Abstract
A new huanglongbing (HLB) “Candidatus Liberibacter” species is genetically characterized, and the bacterium is designated “Candidatus Liberibacter psyllaurous.” This bacterium infects the psyllid Bactericera cockerelli and its solanaceous host plants potato and tomato, potentially resulting in “psyllid yellowing.” Host plant-dependent HLB transmission and variation in psyllid infection frequencies are found.
Huanglongbing (HLB) is a serious disease found in citrus (3) and is vectored by two psyllid species, Diaphorina citri Kuwamaya and Trioza erytreae (Del Guercio) (4, 17). HLB is caused by a phloem-restricted, nonculturable bacterium that belongs to the genus “Candidatus Liberibacter,” which belongs to the Alphaproteobacteria (12). Currently, the genus “Candidatus Liberibacter” is composed of three known species that cause disease, primarily in citrus (3): “Candidatus Liberibacter asiaticus” (12, 13), “Candidatus Liberibacter africanus” (9, 12, 13, 20), and “Candidatus Liberibacter americanus” (26, 27). In this study, we report a new HLB “Candidatus Liberibacter” species which is unique from the other three species because it causes disease in solanaceous plants, such as tomato and potato, and is vectored by the psyllid species Bactericera cockerelli (Sulc).
The psyllid B. cockerelli is found in western North America (16). B. cockerelli is a polyphagous phloem feeder, can successfully reproduce on a wide variety of host plant species, and has been a pest of potato (Solanum tuberosum L.) and tomato (Solanum lycopersicon L.) for many years (21, 29). The salivary secretion of B. cockerelli causes a debilitating yellowing plant condition in tomato and potato called “psyllid yellows” (5, 14). The factor in psyllid secretions that causes psyllid yellows was previously unknown and thought to be a toxin produced by the psyllid (2, 6, 22). In this study, we characterize and describe the potential causative bacterial agent of psyllid yellows disease.
Detection and characterization of bacteria.
Detection and characterization of the HLB symbiont involved several techniques, including PCR, gel electrophoresis, cloning, primer walking, and sequencing. Insect DNA was isolated from individual specimens using two different kits. Initial extractions for cloning and sequencing were performed using the Gentra Puregene cell and tissue kit (Qiagen, Valencia, CA). DNA extractions for determining the presence and absence of the new HLB bacterial symbiont within all psyllid life stages were performed using the EDNA HiSpEx tissue kit (Saturn Biotech, Perth, Australia). This kit enabled the extraction of amplifiable DNA from all psyllid life stages better than the Gentra kit (A. K. Hansen, unpublished data). Plant DNA was extracted using a DNeasy Plant mini extraction kit (Qiagen).
Initial screening of bacteria in psyllids began with eubacterium-specific primers (standard primer 10F and the universal reverse primer 480R) (24, 25) with Phusion high-fidelity DNA polymerase (Finnzymes Oy, Espoo, Finland). PCR concentrations, conditions, and electrophoresis are described by Hansen et al. (11). In all cases (of infected psyllids), this yielded a single visible band of ∼2.6 kb in length. To ensure that one bacterial symbiont was present (7, 11), 10F and 480R PCR products of an HLB-infected psyllid individual (from the Texas colony [see below]) were run out on a 0.7% agarose gel at ∼1.7 V/cm. After 14 h, only one band was present (∼2.6 kb in length). This PCR product was then cloned using the GeneJET PCR cloning kit (Fermentas International Inc., Burlington, Ontario, Canada).
Four sets of overlapping primers were designed (Table 1) by primer walking to amplify clone fragments of <1,000 bp for direct sequencing. The same PCR conditions used above with Phusion polymerase were used for the four primer sets, but with an annealing temperature of 60°C. Amplified DNA was cleaned using the Wizard PCR Preps DNA purification system (Promega, Madison, WI) and directly sequenced in both directions. With the 10F and 480R primers on tomato and potato plants, chloroplast DNA was preferentially amplified. Consequently, the four overlapping sets of primers designed by primer walking (see above and Table 1) were used to amplify and subsequently sequence HLB bacteria DNA from plants. Since fragment 4 (Table 1) spans a variable intergenic spacer region (ISR) (from the end of the 16S rRNA through the ISR to the beginning of the 23S rRNA gene) and always amplified HLB bacteria during optimization assays (checked by sequencing), we used the fourth primer set to detect the presence of the bacterial symbiont in the plant and insect. To control against false negatives, for insects, primers that are specific to the 28S D2 rRNA of B. cockerelli were developed (Table 1), and for plants, extracted DNA was visualized after electrophoresis on a 1% agarose gel stained with ethidium bromide. Known positive and negative controls were also included in all assays. Also, since two B. cockerelli mitochondria haplotypes are known in North America (15), 10 psyllids representing each haplotype (Weslaco City, TX, colony and the Ventura, CA, colony) were screened for HLB infection with 10F and 480R primers. This 2.6-kb fragment was then cloned and subsequently sequenced with the overlapping four primer sets (Table 1).
TABLE 1.
Diagnostic primers used in this study for screening eubacteria (excluding the primary symbionts Carsonella spp.) and symbionts of Bactericera (Paratrioza) cockerelli (Sulc)
| Target DNA | Target | Primer name | Primer sequence (5′-3′) | Product size (bp) | Reference |
|---|---|---|---|---|---|
| All eubacteria | 16S-23S rRNAs | 10F | AGTTTGATCATGGCTCAGATTG | ∼2,600 | 24 |
| 480R | CACGGTACTGGTTCACTATCGGTC | 24 | |||
| “Candidatus Liberibacter psyllaurous” | 16S rRNA | Lp Frag 1-(25F) | CTGATCATGGCTCAGAACGA | 400 | This study |
| Lp Frag 1-(427R) | CGGCGAAAGAGCTTTACAAC | This study | |||
| 16S rRNA | Lp Frag 2-(266F) | AGGCCTACCAAGGCTACGAT | 872 | This study | |
| Lp Frag 2-(1138R) | CACCTTCCTCCGGATTATCA | This study | |||
| 16S rRNA-ISR | Lp Frag 3-(1024F) | CAGCTCGTGTCGTGAGATGT | 662 | This study | |
| Lp Frag 3-(1686R) | TTCGAACTACCGACCTCACC | This study | |||
| 16S rRNA-ISR-partial | Lp Frag 4-(1611F) | GGTTGATGGGGTCATTTGAG | 918 | This study | |
| 23S rRNA | LP Frag 4-480R | CACGGTACTGGTTCACTATCGGTC | This study | ||
| Bactericera cockerelli | 28S D2 rRNA | D2 BC F | GCGAGGACTCAGTTTCGTGT | 175 | This study |
| D2 BC R | AGAGCTCGACTCGGATTGTC | This study |
Phylogenetic tree analysis.
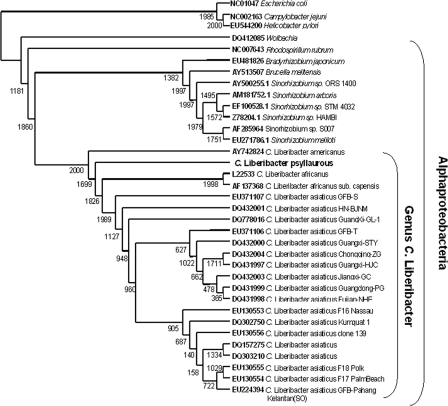
A BLAST search in May 2008 (1) with 1,156 bp of the new 16S rRNA HLB bacterium (isolated from the Texas psyllid colony [GenBank accession no. EU812556]) was conducted. All unique sequences that possessed 90% sequence similarity or more and covered the entire DNA fragment were used to construct the sequence matrix. Seven outgroups (Brucella melitensis [GenBank accession no. AY513507], Bradyrhizobium japonicum [EU481826], Caulobacter crescentus [NC_002696], Rhodospirillum rubrum [NC_007643], Wolbachia spp. [DQ412085], Escherichia coli [NC_010473], Campylobacter jejuni [NC_002163], Helicobacter pylori [EU544200]) were also included within the sequence matrix. ClustalX (28) was used to construct the multiple-sequence alignment and the neighbor-joining phylogeny. Bootstrapping with 2,000 replicates was preformed to achieve 95% reproducibility (10). TREEVIEW 1.6.6 (19) was used to draw the phylogenetic tree. In an additional analysis, we found the sequences showing the most similarity to 1,169 bp of the 16S rRNA of the new HLB bacterium (isolated from the Texas psyllid colony spanning bp 76 to bp 1, 245, since this fragment size has 100% coverage with all HLB “Candidatus Liberibacter” species in GenBank as of June 2008) by a BLAST search (1).
Characterization of the HLB bacterium.
The same HLB 16S-ISR-partial 23S rRNA sequence was found in the psyllid populations from both Texas and California (GenBank accession no. EU812556 and EU812557) and in the bacterial DNA from infected plants (GenBank accession no. EU812558 and EU812559).
Based on the neighbor-joining analysis, the new HLB bacterium is nested within the genus “Candidatus Liberibacter” (Fig. 1). The 16S rRNA sequence most similar to this new “Candidatus Liberibacter” species is sequence DQ471901, “Candidatus Liberibacter asiaticus” from Brazil, which possesses 97% sequence similarity to the new bacterium, with a total BLAST score of 1,965. Overall, there are 26 base pair changes between the sequences and five insertion/deletion events. Based on the genetic and ecological characterization (see below) described herein, we tentatively designate this bacterium “Candidatus Liberibacter psyllaurous” (meaning psyllid yellows).
FIG. 1.
Neighbor-joining phylogenetic tree of 16S rRNAs. Numbers on the inside of nodes represent bootstrap values out of 2,000 iterations. Numbers on the right side of branches indicate GenBank accession numbers.
Insect colonies and transmission trials.
Hundreds of adult and nymph B. cockerelli organisms were collected from fresh market tomato plants in Weslaco City, TX, in June 2005. The colony was maintained on a variety of solanaceous host plants at 25 ± 1°C with a photoperiod of 14 h of light and 10 h of darkness. For all trials, psyllids were reared separately on potato plants and tomato plants in separate cages. The infection status of psyllid eggs, nymphal instars, and adults was tested by PCR for HLB bacterial symbionts from both the tomato and potato colonies (see Table 2 for sample sizes). Before eggs were screened for infection with PCR, the leaves and attached eggs were surface sterilized with 10% bleach (Clorox, Oakland, CA) for 5 minutes and then rinsed three times with sterilized double-distilled water. Eggs were then dissected from the leaves using heat-sterilized insect pins under a dissecting microscope. Consequently, the detection of the transovarial transmission of bacteria was conducted by PCR screening of bleach-treated eggs (Table 2) and also by isolating bleach-treated eggs on six tomato plants, all of which were isolated inside a single mesh cage. Twenty-five emerging adult psyllids were then screened for the HLB bacterium. Of the 25 adults resulting from eggs individually placed on tomato plants, 20 were infected with “Candidatus Liberibacter psyllaurous.” For PCR infection screening of different nymphal instars, we assigned individual nymphs to instars (21, 23). Based on the PCR detection of “Candidatus Liberibacter psyllaurous,” levels of HLB infection are significantly different between psyllid life stages among potato-reared psyllids (Kruskal-Wallis chi-square test score, 583; df = 6; P < 0.001) and between psyllid life stages among tomato-reared psyllids (Kruskal-Wallis chi-square test score, 583; df = 6; P < 0.001) (Table 2). In addition, nymphal-instar infection frequencies between host plants (potato and tomato) differed significantly from one another (by the Wilcoxon signed-rank test, z was −13.203; by the two-tailed t test, P < 0.001) (Table 2). HLB was detected at higher infection frequencies in eggs, first instars, and second instars isolated from potato host plants than in those isolated from tomato host plants (Table 2). On potato, psyllids were fixed for infection from the first instar to the adult stage (Table 2). However, on tomato, the infection frequency of psyllids was initially very low (egg, first instar) but then increased substantially in the second instar, becoming fixed for infection at the third instar (Table 2). Thus, our PCR screening of eggs and the egg transfer experiments show that “Candidatus Liberibacter psyllaurous” is vertically transmitted but at different rates, depending on the host plant on which the psyllid is reared (Table 2). Horizontal transmission through host feeding (Table 2) may also be important since HLB infection increases as the psyllid life stages progress (particularly on tomato).
TABLE 2.
PCR detection of “Candidatus Liberibacter psyllaurous” throughout B. cockerelli life stages reared on two different solanaceous host plants, tomato and potatoa
| Insect stage | Potato
|
Tomato
|
||||
|---|---|---|---|---|---|---|
| n | No. of psyllids infected with Lp | Infection frequency | n | No. of psyllids infected with Lp | Infection frequency | |
| Egg (10 pooled) | 3 | 3 | 1 | 3 | 0 | 0 |
| Egg (single) | 19 | 9 | 0.474 | 26 | 4 | 0.154 |
| Nymph, 1st instar | 11 | 11 | 1 | 23 | 5 | 0.217 |
| Nymph, 2nd instar | 11 | 11 | 1 | 16 | 12 | 0.75 |
| Nymph, 3rd instar | 10 | 10 | 1 | 11 | 11 | 1 |
| Nymph, 4th instar | 11 | 11 | 1 | 11 | 11 | 1 |
| Nymph, 5th instar | 12 | 12 | 1 | 12 | 12 | 1 |
| Adult | 10 | 10 | 1 | 11 | 11 | 1 |
n, number of 28S rRNA D2-positive psyllid individuals screened, confirming successful DNA extraction; Lp, “Candidatus Liberibacter psyllaurous.”
HLB transmission from psyllids to plants was studied by placing 20 psyllid adults from the HLB-infected colony on five potato plants and five tomato plants (20 psyllids/plant). Each plant was isolated in a mesh cage and was PCR screened for HLB before and after inoculation. All treatments were done in the same rearing room under the same conditions used for colony rearing (see above). One week later, samples were screened by PCR for infection by taking 100 mg of leaf mid-veins per replicate from the branch on which psyllids were released (or not released for the control) for each replicate. All five potato plants and all five tomato plants inoculated with HLB-infected psyllids (reared on potato) were positive for “Candidatus Liberibacter psyllaurous” infection. Five control tomato and potato plants (without insect inoculation) were kept in mesh cages as controls and tested negative for infection. Five psyllids from each plant were PCR screened for infection at the end of each trial, and all psyllids screened were positive for HLB. Plants were visually screened qualitatively for yellowing of psyllid-inoculated leaves. Qualitatively, tomato leaves did not show signs of yellowing until 3 to 4 weeks after the trials. After 2.5 months, all tomato leaves became yellow and displayed reduced and stunted growth. In potato, the yellow symptoms showed up a week and a half after adult psyllids inoculated plants. All potato plants died after 1 month of psyllid inoculation. Control plant leaves did not yellow or suffer any mortality during the duration of this experiment. These results for potato are consistent with the report by Munyaneza et al. (18) noting that potatoes grown in the absence of B. cockerelli do not develop yellowing symptoms. Two publications in the literature (8, 18) mention that grafting can transmit the yellowing disease, showing that the direct participation of psyllids is not required for the acquisition of the yellowing disease. We therefore conclude that “Candidatus Liberibacter psyllaurous” is likely the causative agent of this disease. The discovery of “Candidatus Liberibacter psyllaurous” can help plant breeders directly select for resistant cultivars of potato and tomato.
Nucleotide sequence accession numbers.
The GenBank accession numbers for DNA primer walking fragments resulting from the 10F and 480R rRNA operon clones isolated from Texas and California tomato psyllids are EU812556 and EU812557, respectively. The GenBank accession numbers for primer walking fragments isolated from tomato and potato plants are EU812558 and EU812559, respectively. The GenBank accession number for the 28S D2 rRNA of B. cockerelli is EU812555.
Acknowledgments
We thank Paul Rugman-Jones for molecular advice, help, and critique of the manuscript and J. Munyaneza and T. X. Liu for providing psyllids used to start a colony from Texas. We also thank Mark Hansen for his help in the greenhouse.
Footnotes
Published ahead of print on 1 August 2008.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blood, H. L., B. L. Richards, and F. B. Wann. 1933. Studies of psyllid yellows of tomato. Phytopathology 23:930. [Google Scholar]
- 3.Bové, J. M. 2006. Huanglongbing: a destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 88:7-37. [Google Scholar]
- 4.Capoor, S. P., D. G. Rao, and S. M. Viswanath. 1974. Greening disease of citrus in the Deccan trap country and its relationship with the vector Diaphorina citri Kuwayama, p. 43-49. In L. G. Weathers and M. Cohen (ed.), Proceedings of the 6th Conference of the International Organization of Citrus Virologists. University of California, Davis.
- 5.Carter, R. D. 1950. Toxicity of Paratrioza cockerelli (Sulc) to certain solanaceous plants. Ph.D. dissertation. University of California, Berkeley.
- 6.Carter, W. 1939. Injuries to plants caused by insect toxins. Bot. Rev. 5:273-326. [Google Scholar]
- 7.Dale, C., M. Beeton, C. Harbison, T. Jones, and M. Pontes. 2006. Isolation, pure culture, and characterization of “Candidatus Arsenophonus arthropodicus,” an intracellular secondary endosymbiont from the hippoboscid louse fly Pseudolynchia canariensis. Appl. Environ. Microbiol. 72:2997-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniels, L. B. 1954. The nature of the toxicogenic condition resulting from the feeding of the tomato psyllid Paratrioza cockerelli (Sulc). Ph.D. dissertation. University of Minnesota.
- 9.Garnier, M., S. Jagoueix-Eveillard, P. Cronje, H. Le Roux, and J. M. Bové. 2000. Genomic characterization of a liberibacter present in an ornamental rutaceous tree, Calodendrum capense, in the Western Cape province of South Africa. Proposal of “Candidatus Liberibacter africanus subsp. capensis.” Int. J. Syst. Evol. Microbiol. 50:2119-2125. [DOI] [PubMed] [Google Scholar]
- 10.Hall, B. G. 2005. Phylogenetic trees made easy, 2nd ed. Sinauer Associates, Inc., Sunderland, MA.
- 11.Hansen, A. K., G. Jeong, T. D. Paine, and R. Stouthamer. 2007. Frequency of secondary symbiont infection in an invasive psyllid relates to parasitism pressure on a geographic scale in California. Appl. Environ. Microbiol. 73:7531-7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jagoueix, S., J. M. Bové, and M. Garnier. 1994. The phloem-limited bacterium of greening disease of citrus is a member of the α subdivision of the Proteobacteria. Int. J. Syst. Bacteriol. 44:379-386. [DOI] [PubMed] [Google Scholar]
- 13.Jagoueix, S., J. M. Bové, and M. Garnier. 1996. PCR detection of the two ‘Candidatus’ Liberobacter species associated with greening disease of citrus. Mol. Cell. Probes 10:43-50. [DOI] [PubMed] [Google Scholar]
- 14.List, G. M. 1939. The effect of temperature upon egg deposition, egg hatch, and nymphal development of Paratrioza cockerelli (Sulc). J. Econ. Entomol. 32:30-36. [Google Scholar]
- 15.Liu, D., J. T. Trumble, and R. Stouthamer. 2006. Molecular characterization indicates recent introductions of tomato psyllid (Bactericera cockerelli) into western North America are genetically different from eastern populations. Entomol. Exp. Appl. 118:177-183. [Google Scholar]
- 16.Liu, D., and J. T. Trumble. 2007. Comparative fitness of invasive and native populations of the potato psyllid (Bactericera cockerelli). Entomol. Exp. Appl. 123:35-42. [Google Scholar]
- 17.McClean, A. P. D., and P. C. J. Oberholzer. 1965. Citrus psylla, a vector of the greening disease of sweet orange. S. Afr. J. Agric. Sci. 8:297-298. [Google Scholar]
- 18.Munyaneza, J. E., J. M. Crosslin, and J. E. Upton. 2007. Association of Bactericera cockerelli (Homoptera: Psyllidae) with “zebra chip,” a new potato disease in southwestern United States and Mexico. J. Econ. Entomol. 100:656-663. [DOI] [PubMed] [Google Scholar]
- 19.Page, R. D. M. 1996. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 20.Planet, P., S. Jagoueix, J. M. Bové, and M. Garnier. 1995. Detection and characterization of the African citrus greening liberobacter by amplification, cloning and sequencing of the rplKAJL-rpoBC operon. Curr. Microbiol. 30:137-141. [DOI] [PubMed] [Google Scholar]
- 21.Pletsch, D. J. 1947. The potato psyllid Paratrioza cockerelli (Sulc), its biology and control. Mont. Agric. Exp. Stn. Bull. 446:95. [Google Scholar]
- 22.Richards, F. B., and H. L. Blood. 1933. Psyllid yellows of the potato. J. Agric. Res. 46:189-216. [Google Scholar]
- 23.Rowe, J. A., and G. F. Knowlton. 1935. Studies upon the morphology of Paratrioza cockerelli (Sulc). Proc. Utah Acad. Sci. Arts Lett. B 12:233-239. [Google Scholar]
- 24.Russell, J. A., A. Latorre, B. Sabater-Munoz, A. Moya, and N. A. Moran. 2003. Side-stepping secondary symbionts: widespread horizontal transfer across and beyond the Aphidoidea. Mol. Ecol. 12:1061-1075. [DOI] [PubMed] [Google Scholar]
- 25.Sandström, J. A., J. Russell, J. P. White, and N. A. Moran. 2001. Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol. Ecol. 10:217-228. [DOI] [PubMed] [Google Scholar]
- 26.Teixeira, D. D. C., C. Saillard, S. Eveillard, J. L. Danet, P. I. da Costa, A. J. Ayres, and J. M. Bové. 2005. “Candidatus Liberibacter americanus,” associated with citrus huanglongbing (greening disease) in São Paulo State, Brazil. Int. J. Syst. Evol. Microbiol. 55:1857-1862. [DOI] [PubMed] [Google Scholar]
- 27.Teixeira, D. D. C., J. L. Dane, S. Eveillard, E. C. Martins, W. C. de Jesus Junior, P. T. Yamamoto, S. A. Lopes, R. B. Bassanezi, A. J. Ayres, C. Saillard, and J. M. Bové. 2005. Citrus huanglongbing in São Paulo State, Brazil: PCR detection of the “Candidatus” Liberibacter species associated with the disease. Mol. Cell. Probes 19:173-179. [DOI] [PubMed] [Google Scholar]
- 28.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallis, R. L. 1955. Ecological studies on the potato psyllid as a pest of potatoes. USDA Tech. Bull. 1107:25. [Google Scholar]