Abstract
Exposure to endotoxin in home environments has become a key issue in asthma and allergy research. Most studies have analyzed floor or mattress dust endotoxin, but its validity as a proxy for airborne exposure is unknown, while active airborne dust sampling is not feasible in large-scale population studies because of logistic and financial limitations. We therefore developed and evaluated a simple passive airborne dust collection method for airborne endotoxin exposure assessment. We explored an electrostatic dust fall collector (EDC), consisting of a 42- by 29.6-cm-sized folder with four electrostatic cloths exposed to the air. The EDC was tested during two 14-day periods in seven nonfarm and nine farm homes and in farm stables. In parallel, active airborne dust sampling was performed with Harvard impactors and floor dust collected by vacuuming, using nylon sampling socks. The endotoxin levels could be measured in all EDC cloth extracts. The levels (in EU/m2) between EDCs used simultaneously or in different sampling periods in the same home correlated strongly (r > 0.8). EDC endotoxin also correlated moderately to strongly (r = 0.6 to 0.8) with the endotoxin measured by active airborne dust sampling and living room floor dust sampling and—in farm homes—with the endotoxin captured by the EDC in stables. In contrast, endotoxin levels measured by floor dust sampling showed only a poor correlation with the levels measured by active airborne dust sampling. We therefore conclude that measuring endotoxin levels with the EDC is a valid measure of average airborne endotoxin exposure, while reproducibility over time is at least equivalent to that of reservoir dust analyses.
Indoor exposure to allergens, viable microorganisms, microbial cell debris, and other particles in house dust has become a key issue in asthma and allergy research. Many different sampling methods to measure these exposures are available and some are widely used (6, 15, 21). In larger population studies, dust sampling from floors or mattresses with a vacuum cleaner is the most common method since it is easily applied and inexpensive. It is possible to measure a variety of relevant components in these samples, like mite and pet allergens, endotoxins, and β-1,3-glucans (4, 14, 16, 17). However, part of the collected dust fraction consists of large or heavy particles that may never become airborne. Moreover, the dust composition of the samples might depend on the power of the vacuum cleaner, the sampling device trapping the dust (e.g., filters mounted in sampling heads from ALK or nylon-sock samplers [17]), the size of the area sampled, and the sampling time. Although significant associations have been shown between house dust components measured with these methods in reservoir dust and various health effects, it may be argued that airborne dust samples may be more representative of inhaled particle exposures related to asthma and allergies than “reservoir” dust from floors or mattresses.
Several studies have used airborne dust sampling methods, such as active airborne dust sampling with an ion charge device (2, 13) and passive airborne sampling with a petri dish (8, 9, 21) or dust fall collector (6, 24). Of these, the active airborne dust sampling and ion charge device methods have high logistic and equipment costs and have as yet been applied only in short-term and small experimental studies, e.g., to compare with other dust sampling methods (3, 6, 11, 21). The petri dish method is simple and inexpensive but collects dust on a small surface and therefore needs long sampling times (8, 9, 21). The aim of this project was therefore to develop a rigorous, low-cost passive sampling technique for airborne dust in order to assess exposure to airborne microbial and allergenic components in the home environment. Würtz et al. (24) developed a passive sampling technique, the dust fall collector, using an aluminum foil-covered inner surface of a pizza box to collect settling dust in school classrooms. For the measurement of endotoxin on surfaces, Thorne et al. (19) used electrostatic wiping cloths (cloths), which may also be used to collect settled dust from horizontal surface areas. Both methods yielded sufficient dust for endotoxin measurement, but the dust fall collector cannot easily be sent by regular mail before and after sampling, and both methods are rather difficult to handle carefully, without causing contamination, by home residents. We therefore designed a new electrostatic dust fall collector (EDC) by combining several of their features. The EDC consists of a custom-fabricated polypropylene sampler that has electrostatic cloths attached to it to provide a sampling surface. Airborne dust settles on this surface and is captured by the electrostatic properties of the cloth. The feasibility of this method for endotoxin measurements was evaluated in a validation study in urban and farm homes. The comparability of the new method to active airborne measurements as well as to floor dust sampling was investigated, and reproducibility within homes and between two sampling periods was studied.
MATERIALS AND METHODS
Study design.
The study was conducted in 16 homes. To ensure sufficient variation in airborne endotoxin levels, nine farm homes and seven nonfarm homes were included. Dust was collected in each home using three different dust collection methods. Floor dust samples were taken with a vacuum cleaner equipped with a nylon sample sock (17). Active airborne dust samples were collected with PM10 Harvard impactors (7, 18), and the new method was applied, which collected airborne dust settling on the surface of a cloth. In each sampling period, the EDC sampling started on day 0 and ended on day 14, and active airborne sampling started on day 0 and ended on day 6 or 8, depending on the number of filters sampled (see the sampling procedure below). Two floor dust samples were taken either from two adjacent sites in the same room on day 0 or twice from the same site on days 0 and 6. In stables of seven of the farms, one EDC was used for 14 days and crude stable dust was collected on day 0 by wiping dust from a 20- by 30-cm area on window sills or other dry surfaces into a Ziploc bag (16). Reproducibility over time was tested by repeating all measurements in both living rooms and stables 3 weeks after the first sampling period. Short questionnaires were used to collect information about smoking, carpets, animals, mold growth, and other factors that could influence the outcomes. Of the nine farm homes, six reported mold growth, seven had pets, and two reported smoking in the home; in six farms, the floor dust sample was taken from a carpet or rug, while the other three had a completely smooth floor. Of the seven nonfarm homes, two reported mold growth, three had pets, and none reported smoking; in four, the floor dust sample was taken from a carpet or rug and in three from a completely smooth floor.
Sampling procedures. (i) Floor dust sampling.
Floor dust samples were collected by home residents in their living rooms as described previously (17) using a vacuum cleaner equipped with a 25-μm mesh nylon sample sock (Allied Filter Fabrics, Sydney, Australia). The sampling area was 1 m2 if there was a carpet or rug with a >4-m2 surface. In homes with a smooth floor or small rug, a 4-m2 surface area of the smooth floor was sampled. The sampling time was always 2 min. The whole procedure was repeated in the second period, and thus four living room floor samples were collected per home.
(ii) Active airborne dust sampling.
Active airborne dust samples were collected over 6 or 8 days. Harvard impactors (Air Diagnostics and Engineering, Inc., Naples, ME) were placed by a field worker at an approximately 1.5-m height, and the pump was run at a flow of 10 liters/min. The flow was calibrated before and after sampling, and no significant drop in the flow rate was noted for any of the measurements. Dust was collected on 37-mm Teflon filters (Anderson, Smyrna, GA) with a 2-μm pore size in three or four Harvard impactors, with a sample changer switching between the impactors after each 24-h period. Each filter was thus loaded with dust from two 24-h sampling periods to prevent possible overloading of the filters and to average out day-to-day variations. The whole procedure was repeated in the second period. In total, 6 or 8 samples were collected per home.
(iii) Electrostatic dust fall collector.
Passive airborne dust samples were collected in each home and in seven stables with the newly developed EDC. In this evaluation study, prototype cardboard samplers were used that were covered with a plastic film and four electrostatic cloths, each with an area of 0.032 m2 (Zeeman, Utrecht, The Netherlands), attached to their surfaces by aluminum-foil-covered frames. The cloths were rendered pyrogen free before use by heating overnight at 200°C. Each EDC contained four cloths. The EDC was opened horizontally to expose the cloths to the air, allowing the collection of settling dust for 14 days. Two EDCs were placed on top of a bookshelf or a like surface at least 1.50 m above the floor by the field worker visiting the home on day 0 (Fig. 1). At each of the farms, one EDC was placed in the stable, at a site where the animals could not reach it and where it was exposed to as little air disturbance as possible, such as away from doors or windows. The home residents were instructed to close the EDC carefully without touching the cloths after the sampling period. The EDCs were returned to the laboratory in a preaddressed envelope. The total number of cloths was 16 per home—2 sampling periods × 2 EDCs × 4 cloths per EDC—and 8 per stable.
FIG. 1.
The EDC. The sampler consists of four electrostatic cloths mounted in a 40- by 30-cm plastic folder that is left for 14 days in the horizontal position with the cloths exposed to the air. The folder is kept closed before and after sampling and during transport and storage.
Extraction of samples.
Air-sampling filters were weighed prior to and after sampling to assess the dust load. The weighing took place in an acclimatized room (temperature, 23°C; relative humidity, 34.5%; air pressure, 1,008 mbar), where all filters were conditioned for 24 h prior to weighing. The dust load of the EDC cloths could not be reliably measured since the weighing error, as assessed by the repeated weighing of the control cloths, was relatively large (2 to 3 mg). After weighing, all samples were stored at −20°C until extraction, which was done within 4 weeks. The extraction of the socks and crude stable dust was conducted as in previous studies (16, 17). Briefly, endotoxin was extracted from each filter in 5 ml pyrogen-free water (B. Braun NPBI, Oss, The Netherlands) plus 0.05% Tween 20 (Merck, Darmstadt, Germany). For the electrostatic cloths, the protocol of Thorne et al. (19) was adapted for our purposes. Based on preliminary tests, we extracted endotoxin from each single cloth in 20 ml pyrogen-free water plus 0.05% Tween 20 with shaking for 1 h at room temperature. From the extracts, 2 ml was harvested and centrifuged at 1,000 × g for 1 h, and the supernatant was stored in aliquots at −20°C until analysis.
Analysis of samples.
Endotoxin analysis was performed in a suitable dilution in pyrogen-free water with the quantitative kinetic chromogenic Limulus amoebocyte lysate (LAL) assay (Lonza; LAL-Lysate lot EL004V; standard Escherichia coli O55:B5 lot 4L3560; reference standard endotoxin/control standard endotoxin ratio, 18 EU/ng). Each single filter or cloth extract from a home was measured in a 1/50 dilution and each floor dust sample at 1/5,000. The cloth extracts from the stable EDCs were tested in a 1/1,000 dilution and the crude stable dust extracts in a 1/20,000 dilution. The samples were retested at a more appropriate dilution when necessary due to too high or low values at the outer ends of the calibration line. No sample was diluted lower than 1/25. In the whole series of sample extracts, only seven (three from filters and four from cloths) had an endotoxin concentration below the limit of detection (LOD; 0.25 EU/ml for a 1/25 dilution) and were assigned the value of the LOD.
Statistical analyses.
First, arithmetic mean endotoxin loads per period per home were computed for all methods. For the EDC, this included the average of values measured in the four cloths per EDC and the average of the two EDCs used in the same period per home. In subsequent analyses, correlations were calculated between endotoxin levels in cloths from the same EDC, between mean values for duplicate EDCs used in parallel in the same period, between different sampling periods, and between the various sampling methods. Since data showed a log-normal distribution, Pearson correlation coefficients (r) and geometric means (GM) and geometric standard deviations (GSD) were calculated based on ln-transformed values.
We then explored the variability over time and between homes in endotoxin exposures by performing mixed-effect regression analyses. The home ID was included as a random factor to correct for correlations between repeated measurements. A factor identifying farm/nonfarm homes was introduced as a fixed effect. The analyses were conducted with the ln-transformed arithmetic mean endotoxin load per period and per home for all sampling methods (active airborne, n = 3 or 4 filters; passive airborne, n = 8 cloths; floor dust, n = 2 sample socks). Statistical analyses were performed with SAS statistical software (version 9.1; SAS Institute, Cary, NC).
RESULTS
Dust weights.
Control filters for active airborne dust samples showed on average a difference between pre- and postweights of only 0.01 mg. For the farms, an average net dust weight on the filters of 1.15 mg was measured, and for the nonfarm homes, it was 0.78 mg. These values yielded airborne dust levels averaging 40 and 27 μg/m3, respectively. These low concentrations explain why we encountered problems in estimating dust weights on the cloths where the average weighing error was in the range of the sampled dust weight.
Endotoxin measurement.
Endotoxin could be measured in all but 4 of the 256 electrostatic dust fall sampling cloths from farm and nonfarm living rooms and in 127 of the 130 filter extracts. Thus, airborne dust endotoxin could be readily detected and quantified after recovery, not only from filters but also from EDC cloths exposed to the air for 14 days. All EDC cloths from stables, all crude stable dust, and all living-room floor dust samples yielded detectable endotoxin levels. Field control cloths, treated similarly as the sample cloths (heated to 200°C and attached to an EDC for 14 days but without exposure to the air), were all negative. The LOD was 213 EU/m2 for the EDC sampling and 0.05 EU/m3 for the active airborne dust sampling. For the floor dust sampling, the LOD varied—depending on the sampling area and extraction volume—from 62.5 to 2,000 EU/m2.
Table 1 presents the geometric mean endotoxin levels for farm and nonfarm homes separately, as measured with all three sampling methods. The mean endotoxin levels measured in the different types of samples were all far above the respective detection limits. The levels in the samples from farms were consistently higher than those for nonfarms, with GM ratios between the farm and nonfarm being lowest for the floor dust samples (2.5), slightly higher for active airborne samples (2.9), and highest for the EDC samples (3.3).
TABLE 1.
Indoor home endotoxin geometric means (GSDs) measured with different dust sampling methodsa
| Sample type | Nonfarm home endotoxin level
|
Farm home endotoxin level
|
||||
|---|---|---|---|---|---|---|
| Geometric mean (GSD) | Minimum | Maximum | Geometric mean (GSD) | Minimum | Maximum | |
| Electrostatic dust fall dust | 3,000 (2.19) | 900 | 9,000 | 10,000 (2.4) | 2,500 | 41,000 |
| Active airborne dust | 0.36 (2.33) | 0.07 | 2.00 | 1.04 (2.84) | 0.15 | 6.14 |
| Floor dust | 11,500 (4.98) | 400 | 110,800 | 28,400 (4.49) | 2,500 | 1,500,000 |
Values for the electrostatic dust fall and floor dust samples are given in EU/m2, and values for the active airborne dust samples are given in EU/m3.
The mean interday coefficient of variation (CV) of duplicate measurements of the same extracts, and thus the analytical error of the LAL assay, was 35% (range, 3% to 121%). The endotoxin levels on different cloths within the same EDC showed an average CV of 35% (range, 5% to 182%). It therefore can be concluded that the actual sampling error must be small. Accordingly, the correlation coefficient for endotoxin measured on two different cloths from the same EDC was 0.82, and for single cloths from the two different EDCs used in parallel on the same sampling site, a mean correlation coefficient of 0.85 was found. The comparison of the average levels of two EDCs used in parallel resulted in a correlation coefficient of 0.84.
Correlation between sampling periods.
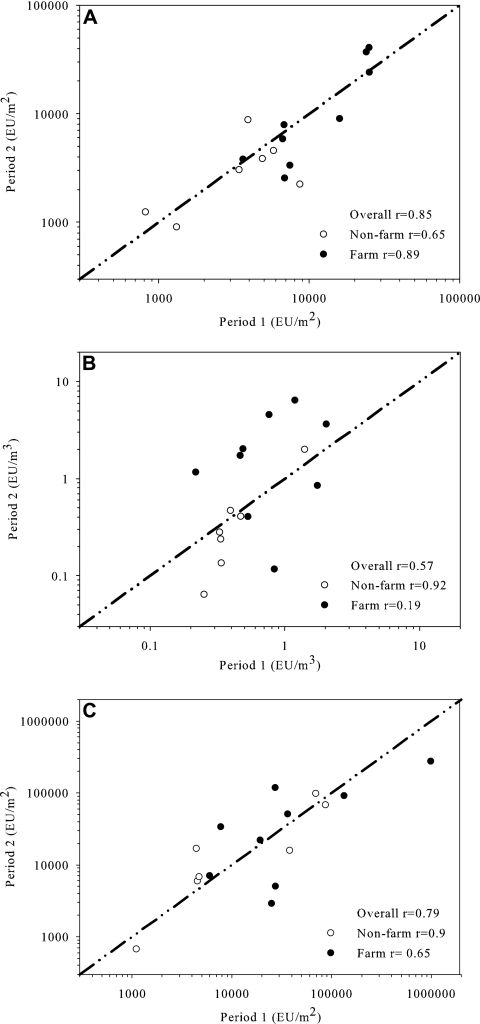
The mean endotoxin levels measured over two 14-day periods in the same home correlated very well for the EDC (r = 0.85) as shown in Fig. 2. The correlation coefficient was very similar for the floor dust sampling (r = 0.79) but weaker for the active airborne dust sampling (r = 0.57). The lower correlation for active airborne dust sampling was completely due to a much lower correlation for the samples from farms (r = 0.19) than for the nonfarm samples (r = 0.92). No such differences between farms and nonfarms were seen in the EDC or floor dust samples.
FIG. 2.
Comparison of the endotoxin levels measured in the same homes over two sampling periods by electrostatic dust fall sampling (A), active airborne dust sampling (B), and floor dust sampling (C).
Comparison between dust sampling methods.
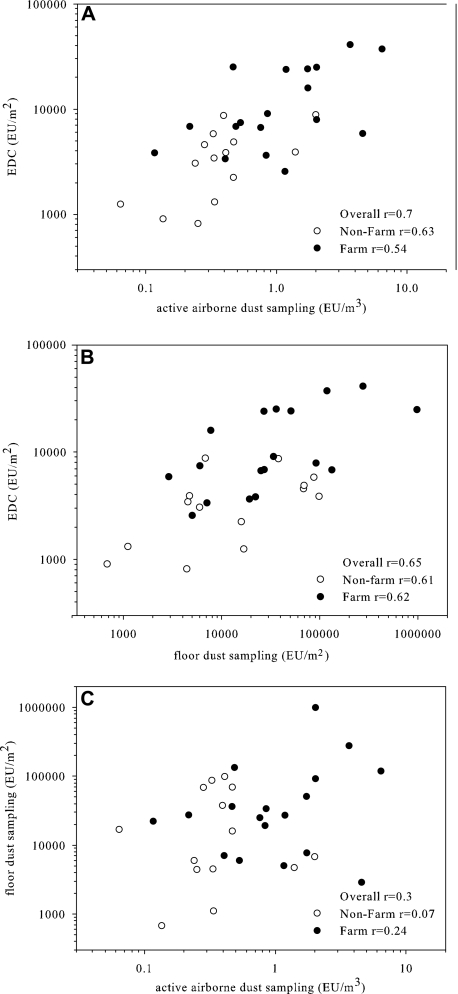
The EDC results correlated well with those of the active airborne dust sampling (r = 0.7; Fig. 3A) and with those of the floor dust sampling (r = 0.65; Fig. 3B). In contrast, the levels measured by the active airborne and floor dust sampling methods were only poorly correlated (r = 0.3; Fig. 3C).
FIG. 3.
Comparison of the endotoxin levels measured by different dust sampling methods: active airborne dust sampling versus EDC sampling (A), floor dust sampling versus EDC sampling (B), and active airborne dust sampling versus floor dust sampling (C).
Characterizing homes.
To assess whether the EDC and, for comparison, the other sampling methods could be used to estimate the average home-associated endotoxin exposure for a longer period, we investigated the within- and between-home variances using a mixed-effect regression analysis. This was performed without (model 1) and with (model 2) taking the farm/nonfarm environment as a fixed effect into account (Table 2).
TABLE 2.
Within- and between-home variance components of ln-transformed endotoxin levels measured in two sampling periods with a 3-week time interval (model 1), and effect of being a farm on measured endotoxin levels (eβ) relative to reference nonfarm homes (intercept eα) (model 2)
| Sampling method | Model 1
|
Model 2a
|
||||
|---|---|---|---|---|---|---|
| Variation within homes | Variation between homes | Variation within homes | Variation between homes | Intercept eα (CI) | Farm eβ (CI) | |
| Electrostatic dust fall dust | 0.21 | 0.98 | 0.21 | 0.62 | 2,782 (1428-5418) | 3.46 (1.5-7.96) |
| Active airborne dust | 0.69 | 0.57 | 0.69 | 0.32 | 0.35 (0.18-0.67) | 2.87 (1.2-6.87) |
| Floor dust | 0.53 | 2.09 | 0.53 | 2 | 11,923 (3,522-40,360) | 2.64 (0.53-13.18) |
CI, confidence interval.
The within-home variance is the result of the analytical error, the sampling error, and the differences between the two periods of sampling. Since the preliminary analyses showed that the sampling error for the EDC was low (see above), it can be assumed that the within-home differences for that sampling method were mainly due to the analytical error and the differences between the two sampling periods. For the floor dust and active airborne dust samplings, we could not easily estimate the contribution of the sampling error due to the small sample size (two floor dust samples and three or four filters per home and period). Moreover, the single filters from the active airborne sampling were sampled on different days and therefore not parallel samples.
The endotoxin levels measured with the EDC or with floor dust sampling showed a four- to fivefold larger between- than within-home variance (Table 2, model 1), whereas for active airborne dust sampling, the within-home variance was larger than the between-home variance. When the farm/nonfarm factor was taken into account, the between-home variance decreased for all three methods, as part of the between-home difference is due to whether the home is a farm or not (Table 2, model 2). The between/within-home variance ratio for active airborne sampling decreased from 0.82 to 0.46 and for the EDC sampling from 4.64 to 2.94. This ratio was least affected for the floor dust sampling, changing from 3.95 to 3.77. Apparently, for the floor dust sampling, the farm/nonfarm environment factor did not influence the between-home variance as much as for both the airborne dust sampling methods. The factor difference for the farm/nonfarm environment from the mixed-model regression analysis confirmed the conclusion from the crude data analysis (Table 1) that farm homes have approximately three times higher endotoxin levels than nonfarm homes. Additional analyses were performed, including other home characteristics—pets, smoking in the home, reported mold growth, and type of floor cover from which the sample was taken—separately or in combination in the statistical models. In all cases, the difference between farms and nonfarms remained essentially unchanged—e.g., the farm/nonfarm factor for endotoxin measured by the EDC varied from 2.6 to 3.5 in the adjusted models (not shown). Thus, confounding by the other home characteristics could be excluded.
Endotoxin in EDC cloths from stables.
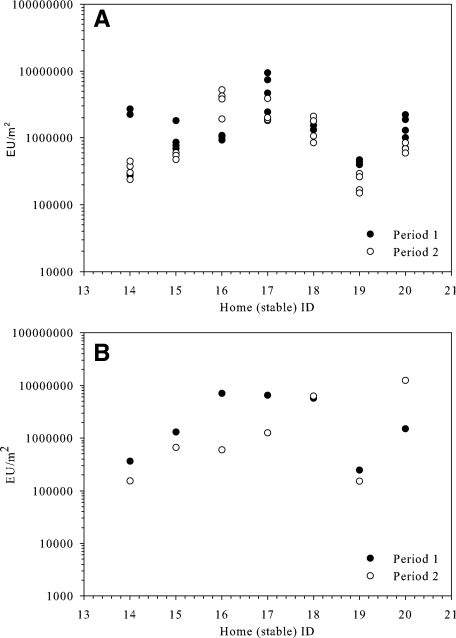
The EDC also proved to be suitable for airborne dust collection and endotoxin measurements in stables (Fig. 4). As expected, high levels for endotoxin were found (GM, 1,030,000 EU/m2). The crude settled dust samples from the stables showed similar endotoxin levels (GM, 1,300,000 EU/m2). These levels are approximately 100 times higher than the levels measured with the EDC in the farm living rooms (10,000 EU/m2; Table 1).
FIG. 4.
Endotoxin in stable dust samples from seven farms. (A) EDC; (B) crude dust.
The endotoxin levels on the four cloths of the EDCs used in stables showed an average CV of 33% with a range of 7% to 93%, which is similar to what we found for the EDCs used in the homes.
The estimated endotoxin levels in stables also showed a moderate to high correlation in time, with a correlation of 0.58 for both the EDC and the crude settled dust samples.
The endotoxin levels of the two types of stable dust samples correlated moderately (r = 0.52). Finally, when comparing stable dust endotoxin levels with the EDC results from the homes corresponding to the stables, we found a moderate correlation (r = 0.55) for the crude settled stable dust and a remarkably high (r = 0.82) correlation between the EDC endotoxin levels measured in the stables and in the living rooms of the same farms.
DISCUSSION
A new method for airborne dust sampling was developed, in which sufficient amounts of endotoxin could be recovered from dust settled on electrostatic cloths and analyzed with the LAL assay, even though only very small dust amounts were captured. These small yields of dust may, however, be a problem for the measurement of other agents, for which only less-sensitive assays are available, like some allergens. Another limitation is that since dust weights could not be assessed with sufficient accuracy, endotoxin levels measured with the EDC can only be presented in EU/m2 and not in EU/g. Since many studies express reservoir dust endotoxin levels in EU/g, this might make comparison with other studies difficult. On the other hand, for floor dust samples, both EU/g and EU/m2 values are generally accepted, while airborne endotoxin measurements are commonly given in EU/m3, not per mg (airborne) dust.
The EDC endotoxin levels measured in living rooms as well as in stables showed good reproducibility between two different sampling periods. This indicates that the method may give a reliable proxy for long-term exposure assessment. Previous reproducibility studies have shown that floor and mattress dust sampling with a vacuum cleaner may result in reproducible proxies for exposures during a period of up to 1 year (1, 5). For the EDC, this needs further investigation since the interval between the sampling periods was only 3 weeks in the present validation study. From the correlation between the results of the two periods for all sampling methods, it seemed that endotoxin levels assessed by active airborne sampling in the farm environment were more susceptible to variation over time compared to the other two methods. Since the active airborne dust sampling selectively captured PM10, this suggests that the PM10 fraction may be more variable in time, but the numbers of observations in this study are too small to draw definite conclusions.
The levels of endotoxin measured with EDC sampling correlated reasonably well with the endotoxin levels measured with active airborne or with floor dust sampling, while the active airborne and floor dust sampling showed only low correlation with each other (r = 0.3). A similar low correlation (r = 0.21) for endotoxin measurement by active airborne and floor dust sampling was reported by Park et al. (12). The results of our study suggest that characteristics of the dust collected with the EDC in homes are somewhere in between the characteristics of the dust collected with the other two methods. Active airborne dust sampling was conducted with PM10 selective filter heads, while the EDC as well as the floor dust sampling are not size-selective methods. Particles captured by the EDC must be airborne to be captured at 1.5-m height and on average must be smaller than floor dust particles; nevertheless, they may be larger than the PM10 particles. Thus, it can be assumed that the EDC collects particles that may be missed by the active airborne dust sampling. Such intermediate dust properties may explain the good correlation of the EDC with both other methods and the much weaker correlation of the active airborne dust sampling with the floor dust sampling. The high correlation between the EDC endotoxin levels measured in stables and in living rooms from the corresponding farms indicates that the levels we found in those homes may be influenced by the 100-times-higher levels found in the stable. It has been described previously that particles may be transported from the workplace into the homes for bakery allergens (22) and animal allergens (10). The results from this small validation study suggest this may also be true for endotoxin. The high correlation to the active airborne dust measurements and the good repeatability over time supports our conclusion that the EDC can serve as a good proxy of airborne exposure to endotoxins.
Cloths within the same EDC or in two EDCs used in parallel in a home gave very similar results. Thus, differences in endotoxin yields between cloths—either due to a different dust-capturing efficacy, a different release of lipopolysaccharides from cloths, and/or analytical errors in the LAL assay—appeared to be small compared to the range of airborne endotoxin levels measured with the EDC. This means that a single cloth from an EDC should be sufficient to characterize a home's airborne endotoxin level. Since with one EDC we collect airborne dust on four parallel cloths, the four cloths may be used separately to measure diverse agents for which different extraction and analysis procedures after sampling are needed. Preliminary studies showed that it is possible to measure β-1,3-glucans or culture-viable molds from the cloths (data not shown). Future work will be directed toward measuring other agents, like fungal extracellular polysaccharide antigens, peptidoglycans, or allergens.
Whether a home was situated on a farm or not determined the level of exposure for all methods. Levels of endotoxins found in farm homes were approximately three times higher than those found in nonfarm homes. Similar differences for the floor dust sampling between farm and nonfarm homes have been reported previously (6, 16, 23).
Very little information is available on airborne endotoxin levels in homes, due to the low levels of airborne endotoxin found in these environments and the high logistic and equipment costs to sample with active airborne sampling methods. Park et al. (11, 12) reported GMs of 0.64 to 0.77 EU/m3 for urban homes, and Thorne et al. (20) performed 24-h inhalable dust sampling in the homes of rural asthmatic children and reported a GM of 6 EU/m3 and a GSD of 2.8. Both studies found higher levels than we found in nonfarm or farm homes. Hyvarinen et al. (6) reported for their active airborne dust sampling endotoxin levels comparable to what we found. In that study, endotoxin was measured with a “pizza box”-like sampler, but the results were only reported in EU/mg (24) and not in EU/m2. Since we could not accurately assess the net dust weight captured on the EDC cloths, we cannot compare our results with that study. Moreover, the sampling period used by Hyvarinen et al. was up to several months, much longer than in our study. This further complicates any comparison of measured endotoxin levels. For other passive or settling airborne dust samples, endotoxin levels have not been reported as yet. The petri dish sampling method (8, 9, 21) collects dust on a much smaller surface; thus, a longer sampling duration would be necessary to measure endotoxin with this method. Another disadvantage of both the petri dish sampling and the dust fall collector (24) is that captured dust must be transferred before extraction and analysis, which might lead to the loss of dust and therefore a loss of precision. In addition, it is not possible to send a petri dish or a dust fall collector after sampling back to the laboratory by regular mail. With the new EDC, this is more than a theoretical option, as shown by our first experiences in the European GABRIEL study. In that multicenter population study, we have distributed several thousand EDCs to study participants, who, with the help of a simple photoillustrated instruction, deploy the EDC sampler themselves and return the sampler together with a completed questionnaire by mail in a simple 20- by 40-cm envelope.
In conclusion, the EDC can be used as a tool to assess airborne endotoxin exposures in home and/or work environments. It is easy to use for the participants of a study, can be sent by mail, and is a cheap and reliable method to collect airborne dust. The electrostatic dust fall collector is a potential alternative to or a complement for vacuum dust sampling in large-scale epidemiological studies.
Acknowledgments
This work was supported by the European Commission as part of GABRIEL, contract number 018996 under the Integrated Program LSH-2004-1.2.5-1. This work was also supported by the EU FP6-funded network of excellence GA2LEN. Peter S. Thorne was supported by NIH P30 ES05605.
We thank the participants of this validation study for their cooperation and James A. Deddens, University of Cincinnati, for his help with the statistical analysis.
Footnotes
Published ahead of print on 1 August 2008.
REFERENCES
- 1.Antens, C. J., M. Oldenwening, A. Wolse, U. Gehring, H. A. Smit, R. C. Aalberse, M. Kerkhof, J. Gerritsen, J. C. de Jongste, and B. Brunekreef. 2006. Repeated measurements of mite and pet allergen levels in house dust over a time period of 8 years. Clin. Exp. Allergy 36:1525-1531. [DOI] [PubMed] [Google Scholar]
- 2.Custis, N. J., J. A. Woodfolk, J. W. Vaughan, and T. A. Platts-Mills. 2003. Quantitative measurement of airborne allergens from dust mites, dogs, and cats using an ion-charging device. Clin. Exp. Allergy 33:986-991. [DOI] [PubMed] [Google Scholar]
- 3.Custovic, A., B. Simpson, A. Simpson, C. Hallam, M. Craven, and A. Woodcock. 1999. Relationship between mite, cat, and dog allergens in reservoir dust and ambient air. Allergy 54:612-616. [DOI] [PubMed] [Google Scholar]
- 4.Fahlbusch, B., A. Koch, J. Douwes, W. Bischof, U. Gehring, K. Richter, H. E. Wichmann, and J. Heinrich. 2003. The effect of storage on allergen and microbial agent levels in frozen house dust. Allergy 58:150-153. [DOI] [PubMed] [Google Scholar]
- 5.Heinrich, J., B. Holscher, J. Douwes, K. Richter, A. Koch, W. Bischof, B. Fahlbusch, R. W. Kinne, and H. E. Wichmann. 2003. Reproducibility of allergen, endotoxin and fungi measurements in the indoor environment. J. Expo. Anal. Environ. Epidemiol. 13:152-160. [DOI] [PubMed] [Google Scholar]
- 6.Hyvarinen, A., M. Roponen, P. Tiittanen, S. Laitinen, A. Nevalainen, and J. Pekkanen. 2006. Dust sampling methods for endotoxin—an essential, but underestimated issue. Indoor Air 16:20-27. [DOI] [PubMed] [Google Scholar]
- 7.Janssen, N. A. H., G. Hoek, H. Harssema, and B. Brunekreef. 1998. Personal sampling of airborne particles: method performance and data quality. J. Expo. Anal. Environ. Epidemiol. 8:37-49. [PubMed] [Google Scholar]
- 8.Karlsson, A. S., M. Hedren, C. Almqvist, K. Larsson, and A. Renstrom. 2002. Evaluation of Petri dish sampling for assessment of cat allergen in airborne dust. Allergy 57:164-168. [DOI] [PubMed] [Google Scholar]
- 9.Karlsson, A. S., A. Renstrom, M. Hedren, and K. Larsson. 2002. Comparison of four allergen-sampling methods in conventional and allergy prevention classrooms. Clin. Exp. Allergy 32:1776-1781. [DOI] [PubMed] [Google Scholar]
- 10.Krop, E. J., G. Doekes, M. J. Stone, R. C. Aalberse, and J. S. van der Zee. 2007. Spreading of occupational allergens: laboratory animal allergens on hair-covering caps and in mattress dust of laboratory animal workers. Occup. Environ. Med. 64:267-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park, J. H., D. L. Spiegelman, H. A. Burge, D. R. Gold, G. L. Chew, and D. K. Milton. 2000. Longitudinal study of dust and airborne endotoxin in the home. Environ. Health Perspect. 108:1023-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park, J. H., D. L. Spiegelman, D. R. Gold, H. A. Burge, and D. K. Milton. 2001. Predictors of airborne endotoxin in the home. Environ. Health Perspect. 109:859-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Platts-Mills, J. A., N. J. Custis, J. A. Woodfolk, and T. A. Platts-Mills. 2005. Airborne endotoxin in homes with domestic animals: implications for cat-specific tolerance. J. Allergy Clin. Immunol. 116:384-389. [DOI] [PubMed] [Google Scholar]
- 14.Platts-Mills, T. A., W. R. Thomas, R. C. Aalberse, D. Vervloet, and M. D. Champman. 1992. Dust mite allergens and asthma: report of a second international workshop. J. Allergy Clin. Immunol. 89:1046-1060. [DOI] [PubMed] [Google Scholar]
- 15.Renstrom, A. 2002. Exposure to airborne allergens: a review of sampling methods. J. Environ. Monit. 4:619-622. [DOI] [PubMed] [Google Scholar]
- 16.Schram, D., G. Doekes, M. Boeve, J. Douwes, J. Riedler, E. Ublagger, E. von Mutius, J. Budde, G. Pershagen, F. Nyberg, J. Alm, C. Braun-Fahrlander, M. Waser, and B. Brunekreef. 2005. Bacterial and fungal components in house dust of farm children, Rudolf Steiner school children and reference children—the PARSIFAL study. Allergy 60:611-618. [DOI] [PubMed] [Google Scholar]
- 17.Schram-Bijkerk, D., G. Doekes, M. Boeve, J. Douwes, J. Riedler, E. Ublagger, E. von Mutius, M. Benz, G. Pershagen, M. Wickman, T. Alfven, C. Braun-Fahrlander, M. Waser, and B. Brunekreef. 2006. Exposure to microbial components and allergens in population studies: a comparison of two house dust collection methods applied by participants and fieldworkers. Indoor Air 16:414-425. [DOI] [PubMed] [Google Scholar]
- 18.Sotiriou, M., S. F. Ferguson, M. Davey, J. M. Wolfson, P. Demokritou, J. Lawrence, S. N. Sax, and P. Koutrakis. 2008. Measurement of particle concentrations in a dental office. Environ. Monit. Assess. 137:351-361. [DOI] [PubMed] [Google Scholar]
- 19.Thorne, P. S., N. Metwali, E. Avol, and R. S. McConnell. 2005. Surface sampling for endotoxin assessment using electrostatic wiping cloths. Ann. Occup. Hyg. 49:401-406. [DOI] [PubMed] [Google Scholar]
- 20.Thorne, P. S., N. Metwali, A. K. W. Kuehl, K. M. Kelly, and E. A. Chrischilles. 2007. Repeated assessment of endotoxin in airborne and settled dust in rural houses. Am. J. Respir. Crit. Care Med. 175:A538. [Google Scholar]
- 21.Tovey, E. R., T. Z. Mitakakis, J. K. Sercombe, C. H. Vanlaar, and G. B. Marks. 2003. Four methods of sampling for dust mite allergen: differences in “dust.” Allergy 58:790-794. [DOI] [PubMed] [Google Scholar]
- 22.Vissers, M., G. Doekes, and D. Heederik. 2001. Exposure to wheat allergen and fungal alpha-amylase in the homes of bakers. Clin. Exp. Allergy 31:1577-1582. [DOI] [PubMed] [Google Scholar]
- 23.Waser, M., R. Schierl, E. von Mutius, S. Maisch, D. Carr, J. Riedler, W. Eder, M. Schreuer, D. Nowak, and C. Braun-Fahrlander. 2004. Determinants of endotoxin levels in living environments of farmers' children and their peers from rural areas. Clin. Exp. Allergy 34:389-397. [DOI] [PubMed] [Google Scholar]
- 24.Wurtz, H., T. Sigsgaard, O. Valbjorn, G. Doekes, and H. W. Meyer. 2005. The dustfall collector—a simple passive tool for long-term collection of airborne dust: a project under the Danish Mould in Buildings Program (DAMIB). Indoor Air 15(Suppl. 9):33-40. [DOI] [PubMed] [Google Scholar]