Abstract
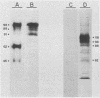
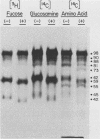
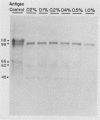
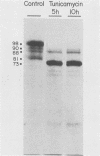
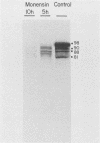
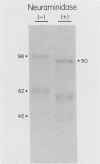
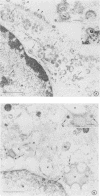
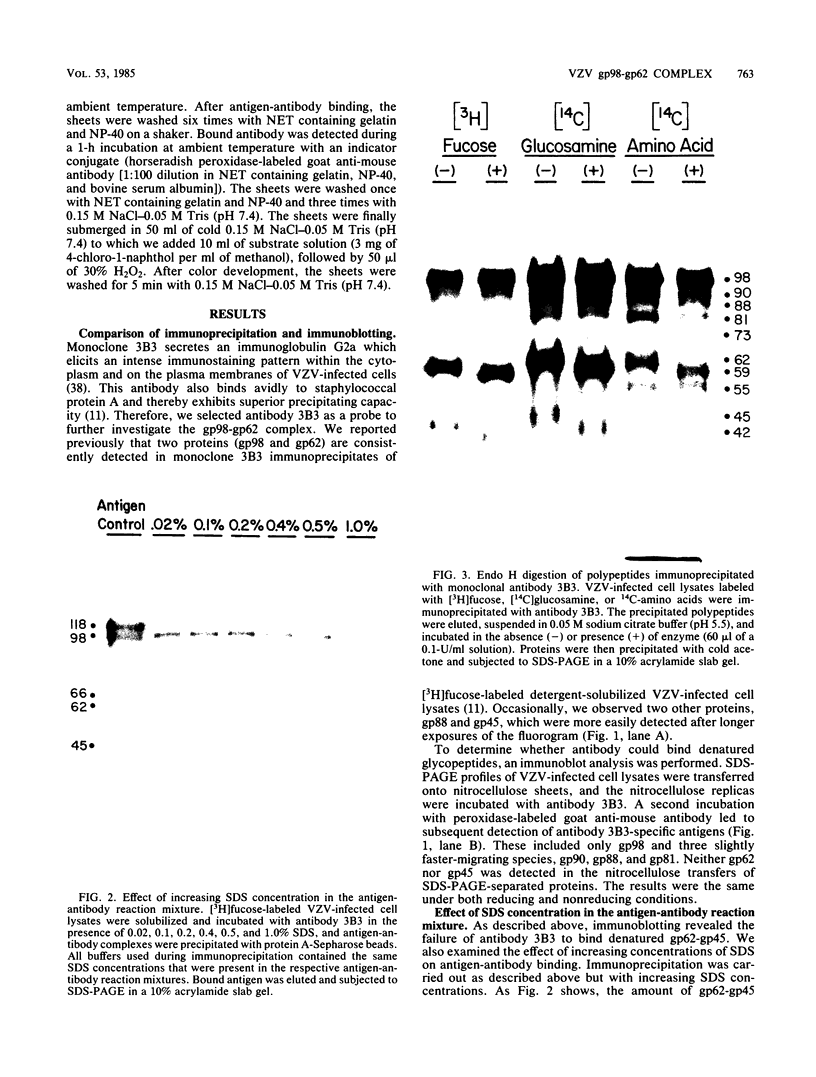
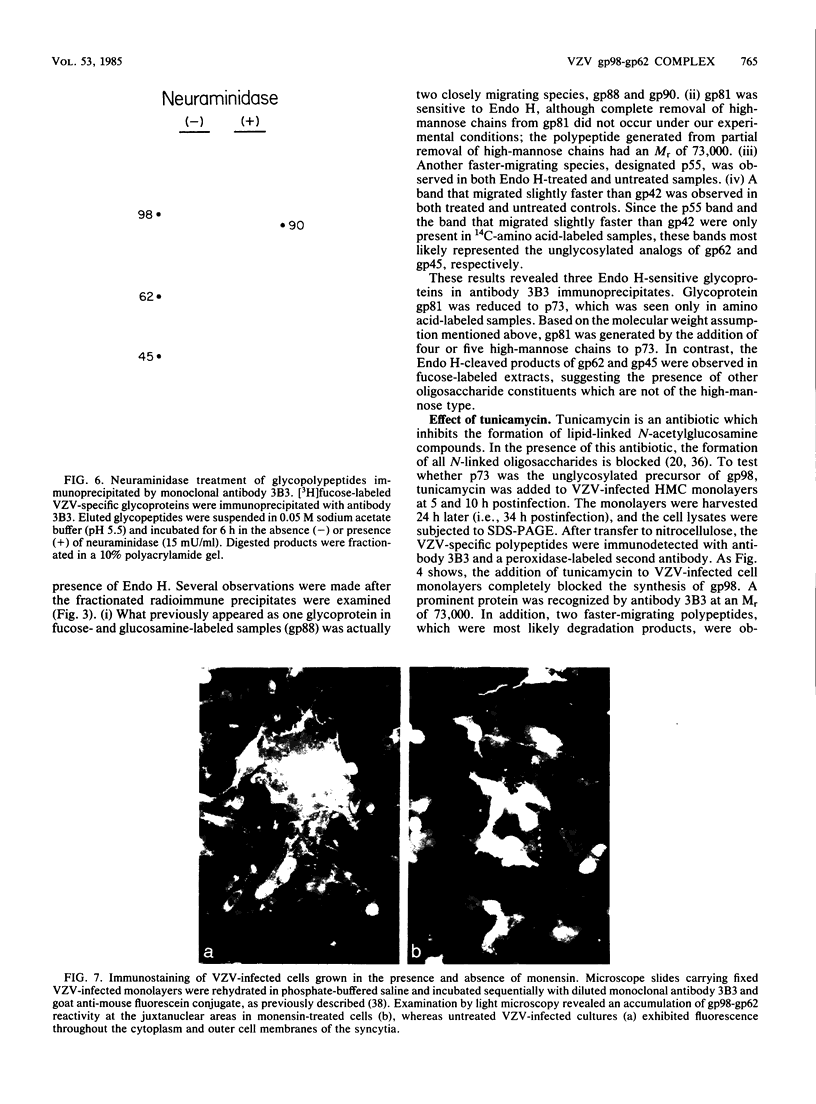
Varicella-zoster virus specifies the formation of several glycoproteins, including the preponderant gp98-gp62 glycoprotein complex in the outer membranes of virus-infected cells. These viral glycoproteins are recognized and precipitated by a previously described monoclonal antibody designated monoclone 3B3. When an immunoblot analysis was performed, only gp98 was reactive with monoclone 3B3 antibody; likewise, titration in the presence of increased concentrations of sodium dodecyl sulfate during antigen-antibody incubations caused selective precipitation of gp98 but not gp62. Further structural analyses of gp98 were performed by using the glycosidases endo-beta-N-acetylglucosaminidase H (endoglycosidase H) and neuraminidase and two inhibitors of glycosylation (tunicamycin and monensin). In addition to gp98, antibody 3B3 reacted with several intermediate products, including gp90, gp88, gp81, and a nonglycosylated polypeptide, p73. Since gp98 was completely resistant to digestion with endoglycosidase H, it contained only complex carbohydrate moieties; conversely, gp81 contained mainly high-mannose residues. Polypeptide p73 was immunodetected in the presence of tunicamycin and designated as a nascent recipient of N-linked sugars, whereas gp88 was considered to contain O-linked oligosaccharides because its synthesis was not affected by tunicamycin. The ionophore monensin inhibited production of mature gp98, but other intermediate forms, including gp90, were detected. Since the latter product was similar in molecular weight to the desialated form of gp98, one effect of monensin treatment of varicella-zoster virus-infected cells was to block the addition of N-acetylneuraminic acid. Monensin also blocked insertion of gp98 into the plasma membrane and, as determined by electron microscopy, inhibited envelopment of the nucleocapsid and its transport within the cytoplasm. On the basis of this study, we reached the following conclusions: the primary antibody 3B3-binding epitope is located on gp98, gp98 is a mature product of viral glycoprotein processing, gp98 contains both N-linked and O-linked oligosaccharide side chains, gp90 is the desialated penultimate form of gp98, gp88 is an O-linked intermediate of gp98, gp81 is the high-mannose intermediate of gp98, and p73 is the unglycosylated precursor of gp98.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achong B. G., Meurisse E. V. Observations on the fine structure and replication of varicella virus in cultivated human amnion cells. J Gen Virol. 1968 Sep;3(2):305–308. doi: 10.1099/0022-1317-3-2-305. [DOI] [PubMed] [Google Scholar]
- Alonso F. V., Compans R. W. Differential effect of monensin on enveloped viruses that form at distinct plasma membrane domains. J Cell Biol. 1981 Jun;89(3):700–705. doi: 10.1083/jcb.89.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergh M. L., Hooghwinkel G. J., van den Eijnden D. H. Biosynthesis of the O-glycosidically linked oligosaccharide chains of fetuin. Indications for an alpha-N-acetylgalactosaminide alpha 2 leads to 6 sialyltransferase with a narrow acceptor specificity in fetal calf liver. J Biol Chem. 1983 Jun 25;258(12):7430–7436. [PubMed] [Google Scholar]
- Beyer T. A., Rearick J. I., Paulson J. C., Prieels J. P., Sadler J. E., Hill R. L. Biosynthesis of mammalian glycoproteins. Glycosylation pathways in the synthesis of the nonreducing terminal sequences. J Biol Chem. 1979 Dec 25;254(24):12531–12534. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Long D., Matthews J. T., May M., Eisenberg R. Glycopeptides of the type-common glycoprotein gD of herpes simplex virus types 1 and 2. J Virol. 1983 Jun;46(3):679–689. doi: 10.1128/jvi.46.3.679-689.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fittschen C., Parmley R. T., Austin R. L., Crist W. M. Vicinal glycol-staining identifies secondary granules in human normal and Chédiak-Higashi neutrophils. Anat Rec. 1983 Mar;205(3):301–311. doi: 10.1002/ar.1092050307. [DOI] [PubMed] [Google Scholar]
- Friedrichs W. E., Grose C. Glycoprotein gp118 of varicella-zoster virus: purification by serial affinity chromatography. J Virol. 1984 Mar;49(3):992–996. doi: 10.1128/jvi.49.3.992-996.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose C., Edwards D. P., Friedrichs W. E., Weigle K. A., McGuire W. L. Monoclonal antibodies against three major glycoproteins of varicella-zoster virus. Infect Immun. 1983 Apr;40(1):381–388. doi: 10.1128/iai.40.1.381-388.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose C., Edwards D. P., Weigle K. A., Friedrichs W. E., McGuire W. L. Varicella-zoster virus-specific gp140: a highly immunogenic and disulfide-linked structural glycoprotein. Virology. 1984 Jan 15;132(1):138–146. doi: 10.1016/0042-6822(84)90098-9. [DOI] [PubMed] [Google Scholar]
- Grose C., Friedrichs W. E., Smith G. C. Purification and molecular anatomy of the varicella-zoster virion. Biken J. 1983 Mar;26(1):1–15. [PubMed] [Google Scholar]
- Grose C., Perrotta D. M., Brunell P. A., Smith G. C. Cell-free varicella-zoster virus in cultured human melanoma cells. J Gen Virol. 1979 Apr;43(1):15–27. doi: 10.1099/0022-1317-43-1-15. [DOI] [PubMed] [Google Scholar]
- Grose C. The synthesis of glycoproteins in human melanoma cells infected with varicella-zoster virus. Virology. 1980 Feb;101(1):1–9. doi: 10.1016/0042-6822(80)90478-x. [DOI] [PubMed] [Google Scholar]
- Hope R. G., Marsden H. S. Processing of glycoproteins induced by herpes simplex virus type 1: sulphation and nature of the oligosaccharide linkages. J Gen Virol. 1983 Sep;64(Pt 9):1943–1953. doi: 10.1099/0022-1317-64-9-1943. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Schlesinger M. J. Vesicular stomatitis virus and sindbis virus glycoprotein transport to the cell surface is inhibited by ionophores. Virology. 1980 Jun;103(2):407–424. doi: 10.1016/0042-6822(80)90200-7. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982 Sep;43(3):1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell. 1983 Mar;32(3):987–997. doi: 10.1016/0092-8674(83)90083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Tunicamycin inhibits glycosylation and multiplication of Sindbis and vesicular stomatitis viruses. J Virol. 1977 Jan;21(1):375–385. doi: 10.1128/jvi.21.1.375-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver R. P., Baenziger J. U., Majerus P. W. Isolation and structural characterization of the polypeptide subunits of membrane glycoprotein IIb-IIIa from human platelets. Blood. 1982 Jan;59(1):80–85. [PubMed] [Google Scholar]
- Nakamura K., Bhown A. S., Compans R. W. Glycosylation sites of influenza viral glycoproteins. Tryptic glycopeptides from the A/WSN (H0N1) hemagglutinin glycoprotein. Virology. 1980 Nov;107(1):208–221. doi: 10.1016/0042-6822(80)90286-x. [DOI] [PubMed] [Google Scholar]
- Nathenson S. G., Uehara H., Ewenstein B. M., Kindt T. J., Coligan J. E. Primary structural: analysis of the transplantation antigens of the murine H-2 major histocompatibility complex. Annu Rev Biochem. 1981;50:1025–1052. doi: 10.1146/annurev.bi.50.070181.005113. [DOI] [PubMed] [Google Scholar]
- Okuno T., Yamanishi K., Shiraki K., Takahashi M. Synthesis and processing of glycoproteins of Varicella-Zoster virus (VZV) as studied with monoclonal antibodies to VZV antigens. Virology. 1983 Sep;129(2):357–368. doi: 10.1016/0042-6822(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Olden K., Pratt R. M., Yamada K. M. Role of carbohydrates in protein secretion and turnover: effects of tunicamycin on the major cell surface glycoprotein of chick embryo fibroblasts. Cell. 1978 Mar;13(3):461–473. doi: 10.1016/0092-8674(78)90320-3. [DOI] [PubMed] [Google Scholar]
- Pizer L. I., Cohen G. H., Eisenberg R. J. Effect of tunicamycin on herpes simplex virus glycoproteins and infectious virus production. J Virol. 1980 Apr;34(1):142–153. doi: 10.1128/jvi.34.1.142-153.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins P. W., Hubbard S. C., Turco S. J., Wirth D. F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977 Dec;12(4):893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Schauer R. Characterization of sialic acids. Methods Enzymol. 1978;50:64–89. doi: 10.1016/0076-6879(78)50008-6. [DOI] [PubMed] [Google Scholar]
- Schwarz R. T., Rohrschneider J. M., Schmidt M. F. Suppression of glycoprotein formation of Semliki Forest, influenza, and avian sarcoma virus by tunicamycin. J Virol. 1976 Sep;19(3):782–791. doi: 10.1128/jvi.19.3.782-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas R. V., Melsen L. R., Compans R. W. Effects of monensin on morphogenesis and infectivity of Friend murine leukemia virus. J Virol. 1982 Jun;42(3):1067–1075. doi: 10.1128/jvi.42.3.1067-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I., Schlesinger S., Kornfeld S. Processing of high mannose oligosaccharides to form complex type oligosaccharides on the newly synthesized polypeptides of the vesicular stomatitis virus G protein and the IgG heavy chain. J Biol Chem. 1978 Feb 10;253(3):716–722. [PubMed] [Google Scholar]
- Tai T., Yamashita K., Kobata A. The substrate specificities of endo-beta-N-acetylglucosaminidases CII and H. Biochem Biophys Res Commun. 1977 Sep 9;78(1):434–441. doi: 10.1016/0006-291x(77)91273-6. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. The release of intact oligosaccharides from specific glycoproteins by endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1974 Feb 10;249(3):818–824. [PubMed] [Google Scholar]
- Tartakoff A., Hoessli D., Vassalli P. Intracellular transport of lymphoid surface glycoproteins. Role of the Golgi complex. J Mol Biol. 1981 Aug 25;150(4):525–535. doi: 10.1016/0022-2836(81)90378-8. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigle K. A., Grose C. Common expression of varicella-zoster viral glycoprotein antigens in vitro and in chickenpox and zoster vesicles. J Infect Dis. 1983 Oct;148(4):630–638. doi: 10.1093/infdis/148.4.630. [DOI] [PubMed] [Google Scholar]
- Weigle K. A., Grose C. Molecular dissection of the humoral immune response to individual varicella-zoster viral proteins during chickenpox, quiescence, reinfection, and reactivation. J Infect Dis. 1984 May;149(5):741–749. doi: 10.1093/infdis/149.5.741. [DOI] [PubMed] [Google Scholar]
- Wenske E. A., Bratton M. W., Courtney R. J. Endo-beta-N-acetylglucosaminidase H sensitivity of precursors to herpes simplex virus type 1 glycoproteins gB and gC. J Virol. 1982 Oct;44(1):241–248. doi: 10.1128/jvi.44.1.241-248.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenske E. A., Courtney R. J. Glycosylation of herpes simplex virus type 1 gC in the presence of tunicamycin. J Virol. 1983 Apr;46(1):297–301. doi: 10.1128/jvi.46.1.297-301.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]