTCTGGRGGAGG and ATTATTATTCAGATTATTAGTGCGG CCGCGTGAGGAGACGGTGACCWGGGTCC, respectively), followed by the use of primers containing the restriction sites for further cloning steps: VHHfor2 (GGCTGAKGTBCAGCTGCAGGCGTCTGGRGGAGG) contained the NcoI and PstI restriction sites, and VHHrev2 (GTTATTATTATTCAGATTATTAGTGCGGCCGCTGGAGACGGTGACCWGGGTCC) contained the NotI restriction site. The final PCR fragments were ligated by using the upstream restriction sites NcoI or PstI and the downstream NotI site into the phagemid vector pAO-Lib, a modified version of pHEN4 (1) carrying a long irrelevant sequence that is removed upon VHH insertion in order to slow down the potential propagation of vector without a VHH insert. Ligated material was transformed into Escherichia coli TG1 cells. The colonies from the plated cells were collected, washed, and stored at −80°C in LB medium supplemented with glycerol (50% final concentration).
Specific VHH were selected from the library using the phage display technology. The VHH library was infected with M13 helper phages (Invitrogen), and phage particles expressing the VHH repertoire were rescued and precipitated with polyethylene glycol as described previously (37). Enrichment in specific binders was performed by three rounds of in vitro selection, i.e., by the so-called “biopanning.” Immunotubes were coated overnight at 4°C with an anti-RV antiserum from guinea pig (1/5,000 dilution in carbonate buffer [pH 9.6]), and 2 × 105 focus-forming units (FFU) of BRV IND (SbI; P[5]G6) were captured after a blocking step. Phages were incubated with the captured BRV IND and washed, and bound phage particles were eluted with 100 mM triethylamine (pH 10.0) and immediately neutralized with Tris (pH 7.4). The eluted phages were used to infect exponentially growing TG1 cells. After the second or third round of biopanning, individual colonies were grown, and the corresponding VHH clones were analyzed by phage ELISA.
Screening for RV and VP6 specific VHH fragments (phage ELISA).
Phages displaying the selected VHH were produced by the individual TG1 E. coli clones as previously described (38). Produced phages were tested by ELISA using two different conditions: a capture ELISA using RV and a direct ELISA using recombinant VP6. MA-104 mock-infected cells and an unrelated protein expressed in baculovirus (BVDV E2) were used as negative antigens. PBS was used as blank, and a normal serum from a nonimmunized guinea pig was used as a negative capture. After the coating step, all plates were blocked with 4% skim milk in 0.5% Tween 20-PBS. Phages were added, followed by incubation at room temperature for 60 min. The assays were developed using a 1/5,000 dilution of a horseradish peroxidase-anti-M13p8 conjugate (Amersham/Pharmacia Biotech) for 40 min at room temperature, followed by H2O2/ABTS [2,2′azinobis(3-ethylbenzthiazolinesulfonic acid)] as a substrate chromogen reagent.
Expression and purification of recombinant VHH.
VHH cDNA of the clones that scored positive in phage ELISA for VP6 and for RV were recloned using the restriction enzymes NcoI and NotI into the expression vector pHEN6 (4), which provides a pelB targeting sequence for the periplasm and a C-terminal His6 tag. Bivalent VHH were constructed by PCR amplification of the VHH sequence using the primers Bivfor2 (CTCGCGGCCCAGCCGGCCATGGCGGATGTGCAGCTTCAGGCGTCTGGG) and Bivrev2 (GCATTGGTTCTGCAGTTGCACATCTGACGGCGGGGTGGACGGAGACGCCGCGGCGGGGTAGACG GGCCCGATGAGGAGACGGTGACCTG) encoding a linker related to the human IgA hinge. The PCR product and the pHEN6 vector containing the template VHH were digested by NcoI and PstI and ligated to produce the pAO-biv vector containing the bivalent VHH. Production of recombinant monovalent or bivalent VHH was performed in shaker flasks by growing cells in Terrific Broth supplemented with 0.1% glucose and ampicillin (50). E. coli XL1-Blue cells were freshly transformed with the different plasmid constructs. VHH expression was then induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 16 h at 27°C. After the cells were pelleted, the periplasmic proteins were extracted by osmotic shock (53). The VHH were purified from this periplasmic extract by using a High-Trap HP Ni-chelating column (Amersham Biosciences).
ELISAs.
The assays were performed in 96-well plates (Nunc Maxisorp), either by direct coating of BRV IND, by capture of BRV IND, or by use of a recombinant VP6 with a polyclonal antiserum to RV made in a germfree pig (20). MA-104 mock-infected cells and an unrelated protein expressed in the baculovirus system (bovine diarrhea virus E2 protein) were used as negative control antigens. PBS was used as blank, and a serum from a nonimmunized guinea pig was used as a negative capture.
The llama serum was tested for anti-RV antibodies as previously described (42) and for anti-VP6 antibodies with a protocol adapted from Fernandez et al. (20). Llama IgG were detected by using a peroxidase-labeled goat anti-llama IgG (H+L; Bethyl Labs, Inc.) at a 1:2,000 dilution.
Purified monovalent VHH molecules with the C-terminal His6 tag were first tested in an ELISA as a RV capture reagent either by direct coating of 10 μg of VHH/ml or by first coating with 10 μg of anti-pentahistidine monoclonal antibody (1/500; Qiagen)/ml, followed by an incubation with 20 μg of VHH/ml. The assays were developed by using an RV polyclonal antiserum made in a colostrum-deprived calf hyperimmunized with BRV IND (1/2,000 dilution) and a peroxidase-labeled anti-bovine IgG (H+L; KPL, Gaithersburg, MD) in a 1/5,000 dilution). Monovalent VHH molecules were also tested as secondary antibodies, and the ELISA was then revealed by the monoclonal anti-pentahistidine antibody and horseradish peroxidase-conjugated goat anti-mouse (1:1,000 dilution; Amersham/Pharmacia Biotech). Bivalent VHH were tested in ELISA as a RV capture reagent at 10 μg/ml.
Western blotting for VHH characterization.
VP6 expressed in Sf9 cells using the baculovirus system and concentrated BRV IND were either resuspended in Laemmli sample buffer and boiled for 10 min or resuspended in nonreducing sample buffer and heated at 65°C for 15 min. Negative controls of concentrated MA-104 cells or wild-type baculovirus-infected Sf9 cells were treated the same way. Samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis in a 12% gel and blotted onto a nitrocellulose membrane (Bio-Rad). The membrane was blocked for 1 h with PBS-Tween (0.05%) containing 3% skim milk. To test the VHH, the membranes were incubated with each VHH (4 μg/ml) in PBS-Tween 20 (0.05%)-skim milk (1%) overnight at 4°C, and then the membranes were washed with PBS-Tween 20 (0.05%) and incubated 1 h at 37°C with the anti-pentahistidine monoclonal antibody at a 1/500 dilution in PBS-Tween 20 (0.05%)-skim milk (1%). A polyclonal serum from a colostrum-deprived calf experimentally infected with RV (42) and monoclonal antibody to VP6 (RG25A10) (9) were used as positive controls. Nonrelated VHH was used as a negative control. Finally, the membranes were incubated with alkaline phosphatase-conjugated anti-mouse or anti-bovine IgG (1/1,000 dilution; KPL) for 1 h at room temperature. The assay was developed by the nitroblue tetrazolium/BCIP (5-bromo-4-chloro-3-indolylphosphate) colorimetric method.
VN test.
Neutralization of RV strains IND, C486, B223, Wa, and H2 was determined in llama serum samples and for purified VHH molecules by a fluorescent focus neutralization test as described previously (55). Briefly, 100 μl of serial dilutions of llama serum or selected purified VHH molecules was mixed with an equal volume of virus in order to have 100 FFU/100 μl of mixture, followed by incubation for 1 h at 37°C. Then, 100 μl of the antibody-virus mixture was plated onto a MA-104 monolayer (four replicates), followed by incubation for 48 h at 37°C. The plates were fixed with 70% acetone, and the assay was developed using a fluorescein isothiocyanate-labeled anti-RV polyclonal antiserum derived from a colostrum-deprived calf by hyperimmunization. VHH VN capacity was expressed as the minimum concentration resulting in a >80% reduction in the number of fluorescent foci. The monovalent or bivalent VHH were assayed from 6.25 to 0.02 μg/well.
Furthermore, to evaluate whether the VHH neutralization activity was associated with VP6 binding, an assay to block VHH neutralization was performed by preincubation of recombinant VP6. Briefly, serial fourfold dilutions of VHH 2KD1, 3A6, and 3B2 (6.25 to 0.02 μg/well) were preincubated with 5 μg of recombinant VP6 produced in Sf9 cells or Sf9 cells infected with wild-type baculovirus for 1 h at 37°C. The residual neutralizing activity of VHH was then tested by VN assay against 100 FFU of BRV C486. An affinity-purified IgG derived from a polyclonal antiserum made in guinea pig with a VN titer of 2,048 to the homologous RV and a monoclonal antibody directed to a neutralizing epitope of VP7 serotype G6 (MA-49 IC3) (35) were used as controls.
RV protection assay in neonatal mice.
Four-day-old BALB/c mouse pups received 100 μl of a solution containing 100 μg of monovalent anti-VP6 VHH, using an intragastric gauge, once a day, starting on day 0 for 5 days. The pups were challenged either with BRV C486 or murine RV ECw. Pup challenge conditions for both strains were standardized in order to produce diarrhea in 100% of the nontreated control pups similarly to a previous report (26, 36). On day 1, at 2 h after the VHH administration pups received 20 μl of 5% bicarbonate solution, they were also challenged by the intragastric route with 100 μl of BRV C486 containing a total of 30 times the dose that caused diarrhea in 50% of suckling mice (DD50) (6 × 105 FFU/mouse) (experiment A) or with 100 μl of murine RV ECw containing 316 DD50 (experiment B). Experiment A was conducted in three independent assays of five mice each, while experiment B was conducted in two independent assays of five mice each. Control groups used in the experiments included (i) RV-inoculated mice and not treated with antibodies and (ii) mice treated with the same amount of a nonrelated VHH (directed against a nonrelated protein). In addition, for experiment A, a control group of mice treated with 450 μg of the affinity-purified IgG derived from a polyclonal antiserum made in guinea pig with a VN titer of 2,048 to the homologous RV was also included. Pups were clinically evaluated for RV-induced diarrhea by direct palpation of the abdomen during the 5 days of the study by a well-trained person working blindly regarding the treatment groups. As murine RV ECw spread in littermates, cases of diarrhea that developed 24 h later or more after the first case in a litter could not be due to the initial inoculum and therefore were not considered (18). For the murine RV challenge, the pups were euthanatized 4 days postinoculation, and the entire small intestines were removed, frozen-thawed, and homogenized in 10% (wt/vol) Hanks balanced salt solution. The homogenates were assayed for detection of RV by ELISA (5). The Fisher exact test was used to compare the proportions of pups with diarrhea among the various groups. The Kruskal-Wallis rank sum (nonparametric) test was used to compare days to onset, mean duration of diarrhea, and mean cumulative diarrhea scores among the treatment groups of experiment A (Statistix 8.0; Analytical Software, Tallahassee, FL). One-way analysis of variance and Tukey's test were used to compare the average values for virus shedding among the groups.
RESULTS
Llama immunization.
To evaluate the llama immune response, antibody titers to RV and VP6 were monitored by ELISA and a VN assay. The number of specific ASC circulating in peripheral blood was followed by ELISPOT assay (Fig. 1). As expected, before the first vaccination dose, at 0 days postinoculation, the llama was already positive for antibodies to RV as determined by ELISA (RV and VP6) and VN assay, indicating a previous contact with the antigen. However, no specific ASC were circulating in blood. At 7 days postinoculation, ELISA antibody titers to IND RV or to VP6 were significantly increased, and a peak of RV-specific ASC was detected in peripheral blood (16 anti-RV IgG ASC/5 × 105 mononuclear cells). After the following boosters the antibody response reached a plateau at 14 days postinoculation, with high ELISA antibody titers for both the whole virus and the VP6 protein. In contrast, the VN titers remained very low for RV (Fig. 1), as expected for an antibody response directed against VP6. Although very high antibody titers were obtained in serum, the number of ASC to RV detected in blood decreased after each booster (Fig. 1). For this reason and in order to give plenty of time to favor antibody affinity maturation, the llama received the final dose of VP6 (Fig. 1) after about 7 months. Finally, the llama was bled 4 days after the last booster, and a very high number of RV-specific plasmocytes was obtained (26.8 anti-RV IgG ASC/5 × 105 mononuclear cells).
From the total 900 ml of blood, 6 × 108 mononuclear cells were extracted (expected to contain at least 32,160 IgG ASC specific for RV according to the ELISPOT result). From the processed RNA (210 μg), a VHH phage display library containing 6 × 107 clones was generated.
Phage display selection of VHH specific for VP6 and RV.
To select phages displaying VHH specific for RV, three rounds of in vitro selection (biopanning) were performed using immobilized BRV IND as an antigen. The VP6 binders in the VHH library showed a large diversity, as judged from a restriction analysis of 192 clones that were randomly chosen after the second and third rounds of biopanning (data not shown). The capacity of the 192 clones to bind RV and VP6 was tested in a phage ELISA. The 10 clones showing the strongest specific binding were subcloned into an expression vector providing a carboxy-terminal His6 tag for purification. From these 10 clones, four VHH, named 2KA4, 2KD1, 3A6, and 3B2, that were produced with the highest yields, between 17 and 52 mg/liter of culture, and that strongly recognized RV strains with different subgroup specificities as determined by ELISA, were selected for further analysis.
Characterization of the specificity of the isolated VHH by Western blotting.
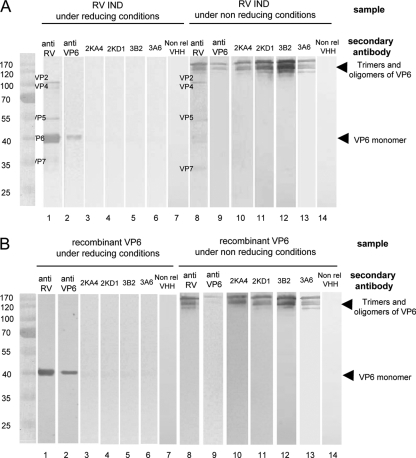
Using Western blot analysis we found that the four VHH selectively recognized bands corresponding to VP6 in samples consisting either of recombinant VP6 or native RV VP6 (Fig. 2). A monoclonal anti-VP6 antibody (RG25A10) was used as a positive control for VP6 detection. Under nonreducing conditions the four VHH recognized the higher-molecular-weight bands that correspond to trimers and oligomers of VP6 (56). The band corresponding to monomeric VP6 obtained under reducing conditions could be detected only using a more sensitive chemoluminescent method (data not shown). This indicates that the four selected VHH recognize conformational epitopes of VP6, in contrast to the monoclonal antibody that recognizes both unfolded and oligomeric VP6. The four tested VHH did not recognize other bands of the typical protein pattern of RV samples, as detected by a polyclonal serum to RV (Fig. 2) and by monoclonal antibodies to VP7 (common 60) and VP5 (BR16D3 F7.G11) (data not shown). In control experiments, no bands were observed in lanes with wild-type baculovirus-infected Sf9 cells and MA-104 cells in both reducing and nonreducing conditions, demonstrating that the anti-VP6 VHH did not detect any cellular proteins (data not shown). As expected, the nonrelated VHH did not recognize any RV protein, demonstrating that recognition of VP6 is not a general property of a VHH.
FIG. 2.
Detection of native and recombinant VP6 protein by Western blot analysis. BRV IND (A) or recombinant VP6 (B) run under reducing conditions or under nonreducing conditions and detected with bovine polyclonal serum anti-group A RV (lanes 1 and 8), anti-VP6 monoclonal antibody (RG25A10) (lanes 2 and 9), VHH 2KA4 anti-VP6 (lanes 3 and 10), VHH 2KD1 anti-VP6 (lanes 4 and 11), VHH 3B2 anti-VP6 (lanes 5 and 12), VHH 3A6 anti-VP6 (lanes 6 and 13), and nonrelated VHH (lanes 7 and 14).
These experiments demonstrate that the four selected VHH specifically recognize VP6.
VP6-specific VHH as broadly reactive reagents for group A RV immunodiagnosis.
Because VP6 is a conserved protein among group A RVs, we assessed the usefulness of the selected VHH as broadly reactive reagents for RV diagnosis. To this end, we tested the use of VHH monomers in three types of ELISAs: (i) VHH directly adsorbed as a capture antibody, (ii) VHH captured by an anti-pentahistidine antibody, and (iii) VHH used as a detection reagent of captured RV. When monomeric VHH were directly adsorbed as capture antibodies, the ELISA results were not reproducible; in contrast, when immobilized by an anti-pentahistidine antibody (Fig. 3a), the four selected VHH were able to detect RV strains of human and animal origin, with different VP6 subgroup specificities and different G and P types. We also constructed expression vectors to produce bivalent anti-VP6 VHH molecules consisting of two identical VHH genes joined by a linker sequence (39). In contrast to the monovalent VHH, bivalent constructs could also be used as a direct coating agent on the ELISA plate, eliminating the need to use an anti-pentahistidine antibody for VHH immobilization. The constructs were also able to detect all of the RV strains tested (Fig. 3b). Taken together, these results indicate that monovalent and bivalent VP6 specific binders can be used as recombinant single-domain antibodies for RV diagnosis.
FIG. 3.
VHH-ELISA detection of RV strains with different subgroup reactivities and G/P-type specificities from different animal species. (a) Antibody-captured monomeric VHH 2KA4, 2KD1, and 3A6 (2 μg/well); (b) direct coating with bivalent VHH biv2KA4, biv2KD1, and biv3A6 (1 μg/well). Tissue culture supernatant of BRV IND (SbI, P[5]G6), C486 (SbI, P[1]G6), and B223 (SbI, P[11]G10), human RV Wa (SbII, P[8]G1), and equine RV H2 (Sb no I no II, P[12]G3). Positive feces, fecal sample corresponding to the peak of virus shedding of a calf experimentally infected with BRV IND; MA-104, supernatant of mock-infected cells; PBS, blank of reaction; negative feces, calf fecal sample negative for RV. Error bars indicate the standard deviation of two independent measurements.
VP6-specific VHH neutralized different RV strains in vitro.
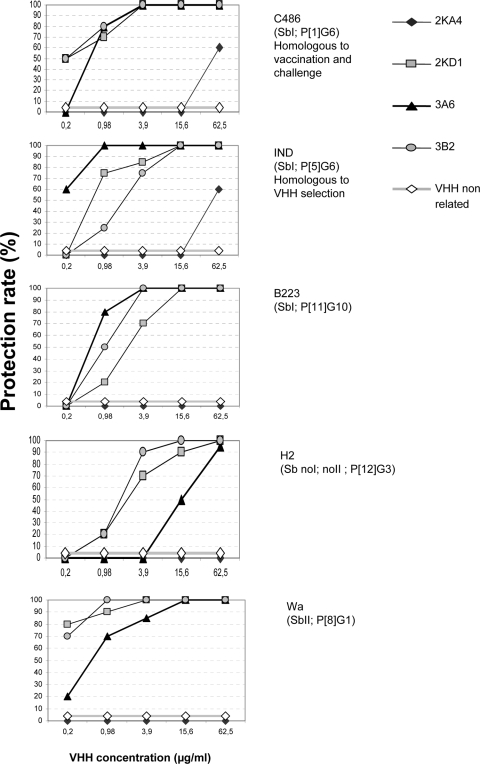
Three of the four monovalent VHH (2KD1, 3A6, and 3B2) directed against VP6 showed broad neutralizing activity in vitro. They completely neutralized the infectivity generated by 100 FFU of RV C486 (P[1]G6), IND (P[5]G6), B223 (P[11]G10, Wa (P[8]G1), and H2 (P[12]G3) strains (Table 1 and Fig. 4). Thus, monovalent VHH directed to VP6 neutralized RV strains belonging to different G/P-type combinations that normally do not show cross neutralization (16). The binder with the highest neutralization capacity was the monovalent 2KD1. In contrast, the monovalent 2KA4 did not neutralize any of the strains tested, although it was able to properly recognize all RVs in the ELISA. This might be due to an increased length of the CDR3 loop in this VHH compared to the other three (14). As expected, a control VHH directed against a nonrelated protein did not neutralize RV infection in vitro at the concentrations tested. We further tested whether bivalency would change the neutralizing activity of the VHH. Consistent with the lack of neutralizing activity of the 2KA4 monomer, its bivalent counterpart did not neutralize any RV strain either. The bivalent VHH Biv3A6 and Biv2KD1 showed lower VN activities than their monovalent counterpart (Table 1).
TABLE 1.
Anti-VP6 VHH neutralizing concentration for different RV strains
| Binder | Neutralizing VHH concna (μg/ml)
|
||||
|---|---|---|---|---|---|
| IND | C486 | B223 | Wa | H2 | |
| Monovalent | |||||
| 2KA4 | Neg | Neg | Neg | Neg | Neg |
| 2KD1 | 0.98 | 0.98 | 0.98 | 0.20 | 3.90 |
| 3A6 | 1.95 | 1.95 | 7.80 | 0.98 | 15.60 |
| 3B2 | 1.95 | 1.95 | 0.98 | 0.20 | 3.90 |
| Unrelated VHH | Neg | Neg | Neg | Neg | Neg |
| Bivalent | |||||
| Biv2KA4 | Neg | Neg | Neg | Neg | Neg |
| Biv2KD1 | 15.60 | 3.90 | 62.50 | 3.90 | 15.60 |
| Biv3A6 | 15.60 | 3.90 | Neg | 3.90 | 15.60 |
That is, the minimum VHH concentration that reduces >80% of FFU generated by 100 FFU of each RV strain. Strains: BRV IND (SbI, P[5]G6), BRV C486 (SbI, P[1]G6), BRV B223 (SbI, P[11]G10), human RV Wa (SbII, P1A[8]G1), equine RV H2 (Sb no I no II; P[12]G3). Neg, no neutralizing activity was observed at the highest concentration tested (62.50 μg/ml).
FIG. 4.
In vitro RV fluorescent focus reduction assay. A fourfold dilution of each monovalent VHH 2KA4, 2KD1, 3A6, and 3B2 was mixed with the same volume of RV containing 100 FFU. The VHH concentration that generates >80% reduction of the infection rate is considered protective. The five panels show, from top to bottom, the results for BRV C486 (homologous to the antigen used in vaccination and mice challenge), BRV IND (homologous to the antigen used in binder selection), BRV B223, equine RV H2, and human RV Wa. The graphs represent the summary results of two independent assays.
To test whether the specific recognition of VP6 is involved in the neutralizing activity of the monovalent 2KD1, 3A6, and 3B2, we performed a blocking neutralization assay in which the VHH were preincubated with recombinant VP6 before the RV neutralization assay. The neutralization activity of the three VHH was reduced by a factor of 4 for 3A6 and 2KD1 and by a factor of 16 for VHH 3B2, whereas no effect was observed on the neutralization activity of a polyclonal IgG to RV or a monoclonal antibody (MA49-IC3) directed to a neutralizing epitope of VP7 (data not shown). The preincubation with wild-type baculovirus-infected Sf9 cells did not modify the neutralizing activity of the VHH or the antibodies used as controls. As an example, Fig. 5 depicts the results for the VHH 3B2 for amounts of VHH ranging from 6.25 to 0.02 μg/well. The results of this assay support the previous observation that the selected VHH are specific for VP6 and suggest that recognition of VP6 is involved in neutralization by monomeric VHH.
FIG. 5.
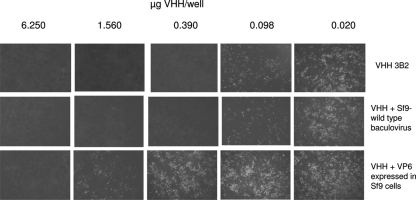
In vitro blocking of RV neutralization for VHH 3B2. Serial fourfold dilutions of VHH 3B2 were preincubated for 1 h at 37°C with a fixed concentration of 5 μg of recombinant VP6 produced in Sf9 cells or with Sf9 cells infected with wild-type baculovirus. The residual neutralizing activity of VHH was then tested by VN against 100 FFU of BRV C486. The VHH alone or the mixes of VHH+VP6 or VHH+Sf9 cells were incubated with 100 FFU of RV C486 for another hour at 37°C. The mixtures were transferred onto confluent MA-104 monolayer. The virus effect was developed by using a fluorescein isothiocyanate-labeled anti-RV IgG. The figure shows the results corresponding to amounts of VHH ranging from 6.25 to 0.02 μg/well. As shown, 5 μg of VP6 was able to block the neutralization effect of 0.39 and 0.098 μg of 3B2 VHH.
Anti-VP6 VHH protects neonatal mice against RV-induced diarrhea.
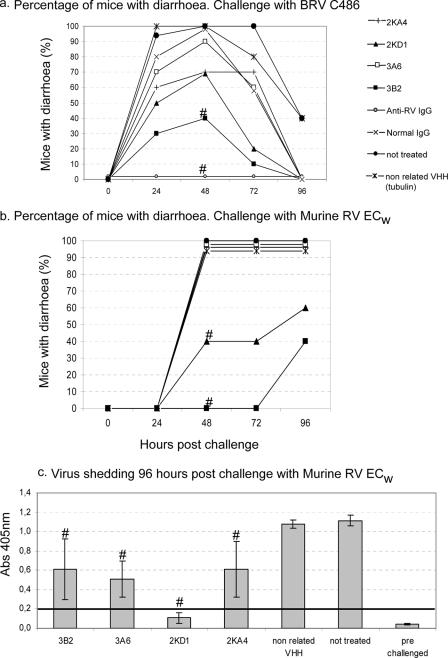
The prophylactic effect of monovalent VHH was evaluated in a neonatal mouse model. Pups received daily a single intragastric dose of 100 μg of VHH for 5 days (day 0 to 4) and were challenged with BRV C486 (experiment A) or murine RV ECw (experiment B) on day 1 (Fig. 6). For experiment A, 61% (9 of 15) of the mice treated with the monovalent 3B2 were completely protected against RV-associated diarrhea. This protection rate was significantly higher than in the different controls (untreated pups or pups treated with the nonrelated VHH), in which all of the pups were affected by diarrhea (Fisher exact test; P < 0.001). In contrast, the percentages of animals with diarrhea in the groups treated with the other VHH (3A6, 2KA4, and 2KD1) were lower than the negative control groups—but not significantly so. Although in the group treated with the monovalent 2KD1 73% of the pups developed diarrhea, the severity and duration of the disease in the affected animals were significantly less than in the nontreated group (as determined by the Kruskal-Wallis rank sum test; P < 0.05). For experiment B, pups treated with the monovalent VHH 3B2 were completely protected from RV-associated diarrhea for up to 72 h postchallenge, and four of ten pups developed diarrhea at 96 h postchallenge. Forty percent (4 of 10) of the suckling mice treated with 2KD1 developed diarrhea 48 h postchallenge, while 100% of the pups treated with VHH 3A6, 2KA4, and nonrelated VHH, together with the nontreated group, developed diarrhea at 48 h postchallenge. At 96 h postchallenge the pups were euthanized, and virus shedding was assayed by ELISA in the 10% (wt/vol) intestine homogenates. The average virus shedding in each group treated with anti-VP6 VHH was significantly lower than in the nontreated group (one-way analysis of variance and Tukey's test, P = 0.001). Furthermore, for the VHH 2KD1-treated group no viral antigen was detected in the 10% intestine homogenates by ELISA.
FIG. 6.
Protection rate against diarrhea achieved by monovalent VHH 2KA4, 2KD1, 3A6, and 3B2 in neonatal mice challenged with RV. Pups were fed 100 μg (100 μl) of each VHH from days 0 to 5 once a day by the intragastric route. At day 1, the pups were challenged intragastrically with RV 2 h after routine feeding. The development of diarrhea was monitored daily for up to 96 h postchallenge. (a) Challenge with 30 DD50 (6 × 105 FFU) of BRV C486. The experiment was carried out in three independent assays with five mice per group. (b) Challenge with 316 DD50 of murine RV ECw. The experiment was carried out in two independent assays with five mice per group. The “#” symbol indicates that the percentage of affected animals differed significantly from the nontreated or challenged group (Fisher exact test, P < 0.05). (c) Virus shedding was quantified by ELISA in the 10% (wt/vol) small intestine homogenates. Bars represent the average of the ELISA absorbances at 405 nm per group. Error bars indicate ± the standard deviation. ELISA cutoff value, 0.200.
DISCUSSION
In this study we demonstrated that recombinant single-domain llama antibody fragments (VHH) directed against the VP6 protein of RV are ideal reagents for RV diagnosis by ELISA. Furthermore, three of the four selected binders were able to neutralize RV infectivity in vitro. To our knowledge, this is the first report of VHH fragments directed to the inner capsid protein VP6 of RV with broad neutralization properties. Furthermore, two VHH conferred 60% protection against RV-induced diarrhea in a neonatal mouse model.
The immunization schedule for the llama was adapted from previous work (39). It was, however, modified according to the monitoring of the number of ASC specific to RV circulating in the blood, a parameter more appropriate for monitoring the active B-cell response to the antigen of interest than the serum antibody titer. The results obtained suggest that a long interval between the last two boosters promoted a higher number of specific ASC, from which the RNA for the VHH library was then extracted. Further experiments with a larger number of llamas and different antigens are needed to generalize this finding. In addition, we confirmed for this llama, as previously described for other animals, that higher amounts of specific ASC are among the circulating lymphocytes at 4 days than at 7 days after the final booster (54).
The goal of our strategy based on immunizing the llama with VP6 protein and using the whole virus for binder selection was to obtain VHH molecules directed to VP6 epitopes shared by all group A RVs and accessible in the intact viral particle. The results obtained from different assays (phage ELISA, ELISAs with different RVs, Western blotting, and VP6-mediated blocking VN assays) indicates that the selected VHH are specific to VP6. The four selected binders (2KA4, 2KD1, 3A6, and 3B2) are most likely directed against conformational epitopes of VP6, given that a strong signal is detected at the corresponding molecular weight of the VP6 trimers and other oligomers in Western blotting under nonreducing conditions. The four VHH recognize RVs from different subgroup specificities, different G/P-type combinations, and different species of origin, by ELISA, indicating that they are directed against shared epitopes that are different from the subgroup-specific ones. Our single-domain antibody fragments are therefore excellent, inexpensive alternatives to the use of mouse monoclonal antibodies for the design of group A RV diagnosis tests and for VP6 or virus affinity purification. We found, furthermore, that bivalent VHH molecules have the advantage over monovalent VHH that they can be used as direct coating for RV capture in ELISA (eliminating the need for additional capture antibodies).
RV neutralization in vitro has been documented for antibodies directed against the outer capsid proteins VP4 and VP7, and it is serotype specific (29). Most conventional antibodies directed against VP6 have not been reported to have extracellular neutralizing activity in vitro (20, 21, 44). For instance, even a monoclonal antibody to VP6 (RV-133) binding both double- and triple-layer particles which induces partial decapsidation did not neutralize RV in vitro (56). On the other hand, a secretory IgA monoclonal antibody to VP6 was reported to mediate protection by intracellular neutralization during transcytosis in mice and shown to inhibit RV replication and transcription (3, 6, 19, 21, 30, 34, 52, 56).
In agreement with the general statement that antibodies to VP6 do not have neutralizing activity in vitro, the baculovirus recombinant VP6 derived from BRV C486, used in our study, was reported to induce partial protection against RV challenge in a neonatal mouse model but did not induce the production of neutralizing antibodies in the vaccinated dams (44). In contrast, monospecific rabbit antiserum to this protein, as well as monoclonal antibodies to a VP6 peptide corresponding to the region from amino acids 40 to 60 of SA11 simian RV, had been reported to have very low neutralization activity in vitro (45, 46). Our discovery of anti-VP6 VHH antibodies with neutralizing activity is in concordance with the latter observation. The recent finding that VP6 is involved in RV cell entry via its binding to the cellular protein hsp70 (22) might be related to the presence of neutralizing epitopes in VP6. Due to their small size, VHH directed to VP6 can, therefore, be expected to access these neutralizing epitopes more easily than the conventional anti-VP6 antibodies, and this highlights the potential use of VHH as excellent tools for studying the role of VP6 in virus-cell interactions.
Interestingly, three of the four monovalent anti-VP6 VHH were able to neutralize all of the RV strains tested independently of their G and P types, demonstrating broad neutralization activity in vitro. In addition, the preincubation of VHH with recombinant VP6 strongly reduced this neutralization activity. This suggests that binding of the VHH to VP6 of the infectious TLP causes neutralization of virus infectivity in a polyreactive way. The capacity of the monomeric VHH to have neutralizing activity could be related to the small size of the VHH molecule. This hypothesis is supported by our observation that the bivalent VHH Biv3A6 and Biv2KD1 showed lower VN activity than their monovalent counterpart.
Several mechanisms could be responsible for the neutralizing activity of the obtained VHH. Their binding could block VP6 interaction with a cellular receptor or induce a conformational change in the viral particle, interfering with virus attachment to the cell.
This hypothesis is supported by previous data demonstrating that VP4 and VP6 bind the hsp70 cellular protein (22). On the other hand, if the VHH-virus complex can attach and enter the cell, virus decapsidation or transcriptional activity and mRNA exit might be blocked. Further studies are needed to elucidate the mechanism behind this neutralizing behavior.
Recently, the generation of a VHH library from lymphocytes of a llama immunized with the RV strain RRV was reported. The binders selected from that library showed high serotype-specific neutralizing activity in vitro (57). The four VP6 specific binders characterized in the present study show similar neutralizing capacity with the advantage of neutralizing group A RV strains of different subgroups and serotypes because they are directed against a highly conserved protein. Interestingly, the highest neutralizing titer was found against the human RV strain (Wa, SbII, P[8]G1), which is the most common strain associated with gastroenteritis in human infants worldwide (29).
The intragastric administration of the VHH 3B2 induced partial protection against RV-induced diarrhea caused by both BRV C486 and by a highly infectious murine RV EC in a neonatal mouse model. Furthermore, for mice treated with VHH 3B2 and challenged with murine EC RV, there was a delay of 2 days in the onset of diarrhea compared to the nontreated group.
In the group treated with the monovalent VHH 2KD1, only 27% of the pups were protected against diarrhea caused by BRV C486. However, after challenge with the RV murine strain, 60% of the pups were protected, and no virus was detected in the intestine homogenates at 96 h postchallenge. This observation is hard to explain but is in agreement with the high neutralization capacity of this VHH. Pups were euthanized at 96 h postinoculation, given that the peak of intestinal virus shedding in suckling mice was reported to be at that point (2). Considering that the virus shedding pattern trough time is highly variable, the absence of virus detection in the 2KD1 VHH-treated group could also be due to a modification in the peak of virus shedding (2). In the present study, challenge of RV was performed by the intragastric route without the need for premixing the virus with the VHH, in contrast to a previous report (57).
The results obtained indicate that the 2KD1 and 3B2 binders are polyreactive tools that could be applied for the preventive or therapeutic treatment of RV-associated diarrhea, avoiding the need to prepare a cocktail of different antibodies directed to the common VP7 and VP4 types. Following these preliminary results obtained in mice, it will be interesting to evaluate the protective capacity of the selected VHH against the virulent HRV Wa in a gnotobiotic pig model for RV infection and disease.
Finally, we described here for the first time a broad neutralization activity of VP6-specific VHH in vitro. The engineered VHH could be applied in group A RV diagnosis and represent excellent tools for the study of RV-cell interaction. Furthermore, if future protection studies are successful, heterologous passive protection treatments based on recombinant llama VP6 VHH might offer promising alternative strategies to prevent RV-induced diarrhea in premature infants and provide other means to complement RV vaccination to reduce diarrhea severity and associated deaths.
Acknowledgments
We are grateful to Diego Franco and Daniela Rodriguez for their contribution to the llama management. We also thank Alejandro Castello, Quilmes University, for providing murine RV strain ECw with the permissions of Ninguo Feng and Harry Greenberg, and Lic Demian Bellido for help with the mouse experiments.
V.P. is member of CONICET Scientist carrier, Argentina.
V.P., L.G., and T.S. received support from the BMBF (grant ARG 05/Z07) and SecyT AL/PA/05/BI6. L.G. was also supported by a fellowship from the German Academic Exchange Service. A.O. was supported by the European Commission (MRTN-CT-2004-512348) and by the German Research Foundation (DFG, Su 175/5).
Footnotes
Published ahead of print on 16 July 2008.
REFERENCES
- 1.Arbabi-Ghahroudi, M., A. Desmyter, L. Wyns, R. Hamers, and S. Muyldermans. 1997. Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Lett. 414521-526. [DOI] [PubMed] [Google Scholar]
- 2.Burns, J. W., A. A. Krishnaney, P. T. Vo, R. V. Rouse, L. J. Anderson, and H. B. Greenberg. 1995. Analyses of homologous rotavirus infection in the mouse model. Virology 207143-153. [DOI] [PubMed] [Google Scholar]
- 3.Burns, J. W., M. Siadat-Pajouh, A. A. Krishnaney, and H. B. Greenberg. 1996. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science 272104-107. [DOI] [PubMed] [Google Scholar]
- 4.Conrath, K. E., M. Lauwereys, M. Galleni, A. Matagne, J. M. Frere, J. Kinne, L. Wyns, and S. Muyldermans. 2001. Beta-lactamase inhibitors derived from single-domain antibody fragments elicited in the Camelidae. Antimicrob. Agents Chemother. 452807-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornaglia, E. M., M. Barrandeguy, M. Fitjman, and A. A. Schudel. 1989. Enzyme linked immunosorbent assay, immunofluorescent test and electrophoresis analysis of rotaviral RNA in the diagnosis and characterization of the bovine rotavirus. Rev. Latinoam. Microbiol. 198959-62. [Google Scholar]
- 6.Corthesy, B., Y. Benureau, C. Perrier, C. Fourgeux, N. Parez, H. Greenberg, and I. Schwartz-Cornil. 2006. Rotavirus anti-VP6 secretory immunoglobulin A contributes to protection via intracellular neutralization but not via immune exclusion. J. Virol. 8010692-10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coste, A., J. Cohen, M. Reinhardt, J. P. Kraehenbuhl, and J. C. Sirard. 2001. Nasal immunization with Salmonella typhimurium producing rotavirus VP2 and VP6 antigens stimulates specific antibody response in serum and milk but fails to protect offspring. Vaccine 194167-4174. [DOI] [PubMed] [Google Scholar]
- 8.Coste, A., J. C. Sirard, K. Johansen, J. Cohen, and J. P. Kraehenbuhl. 2000. Nasal immunization of mice with virus-like particles protects offspring against rotavirus diarrhea. J. Virol. 748966-8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, K. O., O. H. Vandal, L. Yuan, D. C. Hodgins, and L. J. Saif. 2001. Antibody-secreting cell responses to rotavirus proteins in gnotobiotic pigs inoculated with attenuated or virulent human rotavirus. J. Clin. Microbiol. 392807-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, S. C., E. F. Fynan, H. L. Robinson, S. Lu, H. B. Greenberg, J. C. Santoro, and J. E. Herrmann. 1997. Protective immunity induced by rotavirus DNA vaccines. Vaccine 15899-902. [DOI] [PubMed] [Google Scholar]
- 11.Chen, S. C., D. H. Jones, E. F. Fynan, G. H. Farrar, J. C. Clegg, H. B. Greenberg, and J. E. Herrmann. 1998. Protective immunity induced by oral immunization with a rotavirus DNA vaccine encapsulated in microparticles. J. Virol. 725757-5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi, A. H., M. Basu, M. M. McNeal, J. D. Clements, and R. L. Ward. 1999. Antibody-independent protection against rotavirus infection of mice stimulated by intranasal immunization with chimeric VP4 or VP6 protein. J. Virol. 737574-7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi, A. H., K. Smiley, M. Basu, M. M. McNeal, M. Shao, J. A. Bean, J. D. Clements, R. R. Stout, and R. L. Ward. 2007. Protection of mice against rotavirus challenge following intradermal DNA immunization by Biojector needle-free injection. Vaccine 253215-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Genst, E., K. Silence, K. Decanniere, K. Conrath, R. Loris, J. Kinne, S. Muyldermans, and L. Wyns. 2006. Molecular basis for the preferential cleft recognition by dromedary heavy-chain antibodies. Proc. Natl. Acad. Sci. USA 1034586-4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erhard, M. H., E. Gobel, B. Lewan, U. Losch, and M. Stangassinger. 1997. Systemic availability of bovine immunoglobulin G and chicken immunoglobulin Y after feeding colostrum and whole egg powder to newborn calves. Arch. Tierernahr. 50369-380. (In German.) [DOI] [PubMed] [Google Scholar]
- 16.Estes, M. K. 2007. Rotaviruses and their replication, p. 1917-1974. In B. N. Fields, D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 17.Feng, N., J. W. Burns, L. Bracy, and H. B. Greenberg. 1994. Comparison of mucosal and systemic humoral immune responses and subsequent protection in mice orally inoculated with a homologous or a heterologous rotavirus. J. Virol. 687766-7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng, N., M. A. Franco, and H. B. Greenberg. 1997. Murine model of rotavirus infection. Adv. Exp. Med. Biol. 412233-240. [DOI] [PubMed] [Google Scholar]
- 19.Feng, N., J. A. Lawton, J. Gilbert, N. Kuklin, P. Vo, B. V. Prasad, and H. B. Greenberg. 2002. Inhibition of rotavirus replication by a non-neutralizing, rotavirus VP6-specific IgA Mab. J. Clin. Investig. 1091203-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez, F. M., M. E. Conner, D. C. Hodgins, A. V. Parwani, P. R. Nielsen, S. E. Crawford, M. K. Estes, and L. J. Saif. 1998. Passive immunity to bovine rotavirus in newborn calves fed colostrum supplements from cows immunized with recombinant SA11 rotavirus core-like particle (CLP) or virus-like particle (VLP) vaccines. Vaccine 16507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming, F. E., K. L. Graham, K. Taniguchi, Y. Takada, and B. S. Coulson. 2007. Rotavirus-neutralizing antibodies inhibit virus binding to integrins α2β1 and α4β1. Arch. Virol. 1521087-1101. [DOI] [PubMed] [Google Scholar]
- 22.Gualtero, D. F., F. Guzman, O. Acosta, and C. A. Guerrero. 2007. Amino acid domains 280-297 of VP6 and 531-554 of VP4 are implicated in heat shock cognate protein hsc70-mediated rotavirus infection. Arch. Virol. 1522183-2196. [DOI] [PubMed] [Google Scholar]
- 23.Hammarstrom, L. 1999. Passive immunity against rotavirus in infants. Acta Paediatr. Suppl. 88127-132. [DOI] [PubMed] [Google Scholar]
- 24.Harmsen, M. M., C. B. van Solt, H. P. Fijten, L. van Keulen, R. A. Rosalia, K. Weerdmeester, A. H. Cornelissen, M. G. De Bruin, P. L. Eble, and A. Dekker. 2007. Passive immunization of guinea pigs with llama single-domain antibody fragments against foot-and-mouth disease. Vet. Microbiol. 120193-206. [DOI] [PubMed] [Google Scholar]
- 25.Harmsen, M. M., C. B. van Solt, A. Hoogendoorn, F. G. van Zijderveld, T. A. Niewold, and J. van der Meulen. 2005. Escherichia coli F4 fimbriae specific llama single-domain antibody fragments effectively inhibit bacterial adhesion in vitro but poorly protect against diarrhea. Vet. Microbiol. 11189-98. [DOI] [PubMed] [Google Scholar]
- 26.Ijaz, M. K., D. Dent., D. Haines, and L. A. Babiuk. 1989. Development of a murine model to study the pathogenesis of rotavirus infection. Exp. Mol. Pathol. 51186-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iosef, C., T. Van Nguyen, K. Jeong, K. Bengtsson, B. Morein, Y. Kim, K. O. Chang, M. S. Azevedo, L. Yuan, P. Nielsen, and L. J. Saif. 2002. Systemic and intestinal antibody secreting cell responses and protection in gnotobiotic pigs immunized orally with attenuated Wa human rotavirus and Wa 2/6-rotavirus-like-particles associated with immunostimulating complexes. Vaccine 201741-1753. [DOI] [PubMed] [Google Scholar]
- 28.Joosten, V., C. Lokman, C. van den Hondel, and P. J. Punt. 2003. The production of antibody fragments and antibody fusion proteins by yeasts and filamentous fungi. Microb. Cell Factories 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapikian, A. Z. 2001. Rotaviruses, p. 1787-1834. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 30.Klasse, P. J., and Q. J. Sattentau. 2002. Occupancy and mechanism in antibody-mediated neutralization of animal viruses. J. Gen. Virol. 832091-2108. [DOI] [PubMed] [Google Scholar]
- 31.Korhonen, H., P. Marnila, and H. S. Gill. 2000. Bovine milk antibodies for health. Br. J. Nutr. 84(Suppl. 1)S135-S146. [DOI] [PubMed] [Google Scholar]
- 32.Kuroki, M., M. Ohta, Y. Ikemori, F. C. Icatlo, Jr., C. Kobayashi, H. Yokoyama, and Y. Kodama. 1997. Field evaluation of chicken egg yolk immunoglobulins specific for bovine rotavirus in neonatal calves. Arch. Virol. 142843-851. [DOI] [PubMed] [Google Scholar]
- 33.Kuroki, M., M. Ohta, Y. Ikemori, R. C. Peralta, H. Yokoyama, and Y. Kodama. 1994. Passive protection against bovine rotavirus in calves by specific immunoglobulins from chicken egg yolk. Arch. Virol. 138143-148. [DOI] [PubMed] [Google Scholar]
- 34.Lawton, J. A., M. K. Estes, and B. V. Prasad. 1999. Comparative structural analysis of transcriptionally competent and incompetent rotavirus-antibody complexes. Proc. Natl. Acad. Sci. USA 965428-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lucchelli, A., S. Y. Kang, M. K. Jayasekera, A. V. Parwani, D. H. Zeman, and L. J. Saif. 1994. A survey of G6 and G10 serotypes of group A bovine rotaviruses from diarrheic beef and dairy calves using monoclonal antibodies in ELISA. J. Vet. Diagn. Investig. 6175-181. [DOI] [PubMed] [Google Scholar]
- 36.Mackow, E. R., P. T. Vo, R. Broome, D. Bass, and H. B. Greenberg. 1990. Immunization with baculovirus-expressed VP4 protein passively protects against simian and murine rotavirus challenge. J. Virol. 641698-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marks, J. D., H. R. Hoogenboom, T. P. Bonnert, J. McCafferty, A. D. Griffiths, and G. Winter. 1991. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J. Mol. Biol. 222581-597. [DOI] [PubMed] [Google Scholar]
- 38.Muyldermans, S. 2001. Single domain camel antibodies: current status. J. Biotechnol. 74277-302. [DOI] [PubMed] [Google Scholar]
- 39.Olichon, A., and T. Surrey. 2007. Selection of genetically encoded fluorescent single domain antibodies engineered for efficient expression in Escherichia coli. J. Biol. Chem. 28236314-36320. [DOI] [PubMed] [Google Scholar]
- 40.Pant, N., A. Hultberg, Y. Zhao, L. Svensson, Q. Pan-Hammarstrom, K. Johansen, P. H. Pouwels, F. M. Ruggeri, P. Hermans, L. Frenken, T. Boren, H. Marcotte, and L. Hammarstrom. 2006. Lactobacilli expressing variable domain of llama heavy-chain antibody fragments (lactobodies) confer protection against rotavirus-induced diarrhea. J. Infect. Dis. 1941580-1588. [DOI] [PubMed] [Google Scholar]
- 41.Parashar, U. D., C. J. Gibson, J. S. Bresse, and R. I. Glass. 2006. Rotavirus and severe childhood diarrhea. Emerg. Infect. Dis. 12304-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parreno, V., C. Bejar, A. Vagnozzi, M. Barrandeguy, V. Costantini, M. I. Craig, L. Yuan, D. Hodgins, L. Saif, and F. Fernandez. 2004. Modulation by colostrum-acquired maternal antibodies of systemic and mucosal antibody responses to rotavirus in calves experimentally challenged with bovine rotavirus. Vet. Immunol. Immunopathol. 1007-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parreno, V., V. Constantini, S. Cheetham, J. Blanco Viera, L. J. Saif, F. Fernandez, L. Leoni, and A. Schudel. 2001. First isolation of rotavirus associated with neonatal diarrhea in guanacos (Lama guanicoe) in the Argentinean Patagonia region. J. Vet. Med. B Infect. Dis. Vet. Public Health 48713-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Redmond, M. J., M. K. Ijaz, M. D. Parker, M. I. Sabara, D. Dent., E. Gibbons, and L. A. Babiuk. 1993. Assembly of recombinant rotavirus proteins into virus-like particles and assessment of vaccine potential. Vaccine 11273-281. [DOI] [PubMed] [Google Scholar]
- 45.Sabara, M., P. Frenchick, A. Potter, M. K. Ijaz, and J. E. Gilchrist. 1987. Peptides corresponding to antigenic and immunogenic determinants of major neutralizing proteins of rotaviruses. Canada.
- 46.Sabara, M., J. E. Gilchrist, G. R. Hudson, and L. A. Babiuk. 1985. Preliminary characterization of an epitope involved in neutralization and cell attachment that is located on the major bovine rotavirus glycoprotein. J. Virol. 5358-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saif, L. J., and A. Parwani. 1994. Animal rotaviruses, p. 279-367. In D. A. J. Tyrrell and A. Z. Kapikian (ed.), Virus infections of the gastrointestinal tract, 2nd ed. Marcel-Dekker, New York, NY.
- 48.Saif, L. J., D. R. Redman, K. L. Smith, and K. W. Theil. 1983. Passive immunity to bovine rotavirus in newborn calves fed colostrum supplements from immunized or nonimmunized cows. Infect. Immun. 411118-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saif, L. J., P. Weilnau, K. Miller, and L. Stitzlein. 1987. Isotypes of intestinal and systemic antibodies in colostrum-fed and colostrum-deprived calves challenged with rotavirus. Adv. Exp. Med. Biol. 216B1815-1823. [PubMed] [Google Scholar]
- 50.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 51.Sarker, S. A., T. H. Casswall, L. R. Juneja, E. Hoq, I. Hossain, G. J. Fuchs, and L. Hammarstrom. 2001. Randomized, placebo-controlled, clinical trial of hyperimmunized chicken egg yolk immunoglobulin in children with rotavirus diarrhea. J. Pediatr. Gastroenterol. Nutr. 3219-25. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz-Cornil, I., Y. Benureau, H. Greenberg, B. A. Hendrickson, and J. Cohen. 2002. Heterologous protection induced by the inner capsid proteins of rotavirus requires transcytosis of mucosal immunoglobulins. J. Virol. 768110-8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skerra, A., and A. Pluckthun. 1988. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science 2401038-1041. [DOI] [PubMed] [Google Scholar]
- 54.Somroop, S., P. Tongtawe, U. Chaisri, P. Tapchaisri, M. Chongsa-nguan, P. Srimanote, and W. Chaicumpa. 2006. Traffic of antibody-secreting cells after immunization with a liposome-associated, CpG-ODN-adjuvanted oral cholera vaccine. Asian Pac. J. Allergy Immunol. 24229-238. [PubMed] [Google Scholar]
- 55.To, T. L., L. A. Ward, L. Yuan, and L. J. Saif. 1998. Serum and intestinal isotype antibody responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J. Gen. Virol. 79(Pt. 11)2661-2672. [DOI] [PubMed] [Google Scholar]
- 56.Tosser, G., T. Delaunay, E. Kohli, J. Grosclaude, P. Pothier, and J. Cohen. 1994. Topology of bovine rotavirus (RF strain) VP6 epitopes by real-time biospecific interaction analysis. Virology 2048-16. [DOI] [PubMed] [Google Scholar]
- 57.van der Vaart, J. M., D. Wolvers, S. Bezemer, P. W. Hermans, K. Bellamy, S. A. Sarker, C. P. van der Logt, L. Svensson, C. T. Verrips, L. Hammarstrom, and B. J. van Klinken. 2006. Reduction in morbidity of rotavirus induced diarrhea in mice by yeast produced monovalent llama-derived antibody fragments. Vaccine 244130-4137. [DOI] [PubMed] [Google Scholar]
- 58.Yuan, L., L. A. Ward, B. I. Rosen, T. L. To, and L. J. Saif. 1996. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J. Virol. 703075-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]