Abstract
Free-living amoebae are frequent hosts for bacterial endosymbionts. In this study, the symbionts of eight novel environmental Acanthamoeba strains isolated from different locations worldwide were characterized. Phylogenetic analysis revealed that they were related to one of four evolutionary lineages of amoeba symbionts recognized previously. This study provides evidence for the existence of only a small number of phylogenetically well-separated groups of obligate intracellular endosymbionts of acanthamoebae with global distribution.
Free-living amoebae are widespread protozoa, including phylogenetically diverse genera like Acanthamoeba, Hartmanella, and Naegleria. They occur in various habitats, including soil, water, and the air (37, 47), and in many engineered environments, like water supplies and air-conditioning units (42). Free-living amoebae are opportunistic pathogens, causing keratitis or encephalitis, and important predators of prokaryotic and eukaryotic microorganisms with a great influence on microbial community composition (37, 47). By grazing on microbes, free-living amoebae also contribute to plant growth, soil mineralization, and nutrient cycles (9, 11, 47).
Apart from being a food source of free-living amoebae, some bacteria are able to survive phagocytosis and multiply within amoebae. The association between these bacteria and their amoeba hosts can be either transient (in the case of facultative intracellular bacteria) or stable (in the case of obligate intracellular bacteria). A wide range of well-known bacterial and eukaryotic pathogens are able to infect amoebae and exploit them for multiplication (25, 33, 43). Free-living amoebae may, thus, serve as environmental reservoirs and vectors for the transmission of pathogenic bacteria to humans (2, 5) and might represent evolutionary training grounds facilitating the adaptation of bacteria to survival within eukaryotic cells (15, 26, 29, 43, 44).
Stable associations of bacteria with amoebae leading to long-term symbiotic interactions were described for members of four evolutionary lineages within the Alphaproteobacteria (7, 20, 30, 57), the Betaproteobacteria (27, 31), the Bacteroidetes (32, 57), and the Chlamydiae (3, 8, 21, 27, 34). The different lifestyles of these obligate intracellular bacteria—either directly in the amoeba cytoplasm or enclosed in host-derived vacuoles—suggest fundamentally different mechanisms of host-cell interactions. However, with the exception of chlamydia-related amoeba symbionts (22-24, 28, 29), our knowledge about obligate intracellular symbionts of amoebae is still scarce. In this study, novel Acanthamoeba strains and their symbionts were analyzed.
In total, 10 different amoeba strains were isolated from soil and lake sediment samples from Austria, Tunisia, and Dominica, using nonnutrient agar plates seeded with live or heat-inactivated Escherichia coli or Saccharomyces cerevisiae as described previously (Table 1) (27). Amoeba isolates were adapted to axenic culture and tentatively classified as Acanthamoeba spp. based on morphological criteria characteristic for this genus (cell size, contractile vacuole, needle-like pseudopodia, and appearance of the nucleus) (45). Out of these 10 isolates, 8 contained intracellular bacteria as revealed by staining with the fluorescent DNA dye 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI). Isolates EI1, EI2, and EI6 harbored coccoid bacteria, whereas isolates EI3, EI4, EI5, 5a2, and EIDS3 contained rod-shaped bacteria (Table 1). The two Acanthamoeba isolates without intracellular bacteria were not analyzed further.
TABLE 1.
Amoeba isolates and their symbionts analyzed in this study
| Acanthamoeba sp. isolate and ATCC no. | Source | Growth medium and optimal temp | 16S rRNA GenBank accession no. of symbiont | 18S rRNA GenBank accession no. of amoeba host | Highest 16S rRNA sequence similarity toa: | Highest 18S rRNA sequence similarity toa: |
|---|---|---|---|---|---|---|
| EI1 PRA-227 | Soil; Vienna, Austria | TSY, 20°C | AM408788 | AM408796 | Parachlamydia sp. isolate Hall's coccus (99.5%; AF366365) | Acanthamoeba castellanii (99.6%; M13435) |
| EI2 PRA-226 | Soil; lower Austria | TSY, 20°C | AM408789 | AM408797 | Protochlamydia amoebophila UWE25 (98.9%; AF083615) | Acanthamoeba castellanii 4CL (98.9%; AF260724) |
| EI3 PRA-225 | Rainforest soil; Dominica | TSY, 20°C | AM408790 | AM408798 | “Candidatus Paracaedibacter acanthamoebae” (99.7%; AF132137) | Acanthamoeba sp. KA/MSS7 (99.6%; AY173015) |
| EI4 PRA-224 | Garden soil; Vienna, Austria | PYG, 20°C | AM408791 | AM408799 | “Candidatus Amoebophilus asiaticus” TUMSJ-321 (98.3%; AF366581) | Acanthamoeba polyphaga OX-1 (96.6%), AF019051 |
| EI5 PRA-223 | Desert sand, Matmata, Tunisia | TSY, 20°C | AM408792 | AM408800 | “Candidatus Procabacter acanthamoebae” Page23 (97.3%; AF177425) | Acanthamoeba pustulosa (98.0%; AF019050) |
| EI6 PRA-222 | Soil; Schneeberg, lower Austria | TSY, 20°C | AM408793 | AM408801 | Parachlamydia sp. isolate UV-7 (98.9%; AJ715410) | Acanthamoeba castellanii (99.3%; M13435) |
| EIDS3 PRA-221 | Alkaline lake sediment; Darscho Lacke, Burgenland, Austria | PYG, 30°C | AM408794 | AM408802 | “Candidatus Amoebophilus asiaticus” TUMSJ-321 (99%; AF366581) | Acanthamoeba sp. isolate MZOR (99.7%; DQ103890) |
| 5a2 PRA-228 | Lake sediment; Lake Neusiedl, Burgenland, Austria | PYG, 30°C | AM408795 | AM408803 | “Candidatus Amoebophilus asiaticus” TUMSJ-321 (99.3%; AF366581) | Acanthamoeba royreba Oak Ridge ATCC 30884 (98.8%; U07417) |
Data within parentheses are sequence similarities and GenBank accession numbers, respectively.
Simultaneous isolation of DNA from amoeba hosts and their bacterial endosymbionts was performed as described previously (27). The 18S rRNA genes were amplified using primers targeting conserved 18S rRNA gene regions (see Table S1 in the supplemental material), cloned using the Topo TA kit (Invitrogen Life Technologies), and sequenced on an ABI 3130 XL genetic analyzer using the BigDye Terminator kit v3.1. For each isolate, three to six clones were analyzed and found to be identical (99.8 to 100% sequence similarity). The software Pintail (4) indicated that the obtained sequences were not chimeric.
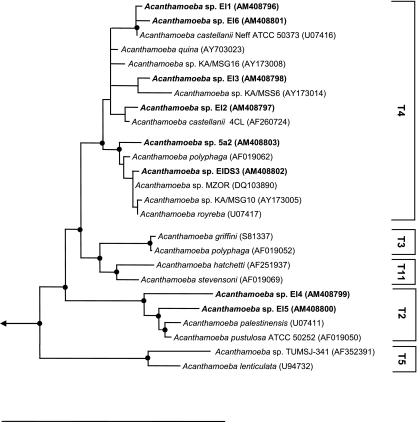
All 18S rRNA sequences showed highest sequence similarity with members of the genus Acanthamoeba (96.6 to 99.7%); similarity values to other genera were below 90% (Table 1). Using the 95% similarity threshold value for the definition of Acanthamoeba 18S rRNA sequence types (51), the Acanthamoeba sp. isolates EI1, EI2, EI3, 5a2, EIDS3, and EI6 could be assigned to the sequence type T4, and Acanthamoeba sp. isolates EI4 and EI5 could be assigned to sequence type T2. Consistently, phylogenetic analysis using the ARB software package (41) revealed well-supported relationships of the new amoeba isolates with the genus Acanthamoeba and the genotypes T2 and T4 (Fig. 1). Acanthamoeba sequence types correlate roughly with morphological groupings and also seem to be in concordance with antigen profiles (38). Bacterial symbionts have been identified previously in Acanthamoeba strains belonging to sequence types T4, T5, and T13 (30, 31); whether the presence of bacterial symbionts is in any way correlated with host sequence types is, however, an open question due to the limited data available. The eight Acanthamoeba isolates containing endosymbionts were deposited in the American Type Culture Collection (Table 1).
FIG. 1.
Phylogenetic relationships of Acanthamoeba host cells. An 18S rRNA-based TREE-PUZZLE tree (HKY nucleotide substitution model) (52) is shown. A filter considering only positions which are conserved in at least 50% of all amoebal 18S rRNA sequences was used for tree calculations. Selected Acanthamoeba 18S rRNA sequence types (51) are indicated. Black dots represent nodes with TREE-PUZZLE support and PHYLIP maximum parsimony bootstrap values (1.000 resampling) (18) greater than 80%. GenBank accession numbers are given in parentheses. The arrow indicates toward the out-group. The bar at the bottom represents 10% of the estimated evolutionary distance.
In order to identify the bacterial endosymbionts of the recovered Acanthamoeba isolates, their near-full-length 16S rRNA gene sequences (1,388 to 1,549 bp) were amplified (see Table S1 in the supplemental material) and cloned. For each symbiont, three to six clones were sequenced and found to be identical (99.9 to 100% sequence similarity); the software Pintail indicated that the obtained 16S rRNA sequences were not chimeric. Comparative sequence analysis revealed that all sequences are highly similar to previously described obligate endosymbionts of free-living amoebae (Table 1).
Three of the identified symbionts (in isolates EI1, EI2, and EI6) showed highest 16S rRNA sequence similarity (98.9 to 99.5%) to members of the Parachlamydiaceae (Table 1) and thus belong to the genera Parachlamydia and Protochlamydia within this family, according to the proposed taxonomy of Chlamydiae (13, 17, 39). Hereafter, these bacteria are accordingly referred to as Parachlamydia sp. isolate EI1, Parachlamydia sp. isolate EI6, and Protochlamydia sp. isolate EI2.
Three Acanthamoeba endosymbionts (in isolates EI4, 5a2, and EIDS3) showed highest 16S rRNA sequence similarity to a group of amoeba symbionts within the Bacteroidetes (98.3 to 99.3%) (Table 1), whose only described representative is “Candidatus Amoebophilus asiaticus” TUMSJ-321 (32). With the exception of a group of arthropod symbionts related to “Candidatus Cardinium hertigii” (58), similarity of these bacteria to other members of the Bacteroidetes was below 85%. These symbionts were thus named “Ca. Amoebophilus” EI4, “Ca. Amoebophilus” 5a2, and “Ca. Amoebophilus” EIDS3.
The endosymbiont of Acanthamoeba isolate EI3 was most similar to the alphaproteobacterial Acanthamoeba symbiont “Candidatus Paracaedibacter acanthamoebae” UWC9 (99.7% sequence similarity) (Table 1) (30); similarity to other members of the Alphaproteobacteria was significantly lower (83 to 92%). The endosymbiont of Acanthamoeba isolate EI3 is therefore tentatively referred to as “Candidatus Paracaedibacter” EI3.
The endosymbiont of Acanthamoeba sp. isolate EI5 had highest similarity with a group of betaproteobacterial endosymbionts of free-living amoebae, particularly with “Candidatus Procabacter acanthamoebae” Page23 (97.3%) (Table 1) (27, 31); similarity to other members of the Betaproteobacteria was below 90%. This symbiont was provisionally named “Candidatus Procabacter” EI5.
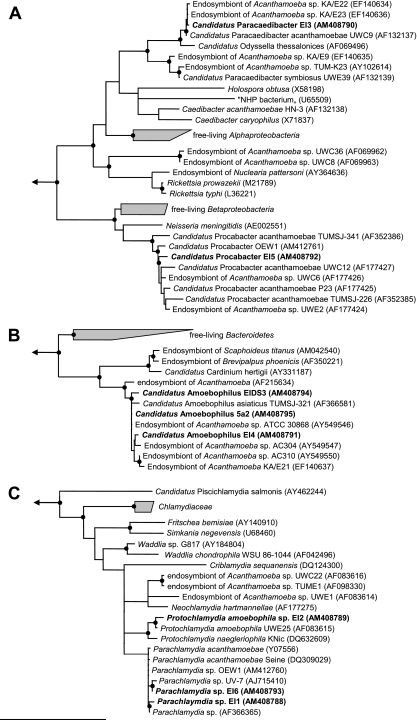
All applied treeing methods used to resolve phylogenetic relationships of the newly identified endosymbionts consistently showed the endosymbionts' affiliation with their respective most-similar sequences, forming stable monophyletic lineages of symbiotic bacteria with high bootstrap and TREE-PUZZLE support within the Proteobacteria, the Chlamydiae, and the Bacteroidetes (Fig. 2).
FIG. 2.
Phylogenetic relationships of Acanthamoeba symbionts. 16S rRNA-based trees calculated using the TREE-PUZZLE algorithm (HKY nucleotide substitution model) (52) are shown for the proteobacterial symbionts (A), the Bacteroidetes symbionts (B) and the chlamydial symbionts (C). A filter considering only positions which are conserved in at least 50% of all Bacteria strains was used for tree calculations. Black dots represent nodes with TREE-PUZZLE support and PHYLIP maximum parsimony bootstrap values (1.000 resampling) (18) greater than 80%. GenBank accession numbers are given in parentheses. Arrows indicate toward the out-groups. The bar at the bottom of the figure represents 10% of the estimated evolutionary distance.
In order to demonstrate the intracellular location of the bacterial symbionts within their Acanthamoeba hosts, fluorescence in situ hybridization (FISH) in combination with confocal laser scanning microscopy was performed. Amoebae were harvested from axenic cultures by centrifugation (4,000 × g; 5 min) and washed with 1× Page's saline (45). After resuspension in 100 μl of 1× Page's saline, 20-μl aliquots of amoebic suspension were incubated on glass slides for 20 min to allow for attachment of amoebae and fixed with 20 μl of 4% paraformaldehyde for 20 min at room temperature. Hybridization was carried out as described elsewhere (14).
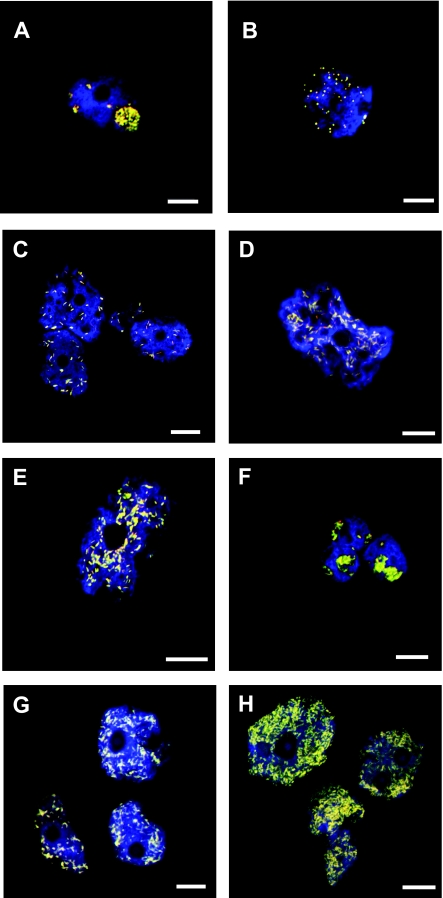
Symbiont-specific probes were selected using probeBase (see Table S1 in the supplemental material) (40) and applied for FISH under the recommended conditions. Positive hybridization reactions for all eight endosymbionts with the specific probes Bn9-658, Aph1180, Proca438, and CC23a were obtained and confirmed the 16S rRNA-based identification and the intracellular location of these symbionts (Fig. 3). Furthermore, the simultaneous hybridization with symbiont-specific probes and the universal bacterial probe set EUB-Mix labeled with different dyes showed that all bacteria within the Acanthamoeba cells were stained by both symbiont-specific probes and EUB-Mix, demonstrating the presence of only a single symbiont phylotype within the respective Acanthamoeba hosts (Fig. 3).
FIG. 3.
Identification and intracellular localization of Acanthamoeba symbionts by FISH. Probes EUK516 labeled with Cy5 (and shown in blue), targeting most Eukarya, and EUB-Mix labeled with Fluos dye (green), targeting most Bacteria strains, were used in all experiments in combination with Cy3-labeled symbiont-specific probes (red) (Table 2); the combined signal from bacterial and symbiont-specific probes appears yellow. At least three independent experiments were performed and ≥100 individual Acanthamoeba host cells were examined, all of which were infected; representative confocal laser scanning micrographs are shown. (A) Parachlamydia sp. isolate EI1 in Acanthamoeba sp. isolate EI1 (probe Bn9-658). (B) Protochlamydia sp. isolate EI2 in Acanthamoeba sp. isolate EI2 (probe Bn9-658). (C) “Candidatus Paracaedibacter” EI3 in Acanthamoeba sp. isolate EI3 (probe Cc23a). (D) “Candidatus Amoebophilus” EI4 in Acanthamoeba sp. isolate EI4 (probe Aph1180). (E) “Candidatus Procabacter” EI5 in Acanthamoeba sp. isolate EI5 (probe Proca438). (F) Parachlamydia EI6 in Acanthamoeba sp. isolate EI6 (probe Bn9-658). (G) “Candidatus Amoebophilus” EIDS3 in Acanthamoeba sp. isolate EIDS3 (probe Aph1180). (H) “Candidatus Amoebophilus” 5a2 in Acanthamoeba sp. isolate 5a2 (probe Aph1180). The white bars in the bottom right corner of each panel represent 10 μm.
The ultrastructure and intracellular niche of the bacterial symbionts within their amoeba host cells were further investigated by transmission electron microscopy. For this analysis, one representative of each evolutionary lineage was selected (Fig. 4). Amoebae were harvested from axenic cultures and directly fixed with 2% glutaraldehyde in 1× Page's amoebic saline for 1 h at room temperature, followed by fixation with 2% osmium tetroxide for 1 h at room temperature and dehydration in an ascending series of acetone. Subsequently, samples were embedded in Spurr resin (Sigma-Aldrich) with polymerization at 60°C for 8 to 12 h. Ultrathin sections were stained with 1% uranyl acetate for 4 min and 0.3% lead citrate for 2 min and examined with a Zeiss CEM 902 transmission electron microscope.
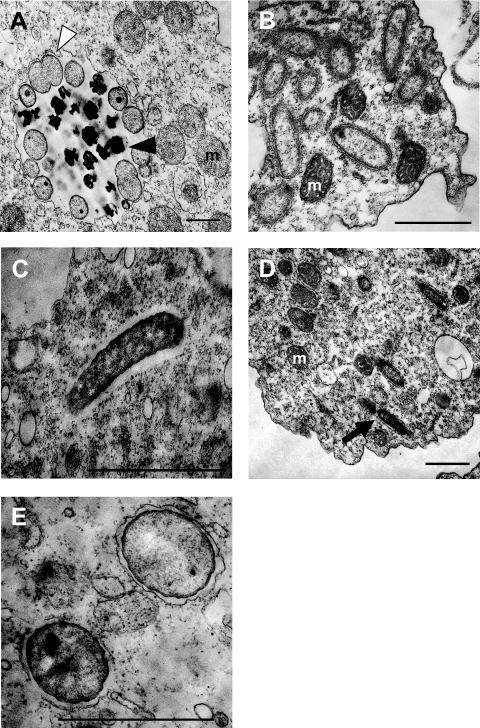
FIG. 4.
Ultrastructure of symbionts within Acanthamoeba host cells. Representatives from each phylogenetic group of symbionts are shown. (A) Parachlamydia sp. isolate EI1. Elementary (black arrowhead) and reticulate (white arrowhead) bodies within the chlamydial inclusion can be seen. (B) “Candidatus Amoebophilus” EI4. (C) “Candidatus Paracaedibacter” EI3. An electron-translucent space, indicative of a capsule or slime layer, surrounding “Candidatus Paracaedibacter” EI3 is clearly visible. (D) “Candidatus Procabacter” EI5 is surrounded by a membrane (black arrow). (E) Protochlamydia sp. isolate EI2. Each Protochlamydia sp. isolate EI2 cell is surrounded by an inclusion membrane. Mitochondria are labeled “m.” The lengths of bars in the bottom right corner of each panel represent 1 μm.
Parachlamydia sp. isolate EI1 showed morphological forms typical of chlamydial developmental stages, consisting of electron-dense elementary bodies and electron-translucent reticulate bodies (1, 13, 21, 24, 35, 56). The diameters of the elementary and reticulate bodies were 0.4 to 0.6 μm and 0.6 to 0.9 μm, respectively (Fig. 4A). The reticulate, but not elementary, bodies were observed undergoing binary fission. Furthermore, Parachlamydia sp. isolate EI1 resided in large vacuoles resembling the host-derived inclusion characteristic for known chlamydiae (19).
“Ca. Amoebophilus” EI4 was rod-shaped (0.3 to 0.5 μm in diameter and 0.7 to 1.4 μm in length) and appeared equally spread throughout the host cytoplasm (Fig. 4B). An association with ribosome-studded host membranes was not as obvious for “Ca. Amoebophilus” EI4 as it was for other “Ca. Amoebophilus asiaticus” strains (32, 57).
“Ca. Paracaedibacter” EI3 had a rod-shaped morphology (0.2 to 0.4 μm in diameter and 0.9 to 1.4 μm in length). These bacteria seemed to be located directly in the host cell cytoplasm, not enclosed in vacuoles but surrounded by an electron-translucent space, indicating a capsule or slime layer similar to that of “Ca. Paracaedibacter acanthamoebae” UWC9 and other similar strains (Fig. 4C) (7, 30, 57).
The betaproteobacterial “Ca. Procabacter” EI5 exhibited rod-shaped morphology (0.3 to 0.4 μm in diameter and 0.8 to 1.3 μm in length) and was equally distributed in the host cytoplasm (Fig. 4D). Interestingly, “Ca. Procabacter” EI5, similar to another Procabacter-related amoeba symbiont described recently (“Candidatus Procabacter” OEW1) (27), was enclosed by a membrane, which contrasts with the original description of its closest relatives, “Ca. Procabacter acanthamoebae” strains Page23, UWC12, and UWE2, that were found directly in the cytoplasm (31).
In light of the ubiquity of acanthamoebae and the numerous reported transient associations between facultative intracellular bacteria and amoebae, it was surprising that all symbionts of the new Acanthamoeba isolates investigated in this study were related to any of the four known groups of obligate amoeba endosymbionts (Fig. 2) (3, 7, 8, 20, 21, 31, 32, 34, 57). This is even more remarkable as none of the Acanthamoeba isolates analyzed here originated from a location sampled previously (Table 2). In fact, for each phylogenetic group of symbionts, amoeba hosts were recovered from different habitats and different locations worldwide. The proteobacterial symbionts, for example, were found in amoebae from America, Europe, Africa, and Asia. This indicates a global distribution of only a small number of phylogenetically distinct groups of amoeba symbionts.
TABLE 2.
Overview of recognized obligate intracellular symbionts of free-living amoebae
| Bacterial lineage | Amoeba symbiont designationa | Country of originb | Source habitat | GenBank 16S rRNA accession no. | Reference or source |
|---|---|---|---|---|---|
| Alphaproteobacteria | “Candidatus Paracaedibacter acanthamoebae” UWC9 | USA | Contact lens case | AF132137 | 30 |
| “Candidatus Paracaedibacter” EI3 | Dominica | Rainforest soil | AM408790 | This study | |
| Endosymbiont of Acanthamoeba sp. isolate KA/E23 | South Korea | Human corneal tissue | EF140636 | 57 | |
| Endosymbiont of Acanthamoeba sp. isolate KA/E22 | South Korea | Human corneal tissue | EF140634 | 57 | |
| “Candidatus Odyssella thessalonicensis” | Greece | Water from air conditioner | AF069496 | 7 | |
| “Candidatus Paracaedibacter symbiosus” E39 | USA (MN) | Soil | AF132139 | 30 | |
| Endosymbiont of Acanthamoeba sp. isolate TUMK-23 | Germany | Activated sludge | AY102614 | 6 | |
| Endosymbiont of Acanthamoeba sp. isolate KA/E9 | South Korea | Human corneal tissue | EF140635 | 57 | |
| Caedibacter acanthamoebae HN-3 | USA | Nasal swab | AF132138 | 30 | |
| Endosymbiont of Acanthamoeba sp. isolate UWC8 | USA | Human corneal tissue | AF069963 | 20 | |
| Endosymbiont of Acanthamoeba sp. isolate UWC36 | USA | Human corneal tissue | AF069962 | 20 | |
| Endosymbiont of Nuclearia pattersoni | Czech Republic | Gills (roach [Rutilus rutilus]) | AY364636 | 16 | |
| “Candidatus Procabacter acanthamoebae” UWC12 | USA | Human corneal tissue | AF177427 | 31 | |
| “Candidatus Procabacter” Page23 | USA (WI) | Freshwater | AF177425 | 31 | |
| “Candidatus Procabacter” TUMSJ-341 | Malaysia | Lake sediment | AF352386 | 31 | |
| “Candidatus Procabacter” TUMSJ-226 | Malaysia | Lake sediment | AF352385 | 31 | |
| “Candidatus Procabacter” UWC6 | USA | Human corneal tissue | AF177426 | 31 | |
| “Candidatus Procabacter” UWE2 | USA (MN) | Soil | AF177424 | 31 | |
| “Candidatus Procabacter” EI5 | Tunisia | Desert sand | AM408792 | This study | |
| “Candidatus Procabacter” OEW1 | Austria | Saline lake sediment | AM412761 | 27 | |
| Bacteroidetes | “Candidatus Amoebophilus asiaticus” TUMSJ-321 | Malaysia | Lake sediment | AF366581 | 32 |
| Endosymbiont of Acanthamoeba sp. isolate KA/E21 | South Korea | Human corneal tissue | EF140637 | 57 | |
| “Candidatus Amoebophilus” EIDS3 | Austria | Alkaline lake sediment | AM408794 | This study | |
| “Candidatus Amoebophilus” EI4 | Austria | Soil | AM408791 | This study | |
| “Candidatus Amoebophilus” 5a2 | Austria | Lake sediment | AM408795 | This study | |
| Chlamydiae | Protochlamydia amoebophila UWE25 | USA (WA) | Soil | AF083615 | 13 |
| Protochlamydia naegleriophila KNic | Germany | Freshwater aquarium water | DQ632609 | 10 | |
| “Candidatus Protochlamydia” EI2 | Austria | Soil | AM408789 | This study | |
| Endosymbiont of Acanthamoeba sp. isolate UWE1 | USA (WA) | Soil | AF083614 | 21 | |
| Parachlamydia acanthamoebae Bn9 | Germany | Nasal swab | Y07556 | 3 | |
| Parachlamydia acanthamoebae Berg17 | Germany | Nasal swab | AM941720 | 3 | |
| Parachlamydia sp. isolate Hall's coccusa | USA (VT) | Water from humidifier | AF366365 | 8 | |
| Parachlamydia sp. isolate EI1 | Austria | Soil | AM408788 | This study | |
| Parachlamydia sp. isolate EI6 | Austria | Soil | AM408793 | This study | |
| Parachlamydia sp. isolate UV-7 | Germany | Activated sludge | AJ715410 | 12 | |
| Parachlamydia sp. isolate Seine | France | Freshwater (Seine river) | DQ309029 | 53 | |
| Parachlamydia sp. isolate OEW1 | Austria | Saline lake sediment | AM412760 | 27 | |
| Neochlamydia hartmannellae | Germany | Water from water conduit | AF177275 | 34 | |
| Endosymbiont of Acanthamoeba sp. isolate TUME1 | Germany | Activated sludge | AF098330 | 21 | |
| Endosymbiont of Acanthamoeba sp. isolate UWC22 | USA | Human corneal tissue | AF083616 | 21 | |
| Criblamydia sequanensis | France | Freshwater (the Seine) | DQ124300 | 53 |
Parachlamydia sp. isolate Hall's coccus, Parachlamydia sp. isolate UV-7, Parachlamydia sp. isolate Seine, and Criblamydia sequanensis were obtained by cocultivation with Acanthamoeba sp. isolates.
USA, United States of America.
Despite the existence of only a few major evolutionary lineages of amoeba symbionts, there is a considerable diversity within some of these lineages. The alphaproteobacterial and the chlamydial symbionts comprise at least four different genera each (Table 2). In addition, two of the bacterial symbionts identified in this study, “Candidatus Amoebophilus” EI4 and “Candidatus Procabacter” EI5, showed a 16S rRNA sequence similarity below the recently proposed thresholds for the discrimination of bacterial species of 98.6 or 98.7% (36, 50) and, thus, represent novel species within the tentative genera “Ca. Amoebophilus” (at least three species in total) and “Ca. Procabacter” (at least four species), respectively (Table 2). This species-level diversity is further supported by differences in ultrastructure and subcellular location observed in this study compared to those in previous reports (27, 31, 32, 57).
One possible explanation for the observed limited phylogenetic diversity of bacterial endosymbionts of Acanthamoeba species might be a potential bias introduced by the isolation procedures and the adaptation to axenic culture conditions. The use of nonnutrient agar plates with E. coli or Enterobacter aerogenes as the food source is currently the standard procedure for isolation of free-living amoebae and was used to recover phylogenetically diverse amoebae (37, 48, 49). From the eight Acanthamoeba isolates analyzed in this study, six belong to Acanthamoeba sequence type T4 (Fig. 1), which is the most abundant genotype in the environment and also comprises most of the pathogenic Acanthamoeba isolates (37, 49, 55), while two belong to sequence type T2. This shows that there is considerable phylogenetic diversity among the isolates obtained with the method applied in this study. However, although unlikely, we cannot exclude that, for some unknown reason, amoebae containing certain types of symbionts are selected for by our isolation procedure. In this context, it seems interesting that the amoeba harboring “Ca. Procabacter” EI5, which is most different from known amoeba symbionts, was recovered from nonnutrient agar plates with Saccharomyces cerevisiae instead of E. coli as the food source. One possibility for isolating free-living amoebae harboring novel bacterial endosymbionts might therefore be to use alternative food sources during isolation.
Another possibility for the discovery of novel intracellular bacteria has been described recently. Cocultivation of environmental samples with (symbiont-free) amoebae was successfully used to identify obligate or facultative intracellular bacteria and to grow them in a surrogate Acanthamoeba host (13, 46, 53, 54). This technique is, by far, less time consuming than the isolation of amoebae and the adaptation to axenic culture conditions by using traditional methods. However, the cocultivation approach bears the disadvantage that the identity of the original host (which does not necessarily have to be an amoeba) remains unknown.
In concert with previous reports (3, 7, 20, 21, 30-32, 34, 57), this study provides evidence for the existence of only a limited number of phylogenetically different groups of obligate bacterial endosymbionts of Acanthamoeba spp., showing a global distribution. This might suggest that adaptation of bacteria to long-term intracellular symbiosis with acanthamoebae has originated only a few times during evolution. The ongoing genome projects of Parachlamydia acanthamoebae UV7, “Candidatus Amoebophilus asiaticus” 5a2, and Acanthamoeba castellanii Neff will help to understand similarities and differences between these symbionts and the interactions with their Acanthamoeba hosts, as well as the role of free-living amoebae as evolutionary training grounds for facultative intracellular bacteria.
Nucleotide sequence accession numbers.
18S and 16S rRNA gene sequences of Acanthamoeba isolates and their symbionts, respectively, were deposited in the EMBL/DDBJ/GenBank data libraries under accession numbers AM408788 to AM408803 (Table 1).
Supplementary Material
Acknowledgments
Work in the laboratory of M.H. and M.W. was funded by grants from the Austrian Science Fund FWF (Y277-B03 and P16566-B14) and the University of Vienna (research focus project FS573001). S.S.-E. is supported by FWF grant P19252-B17.
We gratefully acknowledge Waltraud Klepal and the team of the Ultrastructure Laboratory (University of Vienna) and Kilian Stoecker for advice and assistance with electron microscopy and confocal laser scanning microscopy, respectively. We are grateful to Christian Baranyi for excellent technical assistance.
Footnotes
Published ahead of print on 18 July 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Abdelrahman, Y. M., and R. J. Belland. 2005. The chlamydial developmental cycle. FEMS Microbiol. Rev. 29:949-959. [DOI] [PubMed] [Google Scholar]
- 2.Albert-Weissenberger, C., C. Cazalet, and C. Buchrieser. 2007. Legionella pneumophila—a human pathogen that co-evolved with fresh water protozoa. Cell. Mol. Life Sci. 64:432-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann, R., N. Springer, W. Schonhuber, W. Ludwig, E. N. Schmid, K. D. Muller, and R. Michel. 1997. Obligate intracellular bacterial parasites of acanthamoebae related to Chlamydia spp. Appl. Environ. Microbiol. 63:115-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashelford, K. E., N. A. Chuzhanova, J. C. Fry, A. J. Jones, and A. J. Weightman. 2005. At least 1 in 20 16S rRNA sequence records currently held in public repositories is estimated to contain substantial anomalies. Appl. Environ. Microbiol. 71:7724-7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker, J., and M. R. W. Brown. 1994. Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology 140:1253-1259. [DOI] [PubMed] [Google Scholar]
- 6.Beier, C. L., M. Horn, R. Michel, M. Schweikert, H. D. Gortz, and M. Wagner. 2002. The genus Caedibacter comprises endosymbionts of Paramecium spp. related to the Rickettsiales (Alphaproteobacteria) and to Francisella tularensis (Gammaproteobacteria). Appl. Environ. Microbiol. 68:6043-6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birtles, R. J., T. J. Rowbotham, R. Michel, D. G. Pitcher, B. Lascola, S. Alexiou-Daniel, and D. Raoult. 2000. ‘Candidatus Odyssella thessalonicensis’ gen. nov., sp. nov., an obligate intracellular parasite of Acanthamoeba species. Int. J. Syst. Evol. Microbiol. 50:63-72. [DOI] [PubMed] [Google Scholar]
- 8.Birtles, R. J., T. J. Rowbotham, C. Storey, T. J. Marrie, and D. Raoult. 1997. Chlamydia-like obligate parasite of free-living amoebae. Lancet 349:925-926. [DOI] [PubMed] [Google Scholar]
- 9.Bonkowski, M. 2004. Protozoa and plant growth: the microbial loop in soil revisited. New Phytol. 162:617-631. [DOI] [PubMed] [Google Scholar]
- 10.Casson, N., R. Michel, R. D. Mueller, and G. Greub. 2008. Protochlamydia naegleriophila as etiologic agent of pneumonia. Emerg. Infect. Dis. 14:168-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarholm, M. 2002. Bacteria and protozoa as integral components of the forest ecosystem—their role in creating a naturally varied soil fertility. Antonie van Leeuwenhoek 81:309-318. [DOI] [PubMed] [Google Scholar]
- 12.Collingro, A., S. Poppert, E. Heinz, S. Schmitz-Esser, A. Essig, M. Schweikert, M. Wagner, and M. Horn. 2005. Recovery of an environmental Chlamydia strain from activated sludge by co-cultivation with Acanthamoeba sp. Microbiology 151:301-309. [DOI] [PubMed] [Google Scholar]
- 13.Collingro, A., E. R. Toenshoff, M. W. Taylor, T. R. Fritsche, M. Wagner, and M. Horn. 2005. ‘Candidatus Protochlamydia amoebophila,’ an endosymbiont of Acanthamoeba spp. Int. J. Syst. Evol. Microbiol. 55:1863-1866. [DOI] [PubMed] [Google Scholar]
- 14.Daims, H., K. Stoecker, and M. Wagner. 2005. Fluorescence in situ hybridization for the detection of prokaryotes, p. 213-239. In A. Osborn and C. Smith (ed.), Advanced methods in molecular microbial ecology. Bios-Garland, Abingdon, United Kingdom.
- 15.Darby, A. C., N.-H. Cho, H.-H. Fuxelius, J. Westberg, and S. G. E. Andersson. 2007. Intracellular pathogens go extreme: genome evolution in the Rickettsiales. Trends Genet. 23:511-520. [DOI] [PubMed] [Google Scholar]
- 16.Dykova, I., M. Veverkova, I. Fiala, B. Machackova, and H. Peckova. 2003. Nuclearia pattersoni sp. n. (Filosea), a new species of amphizoic amoeba isolated from gills of roach (Rutilus rutilus), and its rickettsial endosymbiont. Folia Parasitol. (Praha) 50:161-170. [PubMed] [Google Scholar]
- 17.Everett, K. D., R. M. Bush, and A. A. Andersen. 1999. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int. J. Syst. Bacteriol. 49:415-440. [DOI] [PubMed] [Google Scholar]
- 18.Felsenstein, J. 1989. PHYLIP—Phylogeny Inference Package (version 3.2). Cladistics 5:164-166. [Google Scholar]
- 19.Fields, K. A., and T. Hackstadt. 2002. The chlamydial inclusion: escape from the endocytic pathway. Annu. Rev. Cell Dev. Biol. 18:221-245. [DOI] [PubMed] [Google Scholar]
- 20.Fritsche, T. R., M. Horn, S. Seyedirashti, R. K. Gautom, K. H. Schleifer, and M. Wagner. 1999. In situ detection of novel bacterial endosymbionts of Acanthamoeba spp. phylogenetically related to members of the order Rickettsiales. Appl. Environ. Microbiol. 65:206-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fritsche, T. R., M. Horn, M. Wagner, R. P. Herwig, K. H. Schleifer, and R. K. Gautom. 2000. Phylogenetic diversity among geographically dispersed Chlamydiales endosymbionts recovered from clinical and environmental isolates of Acanthamoeba spp. Appl. Environ. Microbiol. 66:2613-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greub, G., J.-L. Mege, and D. Raoult. 2003. Parachlamydia acanthamoeba enters and multiplies within human macrophages and induces their apoptosis. Infect. Immun. 71:5979-5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greub, G., J. L. Mege, J. P. Gorvel, D. Raoult, and S. Meresse. 2005.Intracellular trafficking of Parachlamydia acanthamoebae. Cell. Microbiol. 7:581-589. [DOI] [PubMed] [Google Scholar]
- 24.Greub, G., and D. Raoult. 2002. Crescent bodies of Parachlamydia acanthamoeba and its life cycle within Acanthamoeba polyphaga: an electron micrograph study. Appl. Environ. Microbiol. 68:3076-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greub, G., and D. Raoult. 2004. Microorganisms resistant to free-living amoebae. Clin. Microbiol. Rev. 17:413-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harb, O. S., L.-Y. Gao, and Y. A. Kwaik. 2000. From protozoa to mammalian cells: a new paradigm in the life cycle of intracellular bacterial pathogens. Environ. Microbiol. 2:251-265. [DOI] [PubMed] [Google Scholar]
- 27.Heinz, E., I. Kolarov, C. Kastner, E. R. Toenshoff, M. Wagner, and M. Horn. 2007. An Acanthamoeba sp. containing two phylogenetically different bacterial endosymbionts. Environ. Microbiol. 9:1604-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horn, M. Chlamydiae as symbionts in eukaryotes. Annu. Rev. Microbiol., in press. [DOI] [PubMed]
- 29.Horn, M., A. Collingro, S. Schmitz-Esser, C. L. Beier, U. Purkhold, B. Fartmann, P. Brandt, G. J. Nyakatura, M. Droege, D. Frishman, T. Rattei, H. W. Mewes, and M. Wagner. 2004. Illuminating the evolutionary history of chlamydiae. Science 304:728-730. [DOI] [PubMed] [Google Scholar]
- 30.Horn, M., T. R. Fritsche, R. K. Gautom, K. H. Schleifer, and M. Wagner. 1999. Novel bacterial endosymbionts of Acanthamoeba spp. related to the Paramecium caudatum symbiont Caedibacter caryophilus. Environ. Microbiol. 1:357-367. [DOI] [PubMed] [Google Scholar]
- 31.Horn, M., T. R. Fritsche, T. Linner, R. K. Gautom, M. D. Harzenetter, and M. Wagner. 2002. Obligate bacterial endosymbionts of Acanthamoeba spp. related to the beta-Proteobacteria: proposal of ‘Candidatus Procabacter acanthamoebae’ gen. nov., sp. nov. Int. J. Syst. Evol. Microbiol. 52:599-605. [DOI] [PubMed] [Google Scholar]
- 32.Horn, M., M. D. Harzenetter, T. Linner, E. N. Schmid, K. D. Muller, R. Michel, and M. Wagner. 2001. Members of the Cytophaga-Flavobacterium-Bacteroides phylum as intracellular bacteria of acanthamoebae: proposal of ‘Candidatus Amoebophilus asiaticus.’ Environ. Microbiol. 3:440-449. [DOI] [PubMed] [Google Scholar]
- 33.Horn, M., and M. Wagner. 2004. Bacterial endosymbionts of free-living amoebae. J. Eukaryot. Microbiol. 51:509-514. [DOI] [PubMed] [Google Scholar]
- 34.Horn, M., M. Wagner, K. D. Muller, E. N. Schmid, T. R. Fritsche, K. H. Schleifer, and R. Michel. 2000. Neochlamydia hartmannellae gen. nov., sp. nov. (Parachlamydiaceae), an endoparasite of the amoeba Hartmannella vermiformis. Microbiology 146:1231-1239. [DOI] [PubMed] [Google Scholar]
- 35.Kahane, S., N. Kimmel, and M. G. Friedman. 2002. The growth cycle of Simkania negevensis. Microbiology 148:735-742. [DOI] [PubMed] [Google Scholar]
- 36.Keswani, J., and W. B. Whitman. 2001. Relationship of 16S rRNA sequence similarity to DNA hybridization in prokaryotes. Int. J. Syst. Evol. Microbiol. 51:667-678. [DOI] [PubMed] [Google Scholar]
- 37.Khan, N. A. 2006. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol. Rev. 30:564-595. [DOI] [PubMed] [Google Scholar]
- 38.Köhsler, M., B. Leitner, M. Blaschitz, R. Michel, H. Aspock, and J. Walochnik. 2006. ITS1 sequence variabilities correlate with 18S rDNA sequence types in the genus Acanthamoeba (Protozoa: Amoebozoa). Parasitol. Res. 98:86-93. [DOI] [PubMed] [Google Scholar]
- 39.Kuo, C.-C., M. Horn, and R. S. Stephens. The order Chlamydiales. In B. Hedlund, N. R. Krieg, W. Ludwig, B. J. Paster, J. T. Staley, N. Ward, and W. B. Whitman (ed.), Bergey's manual of systematic bacteriology—the Planctomycetes, Spirochaetes, Fibrobacteres, Bacteriodetes and Fusobacteria, 2nd ed., vol. 4, in press. Springer, New York, NY.
- 40.Loy, A., F. Maixner, M. Wagner, and M. Horn. 2007. probeBase—an online resource for rRNA-targeted oligonucleotide probes: new features 2007. Nucleic Acids Res. 35:D800-D804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Forster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. Konig, T. Liss, R. Lussmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K. H. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marshall, M. M., D. Naumovitz, Y. Ortega, and C. R. Sterling. 1997. Waterborne protozoan pathogens. Clin. Microbiol. Rev. 10:67-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molmeret, M., M. Horn, M. Wagner, M. Santic, and Y. Abu Kwaik. 2005. Amoebae as training grounds for intracellular bacterial pathogens. Appl. Environ. Microbiol. 71:20-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogata, H., B. La Scola, S. Audic, P. Renesto, G. Blanc, C. Robert, P. E. Fournier, J. M. Claverie, and D. Raoult. 2006. Genome sequence of Rickettsia bellii illuminates the role of amoebae in gene exchanges between intracellular pathogens. PLoS Genet. 2:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Page, F. C. 1976. An illustrated key to freshwater and soil amoebae. Freshwater Biological Association, Ambleside, United Kingdom.
- 46.Pagnier, I., D. Raoult, and B. La Scola. 2008. Isolation and identification of amoeba-resisting bacteria from water in human environment by using an Acanthamoeba polyphaga co-culture procedure. Environ. Microbiol. 10:1135-1144. [DOI] [PubMed] [Google Scholar]
- 47.Rodríguez-Zaragoza, S. 1994. Ecology of free living amoebae. Crit. Rev. Microbiol. 20:225-241. [DOI] [PubMed] [Google Scholar]
- 48.Schuster, F. L. 2002. Cultivation of pathogenic and opportunistic free-living amebas. Clin. Microbiol. Rev. 15:342-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schuster, F. L., and G. S. Visvesvara. 2004. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int. J. Parasitol. 34:1001-1027. [DOI] [PubMed] [Google Scholar]
- 50.Stackebrandt, E., and J. Ebers. 2006. Taxonomic parameters revisited: tarnished gold standards. Microbiol. Today November 2006:6-9.
- 51.Stothard, D. R., J. M. Schroeder-Diedrich, M. H. Awwad, R. J. Gast, D. R. Ledee, S. Rodriguez-Zaragoza, C. L. Dean, P. A. Fuerst, and T. J. Byers. 1998. The evolutionary history of the genus Acanthamoeba and the identification of eight new 18S rRNA gene sequence types. J. Eukaryot. Microbiol. 45:45-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strimmer, K., and A. von Haeseler. 1996. Quartet puzzling: a quartet maximum likelihood method for reconstructing tree topologies. Mol. Biol. Evol. 13:964-969. [Google Scholar]
- 53.Thomas, V., N. Casson, and G. Greub. 2006. Criblamydia sequanensis, a new intracellular Chlamydiales isolated from Seine river water using amoebal co-culture. Environ. Microbiol. 8:2125-2135. [DOI] [PubMed] [Google Scholar]
- 54.Thomas, V., K. Herrera-Rimann, D. S. Blanc, and G. Greub. 2006. Biodiversity of amoebae and amoeba-resisting bacteria in a hospital water network. Appl. Environ. Microbiol. 72:2428-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walochnik, J., A. Obwaller, and H. Aspock. 2000. Correlations between morphological, molecular biological, and physiological characteristics in clinical and nonclinical isolates of Acanthamoeba spp. Appl. Environ. Microbiol. 66:4408-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ward, M. E. 1988. The chlamydial developmental cycle, p. 71-95. In A. L. Barron (ed.), Micobiology of chlamydia. CRC Press, Boca Raton, FL.
- 57.Xuan, Y. H., H. S. Yu, H. J. Jeong, S. Y. Seol, D. I. Chung, and H. H. Kong. 2007. Molecular characterization of bacterial endosymbionts of Acanthamoeba isolates from infected corneas of Korean patients. Korean J. Parasitol. 45:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zchori-Fein, E., S. J. Perlman, S. E. Kelly, N. Katzir, and M. S. Hunter. 2004. Characterization of a ‘Bacteroidetes’ symbiont in Encarsia wasps (Hymenoptera: Aphelinidae): proposal of ‘Candidatus Cardinium hertigii.’ Int. J. Syst. Evol. Microbiol. 54:961-968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.