Abstract
The two enteroviral proteinases, 2A proteinase (2Apro) and 3C proteinase (3Cpro), induce host cell translation shutoff in enterovirus-infected cells by cleaving canonical translation initiation factors. Cleavage of poly(A)-binding protein (PABP) by 3Cpro has been shown to be a necessary component for host translation shutoff. Here we show that 3Cpro inhibits cap-independent translation mediated by the poliovirus internal ribosome entry site (IRES) in a dose-dependent manner in HeLa translation extracts displaying cap-poly(A) synergy. This effect is independent of the stimulatory effect of 2Apro on IRES translation, and 3Cpro-induced translation inhibition can be partially rescued by addition of recombinant PABP in vitro. 3Cpro inhibits IRES translation on transcripts containing or lacking poly(A) tails, suggesting that cleavage of PABP and IRES trans-activating factors polypyrimidine tract-binding protein and poly r(C)-binding protein 2 may also be important for inhibition. Expression of 3Cpro cleavage-resistant PABP in cells increased translation of nonreplicating viral minigenome reporter RNAs during infection and also delayed and reduced virus protein synthesis from replicating RNA. Further, expression of cleavage-resistant PABP in cells reduced the accumulation of viral RNA and the output of infectious virus. These results suggest that cleavage of PABP contributes to viral translation shutoff that is required for the switch from translation to RNA replication.
Poliovirus (PV) is a medically important member of the picornavirus genus Enterovirus, which is collectively responsible for millions of human infections per year worldwide. Enteroviruses contain a single-stranded RNA genome that is a direct template for translation by the host cell's translational machinery, and this translation step is required for host morbidity and progeny virus production. The ∼7.5-kb PV genome is comprised of a single open reading frame encoding a 247-kDa polyprotein which is processed to 11 final products, a short 3′ untranslated region (UTR) with defined RNA structure, a poly(A) tail of strain-specific length that is approximately 80 to 120 nucleotides long, and a ∼700-nucleotide 5′ UTR containing an internal ribosome entry site (IRES) for facilitation of viral translation. The extreme 5′ end of the PV genome, known as the cloverleaf or oriL, has roles in nucleic acid stability (5), translation (54), and viral genomic RNA replication (43, 59). Notably, the viral genome has no 7-methylguanosine cap.
The viral IRES is responsible for driving translation, and the 7-methylguanosine cap-binding protein, eukaryotic initiation factor 4E (eIF4E), is not required for IRES-dependent translation. The large scaffolding protein eIF4G is cleaved early in PV infection, and the fragments produced cannot support cap-dependent translation (40); however, cleaved eIF4G stimulates IRES-driven translation more than intact eIF4G (12, 25, 41). The PV IRES is thought to bind eIF4G directly, as it does to related viral IRESs such as that of encephalomyocarditis virus (34). The C-terminal fragment of eIF4G that functions in IRES-dependent translation (p100) is able to recruit eIF3 and the 40S ribosomal subunit to the viral RNA. Other canonical translation factors involved in PV IRES-mediated translation include the DEAD box helicase eIF4A and its activator eIF4B, which also directly binds the IRES (47). The poly(A) tail/poly(A)-binding protein (PABP) interaction with eIF4G is also proposed to stimulate PV IRES translation by mediating a closed-loop structure and enhanced interactions between PABP and eIF4GI (7, 13, 45, 57).
In addition to these canonical translation factors, other cellular proteins, IRES trans-activating factors (ITAFs), stimulate IRES translation. These include polypyrimidine tract-binding protein (PTB) (26, 50), poly r(C)-binding protein 2 (PCBP2) (9, 10), lupus autoantigen (La) (44), and upstream of N-ras (Unr) (16). The ITAFs map to multiple binding sites in enteroviral IRES structures (9, 18, 23) and are proposed to have roles as chaperones to hold the IRES in a translation-competent conformation and possibly to also act as de facto signals for the switch from translation to replication. Additionally, ITAFs may recruit effector proteins to the IRES, as seen in the recent discovery that SRp20 binds PCBP2 and is required for IRES-mediated translation (6).
The two viral proteinases, 2A proteinase (2Apro) and 3C proteinase (3Cpro), cleave several cellular proteins during infection in addition to viral polyprotein precursors. 2Apro cleaves eIF4G, separating the domains of eIF4GI or eIF4GII that bind to eIF4E (and mRNA) and eIF3 (and the 40S ribosome) and inhibits de novo translation initiation by interfering with the ribosome mRNA binding step. 3Cpro cleaves PABP at three sites between its RNA recognition motifs (RRMs) and C-terminal domain (30), and it has been demonstrated that PABP cleavage inhibits cap-dependent translation even in the absence of eIF4G cleavage (38). 2Apro also cleaves PABP; however, it preferentially targets PABP not associated with polysomes, whereas 3Cpro preferentially cleaves polysome-associated PABP (39, 51).
PABP (PABPC1) has four RRMs with unequal binding specificities (21, 33, 37, 46, 55) and a flexible linker leading to a globular C-terminal protein-protein interaction domain (35, 36). PABP interacts with a growing list of proteins, including eIF4G (29), PABP-interacting protein 1 (Paip1) (20), Paip2 (8, 32), deleted in azoospermia-like proteins (DAZL) (19), upstream of N-ras (Unr) (16), eRF3 (28, 58), eIF4B (14), and TOB (48). PABP is recognized as a canonical translation initiation factor (31, 52) and functions in the 60S ribosomal subunit joining step of translation initiation. PABP is cleaved by a large number of viral proteinases, including the 3C proteinases of PV and coxsackievirus (30, 39), feline and human caliciviruses (38), and hepatitis A virus (60) and human immunodeficiency virus proteinase (1). Although the precise sites of cleavage vary, all of these viruses cleave PABP in the flexible linker domain between the RRMs and C-terminal protein interaction domain (42). Thus, focused cleavage of PABP in this small region is an evolutionarily conserved function of a wide range of viruses. We have shown previously that PV 3Cpro preferentially cleaves PABP associated with actively translating polysomes and that these cleavage events, as opposed to cleavage by 2Apro, are determinants for the host cell translation shutoff seen in infection (39). We have also shown that PABP cleavage by viral proteases is regulated by PABP adopting multiple stable conformations and interactions with some PABP-binding proteins and that 3CD precursor and 3Cpro cleave PABP with equivalent efficiency and cleavage site preference (51).
Viral IRES-mediated translation decreases late in infection when the RNA genome is replicated. Viral translation and RNA replication cannot occur simultaneously on the same template; in a situation where either scenario is possible, translation always occurs at the expense of replication (4, 23). Therefore, shutoff of IRES-mediated translation is required to clear ribosomes off the small subset of templates that are entering RNA replication at any one time. The mechanism by which this translation to replication switch occurs is unknown, although several hypotheses have been posed. Gamarnik and Andino have proposed a mechanism by which PCBP2 shifts from an internal binding site in the IRES stem-loop IV to the 5′ cloverleaf in a ternary complex with the viral proteinase 3CDpro, shifting the genome from a translation-competent to a replication-competent state (22, 23). In addition, three important ITAFs, La, PTB, and PCBP2, are modified during infection. La is partly cleaved, releasing a major fragment that redistributes to the cytoplasm and stimulates IRES translation (53). In contrast, PTB is partly cleaved by 3Cpro in PV infection and expression of cleavage-related truncated PTB fragments in cells inhibited PV reporter translation (2), providing another potential mechanism for viral translation shutoff. Similarly, PCBP2 is also partly cleaved late in infection, producing a fragment that will not rescue viral translation in PCBP2-depleted translation extracts (49).
To complement the body of research centering on factors interacting with the 5′ IRES, we investigated the role of PABP cleavage, and thus poly(A) function, in virus translation. Since PABP cleavage inhibits cap-dependent translation and poly(A)/PABP stimulation of viral IRES-mediated translation has been demonstrated, we hypothesized that PABP cleavage would be deleterious for viral translation (7, 45, 57). We show here that 3Cpro inhibits PV IRES-mediated translation in a dose-dependent manner in non-nuclease-treated HeLa lysates displaying cap-poly(A) synergy. This inhibition is partly due to PABP cleavage, and expression of cleavage-resistant PABP in cells prolongs viral translation and interferes with virus replication.
MATERIALS AND METHODS
Nucleic acids.
pT7-PV-IRES-FLuc (originally named pT7-polio-luc) was a gift from Peter Sarnow. The entire PV type I 5′ UTR was cloned in front of the firefly luciferase gene in a pGEM-4 backbone. Prior to transcription, plasmid DNA was linearized with HpaI for polyadenylated transcripts (A30) and with XmaI for non-poly(A) transcripts. pT7-PV5′-FLuc-3′-A71 was derived from pT7-PV-IRES-FLuc in two steps. First, nucleotides 2400 to 2402 of pT7-PV-IRES-Fluc were changed from TAA to CAT via site-directed mutagenesis to create a novel NsiI site using primers for site-directed mutagenesis (sense, 5′-GGAAAGTCCAAATTGTAAAATGCATCTGTATTCAGCGATGACGAAATTCTT AG-3′; antisense, 5′-CTAAGAATTTCGTCATCGCTGAATACAGATGCATTTTACAATTTGGACTTTCC- 3′). Second, nucleotides 7511 to 7533 of pG-A71 containing a portion of PV 3Dpol was amplified by PCR (forward primer, 5′-CCAATGCATTCTAGAAGAGATTCTTCAGGGC-3′; reverse primer, 5′-CGACTCACTAGAGGGCGAATTG-3′), TA cloned into pGEM-TEasy, and then removed by digestion with NsiI and SacI. This fragment was then cloned into mutagenized pT7-PV-IRES-FLuc. Before transcription this plasmid was linearized with HindIII for polyadenylated transcripts (A71) and with BglII for non-poly(A) transcripts. pcDNA3-PABP-hemagglutinin (HA) vectors were created by site-directed of mutagenesis of pSport6-PABP-HA vectors. PABP coding regions in pCMV-Sport6-PABP-HA were mutagenized. Primers for these mutations were as follows: for the 2A cleavage site (G488V), forward primer 5′-CACAGACAATGGTTCCACGTCC and reverse primer 5′-GGACGTGGAACCATTGTCTGTG; for the 3C cleavage site (Q537E), forward primer 5′-GCTGTTCATGTAGAAGGTCAGGAACC and reverse primer 5′-GGTTCCTGACCTTCTACATGAACAGC; and for the 3Calt cleavage site (Q412D), forward primer 5′-ATGGCAGCTATCCCAGACACTCAGAACCGT and reverse primer 5′-ACGGTTCTGAGTGTCTGGGATAGCTGCCAT. Mutations were confirmed by sequencing. pET28a-His-PABP was constructed as described previously (40).
Protein purification.
Coxsackievirus B3 2Apro was purified from pET-Cx2A using ion-exchange chromatography and size exclusion chromatography as previously described (41). PV His-3Cpro was expressed in Escherichia coli from pET-3C and purified using Ni-nitrilotriacetic acid chelating resin (Qiagen) under nondenaturing conditions. His-PABP was expressed in E. coli from pET28a-His-PABP and purified by 30% ammonium sulfate precipitation followed by Ni-nitrilotriacetic acid chelating resin affinity chromatography.
In vitro transcription and translation.
Plasmids were linearized using the appropriate restriction enzyme, phenol-chloroform extracted, precipitated under ethanol, and washed with ethanol. Purified templates were transcribed using T7 polymerase (New England Biolabs) according to the manufacturer's recommendations. Coupled transcription/translation reactions were performed with TNT coupled rabbit reticulocyte lysate (Promega) programmed with pcDNA3-PABP-HA vectors linearized with NotI. Reactions proceeded at 30°C for 90 min in the presence of Tran35S-label (MP Biomedicals) according to the manufacturer's instructions. For luciferase-based in vitro translation reactions, non-nuclease-treated HeLa cytoplasmic translation extract was prepared by hypotonic lysis of HeLa S3 suspension cells grown in Joklik's modified Dulbecco's modified Eagle medium (DMEM), Dounce homogenization, and centrifugation essentially as previously described previously (40). Reaction mixtures contained 50% (vol/vol) lysate, 2 ng/μl reporter RNA, 90 mM potassium acetate, 20 mM MOPS (morpholinepropanesulfonic acid)-KOH, 1 mM MgCl2, 15 mM creatine phosphate, 50 μg/ml creatine kinase, 4 mM dithiothreitol (DTT), 0.5 mM ATP, 0.1 mM GTP, and 0.1 mM full amino acid mixture. Reaction mixtures were incubated at 35°C for 30 min or as described in the figure legends.
In vitro cleavage reactions.
Reaction mixtures were comprised of 1 μl of reticulocyte lysate translation product, 20 mM HEPES (pH 7.4), 1 mM DTT, 150 mM potassium acetate, and 2Apro or 3Cpro at the concentrations indicated in the figure legends and were incubated at 37°C for 30 min.
Sucrose density gradients.
Sucrose gradients (10 to 50%, wt/vol) were prepared by addition of successive layers of 10 mM Tris (pH 7.2), 140 mM NaCl, 1.5 mM MgCl2, and 50, 40, 30, 20, or 10% sucrose which were frozen at −70°C between layer additions. Preparations were thawed overnight to produce continuous gradients, and samples were loaded in the presence of cycloheximide, heparin, DTT, and phenylmethylsulfonyl fluoride as previously described (17). Gradients were centrifuged at 200,000 × g for 3 hours at 4°C.
Cell culture and transfections.
293T cells were grown in six-well plates in DMEM at 37°C and 5% CO2. DNA transfections were performed using Lipofectamine (Invitrogen) and Fugene-6 (Roche) according to the manufacturer's directions. RNA transfections were performed using DMRIE-C (Invitrogen) according to the manufacturer's directions. Purified PV type 1 (Mahoney) from a cesium chloride stock was used to infect cells at a multiplicity of infection (MOI) of 40 as determined by UV spectrophotometry. Infections were performed in serum-free DMEM, which was replaced by 2% fetal bovine serum (FBS)-DMEM at 1 h postinfection (hpi). Pulse-labeling of transfected cells was performed by incubating cells with 30 μCi/ml Tran35S-label in 2% FBS-DMEM for 1 h. Densitometric analysis of band intensity on autoradiograms was performed with Image J software.
Reverse transcription-PCR (RT-PCR).
RNA was extracted from 293T cells using Trizol reagent (Invitrogen) according to the manufacturer's directions. RNA was reverse transcribed using the ImProm-II avian myeloblastosis virus reverse transcriptase kit (Promega) according to the manufacturer's directions using the provided oligo(dT) primer. The resulting cDNA was amplified using primers specific to endogenous glyceraldehyde 3-phosphate dehydrogenase (forward primer, CGTCATGGGTGTGAACCATGAG; reverse primer, CAGCTCAGGGATGACCTTGC) and firefly luciferase (forward primer, TCTGATTACACCCGAGGGGG; reverse primer, CGGTAAGACCTTTCGGTACTTCGTC) for 25 PCR cycles.
Viral RNA synthesis.
293T cells transfected with plasmids expressing PABP or green fluorescent protein were infected at 48 h posttransfection. Virus was removed at 1 hpi, and cells were overlaid with medium containing 2% serum, actinomycin D (5 μg/ml), and 6.3 μCi/ml [3H-5,6]uridine (42 Ci/mmol; MP Biomedicals). At various time points, cell lysates were harvested in triplicate, lysed in water, and trichloroacetic acid precipitated on glass microfiber discs (Fisher) before analysis by scintillation counting. Mock-infected cells were treated similarly.
Plaque assays.
Monolayer HeLa cells were grown in 12-well plates in DMEM and infected at near confluence for 1 h at 37°C using log10 dilutions of infected 293T cell lysate. Cells were overlaid with 0.5% methylcellulose in 1× Iscove's DMEM with 2% FBS. At 48 to 72 hpi, the overlay was removed and wells were flooded with 1% crystal violet stain for visualization.
RESULTS
Kinetics of cleavage of eIF4G, PABP, and ITAFs in HeLa translation lysates.
In order to evaluate PV IRES-mediated translation in the presence or absence of the enteroviral proteinases, we utilized a non-nuclease-treated HeLa translation extract displaying cap-poly(A) synergy (7). This system is advantageous over a nuclease-treated system because it subjects the reporter RNA to endogenous translation factor availability and competitor RNA levels that would be present upon PV infection. The extent and kinetics of cleavage of eIF4G and PABP by recombinant enteroviral proteinases in these translation reactions are shown in Fig. 1. As previously reported (39), recombinant PV His-3Cpro cleaved PABP at two major sites termed 3C and 3Calt (Fig. 1A). Cleavage of PABP in these reactions by 3Cpro was a time- and dose-dependent process, as previously noted. Also, as in vivo, PABP cleavage in the reactions never proceeded to completion, though 3Cpro cleaved another cellular protein, G3BP, to completion in 10 minutes (not shown) (51). In contrast, the cleavage of eIF4G by recombinant coxsackievirus B3 2Apro in these reactions was rapid and profound (Fig. 1B); low concentrations of 2Apro produced complete cleavage of eIF4GI in less than 5 minutes, producing several cleavage products from the multiple eIF4GI isoforms (15). Notably, cleavage of PABP by 2Apro (Fig. 1C) is also incomplete; a single 2Apro cleavage product can be observed late in the reaction, and eightfold-higher levels of 2A protease produce only marginally higher levels of cleavage (51) (data not shown). The cleavage patterns of eIF4G and PABP in the translation reactions approximate those seen in vivo previously and demonstrate that only a fraction of PABP exists in a molecular compartment or conformation that is readily susceptible to cleavage by either protease (51). We also examined the extents of cleavage of two ITAFs that are required for PV translation. Addition of 3Cpro resulted in rapid and efficient cleavage of all isoforms of PTB in this system (Fig. 1D). In contrast, PCBP2 was cleaved more inefficiently and to limited extents, similar to the case for PABP (Fig. 1E).
FIG. 1.
Cleavage of translation factors and ITAFs in HeLa translation lysates. (A) PABP immunoblot showing the time course of PABP cleavage after incubation of the indicated concentrations of 3Cpro with translation lysates. Incubation times are listed above the panel. PABP and cleavage products (cp) are denoted on the right, and migrations of molecular mass standards are shown on the left. (B) Immunoblot analysis of eIF4GI cleavage by 2Apro. (C) Cleavage of PABP by 2Apro shown by immunoblot analysis at a protease concentration matched to that in panel B. The image is overexposed to visualize cleavage product. (D) Cleavage of PTB by 3Cpro shown by immunoblot analysis. (E) Cleavage of PCBP by 3Cpro shown by immunoblot analysis. The asterisk indicates a nonspecific cross-reactive protein. Panels B to E are labeled as described for panel A.
3Cpro inhibits PV IRES-mediated translation in vitro.
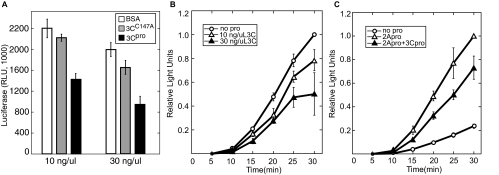
To determine the effect of 3Cpro alone on PV IRES-mediated translation, an uncapped firefly luciferase reporter RNA consisting of the entire PV 5′ UTR, the firefly luciferase open reading frame, and an A30 tail was used to assess PV IRES-mediated translation. Compared to control translation reactions, addition of 3Cpro resulted in inhibition of PV IRES-mediated translation in a dose-dependent manner, reaching a twofold inhibition (Fig. 2A). When kinetics of translation were examined in similar reactions (Fig. 2B), the inhibitory effect of 3Cpro became more pronounced at later time points, indicating that the mechanism of inhibition exerted an increasing effect on translation efficiency. Catalytically inactive 3C proteinase (3CC147A) had only a modest effect on translation when high doses were added to lysates, and proteinase that was heat inactivated at 70°C for 15 min had no significant effect on translation (data not shown).
FIG. 2.
3Cpro inhibits PV IRES-mediated translation. (A) PV IRES-FLuc reporter RNA was translated in vitro in HeLa lysates. Lysates were treated with BSA, inactive 3CC147A, or active 3Cpro for 5 min before addition of RNA and translation energy cocktail as described in Materials and Methods. Aliquots were removed at 30 min and assessed for FLuc activity. RLU, relative light units. (B) Kinetics of translation inhibition by 3Cpro. Lysates were treated as described for panel A and samples removed at the indicated time points for FLuc analysis. Addition of buffer or heat-inactivated 3Cpro had no effect on translation (data not shown). (C) Lysates were treated with 3Cpro (30 ng/μl) or 2Apro (4 ng/μl) as indicated for 5 min before addition of RNA and commencement of translation assay. Aliquots were removed as indicated and FLuc activity determined. In Panels B and C relative light units were normalized to control translation values at 30 min. Error bars indicate standard deviations.
Combined 2Apro and 3Cpro result in a net stimulation of PV IRES-mediated translation.
Early in PV infection, PV IRES-mediated translation is robust while cap-dependent translation is drastically inhibited. To reconcile the inhibitory effects of 3Cpro on PV IRES-mediated translation with the known situation during infection, we tested the effects of 3Cpro in the presence of 2Apro, as both proteinases are present and active during infection. 2Apro has previously been shown to stimulate PV IRES-mediated translation due to cleavage of eIF4G (61), and this effect was observed (Fig. 2C). When in vitro translation reaction mixtures were preincubated with both proteinases, the resulting rate of translation was about threefold greater than the control value but not as great as the fourfold stimulation of translation from preincubation with 2Apro alone (Fig. 2C). Thus, the effects of the two proteinases on PV translation in vitro are opposing and additive.
3Cpro inhibits IRES-mediated translation of poly(A)+ and poly(A)− RNAs.
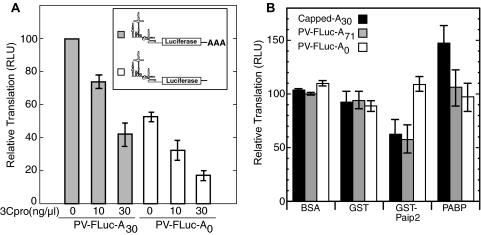
Capped RNAs containing a poly(A) tail are inhibited twofold by 3Cpro in vitro; however, capped RNAs lacking a poly(A) tail are relatively insensitive to translational inhibition by 3Cpro (40). Other than PABP, 3Cpro does not cleave other factors required for capped translation. In contrast, 3Cpro is also known to cleave PV ITAFs such as PCBP2, PTB, and La in the course of an infection. Thus, it is possible that inhibition of PV IRES-mediated translation by 3Cpro is accomplished through cleavage of both the canonical translation factor PABP and ITAFs. To determine whether 3Cpro-mediated inhibition of PV IRES-mediated translation in our system was due to cleavage of PABP or ITAFs, PV-IRES-FLuc transcripts with or without an A30 tail were translated in vitro in the presence or absence of 3Cpro for 30 min (Fig. 3A). Contrary to previous results with capped RNAs (40), PV IRES-mediated transcripts containing or lacking a poly(A) tail were both inhibited by 3Cpro in a dose-dependent manner. This suggested that PABP cleavage is not the sole determinant of 3Cpro-mediated inhibition of PV IRES transcripts. The poly(A) tail did stimulate PV translation in this system, since polyadenylated RNA consistently translated more efficiently under identical conditions (Fig. 3A).
FIG. 3.
(A) 3Cpro inhibits IRES-mediated translation of both poly(A)+ and poly(A)− RNAs. Equivalent amounts of PV-FLuc-A30 or PV-FLuc-A0 RNA was translated in HeLa extracts that were preincubated with proteinase for 5 min. Aliquots were removed for FLuc analysis at 30 min. The results show data from three independent experiments plotted as percent translation (relative light units [RLU]) relative to untreated controls. (B) PABP bound on poly(A) stimulates PV IRES translation in vitro. FLuc reporter RNA with a normal 5′ m7GTP and 3′ poly(A) tail (capped-A30) (0.3 ng/μl), PV5′-FLuc-3′-A71 (2 ng/μl), or PV5′-FLuc-3′-A0 (4 ng/μl) RNA was translated for 30 min in HeLa lysates containing BSA, GST or GST-Paip2 (3 ng/μl), or His-PABP3C/3Calt-HA(3 ng/μl). Results show data from three independent experiments plotted as percent translation (relative light units) relative to PV5′-FLuc-3′-A71 RNA treated with control BSA. Error bars indicate standard deviations of the means.
Paip2 inhibits the translation of polyadenylated transcripts.
To further delineate the importance of the poly(A) tail and PABP's effects on the efficacy of PV IRES-mediated translation in vitro, PV5′-FLuc-3′-A71 [a PV minigenome with a longer poly(A) tract and authentic 5′ and 3′ UTRs] and PV-FLuc-3′-A0 were translated in the presence of Paip2. Since PV reporter RNA containing poly(A) segments translated approximately twofold more efficiently than reporter RNA with A0, more PV-FLuc-3′-A0 RNA was used to equalize product luciferase in these assays. Paip2 removes PABP from poly(A) RNA and has been shown to negatively affect cap-dependent and IRES-dependent translation in other systems (32). As shown in Fig. 3B, treatment of the translation reaction mixtures with glutathione S-transferase (GST)-Paip2 but not GST alone inhibited translation of both capped, polyadenylated RNA and PV5′-FLuc-3′-A71 but not PV5′-FLuc-3′-A0, which has no poly(A) tail. The degree of inhibition approached 40%, similar to previously reported effects of GST-Paip2 on IRES translation in vitro (32, 56). To test the importance of PABP to translation of these reporters, recombinant PABP was added to supplement endogenous PABP in these reactions. Additional PABP enhanced translation of capped, polyadenylated RNA but had no effect on translation of polyadenylated or nonpolyadenylated PV IRES reporters. This suggests that the concentration of PABP in the translation reaction mixtures was saturating for PV IRES translation but not saturating for cap-dependent translation. However, sequestration of PABP by Paip2 did inhibit PV IRES translation, thus supporting a stimulatory role for PABP-poly(A) tail interaction in PV IRES-mediated translation in this system.
Recombinant His-PABP rescues 3Cpro inhibition of PV-IRES-FLuc-A30 translation in vitro.
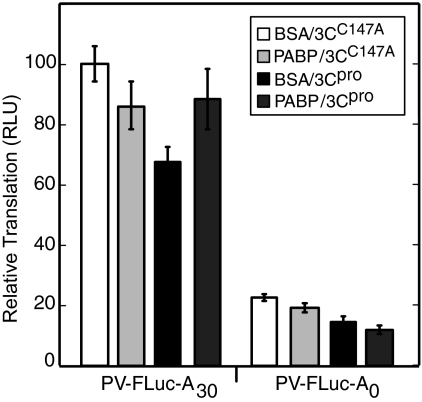
To further clarify the role of PABP cleavage in 3Cpro-mediated inhibition of PV IRES-dependent translation, we incorporated exogenous PABP into translation reactions during ongoing 3Cpro cleavage. Bovine serum albumin (BSA) or His-PABP was added to in vitro translation reaction mixtures containing 3Cpro or 3CC147A, a catalytically inactive mutant of 3Cpro. Addition of 1 ng/μl His-PABP slightly depressed translation of PV IRES-mediated transcripts regardless of poly(A) status (Fig. 4), and addition of 3Cpro, but not 3CC147A, inhibited translation of both transcripts in a similar manner as observed in previous experiments. Despite the negative effect of His-PABP seen in this assay, the recombinant protein was able to rescue translation of a PV-IRES-FLuc-A30 transcript in lysates treated with 3Cpro (Fig. 4, left). In contrast, His-PABP was unable to rescue translation of a PV-IRES-FLuc transcript lacking a poly(A) tail (Fig. 4 right). This indicates that PABP functions at the poly(A) tail to promote PV IRES-mediated translation and that a significant portion of 3Cpro-mediated translation inhibition is due to PABP cleavage.
FIG. 4.
Recombinant His-PABP rescues translation of polyadenylated PV IRES transcripts. PV-IRES-FLuc-A30 or PV-IRES-FLuc-A0 (2 ng/μl) was preincubated with 8 ng/μl BSA or with 1 ng/μl PABP plus 7 ng/μl BSA on ice for 2 min and translated in HeLa extracts that were preincubated with 3 μg 3Cpro or 3CC147A for 2 min at 35°C. Translation was allowed to proceed for 30 min in the presence of the recombinant proteins, and aliquots were removed for FLuc analysis. The results show data from three independent experiments plotted as percent translation relative to PV-IRES-FLuc-A30 RNA treated with control BSA and catalytically inactive 3Cpro. Error bars indicate standard deviations of the means. RLU, relative light units.
Point mutants of PABP are resistant to proteinase cleavage.
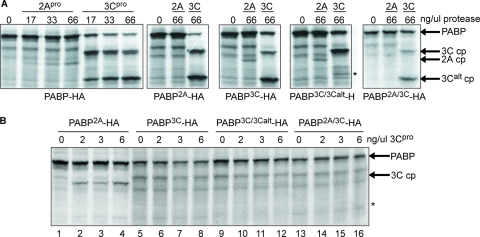
To examine the effects of PABP cleavage on PV infection, we created several point mutations of HA-tagged PABP that inhibited the protein's cleavage by viral proteinases. These mutants were expressed in 35S-labeled coupled transcription and translation reactions for in vitro assessment of cleavage in a eukaryotic system (Fig. 5). The normal extent of cleavage by the recombinant proteinases in this system was assessed by subjecting wild-type PABP-HA to cleavage (Fig. 5, first panel). A dose-response series shows that both 2Apro and 3Cpro cleave PABP-HA in a dose-dependent manner. The PABP2A mutation abolished PABP cleavage by 2Apro (Fig. 5A, second panel), and the PABP3C mutation reduced cleavage of PABP at the 3C cleavage site but did not eliminate it (Fig. 5A, third panel). PABP3C/3Calt, a double mutant compromising both the 3C and 3Calt cleavage sites, nearly abolished cleavage at the 3Calt site but allowed a robust level of cleavage at the mutated 3C cleavage site. Thus, the 3C site mutation was leaky at high proteinase concentrations, allowing some processing to occur. At low proteinase concentration cleavage at the 3Calt site was no longer detectable in wild-type PABP-HA; however, processing at the 3C cleavage site was evident (Fig. 5B, lanes 2 to 4). However, at lower proteinase concentrations processing was effectively blocked by the 3C site mutation in all combinations (Fig. 5B, lanes 5 to 16), and overall the mutations provided moderate to high resistance to proteinase cleavage, depending on the site.
FIG. 5.
PABP-HA mutants are resistant to proteinase cleavage. Coupled transcription/translation rabbit reticulocyte lysates were programmed with pcDNA3 plasmids encoding PABP-HA and various point mutants thereof and translated in the presence of Tran35S label. Lysates were incubated with recombinant 2Apro and 3Cpro for 30 min at 35°C and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. (A) Responses of PABP-HA proteins to high doses of enteroviral proteinases. First panel, cleavage of wild-type PABP-HA by 2Apro and 3Cpro shows that cleavage is dose dependent. Subsequent panels, PABP point mutants exhibit reduced cleavage compared to wild-type PABP. The asterisk indicates the appearance of rare 3Calt′ cleavage as previously described (39). (B) Responses of various PABP-HA mutants to low doses of 3Cpro. The asterisk in the lower panel indicates the expected migration of the potential 3Calt cleavage product (cp).
Cleavage-resistant PABP-HA prolongs PV IRES-mediated translation during infection.
To investigate the in vivo effects of the cleavage-resistant PABP-HA mutants, 293T cells were transfected with the pcDNA3-PABP-HA constructs. To ensure that this epitope tag did not interfere with the functionality of PABP, we analyzed the distribution of ectopically expressed PABP-HA in polysome sucrose gradients. Immunoblot analysis of fractions confirmed that transgenic PABP-HA was present throughout the gradient, including the 80S ribosomal peak and polysome gradients (Fig. 6A), similar to the case for endogenous PABP (data not shown).
FIG. 6.
Expression of cleavage-resistant PABP in PV-infected cells stimulates translation of PV minigenome RNA. (A) HA-tagged PABP cosediments with polysomes. An HA-immunoblot (upper panel) and a UV trace of the polysome sucrose gradient (lower panel) used to pool the indicated fractions for analysis are shown. OD260, optical density at 260 nm. (B) Translation of PV-FLuc-minigenome RNA in PV-infected cells. Cells were transfected with sheared salmon sperm DNA (sss) or the indicated PABP expression plasmids for 48 h before transfection of reporter RNA and virus infection (MOI = 10). Transient-transfection rates were approximately 65%. Times indicate hours postinfection. Infected cells were harvested and lysates assessed for FLuc activity. Three independent experiments are represented, and results are plotted as percent reporter translation (relative light units [RLU] at 5 hpi) compared to cells transfected with wild-type (wt) PABP. Error bars indicate the standard deviations of the means. (C) Immunoblot showing total PABP in transfected/infected cells determined with anti-PABP antiserum. The lower panel shows a GAPDH immunoblot of the same samples. (D) Stability of minigenome RNA in transfected/infected cells. RT-PCR analysis of FLuc minigenome RNA (top band) and GAPDH endogenous mRNA in transfected/infected cells in samples harvested at 5 hpi is shown.
We used reporter PV RNA in cells as a measurement of viral translation since reporters are fully uncoupled from confounding viral RNA replication effects. Plasmids expressing cleavage-resistant PABP-HA were transfected into cells, and at 48 h posttransfection, cells were transfected with PV5′-FLuc-3′-A71 minigenome reporter RNA. Two hours after reporter RNA transfection, cells were infected with PV. Typically, FLuc expression in these infected/transfected cells increased from 3 to 6 h of infection and then began to decline due to effects from the infection (Fig. 6 and data not shown). As shown in Fig. 6B, the expression of wild-type PABP-HA in infected cells stimulated PV-IRES-FLuc translation significantly over that of control cells transfected with sheared salmon sperm DNA. Expression of PABP2A-HA stimulated PV IRES-mediated translation in the same manner. Significantly, expression of all three forms of 3Cpro-resistant PABP-HA stimulated PV IRES-mediated translation beyond the level observed with wild-type PABP-HA. The gap between 3Cpro-resistant PABP-HA stimulation and wild-type PABP-HA stimulation was not discernible at 3 hpi (data not shown) and grew more pronounced with time. These results suggest that PABP cleavage during PV infection is a determinant for PV translation inhibition. When global PABP cleavage in these cells was ascertained by PABP-specific immunoblotting (Fig. 6C), no reproducible differences between cells expressing wild-type or cleavage-resistant PABP could be found, and observed variances could not be attributed to the species of cleavage-resistant PABP expressed. This was likely because ectopic PABP could not be expressed to high levels in cells; they usually were not more than ∼15% of endogenous PABP levels (data not shown). Attempts to monitor cleavage of ectopic PABP-HA with HA antibody were unsuccessful, potentially due to instability of C-terminal PABP cleavage fragments (data not shown). RT-PCR of FLuc RNA transfected into cells was performed to ensure that the disparate FLuc levels measured were not due to different rates of RNA degradation (Fig. 6D), but no difference in the FLuc/GAPDH (glyceraldehyde-3-phosphate dehydrogenase) ratio was observed. Taken together, these data suggest that even modest levels of ectopic expression of PABP resistant to 3Cpro cleavage can enhance or extend the duration of PV IRES-mediated translation.
Cleavage-resistant PABP-HA decreases virus gene expression.
If expression of 3Cpro cleavage-resistant PABP prolonged viral translation in PV-infected cells, we reasoned that it may alter viral RNA replication via interference with the switch to RNA replication. We performed pulse-label analysis of viral protein expression at several time points during the infections. The appearance of high levels of viral protein expression is dependent on RNA replication to produce viral mRNA. Figure 7 shows that ectopic PABP expression produced a modest but consistent decrease in viral production in the cell population, despite the fact that approximately 30% of the cells were not transfected with vectors. Expression of PABP3C/3Calt-HA produced the greatest decrease in viral protein expression. Expression of PABP also delayed the onset of viral protein production (Fig. 7, compare 2C or VP3 bands at 3.5 h). Thus, ectopic cleavage-resistant PABP induced a decrease in protein production that was linked to virus RNA replication but induced an increase in translation of virus RNA that was not linked to RNA replication (Fig. 6B).
FIG. 7.
Expression of cleavage-resistant PABP in PV-infected cells inhibits viral expression. (A) 293T cells transfected with sheared salmon sperm (sss) DNA or PABP expression vectors were infected at 48 h posttransfection and virus replication assessed by pulse-labeling followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography. Transient-transfection rates were approximately 65%, and cells were infected at an MOI of 10. (B) Densitometric analysis of 2C protein expression at 3.5 h and 5 h, plotted as relative band intensity.
Cleavage-resistant PABP-HA decreases progeny virion production.
Since expression of 3Cpro cleavage-resistant PABP prolonged viral translation in PV-infected cells, we reasoned that it may also alter RNA and virion output. To test this hypothesis, we analyzed accumulation of viral RNA in infected cells expressing PABP mutants by labeling with [3H]uridine. Figure 8A shows that early in infection, accumulation of labeled viral RNA was not different in cells expressing mutant or wild-type PABP. However, by 5.2 or 6 hpi, accumulation of viral RNA lagged behind in cells expressing cleavage-resistant PABP3C/3Calt-HA. In separate experiments we also tested whether virion output was similarly affected; thus, intracellular virus was harvested at 5 hpi from cells expressing exogenous PABP-HAs. Plaque assays revealed that expression of wild-type PABP-HA did not significantly affect virion production compared to mock-transfected cells (Fig. 8B), even though these cells displayed stimulated minigenome translation. PABP2A-HA expression resulted in a decrease in infectious particle production, and expression of PABP-HA resistant to 3Cpro resulted in an even greater decrease in progeny virus output. The decreased RNA and viral titer from cells expressing PABP-HA resistant to 3Cpro suggests that this environment may produce a defect in the translation-replication switch for PV.
FIG. 8.
Expression of cleavage-resistant PABP interferes with viral RNA synthesis and infectious particle production. (A) Cells transfected with green fluorescent protein (GFP) or PABP expression vectors were infected at 48 h posttransfection and virus replication assessed by treatment with actinomycin D (5 μg/ml) and labeling of viral RNA replication with [3H]uridine, trichloroacetic acid precipitation, and scintillation counting. (B) 293T cells transfected with various pcDNA3-PABP-HA vectors or mock transfected were infected at 48 h posttransfection (MOI = 40). At 5 hpi, quadruplicate parallel cultures were lysed and intracellular virions assayed by plaque assay. WT, wild type. Error bars indicate standard deviations.
Comparison of cleavage of factors in infected cells.
Previously, cleavages of PTB, PCBP2, and PABP in PV-infected cells have been examined separately. We investigated the cleavage of PTB, PCBP2, and PABP simultaneously in PV infections to assess relative levels of cleavage of each of these factors in vivo. Figure 9 shows that all three factors are diminished in concentration by 4 hpi and by 7 hpi; PABP levels were reduced the greatest extent. Previously described cleavage products of PTB migrating near 40 and 25 kDa were observed (2), which did not accumulate to higher levels late in infection, similar to the case for PABP cleavage products. The previously described PCBP2 cleavage product migrating near 26 kDa was not observed (49), perhaps due to greater instability in vivo, though a potential unstable 38-kDa cleavage product was transiently observed (Fig. 9). This 26-kDa cleavage product was produced during in vitro cleavage reactions (Fig. 1), indicating that the antibody is capable of detecting this fragment.
FIG. 9.
Cleavage of endogenous factors during PV infection. Immunoblot analysis showing relative cleavage and degradation of PABP (left panel), PTB (center panel), and PCBP2 (right panel) in the same infected cells is shown. In the left and center panels, arrowheads indicate cleavage products produced by 3Cpro. In the right panel, the empty arrowhead indicates the expected migration of the 26-kDa PCBP2 3Cpro cleavage product, the filled arrowhead indicates a putative 3Cpro cleavage product, and the asterisk indicates a cross-reactive protein.
DISCUSSION
Much of the study of enteroviral translational control has centered on effects of viral infection on the host cell's cap-dependent translation. Such studies illuminated the differential effects of 2Apro-mediated eIF4G cleavage on cap-dependent and IRES-mediated translation, showed that 3Cpro-mediated PABP cleavage inhibited cap-dependent translation, and provided a two-step mechanism for host translation shutoff. The mechanism of inhibition of viral IRES-mediated translation to prepare for genomic RNA replication is multifactorial and less well understood. Previously, 3CD-mediated repositioning of PCBP2 from stem-loop IV of the IRES to the cloverleaf was implicated in downregulation of PV translation, as well as cleavage of PTB by 3Cpro. Both steps remove functional ITAFs from the IRES. A recent study showed that PCBP2 cleavage by 3Cpro may facilitate this process by altering PCBP2-RNA binding properties (49), and the present study provides another complementary mechanism for the priming of the translation-replication switch. Here we have studied the functional effects of cleavage of PABP in the small linker domain region, which is an evolutionarily conserved function of a wide range of viruses. We have provided evidence that cleavage of PABP also diminishes PV translation, similar to its negative effect on cap-dependent translation, and have isolated the effects of PABP cleavage from PTB and PCBP2 cleavage via restoration experiments and expression of cleavage-resistant PABP during viral infections.
To study the effects of 3Cpro on PV IRES-mediated translation without the influence of 2Apro, a non-nuclease-treated cell-free HeLa lysate was used. Recombinant 3Cpro added to this system inhibited the translation of a PV IRES-driven luciferase reporter in a dose-dependent manner and to a similar extent as the inhibition of capped RNAs by 3Cpro seen earlier (40), suggesting that there may be common mechanisms between 3Cpro-mediated inhibition of capped and PV IRES-driven transcripts. However, in contrast to the case for cap-dependent transcripts, the inhibition was observed in poly(A)+ and poly(A)− trials, indicating that PABP cleavage was not the sole determinant of 3Cpro-mediated inhibition of PV IRES transcripts. Several lines of evidence support PABP/poly(A) stimulation of PV IRES translation. First, nonpolyadenylated RNAs translate two- to fivefold less efficiently than polyadenylated RNAs (Fig. 3A and B and 4), and lack of a poly(A) tail does not contribute significantly to increased RNase degradation of transcripts in cell lysates (data not shown) (7, 40). Second, Paip2 inhibited translation on poly(A)+ but not poly(A)− transcripts, suggesting that PABP stimulated poly(A)-dependent PV IRES-mediated translation in this system. Further, addition of recombinant His-PABP to these reactions partially rescued the inhibitory effect of 3Cpro on poly(A)+ but not poly(A)− transcripts, reinforcing the paradigm of PABP being a canonical initiation factor functioning at poly(A) tails, whether they are on capped or IRES-dependent transcripts (7, 31, 32). Even though significant cleavage of PTB and PCBP2 occurred in rescue experiments, the presence of additional PABP offset the effects of ITAF cleavage, suggesting that PABP cleavage is a contributor to the shutoff of virus translation.
A panel of cleavage-resistant PABP mutants were generated and expressed in 293T cells to assess their effects on PV IRES-mediated translation during PV infection when PTB and PCBP2 cleavage would be unabated. If cleavage of PABP was involved in regulation of the translation-replication switch during PV infection, the cleavage-resistant PABP constructs could potentially exhibit two effects: (i) stimulation of viral translation of transcripts not linked to viral replication and (ii) due to delay of the RNA replication switch, fewer translation products from RNAs produced via RNA replication. Both effects were observed in these studies. Nonreplicating FLuc-minigenome translation in cells expressing 3Cpro-resistant PABP was fairly similar to that in control cells early in infection but became more disparate as the infection progressed and exhibited enhanced translation at later time points. This suggests that PABP cleavage is an integral step in viral translation shutoff and that the accumulated effects of PABP cleavage occur later in infection. PABP-HA that was resistant to 2Apro conferred no translational advantage over wild-type PABP-HA, suggesting that 2Apro-mediated cleavage of PABP is not directly related to shutoff of viral translation. This was not surprising, as 2Apro is known to preferentially target free PABP that is not associated with actively translating RNA (39). In contrast, production of viral proteins from replicating viral RNA was delayed and diminished in cells expressing PABP3C/3Calt-HA. No modifications in processing of viral polyprotein precursors were noted in cells expressing mutant PABP. Additionally, the 3Cpro cleavage-resistant PABP reduced the accumulation of viral RNA and the number of infectious particles produced during infection at the 5-h time point (Fig. 8). These results, taken together, suggest that 3Cpro-mediated cleavage of PABP is an event that facilitates the translation-to-replication switch on the PV genome during PV infection.
PABP is one of the most abundant proteins in the cell, with the cytoplasmic PABPC1 concentration reaching 4 μM (24). Even with a cytomegalovirus promoter driving the transgene, the proportion of PABP-HA to endogenous PABP in transfected 293T cells was estimated to be no more than 1:6 (data not shown). Additionally, overexpression of PABP-HA did not noticeably change overall PABP levels in the cell (Fig. 6C), possibly due to an autoregulatory sequence in the endogenous PABPC1 5′ UTR that is able to bind PABP, which can suppress its translation (3). Also, since transgene expression was not high compared to that for endogenous PABP, the global PABP cleavage patterns during PV infection remained essentially unchanged regardless of whether or not cleavage-resistant PABP was being expressed. This likely reflects the protease accessibility of the large fraction of endogenous PABP. As the C-terminal HA tag still allowed for cosedimentation of PABP-HA with endogenous PABP, it was assumed that the two proteins behaved similarly; however, it remains possible that the transgene performs in only a subset of normal PABP functions. Additionally, several sets of small interfering RNA and short hairpin RNA knockdowns targeted to the UTRs of endogenous PABP proved ineffective, so it was impossible to express the transgenes in excess of endogenous PABP.
We have previously developed a two-step model for host-cell translation shutoff (11, 40, 42) where cleavage of eIF4G by 2Apro is necessary to block de novo translation initiation on cellular transcripts and cleavage of PABP by 3Cpro clears preexisting polysomes, possibly by blocking 3′-to-5′ ribosome recycling, to effect complete shutoff. Similarly, we hypothesize a two-step model for the inhibition of viral translation late in infection. Cleavage of the ITAFs PTB (2) and PCBP2 (49) together with movement of PCBP2 from the IRES to the cloverleaf could function in a manner analogous to that for the eIF4G cleavage and interrupt de novo initiation on the genomic RNA as the first step. The second step involves PABP cleavage, attacking 3′ poly(A) tail function and potential 3′-to-5′ recycling of viral polysomes. PABP is known to stimulate PV translation, both in HeLa lysate and in rabbit reticulocyte lysate (7, 13, 45, 57), and PABP-mediated stimulation of eIF4G function at the IRES was implicated in these earlier studies. Cleavage of eIF4G by 2Apro in cells potentially removes the N-terminal domain of eIF4GI that interacts with PABP from the C-terminal that binds the IRES; however, we have shown that PABP and the N-terminal cleavage fragment of eIF4G still interact (40). Thus, it is unclear whether eIF4G cleavage would negate such a PABP stimulatory effect early during infection. Our experiments show that PABP cleavage by 3Cpro in the presence or absence of 2Apro abrogates this PABP translation stimulation. This suggests that a different PABP function not involving PABP-eIF4GI interaction may be important for viral RNA translation. Cleavage of PABP separates the C-terminal peptide interaction motif that binds to eIF4B, eRF3, and several other host factors from the RNA-binding RRMs. Further, such interactions may not be affected by eIF4G cleavage, since eIF4B binds PV IRES directly (47) and eRF3-PABP interaction is eIF4G independent. Complementary work (60) suggested that the N-terminal cleavage-like fragments of PABP have a dominant-negative effect on hepatitis A virus IRES-mediated translation.
Shutoff of viral translation appears to be tightly coupled to negative-strand RNA synthesis. The previously described shutoff mechanisms of cleavage of PTB, PCBP2, binding of PCBP2 to the cloverleaf, and now PABP cleavage are not mutually exclusive and are likely complementary. More recently, PABP was reported to not be required for viral RNA replication in PABP depletion experiments, though depletion did decrease viral IRES translation (56). In other work, we have shown that PABP inhibits 3Dpol activity on viral RNA poly(A) templates, which complements these results. Full-length PABP inhibits PV 3Dpol activity in negative-strand synthesis in vitro, while a truncated PABP mimicking the N-terminal 3Calt cleavage product does not inhibit 3Dpol activity to the same extent (C. Rivera and R. Lloyd, submitted for publication). Thus, intact PABP may have both stimulatory and inhibitory effects on PV translation/replication. Likewise, cleavage of PABP can be implicated as both a factor in viral translation inhibition and a gatekeeper for allowance of negative-strand RNA synthesis. Both 3Cpro and 3CDpro may be involved in this process. The interaction of 3CDpro with the 5′ cloverleaf structure of the PV genome may be an effective mechanism to target the proteinase to the genome's PABP, as 3CDpro and PCBP2 may be able to form a ternary complex on the 5′ cloverleaf of the genome (22, 49). Such a model has been proposed as a mechanism to preferentially cleave the 5′-most PABP on the genomic poly(A) tail (Rivera and Lloyd, submitted). Cleavage of PABP may disrupt circularization of the PV genome, which is postulated to be necessary for negative-strand synthesis (27), but it is possible that the RNA synthesis step itself may lead to disruption of circularization.
In summary, PABP, via binding the viral poly(A) tail, has been postulated to stimulate IRES-dependent translation, though the precise mechanisms remain unclear. The data presented here suggest that cleavage of PABP is associated with loss of that stimulation and is a necessary step to prime RNA replication.
Acknowledgments
We thank Peter Sarnow for plasmids; Bert Semler for anti-PCBP antibody; Patrick Younan, Carlos Rivera, and Kyle Sherrill for technical expertise; and the members of the Lloyd lab for helpful discussions.
This work was supported by NIH grants AI50237 and GM59803.
Footnotes
Published ahead of print on 16 July 2008.
REFERENCES
- 1.Alvarez, E., A. Castello, L. Menendez-Arias, and L. Carrasco. 2006. HIV protease cleaves poly(A)-binding protein. Biochem. J. 396219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Back, S. H., Y. K. Kim, W. J. Kim, S. Cho, H. R. Oh, J. E. Kim, and S. K. Jang. 2002. Translation of polioviral mRNA is inhibited by cleavage of polypyrimidine tract-binding proteins executed by polioviral 3C(pro). J. Virol. 762529-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bag, J., and J. Wu. 1996. Translational control of poly(A)-binding protein expression. Eur. J. Biochem. 237143-152. [DOI] [PubMed] [Google Scholar]
- 4.Barton, D. J., B. J. Morasco, and J. B. Flanegan. 1999. Translating ribosomes inhibit poliovirus negative-strand RNA synthesis. J. Virol. 7310104-10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barton, D. J., B. J. O'Donnell, and J. B. Flanegan. 2001. 5′ cloverleaf in poliovirus RNA is a cis-acting replication element required for negative-strand synthesis. EMBO J. 201439-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedard, K. M., S. Daijogo, and B. L. Semler. 2007. A nucleo-cytoplasmic SR protein functions in viral IRES-mediated translation initiation. EMBO. J. 26459-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergamini, G., T. Preiss, and M. W. Hentze. 2000. Picornavirus IRESes and the poly(A) tail jointly promote cap-independent translation in a mammalian cell-free system. RNA 61781-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berlanga, J. J., A. Baass, and N. Sonenberg. 2006. Regulation of poly(A) binding protein function in translation: characterization of the Paip2 homolog, Paip2B. RNA 121556-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blyn, L. B., K. M. Swiderek, O. Richards, D. C. Stahl, B. L. Semler, and E. Ehrenfeld. 1996. Poly(rC) binding protein 2 binds to stem-loop IV of the poliovirus RNA 5′ noncoding region: identification by automated liquid chromatography-tandem mass spectrometry. Proc. Natl. Acad. Sci. USA 9311115-11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blyn, L. B., J. S. Towner, B. L. Semler, and E. Ehrenfeld. 1997. Requirement of poly(rC) binding protein 2 for translation of poliovirus RNA. J. Virol. 716243-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonderoff, J. M., and R. E. Lloyd. 2008. CVB translation: lessons from the polioviruses. Curr. Top. Microbiol. Immunol. 323123-147. [DOI] [PubMed] [Google Scholar]
- 12.Borman, A. M., R. Kirchweger, E. Ziegler, R. E. Rhoads, T. Skern, and K. M. Kean. 1997. elF4G and its proteolytic cleavage products: effect on initiation of protein synthesis from capped, uncapped, and IRES-containing mRNAs. RNA 3186-196. [PMC free article] [PubMed] [Google Scholar]
- 13.Bradrick, S. S., E. Y. Dobrikova, C. Kaiser, M. Shveygert, and M. Gromeier. 2007. Poly(A)-binding protein is differentially required for translation mediated by viral internal ribosome entry sites. RNA 131582-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bushell, M., W. Wood, G. Carpenter, V. M. Pain, S. J. Morley, and M. J. Clemens. 2001. Disruption of the interaction of mammalian protein synthesis initiation factor 4B with the poly(A) binding protein by caspase- and viral protease-mediated cleavages. J. Biol. Chem. 27623922-23928. [DOI] [PubMed] [Google Scholar]
- 15.Byrd, M. P., M. Zamora, and R. E. Lloyd. 2005. Translation of eIF4GI proceeds from multiple mRNAs containing a novel cap-dependent IRES that is active during poliovirus infection. J. Biol. Chem. 28018610-18622. [DOI] [PubMed] [Google Scholar]
- 16.Chang, T. C., A. Yamashita, C. Y. Chen, Y. Yamashita, W. Zhu, S. Durdan, A. Kahvejian, N. Sonenberg, and A. B. Shyu. 2004. UNR, a new partner of poly(A)-binding protein, plays a key role in translationally coupled mRNA turnover mediated by the c-fos major coding-region determinant. Genes Dev. 182010-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, C.-Y. A., N. Xu, and A.-B. Shyu. 1995. mRNA decay mediated by two distinct AU-rich elements from c-fos and granulocyte-macrophage colony-stimulating factor transcripts: different deadenylation kinetics and uncoupling from translation. Mol. Cell. Biol. 155777-5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung, P., M. Zhang, J. Yuan, D. Chau, B. Yanagawa, B. McManus, and D. Yang. 2002. Specific interactions of HeLa cell proteins with Coxsackievirus B3 RNA: La autoantigen binds differentially to multiple sites within the 5′ untranslated region. Virus Res. 9023-36. [DOI] [PubMed] [Google Scholar]
- 19.Collier, B., B. Gorgoni, C. Loveridge, H. J. Cooke, and N. K. Gray. 2005. The DAZL family proteins are PABP-binding proteins that regulate translation in germ cells. EMBO J. 242656-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craig, A. W. B., A. Haghighat, A. T. K. Yu, and N. Sonenberg. 1998. Interaction of polyadenylate-binding protein with the eIF4G homologue PAIP enhances translation. Nature 392520-523. [DOI] [PubMed] [Google Scholar]
- 21.Deo, R. C., J. B. Bonanno, N. Sonenberg, and S. K. Burley. 1999. Recognition of polyadenylate RNA by the poly(A)-binding protein. Cell 98835-845. [DOI] [PubMed] [Google Scholar]
- 22.Gamarnik, A., and R. Andino. 2000. Interactions of viral protein 3CD and poly(rC)-binding protein with the 5′ untranslated region of the poliovirus genome. J. Virol. 7422219-22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gamarnik, A., and R. Andino. 1998. Switch from translation to RNA replication in a positive-stranded RNA virus. Genes Dev. 122293-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorlach, M., C. G. Burd, and G. Dreyfuss. 1994. The mRNA poly(A)-binding protein: localization, abundance, and RNA-binding specificity. Exp. Cell Res. 211400-407. [DOI] [PubMed] [Google Scholar]
- 25.Hambidge, S. J., and P. Sarnow. 1992. Translational enhancement of the poliovirus 5′ noncoding region mediated by virus-encoded polypeptide-2A. Proc. Natl. Acad. Sci. USA 8910272-10276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hellen, C. U. T., G. W. Witherell, M. Schmid, S. H. Shin, T. V. Pestova, A. Gil, and E. Wimmer. 1993. A cytoplasmic 57-kDa protein that is required for translation of picornavirus RNA by internal ribosomal entry is identical to the nuclear pyrimidine tract-binding protein. Proc. Natl. Acad. Sci. USA 907642-7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herold, J., and R. Andino. 2001. Poliovirus RNA replication requires genome circularization through a protein-protein bridge. Mol. Cell 7581-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoshino, S., M. Imai, T. Kobayashi, N. Uchida, and T. Katada. 1999. The eukaryotic polypeptide chain releasing factor (eRF3/GSPT) carrying the translation termination signal to the 3′-poly(A) tail of mRNA. Direct association of erf3/GSPT with polyadenylate-binding protein. J. Biol. Chem. 27416677-16680. [DOI] [PubMed] [Google Scholar]
- 29.Imataka, H., A. Gradi, and N. Sonenberg. 1998. A newly identified N-terminal amino acid sequence of human eIF4G binds poly(A)-binding protein and functions in poly(A)-dependent translation. EMBO J. 177480-7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joachims, M., P. C. van Breugel, and R. E. Lloyd. 1999. Cleavage of poly(A)-binding protein by enterovirus proteases concurrent with inhibition of translation in vitro. J. Virol. 73718-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahvejian, A., Y. V. Svitkin, R. Sukarieh, M. N. M'Boutchou, and N. Sonenberg. 2005. Mammalian poly(A)-binding protein is a eukaryotic translation initiation factor, which acts via multiple mechanisms. Genes Dev. 19104-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khaleghpour, K., Y. V. Svitkin, A. W. Craig, C. T. DeMaria, R. C. Deo, S. K. Burley, and N. Sonenberg. 2001. Translational repression by a novel partner of human poly(A) binding protein, Paip2. Mol. Cell 7205-216. [DOI] [PubMed] [Google Scholar]
- 33.Khanam, T., R. S. Muddashetty, A. Kahvejian, N. Sonenberg, and J. Brosius. 2006. Poly(A)-binding protein binds to A-rich sequences via RNA-binding domains 1+2 and 3+4. RNA Biol. 3170-177. [DOI] [PubMed] [Google Scholar]
- 34.Kolupaeva, V. G., T. V. Pestova, C. U. Hellen, and I. N. Shatsky. 1998. Translation eukaryotic initiation factor 4G recognizes a specific structural element within the internal ribosome entry site of encephalomyocarditis virus RNA. J. Biol. Chem. 27318599-18604. [DOI] [PubMed] [Google Scholar]
- 35.Kozlov, G., G. De Crescenzo, N. S. Lim, N. Siddiqui, D. Fantus, A. Kahvejian, J. F. Trempe, D. Elias, I. Ekiel, N. Sonenberg, M. O'Connor-McCourt, and K. Gehring. 2004. Structural basis of ligand recognition by PABC, a highly specific peptide-binding domain found in poly(A)-binding protein and a HECT ubiquitin ligase. EMBO J. 23272-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozlov, G., J.-F. Trempe, K. Khaleghpour, A. Kahvejian, I. Ekiel, and K. Gehring. 2001. Structure and function of the C-terminal PABC domain of human poly(A)-binding protein. Proc. Natl. Acad. Sci. USA 984409-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuhn, U., and T. Pieler. 1996. Xenopus poly(A) binding protein: functional domains in RNA binding and protein-protein interaction. J. Mol. Biol. 25620-30. [DOI] [PubMed] [Google Scholar]
- 38.Kuyumcu-Martinez, M., G. Belliot, S. V. Sosnovtsev, K. O. Chang, K. Y. Green, and R. E. Lloyd. 2004. Calicivirus 3C-like proteinase inhibits cellular translation by cleavage of poly(A)-binding protein. J. Virol. 788172-8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuyumcu-Martinez, N. M., M. Joachims, and R. E. Lloyd. 2002. Efficient cleavage of ribosome-associated poly(A)-binding protein by enterovirus 3C protease. J. Virol. 762062-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuyumcu-Martinez, N. M., M. E. Van Eden, P. Younan, and R. E. Lloyd. 2004. Cleavage of poly(A)-binding protein by poliovirus 3C protease inhibits host cell translation: a novel mechanism for host translation shutoff. Mol. Cell. Biol. 241779-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liebig, H. D., E. Ziegler, R. Yan, K. Hartmuth, H. Klump, H. Kowalski, D. Blaas, W. Sommergruber, L. Frasel, B. Lamphear, R. Rhoads, E. Kuechler, and T. Skern. 1993. Purification of two picornaviral 2A proteinases—interaction with eIF-4g and influence on in vitro translation. Biochemistry 327581-7588. [DOI] [PubMed] [Google Scholar]
- 42.Lloyd, R. 2006. Translational control by viral proteinases. Virus Res. 11976-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyons, T., K. E. Murray, A. W. Roberts, and D. J. Barton. 2001. Poliovirus 5′-terminal cloverleaf RNA is required in cis for VPg uridylylation and the initiation of negative-strand RNA synthesis. J. Virol. 7510696-10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meerovitch, K., J. Pelletier, and N. Sonenberg. 1989. A cellular protein that binds to the 5′-noncoding region of poliovirus RNA: implications for internal translation initiation. Genes Dev. 31026-1034. [DOI] [PubMed] [Google Scholar]
- 45.Michel, Y. M., A. M. Borman, S. Paulous, and K. M. Kean. 2001. Eukaryotic initiation factor 4G-poly(A) binding protein interaction is required for poly(A) tail-mediated stimulation of picornavirus internal ribosome entry segment-driven translation but not for X-mediated stimulation of hepatitis C virus translation. Mol. Cell. Biol. 214097-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nietfeld, W., H. Mentzel, and T. Pieler. 1990. The Xenopus laevis poly(A) binding protein is composed of multiple functionally independent RNA binding domains. EMBO J. 93699-3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ochs, K., L. Saleh, G. Bassili, V. H. Sonntag, A. Zeller, and M. Niepmann. 2002. Interaction of translation initiation factor eIF4B with the poliovirus internal ribosome entry site. J. Virol. 762113-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okochi, K., T. Suzuki, J. Inoue, S. Matsuda, and T. Yamamoto. 2005. Interaction of anti-proliferative protein Tob with poly(A)-binding protein and inducible poly(A)-binding protein: implication of Tob in translational control. Genes Cells 10151-163. [DOI] [PubMed] [Google Scholar]
- 49.Perera, R., S. Daijogo, B. L. Walter, J. H. Nguyen, and B. L. Semler. 2007. Cellular protein modification by poliovirus: the two faces of poly(rC)-binding protein. J. Virol. 818919-8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pestova, T. V., C. U. T. Hellen, and E. Wimmer. 1991. Translation of poliovirus RNA: role of an essential cis-acting oligopyrimidine element within the 5′ nontranslated region and involvement of a cellular 57-kilodalton protein. J. Virol. 656194-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rivera, C. I., and R. E. Lloyd. 2008. Modulation of enteroviral proteinase cleavage of poly(A)-binding protein (PABP) by conformation and PABP-associated factors. Virology 37559-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sachs, A., and R. Davis. 1989. The poly(A)-binding protein is required for poly(A) shortening and 60S ribosomal subunit-dependent translation initiation. Cell 58857-867. [DOI] [PubMed] [Google Scholar]
- 53.Shiroki, K., T. Isoyama, S. Kuge, T. Ishii, S. Ohmi, S. Hata, K. Suzuki, Y. Takasaki, and A. Nomoto. 1999. Intracellular redistribution of truncated La protein produced by poliovirus 3Cpro-mediated cleavage. J. Virol. 732193-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simoes, E. A. F., and P. Sarnow. 1991. An RNA hairpin at the extreme 5′ end of the poliovirus RNA genome modulates viral translation in human cells. J. Virol. 65913-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sladic, R. T., C. A. Lagnado, C. J. Bagley, and G. J. Goodall. 2004. Human PABP binds AU-rich RNA via RNA-binding domains 3 and 4. Eur. J. Biochem. 271450-457. [DOI] [PubMed] [Google Scholar]
- 56.Svitkin, Y. V., M. Costa-Mattioli, B. Herdy, S. Perreault, and N. Sonenberg. 2007. Stimulation of picornavirus replication by the poly(A) tail in a cell-free extract is largely independent of the poly(A) binding protein (PABP). RNA 132330-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Svitkin, Y. V., H. Imataka, K. Khaleghpour, A. Kahvejian, H.-D. Liebig, and N. Sonenberg. 2001. Poly(A)-binding protein interaction with eIF4G stimulates picornavirus IRES-dependent translation. RNA 71743-1752. [PMC free article] [PubMed] [Google Scholar]
- 58.Uchida, N., S. Hoshino, H. Imataka, N. Sonenberg, and T. Katada. 2002. A novel role of the mammalian GSPT/eRF3 associating with poly(A)-binding protein in Cap/Poly(A)-dependent translation. J. Biol. Chem. 27750286-50292. [DOI] [PubMed] [Google Scholar]
- 59.Xiang, W. K., K. S. Harris, L. Alexander, and E. Wimmer. 1995. Interaction between the 5′-terminal cloverleaf and 3AB/3CD(pro) of poliovirus is essential for RNA replication. J. Virol. 693658-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang, B., G. Morace, V. Gauss-Muller, and Y. Kusov. 2007. Poly(A) binding protein, C-terminally truncated by the hepatitis A virus proteinase 3C, inhibits viral translation. Nucleic Acids Res. 355975-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ziegler, E., A. M. Borman, F. G. Deliat, H. D. Liebig, D. Jugovic, K. M. Kean, T. Skern, and E. Kuechler. 1995. Picornavirus 2A proteinase-mediated stimulation of internal initiation of translation is dependent on enzymatic activity and the cleavage products of cellular proteins. Virology 213549-557. [DOI] [PubMed] [Google Scholar]