Abstract
Peripheral blood T-cell responses are used as biomarkers in hepatitis C virus (HCV) vaccine trials. However, it is not clear how T-cell responses in the blood correlate with those in the liver, the infection site. By studying serial liver and blood samples of five vaccinated and five mock-vaccinated control chimpanzees during acute HCV infection, we demonstrate a correlation between HCV-specific CD8 T-cell responses in the blood and molecular and functional markers of T-cell responses in the liver. Thus, HCV-specific CD8 T-cell responses in the blood are valid markers for intrahepatic T-cell activity.
Hepatitis C virus (HCV) infection is a leading cause of chronic liver disease. HCV infection is associated with an increased risk of liver cirrhosis and hepatocellular carcinoma and has become the main reason for liver transplantation in Western countries (2). Spontaneous and vaccine-induced resolution of acute HCV infection depends on vigorous and sustained HCV-specific T-cell responses (5, 10, 14, 16, 18, 20). Because of progress in the development of candidate vaccines (6, 7, 15), the need for standardized monitoring of vaccine-induced T-cell responses is increasing (13).
CD8 T cells are thought to be the main effector cells in acute HCV infection (5, 10, 14, 20). The appearance of HCV-specific T cells in the liver is temporally related to the decrease in HCV RNA titer (6, 7, 15), and T-cell-mediated immune protection has been demonstrated in recovered chimpanzees upon rechallenge with homologous (3, 11, 16) and heterologous (6, 9) virus. However, it is not possible to prospectively monitor HCV-specific intrahepatic T-cell responses in humans because of clinical and ethical concerns. All published studies on the immune response of patients with acute HCV infection are therefore based on HCV-specific T-cell activity in the peripheral blood (5, 10, 14, 16, 20). Even in chimpanzee studies, investigation of intrahepatic T-cell responses is limited by the small size of liver biopsy samples, which necessitates several weeks of in vitro expansion of intrahepatic T cells prior to functional assays (8).
It is therefore not clear whether the results of peripheral blood T-cell analysis accurately reflect the onset and vigor of intrahepatic responses after vaccination. Studying vaccinated and nonvaccinated chimpanzees throughout the first 6 months of acute HCV infection, we here addressed this issue by comparing (i) the results of molecular ex vivo analysis of intrahepatic T-cell responses to those obtained by functional analysis of in vitro-expanded intrahepatic T cells and (ii) the results of molecular and functional analyses of T-cell responses in the liver to those of HCV-specific T-cell responses in the blood.
Ten chimpanzees were studied at biweekly intervals throughout the first 6 months of HCV genotype 1a infection. As previously described (6), five chimpanzees (95A005, 95A014, 97A005, 97A006, and 96A022) had been vaccinated with recombinant adenovirus and DNA vaccines encoding HCV nonstructural regions NS3 to NS5 prior to HCV infection and five control chimpanzees (97A009, 97A014, 98A005, 97A015, and 98A002) had been mock vaccinated prior to HCV infection (6). Detailed information on the vaccine regimen, the HCV inoculum, and the clinical course of the infection was previously reported (6).
To analyze T-cell responses in the liver with ex vivo techniques that do not require weeks of in vitro stimulation, total RNA isolated from the liver biopsy cDNA was synthesized and TaqMan real-time PCR was performed in duplicate to determine the mRNA levels of gamma interferon (IFN-γ), CD8β, and CD4 using primers and probes of TaqMan Gene Expression Assays (Applied Biosystems). The amount of specific mRNA was normalized to endogenous references (β-actin, glyceraldehyde-3-phosphate dehydrogenase, and proteasome β7 subunit mRNA) and presented as increase over baseline, which was the median value of preinfection samples of five mock-vaccinated chimpanzees.
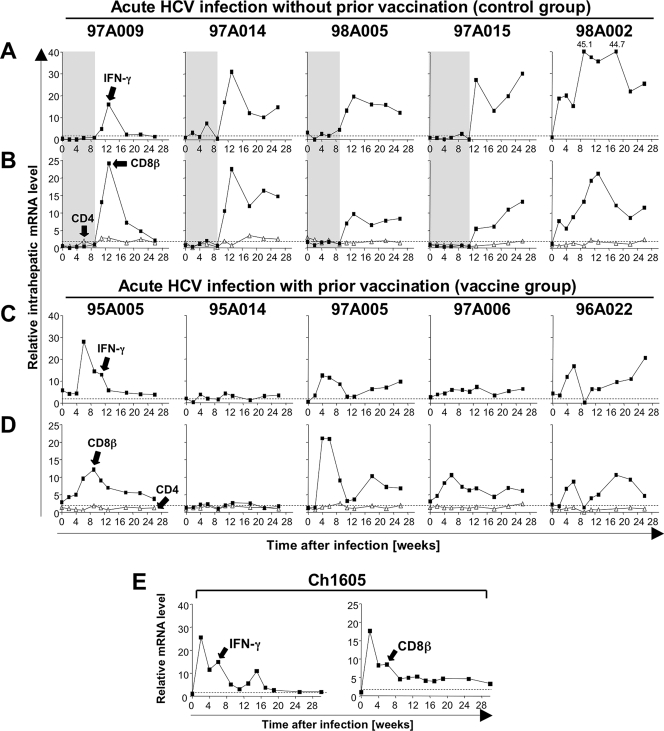
Intrahepatic CD8 and IFN-γ mRNA levels did not increase during the first 8 to 10 weeks of HCV infection in the mock-vaccinated control chimpanzees 97A009, 97A014, 98A005, and 97A015 (Fig. 1A and B). Both parameters were significantly increased 12 to 14 weeks after infection (Fig. 1A and B), concomitant with maximal alanine aminotransferase levels and a decline of HCV RNA titers in the blood (6). This pattern was consistent with published microarray studies (4, 17). Chimpanzee 98A002 was the only chimpanzee in this control group that exhibited a much earlier increase in intrahepatic CD8 and IFN-γ mRNA levels. The unusual CD8 and IFN-γ pattern of this chimpanzee may evidence potential prior priming of T cells, because it resembled the CD8 and IFN-γ mRNA kinetics of the previously recovered and rechallenged chimpanzee Ch1605 (Fig. 1E). Consistent with this notion, early CD8 and IFN-γ mRNA responses starting 4 to 6 weeks after HCV infection were also observed in the five vaccinated chimpanzees (Fig. 1C and D).
FIG. 1.
Intrahepatic IFN-γ, CD8β, and CD4 mRNA levels during acute HCV infection. Five mock-vaccinated control chimpanzees (A and B), five vaccinated chimpanzees (C and D), and one spontaneously recovered chimpanzee (E) were intravenously challenged with 100 50% chimpanzee infectious doses of HCV H77 (genotype 1a) (6, 11). Chimpanzees were housed at the New Iberia Research Center, LA, or the Food and Drug Administration, Bethesda, MD, and the studies were approved by the institutions' Animal Care and Use Committees. Serial liver biopsy samples were analyzed for IFN-γ (A, C, and left side of E), CD8β (filled squares in B, D, and right side of E), and CD4 (open triangles in B and D) mRNA levels by TaqMan real-time PCR. Each gene's mRNA level was normalized to endogenous references and expressed as an increase over baseline. Baseline mRNA levels represent the median value of preinfection samples of five mock-vaccinated chimpanzees. The horizontal dashed line represents the cutoff levels to define a positive response, defined as more than twofold over preinfection levels. Gray areas in panels A and B represent the period prior to substantial increases in intrahepatic IFN-γ and CD8β mRNA levels.
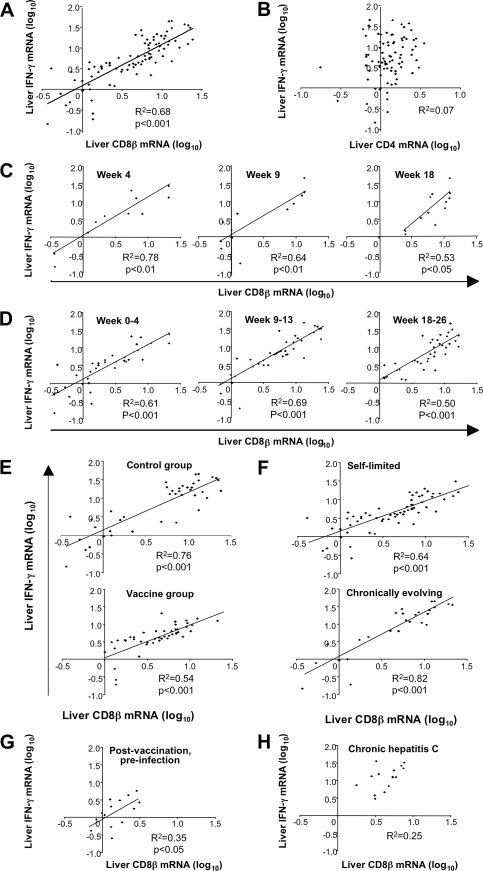
In contrast to CD8β mRNA levels, intrahepatic CD4 mRNA levels remained relatively stable throughout the first 6 months of HCV infection in all chimpanzees (Fig. 1B and D). These findings and the fact that CD8β is expressed only by T cells and not by NK cells (the latter express a CD8α/α homodimer [1, 12]) suggest that liver-infiltrating CD8 rather than CD4 T cells or NK cells contributed to IFN-γ production in the acutely HCV-infected liver. This is consistent with the observation that the increase in intrahepatic IFN-γ and CD8β mRNA levels coincides with the first appearance of HCV-specific, tetramer+ CD8 T cells in the blood during acute hepatitis C (E.-C. Shin and B. Rehermann, unpublished data). Indeed, intrahepatic CD8β and IFN-γ mRNA levels correlated significantly (Fig. 2A; R2 = 0.68, P < 0.001), whereas CD4 and IFN-γ mRNA levels did not (Fig. 2B). To avoid potential effects of a large number of repeated measurements during the whole phase of acute HCV infection, we studied the correlation between intrahepatic CD8β and IFN-γ mRNA levels for single time points (Fig. 2C) or for shorter time periods within the acute phase of hepatitis (Fig. 2D). In each analysis, the statistical significance of the correlation between intrahepatic CD8β and IFN-γ mRNA levels was preserved.
FIG. 2.
Correlation between intrahepatic CD8 and IFN-γ mRNA levels in acute HCV infection. Coefficients of determination (R2) and P values are shown. Only significant P values of less than 0.05 are indicated. Linear regression analysis and calculation of the coefficients of determination (R2) were performed with Microsoft Excel software. A P value of <0.05 was considered statistically significant. (A and B) Regression analysis for intrahepatic CD8β and IFN-γ mRNA levels (A) and for intrahepatic CD4 and IFN-γ mRNA levels (B) during the first 6 months of infection, using data from all 10 chimpanzees. (C and D) Regression analysis for intrahepatic CD8β and IFN-γ mRNA levels using data from individual time points (C) and short time periods during the time of infection (D). (E and F) Regression analysis for intrahepatic CD8β and IFN-γ mRNA levels for the subgroups of control chimpanzees with acute HCV infection (97A009, 97A014, 98A005, 97A015, and 98A002) and vaccinated chimpanzees with acute HCV infection (95A005, 95A014, 97A005, 97A006, and 96A022) (E) and chimpanzees with self-limited acute HCV infection (97A009, 97A014, 98A005, 95A005, 95A014, 97A005, and 97A006) and chimpanzees with chronically evolving acute HCV infection (97A015, 98A002, and 96A022) (F). (G) Regression analysis for intrahepatic CD8β and IFN-γ mRNA levels in vaccinated but not yet HCV-infected chimpanzees. (H) Regression analysis for intrahepatic CD8β and IFN-γ mRNA levels after 6 months of HCV infection (weeks 33 to 73) in chimpanzees 97A015, 98A002, and 96A022.
The correlation between intrahepatic CD8β and IFN-γ mRNA levels was also preserved when individual subgroups, e.g., control chimpanzees, vaccinated chimpanzees, chimpanzees with self-limited infection, and chimpanzees with chronically evolving infection were studied separately (Fig. 2E and F). Furthermore, the correlation between intrahepatic CD8β and IFN-γ mRNA levels was also observed for vaccinated but not yet infected chimpanzees (Fig. 2G), providing evidence of vaccine-induced changes in the intrahepatic immunological milieu. In contrast, no such correlation was evident in the chronic phase of HCV infection (Fig. 2H).
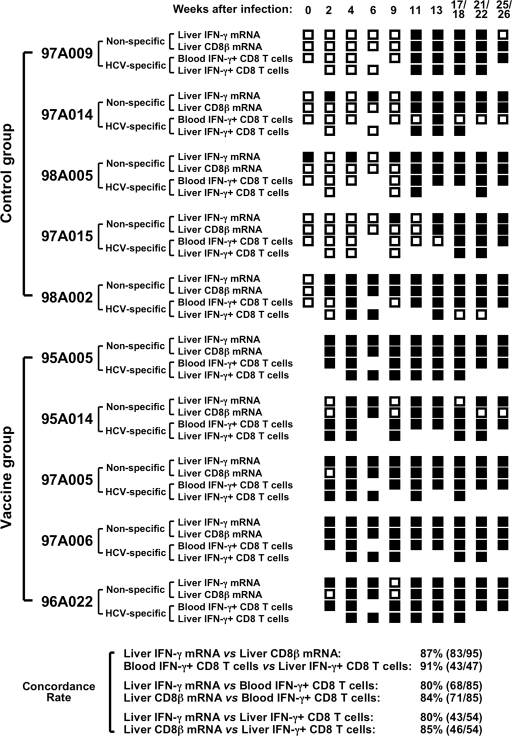
Next, the results of the molecular analysis of CD8 T-cell responses in the liver were correlated with the results of a functional analysis of HCV-specific CD8 T-cell responses in the liver and the blood. Specifically, intrahepatic IFN-γ and CD8β mRNA levels were compared to previously published data on the frequency of IFN-γ-producing CD8 T cells in the liver (determined by intracellular cytokine staining of in vitro expanded intrahepatic CD8 T cells) and blood (determined by ex vivo intracellular cytokine staining in response to peptides covering the HCV nonstructural regions) (6). To define the onset of each of these responses, the presence or absence of each response was evaluated at each study time point. With the exception of chimpanzee 97A014, which displayed a positive HCV-specific CD8 T-cell response in the blood at a single time point, all chimpanzees tested positive at multiple time points throughout the course of acute HCV infection irrespective of which immune response parameter was analyzed. As shown in Fig. 3, the concordance rate between the results obtained with different immunological readouts was higher than 80%. Taken together, these results suggest that all examined immunological readouts are adequate to determine the onset of vaccine- or infection-induced CD8 T-cell responses.
FIG. 3.
Use of molecular (CD8β and IFN-γ mRNA levels) and functional (IFN-γ production) assays to assess the onset of HCV-specific T-cell responses in blood and liver. Intrahepatic IFN-γ and CD8β mRNA levels during acute HCV infection (defined as “non-specific” in the figure) were considered positive if they were more than twofold over preinfection levels. The frequency of HCV-specific, IFN-γ-producing CD8 T cells in blood and liver (defined as “HCV-specific” in the figure) was determined by intracellular cytokine staining using peptides overlapping the HCV nonstructural proteins as previously reported (6). HCV-specific IFN-γ responses of ex vivo-analyzed peripheral blood CD8 T cells were considered positive if greater than 100 HCV-specific IFN-γ+ CD3+ CD8+ cells/106 lymphocytes were detected (6). IFN-γ responses of in vitro-expanded intrahepatic CD8 T cells were considered positive if the response in the presence of overlapping HCV peptides was more than three times higher than the response in the absence of peptides (6). Positive responses are indicated by filled squares and negative responses are indicated by open squares for each individual chimpanzee and time point throughout the study. Concordance rates indicate the number of concordant immune response parameters/(number of total comparisons between two immune response parameters) × 100, where concordance means that two immune response parameters are either both positive or both negative at the same time point.
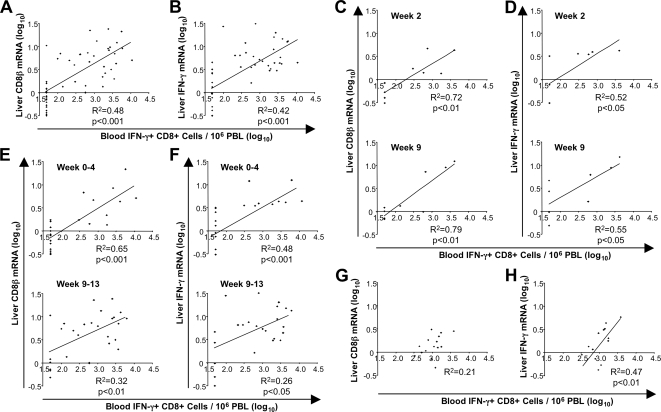
To analyze whether the strength of the HCV-specific CD8 T-cell response in the blood correlated with the strength of the CD8 T-cell response in the liver, regression analysis was performed. As shown in Fig. 4A and B, the frequency of HCV-specific, IFN-γ-producing CD8 T cells in the blood significantly correlated with the expression level of CD8β (R2 = 0.48, P < 0.001) (Fig. 4A) and IFN-γ (R2 = 0.42, P < 0.001) (Fig. 4B) mRNA in the liver. To avoid potential effects of a large number of repeated measurements during the whole phase of acute HCV infection, we studied the correlation between the two readouts for single time points (Fig. 4C and D) and for short time periods within the acute phase of hepatitis (Fig. 4E and F). In each analysis, the statistical significance of the correlation was preserved. Thus, the vigor of the HCV-specific CD8 T-cell activity in the blood correlated with the vigor of the total CD8 T-cell response in the liver during acute HCV infection. For vaccinated but not yet infected chimpanzees, HCV-specific CD8 T-cell responses correlated better with intrahepatic IFN-γ (Fig. 4H; R2 = 0.47, P < 0.01) than with CD8β mRNA levels (Fig. 4G).
FIG. 4.
Correlation between molecular assessment (CD8β and IFN-γ mRNA levels) of the total T-cell response in the liver and functional assessment (IFN-γ production) of HCV-specific T-cell responses in the blood. Coefficients of determination (R2) and P values are shown. Only significant P values of less than 0.05 are indicated. Linear regression analysis and calculation of the coefficients of determination (R2) were performed with Microsoft Excel software. A P value of <0.05 was considered statistically significant. (A and B) Regression analysis between intrahepatic CD8β (A) and intrahepatic IFN-γ (B) mRNA levels in the liver and the frequency of HCV-specific, IFN-γ-producing CD8 T cells in the blood during the first 6 months of the infection in all chimpanzees except chimpanzee 98A002, which displayed an atypically early intrahepatic immune response during infection suggestive of prior exposure. The correlation remained significant but less strong even if this chimpanzee was included in the analysis (R2 = 0.30, P < 0.001 for CD8β mRNA levels, and R2 = 0.20, P < 0.001 for IFN-γ mRNA levels). (C to F) Regression analysis for the same immune parameters as those in panels A and B using data from individual time points (C and D) and short time periods during the time of infection (E and F). (G and H) Regression analysis of intrahepatic CD8β mRNA levels (G) or intrahepatic IFN-γ mRNA levels (H) and the frequency of HCV-specific, IFN-γ-producing CD8 T cells in the blood of vaccinated chimpanzees 95A005, 95A014, 97A005, 97A006, and 96A022 prior to HCV infection. PBL, peripheral blood lymphocytes.
In summary, the present study demonstrates that the results of functional assays that monitor the onset and the vigor of HCV-specific CD8 T-cell activity in the blood correlate significantly with (i) the results of ex vivo molecular analysis of the total CD8 T-cell response in the liver, the site of HCV infection, and with (ii) the results of functional assays with HCV-specific T cells isolated from the liver. It is important to note that the use of large pools of overlapping peptides covering the nonstructural HCV polyprotein sequences likely increased the sensitivity in detecting HCV-specific CD8 T-cell responses in the blood because earlier studies with smaller sets of minimal optimal peptides did not detect HCV-specific CD8 T cells without in vitro expansion of peripheral blood mononuclear cells (19). In chimpanzee studies, where serial liver biopsy samples are available, quantitation of intrahepatic IFN-γ and CD8β mRNA levels may be the most feasible approach. In human studies, where liver biopsy samples cannot easily be obtained, quantification of IFN-γ-producing CD8 T cells in the blood may be the method of choice. Thus, HCV-specific CD8 T-cell activity in the blood may be monitored as a surrogate marker for intrahepatic CD8 T-cell responses in natural history studies and vaccine trials.
Acknowledgments
We thank M.-H. Sung (National Cancer Institute, NIH) for helpful advice on statistical analysis.
This work was supported in part by the NIDDK, NIH intramural research program.
Footnotes
Published ahead of print on 30 July 2008.
REFERENCES
- 1.Addison, E. G., J. North, I. Bakhsh, C. Marden, S. Haq, S. Al-Sarraj, R. Malayeri, R. G. Wickremasinghe, J. K. Davies, and M. W. Lowdell. 2005. Ligation of CD8alpha on human natural killer cells prevents activation-induced apoptosis and enhances cytolytic activity. Immunology 116354-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alter, H. J., and L. B. Seeff. 2000. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin. Liver Dis. 2017-35. [DOI] [PubMed] [Google Scholar]
- 3.Bassett, S. E., B. Guerra, K. Brasky, E. Miskovsky, M. Houghton, G. R. Klimpel, and R. E. Lanford. 2001. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology 331479-1487. [DOI] [PubMed] [Google Scholar]
- 4.Bigger, C. B., K. M. Brasky, and R. E. Lanford. 2001. DNA microarray analysis of chimpanzee liver during acute resolving hepatitis C virus infection. J. Virol. 757059-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diepolder, H. M., R. Zachoval, R. M. Hoffmann, A. Wierenga, T. Santantonio, M.-C. Jung, D. Eichenlaub, and G. R. Pape. 1995. Possible mechanism involving T lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet 3461006-1007. [DOI] [PubMed] [Google Scholar]
- 6.Folgori, A., S. Capone, L. Ruggeri, A. Meola, E. Sporeno, B. B. Ercole, M. Pezzanera, R. Tafi, M. Arcuri, E. Fattori, A. Lahm, A. Luzzago, A. Vitelli, S. Colloca, R. Cortese, and A. Nicosia. 2006. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat. Med. 12190-197. [DOI] [PubMed] [Google Scholar]
- 7.Houghton, M. 2000. Strategies and prospects for vaccination against the hepatitis C viruses. Curr. Top. Microbiol. Immunol. 242327-340. [DOI] [PubMed] [Google Scholar]
- 8.Koziel, M. J., D. Dudley, J. T. Wong, J. Dienstag, M. Houghton, R. Ralston, and B. D. Walker. 1992. Intrahepatic cytotoxic T lymphocytes specific for hepatitis C virus in persons with chronic hepatitis. J. Immunol. 1493339-3344. [PubMed] [Google Scholar]
- 9.Lanford, R. E., B. Guerra, D. Chavez, C. Bigger, K. M. Brasky, X. H. Wang, S. C. Ray, and D. L. Thomas. 2004. Cross-genotype immunity to hepatitis C virus. J. Virol. 781575-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 1911499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nascimbeni, M., E. Mizukoshi, M. Bosmann, M. Major, K. Mihalik, C. Rice, S. Feinstone, and B. Rehermann. 2003. Kinetics of CD4+ and CD8+ memory T-cell responses during hepatitis C virus rechallenge of previously recovered chimpanzees. J. Virol. 774781-4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parnes, J. R. 1989. Molecular biology and function of CD4 and CD8. Adv. Immunol. 44265-311. [DOI] [PubMed] [Google Scholar]
- 13.Rehermann, B., and N. V. Naoumov. 2007. Immunological techniques in viral hepatitis. J. Hepatol. 46508-520. [DOI] [PubMed] [Google Scholar]
- 14.Rehermann, B., and M. Nascimbeni. 2005. Immunology of hepatitis B virus and hepatitis C virus infection. Nat. Rev. Immunol. 5215-229. [DOI] [PubMed] [Google Scholar]
- 15.Shiina, M., and B. Rehermann. 2006. Hepatitis C vaccines: inducing and challenging memory T cells. Hepatology 431395-1398. [DOI] [PubMed] [Google Scholar]
- 16.Shoukry, N., A. Grakoui, M. Houghton, D. Chien, J. Ghrayeb, K. Reimann, and C. Walker. 2003. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J. Exp. Med. 1971645-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su, A. I., J. P. Pezacki, L. Wodicka, A. D. Brideau, L. Supekova, R. Thimme, S. Wieland, J. Bukh, R. H. Purcell, P. G. Schultz, and F. V. Chisari. 2002. Genomic analysis of the host response to hepatitis C virus infection. Proc. Natl. Acad. Sci. USA 9915669-15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takaki, A., M. Wiese, G. Maertens, E. Depla, U. Seifert, A. Liebetrau, J. L. Miller, M. P. Manns, and B. Rehermann. 2000. Cellular immune responses persist, humoral responses decrease two decades after recovery from a single source outbreak of hepatitis C. Nat. Med. 6578-582. [DOI] [PubMed] [Google Scholar]
- 19.Thimme, R., J. Bukh, H. C. Spangenberg, S. Wieland, J. Pemberton, C. Steiger, S. Govindarajan, R. H. Purcell, and F. V. Chisari. 2002. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc. Natl. Acad. Sci. USA 9915661-15668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thimme, R., D. Oldach, K. M. Chang, C. Steiger, S. C. Ray, and F. V. Chisari. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 1941395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]