Abstract
Geothermal waters contain numerous potential electron donors capable of supporting chemolithotrophy-based primary production. Thermodynamic predictions of energy yields for specific electron donor and acceptor pairs in such systems are available, although direct assessments of these predictions are rare. This study assessed the relative importance of dissolved H2 and H2S as energy sources for the support of chemolithotrophic metabolism in an acidic geothermal spring in Yellowstone National Park. H2S and H2 concentration gradients were observed in the outflow channel, and vertical H2S and O2 gradients were evident within the microbial mat. H2S levels and microbial consumption rates were approximately three orders of magnitude greater than those of H2. Hydrogenobaculum-like organisms dominated the bacterial component of the microbial community, and isolates representing three distinct 16S rRNA gene phylotypes (phylotype = 100% identity) were isolated and characterized. Within a phylotype, O2 requirements varied, as did energy source utilization: some isolates could grow only with H2S, some only with H2, while others could utilize either as an energy source. These metabolic phenotypes were consistent with in situ geochemical conditions measured using aqueous chemical analysis and in-field measurements made by using gas chromatography and microelectrodes. Pure-culture experiments with an isolate that could utilize H2S and H2 and that represented the dominant phylotype (70% of the PCR clones) showed that H2S and H2 were used simultaneously, without evidence of induction or catabolite repression, and at relative rate differences comparable to those measured in ex situ field assays. Under in situ-relevant concentrations, growth of this isolate with H2S was better than that with H2. The major conclusions drawn from this study are that phylogeny may not necessarily be reliable for predicting physiology and that H2S can dominate over H2 as an energy source in terms of availability, apparent in situ consumption rates, and growth-supporting energy.
Thermophiles dominate the deepest and shortest branches of the Bacteria and Archaea domains in the tree of life, suggesting that they are likely ancestors of Earth's contemporary microbial populations (8, 35). Consequently, these organisms have attracted considerable attention due to interest in the origin of enzymes and metabolic pathways that are thought to have evolved from such organisms. Chemolithotrophic metabolism is foundational to primary productivity in geothermal environments where temperatures exceed the limit of photosynthesis. The bioenergetics of such systems have been examined from the perspective of theoretical energy yield as a way of discussing the relative importance of the various electron donors and acceptors that could support primary productivity (3-5, 22). Other studies have sought to link the inferred physiology of microbial populations with the predicted energy yields obtainable from the inorganic constituents present (4, 18, 26, 28, 33).
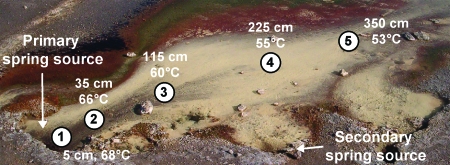
Geothermal features vary considerably with respect to temperature, pH, and the presence and concentrations of energy sources such as H2, H2S, S0, Fe(II) and As(III) (18, 29). Acid-sulfate-chloride (ASC) springs (e.g., Fig. 1) are common throughout the Yellowstone geothermal complex, although they are considerably more concentrated in and around the Norris Geyser Basin area. ASC springs are intriguing from a bioenergetics standpoint because they offer a virtual buffet of energy sources for chemolithoautotrophs, including a constant flux of μM concentrations of dissolved H2S, Fe(II), and As(III), nM concentrations of H2, conspicuous amounts of S0, and often-supersaturating levels of CO2 (10, 18, 21, 26, 37). Discussions regarding the relative importance of these electron donors in supporting primary production in such systems sometimes center on comparisons of potential energy released from their oxidation, with the H2/O2 couple perhaps being favored (37), whereas others urge caution in making such predictions, pointing out that in low-pH systems, the oxidation of H2S could yield nearly as much energy (18).
FIG. 1.
Color image of Dragon Spring in Norris Geyser Basin. The picture depicts changes in the spring outflow channel structure relative to that in previous studies (10, 21), including additional sources emerging downstream from the main source. Sampling transect sites are numbered (starting at the spring source), and representative temperatures occurring during the present study reflect the thermal gradient.
ASC springs are clearly in the latter category and are the focus of the current study, in which we examined microbial utilization of H2S and H2 by using a combination of in-field ex situ assays, microelectrodes, and gas chromatography to document the presence, concentrations, gradients, and consumption of these energy sources. These analyses were combined with molecular-community analysis and with the cultivation of ecologically relevant organisms that represent the dominant phylotypes. Results from these experiments can be summarized by two important conclusions: (i) microbial phylogeny cannot necessarily be relied upon to predict physiology, and (ii) H2S can dominate over H2 in terms of availability, apparent consumption rates, and growth-supporting energy.
MATERIALS AND METHODS
Study site and in situ chemical analysis.
Field experiments were conducted at Dragon Spring (44°43′54.8′′ N, 110°42′39.9′′W, spring number NHSP106 in the Yellowstone National Park [YNP] thermal inventory), located in Norris Geyser Basin, YNP. Water temperature and pH were routinely monitored throughout the 2-year study. The pH of the source water was 3.1 on all but two occasions, on which it was found to be 2.8, and the temperatures ranged from 68 to 72°C. Dissolved H2S was measured by using the methylene blue method (9). Spectrophotometric analysis was performed at springside, using a USB2000 portable spectrophotometer (Ocean Optics, Dunedin, FL). The presence of H2 was determined by using a portable Varian gas chromatograph (model CP-2900) with Ar and N2 as carrier gases. The concentration of aqueous H2 [H2(aq)] in each sample was determined by using the headspace gas chromatography method and temperature-corrected Henry's law constants described previously (18).
Ex situ measurements of H2S and H2 consumption activity.
Mat samples were collected by using sterile wide-bore pipette tips and were placed into sterile 15-ml conical tubes, homogenized by mixing, and split into two subsamples. One subsample was maintained at in situ temperature and was used to assay for microbial H2 or H2S consumption, while the other was boiled for 20 min to serve as a killed control. Subsamples of live and heat-treated mat material were aseptically transferred into autoclaved 150-ml serum bottles containing 40 ml of filter-sterilized spring water. The assay bottles were immediately closed with rubber septa, pressure sealed with aluminum rings, and incubated at in situ temperatures by placing the bottles in the spring at the same location from which the mat material was sampled. H2O samples were withdrawn at timed intervals to be assayed for H2S(aq), whereas headspace gas samples were taken to measure H2. Data for dissolved H2S and H2 levels were normalized based on the mat sample dry weight, which was determined by collecting all mat material from each serum bottle onto preweighed 0.2-μm filters, drying it overnight at 65°C, and then weighing it. The maximum mat sample dry weight variation between samples was 4.1%.
Cultivation and isolation.
Mat samples were collected from sites 35, 115, and 350 cm from the discharge point of the spring. The mat material was stored for transportation in 15-ml conical tubes and maintained at 65°C in a Thermos bottle filled with water from the site. Mat samples were resuspended in 10 ml of spring water collected directly above the mat-sampling site, and 100 μl of this suspension was used to inoculate 16-ml serum bottles sealed with Teflon-coated butyl rubber septa (National Scientific, Rockwood, TN). For aerobic H2S chemolithoautotroph enrichment experiments, the serum bottles contained 4.9 ml of filter-sterilized spring water amended with 200 μM Na2S and buffered to pH 3.1 with 5 mM citric acid and a headspace containing 50% ambient air and 50% CO2 for aerobic culture conditions or 90% CO2 and 10% ambient air for microaerobic conditions. For aerobic H2 chemolithoautotroph enrichment experiments, the serum bottle setup was the same, except that Na2S was omitted and headspace gas composition consisted of 50% CO2, 25% H2, and 25% ambient air. Microaerobic H2 enrichments were the same, except that the headspace composition was 50% CO2, 40% H2, and 10% ambient air. Enrichment cultures were incubated at temperatures ranging from 55 to 70°C, corresponding to in situ temperatures where the mat sample inoculum was collected. At monthly intervals, 100 μl of culture was transferred to similarly prepared serum bottles, and the enrichment cultures were monitored for growth by phase-contrast microscopy. Progress toward a clonal culture was monitored by using denaturing gradient gel electrophoresis (DGGE; see below). After 120 days (i.e., after four enrichment subcultures), cultures were diluted to ∼0.5 cells·ml−1, with 1.0 ml of this dilution used as inoculum for each of 20 similarly prepared serum bottles. These bottles were monitored for growth as above, and positive cultures (∼40% to 50% of the bottles) were again diluted and used as inocula for two additional rounds of enrichment initiated from ∼0.5 cells·ml−1 dilutions. The resulting cultures were considered clonal based on the following criteria: (i) direct sequencing of whole-culture PCR amplicons produced sequencing chromatograms showing no evidence of multiple templates, and (ii) the sequence derived from the whole-culture PCR product exactly matched that of individual sequences derived from a minimum of 20 clones.
DNA extraction, PCR, DGGE, and clone libraries.
Total DNA was extracted from enrichment cultures and isolates by using the method of Pitcher et al. (31). Nearly full-length 16S rRNA genes were amplified and cloned, using PCR primers and protocols we have previously described (11, 19). Sequencing for these clones as well as all other clones (below) was carried out at the Ohio State University Plant Microbe Genomics Facility. DGGE analysis of enrichment cultures was also carried out as previously described (11). Internally transcribed spacer (ITS) regions were amplified by using the primers and cycling conditions previously described (14).
16S rDNA clone libraries were constructed from DNA extracted (6) from mat material taken from the Dragon Spring S0 deposition zone (yellow area in Fig. 1) at sites 35 and 115 cm from the point of discharge. Nearly full-length 16S rRNA genes were amplified as described above and cloned into pCR2.1 (Invitrogen, Carlsbad, CA), using the manufacturer's suggested protocols. For each site, 45 clones were sequenced. Sequences were aligned by using ClustalX (40) and were edited and trimmed by using Se-Al v2.0a11 (http://tree.bio.ed.ac.uk/software/seal/). Phylogenetic analysis of the aligned sequences was performed, using the maximum likelihood algorithm, and bootstrap values for 100 pseudoreplicates were generated by using the PAUP* v4.0b10 software package (39). Hydrogenothermus marinus (GenBank accession number AJ292525.1), a member of the family Aquificaceae, was used as the out-group.
Pure-culture studies.
An isolate representative of the major 16S rRNA gene phylotype that was recovered from the spring was used for studies that compared growth on H2S and H2. Serum bottles were set up as described above, with cell growth rates on these substrates determined by phase-contrast microscopy cell counts. H2S and H2 consumption rates were determined by pregrowing separate cultures on either H2S or H2 to acclimate the cells to a single energy source and then transferring 2 × 106 cells from late-log-phase cultures to 10-ml sealed serum bottles containing 5 ml of filter-sterilized, degassed spring water buffered at pH 3.0 with 5 mM citric acid and containing spring-relevant concentrations of H2S [added as 30 μM Na2S(aq)] and H2 (30 nM aqueous; provided by amending the headspace to 0.15% H2 and allowing the bottles to equilibrate at 55°C in the laboratory with periodic shaking for 24 h). Upon inoculation, H2 consumption was measured immediately for a time zero (t = 0) sample and then at 20-min intervals, and the samples were analyzed on a Shimadzu GC-8A electron capture detector with a stainless steel column (Shimadzu Scientific Instruments, Inc., Columbia, MD). Analyses of the levels of dissolved H2S were performed as described above on 500-μl samples taken at 15-min intervals. Killed controls were obtained by autoclaving cultures at 121°C for 45 min. The amounts of growth of the cultures with H2S and H2 were determined with the same culturing set up, except that headspace gases were replaced two to four times daily (depending on cell number) and Na2S was added as a drip flow via a peristaltic pump.
In-field microelectrode assays.
Electrochemical field analysis of H2S concentrations was accomplished by using a portable DLK-60 potentiostat controlled by a laptop computer (Analytical Instrument Systems, Inc.) and micromanipulator. Voltammetry scans (1,000 mV/sec; initial holding potential of −0.1 V between −0.1 and −1.8 V [versus that for Ag/AgCl]) were acquired via a glass Au-amalgam solid-state working electrode, a platinum wire counter electrode, and a Ag/AgCl reference electrode constructed like that of Brendel and Luther (7). The H2S signal assignment and calibrations were conducted by using spring water doped with freshly prepared standards (16). Vertical measurements of O2 were carried out with a Clark-type microsensor and micromanipulator, using field methods, calibration techniques, and temperature compensation as described previously (32).
Scanning electron microscopy/energy-dispersive X-ray spectroscopy and sulfate analyses.
Cultures were grown under the conditions described above, and the yellow precipitate that is common to these cultures was removed and separated from the culture media by centrifugation at 13,000 × g for 5 min and then dried under vacuum conditions. The resulting material was applied to carbon tape on a sample stage and analyzed on a JEOL model 6100 scanning electron microscope (JEOL, Tokyo, Japan) equipped with an X-ray detector. The sample was subjected to 20 keV of incident energy, and measurements were made over a 50-s interval.
Production levels of sulfate by isolate 3684 were monitored in vitro by growing 10 ml of cells under standard conditions to 5·105 cells ml−1 and then concentrating the cells by centrifugation at 5, 000 × g for 5 min. Cells were resuspended in 1 ml of a synthetic medium [10 mM (NH4)2PO4, 0.2 mM KH2PO4, 2 mM NaCl, 1 mM MgCl, 0.5 mM CaCl2, 50 μM (NH4)2SO4, 1.2 μM FeCl3, 0.5 ml trace element solution (2), 5 mM citric acid, pH 3.0] and used to inoculate 59 ml of the same medium. Na2S was added to a final concentration of 200 μM, and 5.5-ml samples were removed at 12-h intervals and measured for H2S utilization as described above, with 5.0 ml filtered through a 0.2-μm polytetrafluoroethylene filter and measured for SO4 by using a Dionex ion chromatograph.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences described in this study can be found as GenBank accession numbers EU545989 to EU545991 and the ITS sequences as accession numbers EU836320 to EU836324.
RESULTS
Spring chemistry.
In a preceding study, sulfide constraints on the population distribution of an As(III) chemolithotroph were studied (10). Mapping H2S gradients showed that measurable H2S was primarily found in the yellow solid-phase zone (Fig. 1), which is a microbial mat comprised of filamentous microorganisms (primarily Hydrogenobaculum [19]) interwoven with a mineral phase made of up S0 (21). At the point of discharge of the spring, H2S(aq) was found to be 75 to 80 μM; it declined to 5 to 10 μM at a distance of 3.5 m in the outflow channel at transect position 5 (Fig. 1) and was below detection at approximately 5 m (10). In the same study, O2 gradients were also measured, and concentrations were found to be essentially opposite to those of H2S; i.e., O2(aq) was undetectable in the first 100 cm in the outflow channel but then increased rapidly from there on.
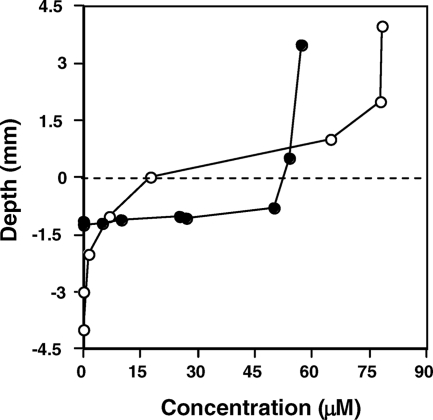
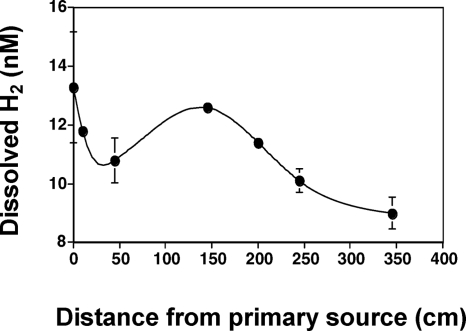
The study described herein occurred approximately 18 months subsequent to that study, and H2S levels were spot-checked to verify that the same gradient patterns were still present (results not shown). In addition, to better understand potentially important geochemical gradients that may be of value for understanding in situ microbial metabolism and for designing ecologically relevant cultivation conditions, microelectrodes were used to examine H2S and O2 concentrations in the vertical dimension (Fig. 2). At a location approximately corresponding to transect site 3 (Fig. 1), H2S concentrations in the water and within the upper 1 mm of mat appeared to remain nearly constant at ∼ 50 μM but then decreased sharply to the detection limit within ∼2.5 mm (Fig. 2A). Vertical O2 profiles were similarly steep, with O2 levels being saturated 4 mm above the mat surface and rapidly changing to anaerobic conditions 2 to 3 mm inside the mat (Fig. 2B). Dissolved H2 concentrations at the spring source were ∼13 nM and decreased rapidly for approximately 50 cm. Aqueous H2 then increased briefly, due to mixing with geothermal water from a second downstream source (Fig. 1), but again declined with distance (Fig. 3), presumably due to off-gassing and or microbial consumption.
FIG. 2.
Vertical H2S and O2 gradients in the microbial mats. Cyclic voltammetry was used to estimate H2S concentrations in the center of the outflow channel approximately 100 cm downstream of the spring source, as shown by filled circles (•). The vertical profile of dissolved O2 at a point 100 cm downstream of the spring source is shown by open circles (○). A dashed line represents the approximate upper boundary of the microbial mat.
FIG. 3.
Aqueous H2 concentrations measured along the center of the outflow channel. The increase at 150 cm corresponds to input from a secondary spring source that contributes to the Dragon Spring outflow channel at approximately 140 cm. Data points indicate the means of three samples at each location. Error bars represent one standard deviation.
Ex situ assays.
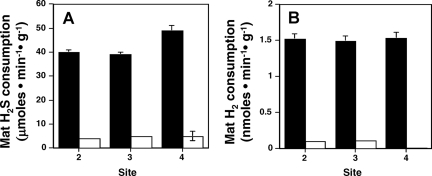
H2S and H2 consumption rates were studied to determine their relative importance as electron donors to the microorganisms inhabiting the S0 deposition zone. Rates of H2S loss (i.e., apparent consumption) in the sealed assay containers ranged from 35 to 50 μmol·min−1·g dry mat−1, whereas H2 consumption rates were approximately three orders of magnitude lower: 1 to 4 nmol·min−1·g dry mat−1 (Fig. 4; see also Fig. S1 in the supplemental material). In ex situ assays using heat-treated mat samples, the apparent H2 and H2S consumption was much lower or absent. For H2S consumption results in heat-killed samples, incomplete heat killing cannot be ruled out, although much-slower abiotic oxidation likely also occurred due to the presence of oxygen unavoidably introduced during sample preparation at springside.
FIG. 4.
Ex situ assays of apparent H2S (A) and H2 (B) consumption by whole-mat samples. Solid bars represent the activity of untreated mat material maintained at in situ temperatures. Open bars represent assays with heat-treated mat material. Site numbers refer to three discreet mat-sampling locations in the center of the outflow channel with respect to the distance from the spring source (2 = 35 cm, 3 = 115 cm, 4 = 225 cm). Each error bar represents one standard deviation of the mean from three replicate assays.
Phylogenetic analysis.
A previous phylogenetic survey of the spring that included S0 mat material from a position equivalent to site 5 (Fig. 1) found that Hydrogenobaculum-like signatures dominated the 16S rDNA clone libraries (19). To better understand the microbial community structure in the regions of the spring where H2S and H2 consumption were examined in the current study, additional 16S rRNA gene clones were amplified from mat DNA extracted from transect sites 2 and 4 within the central flow channel of the S0 deposition zone (Fig. 1). Of the 90 nearly full-length clones sequenced, 94% represented Hydrogenobaculum-like organisms (>99% identity to Hydrogenobaculum acidophilum, GenBank accession number D16296), falling into two distinct phylotypes with >99.9% nucleotide identity to one another; phylotype I comprised 71% of the Hydrogenobaculum-like clones, and the remainder were referred to as phylotype II.
Culturing and isolation.
Filter-sterilized spring water was used as a basal medium in cultivation experiments aimed at isolating H2S and H2 chemolithoautotrophs from the mat exhibiting H2S and H2 consumption. Though the O2 electrode experiments (Fig. 2B) indicated a continuum of aerobic, microaerobic, and anaerobic environments, only aerobic and microaerobic enrichments resulted in growth. Following several subcultures, clonal cultures were derived from positive enrichments by three rounds of dilution to a theoretical 0.5 cell·ml−1. DGGE analysis was used to monitor isolation progress throughout; upon continued subculture, DGGE profiles were progressively less complex, reduced to a single band in each of the late-stage enrichment subcultures and remaining as such following dilution to extinction subcultures. A total of 30 isolates that were phylogenetically very similar to Hydrogenobaculum (>99% identity to H. acidophilum; GenBank accession number D16296) and that formed three distinct 16S phylotypes (see Fig. S2 in the supplemental material) were obtained. Isolate phylotypes I and II were identical to the above-mentioned phylotypes amplified from total mat DNA, whereas the 16S rRNA signature of phylotype III isolates was novel relative to amplicons derived from total mat DNA. Phylotypes I and III deviated from each other by 2 nucleotides, whereas phylotype II deviated from phylotypes I and III by 8 nucleotides over a 1,431-nucleotide region of the 16S gene.
Within each phylotype, cell morphology was invariable, and all isolates were identical with respect to 16S rRNA gene sequences and the cloned 350-bp ITS region. However, within each phylotype, the isolates displayed widely varying growth phenotypes. Some isolates would grow only on H2S and some only on H2, whereas others could grow with either energy source (Table 1). Further, some were found to grow best under microaerobic conditions, whereas others preferred fully aerobic conditions. Phylotype II contained isolates having all combinations of H2S/H2/O2 utilization/requirement patterns. An aerobic isolate (strain YNP3684) capable of growth on either electron donor was maintained on sulfide for a period of 2 years, encompassing 32 culture transfers (1:50 dilution each) in the absence of H2 (effective dilution = 1032). Following this period of extended dilution and culturing, YNP3684 retained its ability to grow autotrophically on H2, providing evidence that it was not a mixed culture.
TABLE 1.
Metabolic differences among Hydrogenobaculum-like isolates
| 16S phylotype | No. of isolates | No. of isolates capable of using indicated electron donor:
|
No. of microaerobic isolatesa | No. of aerobic isolatesa | ||
|---|---|---|---|---|---|---|
| H2S | H2 | H2S/H2 | ||||
| I | 9 | 3 | 2 | 4 | 0 | 9 |
| II | 18 | 15 | 0 | 3 | 6 | 12 |
| III | 3 | 2 | 0 | 1 | 0 | 3 |
| Total | 30 | 20 | 2 | 8 | 6 | 24 |
Isolates listed as microaerobic were incapable of growth with a headspace O2 content of >2%, while aerobic isolates exhibited the best growth at a headspace O2 content of 5.5% and are capable of growth with >10% O2.
Pure culture studies.
Given that YNP3684 was phylogenetically identical to the dominant community sequence type and was capable of growth using either H2S or H2 as an electron donor and CO2 as a carbon source, it was viewed to be ecologically relevant and ideal for modeling studies that would more closely examine H2S or H2 utilization under spring-relevant conditions. One set of experiments examined whether H2S or H2 exerted catabolite regulatory controls over the other and thus potentially influenced in situ utilization patterns. YNP3684 was first cultured separately with H2S or H2 (in filter-sterilized spring water) to acclimate the cells to a single energy source, and then late-log-phase cells were transferred to the same medium that now contained H2S (30 μM) and H2 (25 nM). In such experiments, consumption of both H2S and H2 was immediate and without a discernible lag (Fig. 5A). Initial consumption rates (first three time points) were 0.25 μmol·min−1·106 cells and 0.32 nmol·min−1·1106 cells for H2S and H2, respectively. H2S consumption appeared to remain nearly linear for 50 min (∼5 μM), whereas H2 consumption declined after 30 min (0.03 nmol·min−1·106 cells), when H2(aq) decreased to <15 nM, a concentration roughly equal to that encountered in the spring source waters (Fig. 3).
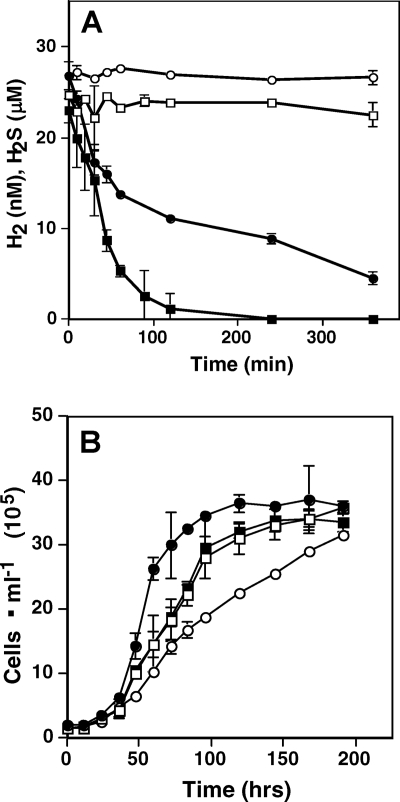
FIG. 5.
H2 and H2S consumption and growth by Hydrogenobaculum strain 3684. (A) Time course consumption assay. Dissolved H2 after the addition of autoclave-killed YNP3684 cells is shown by open circles (○), dissolved H2S after the addition of autoclave-killed YNP3684 cells is shown by open squares (□), dissolved H2 after inoculation with 2 × 106 cells ml−1 is shown by filled circles (•), and dissolved H2S following inoculation with 2 × 106 cells ml−1 is shown by filled squares (▪). (B) Growth of isolate YNP3684. H2 (726 nM) is shown by filled circles (•), 30 nM H2 + 60 μM H2S is shown by filled squares (▪), 60 μM H2S is shown by open squares (□), and 30 nM H2 is shown by open circles (○). Data are the means of triplicate samples. Error bars represent one standard deviation.
Subsequent experiments then examined YNP3684 growth with H2S and H2 separately and in combination. As calculated at early time points (36 to 72 h) when cell concentrations were low and substrates were not limiting, the best growth rate (doubling time of ∼7.3 ± 0.9 h, n = 3) was observed with saturating levels of H2 (726 nM) (Fig. 5B), although the overall-poorest growth rate (doubling time of ∼18.8 ± 0.8 h) occurred with 30 nM H2, a concentration that still exceeded in situ levels in Dragon Spring by approximately a factor of 2. Growth rates with spring-relevant levels of H2S were intermediate between these extremes (doubling time of ∼13.7 ± 1.8 h), and growth with H2S and H2 (doubling time of ∼12.9 ± 0.9 h) did not significantly improve growth statistically beyond that which occurred with H2S alone (Fig. 5B).
Because background levels of S0 (solid phase) (Fig. 1) and SO4 (1.2 mM) (21) in the spring prohibited experiments designed to track the fate of H2S in the ex situ assays, experiments were undertaken to determine the fate of H2S in pure culture. Ion chromatography of culture fluids indicated that ∼80% of added H2S was converted to sulfate after 24 h of incubation with a cell culture at 1·105 cells ml−1 (see Fig. 3A in the supplemental material). YNP3684 culture fluids were visibly yellow when grown on H2S, whereas uninoculated media remained clear, suggesting that S0 may occur as an intermediate during enzymatic oxidation by this organism. Scanning electron microscopy/energy-dispersive X-ray spectroscopy analysis of solid phase culture constituents confirmed the presence of a sulfur-containing solid phase, consistent with the oxidation of H2S to S0 (see Fig. 3B in the supplemental material).
DISCUSSION
Predictive studies concerning the energy sources that fuel primary production in geothermal environments have been very useful as first approximations. Molecular (16S rDNA) approaches estimate in situ metabolism based on physiologies inferred from phylogenetically closely related organisms that have been characterized (28, 33, 37), and thermodynamic predictions have been derived from free-energy yields calculated from the ion activities of electron donors and acceptors found in geothermal systems (4, 5, 18, 27, 33, 37). The present study had similar goals, while focusing on H2 and H2S; however, the experiments were designed to take a more-directed approach: i.e., quantifying presence, measuring utilization, and examining the characteristics of microorganisms capable of growth on these highly exergonic chemolithotrophic substrates.
A previous aqueous chemical analysis (10) agrees with the data obtained for this report (Fig. 2A), with both demonstrating significant levels of H2S, and time course ex situ assays provide evidence of rapid microbe-based H2S consumption (Fig. 4A). H2S- and O2-sensitive microelectrode experiments illustrated that H2S penetration into the mat was limited, rapidly decreasing to below detection within the oxygenated region of the mat (Fig. 2), suggesting that H2S consumption is linked to the presence of O2. Similar experiments also demonstrated H2 gradients (Fig. 3) and microbial consumption (Fig. 4B). The vertical and horizontal gradients of H2S, H2, and O2 suggest a continuum of chemical energy gradients, providing numerous niche opportunities for populations having specialized or flexible metabolic needs. The latter was confirmed by the organisms obtained in the isolation exercises (Table 1) and, indeed, demonstrated optimum O2 requirements exceeding that previously documented for Hydrogenobaculum (1, 12, 36).
The Hydrogenobaculum 16S rDNA signatures that dominated the community clone libraries were ∼99% identical to those of H. acidophilum, which was characterized as being a microaerophile requiring H2 and S0 for growth (36). Thus, physiologic inference would predict that organisms inhabiting the yellow S0 deposition zone would be engaged in H2 oxidation and utilize the abundant S0 present in this region of the spring outflow channel (Fig. 1) (21). Results of the cultivation work, however, illustrate the potential problems associated with physiologic inference in general and, more specifically, the incorrect conclusions drawn if it is applied to the Hydrogenobaculum-like populations inhabiting this spring. Isolates sharing identical 16S rRNA gene sequences (and, indeed, ITS sequences) differed in important ways with respect to the ecologically relevant electron donors they were capable of utilizing (Table 1). Such observations affirm those previously reported for organisms that are phylogenetically closely related (38) or, indeed, identical (20) but which exhibit widely varying physiologies.
The isolates obtained in this study were also important for other reasons. First, we drew attention to the observation that the overwhelming majority (90%) of the Hydrogenobaculum isolates were phylogenetically identical to the two major phylotypes (94% of all PCR clones) obtained from total community DNA. The use of filter-sterilized spring water as the basal growth medium along with O2 concentrations predicted from the microelectrode work likely provided a more natural growth environment than synthetic media, which has historically tended to exclude the growth of ecologically relevant microorganisms (for an example, see reference 13).
Such isolates are also useful for pure-culture modeling studies, where results of ex situ experiments can be studied in more detail and under more-controlled conditions. When isolate YNP3684 (representing the dominant mat phylotype) was cultured in spring water with an average spring concentration of H2S (30 μM) but H2 levels (30 nM) considerably exceeding the maximum value observed at any location in the spring, H2S consumption rates exceeded those of H2 by three orders of magnitude, an observation completely consistent with the ex situ measurements (Fig. 4). Regardless of the substrate to which the cells had been preconditioned, utilization of both substrates was immediate and without a lag phase (Fig. 5A), suggesting constitutive expression of the enzymes involved. In some geothermal systems where microorganisms can be bathed in a continuous flux of multiple energy sources, constitutive utilization of several available energy sources may be the norm.
Another important observation derived from the pure-culture experiments speaks to the significance of enzyme kinetics when considering the relative importance of various energy sources in natural settings. Theoretical energy yields derived from calculating ΔG°rxn fail to account for enzyme properties, the importance of which cannot be overstated. H2 concentrations in the range of 5 to 10 nM have been shown to support microbial growth (24, 25, 37) and are similar to the concentrations observed in several Yellowstone geothermal features (Fig. 3) (37). However, these concentrations are considerably lower than the published Kms derived from purified uptake hydrogenases (e.g., 0.92 μM H2 for Pyrodictium brockii [30]; 19 μM for an Anabaena sp. [17]). The H2 and H2S consumption profiles exhibited by YNP3684 (Fig. 5A) suggest that over the range of concentrations of both substrates directly measured in the spring, utilization of H2S surpassed that of H2. Further, when spring-relevant concentrations of H2 and H2S were provided, consumption rates appeared closely related to actual growth, with this particular organism growing better with H2S (Fig. 5B). Regular analysis of headspace gases in these experiments showed that the H2-cultured cells never experienced H2 concentrations lower than what were measured in the yellow S0 deposition zone (Fig. 3), indicating that the observed culture growth rates may exceed even that occurring in situ. Superior growth with H2 was observed only with saturating concentrations of H2, levels that should be considered unrealistic given the concentrations measured in Dragon Spring and elsewhere in Yellowstone (29, 37).
While it is clear that the aerobic respiration of H2 can provide substantial energy to chemolithotrophs, the potential energy gained from the oxidation of sulfide depends on the extent to which the sulfur is oxidized and may, in fact, be quite similar to that gained through H2 oxidation under the spring conditions examined in this study (18). Several organisms, including Thiobacillus thioparus (23, 41) and Desulfobulbus propionicus (15), are capable of oxidizing H2S to SO4 under aerobic conditions. S0 production by this Hydrogenobaculum isolate (see Fig. S3B in the supplemental material) appears to be intermediate, as SO4 production was also observed (see Fig. S3A in the supplemental material). While Rowe et al. (34) suggested that the presence of elemental sulfur was a major niche determinant for Hydrogenobaculum-like organisms they encountered in thermal tributaries feeding Lemonade Creek, our results indicate that the occurrence of S0 is due, at least in part, to the activity of the organisms present, with rates of abiotic H2S oxidation apparently being a relatively minor contributor (Fig. 4).
In summary, this study directly examined the relative importance H2S and H2 as energy sources in support of primary productivity in a geothermal spring. Field experiments quantified both and measured consumption rates and were combined with cultivation and pure-culture assays to determine the dynamics of electron donor usage by an environmentally relevant isolate. The major conclusions drawn from this study are that phylogeny may not necessarily be a reliable predictor of physiology and that regardless of thermodynamic estimates, H2S can dominate H2 as an energy source in terms of availability, apparent in situ consumption rates, and growth-supporting energy.
Supplementary Material
Acknowledgments
This study was primarily supported by a grant (MCB-0132022) from the National Science Foundation Microbial Observatories Program to T.R.M. Additional support to T.R.M. was provided by the National Aeronautics and Space Administration (NAG 5-8807) and the Montana Agricultural Experiment Station (911310). Support from the American Chemical Society Petroleum Research Fund (43356-GB2) to G.D. and from the Danish Natural Science Research Council to M.K. is also acknowledged. The Thermal Biology Institute, MSU Bozeman, funded summer visiting scholarships for M.K. and G.D. during part of this study.
We thank John Varley and Christie Hendrix of the Yellowstone Center for Resources, YNP, Wyoming, for assistance with permits.
Footnotes
Published ahead of print on 18 July 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Aguiar, P., T. J. Beveridge, and A. L. Reysenbach. 2004. Sulfurihydrogenibium azorense, sp. nov., a thermophilic hydrogen-oxidizing microaerophile from terrestrial hot springs in the Azores. Int. J. Syst. Evol. Microbiol. 54:33-39. [DOI] [PubMed] [Google Scholar]
- 2.Allen, M. B. 1959. Studies with Cyanidium caldarium, an anomalously pigmented chlorophyte. Arch. Microbiol. 32:270-277. [DOI] [PubMed] [Google Scholar]
- 3.Amend, J. P., and A. V. Plyasunov. 2001. Carbohydrates in thermophile metabolism: calculation of the standard molal thermodynamic properties of aqueous pentoses and hexoses at elevated temperatures and pressures. Geochim. Cosmochim. Acta 65:3901-3917. [Google Scholar]
- 4.Amend, J. P., K. L. Rogers, E. L. Shock, S. Gurrieri, and S. Inguaggiato. 2003. Energetics of chemolithoautotrophy in the hydrothermal system of Vulcano Island, southern Italy. Geobiology 1:37-58. [Google Scholar]
- 5.Amend, J. P., and E. L. Shock. 2001. Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiol. Rev. 25:175-243. [DOI] [PubMed] [Google Scholar]
- 6.Botero, L. M., K. B. Brown, S. Brumefield, M. Burr, R. W. Castenholz, M. Young, and T. R. McDermott. 2004. Thermobaculum terrenum gen. nov., sp. nov.: a non-phototrophic gram-positive thermophile representing an environmental clone group related to the Chloroflexi (green non-sulfur bacteria) and Thermomicrobia. Arch. Microbiol. 181:269-277. [DOI] [PubMed] [Google Scholar]
- 7.Brendel, P. J., and G. W. Luther. 1995. Development of a gold amalgam voltammetric microelectrode for the determination of dissolved Fe, Mn, O2 and S(-II) in porewaters of marine and freshwater sediments. Environ. Sci. Technol. 29:751-761. [DOI] [PubMed] [Google Scholar]
- 8.Caetano-Anollés, G. 2002. Evolved RNA secondary structure and the rooting of the universal tree of life. J. Mol. Evol. 54:333-345. [DOI] [PubMed] [Google Scholar]
- 9.Clesceri, N. L. 1998. Special issue: monitoring and characterization techniques for contaminants in the subsurface. J. Environ. Eng. 124:489. [Google Scholar]
- 10.D'Imperio, S., C. R. Lehr, M. Breary, and T. R. McDermott. 2007. Autecology of an arsenite chemolithotroph: sulfide constraints on function and distribution in a geothermal spring. Appl. Environ. Microbiol. 73:7067-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donahoe-Christiansen, J., S. D'Imperio, C. R. Jackson, W. P. Inskeep, and T. R. McDermott. 2004. Arsenite-oxidizing Hydrogenobaculum strain isolated from an acid-sulfate-chloride geothermal spring in Yellowstone National Park. Appl. Environ. Microbiol. 70:1865-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eder, W., and R. Huber. 2002. New isolates and physiological properties of the Aquificales and description of Thermocrinis albus sp. nov. Extremophiles 6:309-318. [DOI] [PubMed] [Google Scholar]
- 13.Felske, A., A. Wolterink, R. van Lis, W. M. de Vos, and A. D. L. Akkermans. 1999. Searching for predominant soil bacteria: 16S rDNA cloning versus strain cultivation. FEMS Microbiol. Ecol. 30:137-145. [DOI] [PubMed] [Google Scholar]
- 14.Ferrera, I., S. Longhorn, A. B. Banta, Y. Liu, D. Preston, and A. L. Reysenbach. 2007. Diversity of 16S rRNA gene, ITS region and aclB gene of the Aquificales. Extremophiles 11:57-64. [DOI] [PubMed] [Google Scholar]
- 15.Fuseler, K., and H. Cypionka. 1995. Elemental sulfur as an intermediate of sulfide oxidation with oxygen by Desulfobulbus propionicus. Arch. Microbiol. 164:104-109. [Google Scholar]
- 16.Holak, W. 1980. Determination of arsenic by cathodic stripping voltammetry with a hanging mercury drop electrode. Anal. Chem. 52:2189-2192. [Google Scholar]
- 17.Houchins, J. P., and R. H. Burris. 1981. Comparative characterization of two distinct hydrogenases from Anabaena sp. strain 7120. J. Bacteriol. 146:215-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inskeep, W. P., G. G. Ackerman, W. P. Taylor, M. Kozubal, S. Korf, and R. E. Macur. 2005. On the energetics of chemolithotrophy in nonequilibrium. Geobiology 3:297-317. [Google Scholar]
- 19.Jackson, C. R., H. W. Langner, J. Donahoe-Christiansen, W. P. Inskeep, and T. R. McDermott. 2001. Molecular analysis of microbial community structure in an arsenite-oxidizing acidic thermal spring. Environ. Microbiol. 3:532-542. [DOI] [PubMed] [Google Scholar]
- 20.Jaspers, E., and J. Overmann. 2004. Ecological significance of microdiversity: identical 16S rRNA gene sequences can be found in bacteria with highly divergent genomes and ecophysiologies. Appl. Environ. Microbiol. 70:4831-4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langner, H. W., C. R. Jackson, T. R. McDermott, and W. P. Inskeep. 2001. Rapid oxidation of arsenite in a hot spring ecosystem, Yellowstone National Park. Environ. Sci. Technol. 35:3302-3309. [DOI] [PubMed] [Google Scholar]
- 22.Larowe, D. E., and H. C. Helgeson. 2007. Quantifying the energetics of metabolic reactions in diverse biogeochemical systems: electron flow and ATP synthesis. Geobiology 5:153-168. [Google Scholar]
- 23.Lizama, H. M., and B. M. Sankey. 1993. Conversion of hydrogen sulfide by acidophilic bacteria. Appl. Microbiol. Biotechnol. 40:438-441. [Google Scholar]
- 24.Lovley, D. R., D. F. Dwyer, and M. J. Klug. 1982. Kinetic analysis of competition between sulfate reducers and methanogens for hydrogen in sediments. Appl. Environ. Microbiol. 43:1373-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovley, D. R., and S. Goodwin. 1988. Hydrogen concentrations as an indicator of the predominant terminal electron-accepting reactions in aquatic sediments. Geochim. Cosmochim. Acta 52:2993-3003. [Google Scholar]
- 26.Macur, R. E., C. R. Jackson, L. M. Botero, T. R. McDermott, and W. P. Inskeep. 2004. Bacterial populations associated with the oxidation and reduction of arsenic in an unsaturated soil. Environ. Sci. Technol. 38:104-111. [DOI] [PubMed] [Google Scholar]
- 27.McCollom, T. M., and J. P. Amend. 2005. A thermodynamic assessment of energy requirements for biomass synthesis by chemolithoautotrophic micro-organisms in oxic and anoxic environments. Geobiology 3:135-144. [Google Scholar]
- 28.Meyer-Dombard, D. R., E. L. Shock, and J. P. Amend. 2005. Archaeal and bacterial communities in geochemically diverse hot springs of Yellowstone National Park, USA. Geobiology 3:211-227. [Google Scholar]
- 29.Nordstrom, D., J. Ball, and R. McCleskey. 2005. Ground water to surface water: chemistry of thermal outflows in Yellowstone National Park, p. 143-162. In W. P. Inskeep and T. R. McDermott (ed.), Geothermal biology and geochemistry in Yellowstone National Park. Montana State University, Bozeman.
- 30.Pihl, T., and R. Maier. 1991. Purification and characterization of the hydrogen uptake hydrogenase from the hyperthermophilic archaebacterium Pyrodictium brockii. J. Bacteriol. 173:1839-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitcher, D. G., N. A. Saunders, and R. J. Owen. 1989. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 8:151-156. [Google Scholar]
- 32.Revsbech, N. P., B. B. Jorgensen, T. H. Blackburn, and Y. Cohen. 1983. Microelectrode studies of the photosynthesis and O2, H2S, and pH profiles of a microbial mat. Limnol. Oceanogr. 28:1062-1074. [Google Scholar]
- 33.Rogers, K. L., and J. P. Amend. 2005. Archaeal diversity and geochemical energy yields in a geothermal well on Vulcano Island, Italy. Geobiology 3:319-332. [Google Scholar]
- 34.Rowe, O. F., J. Sánchez-España, K. B. Hallberg, and D. B. Johnson. 2007. Microbial communities and geochemical dynamics in an extremely acidic, metal-rich stream at an abandoned sulfide mine (Huelva, Spain) underpinned by two functional primary production systems. Environ. Microbiol. 9:1761-1771. [DOI] [PubMed] [Google Scholar]
- 35.Schwartzman, D. W., and C. H. Lineweaver. 2004. The hyperthermophilic origin of life revisited. Biochem. Soc. Trans. 32:168-171. [DOI] [PubMed] [Google Scholar]
- 36.Shima, S., and K. I. Suzuki. 1993. Hydrogenobacter acidophilus sp. nov, a thermoacidophilic, aerobic, hydrogen-oxidizing bacterium requiring elemental sulfur for growth. Int. J. Syst. Bacteriol. 43:703-708. [Google Scholar]
- 37.Spear, J. R., J. J. Walker, T. M. McCollom, and N. R. Pace. 2005. Hydrogen and bioenergetics in the Yellowstone geothermal ecosystem. Proc. Nat. Acad. Sci. USA 102:2555-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stackebrandt, E., and O. Pauker. 2005. Gene sequence heterogeneity of Corallococcus coralloides strains isolated from geographically diverse locations. Environ. Microbiol. 7:1017-1023. [DOI] [PubMed] [Google Scholar]
- 39.Swofford, D. L. 2002. PAUP*. Phylogenetic analysis using parsimony (*and other methods), 4.0 ed. Sinauer Associates, Sunderland, MA.
- 40.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van den Ende, F. P., and H. Vangemerden. 1993. Sulfide oxidation under oxygen limitation by a Thiobacillus thioparus isolated from a marine microbial mat. FEMS Microbiol. Ecol. 13:69-77. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.