Abstract
Decay rates of bacteriophage P22 and Staphylococcus aureus on six types of common household inanimate surfaces were evaluated based on cultivation and quantitative PCR. A much higher level of inactivation was observed using the plate assay, suggesting that detection of the pathogen genome in samples from fomites does not necessarily imply a health risk to humans.
Fomites are a potential vehicle of infectious pathogens in the environment (4, 6). Nonporous fomites are particularly important in fomite-mediated disease transmission because they appear to have more capability to transfer pathogens to hands than porous fomites (8). Molecular detection methods are being accepted in environmental monitoring of air, water, and now fomites, especially for unculturable pathogens, such as noroviruses, but attenuation of pathogen genome on dried fomites has not been evaluated in detail, nor has it been compared with culture-based inactivation rates.
Quantitative microbial risk assessment frameworks are now being used to evaluate the health risks caused by pathogens via fomites. Microbial attenuation on fomites over time is critical in the analysis. However, data on survival times and decay rates on fomite are limited, and it has been difficult to incorporate microbial decay into quantitative microbial risk assessment frameworks and disease transmission models.
Here we investigated microbial decay rates of bacteriophage P22 and Staphylococcus aureus on nonporous fomite materials using both plate assay and quantitative PCR methods. Bacteriophage P22, provided by Charles P. Gerba (University of Arizona), and S. aureus (ATCC number 25923) were used as surrogates for viruses and non-spore-forming bacteria, respectively.
Six types of nonporous fomite materials (surface areas, 10 cm2), including aluminum, ceramic, glass, plastic, stainless steel, and laminated wood, were inoculated with P22 and S. aureus in 10-μl droplets (approximately 107 PFU/cm2 of P22 and 106 CFU/cm2 of S. aureus). The fomite materials were kept at a room temperature of 25°C and ambient relative humidity of 50% until analysis. The inoculated droplets became visibly dried in 30 min. After 0, 22, 41, 66, 115, 162, 332, 552, and 859 h from inoculation, three plates for each material were collected and P22 and S. aureus were recovered from the plate using a wipe method with premoistened antistatic wipes and 150 mM phosphate-buffered saline with 0.01% Tween 80 as an extraction reagent (T. Shibata, Y. Masago, D. L. Cologgi, and J. B. Rose, submitted for publication). The P22 plaque assay was conducted using EPA method 1602 (9) with some minor modifications. For S. aureus, an aliquot of 100 μl of the eluate or dilutions with phosphate-buffered saline was spread onto BBL mannitol salt agar plates (Becton Dickinson, Sparks, MD) using a flame-sterilized bent glass rod and incubated at 35 ± 2°C for 48 h.
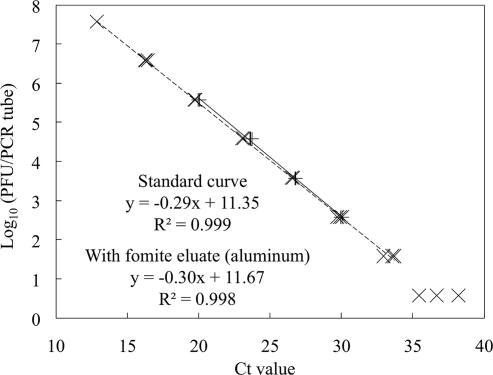
The genome DNA was extracted from 200 μl of the eluates using the QIAamp DNA minikit (Qiagen, Valencia, CA) and detected using the LightCycler 1.5 system (Roche Applied Science, Indianapolis, IN). Primers and a TaqMan probe for P22 detection were designed using the Primer Express 2.0 software program (Applied Biosystems, Foster City, CA) with the whole-genome sequence of the bacteriophage P22 strain used in this study (accession number AB362338) (Table 1). Specificity of the primers and the probe was tested by homology search using BLASTN (3) to confirm nontarget DNA could not be amplified. PCR mixtures (20 μl in total) contained 2 μl of DNA sample, 4 μl of 5× PCR master mix (LightCycler TaqMan Master; Roche Applied Science, Indianapolis, IN), 500 nM each of forward and reverse primers, and 150 nM of the TaqMan probe. Amplification was initiated using the hot start method at 95°C for 10 min; 45 cycles of 95°C for 10 s, 60°C for 20 s, and 72°C for 10 s; and cooling at 40°C for 30 s. Sensitivity analysis (Fig. 1) showed the detection limit of the assay was 3.8 PFU/tube, corresponding to 9.5 × 102 PFU/ml eluate. The genome DNA of S. aureus was detected by following the protocol of Goto et al. (5) (Table 1). The detection limit for S. aureus DNA was 7.3 CFU/PCR tube or 1.8 × 103 CFU/ml eluate. Spiking 2 μl of extracts of fomite eluates (aluminum) in the PCR standard did not change the threshold cycle values of the real-time PCR standard (Fig. 1), showing no inhibitory effect from environmental washings on the quantification of the P22 genome DNA.
TABLE 1.
Primer and TaqMan probe sequences for detection of P22 and S. aureus
| Target | Name | Sequence | Reference |
|---|---|---|---|
| P22 | P22-2F | CTT AAC AAG CTC TGA CTG CTC ATC A | This study |
| P22-2R | CCA TCG CCT GTG ACT GGA T | ||
| P22-2P | FAM-TCG CAA CGA TGC AGA ACG ACT CG-TAMRA | ||
| S. aureus | Sa0836-F | TCG AAA TTA AAT GTT GTC GTG TCT TC | 13 |
| Sa0836-R | TCA TTT TTG ACA TGR AGA GAA ACA TC | ||
| Sa0836-P | FAM-TCG CGA CAT TCA TTA TGC CCA AAT TTT TAA-TAMRA |
FIG. 1.
Standard curves for P22 qPCR detection with or without environmental contaminants (n = 3 for each concentration). The dashed line is the standard curve for P22 quantification, and the solid line is the standard curve with 2 μl of fomite eluates (aluminum) in the PCR reagent.
Microbial attenuation rates on dried fomites were expressed using the equation Nt = N0 × 10−kt, where N0 (CFU or PFU) represents the initial numbers of microorganisms, Nt (CFU or PFU) represents the numbers of microorganism at time t (hour), and parameter k [log10(CFU or PFU)/hour] is the decay rate. Data collected at time zero were not included in the inactivation model because there were still spiked droplets on fomites at that time and a dried environment was not represented.
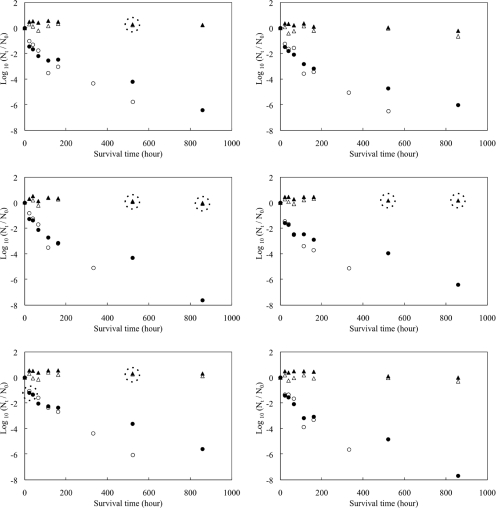
P22 survived for 859 h (36 days), while culturable S. aureus was detected at 552 h but not detected at 859 h from any types of fomites (Fig. 2). The inactivation rates of both microbes, evaluated using plate assays, were much higher than the DNA degradation rates in real-time PCR (Table 2) (P < 0.001 by t test for all microbes and fomite types). DNA degradation of S. aureus was almost undetectable, suggesting that the genomes can exist for a very long period of time after the cell has lost its viability. This result clearly showed that detection of pathogen genome on fomites indicates possible contamination of the fomites in the past, but it does not necessarily imply a health risk to humans. Use of traditional cultivation methods is still suitable for quantitative investigations of microbial fomite contamination associated with the need to evaluate the probability of infection.
FIG. 2.
Survival of P22 and S. aureus on various types of fomite surfaces over time (each plot is the geometric mean for triplicated fomite samples). The y axis shows normalized concentrations in log10(Nt/N0), where Nt is the concentration at sampling (time t) and N0 is the concentration at time zero. Dotted circles surrounding plots indicate that white plots (S. aureus) are hidden behind the black plots (P22).
TABLE 2.
Decay rates (k) of P22 and S. aureus on various types of nonporous fomites
| Organism | Fomite | Decay rate, k [log10(PFU or CFU)/h]
|
||
|---|---|---|---|---|
| Inactivation | DNA degradation | Ratio | ||
| P22 | Aluminum | 5.5 × 10−3 | 3.7 × 10−4 | 15 |
| Ceramic tiles | 5.1 × 10−3 | 6.2 × 10−4 | 8.2 | |
| Glass | 6.8 × 10−3 | 5.4 × 10−4 | 13 | |
| Plastic | 5.2 × 10−3 | 3.5 × 10−4 | 15 | |
| Stainless steel | 4.8 × 10−3 | 3.1 × 10−4 | 15 | |
| Laminated wood | 6.9 × 10−3 | 6.1 × 10−4 | 11 | |
| Pooledb | 5.2 × 10−3 | 4.5 × 10−4 | 12 | |
| S. aureus | Aluminum | 9.0 × 10−3 | −1.3 × 10−4 | NAa |
| Ceramic tiles | 1.0 × 10−2 | 4.7 × 10−4 | 22 | |
| Glass | 1.3 × 10−2 | 2.3 × 10−4 | 59 | |
| Plastic | 1.2 × 10−2 | −9.8 × 10−6 | NA | |
| Stainless steel | 9.9 × 10−3 | −7.0 × 10−5 | NA | |
| Laminated wood | 1.4 × 10−2 | 3.1 × 10−4 | 46 | |
| Pooledb | 7.2 × 10−3 | 8.7 × 10−5 | 86 | |
NA, not available, since no decay rates for genome DNA were observed.
Calculated using geometric mean concentrations of P22 or S. aureus on all fomite types.
As reported in other studies (1, 2, 7), faster inactivation was observed in the first 22 h, which includes a period of desiccation, although these microbes are more stable in liquid media than on dried fomites. Further study is necessary to investigate inactivation kinetics during desiccation. Such information would be particularly useful for fomite-mediated disease transmission models where inoculation of pathogens on fomites is usually in liquid phase, such as contaminated water, nasal secretions, and watery feces.
Acknowledgments
We acknowledge the U.S. Environmental Protection Agency and Department of Homeland Security as co-funding agencies for the Center for Advancing Microbial Risk Assessment (EPA grant RD832262).
We thank Rebecca Ives, Ai Masago, Sangeetha Srinivasan, Theng Theng Fong, and Lauren Bellows at Michigan State University for their technical support.
Footnotes
Published ahead of print on 11 July 2008.
REFERENCES
- 1.Abad, F. X., R. M. Pinto, and A. Bosch. 1994. Survival of enteric viruses on environmental fomites. Appl. Environ. Microbiol. 60:3704-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abad, F. X., C. Villena, S. Guix, S. Caballero, R. M. Pinto, and A. Bosch. 2001. Potential role of fomites in the vehicular transmission of human astroviruses. Appl. Environ. Microbiol. 67:3904-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. H. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boone, S. A., and C. P. Gerba. 2007. Significance of fomites in the spread of respiratory and enteric viral disease. Appl. Environ. Microbiol. 73:1687-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goto, M., H. Takahashi, Y. Segawa, H. Hayashidani, K. Takatori, and Y. Hara-Kudo. 2007. Real-time PCR method for quantification of Staphylococcus aureus in milk. J. Food Prot. 70:90-96. [DOI] [PubMed] [Google Scholar]
- 6.Hall, C. B., R. G. Douglas, and J. M. Geiman. 1980. Possible transmission by fomites of respiratory syncytial virus. J. Infect. Dis. 141:98-102. [DOI] [PubMed] [Google Scholar]
- 7.Panagea, S., C. Winstanley, M. J. Walshaw, M. J. Ledson, and C. A. Hart. 2005. Environmental contamination with an epidemic strain of Pseudomonas aeruginosa in a Liverpool cystic fibrosis centre, and study of its survival on dry surfaces. J. Hosp. Infect. 59:102-107. [DOI] [PubMed] [Google Scholar]
- 8.Rusin, P., S. Maxwell, and C. Gerba. 2002. Comparative surface-to-hand and fingertip-to-mouth transfer efficiency of gram-positive bacteria, gram-negative bacteria, and phage. J. Appl. Microbiol. 93:585-592. [DOI] [PubMed] [Google Scholar]
- 9.U.S. EPA. 2001. Method 1602: male-specific (F+) and somatic coliphage in water by single agar layer (SAL) procedure. EPA 821-R-01-029. U.S. Environmental Protection Agency, Washington, DC.