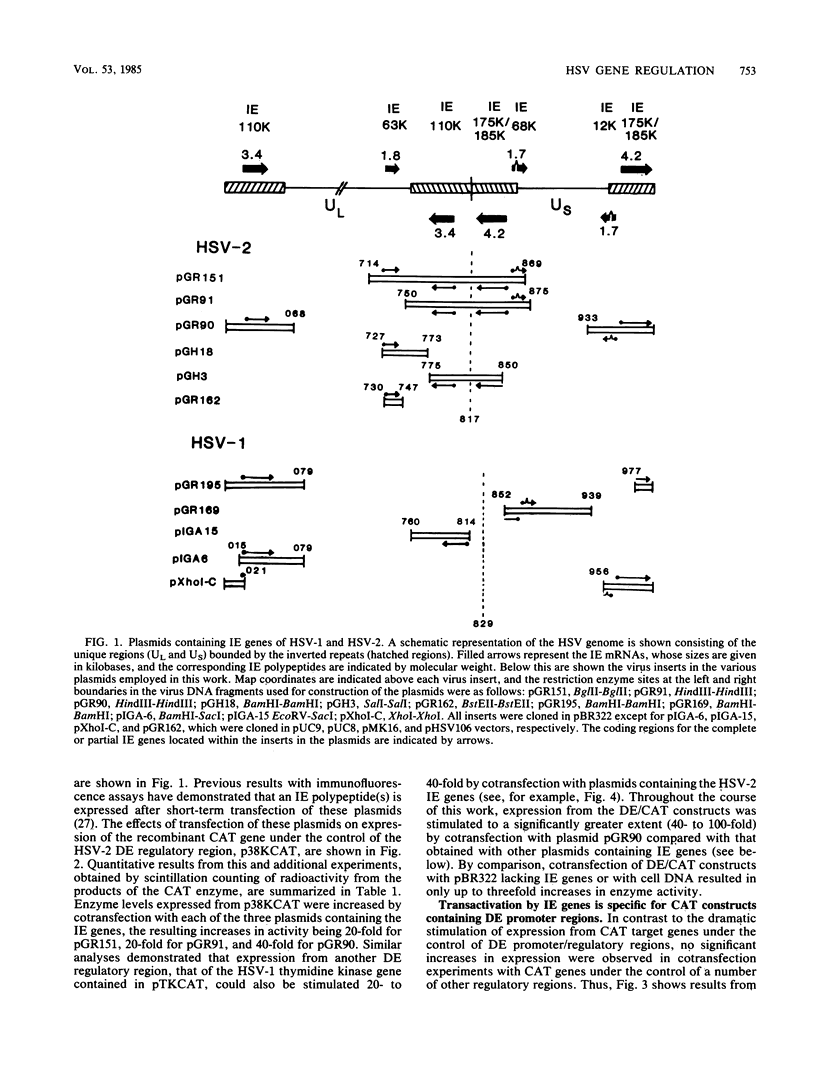
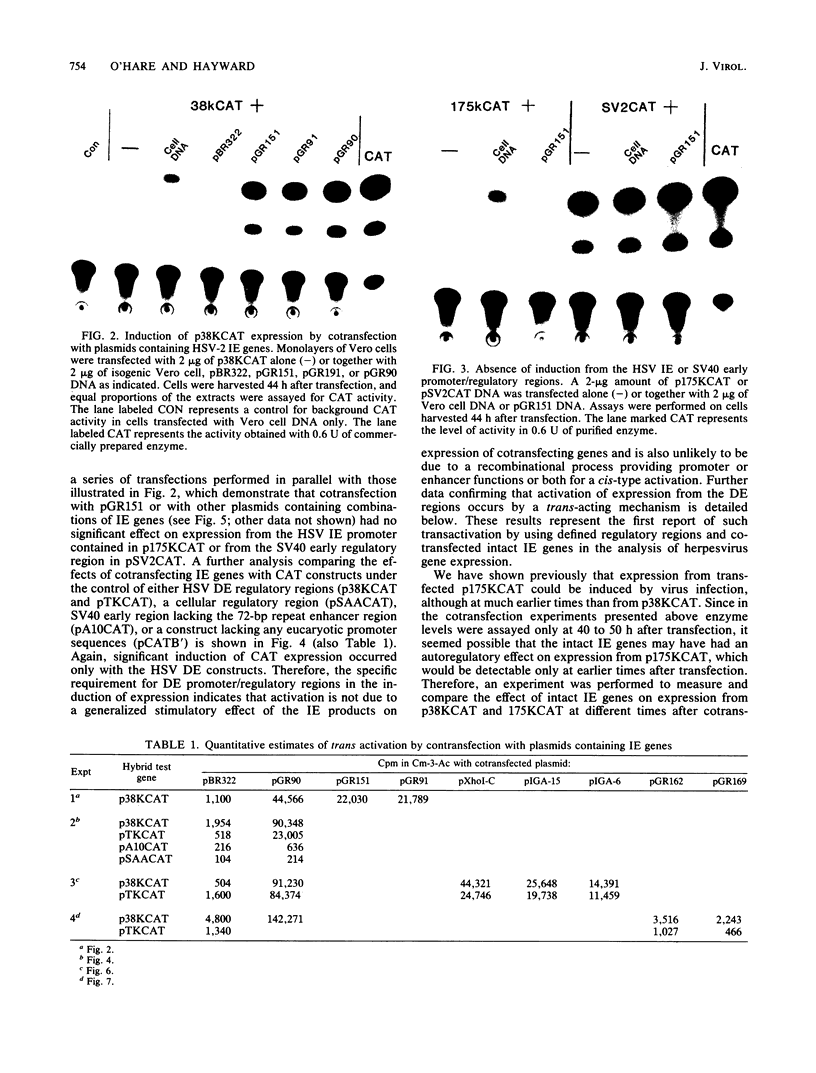
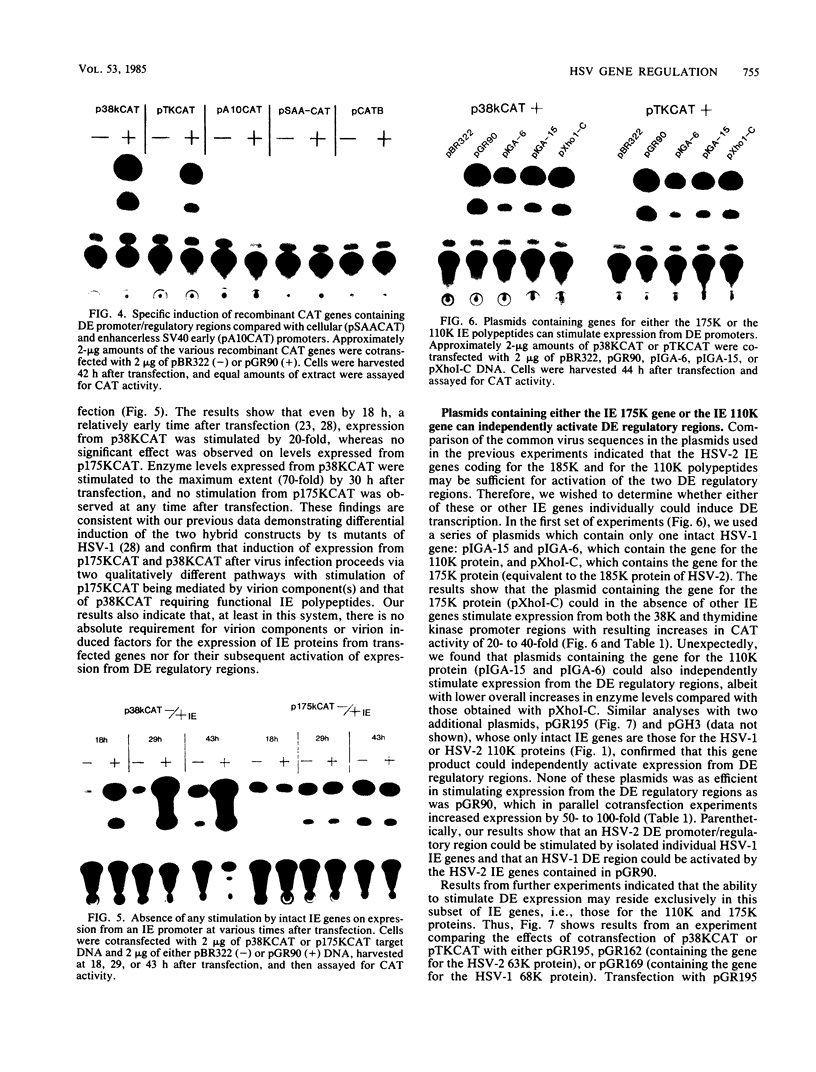
Abstract
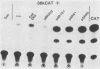
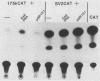
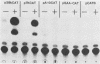
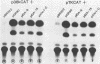
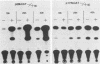
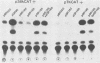
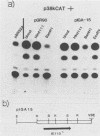
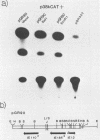
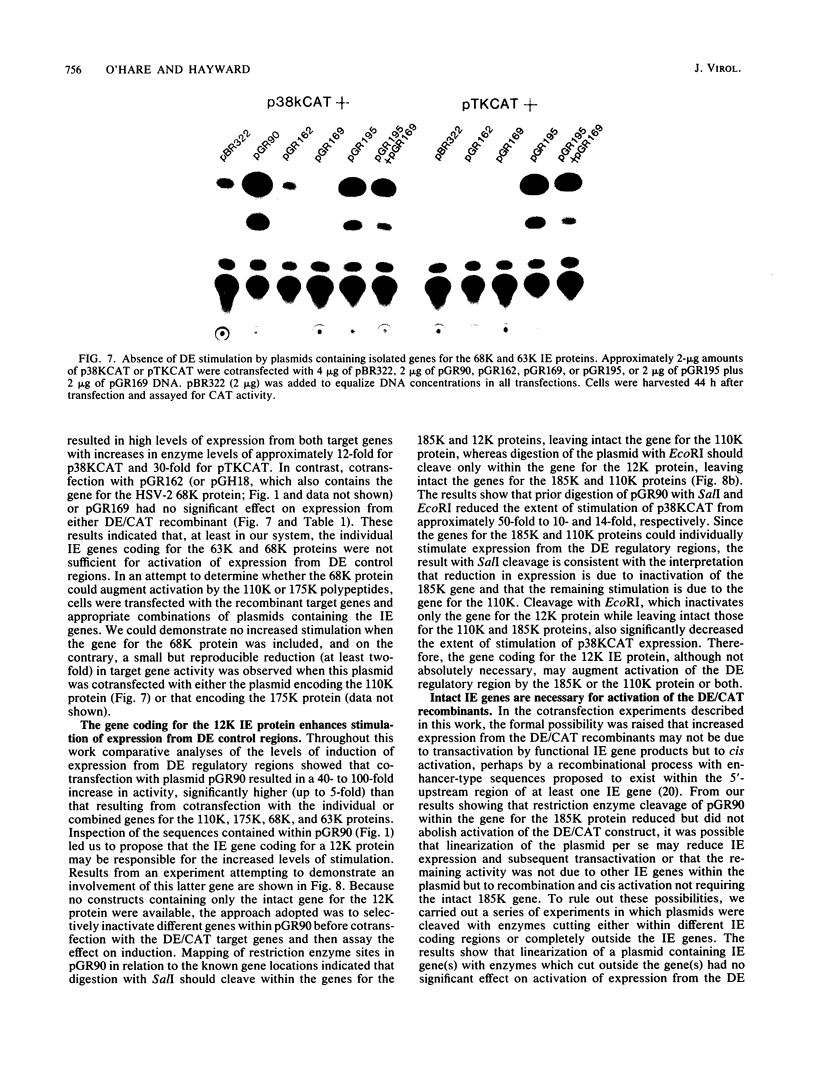
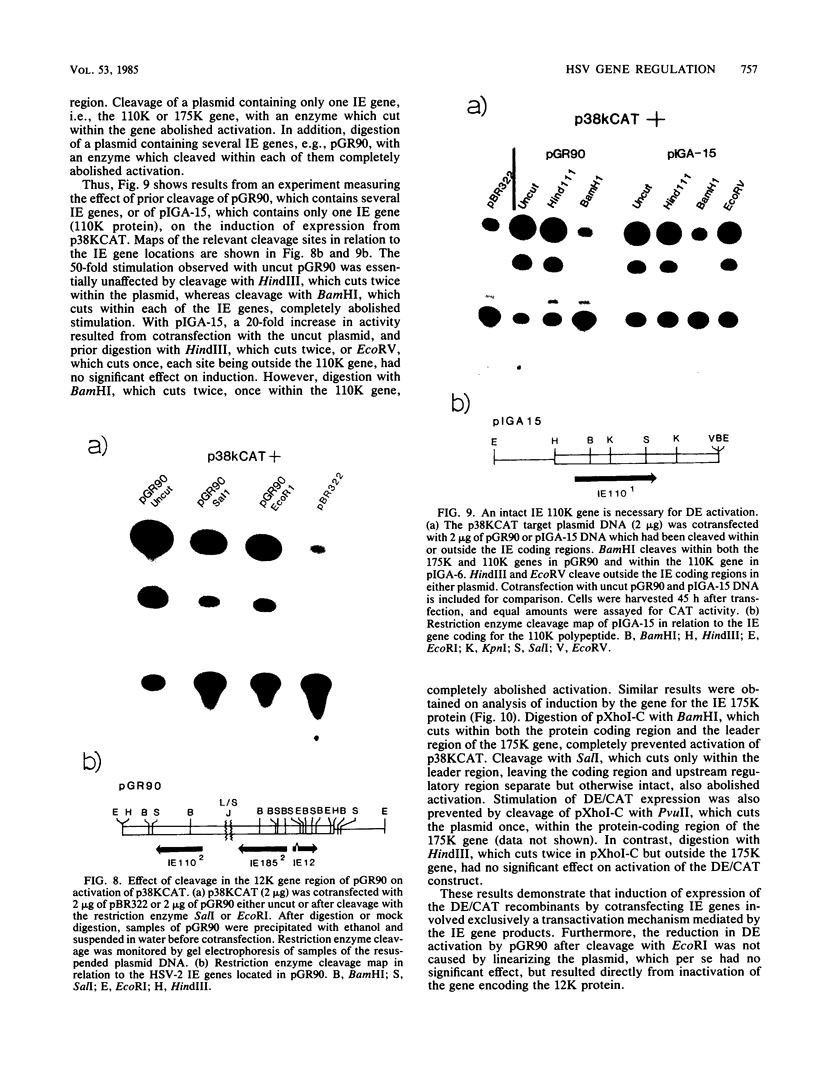
We reconstructed the regulated induction of delayed-early (DE) transcription that occurs during herpes simplex virus (HSV) infection by using a transient expression system in which recombinant target genes were cotransfected into Vero cells together with intact activating genes. Plasmids containing cloned HSV-1 or HSV-2 immediate-early (IE) genes stimulated by up to 100-fold the expression from recombinant constructs containing the bacterial chloramphenicol acetyltransferase (CAT) gene under the control of the DE promoter/regulatory region from the genes for an HSV-2 38,000-molecular-weight (38K) protein and the HSV-1 thymidine kinase. This activation was specific to hybrid genes containing DE regulatory regions since no significant increases in expression were observed in cotransfection experiments with the CAT gene without any promoter region or under the control of a number of other regulatory regions, including an HSV-1 IE regulatory region, the complete or enhancerless early regulatory region of simian virus 40, and an inducible cellular promoter/regulatory region. By using a variety of cotransfected plasmids containing individual or different combinations of HSV-1 or HSV-2 IE genes, we show that of the five known IE genes, two, those coding for the 175K and 110K polypeptides, each possessed the ability to stimulate expression from both DE promoters. Cleavage of the input plasmids within the known coding regions for the 175K and 110K proteins abolished stimulation of DE/CAT gene expression, whereas cleavage outside the coding regions had no effect on stimulation. We conclude that stimulation of CAT expression occurred exclusively by a transactivation mechanism in which the products encoded by these IE genes acted on the DE hybrid constructs at the transcription level. No transcriptional stimulatory function was demonstrated for the IE 68K and 63K proteins, although our results indicate that the IE 12K protein may augment the DE stimulatory activity of the 175K and 110K proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. P., Costa R. H., Holland L. E., Wagner E. K. Characterization of herpes simplex virus type 1 RNA present in the absence of de novo protein synthesis. J Virol. 1980 Apr;34(1):9–27. doi: 10.1128/jvi.34.1.9-27.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. M., Harland J., Subak-Sharpe J. H. Isolation of restriction endonuclease site deletion mutants of herpes simplex virus. J Gen Virol. 1984 Jun;65(Pt 6):1053–1068. doi: 10.1099/0022-1317-65-6-1053. [DOI] [PubMed] [Google Scholar]
- Clements J. B., Watson R. J., Wilkie N. M. Temporal regulation of herpes simplex virus type 1 transcription: location of transcripts on the viral genome. Cell. 1977 Sep;12(1):275–285. doi: 10.1016/0092-8674(77)90205-7. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Draper K. G., Banks L., Powell K. L., Cohen G., Eisenberg R., Wagner E. K. High-resolution characterization of herpes simplex virus type 1 transcripts encoding alkaline exonuclease and a 50,000-dalton protein tentatively identified as a capsid protein. J Virol. 1983 Dec;48(3):591–603. doi: 10.1128/jvi.48.3.591-603.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo F., Campadelli-Fiume G., Foa-Tomasi L., Cassai E. Evidence that herpes simplex virus DNA is transcribed by cellular RNA polymerase B. J Virol. 1977 Mar;21(3):996–1001. doi: 10.1128/jvi.21.3.996-1001.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca N. A., Courtney M. A., Schaffer P. A. Temperature-sensitive mutants in herpes simplex virus type 1 ICP4 permissive for early gene expression. J Virol. 1984 Dec;52(3):767–776. doi: 10.1128/jvi.52.3.767-776.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Schaffer P. A. Fine-structure mapping and functional analysis of temperature-sensitive mutants in the gene encoding the herpes simplex virus type 1 immediate early protein VP175. J Virol. 1980 Oct;36(1):189–203. doi: 10.1128/jvi.36.1.189-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper K. G., Frink R. J., Wagner E. K. Detailed characterization of an apparently unspliced beta herpes simplex virus type 1 gene mapping in the interior of another. J Virol. 1982 Sep;43(3):1123–1128. doi: 10.1128/jvi.43.3.1123-1128.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D. A detailed analysis of an HSV-1 early promoter: sequences involved in trans-activation by viral immediate-early gene products are not early-gene specific. Nucleic Acids Res. 1984 Apr 11;12(7):3037–3056. doi: 10.1093/nar/12.7.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D. DNA sequence elements required for regulated expression of the HSV-1 glycoprotein D gene lie within 83 bp of the RNA capsites. Nucleic Acids Res. 1983 Oct 11;11(19):6647–6666. doi: 10.1093/nar/11.19.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff C. G. Chemical structure of a modification of the Escherichia coli ribonucleic acid polymerase alpha polypeptides induced by bacteriophage T4 infection. J Biol Chem. 1974 Oct 10;249(19):6181–6190. [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Holland L. E., Anderson K. P., Shipman C., Jr, Wagner E. K. Viral DNA synthesis is required for the efficient expression of specific herpes simplex virus type 1 mRNA species. Virology. 1980 Feb;101(1):10–24. doi: 10.1016/0042-6822(80)90479-1. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XI. Identification and relative molar rates of synthesis of structural and nonstructural herpes virus polypeptides in the infected cell. J Virol. 1973 Dec;12(6):1347–1365. doi: 10.1128/jvi.12.6.1347-1365.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz H. R. Control by bacteriophage T4 of two sequential phosphorylations of the alpha subunit of Escherichia coli RNA polymerase. J Mol Biol. 1974 Dec 25;90(4):727–738. doi: 10.1016/0022-2836(74)90536-1. [DOI] [PubMed] [Google Scholar]
- Imperiale M. J., Feldman L. T., Nevins J. R. Activation of gene expression by adenovirus and herpesvirus regulatory genes acting in trans and by a cis-acting adenovirus enhancer element. Cell. 1983 Nov;35(1):127–136. doi: 10.1016/0092-8674(83)90215-5. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. Evidence for translational regulation of herpes simplex virus type 1 gD expression. J Virol. 1984 Aug;51(2):389–394. doi: 10.1128/jvi.51.2.389-394.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J. C., Spandidos D. A., Wilkie N. M. Transcriptional regulation of a herpes simplex virus immediate early gene is mediated through an enhancer-type sequence. EMBO J. 1984 Feb;3(2):389–395. doi: 10.1002/j.1460-2075.1984.tb01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff T., Elkaim R., Goding C. R., Jalinot P., Sassone-Corsi P., Perricaudet M., Kédinger C., Chambon P. Individual products of the adenovirus 12S and 13S EIa mRNAs stimulate viral EIIa and EIII expression at the transcriptional level. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4381–4385. doi: 10.1073/pnas.81.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W. C., Dimock K., Smiley J. R., Bacchetti S. Herpes simplex virus thymidine kinase transcripts are absent from both nucleus and cytoplasm during infection in the presence of cycloheximide. J Virol. 1980 Nov;36(2):361–365. doi: 10.1128/jvi.36.2.361-365.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W., Sollner-Webb B. High level transient expression of a chloramphenicol acetyl transferase gene by DEAE-dextran mediated DNA transfection coupled with a dimethyl sulfoxide or glycerol shock treatment. Nucleic Acids Res. 1984 Jul 25;12(14):5707–5717. doi: 10.1093/nar/12.14.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackem S., Roizman B. Regulation of herpesvirus macromolecular synthesis: temporal order of transcription of alpha genes is not dependent on the stringency of inhibition of protein synthesis. J Virol. 1981 Oct;40(1):319–322. doi: 10.1128/jvi.40.1.319-322.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackem S., Roizman B. Regulation of herpesvirus macromolecular synthesis: transcription-initiation sites and domains of alpha genes. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7122–7126. doi: 10.1073/pnas.77.12.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLauchlan J., Clements J. B. Organization of the herpes simplex virus type 1 transcription unit encoding two early proteins with molecular weights of 140000 and 40000. J Gen Virol. 1983 May;64(Pt 5):997–1006. doi: 10.1099/0022-1317-64-5-997. [DOI] [PubMed] [Google Scholar]
- Middleton M. H., Reyes G. R., Ciufo D. M., Buchan A., Macnab J. C., Hayward G. S. Expression of cloned herpesvirus genes. I. Detection of nuclear antigens from herpes simplex virus type 2 inverted repeat regions in transfected mouse cells. J Virol. 1982 Sep;43(3):1091–1101. doi: 10.1128/jvi.43.3.1091-1101.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Hayward G. S. Expression of recombinant genes containing herpes simplex virus delayed-early and immediate-early regulatory regions and trans activation by herpesvirus infection. J Virol. 1984 Nov;52(2):522–531. doi: 10.1128/jvi.52.2.522-531.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Wolff M. H., Fenwick M., Roizman B. Regulation of herpesvirus macromolecular synthesis. V. Properties of alpha polypeptides made in HSV-1 and HSV-2 infected cells. Virology. 1977 Apr;77(2):733–749. doi: 10.1016/0042-6822(77)90495-0. [DOI] [PubMed] [Google Scholar]
- Post L. E., Mackem S., Roizman B. Regulation of alpha genes of herpes simplex virus: expression of chimeric genes produced by fusion of thymidine kinase with alpha gene promoters. Cell. 1981 May;24(2):555–565. doi: 10.1016/0092-8674(81)90346-9. [DOI] [PubMed] [Google Scholar]
- Post L. E., Roizman B. A generalized technique for deletion of specific genes in large genomes: alpha gene 22 of herpes simplex virus 1 is not essential for growth. Cell. 1981 Jul;25(1):227–232. doi: 10.1016/0092-8674(81)90247-6. [DOI] [PubMed] [Google Scholar]
- Preston C. M. Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature-sensitive mutant tsK. J Virol. 1979 Jan;29(1):275–284. doi: 10.1128/jvi.29.1.275-284.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W. D., Westphal H. Requirement for either early region 1a or early region 1b adenovirus gene products in the helper effect for adeno-associated virus. J Virol. 1984 Aug;51(2):404–410. doi: 10.1128/jvi.51.2.404-410.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rixon F. J., McGeoch D. J. A 3' co-terminal family of mRNAs from the herpes simplex virus type 1 short region: two overlapping reading frames encode unrelated polypeptide one of which has highly reiterated amino acid sequence. Nucleic Acids Res. 1984 Mar 12;12(5):2473–2487. doi: 10.1093/nar/12.5.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Clements J. B. A herpes simplex virus type 1 function continuously required for early and late virus RNA synthesis. Nature. 1980 May 29;285(5763):329–330. doi: 10.1038/285329a0. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Clements J. B. Characterization of transcription-deficient temperature-sensitive mutants of herpes simplex virus type 1. Virology. 1978 Dec;91(2):364–379. doi: 10.1016/0042-6822(78)90384-7. [DOI] [PubMed] [Google Scholar]
- Weeks D. L., Jones N. C. E1A control of gene expression is mediated by sequences 5' to the transcriptional starts of the early viral genes. Mol Cell Biol. 1983 Jul;3(7):1222–1234. doi: 10.1128/mcb.3.7.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox K. W., Kohn A., Sklyanskaya E., Roizman B. Herpes simplex virus phosphoproteins. I. Phosphate cycles on and off some viral polypeptides and can alter their affinity for DNA. J Virol. 1980 Jan;33(1):167–182. doi: 10.1128/jvi.33.1.167-182.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]