Abstract
We constructed self-cloning diploid baker's yeast strains by disrupting PUT1, encoding proline oxidase, and replacing the wild-type PRO1, encoding γ-glutamyl kinase, with a pro1(D154N) or pro1(I150T) allele. The resultant strains accumulated intracellular proline and retained higher-level fermentation abilities in the frozen doughs than the wild-type strain. These results suggest that proline-accumulating baker's yeast is suitable for frozen-dough baking.
Ordinary commercial baker's yeast (mostly strains of Saccharomyces cerevisiae) is generally susceptible to damage during the freeze-thaw process and frozen storage and does not retain sufficient leavening ability after thawing (8, 11). Freeze tolerance is a necessary characteristic of baker's yeast used in frozen doughs because postthaw leavening activity is essential prior to baking. It is known that the laboratory yeast S. cerevisiae induces trehalose synthesis during various stresses (1, 10, 34) and that induced trehalose functions as a stress protectant (7, 16, 23).
We previously found that proline and charged amino acids, such as arginine and glutamate, have cryoprotective activities nearly equal to that of glycerol or trehalose in baker's yeast (26). It was shown previously that yeast cells with higher levels of proline are more tolerant of freezing than wild-type cells (13, 14, 22, 26, 28, 30). Proline and trehalose were reported to preserve membrane structure and function during freezing (20). It has been suggested that proline can prevent ice nucleation and dehydration by forming strong hydrogen bonds with intracellular free water. γ-Glutamyl kinase (GK; the PRO1 gene product) is the key enzyme in proline biosynthesis in S. cerevisiae, and GK activity is subjected to feedback inhibition by proline (22, 32). Proline oxidase (PO; the PUT1 gene product) catalyzes the first step of the proline degradation pathway in S. cerevisiae (36). Interestingly, the D154N and I150T mutant GKs are less sensitive to proline feedback inhibition than the wild-type enzyme, and yeast cells expressing these mutated GKs accumulate proline and show higher tolerance for freezing than wild-type cells (22). With respect to industrial yeast, we previously constructed novel proline-accumulating sake yeast by disrupting the PUT1 gene and introducing the pro1(D154N) mutant allele and found that the resultant strain accumulated proline and was more tolerant of ethanol stress than the control strain (29). Furthermore, self-cloning (SC) diploid sake yeasts that accumulate proline have been developed, and their fermentation profiles during sake brewing have been analyzed (27). For the application of recombinant yeasts for commercial use, an SC yeast, which has no foreign genes or DNA sequences except for yeast DNA, may be more acceptable to consumers than a genetically modified (GM) yeast.
Our objectives in this study were (i) to construct SC diploid baker's yeast strains that accumulate proline and (ii) to determine if intracellular proline enhances the freeze tolerance of baker's yeast strains in doughs. We report here that baker's yeasts that accumulate proline showed increased leavening abilities in the frozen doughs.
Construction of the proline-accumulating baker's yeast.
The baker's yeast strains 3346-ura3 and 3347-ura3 used in this study have only URA3 as a useful marker gene. Therefore, we first integrated a mutated pro1 gene by homologous recombination using URA3 as the selection marker, excised the plasmid region containing the URA3 marker, and then reused this marker with disruption of the PUT1 gene according to the method in our previous study (27). In order to construct a pro1(D154N) or pro1(I150T) mutant strain, the linearized plasmid pRS406-D154NPRO1 or pRS406-I150TPRO1, which expresses the D154N or I150T mutant GK, respectively, with extreme desensitization to feedback inhibition by proline (22), was introduced into strain 3346-ura3 or 3347-ura3. The Ura+ transformants grown on synthetic complete medium lacking uracil (20 g of glucose/liter, 6.7 g of Bacto yeast nitrogen base without amino acids [Difco Laboratories, Detroit, MI]/liter, 2 g of dropout mixture/liter) were cultured in YPD medium (20 g of glucose/liter, 10 g of Bacto yeast extract/liter, 20 g of Bacto peptone/liter) at 30°C for 24 h with shaking, diluted in the same medium, and incubated for several days to obtain strains 3346-D154N-ura3, 3346-I150T-ura3, 3347-D154N-ura3, and 3347-I150T-ura3, which have excised the plasmid and lost one of the two copies of the duplicated region by homologous crossover, from 5-fluoroorotic acid-containing plates (4, 25). The overproduction of proline is believed to dilute the toxic proline analogue azetidine-2-carboxylate (AZC; Sigma-Aldrich, St. Louis, MO) (14, 26), which is incorporated into proteins in competition with proline (18, 33). We examined the growth of yeast strains on agar plates containing SD medium (20 g of glucose/liter, 6.7 g of Bacto yeast nitrogen base without amino acids/liter) with 2 mg of AZC/ml. These strains were capable of growing on SD medium-AZC plates due to proline overproduction. We confirmed the correct gene replacement and the loss of the extraneous plasmid DNA by the direct sequencing of PCR products that contained the PRO1 locus amplified from the chromosomal DNA of these strains.
To disrupt the PUT1 gene on the chromosome of each strain, we amplified the URA3 fragments which had 60-bp PUT1 sequences at both ends by PCR performed with genomic DNA from strains 3346 and 3347 using the PUT1disURA3(+) and PUT1disURA3(−) primers. The sequence of the forward primer [PUT1disURA3(+)] was 5′-GCT TGC TGG GAA CCG AAC ACA AAC TCC ACA AGT CCG TAG CAG CTC TTC TCT TTT GTC TTT AAT GTG GCT GTG GTT TCA GG-3′, and the sequence of the reverse primer [PUT1disURA3(−)] was 5′-GCT ATG GCC TTG ATT AAT GGC CAG CCA TTA TCA GAT CTC ACA GCA TCC CCG TTT TCT TGC GTT CTG GCG AGG TAT TGG AT-3′ (the underlining indicates the sequence 50 bp upstream of the ATG initiation codon and the sequence 30 bp upstream of the TGA termination codon of the PUT1 gene, respectively). The sequences of PUT1 and URA3 were based on the sequences corresponding to GenBank accession numbers M18107 and K02206, respectively. A unique 1.24-kb amplified band containing the URA3 gene was purified and then integrated into the PUT1 locus in strains 3346-D154N-ura3, 3346-I150T-ura3, 3347-D154N-ura3, and 3347-I150T-ura3 by transformation. The transformants, which exhibited the Ura+ phenotype, were selected, and we confirmed that these strains failed to grow on SD medium containing proline (1 g/liter), instead of ammonium sulfate, as the sole source of nitrogen (data not shown). As a result, we constructed the following six haploid strains by an SC method: 3346-D154N/Δput1, 3346-I150T/Δput1, 3347-D154N/Δput1, 3347-I150T/Δput1, 3346-WT/Δput1, and 3347-WT/Δput1. As controls, strains 3346-WT/Δput1 and 3347-WT/Δput1 were constructed from strains 3346-ura3 and 3347-ura3, respectively.
In general, commercial baker's yeast is a diploid or polyploid strain. For industrial applications, the diploid strain is preferable to the haploid strain in terms of its growth characteristics and fermentation abilities (17, 21). For application to dough fermentation and freeze tolerance in doughs, six diploid strains, PRO1-WT-ura3, pro1-D154N-ura3, pro1-I150T-ura3, PRO1-WT/Δput1, pro1-D154N/Δput1, and pro1-I150T/Δput1, were constructed by mating 3346-ura3 and 3347-ura3, 3346-D154N-ura3 and 3347-D154N-ura3, 3346-I150T-ura3 and 3347-I150T-ura3, 3346-WT/Δput1 and 3347-WT/Δput1, 3346-D154N/Δput1 and 3347-D154N/Δput1, and 3346-I150T/Δput1 and 3347-I150T/Δput1, respectively. When the zygote formation was observed with a microscope, the culture was plated onto YPD plates. The larger colonies were selected, and the cells were confirmed to sporulate on sporulation medium at 25°C (19). These diploid strains will be investigated for approval as SC strains, due to a lack of extraneous DNA derived from other organisms (Table 1). As expected, PRO1-WT/Δput1, pro1-D154N/Δput1, and pro1-I150T/Δput1 failed to grow on proline-containing plates, while strains carrying the wild-type PUT1 gene (AY13, PRO1-WT-ura3, pro1-D154N-ura3, and pro1-I150T-ura3) could utilize proline as their nitrogen source (data not shown). Furthermore, we measured the activity of PO, which is the PUT1 gene product (3, 13). The put1-disrupted strains, PRO1-WT/Δput1, pro1-D154N/Δput1, and pro1-I150T/Δput1, exhibited reduced PO activity (>0.2 U/mg of protein), although such activity was readily detected in AY13, PRO1-WT-ura3, pro1-D154N-ura3, and pro1-I150T-ura3 (1.97, 1.23, 1.98, and 1.04 U/mg of protein, respectively). These results showed that the disruption of PUT1 was confirmed on the basis of gene construction, proline utilization, and enzymatic activity. Next, we examined the growth of diploid strains on plates containing SD medium with AZC (2 mg/ml). Strains pro1-D154N-ura3, pro1-I150T-ura3, pro1-D154N/Δput1, and pro1-I150T/Δput1 clearly showed AZC resistance, whereas the rest of the strains were sensitive to AZC (data not shown). To examine whether greater resistance to AZC reflects a higher level of intracellular proline, AY13, PRO1-WT/Δput1, pro1-D154N/Δput1, and pro1-I150T/Δput1 were cultivated in SD liquid medium and the cellular proline levels were examined. The proline content in PRO1-WT/Δput1 (0.069% of the dry weight of cells) was approximately threefold that in the control strain AY13 (0.023%), due to the deficiency of the degradation pathway (28). In agreement with the findings of our previous studies (22, 30), there was significant accumulation of proline in cells of pro1-D154N/Δput1 and pro1-I150T/Δput1 (0.32 and 0.73%, respectively). GK activity could be detected in the crude extracts prepared from yeast cells, but all GKs, even the wild-type enzyme, showed little inhibition in the presence of 50 mM proline (data not shown). Recently, we found that the purified GK is subject to feedback inhibition by proline and that the D154N and I150T mutant GKs are less sensitive than the wild-type enzyme (22). In fact, pro1-D154N/Δput1 and pro1-I150T/Δput1 clearly accumulated larger amounts of proline than PRO1-WT/Δput1 in both SD medium and cane molasses medium (30 g of sugar [calculated as sucrose]/liter, 1.93 g of urea/liter, and 0.46 g of KH2PO4/liter), in proportion to the diminished sensitivities to feedback inhibition by proline. Although the reason for the absence of feedback inhibition in the crude extracts is unclear, we consider that the D154N and I150T mutant GKs are functionally capable of overproducing proline in vivo. These results showed that the baker's yeast strains that carried the mutant PRO1 gene pro1(D154N) or pro1(I150T) and had a disrupted PUT1 gene accumulated large amounts of proline, in a manner similar to that of the laboratory or sake yeast strains (22, 27).
TABLE 1.
S. cerevisiae strains used in this study
| Strain | Genotype | Background and/or description |
|---|---|---|
| 3346 | MATa | Haploid |
| 3347 | MATα | Haploid |
| 3346-ura3 | MATaura3 | Haploid |
| 3347-ura3 | MATα ura3 | Haploid |
| 3346-D154N-ura3 | MATaura3 pro1(D154N) | 3346-ura3; pro1(D154N) mutant |
| 3346-I150T-ura3 | MATaura3 pro1(I150T) | 3346-ura3; pro1(I150T) mutant |
| 3346-WT/Δput1 | MATaput1::URA3 | 3346-ura3; put1 disruptant |
| 3346-D154N/Δput1 | MATapro1(D154N) put1::URA3 | 3346-D154N-ura3; put1 disruptant |
| 3346-I150T/Δput1 | MATapro1(I150T) put1::URA3 | 3346-I150T-ura3; put1 disruptant |
| 3347-D154N-ura3 | MATα ura3 pro1(D154N) | 3347-ura3; pro1(D154N) mutant |
| 3347-I150T-ura3 | MATα ura3 pro1(I150T) | 3347-ura3; pro1(I150T) mutant |
| 3347-WT/Δput1 | MATα put1::URA3 | 3347-ura3; put1 disruptant |
| 3347-D154N/Δput1 | MATα pro1(D154N) put1::URA3 | 3347-D154N-ura3; put1 disruptant |
| 3347-I150T/Δput1 | MATα pro1(I150T) put1::URA3 | 3347-I150T-ura3; put1 disruptant |
| AY13 | MATa/MATα | Diploid derived from 3346 and 3347 |
| PRO1-WT-ura3 | MATa/MATα ura3/ura3 | Diploid derived from 3346-ura3 and 3347-ura3 |
| pro1-D154N-ura3 | MATa/MATα ura3/ura3 pro1(D154N)/pro1(D154N) | Diploid derived from 3346-D154N-ura3 and 3347-D154N-ura3 |
| pro1-I150T-ura3 | MATa/MATα ura3/ura3 pro1(I150T)/pro1(I150T) | Diploid derived from 3346-I150T-ura3 and 3347-I150T-ura3 |
| PRO1-WT/Δput1 | MATa/MATα put1/put1 | Diploid derived from 3346-WT/Δput1 and 3347-WT/Δput1 |
| pro1-D154N/Δput1 | MATa/MATα put1/put1 pro1(D154N)/pro1(D154N) | Diploid derived from 3346-D154N/Δput1 and 3347-D154N/Δput1 |
| pro1-I150T/Δput1 | MATa/MATα put1/put1 pro1(I150T)/pro1(I150T) | Diploid derived from 3346-I150T/Δput1 and 3347-I150T/Δput1 |
Fermentation ability of the proline-accumulating baker's yeast.
We tested the fermentation abilities of diploid baker's yeast strains that accumulate proline for the application of bread baking. To determine the CO2 gas production abilities as indicators of fermentation abilities, seven diploid strains were cultured in cane molasses medium as a carbon source, which simulates the commercial baker's yeast production process, and the amount of CO2 produced by the yeast cells in the dough was measured according to the method of Nishida et al. (15) (Fig. 1). The fermentation abilities differed between the two groups, the Ura− strains and the Ura+ strains. As shown in Fig. 1A, the gassing powers of the Ura− strains, PRO1-WT-ura3, pro1-D154N-ura3, and pro1-I150T-ura3, were approximately 40 to 50% lower than that of the Ura+ strain AY13. In contrast, the Ura+ strains PRO1-WT/Δput1, pro1-D154N/Δput1, and pro1-I150T/Δput1 exhibited fermentation abilities equivalent to that of strain AY13 (Fig. 1B). Incompatible auxotrophic markers among the strains probably affected the cell growth (5). Therefore, we used four Ura+ strains, AY13, PRO1-WT/Δput1, pro1-D154N/Δput1, and pro1-I150T/Δput1, for further analyses.
FIG. 1.
Fermentation abilities of the diploid baker's yeast strains. The bread doughs containing AY13 (closed diamonds), PRO1-WT-ura3 (open squares), pro1-D154N-ura3 (open circles), and pro1-I150T-ura3 (open triangles) (A) or AY13 (closed diamonds), PRO1-WT/Δput1 (closed squares), pro1-D154N/Δput1 (closed circles), and pro1-I150T/Δput1 (closed triangles) (B) were prepared, and then CO2 gas production was monitored every 5 min for 180 min. The data shown are from one experiment. Similar results were seen in replicates of this experiment.
Amino acid content and stress tolerance of the proline-accumulating baker's yeast.
After cultivation in the molasses medium, the amounts of intracellular amino acids in the baker's yeast strains were analyzed by a method described previously (10, 22). As on the SD medium, the put1-disrupted strain PRO1-WT/Δput1 contained a higher proline level (0.20%) than the control strain AY13 (0.096%). Strains pro1-D154N/Δput1 (3.4%) and pro1-I150T/Δput1 (6.3%) accumulated much higher proline levels than AY13, as expected from the above-described results. In contrast, the proline-accumulating strains showed slightly decreased levels of glutamate and arginine, both of which are proline precursors, compared to the wild-type strain (data not shown).
We previously found that yeast strains that accumulate proline are tolerant of osmotic stress factors, such as NaCl and sorbitol, in addition to freezing stress (22, 26). Yeasts used in bread making are exposed to high concentrations of sucrose during sweet-dough fermentation (2). Such high sucrose concentrations exert severe osmotic stress on yeast (35). Therefore, the growth phenotypes of the proline-accumulating baker's yeast strains under freezing and high-sucrose stress conditions were examined. As shown in Fig. 2, the proline-accumulating strains PRO1-WT/Δput1, pro1-D154N/Δput1, and pro1-I150T/Δput1 were more resistant to high-sucrose stress than AY13. Contrary to our expectations, however, there were no significant differences in growth between the proline-accumulating strains and AY13 under freezing stress conditions (data not shown).
FIG. 2.
Growth phenotypes of the diploid baker's yeast strains under sucrose stress. Yeast cells were precultured for 1 day at 30°C in 50 ml of SD medium. Harvested cells were washed, suspended in modified liquid fermentation medium containing (per liter) 500 g of sucrose and 50 g of maltose without glucose (7) at an OD600 of 6 (no stress), and then cultured for 4 days at 30°C with shaking (high-sucrose stress). Approximately 107 cells of each strain and 10−1 to 10−4 serial dilutions (from left to right, as indicated by triangles) were spotted onto YPD agar plates, and the plates were incubated at 30°C for 2 days.
Freeze tolerance of the proline-accumulating baker's yeast in dough.
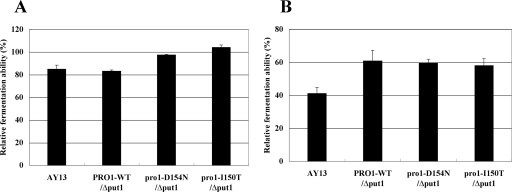
Because freeze tolerance is an important characteristic in recent frozen-dough baking processes (2), we assayed the freeze tolerance of the proline-accumulating baker's yeast in doughs. The formula of dough was 100 g of bread-making flour, 5 g of sucrose, 2 g of NaCl, 4 g of yeast (66% moisture basis), and 68 ml of water. The ingredients were mixed for 3 min with a Swanson type mixer (National Manufacturing Co., Ltd., Sterling, IL). Mixed dough was divided into pieces (40 g each), placed into screw-cap bottles, stored at −20°C without prefermentation before freezing, and kept frozen for 3 weeks. The frozen dough was thawed for 30 min at 30°C, and the fermentation ability was assayed by measuring CO2 gas production using a Fermograph AF-1000 (Atto, Tokyo, Japan). The freeze tolerance was expressed as the percentage of fermentation ability remaining after freezing relative to the ability before freezing (Fig. 3A). We found that pro1-D154N/Δput1 and pro1-I150T/Δput1 retained their fermentation abilities at the same levels as those before freezing, although that of the parent strain AY13 fell to 85% of the prefreezing level. The trehalose contents of AY13, PRO1-WT/Δput1, pro1-D154N/Δput1, and pro1-I150T/Δput1 after cultivation in the molasses medium were 2.7, 4.8, 4.9, and 3.5% of the dry weight, respectively. It appears that there is a weak correlation between the trehalose content and freeze tolerance, because PRO1-WT/Δput1, which contained more trehalose than AY13, exhibited the same level of freeze tolerance as AY13 (Fig. 3A). It is known that yeast cells do not accumulate trehalose in response to freezing stress (12). As for proline, there were no significant differences in freeze tolerance in doughs among the proline-accumulating strains. These results suggest that an appropriate proline level in yeast cells is important to induce a protective effect against freeze-thaw stress.
FIG. 3.
Freeze tolerances of the diploid baker's yeast strains in doughs. Frozen doughs were prepared by using AY13, PRO1-WT/Δput1, pro1-D154N/Δput1, and pro1-I150T/Δput1. The doughs were not prefermented and were frozen at −20°C for 3 weeks (A) or were prefermented for 120 min at 30°C and then frozen at −20°C for 9 days (B). The frozen dough was thawed for 30 min at 30°C, and the remaining CO2 gas production was measured. The gassing power before freezing was defined as 100%. The values are the means and standard deviations of results from three independent experiments.
In commercial frozen-dough processes, prefermentation before freezing is desirable in terms of the texture and taste of the product (9, 31). We therefore performed the same experiment using the doughs prefermented for 120 min at 30°C before freezing, and the dough was kept frozen for 9 days (Fig. 3B). The remaining gassing power of AY13 was dramatically decreased to 41% of that before freezing. It is noteworthy that pro1-D154N/Δput1 and pro1-I150T/Δput1, and even PRO1-WT/Δput1, showed approximately 50% greater fermentation abilities than AY13. Prefermentation is an important process in bread baking, because yeast cells activated during prefermentation produce the metabolites, such as alcohols and organic acids, which influence the taste and flavor of the bread. The reason for the loss of the gassing power remains unclear; however, it is possible that prolonged prefermentation causes serious damage to the membranes of the yeast cells in the dough (11). It is also known that the intracellular amino acid pool decreases through prefermentation (24). Therefore, we consider that intracellular proline, as a cryoprotectant, was not degraded in PRO1-WT/Δput1 during prefermentation, leading to improved tolerance for freezing. These data strongly suggest that proline-accumulating baker's yeast has higher-level freeze tolerance than non-proline-accumulating yeast and is suitable for frozen-dough baking.
The present study is the first to report the construction of diploid baker's yeasts that accumulate proline. It is relatively difficult to breed an industrial baker's yeast strain with higher-level freeze tolerance than that of a laboratory strain. The process that involves adding proline externally to the cell or to the dough remains somewhat troublesome for practical applications. However, yeast strains with comparatively large amounts of proline already in the cells may overcome this problem. Another potential problem is that the strains mentioned above are GM yeasts, because the S. cerevisiae-Escherichia coli shuttle vector was used to complement the auxotrophic marker. According to the Japanese government guidelines, SC processes are considered to be the same as naturally occurring gene conversions, such as recombination, deletion, and transposition; thus, SC yeast need not be treated as recombinant GM yeast (6).
Acknowledgments
This work was supported by a grant to J.S. and H.T. from the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN).
Footnotes
Published ahead of print on 18 July 2008.
REFERENCES
- 1.Attfield, P. V. 1987. Trehalose accumulates in Saccharomyces cerevisiae during exposure to agents that induce heat shock response. FEBS Lett. 225:259-263. [DOI] [PubMed] [Google Scholar]
- 2.Attfield, P. V. 1997. Stress tolerance: the key to effective strains of industrial baker's yeast. Nat. Biotechnol. 15:1351-1357. [DOI] [PubMed] [Google Scholar]
- 3.Brandriss, M. C., and B. Magasanik. 1979. Genetics and physiology of proline utilization in Saccharomyces cerevisiae: enzyme induction by proline. J. Bacteriol. 140:498-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke, D., D. Dawson, and T. Stearns. 2000. Methods in yeast genetics: a Cold Spring Harbor Laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 5.Chopra, R., V. M. Sharma, and K. Ganesan. 1999. Elevated growth of Saccharomyces cerevisiae ATH1 null mutants on glucose is an artifact of nonmatching auxotrophies of mutant and reference strains. Appl. Environ. Microbiol. 65:2267-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hino, A. 2002. Safety assessment and public concerns for genetically modified food products: the Japanese experience. Toxicol. Pathol. 30:126-128. [DOI] [PubMed] [Google Scholar]
- 7.Hino, A., K. Mihara, K. Nakashima, and H. Takano. 1990. Trehalose levels and survival ratio of freeze-tolerant versus freeze-sensitive yeasts. Appl. Environ. Microbiol. 56:1386-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu, K. H., R. C. Hoseney, and P. A. Seib. 1979. Frozen dough. I. Factors affecting stability of yeasted dough. Cereal Chem. 56:419-424. [Google Scholar]
- 9.Hsu, K. H., R. C. Hoseney, and P. A. Seib. 1979. Frozen dough. II. Effects of freezing and storing conditions on stability of yeasted dough. Cereal Chem. 56:424-426. [Google Scholar]
- 10.Kaino, T., and H. Takagi. 2008. Gene expression profiles and intracellular contents of stress protectants in Saccharomyces cerevisiae under ethanol and sorbitol stresses. Appl. Microbiol. Biotechnol. 79:273-283. [DOI] [PubMed] [Google Scholar]
- 11.Kline, L., and T. G. Sugihara. 1968. Factors affecting the stability of frozen bread doughs. I. Prepared by the straight dough method. Baker's Dig. 42:44-69. [Google Scholar]
- 12.Liang, L. K., X. K. Wang, K. L. Zhu, and Z. M. Chi. 2007. Trehalose synthesis in Saccharomycopsis fibuligera does not respond to stress treatments. Appl. Microbiol. Biotechnol. 74:1084-1091. [DOI] [PubMed] [Google Scholar]
- 13.Morita, Y., S. Nakamori, and H. Takagi. 2002. Effect of proline and arginine metabolism on freezing stress of Saccharomyces cerevisiae. J. Biosci. Bioeng. 94:390-394. [DOI] [PubMed] [Google Scholar]
- 14.Morita, Y., S. Nakamori, and H. Takagi. 2003. l-Proline accumulation and freeze tolerance of Saccharomyces cerevisiae are caused by a mutation in the PRO1 gene encoding γ-glutamyl kinase. Appl. Environ. Microbiol. 69:212-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishida, O., S. Kuwazaki, C. Suzuki, and J. Shima. 2004. Superior molasses assimilation, stress tolerance, and trehalose accumulation of baker's yeast isolated from dried sweet potatoes (hoshi-imo). Biosci. Biotechnol. Biochem. 68:1442-1448. [DOI] [PubMed] [Google Scholar]
- 16.Oda, Y., K. Uno, and S. Ohta. 1986. Selection of yeasts for breadmaking by the frozen-dough method. Appl. Environ. Microbiol. 52:941-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohshima, Y., T. Sugaura, M. Horita, and T. Sasaki. 1987. Industrial application of artificially induced diploid strains of Torulaspora delbrueckii. Appl. Environ. Microbiol. 53:1512-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reese, L. M., K. O. Cutler, and C. E. Deutch. 1996. Sensitivity of Escherichia coli to proline analogues during osmotic stress and anaerobiosis. Lett. Appl. Microbiol. 22:202-205. [DOI] [PubMed] [Google Scholar]
- 19.Rose, M. D., F. Winston, and P. Hieter. 1990. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 20.Rudolph, A. S., and J. H. Crowe. 1985. Membrane stabilization during freezing: the role of two natural cryoprotectants, trehalose and proline. Cryobiology 22:367-377. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki, T., and Y. Ohshima. 1987. Induction and characterization of artificial diploids from the haploid yeast Torulaspora delbrueckii. Appl. Environ. Microbiol. 53:1504-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekine, T., A. Kawaguchi, Y. Hamano, and H. Takagi. 2007. Desensitization of feedback inhibition of the Saccharomyces cerevisiae γ-glutamyl kinase enhances proline accumulation and freezing tolerance. Appl. Environ. Microbiol. 73:4011-4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shima, J., A. Hino, C. Yamada-Iyo, Y. Suzuki, R. Nakajima, H. Watanabe, K. Mori, and H. Takano. 1999. Stress tolerance in doughs of Saccharomyces cerevisiae trehalase mutants derived from commercial baker's yeast. Appl. Environ. Microbiol. 65:2841-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shima, J., Y. Sakata-Tsuda, Y. Suzuki, R. Nakajima, H. Watanabe, S. Kawamoto, and H. Takano. 2003. Disruption of the CAR1 gene encoding arginase enhances freeze tolerance of the commercial baker's yeast Saccharomyces cerevisiae. Appl. Environ. Microbiol. 69:715-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sikorski, R. S., and J. D. Boeke. 1991. In vitro mutagenesis and plasmid shuffling: from cloned gene to mutant yeast. Methods Enzymol. 194:302-318. [DOI] [PubMed] [Google Scholar]
- 26.Takagi, H., F. Iwamoto, and S. Nakamori. 1997. Isolation of freeze-tolerant laboratory strains of Saccharomyces cerevisiae from proline-analogue-resistant mutants. Appl. Microbiol. Biotechnol. 47:405-411. [DOI] [PubMed] [Google Scholar]
- 27.Takagi, H., F. Matsui, A. Kawaguchi, H. Wu, H. Shimoi, and Y. Kubo. 2007. Construction and analysis of self-cloning sake yeasts that accumulate proline. J. Biosci. Bioeng. 103:377-380. [DOI] [PubMed] [Google Scholar]
- 28.Takagi, H., K. Sakai, K. Morida, and S. Nakamori. 2000. Proline accumulation by mutation or disruption of the proline oxidase gene improves resistance to freezing and desiccation stresses in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 184:103-108. [DOI] [PubMed] [Google Scholar]
- 29.Takagi, H., M. Takaoka, A. Kawaguchi, and Y. Kubo. 2005. Effect of l-proline on sake brewing and ethanol stress in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 71:8656-8662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terao, Y., S. Nakamori, and H. Takagi. 2003. Gene dosage effect of l-proline biosynthetic enzymes on l-proline accumulation and freeze tolerance in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 69:6527-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teunissen, A., F. Dumortier, M. F. Gorwa, J. Bauer, A. Tanghe, A. Loïez, P. Smet, P. Van Dijck, and J. M. Thevelein. 2002. Isolation and characterization of a freeze-tolerant diploid derivative of an industrial baker's yeast strain and its use in frozen doughs. Appl. Environ. Microbiol. 68:4780-4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomenchok, D. M., and M. C. Brandriss. 1987. Gene-enzyme relationships in the proline biosynthetic pathway of Saccharomyces cerevisiae. J. Bacteriol. 169:5364-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trotter, E. W., L. Berenfeld, S. A. Krause, G. A. Petsko, and J. V. Gray. 2001. Protein misfolding and temperature up-shift cause G1 arrest via a common mechanism dependent on heat shock factor in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 98:7313-7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Dijck, P., D. Colavizza, P. Smet, and J. M. Thevelein. 1995. Differential importance of trehalose in stress resistance in fermenting and nonfermenting Saccharomyces cerevisiae cells. Appl. Environ. Microbiol. 61:109-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verstrepen, K. J., D. Iserentant, P. Malcorps, G. Derdelinckx, P. Van Dijck, J. Winderickx, I. S. Pretorius, J. M. Thevelein, and F. R. Delvaux. 2004. Glucose and sucrose: hazardous fast-food for industrial yeast? Trends Biotechnol. 22:531-537. [DOI] [PubMed] [Google Scholar]
- 36.Wang, S. S., and M. C. Brandriss. 1986. Proline utilization in Saccharomyces cerevisiae: analysis of the cloned PUT1 gene. Mol. Cell. Biol. 6:2638-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]