Abstract
In primary infection, CD8+ T cells are important for clearance of infectious herpes simplex virus (HSV) from sensory ganglia. In this study, evidence of CD4+ T-cell-mediated clearance of infectious HSV type 1 (HSV-1) from neural tissues was also detected. In immunocompetent mice, HSV-specific CD4+ T cells were present in sensory ganglia and spinal cords coincident with HSV-1 clearance from these sites and remained detectable at least 8 months postinfection. Neural CD4+ T cells isolated at the peak of neural infection secreted gamma interferon, tumor necrosis factor alpha, interleukin-2 (IL-2), or IL-4 after stimulation with HSV antigen. HSV-1 titers in neural tissues were greatly reduced over time in CD8+ T-cell-deficient and CD8+ T-cell-depleted mice, suggesting that CD4+ T cells could mediate clearance of HSV-1 from neural tissue. To examine possible mechanisms by which CD4+ T cells resolved neural infection, CD8+ T cells were depleted from perforin-deficient or FasL-defective mice. Clearance of infectious virus from neural tissues was not significantly different in perforin-deficient or FasL-defective mice compared to wild-type mice. Further, in spinal cords and brains after vaginal HSV-1 challenge of chimeric mice expressing both perforin and Fas or neither perforin nor Fas, virus titers were significantly lower than in control mice. Thus, perforin and Fas were not required for clearance of infectious virus from neural tissues. These results suggest that HSV-specific CD4+ T cells are one component of a long-term immune cell presence in neural tissues following genital HSV-1 infection and play a role in clearance of infectious HSV-1 at neural sites, possibly via a nonlytic mechanism.
Herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) are significant human pathogens. An estimated 80% of people are infected with at least one strain (10). It is estimated that 20 to 40% of people in the United States suffer herpetic orolabial lesions due to HSV-1 though the estimated number of those infected ranges from 50 to 80% (9). While seroprevalence of HSV-1 in the United States appears to be declining, the actual number of genital herpes cases attributed to HSV-1 is on the rise (50, 67). Several reports note an increase in the number of genital herpes cases caused by HSV-1, a phenomenon seen in developed nations including the United States (34, 46, 52, 64, 67). Although HSV-2 is typically thought of as the virus responsible for causing genital lesions, the current trend in the United States, especially among adolescents, finds HSV-1 as the most common cause of newly diagnosed genital HSV infections (50). Like HSV-2, HSV-1 has been shown to infrequently result in encephalitis and devastating neural damage (10, 23, 24, 56).
HSV initially infects and undergoes acute replication in epithelial cells and invades local nerve termini (24). The virus travels via retrograde axonal transport and gains access to neuronal cell bodies within the sensory ganglia, thus establishing a lifelong, persistent infection (3, 10, 19, 23, 24, 58). Reactivation from latency can occur during times of emotional or physical stress and can cause recurrent disease (3, 24). During periods of reactivation, the virus is shed from the infected host, sometimes in the absence of clinical symptoms, and thus may have an increased chance of infecting additional susceptible hosts (20, 24).
Candidate vaccines against HSV provided, at best, partial protection in HSV-seronegative women (5, 6, 57). In designing an effective vaccine against HSV, it may be important to examine and consider the various responses of the immune system to natural infection. An effective vaccine against HSV will most likely need to elicit immune responses at both the epithelial and neural sites of infection, as well as from several immune cell types; these are key events that studies of the immune responses to natural infection with HSV may help elucidate.
Cell-mediated immunity has proven important for controlling HSV infection. Both HSV-specific CD4+ and CD8+ T cells have been isolated from the lesions of human patients, and these cells are important for the clearance of virus from the genital epithelium (26, 27, 28). In a murine model of genital HSV-1 infection, it was previously shown that CD4+ T cells are critically important in the prevention of disease (29). In contrast, it has been suggested that CD8+ T cells are predominantly responsible for clearance of infectious HSV-1 from nervous system tissues during a primary HSV infection (54).
In the present study the role of CD4+ T cells at neural sites of infection was examined. In mice inoculated intravaginally (i.vag.) with HSV-1, CD4+ T cells infiltrated the spinal cords and dorsal root ganglia, where they accumulated and persisted. By inoculating mice in which CD8+ T cells were either depleted or genetically absent, we were able to show that CD4+ T cells were sufficient for clearance of HSV-1 from both genital and neural sites after primary infection. Further, by utilizing adoptive transfer models, our data demonstrate that perforin and Fas/FasL were not absolute requirements for the clearance of infectious virus from neural sites. Our results challenge current thinking about clearance of infectious HSV from sensory ganglia and support an important role for CD4+ T cells, in addition to CD8+ T cells, in controlling neural HSV infection.
MATERIALS AND METHODS
Virus.
HSV-1 strain SC16 was obtained from Lawrence Stanberry (Columbia University, New York, NY). Virus stocks were prepared by infection of Vero cell monolayers at a multiplicity of infection of 0.01, as previously described (40, 41). The virus was released from the Vero cells by three freeze-thaw cycles (40, 41). Cell debris was removed by centrifugation, and virus stocks were stored at −80°C (40, 41).
Mice.
C57BL/6J (B6), CD8−/−, perforin-deficient C57BL/6-Pfp−/−tm1Sdz (hereafter, Pfp−/−), C57BL/6-Tg(TcraTcrb)425Cbn/J (OT-II), B6Smn.C3H-Faslgld (B6-gld), and B6.MRL-Faslpr (B6-lpr) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were housed under specific-pathogen-free conditions at the University of Texas Medical Branch at an animal facility approved by the American Association of Laboratory Animal Care. Experiments were conducted with Institutional Animal Care and Use Committee approval.
Virus inoculation and titration.
Mice were inoculated i.vag. as described previously (39). Briefly, prior to inoculation, mice were treated with 2 mg of medroxyprogesterone acetate (Depo-Provera) subcutaneously. Hormonal pretreatment was necessary to induce susceptibility of mice to genital HSV-1 inoculation (38, 40), which may reflect thinning of the genital epithelium (22) or induction of the HSV entry receptor, nectin-1, on vaginal epithelial cells (31). Clearance of infectious virus from spinal cords and dorsal root ganglia and spread to hindbrains were determined by quantification of virus by standard plaque assay, as previously described (40). Briefly, groups of five HSV-1-infected B6 mice were euthanized, and the lumbosacral region of the dorsal root ganglia and the adjacent areas of the spinal cord, as well as hindbrains (including cerebellum, medulla oblongata, and brain stem), were harvested, weighed, and frozen in 1 ml of medium (medium 199 [Gibco], 2% newborn calf serum, 2% penicillin-streptomycin, 2% amphotericin). Tissues were later thawed, homogenized, clarified by centrifugation, and titrated on monolayers of Vero cells. Though PCR has proven highly sensitive for detection of HSV, the current study focused on infectious virus within the spinal cord and sensory ganglia, and therefore the previously established (though less sensitive) method of titration on Vero cell monolayers was employed (15).
Enrichment of CD4+ and CD8+ T cells from neural tissues.
Mice were sacrificed on day 9 after i.vag. inoculation with 105 PFU of HSV-1 strain SC16. Lymphocytes were isolated from pooled spinal cords or dorsal root ganglia by a modification of a method previously described by Marracci et al. (37). Briefly, tissues were pushed through mesh screens and stirred for 30 min in calcium- and magnesium-free phosphate-buffered saline (Invitrogen Corp., Grand Island, NY) at ambient temperature. Samples were then resuspended in a 30% Percoll (Sigma-Aldrich, St. Louis, MO) solution, layered over a 70% Percoll cushion, and centrifuged at 500 × g for 20 min at ambient temperature. After centrifugation, the upper layer containing dispersed neural tissue was removed and again spun over a 70% Percoll cushion. Lymphocytes from the Percoll interface of both preparations were then combined.
Flow cytometry.
Lymphocytes were stained with fluorochrome-labeled antibodies against CD4, CD8, CD25, CD44, or CD69 (Pharmingen, San Diego, CA). Data were acquired on a Becton Dickinson FACSCanto instrument (BD Biosciences, San Diego, CA) at the University of Texas Medical Branch Flow Cytometry Core Facility and analyzed with FlowJo software (Treestar, Inc., Ashland, OR). The number of activated CD4+ T cells in uninfected or HSV-1-infected iliac lymph node, spinal cord, and dorsal root ganglia was determined using FlowJo software and is presented as the number of double-positive cells per 106 total isolated cells.
Depletion of CD8+ T cells in vivo.
Mice received 1 mg of anti-CD8 (2.43) antibody intraperitoneally on the 2 days prior to infection. Following i.vag. inoculation with 106 PFU of HSV-1 SC16, mice also received 0.5 mg of anti-CD8 antibody i.p. every other day throughout the study. Depletion was assessed by flow cytometry and was typically greater than 98%.
ELISPOT analysis.
Enzyme-linked immunospot (ELISPOT) analysis was performed similarly to the procedure described by Milligan et al. (41). B6 mice were infected vaginally with 105 PFU of HSV-1 SC16. Lymphocytes were isolated from iliac lymph nodes, spinal cords, and dorsal root ganglia on days 6, 8, 11, and 14 days postinfection and, for a composite extended time point, at 42, 69, and 168 days postinfection. Iliac lymph nodes were pooled from five animals, and a single-cell suspension was created by pushing the tissues through a mesh screen. Spinal cords and dorsal root ganglia were pooled from five animals and processed as described above. Lymphocytes from all tissues were incubated in anti-gamma interferon (IFN-γ), anti-tumor necrosis factor alpha (TNF-α), anti-interleukin-2 (IL-2), anti-IL-4, or anti-IL-17 antibody-coated ELISPOT plates with 5 × 105 mitomycin C (Sigma-Aldrich)-treated feeder cells per well, with and without UV-killed HSV-1 antigen (41). Plates were incubated for 40 h and developed as previously described by the sequential addition of biotinylated anti-IFN-γ, anti-TNF-α, anti-IL-2, anti-IL-4, or anti-IL-17 (Pharmingen) antibody; avidin-peroxidase (Sigma-Aldrich); and 3-amino-9-ethyl-carbazole plus sodium acetate (13).
Adoptive transfer.
CD4+ T cells from spleens of B6, perforin-deficient, or OT-II (major histocompatibility complex class II [MHC-II] T-cell receptor transgenic mice specific for ovalbumin peptide residues 323 to 339 [OVA323-339]) mice (4) were purified using positive selection magnetic bead separation (Miltenyi Biotech, Auburn, CA). Purity of the CD4+ T-cell isolations was determined by flow cytometric analysis. A total of 4 × 106 CD4+ T cells were injected intravenously in irradiated (900 rads) B6 or B6-lpr mice, thus creating mice that possessed both functional perforin and Fas (Pfp+ Fas+) or neither perforin nor Fas (Pfp− Fas−) and mice that expressed both perforin and Fas but were unable to mount a specific response to infection with HSV-1 (OT-II). Mice were allowed to rest for 7 days, during which time they received one 0.5-mg dose of anti-CD8 antibody to ensure depletion of CD8+ T cells. Mice were then inoculated vaginally with 5 × 103 PFU of HSV-1 SC16.
Statistical analysis.
HSV-1 titers were analyzed by a Mann-Whitney U-test or by one-way analysis of variance with the Bonferoni correction for multiple groups, as appropriate. Analyses were performed using GraphPad Prism, version 4.0, software (San Diego, CA). P values of less than 0.05 were considered statistically significant.
RESULTS
HSV-1 clearance from genital tract, dorsal root ganglia, and spinal cords.
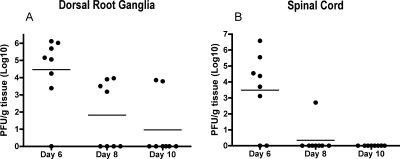
In animal models, cell-mediated immunity has proven important for controlling HSV infection. To examine the kinetics of clearance of HSV-1 from neural tissues, groups of B6 mice were inoculated i.vag. with HSV-1, and infectious virus in the dorsal root ganglia and spinal cords was quantified. Figure 1 demonstrates a typical pattern of clearance of infectious HSV-1 from neural tissues. In the dorsal root ganglia, the mean virus titer declined greatly on days 8 and 10 postinoculation (Fig. 1). Further, the incidence of samples with infectious virus declined over the course of the experiments from seven of eight animals on day 6 to four of eight animals on day 8 and two of eight animals on day 10 postinoculation, demonstrating a trend toward clearance of infectious HSV-1 from dorsal root ganglia. The clearance pattern in spinal cords was even more dramatic. Virus titers greatly decreased between days 6 and 8 and were below the limit of detection by day 10 postinoculation. Further, while six of eight animals contained infectious virus in the spinal cord on day 6 postinoculation, the incidence of samples containing infectious virus decreased to one of eight animals by day 8 and zero of eight animals by day 10 (Fig. 1).
FIG. 1.
HSV-1 clearance from dorsal root ganglia and spinal cord. B6 mice were inoculated i.vag. with HSV-1. Dorsal root ganglia and spinal cords were harvested on the indicated days for quantification of virus as described in Materials and Methods. The decrease in the amount of infectious virus present over the course of the experiment was significant in both dorsal root ganglia (P = 0.0148 from day 6 to day 8 and P = 0.0070 from day 6 to day 10) and spinal cord (P = 0.0312 from day 6 to day 8 and P = 0.0148 from day 6 to day 10). Horizontal lines represent mean virus titers.
CD4+ and CD8+ T cells are present in the HSV-1-infected spinal cord and dorsal root ganglia.
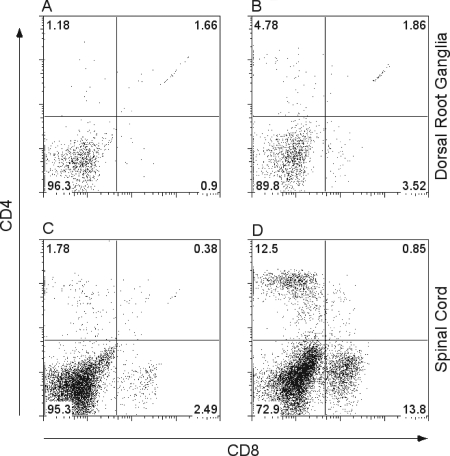
Both HSV-specific CD4+ and CD8+ T cells have been shown to be important for the clearance of HSV-1 and HSV-2 from the genital epithelium (29, 40, 47), and an important role for CD8+ T cells in the protection of nervous system tissues during a primary HSV infection has been suggested (54). To examine the role of T cells in protection of neurons in mice inoculated i.vag. with HSV-1, the presence of CD4+ and CD8+ T-cell subpopulations in neural tissues during the course of HSV-1 infection was assessed. Few CD4+ or CD8+ T cells were detected in the dorsal root ganglia and spinal cords of uninfected mice (Fig. 2A and C). Increased numbers of both CD4+ and CD8+ T cells were detected in the spinal cords and dorsal root ganglia of HSV-1-infected B6 mice on day 8 postinfection (Fig. 2B and D), concurrent with the time of rapid virus clearance (Fig. 1).
FIG. 2.
Presence of CD4+ T cells and CD8+ T cells in HSV-1-infected spinal cords and dorsal root ganglia. B6 mice were infected with HSV-1; these mice and uninfected controls were sacrificed on day 8, and lymphocytes were isolated from pooled spinal cords or pooled dorsal root ganglia and stained with fluorochrome-labeled antibodies against CD4 and CD8. (A) Naïve dorsal root ganglia. (B) HSV-1-infected dorsal root ganglia. (C) Naïve spinal cords. (D) HSV-1-infected spinal cords.
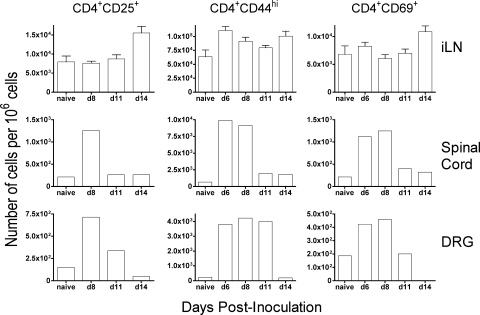
These findings were extended by examining the kinetics of cellular infiltration into draining lymph nodes and infected tissues. The number of CD4+ T cells from iliac lymph nodes, spinal cords, and dorsal root ganglia expressing the activation marker CD25, CD44, or CD69 increased following i.vag. HSV-1 inoculation and generally peaked around day 6 or 8 postinfection (Fig. 3). Importantly, the peak of the activated CD4+ T-cell response correlated with the period of rapid clearance of infectious virus in immunocompetent mice, occurring around day 8 postinoculation (Fig. 1).
FIG. 3.
CD4+ T cells with activated phenotype are present in the spinal cord and dorsal root ganglia (DRG) of HSV-1 inoculated mice. Lymphocytes were isolated from iliac lymph node (iLN), spinal cord, and dorsal root ganglia of HSV-1-infected mice on the indicated days (d). Lymphocytes were stained with fluorochrome-labeled antibodies and analyzed by flow cytometry as described in Materials and Methods. The mean number of total CD4+ T cells isolated from iliac lymph nodes ranged from 7.35 × 104 per 106 cells in naïve animals to 1.30 × 105 per 106 cells at the peak on day 8. The number of total CD4+ T cells isolated from pooled spinal cords ranged from 7.63 × 102 per 106 cells in naïve animals to 1.02 × 104 per 106 cells at the peak on day 6. The number of total CD4+ T cells isolated from pooled dorsal root ganglia ranged from 7.12 × 102 per 106 cells in naïve animals to 4.52 × 103 per 106 cells at the peak on day 8.
HSV-specific effector CD4+ T cells are present in neural tissues during resolution of acute HSV-1 infection.
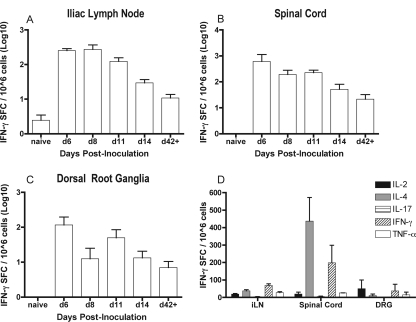
Because CD4+ T cells expressing activation markers indicative of recent antigen exposure infiltrated the spinal cord and dorsal root ganglia of HSV-1-infected mice, the antigen specificity of the CD4+ T cells present at these sites was tested. B6 mice were inoculated i.vag. with HSV-1 and sacrificed, and iliac lymph nodes, spinal cords, and dorsal root ganglia were harvested on various days postinoculation. Lymphocytes were isolated and cultured in the presence and absence of UV-killed HSV-1 antigen. The frequency of HSV-specific (IFN-γ-producing) CD4+ T cells in iliac lymph nodes, spinal cords, and dorsal root ganglia of HSV-infected mice are provided in Fig. 4. In all tissues tested, the number of HSV-specific CD4+ T cells peaked around day 6 or 8 postinfection, followed by a gradual decline at each location examined. Importantly, these HSV-specific CD4+ T cells persisted in the secondary lymphoid and neural tissues tested and were detectable through at least 8 months postinfection.
FIG. 4.
HSV-specific CD4+ T cells in iliac lymph nodes, spinal cords, and dorsal root ganglia after infection with HSV-1. (A to C) B6 mice were inoculated i.vag. with HSV-1 and sacrificed on the indicated days (d), and isolated lymphocytes from these tissues were plated on anti-IFN-γ antibody-coated ELISPOT assay plates. Extended time points labeled as d42+ represent three compiled experiments that were harvested at days 42, 69, and 168 postinoculation. Results are presented as the number of spot forming cells (SFC)/106 lymphocytes. (D) Production of IFN-γ, TNF-α, IL-2, IL-4, and IL-17 by HSV-specific CD4+ T cells in iliac lymph nodes (iLN), spinal cords, and dorsal root ganglia (DRG) after infection with HSV-1. B6 mice were inoculated i.vag. with HSV-1 and sacrificed on day 8 postinfection. Pools of lymphocytes from spinal cords, dorsal root ganglia, or individual lymph nodes were quantified by ELISPOT assay. Mean cell counts of iliac lymph node lymphocytes from individual mice ranged from 1.1 ×106 for naïve mice to 1.0 × 107 at the peak of the response on day 6. Mean cell counts for spinal cord lymphocytes pooled from five mice ranged from 1.1 × 106 to 1.3 × 106. Mean cell counts for dorsal root ganglia lymphocytes pooled from five mice ranged from 4.15 × 105 to 6.07 × 105 cells.
These results were extended by further defining the cytokines secreted by HSV-specific CD4+ T cells in iliac lymph nodes, spinal cord, and dorsal root ganglia. The number of local HSV-specific CD4+ T cells that produced IFN-γ, TNF-α, IL-2, IL-4, or IL-17 was quantified by ELISPOT analysis on day 8 after i.vag. inoculation with HSV-1. This time point corresponds to the peak of HSV-specific CD4+ T-cell infiltration, as well as with rapid clearance of infectious virus (Fig. 1 and 2). HSV-specific CD4+ T cells producing IFN-γ, TNF-α, IL-2, or IL-4 were detected in these tissues on day 8 postinoculation (Fig. 4D). HSV-specific CD4+ T cells producing IL-17 were detected in the iliac lymph nodes but not in the spinal cords or dorsal root ganglia of HSV-1-inoculated mice (Fig. 4D). The frequency of IL-4-producing HSV-specific CD4+ T cells was highest in the spinal cords (Fig. 4D).
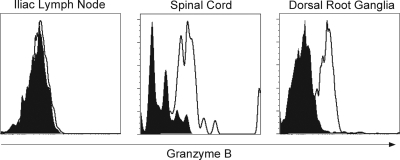
As effector CD4+ T cells have been shown to express cytolytic activity (40, 45, 68) during viral infections, including HSV infections, neural CD4+ T cells in the present study were examined for granzyme B on day 7 postinoculation with HSV-1. Figure 5 demonstrates that CD4+ T cells isolated from the spinal cords and dorsal root ganglia of HSV-1-infected B6 mice expressed granzyme B. In experiments of identical design, detection of FasL on the surface of neural CD4+ T cells was not consistent (data not shown).
FIG. 5.
Granzyme B expression by CD4+ T cells. B6 mice were inoculated i.vag. with HSV-1. Mice were sacrificed on day 8 postinfection, and lymphocytes were isolated from spinal cords, dorsal root ganglia, or iliac lymph nodes. Pools of lymphocytes were stained with fluorochrome-labeled antibodies against CD4 and intracellular granzyme B. Solid histograms represent isotype controls, and open histograms indicate granzyme B staining in HSV-infected tissues.
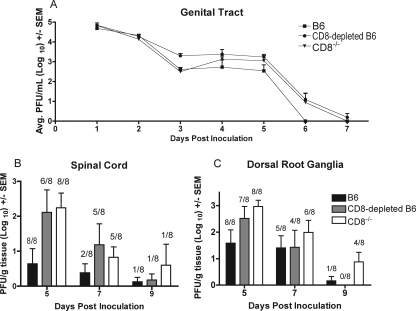
Clearance of acute HSV-1 from genital and neural sites in the absence of CD8+ T cells.
HSV-specific CD4+ T cells infiltrating the spinal cords and sensory ganglia of HSV-1-infected animals were hypothesized to be important for clearance of infectious virus from these sites. To test the ability of CD4+ T cells to clear virus, mice from which CD8+ T cells were either depleted or genetically absent (CD8−/−) were inoculated i.vag. with HSV-1. Wild-type B6 mice cleared infectious virus from the genital tract around day 6 postinfection (Fig. 6A). Both CD8-depleted and CD8−/− animals cleared virus from the genital tract by day 7 postinfection. This 1-day difference in clearance was not statistically significant and may reflect animal-to-animal variability rather than a true biological difference. The amount of infectious virus in the spinal cords and dorsal root ganglia of wild-type B6 mice decreased from day 7 to day 9 postinfection, and the incidence of spinal cords and dorsal root ganglia samples containing infectious HSV-1 also declined over this time from eight of eight mice on day 5 to one of eight by day 9 postinfection (Fig. 6B and C). While both CD8-depleted and CD8−/− mice had higher virus titers in neural tissues on days 7 and 9 than the wild-type B6 mice, these animals also showed a trend toward clearance of infectious virus from both the spinal cord and dorsal root ganglia (Fig. 6B and C). The CD8-depleted animals had a similar pattern of virus clearance from the neural tissues, with six of eight animals having infectious virus in the spinal cord and seven of eight having infectious virus in the dorsal root ganglia on day 5. This was reduced to one of eight (spinal cord) and zero of eight (dorsal root ganglia) animals by day 9 postinfection (Fig. 6). All (eight of eight) CD8−/− animals were found to have infectious HSV-1 in both the spinal cord and dorsal root ganglia on day 5, which was reduced to one of eight (spinal cord) and four of eight (dorsal root ganglia) animals by day 9 postinfection (Fig. 6).
FIG. 6.
CD4+ T-cell-mediated clearance of HSV-1 from the genital tract, sensory ganglia, and spinal cord. B6 mice, CD8-depleted B6 mice, and CD8−/− mice were inoculated i.vag. with HSV-1. Mice were swabbed on the indicated days (A), and spinal cords (B) and dorsal root ganglia (C) were harvested on the indicated days for quantification of virus. Numbers above bars in panels B and C represent the number of samples with infectious virus out of the total number of samples. SEM, standard error of the mean; Avg, average.
CD4+ T-cell clearance of acute HSV from neural tissues does not require a lytic mechanism.
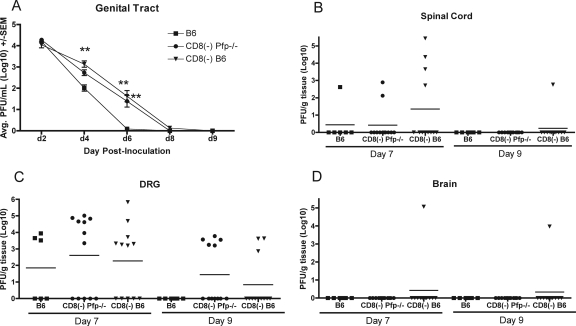
Two approaches were used to test the involvement of cytolytic mechanisms in HSV-1 clearance, including the use of perforin-deficient or FasL-defective mice and the construction of short-term perforin/Fas radiation chimeras. The possible role for perforin in the CD4+ T-cell-mediated clearance of infectious HSV-1 was examined by comparison of mean virus titers in the genital tracts, dorsal root ganglia, spinal cords, and hindbrains of intact B6 mice, CD8-depleted B6, and CD8-depleted Pfp−/− mice. In the genital tract, infectious virus was not detected on day 6 in B6 mice (Fig. 7A). Virus titers significantly higher than those in B6 controls were detected on days 4 and 6 in CD8-depleted mice and on day 6 in CD8-depleted Pfp−/− mice. Virus clearance rates in CD8-depleted Pfp−/− and CD8-depleted B6 mice were very similar, and infectious virus was not detected in either group by day 9 after inoculation. Again, this slight difference in virus titers on day 8 between these two groups may reflect either a limited efficiency of plaque formation on Vero cell monolayers or an inherent variation in virus clearance among individual animals and is most likely not biologically significant. In the dorsal root ganglia, infectious virus was undetectable in B6 mice on day 9, while mean virus titers in both CD8-depleted B6 mice and CD8-depleted Pfp−/− mice dropped by nearly a log between days 7 and 9 (Fig. 7C). In the spinal cord, the B6 and CD8-depleted Pfp−/− mice were able to clear virus by day 9 postinfection, while all but a single CD8-depleted B6 animal (1 of 10) cleared virus by day 9 (Fig. 7B). The infection was apparently well controlled in dorsal root ganglia and spinal cords as virus spread to the hindbrain was detected in only a single CD8-depleted B6 animal (Fig. 7D).
FIG. 7.
CD4+ T-cell-mediated clearance of HSV-1 from the sensory ganglia and spinal cord does not require perforin. (A) Groups of B6, CD8-depleted B6, or CD8-depleted Pfp−/− mice were inoculated i.vag. with HSV-1 and swabbed on the indicated days (d). Spinal cords (B), dorsal root ganglia (DRG; C), and hindbrain (D) were harvested on the indicated days for quantification of virus. **, significantly different compared to B6 controls (P < 0.001). CD8(−), depletion of CD8+ T cells by injection of specific antibody; SEM, standard error of the mean; Avg, average.
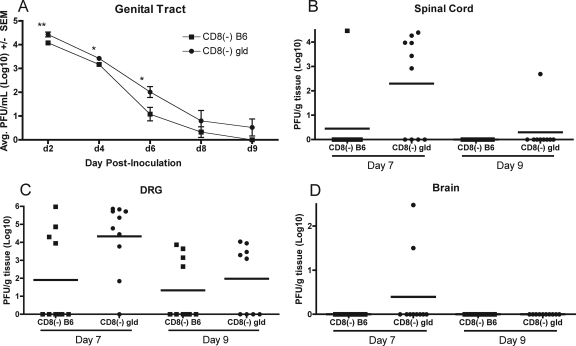
The involvement of the FasL-mediated lytic pathway was examined by comparing the incidence of infection and virus resolution of CD8-depleted B6 and CD8-depleted FasL-defective (B6-gld) mice. Both B6 and B6-gld mice cleared virus from the genital tract, although titers were significantly higher on days 2, 4, and 6 in B6-gld mice (Fig. 8A). Mean virus titers detected in the dorsal root ganglia dropped between days 7 and 9 in both B6 and B6-gld mice (Fig. 8C). The mean HSV-1 titer was much higher in B6-gld mice on day 7 but fell significantly on day 9 (P < 0.05), and infectious virus was detected in fewer mice on day 9 (9/10 on day 7 compared to 5/9 on day 9). Infectious virus was also cleared from the spinal cords of both mouse strains, and infectious virus was detected in only a single CD8-depleted B6-gld mouse on day 9 (Fig. 8C). Again, no virus spread to the hindbrain was detected in either mouse strain on day 9 (Fig. 8D).
FIG. 8.
CD4+ T-cell-mediated clearance of HSV-1 from the sensory ganglia and spinal cord does not require Fas-FasL interaction. (A) Groups of CD8-depleted B6 or CD8-depleted B6-gld (gld) mice were inoculated i.vag. with HSV-1 and swabbed on the indicated days (d). Spinal cords (B), dorsal root ganglia (DRG; C), and hindbrain (D) were harvested on the indicated days for quantification of virus. Significant differences relative to B6 controls are indicated as follows: *, P < 0.05; **, P < 0.005. CD8(−), depletion of CD8+ T cells by injection of specific antibody; SEM, standard error of the mean; Avg, average.
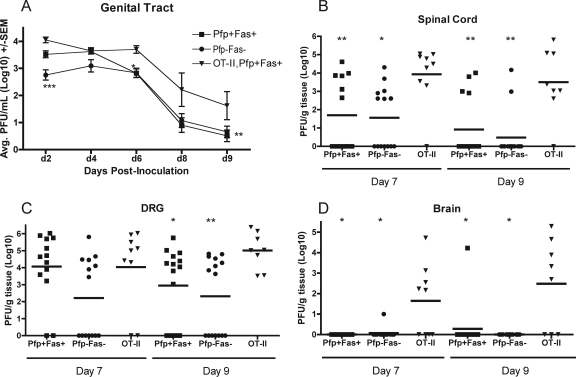
Studies of perforin- and FasL-mediated mechanisms of clearance of infectious HSV-1 were extended by constructing perforin/Fas irradiation chimeras. CD4+ T cells isolated from wild-type B6 (perforin positive), perforin-deficient (Pfp−/−), or, as a control, OT-II (OVA specific) mice were adoptively transferred into groups of irradiated B6 (Fas positive) or Fas-deficient (B6-lpr) mice. This resulted in mice that had both functional perforin and Fas pathways (Pfp+ Fas+), mice that had neither perforin nor Fas pathways (Pfp− Fas−), and mice that had both pathways but whose T cells were unable to recognize the virus (OT-II negative control). Mice were sacrificed on days 7 and 9 after i.vag. challenge with HSV-1, and spinal cords, dorsal root ganglia, and hindbrains were harvested for quantification of infectious virus. Virus titers were similar in the sensory ganglia of Pfp− Fas−, Pfp+ Fas+, and OT-II mice on day 7. However, virus titers in the Pfp− Fas− mice were significantly lower than in controls on day 9 (P < 0.05) (Fig. 9C). HSV-1 titers fell in Pfp− Fas− and Pfp+ Fas+ mice and were significantly lower than in OT-II controls on day 9 (P < 0.01). The incidence of samples containing infectious virus also fell on day 9 in these groups. While the virus infection was not controlled and spread to the hindbrain in OT-II control mice, infectious virus was detected in the hindbrain of only a single Pfp+ Fas+ mouse on day 9 (Fig. 9D).
FIG. 9.
CD4+ T cells reduce infectious virus titers in spinal cords and dorsal root ganglia in the absence of perforin and Fas. (A) Radiation chimeras were constructed as described in Materials and Methods. Mice were challenged i.vag. with HSV-1 and swabbed on the indicated days. Spinal cords (B), dorsal root ganglia (DRG; C), and hindbrain (D) were harvested on indicated days for quantification of virus. Significant differences relative to OT-II controls are indicated as follows: *, P < 0.01; **, P < 0.05; ***, P < 0.001.
DISCUSSION
Cell-mediated immunity is important for controlling HSV infection, and previous studies have demonstrated the importance of CD8+ cytolytic T lymphocytes (CTLs) in the clearance of HSV-2 from the genital epithelium of experimental animals (11, 47) and humans (28, 48). Kuklin et al. demonstrated that CD4+ T cells are critically important in the prevention of disease following vaginal infection of mice with HSV-1 (29). It has been suggested that CD4+ T cells may be an important factor in the control of HSV infection in humans. IFN-γ, which may be supplied by CD4+ T cells, has been shown to reverse the effects of the HSV immediate early protein ICP47, which interferes with the loading of MHC-I molecules with peptides (3, 62). Further, while CD8+ T cells are classically thought to be the cell type responsible for cytolytic effects, there is evidence that cytolytic mechanisms may also be employed by CD4+ T cells in control of various viral infections, including human immunodeficiency virus and hepatitis C (1, 2). An important role for cytolytic CD4+ T cells in infections with HSV has also been suggested (12, 28, 42). Further, HSV-specific CD4+ T cells have been isolated from HSV lesions of humans (26, 28) and the genital epithelium of mice (40), suggesting that they play a role in the clearance of HSV from the genital tract.
HSV is also a neural pathogen, and its replication in immune-competent individuals following primary infection is normally controlled. Simmons and Tscharke presented evidence for CD8+ T-cell-mediated clearance of HSV-1 from thoracic dorsal root ganglia in the murine zosteriform model as mice selectively depleted of CD8+ T cells demonstrated increased neuronal destruction and virus spread (54). In the present study, the increased presence of activated, HSV-specific CD4+ T cells in the spinal cords and dorsal root ganglia of mice in primary HSV-1 infection is clearly demonstrated (Fig. 2, 3, and 4). CD4+ CD25+ T-regulatory cells have previously been shown to be important participants in the T-cell response to HSV (60). While the CD4+ CD25+ T cells noted in the spinal cords and dorsal root ganglia of HSV-1-infected mice in the present study could include some T-regulatory cells, the results of this study demonstrate CD4+ effector T-cell activity (Fig. 4). Further, CD4+ T cells were sufficient for clearance of infectious HSV-1 from the genital tract, as well as possessing the ability to clear infectious virus from the spinal cords and dorsal root ganglia of these mice (Fig. 6). Our data align with one study of ocular HSV-1 infection, in which clearance of infectious HSV-1 from the trigeminal ganglia was apparently achieved by either the CD4+ or the CD8+ T-cell population (16). Although either subset appears sufficient for clearance of infectious HSV, it is important to emphasize that both the CD4+ and the CD8+ T-cell populations are likely to function in clearance in an intact animal. The results from the present study are consistent with this idea in that although CD8-depleted B6 mice cleared virus from sensory ganglia, they did so more slowly than B6 mice containing both CD4+ and CD8+ T-cell subsets (Fig. 6). It is important to note that the ability to detect virus clearance in these studies was limited by the efficiency of plaque formation on Vero cell monolayers. Although some virus may have been present in genital or neural samples beneath our level of detection, the use of this assay nonetheless allowed a quantification of infectious virus for comparison among groups in the context of the inherent limitations of the sensitivity of the assay.
In the genital model of HSV-2 infection, the requirement for either perforin or Fas/FasL cytolytic mechanisms in CD8+ T-cell-mediated clearance from the genital epithelium has been previously demonstrated (11). Perforin is important for CTL-mediated defense against invading pathogens and is thought to have a role in homeostasis, as suggested by the fatal disregulation of the immune system associated with a homozygous deficiency in perforin (36). CTLs can also initiate cell death via a Fas-FasL interaction. FasL on the surface of the CTL interacts with Fas expressed on the target cell, where target cell recognition results in the initiation of cell death pathways (17). In several other viral diseases affecting the central nervous system (CNS), including lymphocytic choriomeningitits, Theiler's, and West Nile viruses, the absence of perforin or Fas/FasL pathways has been correlated with loss of control of the virus and persistence of the virus in the CNS (21, 44, 51, 53). Granule exocytosis is thought to be the main cytotoxic pathway utilized by HSV-specific CTLs. Evidence for the importance of these mechanisms is bolstered by the fact that HSV encodes genes that prevent cytolysis via the Fas/FasL pathway as well as the function of granzyme B (19, 69).
In studies of latently HSV-1-infected human and murine trigeminal ganglia, it was noted that virus-specific CD8+ T cells interacting with the neurons appeared to be capable of cytolysis as they expressed granzyme B and perforin, though the amount of neuronal damage in these tissues was reported as “limited” (59, 65). In the current study, CD4+ T cells within the HSV-1-infected spinal cord and sensory ganglia expressed granzyme B and thus appeared to possess the components necessary for a lytic T cell (Fig. 5). However, through two different experimental designs (specific antibody depletion and radiation chimeras), neither perforin nor Fas-FasL interactions were an absolute requirement for the CD4+ T-cell-mediated clearance of infectious HSV-1 from neural tissues (Fig. 7, 8, and 9). These data do not exclude some role for perforin or Fas-FasL interactions as complete clearance of infectious HSV-1 was not always seen in all animals tested.
In addition to Fas-FasL interactions and granule exocytosis, a CTL can initiate death in a target cell via the release of TNF-α. The release of TNF-α results in the production of caspase-8 and initiation of a caspase cascade that involves the recruitment of TNF receptor 1 (TNF-R1) (a death receptor) and Fas, thus leading to apoptosis of the target cell (25). Thus, in considering the elimination of infectious HSV from neural tissues, TNF-α-mediated apoptosis may prove important. Lundberg et al. previously reported an important role for p55 (TNF-R1) in limiting HSV-1 replication in the eye, ganglia, and brainstem (35). However, an increase in the concentration of TNF-α may result in pathology from destruction of neural tissues in neurodegenerative disorders as well as in response to pathogens (25). Interestingly, in the present study utilizing the genital model of HSV-1 infection in mice, a definitive role for TNF-α in controlling HSV-1 replication in the genital tract, spinal cord, or sensory ganglia using TNF-R1-deficient mice was not demonstrated (data not shown). This is consistent with the observation that relatively few TNF-α-secreting CD4+ T cells infiltrated the HSV-infected dorsal root ganglia and spinal cords in our experiments (Fig. 4).
One noncytolytic mechanism that may be employed by HSV-specific T cells to control virus replication in neural tissues is the release of the cytokine IFN-γ. It has been shown that CD8+ T cells can control HSV infection in neural tissues, as well as prevent reactivation, an effect that may be due to the production and action of IFN-γ (8, 19). IFN-γ directly inhibits virus replication, can upregulate MHC-I and MHC-II expression, and could potentially be involved in recruitment of additional immune cells, such as macrophages, to the site of infection (62). Evidence for IFN-γ-mediated clearance of infectious HSV from neural tissues was previously reported in a study by Lewandowski et al., in which a 10-fold increase in intracerebral IFN-γ correlated with a 5,000-fold increase in the 50% lethal dose, as well as a rapid decrease in HSV titer in neural tissues (30). Thus, the CD4+ T cells found in the HSV-1-infected neural tissues in our experiments may employ IFN-γ in clearance of infectious virus. This is an attractive idea in that a nonlytic mechanism may be preferable in neural tissues that cannot be regenerated. In the trigeminal ganglia, the inflammatory cell infiltrate has been shown to consist largely of CD8+ T cells and a relatively small number of CD4+ T cells, accompanied by both Th1 and Th2 cytokines but in the absence of neuronal destruction (32, 61). Liu et al. hypothesize that the IFN-γ and TNF-α produced in the infected trigeminal ganglia function to control virus replication while the IL-4 and IL-10 found in these tissues limit the infiltration of polymorphonuclear leukocytes and thus destruction of neural tissues (32). This hypothesis is intriguing and is consistent with the IFN-γ- and IL-4-producing CD4+ T cells detected in neural tissues in the current experiments (Fig. 4D). The large number of IL-4-producing cells detected in HSV-infected neural tissues is somewhat surprising as HSV induces a dominant Th1-type response in the draining lymph nodes (40). The reason for this prominent IL-4 response is unclear at present although it should be pointed out that IL-4-secreting cells are commonly detected in secondary lymph nodes and genital tracts, despite the presence of a dominant IFN-γ response (40; also A. J. Johnson, M. D. Bird, M. H. Nelson, C.-F. Chu, and G. N. Milligan, unpublished data).
In the present study, the long-term presence of HSV-specific CD4+ T cells in spinal cord and dorsal root ganglia of mice following HSV-1 inoculation was demonstrated (Fig. 4). It has been previously shown that HSV-specific CD8+ T cells are retained in HSV-infected trigeminal ganglia (33). It has been proposed that the presence of T cells in these neural sites may limit the spread of the virus to additional uninfected neurons, reduce the number of reactivation events, secrete neurotrophic factors, or simply be an effect of repeated reactivation events (18). The mechanism by which these adaptive immune cells are maintained in neural tissues remains to be defined. In murine trigeminal ganglia infected with HSV-1, it has been shown that rare neurons (approximately one neuron every 10 days) within the latently infected ganglia do express productive cycle genes and that these rare neurons appear to be responsible for a local immune response involving the infiltration of inflammatory cells (14). Thus, the maintenance of a T-cell population within HSV-infected neural tissues, as was seen in the current study, may be in response to sporadic translation of viral message during “latent” infection.
Candidate vaccines against HSV have not been completely protective in all groups, suggesting that new strategies should be pursued. In designing an effective vaccine against HSV, it may be useful to examine and consider the various responses of the immune system to natural infection. Our data illustrate the important role of CD4+ T cells in clearance of infectious virus from the genital tract, dorsal root ganglia, and spinal cord during primary infection. In addition to playing an effector role in clearance of HSV from genital and neural sites, CD4+ T cells may also support maintenance, activation, and expansion of other adaptive immune cells at peripheral sites as has been shown in other viral infection models. CD4+ T cells in association with either CD8+ T cells or B cells are required for rapid clearance of measles virus from the CNS (63). A role for CD4+ T cells in supporting protective antibody within the CNS has been shown in studies of West Nile virus (55). In studies of infection with Sindbis virus and mouse hepatitis virus, antibody has been shown to be critical for the prevention of virus reactivation within the CNS (7, 49). In our HSV model, we noted greatly diminished HSV-specific B-cell and CD8+ T-cell responses following inoculation of CD4+ T-cell-depleted mice (data not shown). CD4+ T cells are also known to support activation of sensory ganglia-resident, memory CD8+ T cells (66). Such CD4+ T-cell responses at neural sites of HSV infection might therefore be beneficial to the immunized individual by limiting HSV infection of the sensory ganglia. This, in turn, might result in limitation in the amount of virus establishing latency and reduction in recurrent lesions at the original site of infection, thus impacting the transmission of virus to new hosts, including neonates. The results of the present study demonstrate that HSV-specific CD4+ T cells are recruited to HSV-infected sensory ganglia, where they are maintained long after viral infection, along with HSV-specific CD8+ T cells (32) and plasma cells (43). Our findings further demonstrate an important role for these CD4+ T cells in protection of the sensory ganglia and spinal cord and suggest that an effective HSV vaccine may need to elicit immune responses at both the site of epithelial infection and in the neural tissues.
Acknowledgments
We thank Janice J. Endsley for critical reading of the manuscript, Bradford D. Loucas for assistance with irradiation, and Mark Griffin of the University of Texas Medical Branch Flow Cytometry Core Facility for assistance with flow cytometry.
This work was supported by research grants AI42815 and AI054444 from the National Institutes of Health. A. J. Johnson was supported by a predoctoral fellowship from The Vale-Asche Foundation Fellowships in Infectious Disease Research.
The authors have no conflicting financial interests.
Footnotes
Published ahead of print on 30 July 2008.
REFERENCES
- 1.Appay, V., J. J. Zaunders, L. Papagno, J. Sutton, A. Jaramillo, A. Waters, P. Easterbrook, P. Grey, D. Smith, A. J. McMichael, D. A. Cooper, S. L. Rowland-Jones, and A. D. Kelleher. 2002. Characterization of CD4+ CTLs ex vivo. J. Immunol. 1685954-5958. [DOI] [PubMed] [Google Scholar]
- 2.Aslan, N., C. Yurdaydin, J. Wiegand, T. Greten, A. Ciner, M. F. Meyer, H. Heiken, B. Kuhlmann, T. Kaiser, H. Bozkaya, H. L. Tillman, A. M. Bozdayi, and M. P. Manns. 2006. Cytotoxic CD4+ T cells in viral hepatitis. J. Viral Hepat. 13505-514. [DOI] [PubMed] [Google Scholar]
- 3.Aurelian, L. 2004. Herpes simplex virus type 2 vaccines: new ground for optimism? Clin. Diagn. Lab. Immunol. 11437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnden, M. J., J. Allison, W. R. Heath, and F. R. Carbone. 1998. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 7634-40. [DOI] [PubMed] [Google Scholar]
- 5.BenMohamed, L., G. Bertrand, C. D. McNamara, H. Gras-Masse, J. Hammer, S. T. Wechsler, and A. B. Nesburn. 2003. Identification of novel immunodominant CD4+ Th1-type T-cell peptide epitopes from herpes simplex virus glycoprotein D that confer protective immunity. J. Virol. 779463-9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein, D. I., F. Y. Aoki, S. K. Tyring, L. R. Stanberry, C. St.-Pierre, S. D. Shafran, G. Leroux-Roels, K. Van Herck, A. Bollaerts, G. Dubin, and the GlaxoSmithKline Herpes Vaccine Study Group. 2005. Safety and immunogenicity of glycoprotein D-adjuvant genital herpes vaccine. Clin. Infect. Dis. 401271-1281. [DOI] [PubMed] [Google Scholar]
- 7.Burdeinick-Kerr, R., J. Wind, and D. E. Griffin. 2007. Synergistic roles of antibody and interferon in noncytolytic clearance of Sindbis virus from different regions of the central nervous system. J. Virol. 815628-5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantin, E. M., D. R. Hinton, J. Chen, and H. Openshaw. 1995. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J. Virol. 694898-4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corey, L. 2000. Herpes simplex virus, p. 1762-1780. In G. L. Mandell, G. R. Douglas, J. E. Bennett, and R. Dolan (ed.), Principles and practice of infectious diseases, 5th ed. Churchill Livingstone, Philadelphia, PA.
- 10.Deshpande, S. P., U. Kumaraguru, and B. T. Rouse. 2000. Why do we lack an effective vaccine against herpes simplex virus infections? Microbes Infect. 2973-978. [DOI] [PubMed] [Google Scholar]
- 11.Dobbs, M. E., J. E. Strasser, C.-F. Chu, C. Chalk, and G. N. Milligan. 2005. Clearance of herpes simplex virus type 2 by CD8+ T cells requires gamma interferon and either perforin- or Fas-mediated cytolytic mechanisms. J. Virol. 7914546-14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doymaz, M. Z., C. M. Foster, D. Destephano, and B. T. Rouse. 1991. MHC II-restricted, CD4+ cytotoxic T lymphocytes specific for herpes simplex virus-1: implications for the development of herpetic keratitis in mice. Clin. Immunol. Immunopathol. 61398-409. [DOI] [PubMed] [Google Scholar]
- 13.Dudley, K. L., N. Bourne, and G. N. Milligan. 2000. Immune protection against HSV-2 in B-cell-deficient mice. Virology 270454-463. [DOI] [PubMed] [Google Scholar]
- 14.Feldman, L. T., A. R. Ellison, C. C. Voytek, L. Yang, P. Krause, and T. P. Margolis. 2002. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc. Natl. Acad. Sci. USA 99978-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gebhardt, B. M., and W. P. Halford. 2005. Evidence that spontaneous reactivation of herpes virus does not occur in mice. Virol. J. 267-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghiasi, H., G.-C. Perng, A. B. Nesburn, and S. L. Wechsler. 1999. Either a CD4+ or CD8+ T cell function is sufficient for clearance of infectious virus from trigeminal ganglia and establishment of herpes simplex virus type 1 latency in mice. Microb. Pathog. 27387-394. [DOI] [PubMed] [Google Scholar]
- 17.Hanabuchi, S., M. Koyanagi, A. Kawasaki, N. Shinohara, A. Matsuzawa, Y. Nishimura, Y. Kobayashi, S. Yonehara, H. Yagita, and K. Okumura. 1994. Fas and its ligand in a general mechanism of T-cell-mediated cytotoxicity. Proc. Natl. Acad. Sci. USA 914930-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hüfner, K., T. Derfuss, S. Herberger, K. Sunami, S. Russell, I. Sinicina, V. Arbusow, M. Strupp, T. Brandt, and D. Theil. 2006. Latency of α-herpes viruses is accompanied by a chronic inflammation in human trigeminal ganglia but not dorsal root ganglia. J. Neuropathol. Exp. Neurol. 651022-1030. [DOI] [PubMed] [Google Scholar]
- 19.Jerome, K. R., Z. Chen, R. Lang, M. R. Torres, J. Hofmeister, S. Smith, R. Fox, C. J. Froelich, and L. Corey. 2001. HSV and glycoprotein J. inhibit caspase activation and apoptosis induced by granzyme B or Fas. J. Immunol. 1673928-3935. [DOI] [PubMed] [Google Scholar]
- 20.Jones, C. A., and A. L. Cunningham. 2004. Vaccination strategies to prevent genital herpes and neonatal herpes simplex virus (HSV) disease. Herpes 1112-17. [PubMed] [Google Scholar]
- 21.Kagi, D., F. Vignaux, B. Lederman, K. Burki, V. Depraetere, S. Nagata, H. Hengartner, and P. Golstein. 1994. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science 256528-530. [DOI] [PubMed] [Google Scholar]
- 22.Kaushic, C., A. A. Ashkar, L. A. Reid, and K. L. Rosenthal. 2003. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J. Virol. 774558-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khanna, K. M., A. J. Lepisto, and R. L. Hendricks. 2004. Immunity to latent viral infection: many skirmishes but few fatalities. Trends Immunol. 25230-234. [DOI] [PubMed] [Google Scholar]
- 24.Khanna, K. M., A. J. Lepsito, V. Decman, and R. L. Hendricks. 2004. Immune control of herpes simplex virus during latency. Curr. Opin. Immunol. 16463-469. [DOI] [PubMed] [Google Scholar]
- 25.Kogo, J., Y. Takeba, T. Kumai, Y. Kitaoka, N. Matsumoto, S. Ueno, and S. Kobayashi. 2006. Involvement of TNF-α in glutamate-induced apoptosis in a differentiated neuronal cell line. Brain Res. 1122201-208. [DOI] [PubMed] [Google Scholar]
- 26.Koelle, D. M., H. Abbo, A. Peck, K. Ziegweid, and L. Corey. 1994. Direct recovery of HSV-specific T lymphocyte clones from human recurrent HSV-2 lesions. J. Infect. Dis. 169956-961. [DOI] [PubMed] [Google Scholar]
- 27.Koelle, D. M., L. Corey, R. L. Burke, R. J. Eisenberg, G. H. Cohen, R. Pichyangkura, and S. J. Triezenberg. 1994. Antigenic specificity of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J. Virol. 682803-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koelle, D. M., C. M. Posavad, G. R. Barnum, M. L. Johnson, J. M. Frank, and L. Corey. 1998. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J. Clin. Investig. 1011500-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuklin, N. A., M. Daheshia, S. Chun, and B. T. Rouse. 1998. Role of mucosal immunity in herpes simplex virus infection. J. Immunol. 1605998-6003. [PubMed] [Google Scholar]
- 30.Lewandowski, G., M. Hobbs, and A. Geller. 1998. Evidence that IFN-γ production is a biological basis of herpes simplex virus type-2 neurovirulence. J. Neuroimmunol. 8166-75. [DOI] [PubMed] [Google Scholar]
- 31.Linehan, M. M., S. Richman, C. Krummenacher, R. J. Eisenberg, G. H. Cohen, and A. Iwasaki. 2004. In vivo role of nectin-1 in entry of herpes simplex virus type 1 (HSV-1) and HSV-2 through the vaginal mucosa. J. Virol. 782530-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu, T., Q. Tang, and R. L. Hendricks. 1996. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J. Virol. 70264-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu, T., K. M. Khanna, X.-P. Chen, D. J. Fink, and R. L. Hendricks. 2000. CD8+ T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J. Exp. Med. 1911459-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowhagen, G. B., P. Tunback, K. Andersson, T. Bergstrom, and G. Johannisson. 2000. First episodes of genital herpes in a Swedish STD population: a study of epidemiology and transmission by the use of herpes simplex virus (HSV) typing and specific serology. Sex. Transm. Infect. 76179-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundberg, P., P. V. Welander, C. K. Edwards III, N. van Rooijen, and E. Cantin. 2007. Tumor necrosis factor (TNF) protects resistant C57BL/6 mice against herpes simplex virus-induced encephalitis independently of signaling via TNF receptor 1 or 2. J. Virol. 811451-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maher, K. J., N. G. Klimas, B. Hurwitz, R. Schiff, and M. A. Fletcher. 2002. Quantitative fluorescence measures for determination of intracellular perforin content. Clin. Diagn. Lab. Immunol. 91248-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marracci, G. H., R. E. Jones, G. P. McKeon, and D. N. Bourdette. 2002. Alpha lipoic acid inhibits T cell migration into the spinal cord and suppresses and treats experimental autoimmune encephalomyelitis. J. Neuroimmol. 131104-114. [DOI] [PubMed] [Google Scholar]
- 38.McDermott, M. R., J. R. Smiley, P. Leslie, J. Brais, H. E. Rudzroga, and J. Bienenstock. 1984. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J. Virol. 51747-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milligan, G. N. 1999. Neutrophils aid in protection of the vaginal mucosae of immune mice against challenge with herpes simplex virus type 2. J. Virol. 736380-6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milligan, G. N., and D. I. Bernstein. 1995. Analysis of herpes simplex virus-specific cells in the murine female genital tract following genital infection with herpes simplex virus type 2. Virology 212481-489. [DOI] [PubMed] [Google Scholar]
- 41.Milligan, G. N., K. L. Dudley-McClain, C.-F. Chu, and C. G. Young. 2004. Efficacy of genital T cell responses to herpes simplex virus type 2 resulting from immunization of the nasal mucosa. Virology 318507-515. [DOI] [PubMed] [Google Scholar]
- 42.Milligan, G. N., K. L. Dudley-McClain, C. G. Young, and C.-F. Chu. 2004. T-cell-mediated mechanisms involved in resolution of genital herpes simplex virus type 2 (HSV-2) infection in mice. J. Reprod. Immunol. 61115-127. [DOI] [PubMed] [Google Scholar]
- 43.Milligan, G. N., M. G. Meador, C.-F. Chu, C. G. Young, T. L. Martin, and N. Bourne. 2005. Long-term presence of virus-specific plasma cells in sensory ganglia and spinal cord following intravaginal inoculation of herpes simplex virus type 2. J. Virol. 7911537-11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murray, P. D., D. B. McGavern, X. Lin, M. K. Njenga, J. Leibowitz, L. R. Pease, and M. Rodriguez. 1998. Perforin-dependent neurologic injury in a viral model of multiple sclerosis. J. Neurosci. 187306-7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niemialtowski, M. G., V. L. Godfrey, and B. T. Rouse. 1994. Quantitative studies on CD4+ and CD8+ cytotoxic T lymphocyte responses against herpes simplex virus type 1 in normal and beta 2-m deficient mice. Immunobiology 190183-194. [DOI] [PubMed] [Google Scholar]
- 46.Nilsen, A., and H. Myremel. 2000. Changing trends in genital herpes simplex virus infection in Bergen, Norway. Acta Obstet. Gynecol. Scand. 79693-696. [PubMed] [Google Scholar]
- 47.Parr, M. B., and E. L. Parr. 1998. Mucosal immunity to herpes simplex virus type 2 infection in the mouse vagina is impaired by in vivo depletion of T lymphocytes. J. Virol. 722677-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Posavad, C. M., D. M. Koelle, and L. Corey. 1996. High frequency of CD8+ cytotoxic T-lymphocyte precursors specific for herpes simplex viruses in persons with genital herpes. J. Virol. 708165-8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramakrishna, C., S. A. Stohlman, R. D. Atkinson, M. J. Schlomchik, and C. C. Bergmann. 2002. Mechanisms of central nervous system viral persistence: the critical role of antibody and B cells. J. Immunol. 1681204-1211. [DOI] [PubMed] [Google Scholar]
- 50.Roberts, C. M., J. R. Pfister, and S. J. Spear. 2003. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex. Transm. Dis. 30797-800. [DOI] [PubMed] [Google Scholar]
- 51.Rossi, C. P., A. McAllister, M. Tanguy, D. Kagi, and M. Brahic. 1998. Theiler's virus infection of perforin-deficient mice. J. Virol. 724515-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scoular, A., J. Norrie, G. Gillespie, N. Mir, and W. F. Carman. 2002. Longitudinal study of genital infection by herpes simplex virus type 1 in Western Scotland over 15 years. BMJ 3241366-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shrestha, B., and M. S. Diamond. 2007. Fas ligand interactions contribute to CD8+ T cell-mediated control of West Nile virus infection in the central nervous system. J. Virol. 8111749-11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simmons, A., and D. C. Tscharke. 1992. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J. Exp. Med. 1751337-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sitati, E. M., and M. S. Diamond. 2006. CD4+ T-cell responses are required for clearance of West Nile virus from the central nervous system. J. Virol. 8012060-12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stanberry, L. R., D. M. Jorgensen, and A. J. Nahmias. 1997. Herpes simplex viruses 1 and 2, p. 419-454. In A. S. Evans and R. Kaslow (ed.), Viral infections of humans: epidemiology and control, 4th ed. Plenum Publishers, New York, NY.
- 57.Stanberry, L. R., S. L. Spruance, A. L. Cunningham, D. I. Bernstein, A. Mindel, S. Sacks, S. Tyring, F. Y. Aoki, M. Slaoui, M. Denis, P. Vandepapeliere, and G. Dubin. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 3471652-1661. [DOI] [PubMed] [Google Scholar]
- 58.Stevens, J. G. 1975. Latent herpes simplex virus and the nervous system. Curr. Top. Immunol. 7031-50. [DOI] [PubMed] [Google Scholar]
- 59.Suvas, S., A. K. Azkur, and B. T. Rouse. 2006. Qa-1b and CD94-NKG1a interaction regulate cytolytic activity of herpes simplex virus-specific memory CD8+ T cells in the latently infected trigeminal ganglia. J. Immunol. 1761703-1711. [DOI] [PubMed] [Google Scholar]
- 60.Suvas, S., U. Kumaraguru, C. D. Pack, S. Lee, and B. T. Rouse. 2003. CD4+ CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J. Exp. Med. 198889-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Theil, D., T. Derfuss, I. Paripovic, S. Herberger, E. Meinl, O. Schueler, M. Strupp, V. Arbusow, and T. Brandt. 2003. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am. J. Pathol. 1632179-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tigges, M. A., S. Leng, D. C. Johnson, and R. L. Burke. 1996. Human herpes simplex virus (HSV)-specific CD8+ CTL clones recognize HSV-2-infected fibroblasts after treatment with IFN-γ or when virion host shutoff functions are disabled. J. Immunol. 1563901-3910. [PubMed] [Google Scholar]
- 63.Tishon, A., H. Lewicki, A. Andaya, D. McGavern, L. Martin, and M. B. A. Oldstone. 2006. CD4 T cell control primary measles virus infection of the CNS: regulation is dependent on combined activity with either CD8 T cells or with B cells: CD4, CD8 or B cells alone are ineffective. Virology 347234-245. [DOI] [PubMed] [Google Scholar]
- 64.Tran, T., J. D. Druce, M. C. Catton, H. Kelly, and C. J. Birch. 2004. Changing epidemiology of genital herpes simplex virus infection in Melbourne, Australia, between 1980 and 2003. Sex. Transm. Infect. 80277-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verjans, G. M., R. Q. Hintzen, J. M. van Dun, A. Poot., J. C. Milikan, J. D. Laman, A. W. Langerak, P. R. Kinchington, and A. D. M. E. Osterhaus. 2007. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc. Natl. Acad. Sci. USA 1043496-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wakim, L. M., J. Waithman, N. van Rooijen, W. R. Heath and Carbone, F. R. 2008. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science 319198-202. [DOI] [PubMed] [Google Scholar]
- 67.Xu, F., M. R. Sternberg, B. J. Kottiri, G. M. McQuillan, F. K. Lee, A. J. Nahmias, S. M. Berman, and L. E. Markowitz. 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296864.973. [DOI] [PubMed] [Google Scholar]
- 68.Yap, K. L., and G. L. Ada. 1978. Cytotoxic T cells in the lungs of mice infected with influenza A virus. Scand. J. Immunol. 773-80. [DOI] [PubMed] [Google Scholar]
- 69.Yasukawa, M., H. Ohminami, J. Arai, Y. Kasahara, Y. Ishida, and S. Fujita. 2000. Granule exocytosis, and not the Fas/Fas ligand system, is the main pathway of cytotoxicity mediated by alloantigen-specific CD4+ as well as CD8+ cytotoxic T lymphocytes in humans. Blood 952352-2355. [PubMed] [Google Scholar]