Abstract
Although genomic technologies have advanced the characterization of gene regulatory networks downstream of transcription factors, the identification of pathways upstream of these transcription factors has been more challenging. In this study we present a gene signature-based approach for connecting signaling pathways to transcription factors, as exemplified by p73. We generated a p73 gene signature by integrating whole-genome chromatin immunoprecipitation and expression profiling. The p73 signature was linked to corresponding signatures produced by drug candidates, using the in silico Connectivity Map resource, to identify drugs that would induce p73 activity. Of the pharmaceutical agents identified, there was enrichment for direct or indirect inhibitors of mammalian Target of Rapamycin (mTOR) signaling. Treatment of both primary cells and cancer cell lines with rapamycin, metformin, and pyrvinium resulted in an increase in p73 levels, as did RNA interference-mediated knockdown of mTOR. Further, a subset of genes associated with insulin response or autophagy exhibited mTOR-mediated, p73-dependent expression. Thus, downstream gene signatures can be used to identify upstream regulators of transcription factor activity, and in doing so, we identified a new link between mTOR, p73, and p73-regulated genes associated with autophagy and metabolic pathways.
Mammalian transcription factors can bind to and regulate thousands of sites throughout the genome (19, 104, 106). The sheer complexities of these gene regulatory networks make conventional methods an unsuitable choice for comprehensive analysis. The advent of genomic technologies, in particular DNA microarray profiling and chromatin immunoprecipitation (ChIP) coupled with high-throughput sequencing (ChIPSeq) or ChIP-on-Chip, has led to the identification of target genes and transcriptional networks on a genome-wide scale (42, 57). These techniques have been used to characterize the gene regulatory networks of a growing number of transcription factors (42).
The integral role of p53 in tumor suppression has prompted many laboratories to perform extensive analyses of signaling pathways downstream of the p53 family of sequence-specific DNA binding transcription factors (p53 and its homologs p63 and p73). It is estimated that p53 has 1,600 binding sites in the human genome, only 22% of which are near promoters (19, 104). A more recent study has identified ∼5,800 binding sites for p63 (106). By using integrative genomic tools, hundreds of novel target genes have been identified for all three family members (37, 44, 77, 100, 104, 106). Similar analyses with many transcription factors have led to an explosion of genomic binding site and target gene expression data (42). These data sets hold great potential for much more than the characterization of downstream pathways; in particular we predict that they can be used to define the signaling pathways that reside upstream of transcription factors of interest.
Despite the ability of p73 to regulate many p53 family target genes, little is known about the specific pathways that modulate p73 during development, tumorigenesis, and tumor therapy (67). Unlike p53, which is mutated in more than 50% of human cancers, p73 is not mutated during tumorigenesis but instead can be overexpressed (16, 28, 29, 110, 111). There has been much interest in modulating p73, due to its high expression level in p53-deficient tumors and its ability to activate p53 target genes, leading to apoptosis of tumor cells (2, 9, 22, 36, 59, 68). Given the above, drug-inducible pathways upstream of p73 are of therapeutic interest.
We used gene expression signatures downstream of p73 to identify novel upstream regulators. A p73 gene signature was created using a combination of genomic tools and was queried against a database of drug-related profiles known as the Connectivity Map (48, 61, 105). Pattern-matching software (61) was used to identify potential p73-activating drugs. A link between p73 and mammalian Target of Rapamycin (mTOR), a kinase important in energy homeostasis and tumorigenesis, was identified and validated, demonstrating the utility of this approach to identify critical signaling nodes upstream of transcription factors.
MATERIALS AND METHODS
Cell culture and treatment.
The rhabdomyosarcoma cell line Rh30 was provided by P. Houghton (St. Jude Children's Research Hospital) and cultured in RPMI medium 1640 (Invitrogen, Carlsbad, CA). Human mammary epithelial cells (HMECs) were isolated and cultured as described previously (44, 78). MDA-MB-231 cells (American Type Culture Collection [ATCC], Manassas, VA) were cultured in McCoy's 5A medium (Invitrogen); MDA-MB-468 cells (ATCC) were cultured in 1:1 McCoy's 5A-Dulbecco's modified Eagle medium (Invitrogen); and 293T cells (ATCC), H1299 cells (ATCC), and HaCat cells (kindly provided by P. Boukamp, Deutsches Krebsforschungszentrum, Heidelberg, Germany) were cultured as previously described (7, 11).
Rapamycin (Calbiochem), metformin (Sigma), and pyrvinium (United States Pharmacopeial Convention) were used as described. For experiments involving rapamycin, cells were plated at 3 × 105 to 4 × 105 cells per 10-cm2 dish. (HMECs were plated at 5 × 105 cells per 10-cm2 dish.) After cells had completely attached, medium was changed 12 h prior to addition of drug (to avoid experimental variation due to the effect of medium replacement on mTOR [20]), or drug was added to serum-free medium as described. Medium without antibiotics was used for treatment.
For cell growth experiments, MDA-MB-231 cells were plated in triplicate and treated with short hairpin RNA (shRNA)-expressing lentivirus, as described below. After 2 days, cells were treated with 20 nM rapamycin or vehicle control and total cell numbers were determined at the indicated times.
The status of p63 in breast cancer cell lines was confirmed using adenoviral shRNA kindly provided by L. Ellisen (18).
Cell transfection/infection and shRNA.
The following sequences were used for small interfering RNA: p73-1, 5′-GCAATAATCTCTCGCAGTA-3′; p73-2, 5′-GAGACGAGGACACGTACTA-3′; green fluorescent protein (GFP), 5′-GAAGGTGATACCCTTGTTA-3′; mTOR (FRAP1), 5′-GCATTTACTGCTGCCTCCTAT-3′; p73β, 5′-TCAAGGAGGAGTTCACGGA-3′; and TAp73, 5′-GAACCAGACAGCACCTACT-3′. 293T cells were transfected using Fugene 6 (Roche, Indianapolis, IN). For knockdown of p73, the pSicoR lentivirus system was used (99). Production of virus and transduction were performed as described previously (82). For knockdown of mTOR (FRAP1), the pGIPZ system was used according to the manufacturer's protocol (OpenBiosystems, Huntsville, AL).
For microarray and ChIP assays, MDA-MB-231 cells were infected with adenovirus expressing hemagglutinin-TAp73β (pAdEasy-1:HA-TAp73β) or with a control adenovirus, and the cells were harvested after 80% transduction efficiency was reached, as monitored by GFP fluorescence. The recombinant adenoviruses were generated as previously described (43), pAdEasy was kindly provided by B. Vogelstein (Johns Hopkins University). Overexpression of multiple p73 isoforms was performed using the pcDNA3 backbone (kindly provided by C. Backendorf and G. Melino [24, 73]), and transfection was performed using Lipofectamine (Invitrogen, Carlsbad, CA) as previously described (78).
Protein lysate preparation and Western analysis.
Cells were trypsinized and lysed as previously described (92). Western analysis was done as previously described (35) with the following antibodies: p73 monoclonal antibodies IMG-246, IMG-259, and IMG-313 (Imgenex, San Diego, CA); p73 monoclonal antibody cocktail Ab-4 (Neomarkers, Fremont, CA); mdm2 monoclonal antibody SMP14, β-actin polyclonal antibody I-19, mTOR polyclonal antibody N-19 (α-FRAP), p63 monoclonal antibody 4A4, and p53 monoclonal antibody DO-1 (Santa Cruz Biotechnology, Santa Cruz, CA); glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody MAB374 (Chemicon, Temecula, CA); p21 monoclonal antibody Ab-1 (Calbiochem, San Diego, CA); phospho-4EBP1 Thr37/46 polyclonal antibody, phospho-S6 Ser235/236 polyclonal antibody 2F9, and total S6 monoclonal antibody 54D2 (Cell Signaling Technology, Danvers, MA); MAP1LC3B antibody (Abgent, San Diego, CA); and p73 antibody (Bethyl Laboratories, Montgomery, TX). p73 was immunoprecipitated for ChIP with Ab-4 or p73 antibody using conditions previously described (92). A Fluor-S Max MultiImager (Bio-Rad, Hercules, CA) was used to quantify Western signals.
Flow cytometry.
Flow cytometry was performed as described elsewhere (6, 12).
RNA isolation, microarray experiments, and statistical analyses.
Microarray experiments were performed in duplicate as follows: H1299 cells were infected with adenoviruses expressing GFP or TAp73β for 5 h, and RNA was isolated using the Aurum total RNA minikit (Bio-Rad, Richmond, CA) and submitted to the Vanderbilt-Ingram Cancer Center Microarray Shared Resource for quality control. The RNA was processed, and the microarray was hybridized as previously described (7). Microarray data analyses were performed using the ArrayAssist software platform (Stratagene, La Jolla, CA). Algorithms similar to those described elsewhere (7) were used to create a list of probes with changes in gene expression for p73-overexpressing samples versus GFP controls. The following software programs were used for statistical analyses, gene annotations, and determination of categorical enrichment as indicated: ArrayAssist (Stratagene), WebGestalt (Bioinformatics Resource Center at Vanderbilt University) (114), Ingenuity Pathway Analysis (Ingenuity Systems, Redwood City, CA), NCBI DAVID, and the Connectivity Map (61). KEGG and gene ontology analyses was accessed through WebGestalt, using statistical tests coupled to the WebGestalt interface (114).
Quantitative reverse transcription-PCR.
Total RNA was purified and reverse transcribed, and quantitative real-time PCR was performed as previously described (7); primer sequences are available upon request.
ChIP and ChIPSeq.
Formaldehyde cross-linking and chromatin preparation and immunoprecipitation (ChIP) were carried out as described previously (44, 90, 92). For ChIPSeq and semiquantitative ChIP experiments, cells were cross-linked and submitted to GenPathway, Inc. (San Diego, CA), according to their FactorPath protocol.
Microarray and ChIPSeq data set accession numbers.
All gene expression data from microarray experiments and genomic location information from the ChIPSeq experiment have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO series accession numbers GSE11626 and GSE11672.
RESULTS
Generation of transcription factor gene signatures for use in identifying upstream signaling pathways.
With an overall goal of developing a gene signature-based approach for connecting signaling pathways to transcription factors, we first sought to identify known pharmaceutical activators of transcription factor signaling by using publicly available expression profile data sets and the Connectivity Map resource. Our ultimate goal was to use this strategy to identify novel upstream regulators of p73. However, we thought it prudent to use well-defined transcription factors with known upstream signaling pathways to confirm that such pathways can be identified using the Connectivity Map within reasonable parameters. As a starting point for feasibility testing, we analyzed two publicly available expression profile data sets. In the first analysis, we made use of a list of genes up- or downregulated by zinc-inducible p53 (115). In the second, we generated a gene list from raw microarray data generated from NIH 3T3 cells in which peroxisome proliferator-activated receptor gamma (PPARγ) had been overexpressed (1). The lists of genes both induced and repressed by these transcription factors were queried against the Connectivity Map resource (61). In brief, this resource consists of pattern-matching software that compares an input gene signature to a database of signatures from 164 small-molecule bioactive compounds (dubbed perturbagens), 85 of which are classified as pharmaceutical drugs. A connectivity score from +1 to −1 is assigned based on the degree of similarity or dissimilarity between the two signatures (61). Thus, a drug with a high connectivity score has a gene signature very similar to the query signature and might be hypothesized to act on a pathway in parallel with the transcription factor that generated the query signature. This score allows the user to choose perturbagens irrespective of P value, which is of particular relevance because perturbagens are profiled at different doses, in different cell lines, or for a different number of experimental replicates (61). Both the average connectivity score and the maximum connectivity score (from the best instance of treatment, dose, and cell line) are informative; for example, some cell lines might not express the target of interest, and some doses might not be effective, bringing down the average.
In this manner we evaluated the gene signatures of p53 and PPARγ that we had created to test the feasibility of our approach. Many of the well-studied chemical agents that activate p53 are not included in the Connectivity Map. Nevertheless, when we analyzed the p53 gene signature, two known activators of p53 were identified: nocodazole, a microtubule inhibitor (23), and tioguanine, a chemotherapy drug known to induce p53-mediated autophagy (95, 113). These agents were ranked 6th and 18th, respectively, out of all 85 pharmaceutical perturbagens by average connectivity score (data not shown). Analysis of the PPARγ signature resulted in the identification of the PPARγ activator troglitazone, used to treat diabetes mellitus type 2. Troglitazone was ranked 22nd out of 85 compounds (data not shown). Given these results, we considered the 30 highest-ranking perturbagens to be of likely relevance in terms of modulating a transcription factor of interest.
Having ascertained the feasibility of the in silico arm of our approach, we sought to identify novel signaling upstream of the transcription factor p73. To establish a collection of p73-regulated genes, Affymetrix GeneChips were used to quantify transcript levels after ectopic p73 expression in the H1299 lung carcinoma cell line. This cell line does not have readily detectable expression of any of the p53 family members (65), making it ideal for an analysis of p73 without the confounding effect of its homologs. Since p73 can be expressed as multiple isoforms, we ectopically expressed an isoform designated TAp73β that is capable of strong transactivation (24). p73 transcriptional activity was evidenced by induction of known target genes p21 and mdm2 (Fig. 1A to C), and 11 out of the top 20 regulated genes as shown by microarray are known p53 family targets (Table 1).
FIG. 1.
Generation of a multitiered p73 signature. H1299 cells were transduced with TAp73β- or GFP-expressing adenoviruses. (A) Protein lysates were harvested after transduction, and p73, GAPDH, and downstream targets mdm2 and p21 were analyzed by Western blotting. (B and C) p73 regulates known target genes when expressed in H1299 cells. (B) Total RNA was purified and reverse transcribed, and quantitative real-time PCR was performed with primers for p21 and mdm2. The samples were normalized to GAPDH, and the results are presented as increases over values for GFP control. Error bars represent standard deviations from three experiments. (C) For ChIP analysis, p73 was immunoprecipitated (IP) from formaldehyde-cross-linked H1299 cells transduced with adenoviral p73 or a GFP control. Associated DNA fragments were PCR amplified using primers flanking the p53 family response elements in p21 and mdm2. Nonspecific binding was assayed by immunoprecipitation (IP) of p73 from non-cross-linked lysates or cross-linked lysates with an isotype-matched antibody (−, specific immunoprecipitation). (D) Schematic showing the number of genes that increase or decrease upon p73 overexpression relative to GFP control by microarray alone, the number of genes identified by ChIPSeq analysis alone, and the number of genes that are present in both data sets. (E and F) DNA fragments were created from ChIP as for panel D, and analysis of p73 binding at genomic regions near the indicated genes was performed by semiquantitative PCR for genes showing high levels of binding (E) and lower levels of binding (F). “Neg.” represents a negative-control region. Error bars represent standard deviations from three experiments.
TABLE 1.
Top 20 genes identified by microarray analysis
| Gene name | Gene abbreviation | Change (fold) by microarray analysis | Reference(s) for known targets |
|---|---|---|---|
| Stratifin (14-3-3sigma) | SFN | 275 | 46 |
| IBR domain containing 2 | IBRDC2 (p53RFP) | 99 | 52 |
| S100 calcium binding protein A2 | S100A2 | 61 | 47,93 |
| Transformed 3T3 cell double minute 2 | MDM2 | 53 | 41,69,70 |
| Carboxypeptidase M | CPM | 48 | |
| Microtubule-associated protein 2 | MAP2 | 41 | |
| Basic helix-loop-helix domain containing | BHLHB2 (DEC1) | 34 | 79,94 |
| Jagged 2 | JAG2 | 34 | 17,88 |
| p21 | CDKN1A (p21) | 32 | 30,74 |
| DNA damage-inducible transcript 4 | DDIT4 (REDD1) | 21 | 31 |
| Apoptotic peptidase activating factor 1 | APAF1 | 17 | 39,71 |
| Chromosome 11 open reading frame 17 | C11orf17 | 17 | |
| Pantothenate kinase 1 | PANK1 | 17 | |
| SRY box 7 | SOX7 | 16 | |
| RAP2B, member of RAS oncogene family | RAP2B | 16 | |
| Ferredoxin reductase | FDXR | 15 | 54,65 |
| Dystonin | DST (BPAG-1) | 13 | 76 |
| Angiopoietin-like 4 | ANGPTL4 | 13 | |
| Hypothetical protein MGC5370 | MGC5370 | 12 | |
| Endothelial PAS domain protein 1 | EPAS1 | 11 |
In order to discriminate between direct and indirect regulation by p73, we also performed whole-genome ChIP to identify sequences to which p73 is bound (ChIPSeq), using formaldehyde-cross-linked samples that were replicates of those used for our microarray experiment. Over 4,000 p73-bound DNA sequences were isolated, sequenced, and mapped to the mammalian genome using GenPathway FactorPath Discovery technology. Tags that mapped within 10 kb of genes were considered for further analysis. Overlay of our microarray data set (562 genes that increased or decreased twofold with p73 overexpression) with our whole-genome ChIP data set (2,298 genes) gave a more refined list of candidate p73 target genes (121 genes) (Fig. 1D). In order to validate our technique for identifying novel p73 target genes, we performed semiquantitative ChIP using a different p73 antibody from that in the original ChIPSeq data set to immunoprecipitate DNA-protein complexes. p73 bound to the promoter elements of both p21 and mdm2 (Fig. 1E). Binding levels did not correlate with expression levels for these two genes (Fig. 1B), suggesting that other transcriptional modulators (e.g., transcription factors or cofactors) regulate gene expression in this system, similarly to what has been reported previously (106). We chose 15 additional genes that were present in both the microarray and the ChIPSeq data sets and were able to validate the idea that p73 bound promoter or intronic regions in all 15 genes (Fig. 1E [high level of binding] and F [lower level of binding]).
Perturbagens identified through the Connectivity Map increase p73 levels.
Multiple tiers of information were used to create a rank-ordered list of target genes for further analysis, including (i) presence in microarray data set, (ii) change in expression level with p73 overexpression, (iii) presence in ChIP data set, (iv) presence of a cluster of ChIP tags at gene locus, (v) number of tags per cluster, (vi) cluster length, and (vii) functional annotation by a variety of methods that are described in greater detail below. The microarray data set was used to query the Connectivity Map because it contains a large number of genes analyzed in the proper format. However, we later used all tiers of information to choose target genes for follow-up analysis.
To begin our analysis, genes whose transcript levels increased or decreased twofold after ectopic p73 expression were analyzed using the Connectivity Map. Because we were ultimately interested in connecting p73 to pathways rather than just drugs per se, we focused on the “pharmaceutical” subset of 85 perturbagens, which we found to be better affiliated with known molecular targets and signaling pathways. Five of the top 30 perturbagens predicted to induce p73 activity were either direct or indirect inhibitors of mTOR signaling (Table 2). This includes sirolimus (known as rapamycin), a drug that binds to FKBP12 to form a complex that inhibits mTOR (40), as well as metformin, a drug widely used to treat diabetes mellitus type 2 that has been shown to activate AMP kinase and inhibit mTOR in cell culture (112). We also identified phenformin, a drug that is in the same class as metformin but is no longer used therapeutically; pyrvinium, an inhibitor of the Akt kinase that is an upstream activator of mTOR (32); and dexamethasone, known to inhibit mTOR in muscle cells (103).
TABLE 2.
Top 30 pharmaceutical perturbagens identified through the Connectivity Map that induce a p73 signature
| Pharmaceutical perturbagen | Maximum connectivity scorea | Rankb | Description |
|---|---|---|---|
| Valproic acid | 1 | 10 | Histone deacetylase 1 inhibitor |
| Pyrvinium | 0.813 | 7 | Inhibits carbohydrate metabolismc |
| Prazosin | 0.801 | 8 | Alpha-adrenergic blocker |
| Sirolimus | 0.736 | 19 | mTOR inhibitord |
| Oxaprozin | 0.727 | 1 | COX inhibitor |
| Carbamazepine | 0.694 | 6 | Sodium channel inhibitor |
| Haloperidol | 0.694 | 14 | Antidopaminergic action |
| Sodium phenylbutyrate | 0.662 | 16 | Orphan drug |
| Deferoxamine | 0.656 | 22 | Iron chelator |
| Fulvestrant | 0.641 | 29 | Estrogen receptor antagonist |
| Mercaptopurine | 0.617 | 2 | Antimetabolite |
| Felodipine | 0.602 | 23 | Calcium channel blocker |
| Clozapine | 0.585 | 13 | Antiserotonergic/dopaminergic |
| Diclofenac | 0.519 | 15 | COX inhibitor |
| Exemestane | 0.516 | 3 | Aromatase inhibitor |
| Dopamine | 0.507 | 4 | Neurohormone |
| Copper sulfate | 0.499 | 30 | Copper treatment |
| Clofibrate | 0.487 | 18 | PPAR activator |
| Dexamethasone | 0.469 | 28 | Steroid hormonee |
| Tamoxifen | 0.466 | 17 | Selective estrogen receptor modulator |
| Gefitinib | 0.448 | 5 | Epidermal growth factor receptor inhibitor |
| Chlorpropamide | 0.448 | 20 | Augments beta-cell insulin secretion |
| Cyclosporine | 0.442 | 21 | Inhibits calcineurin |
| Metformin | 0.436 | 26 | Improves insulin sensitivityf |
| Fluphenazine | 0.404 | 24 | GABA agonist |
| Tomelukast | 0.398 | 9 | Leukotriene antagonist |
| Imatinib | 0.38 | 25 | Tyrosine kinase inhibitor |
| Phenformin | 0.35 | 11 | Improves insulin sensitivityg |
| Pentamidine | 0.35 | 12 | Orphan drug—antimicrobial |
| Colforsin | 0.335 | 27 | Stimulates adenylate cyclase |
Annotation of genes in the p73 signature by ontology (function) and pathway analyses generated additional evidence supporting a connection between mTOR and p73. Enrichment of functional categories in the p73 signature was determined by comparing the observed number of genes in that category to the number of genes expected by chance based on sample size (see Materials and Methods). Known p73 functions were enriched in our data sets, including regulation of the cell cycle and cell death (Fig. 2A). Interestingly, the p73 signature also showed enrichment for novel functions such as cellular metabolism (Fig. 2B). The Kyoto Encyclopedia of Genes and Genomes (KEGG), a compendium of genes annotated and organized by signaling pathway (75), was used to annotate p73-regulated genes by pathway. Both the mTOR pathway and the insulin signaling pathway (a canonical pathway upstream of mTOR [5]) were enriched in the p73 signature (Fig. 2C). Taken together, the data from the Connectivity Map and gene annotation analyses led us to hypothesize that mTOR is an upstream regulator of p73.
FIG. 2.
Enrichment of genes by function and signaling pathway in the p73 gene signature. (A and B) Enrichment of major biological processes among genes regulated by p73. Gene ontology enrichment is shown for sets of genes that are both present in the ChIP data set and increased (A) or decreased (B) twofold over GFP values with p73 overexpression in H1299 cells by microarray analysis. Processes with P values by hypergeometric test of less than 0.01 and containing two or more genes as annotated by WebGestalt are graphed. (C) Analysis of KEGG signaling pathways enriched among all genes that were upregulated twofold or more in p73-overexpressing H1299 cells by microarray. Enrichment is shown as the number of observed genes in the data set compared to the expected number of genes as calculated using the WebGestalt software.
To validate p73 modulation by the mTOR inhibitors identified through the Connectivity Map, we focused on two drugs that are widely used therapeutically, rapamycin and metformin. MDA-MB-231 cells were treated with these drugs, and mTOR inhibition was confirmed using phospho-4EBP1 as a marker of mTOR activity. Both agents caused an elevation in p73 protein levels (Fig. 3A). (Unless otherwise noted, p73 refers to the TAp73β isoform throughout.) To determine if the observed effects of these drugs on p73 levels were specific to breast cancer cell lines such as MDA-MB-231, we also treated the rhabdomyosarcoma cell line Rh30 with rapamycin and metformin using phospho-S6 as a marker of drug activity. Unlike MDA-MB-231 cells that express one predominant p73 isoform (TAp73β), Rh30 cells express high levels of two p73 isoforms, TAp73α and TAp73β, that differ only in the presence or absence of a C-terminal sterile alpha motif of unknown function (26). Only the TAp73β isoform is elevated after treatment with rapamycin and metformin in Rh30 cells (Fig. 3B). Pyrvinium pyruvate inhibits mTOR through the upstream kinase Akt (32). In a breast cancer cell line expressing high levels of Akt (MDA-MB-468) (109), we also observed an increase in p73 protein levels 12 h after treatment with pyrvinium (Fig. 3C). Thus, drugs that inhibit mTOR by multiple mechanisms were found to elevate p73 levels.
FIG. 3.
Western analysis of perturbagen effect on p73. (A) p73 levels were increased in MDA-MB-231 cells treated with rapamycin (rap) (left panel) or metformin (met) (right panel) for 24 h. (B) Rh30 cells treated with rapamycin (left panel) or metformin (right panel) for 24 h. (C) MDA-MB-468 cells treated with pyrvinium (pyr) for 36 h. (D) Serum starvation enhances rapamycin-induced regulation of p73. Left panel: 20 nM rapamycin was added to MDA-MB-231 cells 12 h after replacement of medium containing 10% serum. Right panel: 20 nM rapamycin was added to MDA-MB-231 cells in fresh serum-free medium. For all panels, “C” is vehicle control. Protein lysates were analyzed by Western blotting for p73, p4EBP1, pS6, total S6, and actin as indicated. Panels are representative of at least three independent experiments.
Serum starvation is a model for energy depletion in cell culture and leads to changes in mTOR signaling (98) and to differences in the effects of rapamycin on MDA-MB-231 cells (38). When rapamycin was added to MDA-MB-231 cells in medium lacking serum, the elevation of p73 was synergistically enhanced at later time points (Fig. 3D). The observed changes in p73 protein in MDA-MB-231 and Rh30 cells were not due to parallel changes in p73 RNA levels (Fig. 4A and B). In addition, because mutant p53 is a known regulator of p73 through physical interaction (56), we assessed both mutant p53 levels and the potential of mutant p53 to coassociate with p73 in both cell lines. The levels of mutant p53 protein did not change in response to rapamycin, nor was there detectable coassociation between p73 and mutant p53 as assessed by coimmunoprecipitation analysis (data not shown).
FIG. 4.
Changes in p73 protein levels do not correspond to changes in p73 RNA levels. MDA-MB-231 cells (A) and Rh30 cells (B) were treated with rapamycin for 24 h in 10% serum and analyzed by Western blotting for p73, pS6, total S6, and actin. Total RNA was purified 24 h after treatment and reverse transcribed, and quantitative real-time PCR was performed with primers for TAp73. The samples were normalized to GAPDH, and the results are presented as increase over vehicle control (C). Error bars represent standard deviations from three experiments. Densitometry was performed on p73 Western signals, followed by normalization to actin. The increases in TAp73β protein levels over vehicle control were 2.2-fold in panel A and 4.3-fold in panel B.
To determine if the rapamycin-induced elevation of p73 was an indirect consequence of rapamycin-induced cell cycle arrest (45), we treated MDA-MB-231 and Rh30 cells with the cell cycle inhibitors hydroxyurea and mimosine. Cells were treated with the inhibitors for 24 h to achieve a G1/S-phase arrest; however, TAp73β levels remained constant (Fig. 5). These data show that p73 levels are responsive to cellular energy status but not general cell cycle arrest.
FIG. 5.
General cell cycle inhibition does not increase p73 levels. MDA-MB-231 and Rh30 cells were treated with 100 nM hydroxyurea (Hu) or 500 μM mimosine (Mim) or the appropriate vehicle control (C) for 24 h. Protein lysates were harvested and analyzed for p73 and actin by Western blotting, and cells were analyzed by flow cytometry to assess cell cycle profile.
MDA-MB-231 and Rh30 cells do not express detectable levels of p63 by Western blot assay (data not shown), and in both cases p53 is known to be mutant (51, 84). In contrast, experiments within the Connectivity Map were often performed in cell lines that express multiple family members. Because both p53 and p63 can regulate overlapping sets of target genes with p73 (26), we addressed the possibility that rapamycin-induced changes in gene expression are due to modulation of p53 and/or p63. To assess the effect of rapamycin on protein levels of all three family members, we used nontransformed HMECs that express p53, p63, and p73. Only p73 increased in response to rapamycin in HMECs; p53 and p63 levels both decreased slightly (Fig. 6). We tested additional cell lines that express functional p53 or p63 (UM-SCC-012, UM-SCC-6, UM-SCC-17b, and MCF-7); in no case did we observe an increase in p53 or p63 (data not shown).
FIG. 6.

Differential regulation of p53 family members by rapamycin. HMECs were treated with vehicle control (C) or rapamcyin for 12 h. Protein lysates were analyzed by Western blotting for p53, p63, p73, actin, pS6, and total S6. Results are representative of three independent experiments.
mTOR regulates p73 signaling.
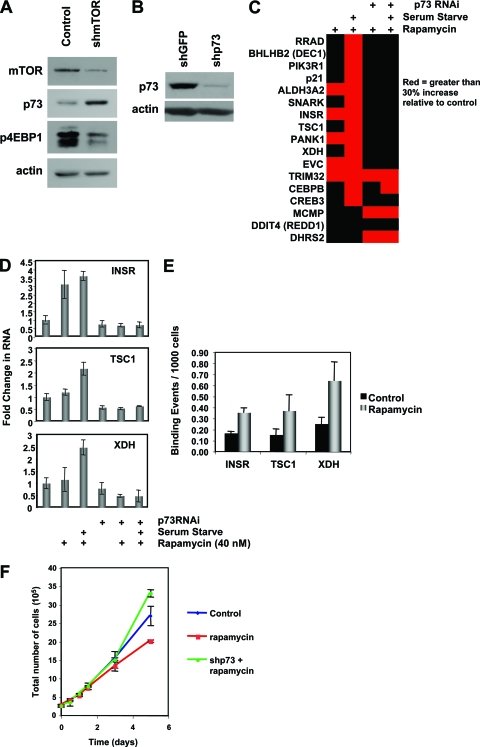
To determine if the modulation of p73 by rapamycin was mTOR dependent and to control for any off-target effects induced by rapamycin and metformin, we inhibited mTOR using a nonpharmaceutical approach. MDA-MB-231 cells were infected for 3 days with lentivirus expressing shRNA that targets mTOR. Expression of the shRNA resulted in a significant decrement in mTOR protein levels and activity and a concomitant increase in p73 protein levels (Fig. 7A).
FIG. 7.
mTOR regulates p73 levels and activity. (A) mTOR knockdown induces p73. MDA-MB-231 cells were transduced with lentivirus engineered to express shRNA against the FRAP1 subunit of mTOR or with control lentivirus. Protein lysates were harvested 3 days after transduction, and mTOR, p73, p4EBP1, and actin were analyzed by Western blotting to demonstrate knockdown of mTOR levels and activity and induction of p73 levels. Western blots are representative of at least three independent experiments. (B) MDA-MB-231 cells were transduced with lentivirus engineered to express shRNA against GFP or p73. Protein lysates were harvested 5 days after transduction, and reduction of p73 levels was confirmed by Western blotting. (C) p73 activity is induced in MDA-MB-231 cells treated with 20 nM rapamycin, with or without concurrent serum starvation, and the result was verified using p73 RNAi. Cells were serum starved by preincubation in serum-free medium overnight before treatment with rapamycin. p73 RNAi was performed by transducing cells with lentivirus engineered to express shRNA against either GFP or p73 72 h before treatment. Total RNA was purified 48 h after treatment and reverse transcribed, and quantitative real-time PCR was performed with primers for the indicated genes. The samples were normalized to GAPDH, and the results are presented as increase over vehicle control values for an average of three experiments. Samples that exhibited a 30% or greater increase relative to control are indicated in red. Twelve of 17 genes exhibited a p73-dependent increase in RNA levels after rapamycin treatment and serum starvation. (D) Change in RNA as in panel C for INSR, TSC1, and XDH shows rapamycin/serum starvation-induced changes that are p73 dependent. Error bars represent standard deviations for three experiments. (E) Semiquantitative ChIP was performed to assess levels of p73 binding to genomic regions in INSR, TSC1, and XDH promoters or introns in Rh30 cells treated with vehicle or 40 nM rapamycin for 24 h. (F) MDA-MB-231 cells were transduced with shRNA lentivirus as for panel B and treated with 20 nM rapamycin or vehicle. At the indicated times cells from treated or control cultures were counted, and changes in cell growth rates due to p73 and rapamycin are shown. Error bars represent standard deviations from three experiments.
Since a signature of p73-regulated genes was the initial query that identified a link to mTOR, we hypothesized that several of these genes should be regulated by mTOR in a p73-dependent manner. To test our hypothesis, MDA-MB-231 cells were infected with lentiviral shRNA targeting p73 (Fig. 7B). RNA was harvested from these cells after treatment with rapamycin in the presence or absence of serum in the medium. The target genes selected for further analysis were genes in the p73 signature with mTOR-related gene ontologies (e.g., involvement in insulin signaling), as well as genes with general metabolic functions if they exhibited a significant change after p73 expression and if one or more ChIP tags mapped to the gene locus. All of the target genes that were selected are shown in Fig. 7C. We found that 12 out of the 17 candidate target genes identified using our ad hoc selection method exhibited an increase in expression after treatment with rapamycin that was abrogated by depletion of p73 (Fig. 7C).
Three target genes further demonstrate our selection methods. The insulin receptor (INSR) was identified by both microarray analysis and ChIP and was selected based on known cellular function. Similarly, tuberous sclerosis 1 (TSC1) was identified by ChIP and is a known component of the mTOR signaling pathway (34). Finally, xanthine dehydrogenase (XDH) is an example of a target gene chosen both for its ontology and for its presence as a cluster of tags in the ChIPSeq data set (data not shown). XDH regulates cellular metabolism and cellular response to reactive oxidative stress (3), a process that can by regulated by mTOR during hypoxia (27).
XDH, TSC1, and INSR are three examples of a larger subset of genes whose regulation by mTOR is p73 dependent at the transcriptional level. An increase in all three genes after treatment with rapamycin in the absence of serum is abrogated by p73 knockdown, as depicted in Fig. 7C and in greater detail in Fig. 7D. In addition, semiquantitative ChIP in Rh30 cells revealed an increase in p73 binding to promoter or intronic sequences in all three genes in response to rapamycin (Fig. 7E). These genes, which were selected using multiple criteria, thus serve as readout of an mTOR-p73 signaling axis.
Also consistent with mTOR being an upstream modifier of p73 signaling, knockdown of p73 prevented a decrease in cell growth rate in MDA-MB-231 cells after treatment with rapamycin (Fig. 7F). These data suggest that cellular processes affecting growth rate are modulated by mTOR and p73.
To assess more-specific functions of p73 that might be regulated by mTOR, we focused on autophagy, a form of degradative cell death in response to energy starvation recently shown to be induced by p73 (21). Because our gene signature was generated using the TAp73β isoform and only TAp73β is induced by rapamycin, we tested the effect of selective knockdown of this isoform on autophagy by using two different RNA interference (RNAi) constructs targeting either the N-terminal TA domain or the C-terminal β domain. In both cases, knockdown abrogated baseline autophagy as measured by detection of the cleaved forms of MAP1LC3 (LC-I and LC-II) by Western blotting (Fig. 8A). MAP1LC3-II is the only protein known to be associated with the completed autophagolysosome and is considered a marker of autophagy (83).
FIG. 8.
Analysis of p73-regulated genes in profiling studies of starvation and starvation-induced autophagy. (A) p73β knockdown decreases levels of autophagy markers. MDA-MB-231 cells were transduced with lentivirus engineered to express shRNA against TAp73 isoforms or against p73β isoforms. Protein lysates were prepared, and p73, actin, and the autophagy markers LC3-I and LC3-II were detected by Western blotting. (B and C) Genes from the p73 signature were assessed using publicly available data sets in which T98G glioblastoma cells were grown asynchronously or serum starved for 3 days before RNA harvest and microarray analysis (15) (B) or in which Awells B-lymphoblastoid cells were serum starved for 6 h or 24 h, inducing autophagy (25) (C). Known p53, p63, and/or p73 target genes indicated in orange are DDIT4 (31), DDB2 (53), DFNA5 (66), CDKN1C (10), GADD45A (49), JAG1 (88), and SESN2 (13). Cell lines are arranged in columns, grouped by treatment as indicated. Genes (annotated from Affymetrix probes) are in rows that are ordered based on hierarchical clustering results (data not shown). Color range shown indicates baseline transformed expression level on a log scale. Genes that are upregulated during autophagy or serum starvation that were selected for additional analysis are in blue. (D) Changes in RNA as in Fig. 4 for KLHL24 and LOC153222 indicate increases in RNA levels after rapamycin treatment in a p73-dependent manner in MDA-MB-231 cells.
We next sought to identify downstream factors that might mediate this effect on autophagy. p73-regulated genes were analyzed using publicly available data sets in which biological states involving mTOR modulation, such as nutrient deprivation and autophagy, were profiled using DNA microarrays (15, 25). Several known p73 target genes showed increased expression in response to starvation and/or starvation-induced autophagy (Fig. 8B and C, orange font). Two unknown genes in the p73 gene signature, Kelch-like 24 (KLHL24) and LOC153222, clustered with the known and functionally better-annotated target genes that were increased in these biological states (Fig. 8B and C, blue font). Both KLHL24 and LOC153222 RNA levels increased in MDA-MB-231 cells treated with rapamycin (Fig. 8D). For both genes, this increase was p73 dependent (Fig. 8D). Thus, our approach resulted in the identification of new p73-regulated genes associated with autophagy and metabolism. A gene signature of p73 target genes, when queried using the Connectivity Map, allowed us to identify mTOR as a key signaling pathway upstream of p73.
DISCUSSION
Through whole-genome ChIP coupled with gene expression profiling, we have created a p73 gene signature that contains multiple tiers of expression and genome location information. This signature was used to identify a novel connection between p73 and the upstream kinase mTOR. Further, select genes from the p73 signature exhibited mTOR-mediated, p73-dependent regulation. Our approach, using a downstream signature to identify upstream regulators, is based on straightforward cell culture methods and should be widely applicable to the study of many transcription factors.
Determinants of our approach include overexpression of the protein of interest in a cell line that does not express high levels of the protein. This should be easily applied to transcription factors that are not essential for cell cycle progression or proliferation. In addition, we predict that modifications of our approach such as using a knockdown instead of an overexpression technique would be successful. One of the striking findings from the completion of the Connectivity Map was the extent to which connections could be made across different cell lines and tissues (60, 61). Likewise, we did not encounter any problems due to the cell lines that we chose for analysis, although this might be a significant issue for a modified knockdown approach. In our study, p73 was regulated by mTOR inhibitors in primary cells as well as multiple cancer cell lines.
The link between mTOR and p73 sheds new light on p73 biology, further demonstrating that p73 can be regulated by upstream pathways outside of canonical p53 signaling (55, 58, 64, 85, 96, 97, 102). For example, it has been shown that Akt can regulate p73 and p73-dependent apoptosis (2, 8). It would be intriguing to determine the role of mTOR (and additional members of the mTOR signaling pathway such as Akt) in canonical p53 family functions such as apoptosis and cell cycle arrest. Because Akt has been shown to regulate an activator of p73, YAP, directly (8), this mechanism may function in parallel with mTOR to affect p73-dependent transcriptional regulation.
Other functions downstream of mTOR may also be mediated by p73. Recent studies show that metformin induces apoptosis specifically in p53-null tumor cells (14). Given our results showing that metformin can increase p73 levels, additional studies are ongoing to test the role of p73 in metformin-induced apoptosis and tumor toxicity, in the presence or absence of p53.
It is the isoform TAp73β that is induced by rapamycin and metformin, with apparent coordinate downregulation of TAp73α (Fig. 3B and 4B). Few proteins that differentially modify the alpha versus beta isoforms of p73 have been identified (50, 72). We identified a putative E3 ubiquitin ligase in our data sets, TRIM32. The RNA levels of TRIM32 increase 142% upon serum-free treatment with rapamycin (Fig. 7C and data not shown). The increase is decreased to 37% after treatment with concomitant p73 RNAi-mediated knockdown (Fig. 7C and data not shown). Perhaps TRIM32 or another regulatory protein differentially modifies TAp73α and TAp73β isoforms in response to signaling from the mTOR pathway.
We demonstrated that endogenous regulation of caspase-independent cell death, known as autophagy, is a specific function of the TAp73β isoform (Fig. 8A). Although the mechanism that links p73 to autophagy is unknown (21), the p73 signature presented in this work contains candidate target genes, including unknown factors such as KLHL24 and LOC153222, that are increased in autophagy by microarray profiling (Fig. 8). KLHL24 contains a BTB/POZ domain that can function in transcriptional repression, and LOC153222 contains a basic leucine zipper domain suggesting transcription factor activity (4). These factors may regulate a transcriptional program inhibited by mTOR but induced by p73 during autophagy.
Our study suggests a larger role for mTOR in regulating the p53 family as a whole. Recent studies have placed p53 upstream of mTOR through regulation of PTEN, TSC1, and IGFBP3 (33), as well as downstream of mTOR in vivo in hamartomas (62). In general the mTOR signaling pathway integrates multiple inputs and can involve alteration of and feedback regulation by Akt and the mTORC2 complex (86, 87, 91, 101). It would be interesting to determine the signaling mechanisms by which p73 connects the p53 family to this pathway. Our data suggest that p73 is regulated by mTOR at the posttranscriptional level, either by altering protein stability or by other mechanisms. For example, recent studies suggest that p53 family transcripts may have internal ribosomal entry sites that would allow for cap-independent translation (80, 89, 107). Cap-independent translation may play a role in mTOR regulation of the p53 family.
As has been suggested by Golub, Lamb, and colleagues, our study demonstrates the potential impact of an expanded Connectivity Map as a community-wide resource that would include many more drugs and perturbagens than were included in the first build (60, 61). Here we show utility for linking the pathways represented by the Connectivity Map to the study of transcription factors in particular, but the power of this approach is dependent on the number of drugs and drug-inducible pathways that are testable through this resource. In our study we chose to assess the top 30 compounds predicted to induce a p73 gene signature. This was based on our pilot study using transcription factors with known activators. In contrast, few compounds were identified that would be predicted to repress p73 (i.e., compounds with a negative connectivity score), and we did not do a formal analysis of these drugs. However, one compound with a negative connectivity score was estradiol (data not shown), a known activator of the mTOR pathway (108). Preliminary data suggest that estradiol can decrease p73 levels (data not shown). Therefore, we predict that using our approach to identify compounds that inhibit transcription factors could also elucidate signaling connections. An expanded Connectivity Map that included more drugs that impact the same pathway at multiple levels would allow for more rigorous statistical testing and thus increase an investigator's ability to look for enrichment of pathways and agents among the top hits.
Already, genomic technologies have created a wealth of information downstream of transcription factors. We propose that this information can be used not only to characterize downstream signaling pathways but also to map upstream drug-inducible pathways. Using a genomic-based screening procedure and cell culture-based validation techniques, we identified a novel link between p73 and mTOR, an important kinase in energy homeostasis and tumorigenesis. Because p73 isoforms are overexpressed in many tumors, they may provide effective targets for cancer treatment (9). Recent studies suggest that p73 may be present in an inactive form in select head and neck squamous cell carcinomas and breast tumors and that activation of p73 would lead to tumor cell apoptosis (28, 63, 81). Thus, our results have implications for ongoing and future clinical trials examining the efficacy of mTOR inhibitors in tumors expressing p73.
Acknowledgments
We thank Christopher Barton for characterizing p63 isoform expression in breast cancer cell lines using adenoviral p63 shRNA kindly provided by Leif Ellisen. We also thank Claude Backendorf and Gerry Melino for providing p73 expression constructs, Tyler Jacks and Earle Burgess for providing and modifying lentivirus packaging vectors for the pSicoR system and p73 shRNA, and Tracy Triplett and Bianca Sirbu for technical assistance. We thank the members of the Pietenpol laboratory for helpful discussions and reading of the manuscript.
This work was supported by the National Institutes of Health grants CA70856 and CA105436 (J. A. Pietenpol), ES00267 and CA68485 (core services), US Army Medical Research and Materiel Command grant W81XWH-04-1-0304 (J. M. Rosenbluth), and GM07347 (MSTP training).
Footnotes
Published ahead of print on 4 August 2008.
REFERENCES
- 1.Akerblad, P., R. Mansson, A. Lagergren, S. Westerlund, B. Basta, U. Lind, A. Thelin, R. Gisler, D. Liberg, S. Nelander, K. Bamberg, and M. Sigvardsson. 2005. Gene expression analysis suggests that EBF-1 and PPARgamma2 induce adipogenesis of NIH-3T3 cells with similar efficiency and kinetics. Physiol. Genomics 23206-216. [DOI] [PubMed] [Google Scholar]
- 2.Amin, A. R., R. K. Paul, V. S. Thakur, and M. L. Agarwal. 2007. A novel role for p73 in the regulation of Akt-Foxo1a-Bim signaling and apoptosis induced by the plant lectin, concanavalin A. Cancer Res. 675617-5621. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, R. F., K. B. Patel, K. Reghebi, and S. A. Hill. 1989. Conversion of xanthine dehydrogenase to xanthine oxidase as a possible marker for hypoxia in tumours and normal tissues. Br. J. Cancer 60193-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apweiler, R., T. K. Attwood, A. Bairoch, A. Bateman, E. Birney, M. Biswas, P. Bucher, L. Cerutti, F. Corpet, M. D. Croning, R. Durbin, L. Falquet, W. Fleischmann, J. Gouzy, H. Hermjakob, N. Hulo, I. Jonassen, D. Kahn, A. Kanapin, Y. Karavidopoulou, R. Lopez, B. Marx, N. J. Mulder, T. M. Oinn, M. Pagni, F. Servant, C. J. Sigrist, and E. M. Zdobnov. 2000. InterPro—an integrated documentation resource for protein families, domains and functional sites. Bioinformatics 161145-1150. [DOI] [PubMed] [Google Scholar]
- 5.Avruch, J., Y. Lin, X. Long, S. Murthy, and S. Ortiz-Vega. 2005. Recent advances in the regulation of the TOR pathway by insulin and nutrients. Curr. Opin. Clin. Nutr. Metab. Care 867-72. [DOI] [PubMed] [Google Scholar]
- 6.Barbieri, C. E., C. E. Barton, and J. A. Pietenpol. 2003. Delta Np63 alpha expression is regulated by the phosphoinositide 3-kinase pathway. J. Biol. Chem. 27851408-51414. [DOI] [PubMed] [Google Scholar]
- 7.Barbieri, C. E., L. J. Tang, K. A. Brown, and J. A. Pietenpol. 2006. Loss of p63 leads to increased cell migration and up-regulation of genes involved in invasion and metastasis. Cancer Res. 667589-7597. [DOI] [PubMed] [Google Scholar]
- 8.Basu, S., N. F. Totty, M. S. Irwin, M. Sudol, and J. Downward. 2003. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol. Cell 1111-23. [DOI] [PubMed] [Google Scholar]
- 9.Bell, H. S., and K. M. Ryan. 2007. Targeting the p53 family for cancer therapy: ‘big brother’ joins the fight. Cell Cycle 61995-2000. [DOI] [PubMed] [Google Scholar]
- 10.Blint, E., A. C. Phillips, S. Kozlov, C. L. Stewart, and K. H. Vousden. 2002. Induction of p57(KIP2) expression by p73beta. Proc. Natl. Acad. Sci. USA 993529-3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boukamp, P., R. T. Petrussevska, D. Breitkreutz, J. Hornung, A. Markham, and N. E. Fusenig. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106761-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown, K. A., M. E. Aakre, A. E. Gorska, J. O. Price, S. E. Eltom, J. A. Pietenpol, and H. L. Moses. 2004. Induction by transforming growth factor-beta1 of epithelial to mesenchymal transition is a rare event in vitro. Breast Cancer Res. 6R215-R231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Budanov, A. V., A. A. Sablina, E. Feinstein, E. V. Koonin, and P. M. Chumakov. 2004. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 304596-600. [DOI] [PubMed] [Google Scholar]
- 14.Buzzai, M., R. G. Jones, R. K. Amaravadi, J. J. Lum, R. J. DeBerardinis, F. Zhao, B. Viollet, and C. B. Thompson. 2007. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 676745-6752. [DOI] [PubMed] [Google Scholar]
- 15.Cam, H., E. Balciunaite, A. Blais, A. Spektor, R. C. Scarpulla, R. Young, Y. Kluger, and B. D. Dynlacht. 2004. A common set of gene regulatory networks links metabolism and growth inhibition. Mol. Cell 16399-411. [DOI] [PubMed] [Google Scholar]
- 16.Cam, H., H. Griesmann, M. Beitzinger, L. Hofmann, R. Beinoraviciute-Kellner, M. Sauer, N. Huttinger-Kirchhof, C. Oswald, P. Friedl, S. Gattenlohner, C. Burek, A. Rosenwald, and T. Stiewe. 2006. p53 family members in myogenic differentiation and rhabdomyosarcoma development. Cancer Cell 10281-293. [DOI] [PubMed] [Google Scholar]
- 17.Candi, E., A. Rufini, A. Terrinoni, A. Giamboi-Miraglia, A. M. Lena, R. Mantovani, R. Knight, and G. Melino. 2007. DeltaNp63 regulates thymic development through enhanced expression of FgfR2 and Jag2. Proc. Natl. Acad. Sci. USA 10411999-12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carroll, D. K., J. S. Carroll, C. O. Leong, F. Cheng, M. Brown, A. A. Mills, J. S. Brugge, and L. W. Ellisen. 2006. p63 regulates an adhesion programme and cell survival in epithelial cells. Nat. Cell Biol. 8551-561. [DOI] [PubMed] [Google Scholar]
- 19.Cawley, S., S. Bekiranov, H. H. Ng, P. Kapranov, E. A. Sekinger, D. Kampa, A. Piccolboni, V. Sementchenko, J. Cheng, A. J. Williams, R. Wheeler, B. Wong, J. Drenkow, M. Yamanaka, S. Patel, S. Brubaker, H. Tammana, G. Helt, K. Struhl, and T. R. Gingeras. 2004. Unbiased mapping of transcription factor binding sites along human chromosomes 21 and 22 points to widespread regulation of noncoding RNAs. Cell 116499-509. [DOI] [PubMed] [Google Scholar]
- 20.Chang, S. B., P. Miron, A. Miron, and J. D. Iglehart. 2007. Rapamycin inhibits proliferation of estrogen-receptor-positive breast cancer cells. J. Surg. Res. 13837-44. [DOI] [PubMed] [Google Scholar]
- 21.Crighton, D., J. O'Prey, H. S. Bell, and K. M. Ryan. 2007. p73 regulates DRAM-independent autophagy that does not contribute to programmed cell death. Cell Death Differ. 141071-1079. [DOI] [PubMed] [Google Scholar]
- 22.Das, S., S. Nama, S. Antony, and K. Somasundaram. 2005. p73 beta-expressing recombinant adenovirus: a potential anticancer agent. Cancer Gene Ther. 12417-426. [DOI] [PubMed] [Google Scholar]
- 23.De Brabander, M., J. De May, M. Joniau, and G. Geuens. 1977. Ultrastructural immunocytochemical distribution of tubulin in cultured cells treated with microtubule inhibitors. Cell Biol. Int. Rep. 1177-183. [DOI] [PubMed] [Google Scholar]
- 24.De Laurenzi, V., A. Costanzo, D. Barcaroli, A. Terrinoni, M. Falco, M. Annicchiarico-Petruzzelli, M. Levrero, and G. Melino. 1998. Two new p73 splice variants, gamma and delta, with different transcriptional activity. J. Exp. Med. 1881763-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dengjel, J., O. Schoor, R. Fischer, M. Reich, M. Kraus, M. Muller, K. Kreymborg, F. Altenberend, J. Brandenburg, H. Kalbacher, R. Brock, C. Driessen, H. G. Rammensee, and S. Stevanovic. 2005. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc. Natl. Acad. Sci. USA 1027922-7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deyoung, M. P., and L. W. Ellisen. 2007. p63 and p73 in human cancer: defining the network. Oncogene 265169-5183. [DOI] [PubMed] [Google Scholar]
- 27.DeYoung, M. P., P. Horak, A. Sofer, D. Sgroi, and L. W. Ellisen. 2008. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 22239-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deyoung, M. P., C. M. Johannessen, C. O. Leong, W. Faquin, J. W. Rocco, and L. W. Ellisen. 2006. Tumor-specific p73 up-regulation mediates p63 dependence in squamous cell carcinoma. Cancer Res. 669362-9368. [DOI] [PubMed] [Google Scholar]
- 29.Dominguez, G., J. M. Silva, J. Silva, J. M. Garcia, A. Sanchez, A. Navarro, I. Gallego, M. Provencio, P. Espana, and F. Bonilla. 2001. Wild type p73 overexpression and high-grade malignancy in breast cancer. Breast Cancer Res. Treat. 66183-190. [DOI] [PubMed] [Google Scholar]
- 30.el-Deiry, W. S., T. Tokino, V. E. Velculescu, D. B. Levy, R. Parsons, J. M. Trent, D. Lin, W. E. Mercer, K. W. Kinzler, and B. Vogelstein. 1993. WAF1, a potential mediator of p53 tumor suppression. Cell 75817-825. [DOI] [PubMed] [Google Scholar]
- 31.Ellisen, L. W., K. D. Ramsayer, C. M. Johannessen, A. Yang, H. Beppu, K. Minda, J. D. Oliner, F. McKeon, and D. A. Haber. 2002. REDD1, a developmentally regulated transcriptional target of p63 and p53, links p63 to regulation of reactive oxygen species. Mol. Cell 10995-1005. [DOI] [PubMed] [Google Scholar]
- 32.Esumi, H., J. Lu, Y. Kurashima, and T. Hanaoka. 2004. Antitumor activity of pyrvinium pamoate, 6-(dimethylamino)-2-[2-(2,5-dimethyl-1-phenyl-1H-pyrrol-3-yl)ethenyl]-1-me thyl-quinolinium pamoate salt, showing preferential cytotoxicity during glucose starvation. Cancer Sci. 95685-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng, Z., W. Hu, E. de Stanchina, A. K. Teresky, S. Jin, S. Lowe, and A. J. Levine. 2007. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 673043-3053. [DOI] [PubMed] [Google Scholar]
- 34.Feng, Z., H. Zhang, A. J. Levine, and S. Jin. 2005. The coordinate regulation of the p53 and mTOR pathways in cells. Proc. Natl. Acad. Sci. USA 1028204-8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flatt, P. M., L. J. Tang, C. D. Scatena, S. T. Szak, and J. A. Pietenpol. 2000. p53 regulation of G2 checkpoint is retinoblastoma protein dependent. Mol. Cell. Biol. 204210-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flores, E. R., K. Y. Tsai, D. Crowley, S. Sengupta, A. Yang, F. McKeon, and T. Jacks. 2002. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature 416560-564. [DOI] [PubMed] [Google Scholar]
- 37.Fontemaggi, G., I. Kela, N. Amariglio, G. Rechavi, J. Krishnamurthy, S. Strano, A. Sacchi, D. Givol, and G. Blandino. 2002. Identification of direct p73 target genes combining DNA microarray and chromatin immunoprecipitation analyses. J. Biol. Chem. 27743359-43368. [DOI] [PubMed] [Google Scholar]
- 38.Gadir, N., D. N. Jackson, E. Lee, and D. A. Foster. 2008. Defective TGF-beta signaling sensitizes human cancer cells to rapamycin. Oncogene 271055-1062. [DOI] [PubMed] [Google Scholar]
- 39.Gressner, O., T. Schilling, K. Lorenz, E. S. Schleithoff, A. Koch, H. Schulze-Bergkamen, A. M. Lena, E. Candi, A. Terrinoni, M. V. Catani, M. Oren, G. Melino, P. H. Krammer, W. Stremmel, and M. Muller. 2005. TAp63alpha induces apoptosis by activating signaling via death receptors and mitochondria. EMBO J. 242458-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guertin, D. A., and D. M. Sabatini. 2007. Defining the role of mTOR in cancer. Cancer Cell 129-22. [DOI] [PubMed] [Google Scholar]
- 41.Harms, K. L., and X. Chen. 2006. p19ras brings a new twist to the regulation of p73 by Mdm2. Sci. STKE 2006pe24. [DOI] [PubMed] [Google Scholar]
- 42.Hawkins, R. D., and B. Ren. 2006. Genome-wide location analysis: insights on transcriptional regulation. Hum. Mol. Genet. 15(Spec. No. 1)R1-R7. [DOI] [PubMed] [Google Scholar]
- 43.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 952509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hearnes, J. M., D. J. Mays, K. L. Schavolt, L. Tang, X. Jiang, and J. A. Pietenpol. 2005. Chromatin immunoprecipitation-based screen to identify functional genomic binding sites for sequence-specific transactivators. Mol. Cell. Biol. 2510148-10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heitman, J., N. R. Movva, and M. N. Hall. 1991. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 253905-909. [DOI] [PubMed] [Google Scholar]
- 46.Hermeking, H., C. Lengauer, K. Polyak, T. C. He, L. Zhang, S. Thiagalingam, K. W. Kinzler, and B. Vogelstein. 1997. 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol. Cell 13-11. [DOI] [PubMed] [Google Scholar]
- 47.Hibi, K., S. Fujitake, T. Takase, Y. Kodera, K. Ito, S. Akiyama, M. Shirane, and A. Nakao. 2003. Identification of S100A2 as a target of the DeltaNp63 oncogenic pathway. Clin. Cancer Res. 94282-4285. [PubMed] [Google Scholar]
- 48.Hieronymus, H., J. Lamb, K. N. Ross, X. P. Peng, C. Clement, A. Rodina, M. Nieto, J. Du, K. Stegmaier, S. M. Raj, K. N. Maloney, J. Clardy, W. C. Hahn, G. Chiosis, and T. R. Golub. 2006. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer Cell 10321-330. [DOI] [PubMed] [Google Scholar]
- 49.Hollander, M. C., M. S. Sheikh, D. V. Bulavin, K. Lundgren, L. Augeri-Henmueller, R. Shehee, T. A. Molinaro, K. E. Kim, E. Tolosa, J. D. Ashwell, M. P. Rosenberg, Q. Zhan, P. M. Fernandez-Salguero, W. F. Morgan, C. X. Deng, and A. J. Fornace, Jr. 1999. Genomic instability in Gadd45a-deficient mice. Nat. Genet. 23176-184. [DOI] [PubMed] [Google Scholar]
- 50.Hosoda, M., T. Ozaki, K. Miyazaki, S. Hayashi, K. Furuya, K. Watanabe, T. Nakagawa, T. Hanamoto, S. Todo, and A. Nakagawara. 2005. UFD2a mediates the proteasomal turnover of p73 without promoting p73 ubiquitination. Oncogene 247156-7169. [DOI] [PubMed] [Google Scholar]
- 51.Hosoi, H., M. B. Dilling, T. Shikata, L. N. Liu, L. Shu, R. A. Ashmun, G. S. Germain, R. T. Abraham, and P. J. Houghton. 1999. Rapamycin causes poorly reversible inhibition of mTOR and induces p53-independent apoptosis in human rhabdomyosarcoma cells. Cancer Res. 59886-894. [PubMed] [Google Scholar]
- 52.Huang, J., L. G. Xu, T. Liu, Z. Zhai, and H. B. Shu. 2006. The p53-inducible E3 ubiquitin ligase p53RFP induces p53-dependent apoptosis. FEBS Lett. 580940-947. [DOI] [PubMed] [Google Scholar]
- 53.Hwang, B. J., J. M. Ford, P. C. Hanawalt, and G. Chu. 1999. Expression of the p48 xeroderma pigmentosum gene is p53-dependent and is involved in global genomic repair. Proc. Natl. Acad. Sci. USA 96424-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hwang, P. M., F. Bunz, J. Yu, C. Rago, T. A. Chan, M. P. Murphy, G. F. Kelso, R. A. Smith, K. W. Kinzler, and B. Vogelstein. 2001. Ferredoxin reductase affects p53-dependent, 5-fluorouracil-induced apoptosis in colorectal cancer cells. Nat. Med. 71111-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Irwin, M., M. C. Marin, A. C. Phillips, R. S. Seelan, D. I. Smith, W. Liu, E. R. Flores, K. Y. Tsai, T. Jacks, K. H. Vousden, and W. G. Kaelin, Jr. 2000. Role for the p53 homologue p73 in E2F-1-induced apoptosis. Nature 407645-648. [DOI] [PubMed] [Google Scholar]
- 56.Irwin, M. S., K. Kondo, M. C. Marin, L. S. Cheng, W. C. Hahn, and W. G. Kaelin, Jr. 2003. Chemosensitivity linked to p73 function. Cancer Cell 3403-410. [DOI] [PubMed] [Google Scholar]
- 57.Johnson, D. S., A. Mortazavi, R. M. Myers, and B. Wold. 2007. Genome-wide mapping of in vivo protein-DNA interactions. Science 3161497-1502. [DOI] [PubMed] [Google Scholar]
- 58.Jones, E. V., M. J. Dickman, and A. J. Whitmarsh. 2007. Regulation of p73-mediated apoptosis by c-Jun N-terminal kinase. Biochem. J. 405617-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jost, C. A., M. C. Marin, and W. G. Kaelin, Jr. 1997. p73 is a simian [correction of human] p53-related protein that can induce apoptosis. Nature 389191-194. [DOI] [PubMed] [Google Scholar]
- 60.Lamb, J. 2007. The Connectivity Map: a new tool for biomedical research. Nat. Rev. Cancer 754-60. [DOI] [PubMed] [Google Scholar]
- 61.Lamb, J., E. D. Crawford, D. Peck, J. W. Modell, I. C. Blat, M. J. Wrobel, J. Lerner, J. P. Brunet, A. Subramanian, K. N. Ross, M. Reich, H. Hieronymus, G. Wei, S. A. Armstrong, S. J. Haggarty, P. A. Clemons, R. Wei, S. A. Carr, E. S. Lander, and T. R. Golub. 2006. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science 3131929-1935. [DOI] [PubMed] [Google Scholar]
- 62.Lee, C. H., K. Inoki, M. Karbowniczek, E. Petroulakis, N. Sonenberg, E. P. Henske, and K. L. Guan. 2007. Constitutive mTOR activation in TSC mutants sensitizes cells to energy starvation and genomic damage via p53. EMBO J. 264812-4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leong, C. O., N. Vidnovic, M. P. DeYoung, D. Sgroi, and L. W. Ellisen. 2007. The p63/p73 network mediates chemosensitivity to cisplatin in a biologically defined subset of primary breast cancers. J. Clin. Investig. 1171370-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lissy, N. A., P. K. Davis, M. Irwin, W. G. Kaelin, and S. F. Dowdy. 2000. A common E2F-1 and p73 pathway mediates cell death induced by TCR activation. Nature 407642-645. [DOI] [PubMed] [Google Scholar]
- 65.Liu, G., and X. Chen. 2002. The ferredoxin reductase gene is regulated by the p53 family and sensitizes cells to oxidative stress-induced apoptosis. Oncogene 217195-7204. [DOI] [PubMed] [Google Scholar]
- 66.Masuda, Y., M. Futamura, H. Kamino, Y. Nakamura, N. Kitamura, S. Ohnishi, Y. Miyamoto, H. Ichikawa, T. Ohta, M. Ohki, T. Kiyono, H. Egami, H. Baba, and H. Arakawa. 2006. The potential role of DFNA5, a hearing impairment gene, in p53-mediated cellular response to DNA damage. J. Hum. Genet. 51652-664. [DOI] [PubMed] [Google Scholar]
- 67.McKeon, F., and G. Melino. 2007. Fog of war: the emerging p53 family. Cell Cycle 6229-232. [DOI] [PubMed] [Google Scholar]
- 68.Melino, G., F. Bernassola, M. Ranalli, K. Yee, W. X. Zong, M. Corazzari, R. A. Knight, D. R. Green, C. Thompson, and K. H. Vousden. 2004. p73 Induces apoptosis via PUMA transactivation and Bax mitochondrial translocation. J. Biol. Chem. 2798076-8083. [DOI] [PubMed] [Google Scholar]
- 69.Michael, D., and M. Oren. 2002. The p53 and Mdm2 families in cancer. Curr. Opin. Genet. Dev. 1253-59. [DOI] [PubMed] [Google Scholar]
- 70.Momand, J., G. P. Zambetti, D. C. Olson, D. George, and A. J. Levine. 1992. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 691237-1245. [DOI] [PubMed] [Google Scholar]
- 71.Moroni, M. C., E. S. Hickman, E. L. Denchi, G. Caprara, E. Colli, F. Cecconi, H. Muller, and K. Helin. 2001. Apaf-1 is a transcriptional target for E2F and p53. Nat. Cell Biol. 3552-558. [DOI] [PubMed] [Google Scholar]
- 72.Munarriz, E., D. Barcaroli, A. Stephanou, P. A. Townsend, C. Maisse, A. Terrinoni, M. H. Neale, S. J. Martin, D. S. Latchman, R. A. Knight, G. Melino, and V. De Laurenzi. 2004. PIAS-1 is a checkpoint regulator which affects exit from G1 and G2 by sumoylation of p73. Mol. Cell. Biol. 2410593-10610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Niemantsverdriet, M., W. P. Vermeij, and C. Backendorf. 2005. RT-PCR analysis of p73 splice variants, ease or tease? Leukemia 191685-1686. [DOI] [PubMed] [Google Scholar]
- 74.Nozell, S., and X. Chen. 2002. p21B, a variant of p21(Waf1/Cip1), is induced by the p53 family. Oncogene 211285-1294. [DOI] [PubMed] [Google Scholar]
- 75.Ogata, H., S. Goto, W. Fujibuchi, and M. Kanehisa. 1998. Computation with the KEGG pathway database. Biosystems 47119-128. [DOI] [PubMed] [Google Scholar]
- 76.Osada, M., Y. Nagakawa, H. L. Park, K. Yamashita, G. Wu, M. S. Kim, A. Fomenkov, B. Trink, and D. Sidransky. 2005. p63-specific activation of the BPAG-1e promoter. J. Investig. Dermatol. 12552-60. [DOI] [PubMed] [Google Scholar]
- 77.Osada, M., H. L. Park, Y. Nagakawa, S. Begum, K. Yamashita, G. Wu, M. S. Kim, B. Trink, and D. Sidransky. 2006. A novel response element confers p63- and p73-specific activation of the WNT4 promoter. Biochem. Biophys. Res. Commun. 3391120-1128. [DOI] [PubMed] [Google Scholar]
- 78.Perez, C. A., J. Ott, D. J. Mays, and J. A. Pietenpol. 2007. p63 consensus DNA-binding site: identification, analysis and application into a p63MH algorithm. Oncogene 267363-7370. [DOI] [PubMed] [Google Scholar]
- 79.Qian, Y., J. Zhang, B. Yan, and X. Chen. 2008. DEC1, a basic helix-loop-helix transcription factor and a novel target gene of the p53 family, mediates p53-dependent premature senescence. J. Biol. Chem. 2832896-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ray, P. S., R. Grover, and S. Das. 2006. Two internal ribosome entry sites mediate the translation of p53 isoforms. EMBO Rep. 7404-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rocco, J. W., C. O. Leong, N. Kuperwasser, M. P. DeYoung, and L. W. Ellisen. 2006. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell 945-56. [DOI] [PubMed] [Google Scholar]
- 82.Rubinson, D. A., C. P. Dillon, A. V. Kwiatkowski, C. Sievers, L. Yang, J. Kopinja, D. L. Rooney, M. Zhang, M. M. Ihrig, M. T. McManus, F. B. Gertler, M. L. Scott, and L. Van Parijs. 2003. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat. Genet. 33401-406. [DOI] [PubMed] [Google Scholar]
- 83.Rubinsztein, D. C., J. E. Gestwicki, L. O. Murphy, and D. J. Klionsky. 2007. Potential therapeutic applications of autophagy. Nat. Rev. Drug Discov. 6304-312. [DOI] [PubMed] [Google Scholar]
- 84.Runnebaum, I. B., M. Nagarajan, M. Bowman, D. Soto, and S. Sukumar. 1991. Mutations in p53 as potential molecular markers for human breast cancer. Proc. Natl. Acad. Sci. USA 8810657-10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sanchez-Prieto, R., V. J. Sanchez-Arevalo, J. M. Servitja, and J. S. Gutkind. 2002. Regulation of p73 by c-Abl through the p38 MAP kinase pathway. Oncogene 21974-979. [DOI] [PubMed] [Google Scholar]
- 86.Sarbassov, D. D., S. M. Ali, S. Sengupta, J. H. Sheen, P. P. Hsu, A. F. Bagley, A. L. Markhard, and D. M. Sabatini. 2006. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 22159-168. [DOI] [PubMed] [Google Scholar]
- 87.Sarbassov, D. D., D. A. Guertin, S. M. Ali, and D. M. Sabatini. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 3071098-1101. [DOI] [PubMed] [Google Scholar]
- 88.Sasaki, Y., S. Ishida, I. Morimoto, T. Yamashita, T. Kojima, C. Kihara, T. Tanaka, K. Imai, Y. Nakamura, and T. Tokino. 2002. The p53 family member genes are involved in the Notch signal pathway. J. Biol. Chem. 277719-724. [DOI] [PubMed] [Google Scholar]
- 89.Sayan, A. E., J. P. Roperch, B. S. Sayan, M. Rossi, M. J. Pinkoski, R. A. Knight, A. E. Willis, and G. Melino. 2007. Generation of DeltaTAp73 proteins by translation from a putative internal ribosome entry site. Ann. N. Y. Acad. Sci. 1095315-324. [DOI] [PubMed] [Google Scholar]
- 90.Schavolt, K. L., and J. A. Pietenpol. 2007. p53 and Delta Np63 alpha differentially bind and regulate target genes involved in cell cycle arrest, DNA repair and apoptosis. Oncogene. 266125-6132. [DOI] [PubMed] [Google Scholar]
- 91.Sun, S. Y., L. M. Rosenberg, X. Wang, Z. Zhou, P. Yue, H. Fu, and F. R. Khuri. 2005. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 657052-7058. [DOI] [PubMed] [Google Scholar]
- 92.Szak, S. T., D. Mays, and J. A. Pietenpol. 2001. Kinetics of p53 binding to promoter sites in vivo. Mol. Cell. Biol. 213375-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tan, M., C. W. Heizmann, K. Guan, B. W. Schafer, and Y. Sun. 1999. Transcriptional activation of the human S100A2 promoter by wild-type p53. FEBS Lett. 445265-268. [DOI] [PubMed] [Google Scholar]
- 94.Thin, T. H., L. Li, T. K. Chung, H. Sun, and R. Taneja. 2007. Stra13 is induced by genotoxic stress and regulates ionizing-radiation-induced apoptosis. EMBO Rep. 8401-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tishler, R. B., D. M. Lamppu, S. Park, and B. D. Price. 1995. Microtubule-active drugs taxol, vinblastine, and nocodazole increase the levels of transcriptionally active p53. Cancer Res. 556021-6025. [PubMed] [Google Scholar]
- 96.Toh, W. H., M. M. Siddique, L. Boominathan, K. W. Lin, and K. Sabapathy. 2004. c-Jun regulates the stability and activity of the p53 homologue, p73. J. Biol. Chem. 27944713-44722. [DOI] [PubMed] [Google Scholar]
- 97.Urist, M., T. Tanaka, M. V. Poyurovsky, and C. Prives. 2004. p73 induction after DNA damage is regulated by checkpoint kinases Chk1 and Chk2. Genes Dev. 183041-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vander Haar, E., S. I. Lee, S. Bandhakavi, T. J. Griffin, and D. H. Kim. 2007. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat. Cell Biol. 9316-323. [DOI] [PubMed] [Google Scholar]
- 99.Ventura, A., A. Meissner, C. P. Dillon, M. McManus, P. A. Sharp, L. Van Parijs, R. Jaenisch, and T. Jacks. 2004. Cre-lox-regulated conditional RNA interference from transgenes. Proc. Natl. Acad. Sci. USA 10110380-10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vigano, M. A., J. Lamartine, B. Testoni, D. Merico, D. Alotto, C. Castagnoli, A. Robert, E. Candi, G. Melino, X. Gidrol, and R. Mantovani. 2006. New p63 targets in keratinocytes identified by a genome-wide approach. EMBO J. 255105-5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wan, X., B. Harkavy, N. Shen, P. Grohar, and L. J. Helman. 2007. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 261932-1940. [DOI] [PubMed] [Google Scholar]
- 102.Wan, Y. Y., and J. DeGregori. 2003. The survival of antigen-stimulated T cells requires NFkappaB-mediated inhibition of p73 expression. Immunity 18331-342. [DOI] [PubMed] [Google Scholar]
- 103.Wang, H., N. Kubica, L. W. Ellisen, L. S. Jefferson, and S. R. Kimball. 2006. Dexamethasone represses signaling through the mammalian target of rapamycin in muscle cells by enhancing expression of REDD1. J. Biol. Chem. 28139128-39134. [DOI] [PubMed] [Google Scholar]
- 104.Wei, C. L., Q. Wu, V. B. Vega, K. P. Chiu, P. Ng, T. Zhang, A. Shahab, H. C. Yong, Y. Fu, Z. Weng, J. Liu, X. D. Zhao, J. L. Chew, Y. L. Lee, V. A. Kuznetsov, W. K. Sung, L. D. Miller, B. Lim, E. T. Liu, Q. Yu, H. H. Ng, and Y. Ruan. 2006. A global map of p53 transcription-factor binding sites in the human genome. Cell 124207-219. [DOI] [PubMed] [Google Scholar]
- 105.Wei, G., D. Twomey, J. Lamb, K. Schlis, J. Agarwal, R. W. Stam, J. T. Opferman, S. E. Sallan, M. L. den Boer, R. Pieters, T. R. Golub, and S. A. Armstrong. 2006. Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell 10331-342. [DOI] [PubMed] [Google Scholar]
- 106.Yang, A., Z. Zhu, P. Kapranov, F. McKeon, G. M. Church, T. R. Gingeras, and K. Struhl. 2006. Relationships between p63 binding, DNA sequence, transcription activity, and biological function in human cells. Mol. Cell 24593-602. [DOI] [PubMed] [Google Scholar]
- 107.Yang, D. Q., M. J. Halaby, and Y. Zhang. 2006. The identification of an internal ribosomal entry site in the 5′-untranslated region of p53 mRNA provides a novel mechanism for the regulation of its translation following DNA damage. Oncogene 254613-4619. [DOI] [PubMed] [Google Scholar]
- 108.Yu, J., and E. P. Henske. 2006. Estrogen-induced activation of mammalian target of rapamycin is mediated via tuberin and the small GTPase Ras homologue enriched in brain. Cancer Res. 669461-9466. [DOI] [PubMed] [Google Scholar]
- 109.Yu, K., L. Toral-Barza, C. Discafani, W. G. Zhang, J. Skotnicki, P. Frost, and J. J. Gibbons. 2001. mTOR, a novel target in breast cancer: the effect of CCI-779, an mTOR inhibitor, in preclinical models of breast cancer. Endocr. Relat. Cancer 8249-258. [DOI] [PubMed] [Google Scholar]
- 110.Zaika, A. I., S. Kovalev, N. D. Marchenko, and U. M. Moll. 1999. Overexpression of the wild type p73 gene in breast cancer tissues and cell lines. Cancer Res. 593257-3263. [PubMed] [Google Scholar]
- 111.Zaika, A. I., N. Slade, S. H. Erster, C. Sansome, T. W. Joseph, M. Pearl, E. Chalas, and U. M. Moll. 2002. DeltaNp73, a dominant-negative inhibitor of wild-type p53 and TAp73, is up-regulated in human tumors. J. Exp. Med. 196765-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zakikhani, M., R. Dowling, I. G. Fantus, N. Sonenberg, and M. Pollak. 2006. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 6610269-10273. [DOI] [PubMed] [Google Scholar]
- 113.Zeng, X., T. Yan, J. E. Schupp, Y. Seo, and T. J. Kinsella. 2007. DNA mismatch repair initiates 6-thioguanine-induced autophagy through p53 activation in human tumor cells. Clin. Cancer Res. 131315-1321. [DOI] [PubMed] [Google Scholar]
- 114.Zhang, B., S. Kirov, and J. Snoddy. 2005. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 33W741-W748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao, R., K. Gish, M. Murphy, Y. Yin, D. Notterman, W. H. Hoffman, E. Tom, D. H. Mack, and A. J. Levine. 2000. Analysis of p53-regulated gene expression patterns using oligonucleotide arrays. Genes Dev. 14981-993. [PMC free article] [PubMed] [Google Scholar]