Abstract
Multidrug resistance in the yeast Saccharomyces cerevisiae is sensitive to the mitochondrial genome status of cells. Cells that lose their organellar genome ([rho0] cells) dramatically induce transcription of multiple or pleiotropic drug resistance genes via increased expression of a zinc cluster-containing transcription factor designated Pdr3. A major Pdr3 target gene is the ATP-binding cassette transporter-encoding gene PDR5. Pdr5 has been demonstrated to act as a phospholipid floppase catalyzing the net outward movement of phosphatidylethanolamine (PE). Since the mitochondrially localized Psd1 enzyme provides a major route of PE biosynthesis, we evaluated the potential linkage between Psd1 function and PDR5 regulation. Overproduction of Psd1 in wild-type ([rho+]) cells was found to induce PDR5 transcription and drug resistance in a Pdr3-dependent manner. Loss of the PSD1 gene from [rho0] cells prevented the normal activation of PDR5 expression. Surprisingly, expression of a catalytically inactive form of Psd1 still supported PDR5 transcriptional activation, suggesting that PE levels were not the signal triggering PDR5 induction. Expression of green fluorescent protein fusions mapped the region required to induce PDR5 expression to the noncatalytic amino-terminal portion of Psd1. Psd1 is a novel bifunctional protein required both for PE biosynthesis and regulation of multidrug resistance.
Coordination between expression of the nuclear and mitochondrial genomes is an essential feature of eukaryotic cells. Mitochondrial-to-nuclear signaling is referred to as retrograde regulation and acts to sense changes in mitochondrial genome content or function. These changes are then communicated to the nuclear genome for an appropriate response. Genetic analysis of the yeast Saccharomyces cerevisiae has provided the clearest picture of retrograde signaling pathways to date. In cells that have lost their mitochondrial genome ([rho0] cells), at least two different pathways producing a mitochondrion-to-nucleus signal are induced. The first is the prototypical retrograde pathway that is triggered in response to depletion of tricarboxylic acid cycle intermediates required for amino acid biosynthesis (reviewed in reference 33). The second is the pleiotropic drug resistance (Pdr) pathway, which induces a suite of genes involved in tolerance to a wide range of toxic agents, including antifungal drugs (reviewed in reference 37). The physiology underlying induction of PDR genes is less well understood but involves the posttranslational activation of a zinc cluster-containing transcription factor called Pdr3 (10, 18, 23, 57). A key downstream target of Pdr3 is the ATP-binding cassette (ABC) transporter-encoding gene, designated PDR5 (2, 5, 19), which encodes a plasma membrane-localized multidrug transporter protein (12).
While the genes that are induced by Pdr3 in [rho0] cells have been catalogued by DNA microarray approaches (11), much less is known about the underlying regulatory circuitry modulating Pdr3 function. Analysis of the spectrum of mitochondrial damage inducing Pdr3 indicates that some unique property of [rho0] mitochondria is required to deliver this signal. Petite mutants that still retain their mitochondrial genome fail to exhibit the elevated multidrug resistance and enhanced Pdr5 expression seen in [rho0] cells (18, 57). Ultrastructural analyses of [rho0] mitochondria demonstrated that the normal morphology of this organelle is grossly disturbed in this type of mutant (40). Instead of the familiar invaginated cristae, a multilamellar structure was observed. This proliferation of membranous structures suggests that movement of substrates out of the mitochondria might be compromised in these [rho0] strains.
A possible link between Pdr5 function and the mitochondria was suggested by earlier experiments in which Pdr5 was associated with the net outward movement, or flop, of the phospholipids phosphatidylethanolamine (PE) and phosphatidylcholine (PC) (27, 43). The major route of PE biosynthesis in S. cerevisiae is catalyzed by the mitochondrial intermembrane space enzyme phosphatidylserine decarboxylase (Psd1) (6, 51). We set out to test whether the altered structure of [rho0] mitochondria disturbs the normal intracellular trafficking of PE and whether this might be the signal detected by Pdr3 that would normally lead to induction of PDR5 transcription.
Overproduction of Psd1 led to an elevation of PDR5 expression in cells that contain normal mitochondria ([rho+]). Induction of PDR5 transcription in these Psd1-overproducing cells exhibits the same genetic requirement for Pdr3 as seen in [rho0] cells, consistent with these different genetic situations defining a common regulatory connection. Loss of PSD1 from [rho0] cells led to a depression in PDR5 activation. Surprisingly, expression of a catalytically inactive form of Psd1 was still able to induce PDR5 expression. Use of fusion proteins consisting of Psd1 and green fluorescent protein (GFP) demonstrated that the amino-terminal domain of Psd1 was sufficient for both mitochondrial localization and activation of PDR5 transcription. These data are consistent with the view that Psd1 is a bifunctional enzyme, since two separable activities have been demonstrated for this protein: PE biosynthesis and regulation of PDR5 expression.
MATERIALS AND METHODS
Yeast strains and media.
The strains used in this study are listed in Table 1. All strains were derived from either SEY6210 or W303. Yeast cells were grown in YPD (1% yeast extract, 2% peptone, and 2% dextrose) or synthetic complete (SC) medium at 30°C with shaking. YPD medium containing either gradient or different concentrations of cycloheximide or cadmium were prepared by addition of the required amounts of drugs, after autoclaving the medium and just before pouring the plates or immediately prior to growth experiment. YPGE (2% yeast extract, 1% peptone, 3% glycerol, and 3% ethanol) medium was used as a nonfermentable carbon source. Yeast transformation was performed using the standard lithium acetate technique (21). For testing drug sensitivity, gradient plate assays (24) were performed as described earlier. Briefly, strains were grown to mid-log phase, and then 1,000 cells were spotted at regular intervals on a petri dish containing a gradient of the indicated drug. Gradient plates used a maximum concentration of 0.25 mg/ml cycloheximide. For routine measurements of lacZ expression, standard β-galactosidase assays (16) were performed as described previously. Detection of the weakly expressed PDR3-lacZ fusion gene was accomplished using a luminescent β-galactosidase assay (Clontech) performed following the manufacturer's guidelines. All assays were done with at least three independent transformants.
TABLE 1.
Strains used in this study
| Strain | Description | Source |
|---|---|---|
| SEY6210 | MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 Mel− | Scott Emr |
| BY4742 | MATα his3-Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | Open Biosystems |
| W303 | MATaade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 | Lab collection |
| PSY4 | MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 psd1Δ:: kanMX2 | This study |
| PSY8 | MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 psd2Δ::natMX4 | This study |
| KGS20 | MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 psd1Δ::TRP1 | This study |
| KGS21 | MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 psd2Δ::HIS3MX6 | This study |
| KGS22 | MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 psd1Δ::KanMX2 psd2Δ::HIS3MX6 | This study |
| KGS23 | MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 PSD1-TAP::HIS3MX6 | This study |
| KGS24 | MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 [rho0] PSD1-TAP::HIS3MX6 | This study |
| KGS29 | MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 cyb2Δ::kanMX2 | This study |
| KGS30 | MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 fzo1Δ::natMX4 cyb2Δ::kanMX2 | This study |
| KGS31 | MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 TDH3-PSD1::KanMX2 | This study |
| KGS32 | MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 KanMX2::TDH3-PSD1-TAP::His3MX6 | This study |
| KGS37 | MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 fzo1Δ::KanMX2 psd1Δ::TRP1 | This study |
| KGS38 | MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 fzo1Δ::kanMX2 psd2Δ::HIS3MX6 | This study |
| KGS39 | MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 [rho0] psd1Δ::kanMX2 psd2Δ::HIS3MX6 | This study |
| KGS40 | MATaade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 psd1Δ::kanMX2 | This study |
| KGS41 | MATaade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 psd2Δ::HIS3MX6 | This study |
| KGS42 | MATaade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 psd1Δ::kanMX2 psd2Δ::HIS3MX6 | This study |
| KGS45 | MATaade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 cyb2Δ::kanMX2 | This study |
| KGS46 | MATaade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 TDH3-PSD1::kanMX2 | This study |
Strain construction.
The PSD1 open reading frame (ORF) was disrupted in SEY6210, SEY6210 pdr1-Δ2:hisG, SEY6210 pdr3-Δ1:hisG, and SEY6210 pdr1-Δ2:hisG pdr3-Δ1:hisG by PCR-mediated gene disruption (53) using a kanMX4 cassette amplified by using the primers Psd1-For1 and Psd1-Rev1, yielding YP4, YP5, YP6, and YP7, respectively. The PSD1 ORF was also disrupted in SEY6210 and SEY6210 [rho0] with a TRP1 cassette using primers PSD1 For1 and PSD1 Tap-Rev, yielding KGS20 and KGS. The PSD2 ORF was also disrupted in SEY6210 using PCR-mediated gene disruption with the natR:MX4 and HIS3MX6 module primers PSD2 Del-For and PSD2-Del-Rev, yielding YP8 and KGS21. The double deletion of PSD1 and PSD2 in SEY6210 was obtained by transforming YP4 with a psd2Δ:HIS3MX6 cassette, yielding KGS22. The PSD1 gene was also disrupted in W303 using PCR-mediated gene disruption with a kanMX4 cassette, yielding KGS40. The double deletion of PSD1 and PSD2 was made in W303 by transformation of the psd2Δ:HIS3MX6 cassette in KGS40, yielding KGS42. All the disruptions were confirmed by PCR. Strains containing tandem affinity purification (TAP) tag fusions of PSD1 in SEY6210 and SEY6210 [rho0] were constructed by transforming these strains with a TAP-HIS3MX6 cassette amplified from the Open Biosystems TAP tag strain collection, yielding KGS23 and KGS24. For constructing strains overproducing Psd1 from the TDH3 promoter, the chromosomal PSD1 promoter was replaced by transformation with a kanMX2-TDH3 cassette amplified from plasmid pYM-N14 (22). In SEY6210, the PSD1 gene was placed under TDH3 promoter control using the kanMX2-TDH3 cassette, yielding KGS31. The strain KGS31 was then transformed with the TAP-HISMX6 cassette to TAP tag PSD1, yielding KGS32. The PSD1 gene was also placed under TDH3 promoter control in W303, by transforming this strain with the same kanMX2-TDH3 cassette, yielding KGS46. The primers used to amplify the kanMX2-TDH3 cassette were PSD1-S1 and PSD1-S4. The genomic structures of all strains were confirmed by PCR. Primer sequences are available on request.
Plasmids.
Plasmids used in this study are listed in Table 2. A 2.7-kb PCR fragment containing the PSD1 gene with 700 bp upstream and 500 bp downstream flanking sequence was cloned in pRS314 as a KpnI-NotI fragment. This plasmid was designated pPS14. This 2.7-kb fragment was then cloned in pRS426 cut with KpnI and NotI to obtain pKGE20. The plasmid pKGE20 was then digested with BamHI and NotI to excise a fragment carrying the PSD1 gene. This fragment was then cloned in pRS425 digested with BamHI and NotI to obtain pKGE21. To construct an N-terminally hemagglutinin (HA)-tagged PSD1 gene under the control of the PGK1 promoter, the 1.5-kb PSD1 ORF was cloned as a BamHI fragment in plasmid pXTZ138 (57) digested with BamHI, yielding pKGE23. The N-terminally truncated PSD1, which had the first 136 amino acids deleted, was also cloned as a BamHI fragment in pXTZ138, yielding pKGE24. The PSD1 gene was C-terminally HA tagged by removing a HpaI-KpnI fragment from plasmid pKGE20 and replacing it with 1.6-kb HpaI-KpnI fragment from a GAL1-PSD1-HA plasmid, yielding KGE26. The GAL1-PSD1-HA plasmid was obtained from Open Biosystems. The C-terminally HA-tagged PSD1 was placed under the control of either the CUP1 or PGK1 promoter by a three-part ligation. The 650-bp CUP1 and PGK1 promoters were amplified as NotI-BamHI fragments. The first 500 bp of the PSD1 ORF were obtained by digesting pKGE23 with BamHI-HpaI, and the remaining C-terminal PSD1 ORF along with the HA tag was obtained by digesting pKGE26 with HpaI-KpnI. These three fragments (650-bp promoter of either CUP1 or PGK1, 1.5-kb N-terminal PSD1, and 1.6-kb C-terminally HA-tagged PSD1) were ligated together and cloned in pRS426 digested with BamHI-KpnI, yielding pKGE27 (PGK-PSD1-HA) and KGE28 (CUP1-PSD1-HA). The truncated M137 PSD1 (with amino acids 1 to 136 deleted) was also cloned under PGK1 and CUP1 promoter control along with the C-terminal HA tag in pRS426 as a BamHI-KpnI fragment, yielding pKGE29 and pKGE30. To generate the catalytically inactive mutant, PCR overlap extension (20) mutagenesis was utilized to change amino acids LGS starting at position 461 to AAA. The resulting PCR fragment was then recombined into the original pKGE26 plasmid. This plasmid was named pJS10. The IM-GFP PSD1 mutant was produced using homologous recombination. The enhanced GFP (eGFP) cassette flanked by targeting sequences was amplified from plasmid pGFP-URA3 (15) with 50 bases corresponding to the N terminus of PSD1 and 50 bases corresponding to the HA tag. This PCR fragment was transformed into cells along with pKGE26, resulting in truncation of PSD1 after amino acid 104 with the eGFP ORF. The recombination retains the C-terminal HA tag, and this plasmid was named pJS11. A similar approach was utilized to insert eGFP into the PSD1 gene to create pJS19. Here, eGFP was amplified with ends that would insert the fluorescent protein between the inner membrane-targeting region and the catalytic region of the enzyme. Plasmid pKGE26 was gapped by digesting with SpeI, and the gap was filled with the resulting PCR product by homologous recombination. Plasmids pJS12 (Candida albicans) and pJS13 (Candida glabrata) were constructed by amplifying the ORF of the homologous PSD1 genes from genomic DNA and recombining the PCR product back into pKGE26 to replace the S. cerevisiae ORF. This led to all fungal Psd enzymes being transcribed by the S. cerevisiae PSD1 promoter and tagged at the C terminus with HA. The control plasmid for a known mitochondrial targeting sequence was obtained from Ben Glick and consists of the Cox4 presequence fused to mCherry (3).
TABLE 2.
Plasmids used in this study
| Plasmid | Descriptiona | Source |
|---|---|---|
| pKGE20 | pRS426 2μm URA3 PSD1 | This study |
| pKGE21 | pRS425 2μm LEU2 PSD1 | This study |
| pKGE23 | pRS315 PGK-HA-PSD1 | This study |
| pKGE24 | pRS315 PGK-HA-M137 PSD1 | This study |
| pKGE25 | pRS426 2μm URA3 PGK-HA-PSD1 | This study |
| pKGE26 | pRS426 2μm URA3 PSD1-HA | This study |
| pKGE27 | pRS426 2μm URA3 PGK-PSD1-HA | This study |
| pKGE28 | pRS426 2μm URA3 CUP1-PSD1-HA | This study |
| pKGE29 | pRS426 2μm URA3 PGK-M137 PSD1-HA | This study |
| pKGE30 | pRS426 2μm URA3 CUP1-M137 PSD1-HA | This study |
| pKGE33 | pRS426 2μm URA3 CUP1-eGFP-PSD1-HA | This study |
| pJS10 | pRS426 2μm URA3 PSD1 LGS461AAA-HA | This study |
| pJS11 | pRS426 2μm URA3 PSD1 (1-104 aa)-eGFP-HA | This study |
| pJS12 | pRS426 2μm URA3 ScPSD1 Prom-CaPSD1-HA | This study |
| pJS13 | pRS426 2μm URA3 ScPSD1 Prom-CgPSD1-HA | This study |
| pJS14 | pRS426 2μm URA3 PSD1 (1-45 aa)-eGFP-HA | This study |
| pJS19 | pRS426 2μm URA3 PSD1 (1-104 aa)-eGFP-(105-500 aa)-HA | This study |
aa, amino acids.
Fluorescence microscopy.
The strains carrying different GFP-tagged versions of Psd1 and the mitochondrially targeted mCherry were grown to saturation. These cultures were then reinoculated at a starting optical density at 600 nm (OD600) of 0.1. Cells were allowed to grow for 3 to 4 h to an approximate OD600 of 0.4 to 0.6, and then 5 μl of the culture was placed on a glass slide, overlaid with a coverslip, and examined under the 100× oil objective of an Olympus BX60 microscope. Images were captured on a Hamamatsu digital camera (C4742-95).
Western analysis.
For Western analysis, cells were grown in 100 ml of YPD or selective medium. Cells were harvested at OD600 of ∼1.0, washed, and lysed by vortexing in the presence of glass beads in a buffer containing 300 mM sorbitol, 100 mM NaCl, 5 mM MgCl2, 10 mM Tris (pH 7.4), and complete protease inhibitors (Boehringer Mannheim). Lysates were cleared by centrifugation, and Laemmli buffer was added prior to electrophoretic analysis. Fractionation of glass bead lysates was accomplished by centrifuging cleared extracts at 10,000 × g. This treatment produced supernatant (S10) and pellet (P10) fractions that were analyzed by Western blotting for the indicated proteins. The Bradford assay was used to determine the concentration of proteins in different samples prepared by a sorbitol lysis method described before (24). In some cases, a trichloroacetic acid extraction method was used. Three to 10 OD600 units of cells were harvested and resuspended in 10% trichloroacetic acid with vortexing. After a 1-min incubation at room temperature, lysates were pelleted and washed twice with ice-cold acetone. Protein pellets were resuspended in 10 μl of Laemmli buffer/OD unit. One molar Tris base was added to neutralize samples when necessary. Glass beads were added and the samples vortexed to resolubilize proteins. Equal amounts of proteins were loaded on a 10% polyacrylamide gel. The proteins were transferred to a nitrocellulose membrane, blocked with 5% nonfat dry milk in phosphate-buffered saline, and then probed with either an anti-HA or anti-TAP antibody. Horseradish peroxidase-conjugated secondary antibody and the ECL kit (Pierce) were used to visualize immunoreactive proteins.
Rhodamine 6G efflux assay in intact cells.
Strains were grown overnight in YPD and then reinoculated into fresh YPD medium to an initial OD600 of 0.1. Cells were allowed to grow for 5 to 6 h at 30°C with shaking. Equal numbers of cells from each culture were harvested and washed twice with water and twice with HEPES buffer (50 mM HEPES-NaOH, pH 7.0). Cells were then resuspended in HEPES buffer with 2-deoxy-d-glucose, and rhodamine 6G was added to a final concentration of 5 mM or 10 μM.
These cells were then kept at 30°C for 2 h. Rhodamine transport from these de-energized cells was initiated by addition of 1 mM glucose. The fluorescence of rhodamine extruded into the supernatant was measured after centrifugation using a Molecular Devices Spectramax microplate fluorimeter. The excitation and emission wavelength were 529 nm and 553 nm, respectively. Transport was measured at time period of 0, 7, and 21 minutes.
RESULTS
Overproduction of mitochondrial PE decarboxylase elevates cycloheximide resistance.
Previous work has demonstrated that the ATP-binding cassette transporter Pdr5 participates in control of phospholipid asymmetry across the plasma membrane by stimulating the net outward movement (flop) of PE (27, 43). To determine whether Pdr5-mediated drug resistance might respond to changes in levels of phospholipid biosynthetic enzymes, we used a collection (14) of GAL-driven fusion plasmids to overproduce several enzymes involved in production of these membrane components. Wild-type yeast cells were transformed with these GAL promoter-regulated plasmids, induced by growth in the presence of galactose, and then tested for the ability to grow on media containing cycloheximide. Increased Pdr5 expression leads to elevated cycloheximide tolerance (36). Overproduction of these GAL-regulated proteins was confirmed by Western blotting (data not shown).
Increased expression of the mitochondrial form of phosphatidylserine decarboxylase Psd1 produced a clear increase in cycloheximide resistance (Fig. 1). Importantly, overproduction of a Golgi/vacuolar-localized form of this enzyme (Psd2) (52) had no effect on cycloheximide resistance. Dpl1 is an endoplasmic reticulum-localized lyase that cleaves phosphorylated long-chain bases into an aldehyde moiety and produces ethanolamine phosphate (47). Increased Dpl1 activity can elevate PE levels in cells (4), but overproduction of this enzyme did not influence cycloheximide tolerance. Overproduction of enzymes (Cho2 or Opi3) involved in PC biosynthesis had no effect on cycloheximide resistance.
FIG. 1.

Overproduction of Psd1 elevates cycloheximide resistance. (A) Biosynthetic pathway leading to production of the three major phospholipids in S. cerevisiae. Phosphatidylserine (PS) is decarboxylated by the enzymes Psd1 or Psd2 to produce PE. PE is then methylated by the Cho2 and Opi3 enzymes to form PC. (B) A wild-type strain was transformed with low-copy-number plasmids carrying transcriptional fusions between the GAL promoter and the indicated ORF. Transformants were grown to mid-log phase in selective medium with galactose as the carbon source and then placed on solid medium containing a gradient of cycloheximide (Cyh) (indicated by the bar of increasing width). Each spot contained 1,000 cells of each indicated transformant strain.
Catalytically inactive Psd1 still elevates cycloheximide resistance.
Since Pdr5 is thought to support outward PE movement in cells (27, 43), we wanted to test whether increased Psd1 levels might elevate intracellular PE levels, leading to increased Pdr5 function. A high-copy-number plasmid expressing an epitope-tagged form of Psd1 was constructed in which Psd1 expression was driven from the wild-type PSD1 promoter. A mutant form of Psd1 that was expected to have no catalytic activity was also constructed to determine whether enzymatically inactive Psd1 could still influence PDR5 expression. Mutations in an LGST sequence located at the C-terminal end of phosphatidylserine decarboxylase proteins prevent autoproteolytic cleavage catalyzed by the serine residue (32). This proteolysis would normally produce large b and small a subunits that constitute the active enzyme (31). We replaced the LGS tripeptide starting at position 461 in the S. cerevisiae enzyme with three alanine residues with the goal of preventing normal enzymatic maturation. This mutant form was designated LGS461AAA and was expressed in a high-copy-number plasmid. The wild-type and mutant forms of Psd1 were transformed into a wild-type strain, tested for the ability to confer drug resistance, and analyzed by Western blotting.
The presence of wild-type PSD1 on a high-copy-number plasmid led to the appearance of an elevated cycloheximide resistance phenotype (Fig. 2A) but did not influence resistance to oligomycin. Oligomycin resistance is influenced by the YOR1 ABC transporter-encoding gene, another target of Pdr pathway regulation (17, 26). This result suggests that, as in the case of [rho0] cells, increased Psd1 levels are primarily responded to by increased PDR5 expression, as YOR1 expression is only weakly elevated in [rho0] cells (18, 50). Surprisingly, the LGS461AAA mutant form of Psd1 was unaffected in its ability to induce cycloheximide resistance (Fig. 2B). This finding argues that catalytic activity of Psd1 and by extension PE levels are not involved in triggering increased drug resistance. The lack of correlation between increased PE biosynthesis and increased drug resistance is consistent with a previous study using PSD1 carried on a high-copy-number plasmid, which did not detect increased intracellular PE (4). The presence of wild-type PSD1 on a low-copy-number plasmid did not influence drug resistance, consistent with a requirement for high-level production of Psd1.
FIG. 2.
Processing and induction of drug resistance by Psd1. (A) A high-copy-number clone (2μm PSD1-HA) expressing epitope-tagged Psd1 was introduced into wild-type cells and tested for the ability to elevate resistance to cycloheximide (CYH) or oligomycin (OLI). Serial dilutions of cells transformed with the vector plasmid alone were used as negative controls. (B) A wild-type strain was transformed with an empty high-copy-number plasmid (vector), a low-copy-number (CEN) or high-copy-number (2μm) plasmid expressing a wild-type form of PSD1-HA, or a high-copy-number plasmid containing a mutant form of PSD1 (LGS461AAA PSD1-HA). Transformants were grown to mid-log phase and placed on rich medium containing a gradient of cycloheximide indicated as described for Fig. 1. (C) The transformants from panel B were analyzed by Western blotting using antibodies against the epitope tag (α-HA) or Vph1 (α-Vph1) as a loading control. Molecular mass standards in kilodaltons are denoted by the numbers at the right.
Western blot analysis (Fig. 2C) of wild-type and LGS461AAA Psd1 indicated that the wild-type enzyme is likely to be cleaved at its C terminus like the bacterial and mammalian enzymes (30, 31). The mutant protein produced no low-molecular-weight peptide, indicating that loss of the LGS motif was sufficient to prevent formation of the small C-terminal a fragment. We believe that the upper form of LGS461AAA Psd1-HA represents the full-length protein while the lower form results from N-terminal processing of localization information, but the key finding is that this mutant form of the enzyme cannot be properly matured. These data are consistent with a recent analysis of Psd1 processing in yeast (39). As expected, failure to process the C terminus of Psd1 causes the LGS461AAA mutant to fail to function biologically in terms of phospholipid biosynthesis (see below).
To confirm that elevated Psd1 levels induce Pdr5 expression and cycloheximide resistance, several different approaches to assess Pdr5 function were used to evaluate functional levels of this membrane transporter.
High levels of Psd1 induce PDR5 expression via the Pdr pathway.
Previous work has established that Pdr5 efficiently mediated the energy-dependent efflux of the fluorescent dye rhodamine G from yeast cells (29). This dye was used to test the membrane transport activity of Pdr5 in the presence of low and high gene dosages of PSD1. A high-copy-number plasmid containing PSD1 or the empty vector was introduced into isogenic wild-type or pdr5Δ cells. Transformants were grown to mid-log phase and rhodamine G efflux measured (Fig. 3A).
FIG. 3.
Induction of Pdr5-dependent transport and expression by overproduction of Psd1. (A) Wild-type (wt) or isogenic pdr5Δ cells were transformed by a high-copy-number vector plasmid or the same plasmid containing the PSD1 gene. These transformants were grown to mid-log phase and then analyzed for rhodamine G efflux activity as described previously (29). Efflux measurements were conducted after de-energizing the cells in the presence or absence of added 1 mM glucose to restore ATP levels. Rhodamine fluorescence in the supernatants of the cells was determined and plotted on the graph. These data are representative values that varied less than 20% over at least two independent trials. (B) The chromosomal PSD1 promoter was replaced by strong glycolytic glyceraldehyde-3-phosphate dehydrogenase (encoded by the TDH3 gene) promoter using a PCR-based recombination approach (22). This otherwise wild-type strain (TDH3-PSD1) was grown along with the isogenic wild-type and pdr5Δ cells to mid-log phase. Whole-cell protein extracts were prepared, resolved by SDS-PAGE, and analyzed by Western blotting. The antibodies used were directed against Pdr5 (α-Pdr5) or Vph1 (α-Vph1).
High-copy-number PSD1 induced more than a twofold increase in rhodamine G transport compared to single-copy gene dosage. This increase in dye transport was dependent on Pdr5, as it did not occur in a pdr5Δ background.
To determine whether the increased Pdr5 transport activity was correlated with an increase in Pdr5 expression, Western blot analysis was carried out using a rabbit anti-Pdr5 antibody (12). A strain in which the PSD1 promoter was replaced with the TDH3 promoter, which provides strong, constitutive expression (35), was constructed to facilitate elevated Psd1 levels. Whole-cell protein extracts were made from this strain and isogenic wild-type or pdr5Δ strains. Equal amounts of protein extracts were then resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blotting using anti-Pdr5 or anti-Vph1 antibodies (Fig. 3B).
Steady-state levels of Pdr5 were elevated in the strain containing the TDH3-PSD1 promoter fusion compared to the wild type. This TDH3-PSD1-containing strain was also highly cycloheximide resistant (data not shown). The increase in cycloheximide resistance seen when Psd1 is overproduced can be simply explained by the observed elevation of Pdr5 expression levels.
Increased Pdr5 expression typically results from increased transcription of the PDR5 gene (reviewed in reference 13, 38). A PDR5-lacZ fusion gene has been extensively documented to serve as an accurate monitor for PDR5 gene transcription (18, 23, 25). Wild-type and TDH3-PSD1-containing cells were transformed with this PDR5-lacZ fusion as well as with PDR15-, SNQ2-, and TRP5-lacZ fusion genes carried on low-copy-number plasmids. PDR15 has previously been found to respond to mitochondrial signals in a fashion similar to that of PDR5, while SNQ2 does not (56). However, both PDR15 and SNQ2, like PDR5, contain Pdr1/Pdr3 response elements in their promoters and respond similarly to other pathway signals such as hyperactive alleles of Pdr1 (9, 34, 55). TRP5 is involved in tryptophan biosynthesis and is independent of Pdr pathway regulation (23).
Both PDR5 and PDR15 were induced by at least 400% in the Psd1-overproducing cells (Fig. 4A). SNQ2 was elevated less than twofold, consistent with the modest activation seen for this gene in [rho0] cells (56). TRP5 exhibited no significant response to high levels of Psd1. This analysis indicates that the pattern of gene regulation induced by Psd1 overproduction closely resembles that seen in [rho0] cells. Previous work has demonstrated that activation of PDR5 transcription in [rho0] cells selectively requires the Pdr3 transcription factor and is independent of the presence of its homologue Pdr1 (18). To determine the genetic requirements for Psd1 induction of PDR5 transcription, an isogenic series of strains that contained variable gene dosages of PDR1 and/or PDR3 was used. These strains were transformed with a high-copy-number plasmid carrying PSD1 or with an empty vector plasmid. Transformants were compared for their ability to tolerate cycloheximide challenge (Fig. 4B).
FIG. 4.
The Pdr pathway responds to elevated Psd1 levels. (A) Wild-type (wt) or isogenic TDH3-PSD1 cells were transformed with low-copy-number plasmids containing the indicated lacZ fusion genes. Transformants were grown to mid-log phase and assayed for β-galactosidase activities as described previously (16). (B) A collection of isogenic strains containing variable dosages of PDR1 and/or PDR3 were transformed with a high-copy-number plasmids containing PSD1 or the empty vector. Transformants were grown to mid-log phase and then placed on medium containing a gradient of cycloheximide (Cyh).
Removal of PDR3 from an otherwise wild-type strain prevented the Psd1-mediated increase in cycloheximide resistance that was induced by the 2μm PSD1 clone. Loss of PDR1 caused cells to become hypersensitive to cycloheximide, as seen before (1), but had no detectable effect on Psd1 induction of drug resistance. A pdr1Δ pdr3Δ double mutant strain was extremely drug sensitive and unresponsive to high-copy-number PSD1.
Together, these data strongly argue that elevation in Psd1 expression triggered a Pdr3-dependent elevation in PDR5 gene transcription. This behavior is strikingly similar to the Pdr3-mediated activation of PDR5 expression that occurs in [rho0] cells (18). A key difference between these situations is all the data reported above were generated from cells with an intact mitochondrial genome, albeit through Psd1 overproduction. To explore the role of Psd1 in regulation of Pdr3 in [rho0] cells, strains that lack their mitochondrial genome and the PSD1 gene were constructed.
PSD1 is required for normal mitochondrial signaling to PDR5.
Previous work has demonstrated that the nuclear protein Lge1 is required for mitochondrial signaling to PDR5 (56). A [rho0] lge1Δ cell was found to have lowered drug resistance and PDR5 expression than an isogenic [rho0] strain. To determine whether PSD1 participated in [rho0] signaling, a set of isogenic [rho0] strains lacking LGE1 and/or PSD1 was constructed. These strains were transformed with the reporter plasmids for PDR5 and PDR15 as described above and tested for their ability to tolerate cycloheximide challenge.
Expression of both PDR5 and PDR15 was reduced in [rho0] cells that lacked PSD1 (Fig. 5A). Elimination of LGE1 from a [rho0] psd1Δ strain did not further lower expression, consistent with the interpretation that Lge1 and Psd1 lie in a common retrograde signaling pathway from the mitochondria. This same behavior was also seen when expression of PDR3 was assessed (Fig. 5B). Activation of PDR3 is required for downstream induction of PDR5 and PDR15 (56), arguing that the defect caused by loss of PSD1 from [rho0] cells prevents increased activity of Pdr3. A [rho0] psd1Δ strain was less resistant to cycloheximide than a [rho0] strain but more drug tolerant than a [rho0] lge1Δ strain (Fig. 5C). A [rho0] psd1Δ lge1Δ strain was equally cycloheximide sensitive as a [rho0] lge1Δ strain, again supporting the conclusion that Psd1 and Lge1 defined a common signaling pathway. An important caveat to the analysis of these lge1Δ mutant strains comes from our previously reported finding that loss of Lge1 causes a reduction in fitness (56), as Lge1 is clearly involved in regulation of expression of many genes and not simply those involved in the Pdr pathway. We interpret these data to argue that loss of Lge1 causes a reduction in growth rate as well as a diminution of Pdr pathway signaling in [rho0] cells, while psd1Δ primarily influences Pdr pathway activity.
FIG. 5.
PSD1 is required for [rho0] signaling to the nucleus. (A) Isogenic [rho+] and [rho0] lacking the indicated nuclear genes were transformed with low-copy-number plasmids carrying either a PDR5- or PDR15-lacZ fusion gene. Transformants were grown and assayed for β-galactosidase levels as described for Fig. 4. (B) A low-copy-number plasmid containing a PDR3-lacZ fusion gene was introduced into the indicated strains. Transformants were assayed for β-galactosidase activity using the fluorescent substrate as described for Fig. 3. (C) Strains containing the relevant genotypes indicated at the left hand were tested for resistance to cycloheximide (Cyh) using a gradient plate assay as described for Fig. 1. wt, wild type.
Two control strains were constructed to probe the specificity of Psd1 signaling. A [rho0] psd2Δ strain was generated and found to exhibit cycloheximide resistance identical to that of a [rho0] strain. A [rho0] cyb2Δ strain was tested for drug resistance to ensure that loss of any protein localized to the mitochondrial intermembrane space did not lower cycloheximide resistance. These data confirm that Psd1 is required to support normal retrograde signaling in [rho0] cells and eliminate any concern that Psd1-mediated activation of PDR5 expression occurs solely as a result of Psd1 overexpression.
The amino terminus of Psd1 is necessary and sufficient to induce PDR5 expression.
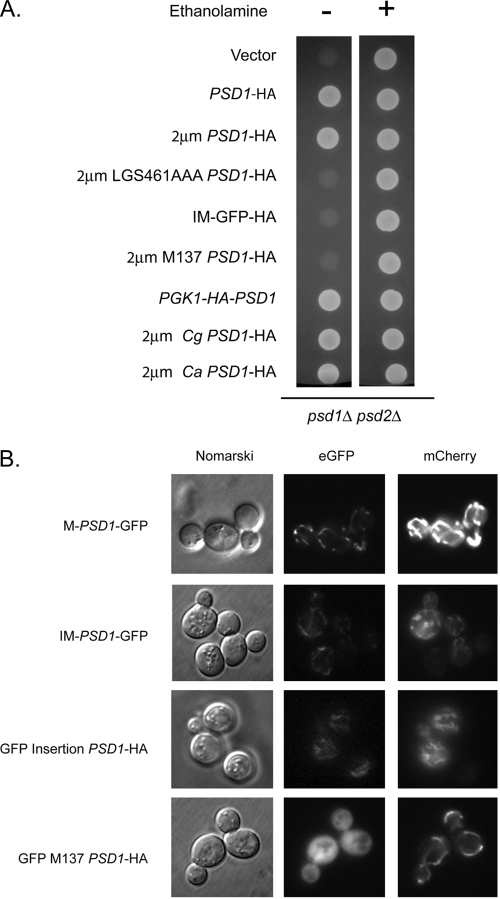
The finding described above that the catalytic activity of Psd1 was not required to induce PDR5 expression prompted us to test truncation derivatives of this enzyme to map the region required to participate in retrograde signaling. Based both on earlier experiments of others and on alignments of various Psd1 homologues (46), the amino-terminal ca. 100 residues were predicted to be involved in mitochondrial targeting of the protein. Since the LGS461AAA Psd1 retained the ability to induce cycloheximide resistance but was unable to complement ethanolamine auxotrophy of a psd1Δ psd2Δ strain (Fig. 6), this suggested that the catalytic function of Psd1 was not required for regulation of PDR5 expression. To further test this idea, the entire catalytic region of Psd1 was replaced by GFP. This construct maintained the original epitope tag and contained the N-terminal information for targeting to Psd1 residue 104. This fusion was designated IM-GFP, since it was expected to be delivered to the usual intermembrane space location of wild-type Psd1.
FIG. 6.
Complementation of ethanolamine auxotrophy by and localization of Psd1 derivatives. (A) Cells lacking phosphatidylserine decarboxylase activity (psd1Δ psd2Δ) were transformed with plasmids expressing the indicated forms of Psd1. Transformants were spotted on medium containing (+) or lacking (−) ethanolamine and then incubated at 30°C. (B) Plasmids expressing the indicated forms of Psd1 were introduced into wild-type cells along with a control plasmid expressing mitochondrially targeted mCherry (3). Transformants were grown to mid-log phase and visualized by differential interference (Nomarski) or fluorescence microscopy for GFP or mCherry.
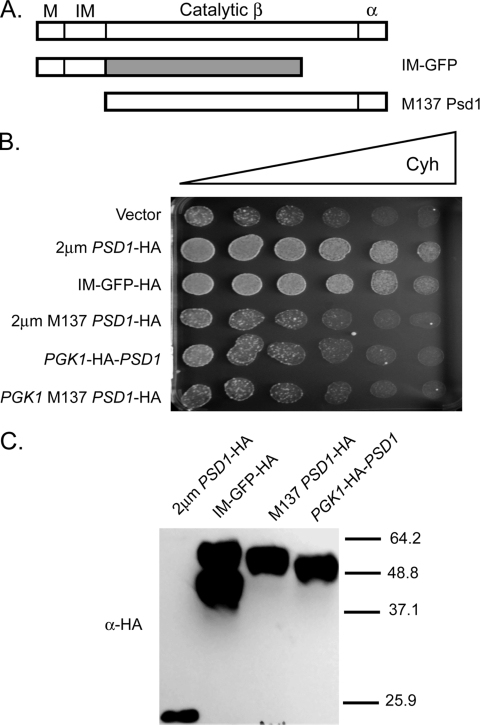
Two other constructs were generated. One expressed residues 137 to 500 (M137 Psd1) but lacked the amino-terminal targeting information. This truncation mutant was expressed with a C-terminal epitope tag or an amino-terminal tag. Wild-type Psd1 was also expressed from the PGK1 promoter with an amino-terminal HA tag as a control. These clones were introduced into wild-type cells and tested for drug resistance, steady-state protein levels, and ability to confer ethanolamine prototrophy (Fig. 6 and 7).
FIG. 7.
The amino terminus of Psd1 is sufficient to induce drug resistance. (A) The potential functional domains of Psd1 are represented along with two mutant constructs used to map the minimal signaling domain. Wild-type Psd1 is shown at the top. M indicates the mitochondrial targeting signal, while IM denotes the inner membrane localization motif. The two catalytic subunits are designated a and b. The small a subunit is generated by autoproteolysis between G462 and S463 in the C terminus of Psd1. The IM-GFP construct replaces the catalytic a and b domains with GFP. The M137 Psd1 derivative deletes the targeting information from the protein and initiates translation at methionine 137. (B) High-copy-number plasmids expressing the indicated forms of Psd1 were tested for the ability to elevate cycloheximide (Cyh) resistance as described for Fig. 1. (C) Whole-cell protein extracts were prepared from wild-type cells expressing the indicated forms of Psd1 and analyzed by Western blotting using an anti-HA antibody. Molecular mass standards are indicated on the right.
Strikingly, the IM-GFP fusion protein was able to drive high-level cycloheximide resistance in a manner indistinguishable from wild-type Psd1 (Fig. 7B). Importantly, introduction of a low-copy-number plasmid expressing IM-GFP into [rho0] psd1Δ cells restored full cycloheximide resistance, indicating that this protein was functional at lower levels of expression (data not shown). Neither form of the M137 polypeptide retained the ability to modulate drug resistance, nor did the N-terminally tagged full-length Psd1. Only the HA-tagged full-length Psd1 was capable of supporting ethanolamine-independent growth (Fig. 6). These data strongly support the beliefs that the catalytic region of Psd1 is not required for signaling and that the wild-type amino-terminal domain of this enzyme is both necessary and sufficient for inducing drug resistance.
Western blot analysis of these constructs provided information on two features of Psd1 processing (Fig. 7C). First, the IM-GFP protein was cleaved into two forms differing by roughly 5 kDa. This is consistent with the upper species representing the primary translation product and the lower form corresponding to Psd1 with the amino-terminal mitochondrial targeting signal removed. Second, the M137 Psd1 form contains a C terminus that is identical to that of wild-type Psd1 (including the LGST processing site), but no detectable fragment a was detected from this construct. This mutant demonstrated that either the amino terminus of Psd1 is required for C-terminal processing or mislocalized Psd1 fails to produce the properly matured C-terminal segment.
Other fungal Psd1 enzymes activate drug resistance.
Previous work has demonstrated that Candida glabrata also exhibits retrograde regulation of multidrug resistance in [rho0] cells (48). Candida albicans is the major human fungal pathogen, but C. glabrata infections have increased in incidence so that this organism now represents nearly 25% of all nosocomial fungal infections (reviewed in reference 41). Both Candida enzymes were expressed in S. cerevisiae to test the possibility that the Psd1 signaling function might be conserved in these pathogenic organisms. The Candida proteins were transcribed from the S. cerevisiae PSD1 promoter as epitope-tagged molecules to allow their expression and processing to be assessed. Plasmids expressing these fungal Psd1 enzymes were introduced into psd1Δ psd2Δ S. cerevisiae cells. Transformants were evaluated for their ability to complement the ethanolamine auxotrophy of this strain as well as to elevate cycloheximide resistance. Western blotting was also carried out to examine the relative expression and processing of each form of Psd1 (Fig. 6 and 8).
FIG. 8.
Overproduction of Candida Psd1 homologues elevate drug resistance. Wild-type cells were transformed with a low-copy-number plasmid expressing epitope-tagged Psd1 (PSD1-HA), an empty high-copy-number vector, or high-copy-number plasmids expressing S. cerevisiae (2μm PSD1-HA), C. glabrata (2μm Cg PSD1-HA), or C. albicans (2μm Ca PSD1-HA) epitope-tagged Psd1 homologues. Transformants were grown to mid-log phase and tested for cycloheximide (Cyh) resistance using a gradient plate assay (A) or Western blotting (B) with the indicated antisera. Molecular mass standards are indicated on the right.
Both Candida proteins were able to restore ethanolamine-independent growth to the psd1Δ psd2Δ strain. Importantly, these Psd1 homologues also stimulated cycloheximide resistance like the S. cerevisiae protein, consistent with these enzymes being able to engage the same signaling apparatus in this foreign environment. Western blot analysis demonstrated that the Candida enzymes were processed at their C termini to produce small a peptides in a manner similar to that for the S. cerevisiae protein. These findings support the belief that Psd1 regulation of multidrug resistance may be conserved across fungal evolution, but direct demonstration of Psd1 signaling in the two Candida species is required to confirm this idea.
Localization determinants of Psd1.
Since correct mitochondrial localization of Psd1 is likely to be required for its normal function, several GFP fusions to different segments of Psd1 were expressed in cells to confirm the predicted location of targeting information in the enzyme. GFP was inserted between residues 104 and 110 in the amino terminus of Psd1 to generate a fully functional GFP insertion Psd1 chimera. This GFP-Psd1 construct was able to confer ethanolamine-independent growth and elevated cycloheximide resistance like the wild-type protein (data not shown). GFP was fused to the M137 Psd1 truncation at the amino terminus. Two other constructs that contain different segments of the Psd1 N terminus fused to GFP were produced: M-GFP and IM-GFP. M-GFP contains the amino-terminal 45 amino acids fused to GFP, while the IM-GFP fusion contains 104 amino-terminal residues. All these fusion proteins were produced from high-copy-number plasmids and were introduced into wild-type cells. A control plasmid expressing a Cox4-mCherry fusion protein was introduced along with each GFP fusion-expressing plasmid to serve as a control for authentic mitochondrial localization (3). Transformants were grown to mid-log phase and visualized by Nomarski optics and fluorescence microscopy (Fig. 6).
The GFP insertion Psd1 fusion protein localized to cytoplasmic structures that were also labeled by the mitochondrially directed mCherry protein, as expected for proteins demonstrating mitochondrial localization. This same colocalization was observed for both the M-GFP and IM-GFP fusion proteins, consistent with these chimeras being localized to the mitochondria. However, the GFP-M137 Psd1 construct showed diffuse cytoplasmic fluorescence, supporting the notion that the localization information for Psd1 was contained within the amino-terminal segment of the protein as expected.
Signaling by Psd1 correlates with changes in fractionation properties.
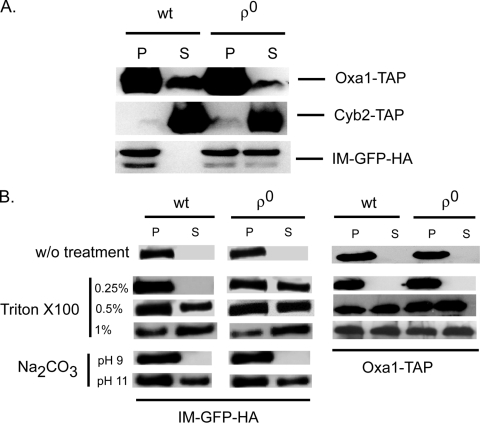
The dramatic change in membrane structure seen in [rho0] cells (40) suggested the possibility that the submitochondrial location of Psd1 may be critical in determining whether this protein engages the signaling pathway leading to PDR5 activation. To explore this possibility, we examined the behavior of the IM-GFP fusion protein in [rho+] and [rho0] cells to determine if this change in mitochondrial genome status influenced the biochemical fractionation of this signaling-competent mutant form of Psd1. Cells were broken by glass bead lysis in the presence of sorbitol and centrifuged at 10,000 × g to produce soluble (S10) and pellet (P10) fractions. Aliquots of each fraction were analyzed by Western blotting for the presence of the IM-GFP protein as well as marker proteins (Fig. 9).
FIG. 9.
Fractionation of Psd1 is altered in [rho0] cells. Isogenic [rho+] or [rho0] strains were transformed with cassettes expressing TAP fusion proteins (Oxa1-TAP or Cyb2-TAP) or a low-copy-number plasmid producing the IM-GFP-HA form of Psd1. Transformants were grown in selective minimal medium to mid-log phase. (A) Whole-cell protein extracts were generated by glass bead lysis using a sorbitol-containing buffer (54), and these extracts were separated into P10 (P) and S10 (S) fractions by centrifugation at 10,000 × g. Equal amounts of protein from each fraction were then resolved on SDS-PAGE and analyzed by Western blotting with anti-TAP or anti-HA antibodies. (B) The P10 fraction from panel A was resuspended in sorbitol buffer (without [w/o] treatment) or in sorbitol buffer containing the indicated concentrations of Triton X-100 or Na2CO3 added to raise the pH to the value noted. These samples were then mixed and centrifuged again at 10,000 × g. These P10 and S10 fractions were Western blotted as described for panel A. wt, wild type.
IM-GFP moves from a strict enrichment in the P10 fraction in [rho+] cells to a nearly equal distribution in both the S10 and P10 fractions upon loss of the mitochondrial genome. This is consistent with the idea that IM-GFP submitochondrial localization has changed in [rho0] cells, permitting generation of the signal that will ultimately induce PDR5 transcription in these cells. Importantly, blotting for control proteins confirmed that the observed redistribution of IM-GFP was not also seen in other mitochondrial proteins. A TAP-tagged version of Oxa1 (Oxa1-TAP), which is a component of the mitochondrial inner membrane (28), is found exclusively in the P10 fractions in both [rho+] and [rho0] cells. Similarly, Cyb2-TAP, a protein normally found in the mitochondrial intermembrane space (45), is found in the S10 fractionation irrespective of organellar genome status. We also performed this fractionation with full-length Psd1 containing a C-terminal epitope tag and found the same redistribution to the S10 fractions (data not shown).
To further examine the membrane association of IM-GFP in [rho+] and [rho0] cells, the P10 fractions were isolated and treated with various concentrations of Triton X-100 detergent. A low level of detergent was able to release nearly 50% of IM-GFP from its association with the P10 fraction of [rho0] cells, while at least 0.5% Triton X-100 was necessary to solubilize significant levels of IM-GFP from [rho+] cells. A control protein (Oxa1-TAP) was solubilized by 0.5% Triton X-100 in both [rho+] and [rho0] cells. These data are consistent with the view that Psd1 membrane association is altered in [rho0] cells, an alteration that may explain the Psd1-dependent activation of PDR5 expression in this genetic background.
DISCUSSION
This work has provided new insight into the retrograde signaling pathway connecting the mitochondria to expression of PDR genes in yeast. Previous experiments with both S. cerevisiae and C. glabrata indicated that the multidrug resistance phenotype was elevated through increased expression of ABC transporter genes in [rho0] cells from both organisms (18, 48, 50). Here we identify the first signal in a [rho+] genetic background that triggers a similar regulatory pathway. Although overproduction of Psd1 is sufficient to induce PDR5 expression and multidrug resistance in [rho+] cells, this enzyme is also required for full activation of these same parameters in [rho0] cells. S. cerevisiae and C. glabrata share the ability to tolerate loss of their mitochondrial genome to give rise to [rho0] strains. Our finding that overproduction of the C. albicans Psd1 in S. cerevisiae is capable of participating in retrograde regulation in this heterologous environment suggests that this pathway might be conserved in petite negative yeasts. Eukaryotic microorganisms that do not normally tolerate loss of their mitochondrial genome are called petite negative (reviewed in reference 7). C. albicans is a petite-negative yeast and has multidrug resistance genes encoding proteins very similar to Pdr5 from S. cerevisiae (44, 49). The C. albicans Pdr5 homologue CDR1 is transcriptionally controlled by a zinc cluster protein called Tac1 that resembles Pdr1 and Pdr3 among other S. cerevisiae transcription factors of this class (8). While understanding of C. albicans multidrug resistance gene regulation is at an early stage, no evidence for clear conservation of retrograde signaling has yet been obtained. We are currently evaluating the possibility that Psd1 still serves as a regulatory signal for multidrug resistance in this important human pathogen.
Previously, we identified the nuclear factor Lge1 as an upstream component of the retrograde pathway inducing PDR3 expression and ultimately multidrug resistance (56). The finding that Psd1 also lies upstream from Pdr3 prompted us to examine the epistasis between LGE1 and PSD1 in retrograde signaling. Our analysis indicated that these two genes defined a common pathway leading to activation of PDR5 transcription. Importantly, even in the lge1Δ psd1Δ [rho0] strain, a lower but significant level of PDR5 induction was observed (Fig. 5). This result argues that at least two different signaling pathways that affect Pdr3 function are activated in [rho0] cells. One of these proceeds through Psd1 to Lge1, while the other involves as-yet-unknown factors. Identification of components in this second retrograde-responsive pathway is a central experimental goal of current work.
The finding that both a catalytically inactive form of wild-type Psd1 and the amino-terminal 104 residues fused to GFP were sufficient to induce PDR5 expression was unexpected. The original rationale driving the examination of Psd1 as a possible candidate involved in PDR5 signaling came from the role of this mitochondrial enzyme in producing PE, which in turn could be transported by Pdr5 (27, 43). Mapping the minimum required signaling domain of Psd1 to its amino-terminal 104 residues strongly suggests that this protein may interact through this domain with some other factor to control PDR5 expression. We believe that this interaction is likely controlled by the localization of Psd1, since overproduction in [rho+] cells or the absence of Psd1 in [rho0] cells influences PDR5 expression. Our working hypothesis is that loss of normal Psd1 localization within the mitochondria due to overproduction or in [rho0] cells allows this enzyme to interact with another molecule, which in turn generates a signal that is finally detected by Pdr3. However, Psd1 or mutant derivatives must be delivered to the mitochondria to function in retrograde signaling, as overproduction of a form of Psd1 lacking mitochondrial localization signals fails to induce drug resistance (Fig. 7). We have also found that overproduction of Psd1 in [rho+] cells leads to an increase in Psd1 found in the S10 fraction, using an analysis similar to that for Fig. 9 (data not shown). Together, these data suggest that in both situations in which Psd1 signals to PDR5, Psd1 membrane attachment is weakened. We are currently examining submitochondrial localization of Psd1 in detail to provide details underlying the observed difference in biochemical fractionation of signaling-active Psd1.
These data also provide new insight into the proteolytic processing of phosphatidylserine decarboxylase enzymes. In E. coli, Psd is produced and processed in the cytoplasm to form the mature α and β chains (31). Mammalian Psd is localized to the mitochondria and processed in a fashion similar to that for the yeast enzyme, but where the proteolytic cleavage occurs is not known (30). Here we provide the first demonstration that mitochondrial localization of Psd1 is obligatory for cleavage of the a and b chains of this eukaryotic enzyme. The M137 form of Psd1 is produced to a high level in cells, but no detectable cleavage of its wild-type C terminus can be seen (Fig. 7). Since this protein accumulates in the cytoplasm, we cannot determine if the amino terminus is required for processing or if the lack of mitochondrial accumulation elicits this processing failure. Irrespective of the precise cause, the effect of preventing cleavage at the LGST site is clear for this mutant. To our knowledge, this is the first example of failure of a Psd protein containing a wild-type C terminus to process. Our data argue against the possibility that production of the intact b-a monomer is sufficient to produce a processed form of this enzyme. It is possible that either another protein and/or the proper internal mitochondrial residence is required for faithful production of the processed Psd1.
Production of the two Candida proteins in S. cerevisiae indicates that these Psd1 homologues can replace the function of the endogenous protein in both biosynthesis of PE and induction of PDR5 expression. We have recently found that overproduction of the C. glabrata enzyme in this pathogenic organism leads to induction of azole resistance (J. Schmidt, unpublished data). This finding supports the idea that Psd1 localization is a regulatory determinant of multidrug resistance, at least this Candida species. We are in the process of assessing the possible conservation of this signaling axis in C. albicans. Mammals also express a mitochondrial Psd1 (30), and at least one report describes [rho0] hepatoma cells that overproduce an important multidrug-resistant ABC transporter, ABCB1 (P-glycoprotein) (42). These observations provide a starting point to test the possibility that a similar retrograde pathway might be present in mammalian cells, linking phosphatidylserine decarboxylase to multidrug resistance gene expression.
Acknowledgments
This work was supported by NIH grants GM49825 and GM75120.
We thank Wayne Rieckhof and Dennis Voelcker for useful discussions, Ben Glick for providing the mitochondrially targeted mCherry plasmid, and Karl Kuchler for the Pdr5 antiserum.
Footnotes
Published ahead of print on 21 July 2008.
REFERENCES
- 1.Balzi, E., W. Chen, S. Ulaszewski, E. Capieaux, and A. Goffeau. 1987. The multidrug resistance gene PDR1 from Saccharomyces cerevisiae. J. Biol. Chem. 26216871-16879. [PubMed] [Google Scholar]
- 2.Balzi, E., M. Wang, S. Leterme, L. Van Dyck, and A. Goffeau. 1994. PDR5: a novel yeast multidrug resistance transporter controlled by the transcription regulator PDR1. J. Biol. Chem. 2692206-2214. [PubMed] [Google Scholar]
- 3.Bevis, B. J., and B. S. Glick. 2002. Rapidly maturing variants of the Discosma red fluorescent protein (DsRed). Nat. Biotechnol. 2083-87. [DOI] [PubMed] [Google Scholar]
- 4.Birner, R., M. Burgermeister, R. Schneiter, and G. Daum. 2001. Roles of phosphatidylethanolamine and of its several biosynthetic pathways in Saccharomyces cerevisiae. Mol. Biol. Cell 12997-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bissinger, P. H., and K. Kuchler. 1994. Molecular cloning and expression of the S. cerevisiae STS1 gene product. J. Biol. Chem. 2694180-4186. [PubMed] [Google Scholar]
- 6.Clancey, C. J., S. C. Chang, and W. Dowhan. 1993. Cloning of a gene (PSD1) encoding phosphatidylserine decarboxylase from Saccharomyces cerevisiae by complementation of an Escherichia coli mutant. J. Biol. Chem. 26824580-24590. [PubMed] [Google Scholar]
- 7.Contamine, V., and M. Picard. 2000. Maintenance and integrity of the mitochondrial genome: a plethora of nuclear genes in the budding yeast. Microbiol. Mol. Biol. Rev. 64281-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coste, A. T., M. Karababa, F. Ischer, J. Bille, and D. Sanglard. 2004. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot. Cell 31639-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decottignies, A., L. Lambert, P. Catty, H. Degand, E. A. Epping, W. S. Moye-Rowley, E. Balzi, and A. Goffeau. 1995. Identification and characterization of SNQ2, a new multidrug ABC transporter of the yeast plasma membrane. J. Biol. Chem. 27018150-18157. [DOI] [PubMed] [Google Scholar]
- 10.Delaveau, T., A. Delahodde, E. Carvajal, J. Subik, and C. Jacq. 1994. PDR3, a new yeast regulatory gene, is homologous to PDR1 and controls the multidrug resistance phenomenon. Mol. Gen. Genet. 244501-511. [DOI] [PubMed] [Google Scholar]
- 11.Devaux, F., E. Carvajal, S. Moye-Rowley, and C. Jacq. 2002. Genome-wide studies on the nuclear PDR3-controlled response to mitochondrial dysfunction in yeast. FEBS Lett. 51525-28. [DOI] [PubMed] [Google Scholar]
- 12.Egner, R., Y. Mahé, R. Pandjaitan, and K. Kuchler. 1995. Endocytosis and vacuolar degradation of the plasma membrane-localized Pdr5 ATP-binding cassette multidrug transporter in Saccharomyces cerevisiae. Mol. Cell. Biol. 155879-5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ernst, R., R. Klemm, L. Schmitt, and K. Kuchler. 2005. Yeast ATP-binding cassette transporters: cellular cleaning pumps. Methods Enzymol. 400460-484. [DOI] [PubMed] [Google Scholar]
- 14.Gelperin, D. M., M. A. White, M. L. Wilkinson, Y. Kon, L. A. Kung, K. J. Wise, N. Lopez-Hoyo, L. Jiang, S. Piccirillo, H. Yu, M. Gerstein, M. E. Dumont, E. M. Phizicky, M. Snyder, and E. J. Grayhack. 2005. Biochemical and genetic analysis of the yeast proteome with a movable ORF collection. Genes Dev. 192816-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerami-Nejad, M., J. Berman, and C. A. Gale. 2001. Cassettes for PCR-mediated construction of green, yellow, and cyan fluorescent protein fusions in Candida albicans. Yeast 18859-864. [DOI] [PubMed] [Google Scholar]
- 16.Guarente, L. 1983. Yeast promoter and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 101181-191. [DOI] [PubMed] [Google Scholar]
- 17.Hallstrom, T. C., and W. S. Moye-Rowley. 1998. Divergent transcriptional control of multidrug resistance genes in Saccharomyces cerevisiae. J. Biol. Chem. 2732098-2104. [DOI] [PubMed] [Google Scholar]
- 18.Hallstrom, T. C., and W. S. Moye-Rowley. 2000. Multiple signals from dysfunctional mitochondria activate the pleiotropic drug resistance pathway in Saccharomyces cerevisae. J. Biol. Chem. 27537347-37356. [DOI] [PubMed] [Google Scholar]
- 19.Hirata, D., K. Yano, K. Miyahara, and T. Miyakawa. 1994. Saccharomyces cerevisiae YDR1, which encodes a member of the ATP-binding cassette (ABC) superfamily, is required for multidrug resistance. Curr. Genet. 26285-294. [DOI] [PubMed] [Google Scholar]
- 20.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 7751-59. [DOI] [PubMed] [Google Scholar]
- 21.Ito, H., Y. Fukuda, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janke, C., M. M. Magiera, N. Rathfelder, C. Taxis, S. Reber, H. Maekawa, A. Moreno-Borchart, G. Doenges, E. Schwob, E. Schiebel, and M. Knop. 2004. A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast 21947-962. [DOI] [PubMed] [Google Scholar]
- 23.Katzmann, D. J., P. E. Burnett, J. Golin, Y. Mahe, and W. S. Moye-Rowley. 1994. Transcriptional control of the yeast PDR5 gene by the PDR3 gene product. Mol. Cell. Biol. 144653-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katzmann, D. J., E. A. Epping, and W. S. Moye-Rowley. 1999. Mutational disruption of plasma membrane trafficking of Saccharmyces cerevisiae Yor1p, a homologue of mammalian multidrug resistance protein. Mol. Cell. Biol. 192998-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katzmann, D. J., T. C. Hallstrom, Y. Mahe, and W. S. Moye-Rowley. 1996. Multiple Pdr1p/Pdr3p binding sites are essential for normal expression of the ATP binding cassette transporter protein-encoding gene PDR5. J. Biol. Chem. 27123049-23054. [DOI] [PubMed] [Google Scholar]
- 26.Katzmann, D. J., T. C. Hallstrom, M. Voet, W. Wysock, J. Golin, G. Volckaert, and W. S. Moye-Rowley. 1995. Expression of an ATP-binding cassette transporter-encoding gene (YOR1) is required for oligomycin resistance in Saccharomyces cerevisiae. Mol. Cell. Biol. 156875-6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kean, L. S., A. M. Grant, C. Angeletti, Y. Mahé, K. Kuchler, R. S. Fuller, and J. W. Nichols. 1997. Plasma membrane translocation of fluorescent-labeled phosphatidylethanolamine is controlled by transcription regulators, PDR1 and PDR3. J. Cell Biol. 138255-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kermorgant, M., N. Bonnefoy, and G. Dujardin. 1997. Oxa1p, which is required for cytochrome c oxidase and ATP synthase complex formation, is embedded in the mitochondrial inner membrane. Curr. Genet. 31302-307. [DOI] [PubMed] [Google Scholar]
- 29.Kolaczkowski, M., M. van der Rest, A. Cybularz-Kolaczkowski, J.-P. Soumillion, W. N. Konings, and A. Goffeau. 1996. Anticancer drugs, ionophoric peptides and steroids as substrates of the yeast multidrug transporter Pdr5p. J. Biol. Chem. 27131543-31548. [DOI] [PubMed] [Google Scholar]
- 30.Kuge, O., K. Saito, M. Kojima, Y. Akamatsu, and M. Nishijima. 1996. Post-translational processing of the phosphatidylserine decarboxylase gene product in Chinese hamster ovary cells. Biochem. J. 31933-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, Q. X., and W. Dowhan. 1988. Structural characterization of Escherichia coli phosphatidylserine decarboxylase. J. Biol. Chem. 26311516-11522. [PubMed] [Google Scholar]
- 32.Li, Q. X., and W. Dowhan. 1990. Studies on the mechanism of formation of the pyruvate prosthetic group of phosphatidylserine decarboxylase from Escherichia coli. J. Biol. Chem. 2654111-4115. [PubMed] [Google Scholar]
- 33.Liu, Z., and R. A. Butow. 2006. Mitochondrial retrograde signaling. Annu. Rev. Genet. 40159-185. [DOI] [PubMed] [Google Scholar]
- 34.Mahe, Y., A. Parle-McDermott, A. Nourani, A. Delahodde, A. Lamprecht, and K. Kuchler. 1996. The ATP-binding cassette multidrug transporter Snq2 of Saccharomyces cerevisiae: a novel target for the transcription factors Pdr1 and Pdr3. Mol. Microbiol. 20109-117. [DOI] [PubMed] [Google Scholar]
- 35.McAlister, L., and M. J. Holland. 1985. Differential expression of the three yeast glyceraldehyde-3-phosphate dehydrogenase genes. J. Biol. Chem. 26015019-15027. [PubMed] [Google Scholar]
- 36.Meyers, S., W. Schauer, E. Balzi, M. Wagner, A. Goffeau, and J. Golin. 1992. Interaction of the yeast pleiotropic drug resistance genes PDR1 and PDR5. Curr. Genet. 21431-436. [DOI] [PubMed] [Google Scholar]
- 37.Moye-Rowley, W. S. 2005. Retrograde regulation of multidrug resistance in Saccharomyces cerevisiae. Gene 35415-21. [DOI] [PubMed] [Google Scholar]
- 38.Moye-Rowley, W. S. 2003. Transcriptional control of multidrug resistance in the yeast Saccharomyces. Prog. Nucleic Acids Res. Mol. Biol. 73251- 279. [DOI] [PubMed] [Google Scholar]
- 39.Nebauer, R., I. Schuiki, B. Kulterer, Z. Trajanoski, and G. Daum. 2007. The phosphatidylethanolamine level of yeast mitochodria is affected by the mitochondrial components Oxa1p and Yme1p. FEBS J. 2746180-6190. [DOI] [PubMed] [Google Scholar]
- 40.Paumard, P., J. Vaillier, B. Coulary, J. Schaeffer, V. Soubannier, D. M. Mueller, D. Brethes, J.-P. di Rago, and J. Velours. 2002. The ATP synthase is involved in generating mitochondrial cristae morphology. EMBO J. 21221-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfaller, M. A., D. J. Diekema, R. N. Jones, H. S. Sader, A. C. Fluit, R. J. Hollis, and S. A. Messer. 2001. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J. Clin. Microbiol. 393254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pillay, V., R. D. Martinus, J. S. Hill, and D. R. Phillips. 1998. Upregulation of P-glycoprotein in rat hepatoma rho(o) cells: implications for drug-DNA interactions. J. Cell Biochem. 69463-469. [PubMed] [Google Scholar]
- 43.Pomorski, T., R. Lombardi, H. Riezman, P. F. Devaux, G. van Meer, and J. C. Holthuis. 2003. Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol. Biol. Cell 141240-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prasad, R., P. Dewergifosse, A. Goffeau, and E. Balzi. 1995. Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr. Genet. 27320-329. [DOI] [PubMed] [Google Scholar]
- 45.Reid, G. A., T. Yonetani, and G. Schatz. 1982. Import of proteins into mitochondria. Import and maturation of the mitochondrial intermembrane space enzymes cytochrome b2 and cytochrome c peroxidase in intact yeast cells. J. Biol. Chem. 25713068-13074. [PubMed] [Google Scholar]
- 46.Rontein, D., W. I. Wu, D. R. Voelker, and A. D. Hanson. 2003. Mitochondrial phosphatidylserine decarboxylase from higher plants. Functional complementation in yeast, localization in plants, and overexpression in Arabidopsis. Plant Physiol. 1321678-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saba, J. D., F. Nara, A. Bielawska, S. Garrett, and Y. A. Hannun. 1997. The BST1 gene of Saccharomyces cerevisiae is the sphingosine-1-phosphate lyase. J. Biol. Chem. 27226087-26090. [DOI] [PubMed] [Google Scholar]
- 48.Sanglard, D., F. Ischer, and J. Bille. 2001. Role of ATP-binding cassette transporter gene in high-frequency acquisition of resistance to azole antifungals in Candida glabrata. Antimicrob. Agents Chemother. 451174-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanglard, D., F. Ischer, M. Monod, and J. Bille. 1997. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology 143405-416. [DOI] [PubMed] [Google Scholar]
- 50.Traven, A., J. M. Wong, D. Xu, M. Sopta, and C. J. Ingles. 2001. Interorganellar communication. Altered nuclear gene expression profiles in a yeast mitochondrial DNA mutant. J. Biol. Chem. 2764020-4027. [DOI] [PubMed] [Google Scholar]
- 51.Trotter, P. J., J. Pedretti, and D. R. Voelker. 1993. Phosphatidylserine decarboxylase from Saccharomyces cerevisiae. Isolation of mutants, cloning of the gene, and creation of a null allele. J. Biol. Chem. 26821416-21424. [PubMed] [Google Scholar]
- 52.Trotter, P. J., and D. R. Voelker. 1995. Identification of a non-mitochondrial phosphatidylserine decarboxylase activity (PSD2) in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2706062-6070. [DOI] [PubMed] [Google Scholar]
- 53.Wach, A., A. Brachat, R. Pohlmann, and P. Philippsen. 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 101793-1808. [DOI] [PubMed] [Google Scholar]
- 54.Wemmie, J. A., and W. S. Moye-Rowley. 1997. Mutational analysis of the Saccharomyces cerevisiae ATP-binding cassette transporter protein Ycf1p. Mol. Microbiol. 25683-694. [DOI] [PubMed] [Google Scholar]
- 55.Wolfger, H., Y. Mahé, A. Parle-McDermott, A. Delahodde, and K. Kuchler. 1997. The yeast ATP binding cassette (ABC) protein genes PDR10 and PDR15 are novel targets for the Pdr1 and Pdr3 transcriptional regulators. FEBS Lett. 418269-274. [DOI] [PubMed] [Google Scholar]
- 56.Zhang, X., A. Kolaczkowska, F. Devaux, S. L. Panwar, T. C. Hallstrom, C. Jacq, and W. S. Moye-Rowley. 2005. Transcriptional regulation by Lge1p requires a function independent of its role in histone H2B ubiquitination. J. Biol. Chem. 2802759-2770. [DOI] [PubMed] [Google Scholar]
- 57.Zhang, X., and W. S. Moye-Rowley. 2001. Saccharomyces cerevisiae multidrug resistance gene expression inversely correlates with the status of the Fo component of the mitochondrial ATPase. J. Biol. Chem. 27647844-47852. [DOI] [PubMed] [Google Scholar]