Abstract
The species structure of an ectomycorrhizal (ECM) community was assessed monthly for 15 months in the two horizons (A1 and A2) of an oak temperate forest in northeastern France. Ectomycorrhizal species were identified each month by internal transcribed spacer sequencing. Seventy-five fungal symbionts were identified. The community was dominated by Tomentellaceae, Russulaceae, Cortinariaceae, and Boletales. Four species are abundant in the study site: Lactarius quietus, Tomentella sublilacina, Cenococcum geophilum, and Russula sp1. The relative abundance of each species varied depending on the soil horizon and over time. Some species, such as L. quietus, were present in the A1 and A2 horizons. C. geophilum was located particularly in the A2 horizon, whereas T. sublilacina was more abundant in A1. Some species, such as Clavulina sp., were detected in winter, while T. sublilacina and L. quietus were present all year long. Our results support the hypothesis that a rapid turnover of species composition of the ECM community occurs over the course of a month. The spatial and temporal unequal distribution of ECM species could be explained by their ecological preferences, driven by such factors as root longevity, competition for resources, and resistance to environmental variability.
The fine roots of social tree species in temperate and boreal forests are symbiotically associated with fungi (52), forming composite organs called ectomycorrhizas (ECM). The ECM fungi play a crucial role in tree health by enhancing the nutrient acquisition, drought tolerance, and pathogen resistance of their hosts. ECMs efficiently take up water and organic and inorganic nutrients from the soil via the extramatricial mycelium and translocate these to colonized tree roots, receiving carbohydrates from the host in return (52). Most of the ectomycorrhizal roots are located in the top 20 centimeters of the soil, an area which is enriched in organic matter and where nutrients are concentrated (50). The ECM fungal community is species rich at the forest stand level, where hundreds of different fungal symbionts can be identified by morphotyping and DNA-based molecular methods (13, 17, 36, 56).
Beside its species composition, the structure is an important characteristic of the ECM community. Differences in ECM community structure on different scales are well documented: on the ecosystem scale (postdisturbance or postplanting successions) and along forest dynamics (4, 28, 56, 65), on the seasonal scale (6, 8, 20, 33, 54), and along spatial dimensions (vertical scale [13, 14, 24, 49] and horizontal scale [36, 58]). In a microsite or on a forest strand scale, species are distributed neither uniformly nor randomly but rather are aggregated in patches or distributed along gradients (9, 12, 19, 24, 44). The spatial heterogeneity of communities is important in terms of succession, adaptation, maintenance of species diversity, interspecific competition, and community stability (38). A spatial niche differentiation of ECM species and of ECM exploration types (1) could be due to specific physicochemical properties of soil horizons (47) and to differential resource utilization (35, 39). However, despite acknowledgment of the functional importance of ECM fungi in host tree nutrition, very little is known about the distribution and abundance of ECM and about the spatiotemporal structure of the ECM community in forest soil (13, 25, 29, 37, 58).
The present work addressed these issues for an oak forest in northeastern France by monthly sampling of fine roots in two soil horizons for 15 months and characterization of the structure and relative abundance of species of the ECM community in each sample. The objectives of the study were to describe the ECM community structure in time and space to obtain information about the spatiotemporal partitioning of the ECM species.
MATERIALS AND METHODS
Site and forest stand.
The experimental site is a 100-year-old oak forest with a continuous canopy and a hornbeam understory (Quercus petraea (Mattuschka) Liebl., Quercus robur Ehrh., and Carpinus betulus L.) in northeastern France (48°75′N, 6°35′E; altitude, 250 m). The luvic cambisol (pH [H2O] 4.6) has a loamy texture in the A1 (0 to 5 cm; P, 0.3 g kg−1 according to the method described in reference 15; total C, 26.7 g kg−1; total N, 1.9 g kg−1; C/N, 14.6) and depth range for the A2 horizon (P, 0.39 g kg−1; total C, 26.3 g kg−1; total N, 1.94 g kg−1; C/N, 13.4). The forest floor is flat, with scarce vegetation (oak seedlings, Convallaria majalis L., and Deschampsia cespitosa L.). Soil temperature (°C) and water potential (in MPa) were measured three times a month with 20 psychrometric probes (Wescor PST-55-15-SF) (10 cm deep in the A2 horizon) and a millivoltmeter (Wescor HR-33T; Logan, UT).
Root sampling and identification of ECM species.
In the experimental site, the sampling area (24 by 24 m) was fenced against digging by wild boars. In this area, six blocks (5 by 3 m) were materialized. We determined the size of the blocks by considering the number of samples needed from each block throughout the duration of the study (15) and by taking into account that two samples should be at least 1 m apart. Each block was placed at least 1.5 m from oak trees to prevent the bias due to stem flow. From July 2004 to September 2005, one soil core (10 cm in diameter and 25 cm deep) was randomly sampled monthly in each of the six blocks. The number and size of soil samples were chosen in accordance with previous studies (13, 51, 55, 62). Soil samples were immediately transported to the nearby laboratory and processed on the same day. The soil cores were sliced into two samples: the top (0 to 5 cm), corresponding to the A1 horizon, enriched in organic matter and containing densely packed fine roots, and the 5-to-25-cm layer, corresponding to the top of the A2 horizon. Roots were soaked in tap water for 15 min before being gently washed. Fine roots were observed in water with a stereomicroscope (magnification, ×40). First, ECM morphotypes (ectomycorrhizae sharing common morphological features) were characterized according to the method of Agerer (3). ECM morphotypes were also superficially classified at the genus level (e.g., Russula, Lactarius, Cortinarius, Boletus, and Tomentella spp.) or as “unidentified morphotype.”
Each month and for each encountered morphotype, a few tips (three to five) were kept at −20°C in Eppendorf tubes. Subsequently, the fungal symbiont of the frozen root tips was identified by sequencing the internal transcribed spacer (ITS) region of its ribosomal DNA as follows. The DNA from the pooled three to five ECM tips kept frozen for each morphotype at each sampling date was extracted using the DNeasy Plant Mini kit (Qiagen SA, Courtaboeuf, France). PCR amplification was performed using a GeneAmp 9600 thermocycler (Perkin-Elmer Instruments, Connecticut) for the ITS region of nuclear ribosomal DNA, using the fungus-specific primer pair ITS1F and ITS4 (17). Successful PCRs resulted in a single band on a 0.8% agarose gel (Bioprobe, QBiogene) in 1% Tris buffer-EDTA and stained with ethidium bromide (2 μg/ml; Roche, France). The size of the band was estimated with a 1-kb ladder (Gibco BRL, France). Amplified products were purified using the Multiscreen-PCR plate system (Millipore Corporation, Massachusetts) according to the manufacturer's instructions. The DNA concentration was estimated with a Low DNA Mass ladder (Invitrogen, France). The sequencing of the amplicons from the ITS PCR was performed on the eight-capillary sequencer CEQ 2000XL (Beckman, California). Two nanograms of purified template DNA was labeled during a cycle of sequencing reaction with 5 ng CEQ DTCS-Quick Start kit (Beckman, California) in a GeneAmp 9600 thermocycler (Perkin Elmer Instruments, Connecticut). All samples were sequenced with the primers ITS1F and ITS4 (17).
Sequences were visually checked and manually corrected after identification of machine errors. Sequences showing a potential species mixture (superimposed chromatograms suggesting the presence of more than one ITS sequence) were eliminated. Sequences were grouped by similarity (>99% sequence identity) using the Sequencher 4.2 software program (Gene Codes, Ann Arbor, MI). To reveal the taxonomic affinities of fungal species, BLASTN searches were carried out against the NCBI (http://www.ncbi.nlm.nih.gov/) and UNITE (http://unite.ut.ee [34]) public sequence databases. The relevance of species identifications was assessed according to the geographic origin of the species given by similarity research. We consider the cutoff similarity point for between-species distinctions to be about 98% (59). Results of sequence analyses are given in Table S1 in the supplemental material. This step allows a species name to be assigned for each morphotype. It also allows the grouping of morphotypes we first considered to be different. As a consequence, 539 sequences were obtained from the ECMs studied during the 15 months. Only 4 of the 539 sequences showed species mixture (less than one percent of the total number of sequences).
Calculations and statistical analysis.
The total monthly abundance (total number of living ECM per soil sample) was recorded for each morphotype in the two horizons. The relative morphotype abundance in a sample (proportion of ECM, expressed as the percentage of each morphotype per total number of living ECM root tips in the sample) was then calculated. The abundance of ECM species was analyzed with two-way analysis of variance (ANOVA) using the S-PLUS software program (64); the two factors of interest were species and date of sampling, species and horizon, or horizon and date of sampling (differences are significant for P values of <0.05). We also analyzed the variation between the different samples at the same sampling date (interblock variation). Accordingly, to explore the response of the variable “abundance of ECM species,” significant differences following the ANOVA were analyzed with Tukey's honestly significant difference test.
A principal component analyses (PCA) of the relative species abundance was used to analyze the influence of the sampling date. Subsequently, ECMs were classified into exploration types (1). The relative proportions of the different exploration types found in the A1 horizon and the A2 horizon were compared using a χ2 test (P < 0.05).
Diversity analyses.
We used the equitability index (40) as a diversity descriptor to characterize monthly the ECM community in the two horizons. Equitability is the property of a community assemblage that relates to evenness of distribution of the species or their relative abundances: maximum equitability indicates that all species are represented by a similar number of individuals and minimum equitability that one species only is dominant and all others are sparsely represented. The equitability index (J′) is the component of species diversity that measures the relative abundance of species and was calculated as follows: J′ = H′/log(S), where S is the number of species and H′ the Shannon-Weiner index. The Shannon-Weiner index is a measure of the information content that accounts for evenness and richness. It was used as an index of biodiversity in a sample: H′ = −Σ[Pilog(Pi)], where Pi is the probability that one apex belongs to ECM species i and can be derived from the equation Pi = Ni/N, where Ni is the number of apexes in ECM species i and N the total number of apexes (45, 57).
RESULTS
The mean temperature and mean water potential measured during the 15-month sampling period were 11.2°C and −0.79 MPa, respectively (data not shown). Soil temperature reached a maximum of 21°C in August 2005 and a minimum of 1.4°C in March 2005. The soil water potential reached a maximum of −0.18 MPa in winter and a minimum of −2.13 MPa in August 2005. The temperature stayed above the mean value from July 2004 to November 2004 and from May 2005 to September 2005. The water soil potential stayed below the mean value from July 2004 to November 2004 and from July 2005 to September 2005.
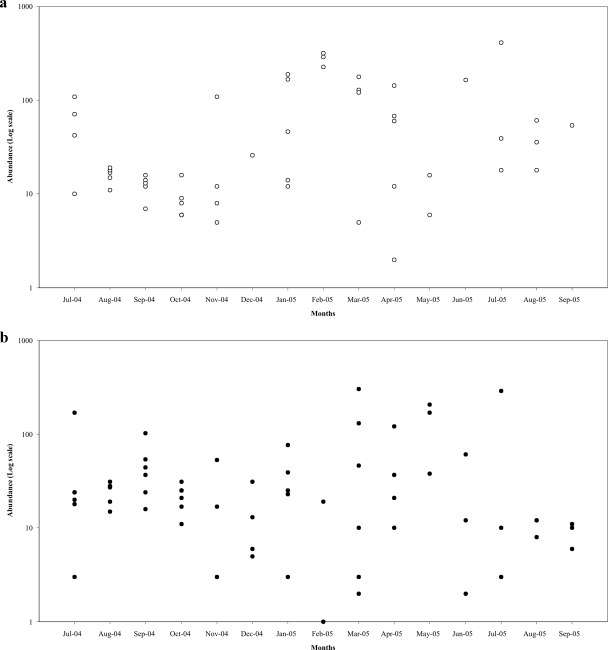
Figure 1 represents the number of ectomycorrhizal root tips counted in each of the six soil samples throughout the year for the two species most frequently found in the A1 horizon, Lactarius quietus and Tomentella sublilacina. We observed that these ectomycorrhizas were not found in the six soil samples at each sampling date (i.e., in November and December 2004 for L. quietus and T. sublilacina). Moreover, the number of tips counted in each soil sample varied widely (up to 2 orders of magnitude) among the replicates at a given sampling date. But the variation between the different samples at the same sampling date (interblock variation) was not significant (df = 6; F = 0.920; P = 0.46). The result of the ANOVA has shown that the species (df = 74; F = 8.371; P < 10−6), the sampling date (df = 14; F = 4.690; P < 10−6), and the horizon (df = 2; F = 41.611; P < 10−6) factors had a significant effect on the abundance of the species (P < 0.001).
FIG. 1.
Number of L. quietus (a) or T. sublilacina (b) root tips in each sample (log scale) at each date of sampling from July 2004 to September 2005 in the A1 horizon. L. quietus and T. sublilacina are the two most abundant ECM species in the A1 horizon.
Distribution of ECM species in two horizons of the study site.
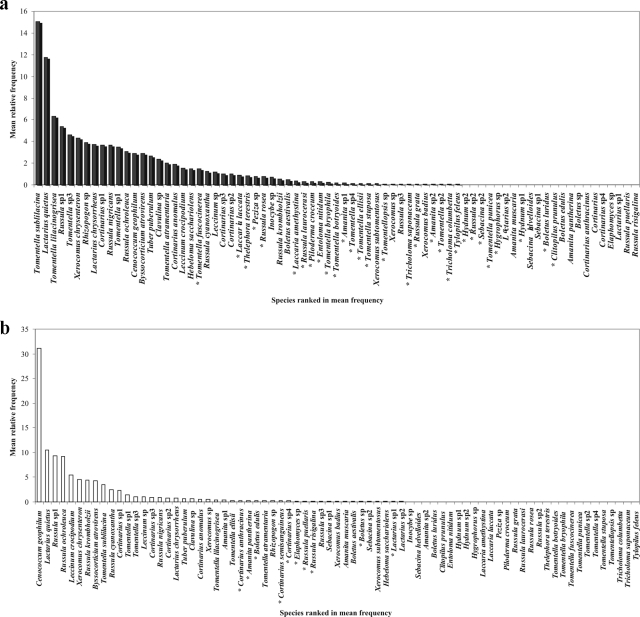
A total of 90 soil cores and 180 samples were collected from July 2004 to September 2005. Seventy-five different ECM species were distinguished, based on molecular analysis of ECM root tips (see Table S1 in the supplemental material). Among these, one species, Cortinarius sp4, was identified solely based on morphological aspect, because sequencing failed. Based on the high similarity to databases sequences, Russula, Lactarius, Cortinarius, Boletus, and Tomentella spp. were easily identified at the species level. Ninety-eight percent identity is commonly taken to be the cutoff point for species delimitations (59). With the 15 sampling dates pooled together, this study revealed a few abundant and many rare species (Fig. 2). Tomentella sublilacina, Lactarius quietus, Tomentella lilacinogrisea, and Russula sp1 were the most common species in the A1 horizon (15, 11.8, 6.3, and 5.4%, respectively) (Fig. 2a), while Cenococcum geophilum, L. quietus, Russula sp1, and Russula ochroleuca were most common in the A2 horizon (31.2, 10.5, 9.4 and 9.2%, respectively) (Fig. 2b). Among the rare fungi, 50 and 61 species out of the 75 species present in the A1 and A2 horizon, respectively, had a mean relative frequency below one percent. The two soil horizons shared 38 species (48%) (e.g., C. geophilum, L. quietus, and T. sublilacina). Some species were present only in either the A1 horizon (27 species, e.g., Entoloma nitidum, Laccaria amethystina, and Piloderma croceum) or in the A2 horizon (10 species, e.g., Amanita pantherina, Cortinarius anthracinus, Russula puellaris) (see Table S1 in the supplemental material). The result of Tukey's test (data not shown) on the abundance between species showed that the abundances of L. quietus, T. sublilacina, Russula sp1, and C. geophilum were significantly higher than those of the 71 remaining species. The result of the two-way ANOVA between species and horizon factors highlights that the interaction between the two factors was significant (df = 74; F = 4.144; P < 10−6).
FIG. 2.
Mean relative abundance of the 75 ectomycorrhizal species in the A1 horizon (a) or in the A2 horizon (b). Species are ranked according to their mean frequencies. An asterisk indicates an ectomycorrhizal species present in only one of the two horizons. Twenty-seven species were observed only in the A1 horizon and ten only in the A2 horizon. Thirty-eight species were present in both horizons.
Based on the ECM exploration type classification according to the method of Agerer (1), contact (C), short-distance (SD), medium-distance (MD), and long-distance (LD) types could be found. The distribution of the 75 species into the exploration types is shown in Table S1 in the supplemental material. Species were distributed with different proportions in the four different groups: C (23 spp.), SD (11 spp.), MD (29 spp.), and LD (12 spp.). In the A1 horizon, the most abundant species belonged to the C (e.g., Russula sp1, T. sublilacina, and T. lilacinogrisea), MD (e.g., Cortinarius sp1, L. quietus, and Lactarius chrysorrheus), and LD (e.g., Xerocomus chrysenteron) types. In the A2 horizon, the most abundant species belonged to the C (e.g., Russula sp1 and R. ochroleuca), SD (e.g., C. geophilum), MD (e.g., L. quietus), and LD (e.g., Leccinum crocipodium and X. chrysenteron) types. The C and MD types were significantly more abundant in the A1 horizon (49.3 and 31.9%, respectively) than in A2 (33.5 and 22.8%, respectively). The SD type was significantly more abundant in the A2 horizon than in A1 (33.5 and 8.3%, respectively). No differences were observed for the LD type (10.5 and 10.2% in the A1 and A2 horizons, respectively).
Analysis of diversity indicators of the ECM community.
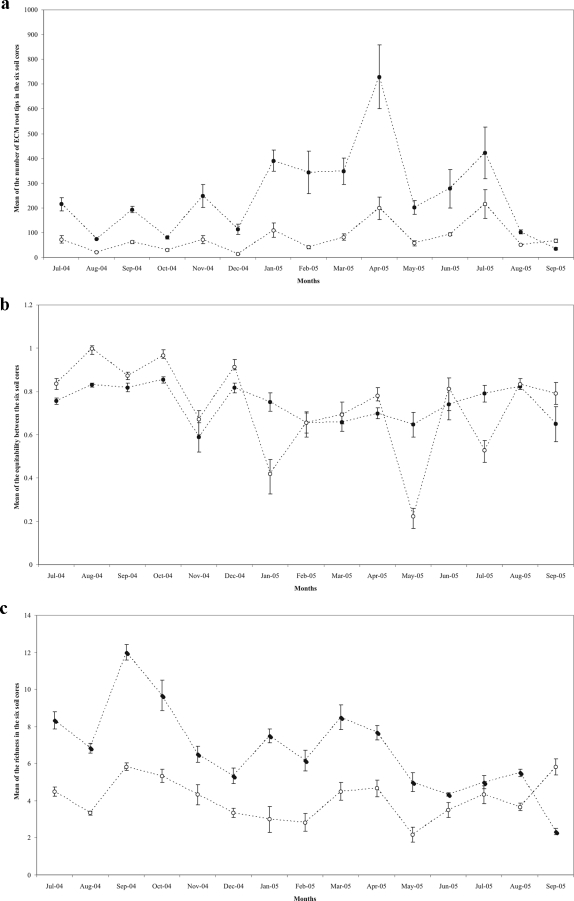
With the exception of September 2005, the mean of species richness in the six soil cores per date was higher in the A1 horizon than in the A2 horizon (Fig. 3c).
FIG. 3.
Parameters characterizing the ectomycorrhizal community followed during 15 months, from July 2005 to September 2006, in the A1 (filled circles) or A2 (open circles) horizon. (a) Monthly mean total number of ECM root tips in the six samples. (b) Monthly mean equitability [J′ = H′/log(S), where S is the number of species and H′ the Shannon-Weiner index]. The Shannon-Weiner index is a measure of the information content that accounts for evenness and richness. It was used as an index of biodiversity in a sample: H′ = −Σ[Pilog(Pi)], where Pi is the probability that one apex belongs to ECM species i and can be derived from Pi = Ni/N, where Ni is the number of apexes in ECM species i and N the total number of apexes. (c) Monthly mean of species richness. Bars represent standard errors (n = 6).
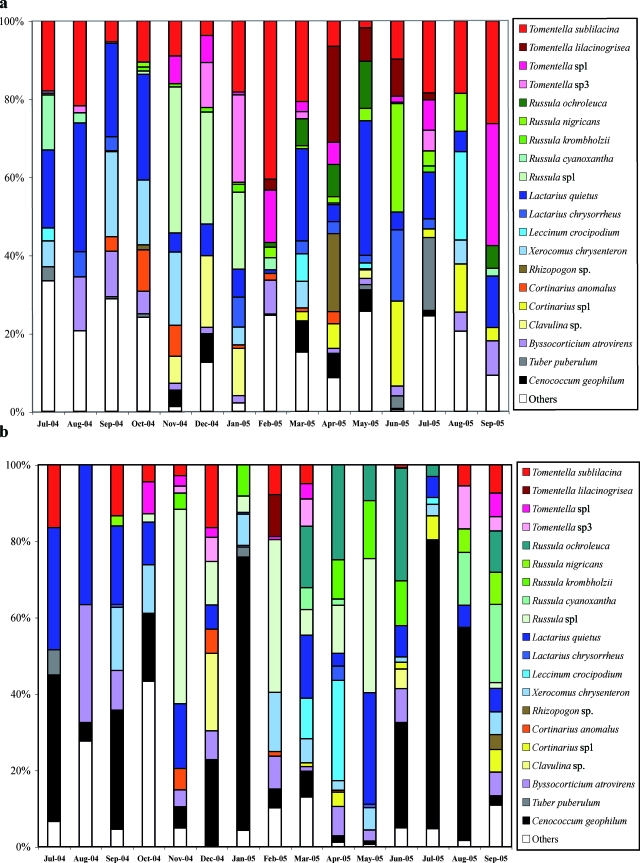
The equitability index was significantly the lowest in the A2 horizon in January 2005, May 2005, and July 2005 (Fig. 3b). For these dates, one or two ectomycorrhizal species, exceptionally more abundant, were responsible for the low equitability index in the A2 horizon: C. geophilum in January, Russula sp1 and L. quietus in May, and C. geophilum in July (Fig. 4). No differences were observed in equitability in February 2005, March 2005, or June and July 2005. From July 2004 to October 2004 and in December 2004, April 2005, and September 2005, the equitability was significantly higher in the A2 horizon.
FIG. 4.
Relative monthly abundances of the 20 most abundant ectomycorrhizal species in the A1 horizon (a) or in the A2 horizon (b). The other morphotypes are pooled under the term “others.” Orange, Cortinarius species; light blue, Boletus species; dark blue, Lactarius species; green, Russula species; red, Tomentella species.
Temporal changes of ECM species in the study site.
With the exception of September 2005, the mean number of ECM root tips in the six soil cores was higher in the A1 horizon than in the A2 horizon due to a higher density of roots in the A1 horizon. A peak was observed in April 2005 in the A1 horizon and a less accentuated one at the same date in the A2 horizon (Fig. 3a). The result of Tukey's test (data not shown) on the abundance between dates has shown that April 2005 was the date significantly different from the others (P < 0.005). This date corresponds to the beginning of spring and the budbreak. The result of the two-way ANOVA between species and date factors highlights that the interaction between the two factors was significant (df = 1036; F = 1.540; P < 10−6).
The different ECM species encountered grouped according to their presence, abundance, and frequency for the whole year in each horizon. Some species, such as L. quietus and T. sublilacina, were present and abundant all year long (Fig. 4a and b). C. geophilum was present all year long but abundant only in summer (July and August 2004 and 2005) and in winter from December 2004 to January 2005. This ECM species was found in the two horizons but especially in the A2 horizon. Russula sp1 was present all year long but was abundant from November 2004 to January 2005 in the A1 horizon and from November 2004 to May 2005 in the A2 horizon. Some species, such as Clavulina sp., were present as ECM only during a few months. Clavulina sp. was present in winter from November 2004 to January 2005 and in May, just at the beginning of the spring. A fourth type of pattern was noted for Entoloma nitidum (an ECM morphotype first found in our sampling site [41]). It was present only in July, when the pedoclimatic conditions become critical, with high temperature and low water potential: we made this observation every year in July 2003, 2004 and 2005 (data not published).
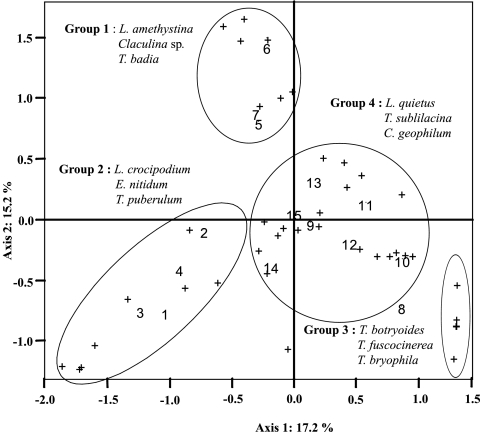
The graphic overlay of the PCA (Fig. 5) depicting the distribution of the ECM species across time resulted from the relative abundance data measured monthly in the A1 horizon. The first two axes explained 17.2 and 15.2% of the variance, respectively. Four principal groups of ECM species were noted, corresponding to fungi present all year long or only during a specific period. The group numbered 1 corresponded to ECM species present in summer, when soil water potential was very negative (i.e., E. nitidum, L. crocipodium, and Tuber puberulum). The second group corresponded to fungi specific to the winter period (i.e., L. amethystina, Clavulina sp., and Tomentella badia). The third group pooled ECM species present only in February and which contained only Tomentella species (i.e., T. botryoides, T. bryophyla, and T. fuscocinerea). The last group corresponded to ECM fungi present all year long with fluctuating abundance (i.e., L. quietus, T. sublilacina, and C. geophilum). No such seasonal structuration of the ECM community appeared when the PCA was done on the data from the A2 horizon.
FIG. 5.
Principal component analysis indicating the effect of sampling date on ectomycorrhizal fungal community structure in the A1 horizon. Crosses correspond to ECM species. Three representative species are given for each group. Numbers correspond to sampling dates (1, July 2004; 2, August 2004; 3, September 2004; 4, October 2004; 5, November 2004; 6, December 2004; 7, January 2005; 8, February 2005; 9, March 2005; 10, April 2005; 11, May 2005; 12, June 2005; 13, July 2005; 14, August 2005; 15, September 2005).
DISCUSSION
Overall structure of the ECM community.
The ECM community described here in a mature oak stand is dominated by Tomentella, Russula, Lactarius, Boletoid, and Cortinarius species. A high frequency of such species on root tips is common for many ECM communities with different hosts. Moreover, the ECM mean abundance graph showed a high similarity to those of other studies (8, 58, 59). In our study, four ECM species are significantly more abundant than the others: L. quietus, T. sublilacina, C. geophilum, and Russula sp1. In all the ECM communities described in the literature, two or three dominant ECM species are always most abundant: Russula amoenolens and a boletoid type in a pine forest in California (18), C. geophilum and Inocybe maculata in a mixed deciduous forest (59), and Lactarius subdulcis and Clavulina cristata in a beech forest in northeastern France (8).
In our study, the relative abundance of the different ECM species was highly variable with the soil horizon. Differences in the frequency of occurrence of different species in different layers of the soil profile were shown by using terminal restriction fragment length polymorphism analysis or by sequencing the ribosomal DNA of the ITS region (13, 49). In our study, as in a previous one (5), the number of ECM root tips was higher in the A1 horizon than in the A2 horizon, where roots are more concentrated (50), where nutrients are abundant, and where the processes of mineralization and mobilization are more intense (12, 48). In the present study, L. quietus was present in the same proportion in the two horizons whereas T. sublilacina was mostly concentrated in the A1 horizon (0 to 5 cm) and C. geophilum in the A2 horizon (5 to 25 cm). The apparent preference of T. sublilacina mycorrhizae for the superficial (A1) horizon has been noted previously for a California ponderosa pine forest (53) and with another Tomentella species in Canadian jack pine forests (65). C. geophilum has already been described as abundant in mineral horizons (22, 49).
L. quietus, T. sublilacina, and C. geophilum, the three most abundant ECM species in our study, are commonly associated with Quercus sp. L. quietus is associated principally with Quercus and especially with Q. petraea under different soil conditions (63). T. sublilacina is a dominant species associated with Pinus muricata, Pseudotsuga menziesii, or Quercus sp. (18, 23, 56). C. geophilum, an asexual ascomycete, has been described in different reports as a ubiquist ECM fungi and is known for its wide host and habitat range (12, 26).
Factors driving temporal changes in the ECM community.
Ectomycorrhizal root colonization varies across time and depends on different factors, such as resistance to the fluctuation of soil parameters (resource availability and temperature) and competition between ECM species for soil/tree resources.
Here we observed a higher number of ECM root tips in April and a peak of mean species richness in September. This is consistent with a previous study (60), where authors showed that the rate of root elongation varied seasonally, with major periods of growth occurring at budburst in the spring (April) and after leaf senescence (September).
In our study, C. geophilum was the most abundant species in the A2 horizon in winter but also in summer (i.e., in August 2005, when the soil water potential reached a minimum of −2.13 MPa) (Fig. 4). Its dominance in summer could be explained by a better ability to resist water stress. as suggested by previous studies (27, 46). The dominance of C. geophilum in winter has also been reported (5). The survival of mycorrhizal roots and particularly roots mycorrhized by C. geophilum, which remain vital over winter, enhances water and nutrient uptake.
L. quietus was found to be one of the most abundant species in our sampling site. In a previous study, we hypothesized that L. quietus contributes to providing oak trees with carbon at budbreak (April and May) through enzymatic activities before photosynthesis begins (11). Clavulina sp. and L. amethystina were found in winter in our sampling site. In a recent study (8), the authors showed the seasonal dominance of L. amethystina, C. cristata, and Russula mairei in winter in a beech forest. In our study, the PCA and the result of the two-way ANOVA between species and date factors highlights that the interaction between the two factors was significant. So, our results also suggest that some species were present under specific conditions. However, since the study lasted only 15 months, we cannot show with evidence that taxa adapted to winter or spring conditions, as shown by others (51).
Factors driving spatial distribution of ECMs.
In our sampling site, we observed a high spatial variability in the abundance of ECM root tips at the same date of sampling, particularly in the cases of T. sublilacina and L. quietus, the most abundant species in the A1 horizon. Previous studies have shown that ECMs are frequently patchily distributed (19, 24, 25, 55) and often form dense systems in small areas due to the heterogeneity in the distribution of nutrients, in moisture, or in the composition of the microfauna (2, 7, 42, 43). In our study, the results of the ANOVA show that the sampling effort was sufficient to detect trends of interest, because part of the variation in the abundance of ECMs could be significantly explained by the factors date of sampling, horizon, and species.
As proposed by Agerer's exploration type approach (1), the anatomical characteristics of ECM morphotypes represent distinct ecophysiological strategies for nutrient uptake and transport. Here, in the A1 horizon, the C exploration type (e.g., Tomentella and Russula spp.) and the MD exploration type e.g., Lactarius spp.) are associated. According to reference 1, the C type (smooth mantle with few emanating hyphae) and the MD type, especially Lactarius spp. (smooth type with few emanating hyphae and undifferentiated rhizomorphs), grow frequently in close contact with the substrate and then increase their access to nitrogen contained in organic compounds (lignin and humic compounds [21, 61]).
The differences we observed in the distribution of exploration types between the A1 (C and MD) and A2 (SD) horizons are in agreement with findings of a previous study (5), which suggested that MD and SD exploration strategies corresponded to the different distributions of organic matter in the two horizons: coarse and loose debris in A1, as opposed to diffuse in A2.
Concerning the LD exploration type (see Table S1 in the supplemental material), especially Bolletoid species, such as X. chrysenteron, they were present in the same proportion in our two soil horizons and they have rhizomorphs optimized for long-distance transport of solutes.
Potential of soil exploration by ECM fungi.
In our study, the molecular identification of the fungal partner combined with abundance measurements provided information on the ECM community structure. The distribution of an ECM species may not be independent of that of the others, but it basically results from its ecological preferences, which delineate its potential niche, as well as the interactions between species that limit a species in realizing its niche (31, 32). Concerning the spatial distribution of the extramatricial mycelium in the soil, our study does not provide direct information, as do some studies using soil DNA extraction (16, 19, 30, 32). However, a previous study (30) on the disproportionate abundance between ECM root tips and their associated mycelia showed that russuloid and Cortinarius species are more frequent as root tips than as mycelia, that Tomentella species were equally frequent as root tips and as mycelia, and that Boletoid species were less dominant as root tips than as mycelia. As a consequence, since russuloid, Cortinarius, and Tomentella species represent more than 75% of all species in our study, we can assume that our approach provided a good image of the spatial repartition and of the exploration potential of ECM species in the forest soil we studied.
However, it is now necessary to complete community structure studies with functional studies (10) to understand the spatiotemporal variations of the community structure and to understand the role of the ECM community in nutrient cycles in forest stands.
Supplementary Material
Acknowledgments
The Ph.D. scholarship of the first author was funded by grants from the French Ministry of Ecology and Sustainable Development; part of this research was supported by the Biological Invasions program of the same ministry.
We are grateful to the Office National des Forêts for permitting sampling in the Champenoux State Forest.
Footnotes
Published ahead of print on 25 July 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Agerer, R. 2001. Exploration types of ectomycorrhizae: a proposal to classify ectomycorrhizal mycelial systems according to their patterns of differentiation and putative ecological importance. Mycorrhiza 11:107-114. [Google Scholar]
- 2.Agerer, R., and A. Göttlein. 2003. Correlations between projection area of ectomycorrhizae and H2O extractable nutrients in organic soil layers. Mycol. Prog. 2:45-52. [Google Scholar]
- 3.Agerer, R. 1987-1998. Colour atlas of ectomycorrhizae. Einhorn-Verlag Eduard Dietenberger, Munich, Germany.
- 4.Baar, J., J. R. Horton, A. Kretzer, and T. D. Bruns. 1999. Mycorrhizal recolonization of Pinus muricata from resistant propagules after a strand-replacing wildfire. New Phytol. 143:409-418. [Google Scholar]
- 5.Baier, R., J. Ingenhaag, H. Blaschke, A. Göttlein, and R. Agerer. 2006. Vertical distribution of an ectomycorrhizal community in upper soil horizons of a young Norway spruce (Picea abies [L] Karst.) stand of the Bavarian Limestone Alps. Mycorrhiza 16:197-206. [DOI] [PubMed] [Google Scholar]
- 6.Blasius, D., I. Kottke, and F. Oberwinkler. 1989. Spatial and seasonal dynamics of ectomycorrhizae of Picea abies (L.) Karst. in the Black Forest. Agric. Ecosys. Environ. 28:27-30. [Google Scholar]
- 7.Bruns, T. D. 1995. Thoughts of the processes that maintain local species diversity of ectomycorrhizal fungi. Plant Soil 170:63-73. [Google Scholar]
- 8.Buée, M., D. Vairelles, and J. Garbaye. 2005. Year-round monitoring of diversity and potential metabolic activity of the ectomycorrhizal community in a beech (Fagus sylvatica) forest subjected to two thinning regimes. Mycorrhiza 15:235-245. [DOI] [PubMed] [Google Scholar]
- 9.Clemmensen, K. E., A. Michelsen, S. Jonasson, and G. R. Shaver. 2006. Increased ectomycorrhizal fungal abundance after long-term fertilization and warming of two arctic tundra ecosystems. New Phytol. 171:391-404. [DOI] [PubMed] [Google Scholar]
- 10.Courty, P. E., K. Pritsch, M. Schloter, A. Hartmann, and J. Garbaye. 2005. Activity profiling of ectomycorrhiza communities in two forest soils using multiple enzymatic tests. New Phytol. 167:309-319. [DOI] [PubMed] [Google Scholar]
- 11.Courty, P. E., N. Bréda, and J. Garbaye. 2007. Relation between oak tree phenology and the secretion of organic matter degrading enzymes by Lactarius quietus ectomycorrhizas before and during bud break. Soil Biol. Biochem. 39:1655-1663. [Google Scholar]
- 12.Dalhberg, A. 2001. Community ecology of ectomycorrhizal fungi: an advancing interdisciplinary field. New Phytol. 150:555-562. [Google Scholar]
- 13.Dickie, I. A., B. Xu, and R. T. Koide. 2002. Vertical niche differentiation of ectomycorrhizal hyphae in soil as shown by T-RFLP analysis. New Phytol. 156:527-535. [DOI] [PubMed] [Google Scholar]
- 14.Dickie, I. A. 2007. Host preference, niches and fungal diversity. New Phytol. 174:230-233. [DOI] [PubMed] [Google Scholar]
- 15.Duchaufour, P., and M. Bonneau. 1959. Une nouvelle méthode de dosage du phosphore assimilable dans les sols forestiers. Bull. AFES 41:193-198. [Google Scholar]
- 16.Dunham, S. M., K. H. Larsson, and J. W. Spatafora. 2007. Species richness and community composition of mat-forming ectomycorrhizal fungi in old- and second-growth Douglas fir forests of the HJ Andrews Experimental Forest, Oregon, USA. Mycorrhiza 17:633-645. [DOI] [PubMed] [Google Scholar]
- 17.Gardes, M., and T. D. Bruns. 1993. ITS primers with enhanced specificity for basidiomycetes—application for the identification of mycorrhizae and rusts. Mol. Ecol. 2:113-118. [DOI] [PubMed] [Google Scholar]
- 18.Gardes, M., and T. D. Bruns. 1996. Community structure of ectomycorrhizal fungi in a Pinus muricata forest: above- and belowground views. Can. J. Bot. 74:1572-1583. [Google Scholar]
- 19.Genney, D. R., I. C. Anderson, and I. J. Alexander. 2006. Fine-scale distribution of pine ectomycorrhizas and their extramatrical mycelium. New Phytol. 170:381-390. [DOI] [PubMed] [Google Scholar]
- 20.Harvey, A. E., M. F. Jurgensen, and M. J. Larsen. 1978. Seasonal distribution of ectomycorrhizae in a mature Douglas-fir/larch forest soil in western Montana. Forest Sci. 24:203-208. [Google Scholar]
- 21.Haselwandter, K., O. Bobleter, and D. J. Read. 1990. Degradation of 14C-labelled lignin and dehydropolymer of coniferyl alcohol by ericoid and ectomycorrhizal fungi. Arch. Microbiol. 153:352-354. [Google Scholar]
- 22.Heinonsalo, J., K. S. Jorgensen, and R. Sen. 2001. Microcosm-based analyses of Scots pine seedling growth, ectomycorrhizal fungal community structure and bacterial carbon utilization profiles in boreal forest humus and underlying illuvial mineral horizons. FEMS Microbiol. Ecol. 36:73-84. [DOI] [PubMed] [Google Scholar]
- 23.Horton, T. R., and T. D. Bruns. 1998. Multiple host fungi are the most frequent and abundant ectomycorrhizal types in a mixed stand of Douglas-fir (Pseudotsuga menziesii D. Don) and Bishop pine (Pinus muricata D. Don). New Phytol. 139:331-339. [Google Scholar]
- 24.Horton, T. R., and T. D. Bruns. 2001. The molecular revolution in ectomycorrhizal ecology: peeking into the black box. Mol. Ecol. 10:1855-1871. [DOI] [PubMed] [Google Scholar]
- 25.Izzo, A., J. Agbowo, and T. D. Bruns. 2005. Detection of plot-level changes in ectomycorrhizal communities across years in an old-growth mixed-conifer forest. New Phytol. 166:619-630. [DOI] [PubMed] [Google Scholar]
- 26.Jany, J. L., F. Martin, and J. Garbaye. 2002. Cenococcum geophilum populations show a high degree of genetic diversity in beech forests. New Phytol. 154:651-659. [DOI] [PubMed] [Google Scholar]
- 27.Jany, J. L., J. Garbaye, and F. Martin. 2003. Respiration activity of ectomycorrhizas Cenococcum geophilum and Lactarius sp. in relation to soil water potential in five beech forests. Plant Soil 255:487-494. [Google Scholar]
- 28.Jones, M. D., D. M. Durall, and J. W. G. Cairney. 2003. Ectomycorrhizal fungal communities in young forest stands regenerating after clearcut logging. New Phytol. 157:399-422. [DOI] [PubMed] [Google Scholar]
- 29.Jonsson, L., A. Dahlberg, and T. E. Brandrud. 2000. Spatiotemporal distribution of an ectomycorrhizal community in an oligotrophic Swedish Picea abies forest subjected to experimental nitrogen addition: above- and below-ground views. Forest Ecol. Manag. 132:143-156. [Google Scholar]
- 30.Kjoller, R. 2006. Disproportionate abundance between ectomycorrhizal root tips and their associated mycelia. FEMS Microbiol. Ecol. 58:214-224. [DOI] [PubMed] [Google Scholar]
- 31.Koide, R. T., B. Xu, J. Sharda, Y. Lekberg, and N. Ostiguy. 2005. Evidence of species interactions within an ectomycorrhizal fungal community. New Phytol. 165:305-316. [DOI] [PubMed] [Google Scholar]
- 32.Koide, R. T., B. Xu, and J. Sharda. 2005. Contrasting belowground views of an ectomycorrhizal fungal community. New Phytol. 166:251-262. [DOI] [PubMed] [Google Scholar]
- 33.Koide, R. T., D. L. Shumway, B. Xu, and J. N. Sharda. 2007. On temporal partitioning of a community of ectomycorrhizal fungi. New Phytol. 174:420-429. [DOI] [PubMed] [Google Scholar]
- 34.Kõljalg, U., K. H. Larsson, K. Abarenkov, R. H. Nilsson, I. J. Alexander, U. Eberhardt, S. Erland, K. Hoiland, R. Kjoller, E. Larsson, T. Pennanen, R. Sen, A. F. S. Taylor, L. Tedersoo, T. Vralstad, and B. M. Ursing. 2005. UNITE: a database providing web-based methods for the molecular identification of ectomycorrhizal fungi. New Phytol. 166:1063-1068. [DOI] [PubMed] [Google Scholar]
- 35.Kuyper, T. W., and R. Landeweert. 2002. Vertical niche differentiation by hyphae of ectomycorrhizal fungi in soil. New Phytol. 156:321-326. [DOI] [PubMed] [Google Scholar]
- 36.Landeweert, R., P. Leeflang, T. W. Kuyper, E. Hoffland, A. Rosling, K. Wernars, and E. Smit. 2003. Molecular identification of ectomycorrhizal mycelium in soil horizons. Appl. Environ. Microbiol. 69:327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landeweert, R., P. Leeflang, E. Smit, and T. Kuyper. 2005. Diversity of an ectomycorrhizal fungal community studied by a root tip and total soil DNA approach. Mycorrhiza 15:1-6. [DOI] [PubMed] [Google Scholar]
- 38.Legendre, P., and M. J. Fortin. 1989. Spatial pattern and ecological analysis. Vegetation 80:107-138. [Google Scholar]
- 39.Lilleskov, E. A., and T. D. Bruns. 2003. Root colonization dynamics of two ectomycorrhizal fungi of contrasting life history strategies are mediated by addition of organic nutrient patches. New Phytol. 159:141-151. [DOI] [PubMed] [Google Scholar]
- 40.Lincoln, R., G. Boxshall, and P. Clark. 1998. A dictionary of Ecology, Evolution and Systematics, 2nd ed. Cambridge University press, Cambridge, United Kingdom.
- 41.Montecchio, L., S. Rossi, P. E. Courty, and J. Garbaye. 2006. Entoloma nitidum Quél. Descriptions Ectomycorrhizas 9/ 10:31-36. [Google Scholar]
- 42.Neville, J., J. L. Tessier, I. Morrison, J. Scarrat, B. Canning, and J. N. Klironomos. 2002. Soil depth distribution of ecto- and arbuscular mycorrhizal fungi associated with Populus tremuloides within a 3-year-old boreal forest clear-cut. Appl. Soil Ecol. 19:209-216. [Google Scholar]
- 43.Newell, K. 1984. Interactions between two decomposer basidiomycetes and a collembolan under Sitka spruce: grazing and its potential effects on fungal distribution and litter decomposition. Soil Biol. Biochem. 16:235-239. [Google Scholar]
- 44.Peter, M., F. Ayer, and S. Egli. 2001. Nitrogen addition in a Norway spruce stand altered macromycete sporocarp production and below-ground ectomycorrhizal species composition. New Phytol. 149:311-325. [DOI] [PubMed] [Google Scholar]
- 45.Peter, M., F. Ayer, S. Egli, and R. Honneger. 2001. Above- and below-ground community structure of ectomycorrhizal fungi in three Norway spruce stands in Switzerland. Can. J. Bot. 79:1134-1151. [Google Scholar]
- 46.Pigott, C. D. 1982. Survival of mycorrhiza formed by Cenococcum geophilum Fr in dry soils. New Phytol. 92:513-517. [Google Scholar]
- 47.Read, D. 1991. Mycorrhizas in ecosystems. Experientia 47:376-391. [Google Scholar]
- 48.Read, D. J., and J. Perez-Moreno. 2003. Mycorrhizas and nutrient cycling in ecosystems—a journey towards relevance? New Phytol. 157:475-492. [DOI] [PubMed] [Google Scholar]
- 49.Rosling, A., R. Landeweert, B. D. Lindahl, K. H. Larsson, T. W. Kuyper, A. F. S. Taylor, and R. D. Finlay. 2003. Vertical distribution of ectomycorrhizal fungal taxa in a podzol soil profile. New Phytol. 159:775-783. [DOI] [PubMed] [Google Scholar]
- 50.Schenk, H. J., and R. B. Jackson. 2002. The global biogeography of roots. Ecol. Monogr. 72:311-328. [Google Scholar]
- 51.Smith, M. E., G. W. Douhan, and D. M. Rizzo. 2007. Ectomycorrhizal community structure in a xeric Quercus woodland based on rDNA sequence analysis of sporocarps and pooled roots. New Phytol. 174:847-863. [DOI] [PubMed] [Google Scholar]
- 52.Smith, S. E., and D. J. Read. 1997. Mycorrhizal symbiosis, 2nd ed. Academic Press, New York, NY.
- 53.Stendell, E. R., T. R. Horton, and T. D. Bruns. 1999. Early effects of prescribed fire on the structure of the ectomycorrhizal fungus community in a Sierra Nevada ponderosa pine forest. Mycol. Res. 103:1353-1359. [Google Scholar]
- 54.Swaty, R. L., S. A. Gehring, M. Van Erl, T. C. Theimer, P. Keim, and P. Whitham. 1998. Temporal variation in temperature and rainfall differentially affects ectomycorrhizal colonization at two contrasting sites. New Phytol. 139:733-739. [Google Scholar]
- 55.Taylor, A. F. S. 2002. Fungal diversity in ectomycorrhizal communities: sampling effort and species detection. Plant Soil 244:19-28. [Google Scholar]
- 56.Taylor, D. L., and T. D. Bruns. 1999. Community structure of ectomycorrhizal fungi in a Pinus muricata forest: minimal overlap between the mature forest and resistant propagule communities. Mol. Ecol. 8:1837-1850. [DOI] [PubMed] [Google Scholar]
- 57.Taylor, D. L., and T. D. Bruns. 1999. Population, habitat and genetic correlates of mycorrhizal specialization in the ‘cheating’ orchids Corrallorhiza maculata and C. mertensiana. Mol. Ecol. 8:1719-1732. [DOI] [PubMed] [Google Scholar]
- 58.Tedersoo, L., U. Koljalg, N. Hallenberg, and K. H. Larsson. 2003. Fine scale distribution of ectomycorrhizal fungi and roots across substrate layers including coarse woody debris in a mixed forest. New Phytol. 159:153-165. [DOI] [PubMed] [Google Scholar]
- 59.Tedersoo, L., T. Suvi, E. Larsson, and U. Koljalg. 2006. Diversity and community structure of ectomycorrhizal fungi in a wooded meadow. Mycol. Res. 110:734-748. [DOI] [PubMed] [Google Scholar]
- 60.Teskey, R. O., and T. M. Hinckley. 1981. Influence of temperature and water potential on root growth of white oak. Physiol. Plant. 52:363-369. [Google Scholar]
- 61.Trojanowski, J., K. Haider, and A. Hütterman. 1984. Decomposition of 14C-labelled lignin hemicellulose and lignocellulose by mycorrhizal fungi. Arch. Microbiol. 139:202-206. [Google Scholar]
- 62.Twieg, B. D., D. M. Durall, and S. W. Simard. 2007. Ectomycorrhizal fungal succession in mixed temperate forests. New Phytol. 176:437-447. [DOI] [PubMed] [Google Scholar]
- 63.Urban, U. 1998. Identification and characterization of ectomycorrhizae of oak-species, p. 471. Int. Congr. Mycorrhiza II Book Abstr. Swedish University of Agricultural Sciences, Uppsala, Sweden.
- 64.Venables, W. N., and B. D. Ripley. 1999. Modern applied statistics with S-Plus, 3rd ed. Springer-Verlag, New York, NY.
- 65.Visser, S. 1995. Ectomycorrhizal fungal succession in jack pine stands following wildfire. New Phytol. 129:389-401. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.