Abstract
Trypanosoma brucei, the etiologic agent of African sleeping sickness, divides into insect (procyclic) and bloodstream forms. These two forms are subject to distinct cell cycle regulations, with cytokinesis controlled primarily by basal body/kinetoplast segregation in the procyclic form but by mitosis in the bloodstream form. Polo-like kinases (PLKs), known to play essential roles in regulating both mitosis and cytokinesis among eukaryotes, have a homologue in T. brucei, TbPLK, which regulates only cytokinesis. In our previous study, overexpressed triply hemagglutinin-tagged TbPLK (TbPLK-3HA) in the procyclic form localized to a mid-dorsal point and the anterior tip of the cell along the flagellum attachment zone (FAZ). In our current study, TbPLK-3HA expressed at the endogenous level was identified at the same dorsal location of both procyclic and bloodstream forms, albeit it was no longer detectable at the anterior tip of the cell. Endogenously expressed TbPLK fused with an enhanced yellow fluorescent protein (EYFP) localized to the same dorsal location along the FAZs in living procyclic and bloodstream cells. Fluorescence-activated cell sorter analysis of hydroxyurea-synchronized procyclic cells revealed that TbPLK-EYFP emerges during S phase, persists through G2/M phase, and vanishes in G1 phase. An indicated TbPLK-EYFP association with the FAZs of G2/M cells may thus represent a timely localization to a potential initiation site of cytokinesis, which agrees with the recognized role of TbPLK in cytokinetic initiation.
Trypanosoma brucei is a unicellular protozoan parasite that causes African sleeping sickness in humans and nagana in cattle. Its life cycle can be roughly divided into metacyclic and procyclic forms in tsetse flies and long slender and short stumpy bloodstream forms in mammalian hosts. Only the procyclic form and the long slender bloodstream form can be cultured in vitro, and their mechanisms of cell division during these two stages of development have thus been extensively examined (28). Trypanosomes divide by a binary fission from the anterior to the posterior end. Prior to this, the nucleus, the single mitochondrion, the mitochondrial DNA complex known as the kinetoplast, the basal body, and the flagellum are each duplicated and segregated into two daughter cells (42). The nuclear cycle has the usual G1, S, and G2/M phases (53), which must be coordinated with the kinetoplast cycle for a fruitful cytokinetic initiation (34). Distinctive cell cycle regulations have been observed for the two forms of T. brucei (29). While cytokinesis in the procyclic form is triggered primarily by the segregation of two kinetoplast/basal body pairs (37), it is apparently under the strict control of mitosis in the bloodstream form (15, 48). This change of command during two stages of development of the same species of organism may constitute one of the most intriguing aspects of trypanosome biology. It will be interesting to find out how proteins known to control both mitosis and cytokinesis in eukaryotes would function in trypanosomes.
Polo-like kinase (PLK) is such a protein and plays multiple roles in cell cycle regulation in all eukaryotes examined thus far. Its function is required for G2/M-phase entry (1), metaphase/anaphase transition (2), anaphase release (19), mitotic exit (17), and the initiation of cytokinesis (14). These roles have largely been conserved throughout evolution (10). PLK has unique structural features; it has a serine threonine kinase domain at the N terminus and two polo-box domains at the C terminus of the protein (18, 40). Multiple PLK homologues are present in metazoans (7), but only single PLK homologues have been found in unicellular organisms (21, 32). A well-conserved single PLK homologue was identified in T. brucei (TbPLK), and it contains a conserved kinase domain at the N terminus and two polo-box domains at the C terminus of the protein (13). In an earlier study, we found that TbPLK was capable of complementing Saccharomyces cerevisiae depleted of its own PLK Cdc5, suggesting that TbPLK possesses all the functions of Cdc5 (24). However, through RNA interference (RNAi) knockdown experiments, we found that TbPLK performs only the function of cytokinetic initiation in trypanosomes (24). When TbPLK was depleted, nuclear divisions and multiplications of kinetoplast, basal body, and flagellum continued on, whereas cell division was blocked, resulting in cells with multiple nuclei, kinetoplasts, basal bodies, and flagella. A similar observation was made in a separate study, except that only double kinetoplasts, basal bodies, and flagella were identified in the multinucleate RNAi cells (16). A more recent report (8) indicated that TbPLK depletion also leads to a malformed bilobed structure which is required for regulating Golgi duplication, resulting in an increase in the number of dispersed Golgi structures. By overexpressing a hemagglutinin (HA)-tagged TbPLK in procyclic trypanosome and monitoring its localization by immunofluorescence microscopy, we observed that TbPLK was not localized to the nucleus at all, as in other eukaryotes (24). Instead, TbPLK was identified at a midpoint on the dorsal side of the cell and the anterior tip of the cell. In the cytoskeleton preparation, the subcellular localization of TbPLK coincided with the dorsal-side flagellum attachment zone (FAZ) (24). In a subsequent report, however, the immunofluorescence assay indicated the presence of overexpressed TbPLK in the cytoplasm (16). More recently, however, a third research group used affinity-purified antibodies raised against TbPLK and was able to confirm our results by finding TbPLK at the growing tip of the new FAZ (8). These conflicting claims prompted us to reexamine the localization of TbPLK and repeat our previous observation. We then expressed the HA-tagged TbPLK at endogenous levels in both the procyclic and bloodstream forms of trypanosome and found it localized to the same midpoint of the dorsal side of the cell. When TbPLK was fused with an enhanced yellow fluorescent protein (EYFP) and also expressed at the endogenous levels in both forms of trypanosome, it was also found to be localized at the midpoint of the dorsal side of the cells corresponding to the FAZ.
A difficulty in the localization study was that only about 20% of the cells appeared to express TbPLK, suggesting that the protein is expressed only during a certain phase of the cell cycle. In procyclic cells of T. brucei synchronized by hydroxyurea (6), TbPLK was found to appear in late S phase and persist in G2/M phase but vanish in G1 phase, which agrees with the postulated function of TbPLK in controlling cytokinesis in trypanosomes.
MATERIALS AND METHODS
Cell culture.
Procyclic-form cells of strains 427 and 29-13 (52) were grown at 26°C in Cunningham's medium supplemented with 10% fetal bovine serum (HyClone, UT). Bloodstream-form cells of strains 221 and 90-13 (52) were grown at 37°C with 5% CO2 supplied in HMI-9 medium containing 10% fetal bovine serum (HyClone, UT) and 10% serum plus (SAFC Bioscience Inc., KS). To maintain the T7 RNA polymerase and tetracycline repressor gene constructs within the cells, 15 μg/ml G418 and 50 μg/ml hygromycin B were added to the Cunningham's medium for the 29-13 cell line, whereas 2.5 μg/ml G418 and 5 μg/ml hygromycin B were added to the HMI-9 medium for the 90-13 cell line. Cells were routinely diluted with fresh medium when their density reached 5 × 106 cells/ml.
Synchronization of the culture of procyclic-form cells was performed using 0.2 mM hydroxyurea according to the procedure reported by Chowdhury et al. (6).
Plasmids and cell lines.
For the overexpression of triply HA-tagged TbPLK (TbPLK-3HA) in the procyclic form, the 29-13 cell line harboring pLew100-PLK-3HA was cultured in the presence of 2.5 μg/ml phleomycin (24).
For endogenous tagging with three tandem repeats of HA, a 600-bp fragment from the C-terminal portion of TbPLK was amplified by PCR and cloned into the KpnI-AflII sites of the endogenous 3HA-tagging vector pcNeo-3HA, which was created previously by replacing the PTP tag of pC-PTP-NEO (39) with three tandem repeats of HA. The resulting plasmid, pcNeo-PLK-3HA, was linearized by Eco47III digestion and electroporated into procyclic-form and bloodstream-form cell lines 427 and 221, respectively.
For endogenous tagging with enhanced EYFP, a 600-bp KpnI-AflII fragment from pcNeo-PLK-3HA was cloned into the KpnI-AflII sites of the blasticidin-resistant vector pcBla-3HA, which is a derivative of pRPA31-HA-BLA (31), to yield pcBla-PLK-3HA. Then, a DNA fragment encoding EYFP from pEYFP (Clontech Laboratory Inc., CA) was amplified by PCR and cloned into the AflII-BamHI sites of pcBla-PLK-3HA to replace the 3HA tag. The resulting plasmid, pcBla-PLK-EYFP, was linearized by Eco47III digestion and electroporated into the procyclic-form cell line 29-13 or the bloodstream-form cell line 90-13 cultured in the presence of 10 μg/ml blasticidin.
Western blotting.
Cells were washed twice in phosphate-buffered saline (PBS), boiled for 3 min in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer (Bio-Rad, CA), and centrifuged at 20,000 × g for 10 min. Total protein from the supernatant was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto immunoblot polyvinylidene difluoride membranes (Bio-Rad, CA). These membranes were blocked for 1 h in TBST (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.1% Tween 20) containing 5% skim milk and then incubated for 1 h with anti-HA mouse monoclonal antibody (MAb) conjugated with horseradish peroxidase (H6533; Sigma, MO) at a dilution ratio of 1:30,000 for detecting 3HA-tagged protein. EYFP-fused protein was detected using anti-green fluorescent protein (GFP) MAb JL-8 at a dilution ratio of 1:5,000 (632380; Clontech Laboratory Inc.) and α-tubulin by anti-α-tubulin MAb at a dilution ratio of 1:30,000 (T9026; Sigma, MO). Anti-mouse immunoglobulin G (IgG) conjugated with horseradish peroxidase at a dilution ratio of 1:10,000 (A4416; Sigma, MO) was used as the secondary antibody. Proteins on blots were detected by chemiluminescence (Immobilon Western; Millipore, CA). The signal strength was quantified by using ImageJ software (http://rsb.info.nih.gov/ij/). Coomassie brilliant blue G-250 staining as a loading control was performed using SimplyBlue SafeStain (Invitrogen, CA).
Immunofluorescence staining.
Cells were washed twice in PBS and fixed in 4% paraformaldehyde for 5 min. After being washed, the fixed cells were loaded on poly-l-lysine coverslips for 20 min, treated with cold methanol for 5 min, and rehydrated in PBS for 5 min. The protocol for preparing cytoskeleton from procyclic-form T. brucei was followed for the cytoskeleton from the bloodstream form (24), except that live bloodstream-form cells were loaded on poly-l-lysine-coated coverslips for 20 min and then washed three times in PEME buffer [100 mM piperazine-N,N′-bis(2-ethanesulfonic acid) sesquisodium (PIPES 1.5 Na), 2 mM EGTA, 0.1 mM EDTA, and 1 mM MgSO4]. After the third wash, PEME containing 1% NP-40 was added at room temperature for 5 min and the cells were rewashed in PEME for a cytoskeleton preparation. The cytoskeleton was then fixed using 4% paraformaldehyde and blocked in 2% bovine serum albumin in PBS containing 0.1% Triton X-100. Antibodies were added, the mixture was incubated for an hour, and cells were washed three times in PBS containing 0.1% Triton X-100. Corresponding secondary antibodies conjugated with fluorescent dyes were then added for another hour. Antibodies used in the study included tetramethyl rhodamine isocyanate (TRITC)-conjugated HA probe for 3HA-tagged clone F-7 (sc-7392 TRITC; dilution, 1:500; Santa Cruz Biotechnology, CA) and L3B2 for the FAZ (mouse MAb from Keith Gull, Oxford University; used at a dilution of 1:50 [23]). The secondary antibodies used were anti-mouse IgG conjugated with Alexa488 (A21202; Invitrogen, CA) and anti-mouse IgG conjugated with Texas Red (T862; Invitrogen, CA). Following incubation with the secondary antibodies, cells were washed three times in PBS containing 0.1% Triton X-100. The slides were mounted in Vectashield mounting medium containing 4′,6′-diamino-2-phenylindole (DAPI) (Vector Laboratories Inc., CA) and examined with a fluorescence microscope (IX70; Olympus, Japan). Photographs were taken with an Olympus PlanApo 60× oil Ph3 objective lens (numerical aperture, 1.4). Data were collected with a Photometrics Cool Snap HQ charge-coupled-device camera and processed by use of the software MetaVue version 5.0 and ImageJ.
FACS analysis.
Cell samples for fluorescence-activated cell sorter (FACS) analysis were prepared as described previously (30) with minor modifications. Briefly, T. brucei cells were collected, centrifuged at 2,000 × g for 10 min, and washed twice in PBS. Cell pellets were gently suspended in 100 μl of PBS and mixed with 200 μl of 10% ethanol and 5% glycerol in PBS. They were then mixed with another 200 μl of 50% ethanol and 5% glycerol in PBS prior to incubation on ice for 5 min. One milliliter of 70% ethanol and 5% glycerol in PBS was added. The cells were then suspended in PBS. DNase-free RNase (Roche Diagnosis, IN) and propidium iodide (PI) were added to the suspension to final concentrations of 10 and 20 μg/ml, respectively, and incubated for 30 min at room temperature before the FACS analysis. The DNA content (FL2 channel) and EYFP signal strength (FL1 channel) of PI-stained cells were analyzed with a FACScan analytical flow cytometer using Cellquest software (BD Biosciences, CA).
RESULTS
Overexpressed TbPLK-3HA localizes to the midpoint of the dorsal side and the anterior tips of procyclic cells.
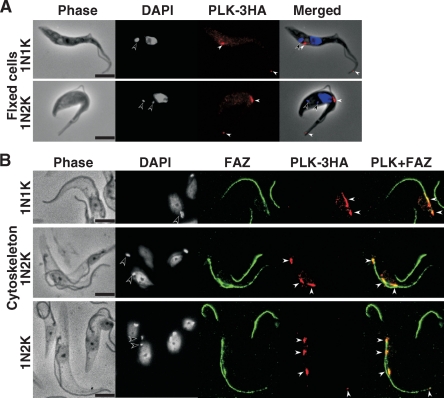
An anti-HA antibody staining of the paraformaldehyde-fixed procyclic cells overexpressing TbPLK-3HA indicated two areas of focal presence of the protein at the anterior tip of the cell and at the midportion on the dorsal side of the cell (Fig. 1A). These results are in agreement with those published previously (24). This localization was also observed for the cells cultured for only 1 day after tetracycline induction instead of 5 days (data not shown). Similar results were obtained from TbPLK tagged with 3HA at either the C terminus (24) or the N terminus, indicating that the tagging position does not affect the localization of the protein (data not shown). The subcellular localization of overexpressed TbPLK-3HA in the procyclic cytoskeleton was also examined using the cells cultured for 5 days after the induction of overexpression, as done in the previous study (24). Mouse L3B2 MAb, which recognizes the FAZ (23) in the cytoskeleton, and anti-HA conjugated with TRITC were used to stain the cytoskeleton. The results (Fig. 1B) indicate that spots of TbPLK-3HA were associated with the FAZ. Although they are not present along the entire length of the FAZ as observed previously, they are always present at the posterior ends of both the old and the new FAZ, which is the originating point of the FAZ (42), and at the anterior end of the new FAZ. For some cells, localization at the anterior end of the old FAZ was also observed. Repeated examinations suggested that the positions of the anterior ends of the new and the old FAZ may correspond to the stained dorsal midpoint and the anterior tip, respectively, of the cells shown in Fig. 1A. Thus, a tentative conclusion one may derive from the data in Fig. 1 is that TbPLK could be associated with both ends of the FAZ when overexpressed in the procyclic form.
FIG. 1.
Overexpressed TbPLK-3HA localized to the ends of the FAZ. (A) Procyclic-form 29-13 cells harboring pLew100-PLK-3HA were cultured for 5 days in the presence of 0.1 μg/ml tetracycline, fixed with 4% paraformaldehyde, and stained with anti-HA antibody (red) and DAPI (blue). (B) The cytoskeleton from the same cell culture was stained with anti-HA antibody for TbPLK-3HA (red), with L3B2 antibody for the FAZ (green), and with DAPI for DNA (blue). Open and closed arrowheads indicate the kinetoplast and TbPLK-3HA signals, respectively. Bars, 5 μm.
TbPLK-3HA expressed at the endogenous level also localizes to the dorsal midpoints of both procyclic and bloodstream cells.
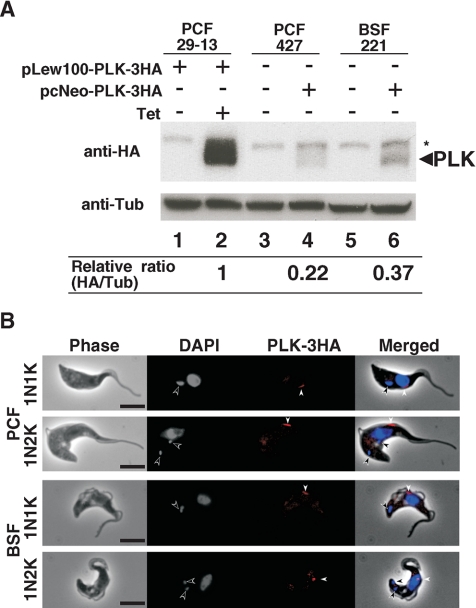
There is always the concern of whether an overexpressed protein can localize to the same place as that protein when it is expressed at the endogenous level. To cope with this uncertainty, the level of overexpressed TbPLK-3HA in the procyclic cells studied as shown in Fig. 1 was analyzed by Western blotting using the anti-HA MAb and given a relative quantity of 100% (Fig. 2A). Linearized DNA encoding TbPLK-3HA, which was integrated into the chromosome through homologous recombination, was expressed in the procyclic and bloodstream forms of trypanosome in the in vitro cultures. The levels of these endogenously expressed proteins in the procyclic and bloodstream forms were also monitored by Western blotting. They turned out to correspond to 22% and 37% of the overexpressed protein, respectively (Fig. 2A). An overexpression of protein could thus exceed the endogenous level four- or fivefold, though it may be possible that the 3HA tag could affect the translational competence of the mRNA or alter the stability of the protein.
FIG. 2.
Endogenous TbPLK-3HA localization in the procyclic and bloodstream forms. (A) Western blotting showing TbPLK-3HA expression levels. For the overexpression of TbPLK-3HA, procyclic-form (PCF) 29-13 cells (lanes 1 and 2) harboring pLew100-PLK-3HA were cultured for 1 day in the absence (lane 1) or presence (lane 2) of 0.1 μg/ml tetracycline. PCF 427 cells (lane 4) and bloodstream-form (BSF) 221 cells (lane 6) harboring pcNeo-PLK-3HA in one of the two alleles of the TbPLK genomic locus were used to examine the endogenous TbPLK-3HA. Wild-type PCF 427 and BSF 221 cells were used as negative controls (lanes 3 and 5). Total proteins from 2.5 × 106 cells were applied to Western analysis with anti-HA antibody (top) or anti-α-tubulin antibody as the loading control (bottom). The asterisk indicates a nonspecific band. The ratio of TbPLK-3HA to α-tubulin (Tub), determined using ImageJ software, is indicated under each lane. (B) Immunostaining of endogenous TbPLK-3HA using anti-HA antibody. The 29-13 cells with the endogenous TbPLK-3HA harboring pcNeo-PLK-3HA were fixed with 4% paraformaldehyde and stained with anti-HA antibody (red) and DAPI (blue). Open and closed arrowheads indicate kinetoplast and TbPLK-3HA signals, respectively. Bars, 5 μm.
The question is then whether the protein expressed at the assumed endogenous level in trypanosome also localizes in the same pattern as the overexpressed one. Immunostainings of the endogenously expressed TbPLK-3HA in procyclic- and bloodstream-form cells were performed. The data showed that the protein localizes to the dorsal midpoints of cells in both forms (Fig. 2B). The only probable distinction from the overexpressed TbPLK was that the spot of the latter seen at the anterior tips of procyclic cells could not be observed in the two forms of cells expressing the protein at the endogenous levels. This could mean that the location at the anterior tip might be an artifact due to the overexpression of the protein. Another possibility could be that since the endogenous levels are lower than those from the overexpression, the signal at the anterior tip might be too weak to be seen. Since the anterior tip of the cell corresponds to the anterior tip of the FAZ, it is not too speculative to assume that TbPLK is localized along the FAZ. When its signal is strong, it can be seen from one end to the other end of the FAZ. When it is relatively weak, it is seen primarily at the origin (the posterior end) of the FAZ. These results are in agreement with those reported very recently for the procyclic form by de Graffenried et al. (8). No signal from the endogenously expressed TbPLK-3HA could be observed in the cytoskeletal preparations from either procyclic or bloodstream cells, a finding which could be attributed to the lower level of expression (see below).
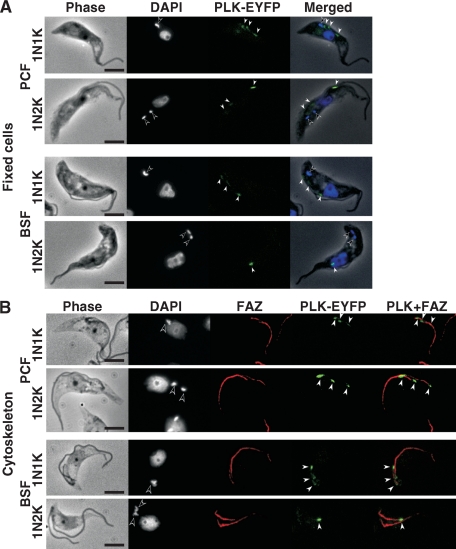
Endogenously expressed TbPLK-EYFP localizes to a few dorsal midpoints of living cells along the FAZs of both procyclic and bloodstream forms. To verify that the localization of TbPLK-3HA was not attributed to unknown artifacts from the procedure of immunostaining, TbPLK was tagged with EYFP at its C terminus and expressed at the endogenous levels in the two forms of the trypanosome. Among the transfected cells, TbPLK-EYFP could be seen in the form of bright spots numbering from one to three and distributed in the midportion along the dorsal side (Fig. 3A). When the cells were converted to cytoskeletal preparations and stained with L3B2 antibody against the FAZ, the few fluorescent spots of TbPLK-EYFP were clearly associated with the FAZ, with one of the spots invariably localized to the anterior end of the new FAZ, whereas the other was always at the posterior end (Fig. 3B). These observations, equally applicable to both procyclic and bloodstream forms of trypanosome (Fig. 3), suggest that TbPLK is associated with the FAZ in both forms of trypanosome. The fact that more discrete spots of TbPLK-EYFP than of TbPLK-3HA could be observed along the FAZ could reflect the higher intensity of EYFP fluorescence.
FIG. 3.
Endogenous TbPLK-EYFP localization in both procyclic and bloodstream forms. (A) Procyclic-form (PCF) 29-13 cells or bloodstream-form (BSF) 90-13 cells harboring pcBla-PLK-EYFP were fixed with 4% paraformaldehyde and stained with DAPI for DNA (blue). TbPLK-EYFP was visualized in green. (B) The cytoskeleton from the same cell culture was stained with L3B2 antibody for the FAZ (red), and with DAPI for DNA (blue). TbPLK-EYFP was visualized in green. Open and closed arrowheads indicate kinetoplast and TbPLK-EYFP signals, respectively. Bars, 5 μm.
TbPLK emerges in S phase, reaches the highest level in G2/M phase, and vanishes in G1 phase.
One of the difficulties we encountered in trying to localize TbPLK in trypanosome cells was that not all the cells were found to express the protein, regardless of what tag was employed. In repeated trials, only about 20% of the procyclic form and 34% of the bloodstream form were found expressing TbPLK tagged with either 3HA or EYFP. These low percentages of cells expressing TbPLK at a given time led us to suspect that TbPLK could be specifically expressed only during a certain phase of the cell cycle in trypanosomes. Trypanosomes are known to have a very long G1 phase (34). We thus anticipated that TbPLK could be absent during that phase but emerge in the S or G2/M phase of the cell cycle.
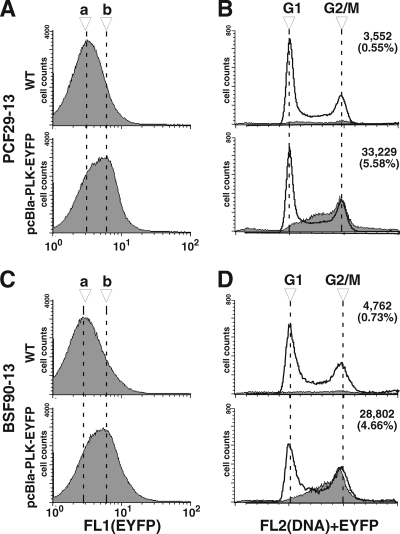
Procyclic and bloodstream form cells expressing TbPLK-EYFP at endogenous levels were each analyzed for DNA content by flow cytometry in the FL2 channel while using the FL1 channel to sort the cells according to the signal strength of EYFP fluorescence (Fig. 4A and C). A significant shift of the EYFP fluorescence peak was detected between the cells having no TbPLK-EYFP expression (peak a) and the cells having TbPLK-EYFP expression (peak b), and the shift was found in both forms of trypanosome. A small population of the cells showing the EYFP signal was then sorted for DNA contents by flow cytometry. The results indicate that for both forms of trypanosome, the G1-phase cells showed no sign of fluorescence, whereas the S-phase cells and G2/M-phase cells showed clear fluorescence signals (Fig. 4B and D).
FIG. 4.
Endogenous TbPLK-EYFP was expressed in S- and G2/M-phase cells. Procyclic-form (PCF) 29-13 cells or bloodstream-form (BSF) 90-13 cells without (wild type [WT]) or with pcBla-PLK-EYFP were stained with PI and analyzed by flow cytometry (one representative experiment out of three). (A and C) Histograms of the cells without or with TbPLK-EYFP monitored by green fluorescence (FL1 channel). Dashed lines a and b indicate the peak locations in the histograms of cells without and with pcBla-PLK-EYFP, respectively. (B and D) Histograms for cells sorted by the DNA contents (FL2 channel) from total cells are indicated by solid lines. To determine the cells showing fluorescence from TbPLK-EYFP, a gate was defined to exclude most of the autofluorescent cells from the group of cells having no TbPLK-EYFP, and the same gate was applied to the cells with TbPLK-EYFP. Histograms of the DNA contents of cells that fell within the gate are indicated by gray color. Of 500,000 events that were analyzed, the number (along with the percentage) of events that fell within the gate is indicated at the top right of each histogram.
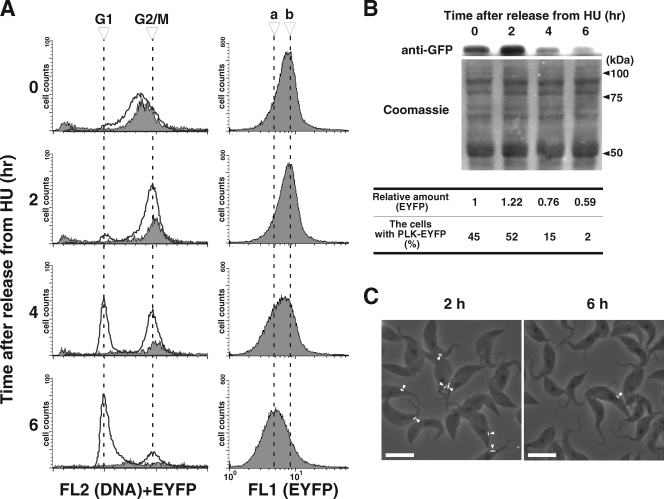
To further clarify that TbPLK is expressed only in the S and G2/M phases of the cell cycle, we utilized a method recently developed by Chowdhury et al. to synchronize the procyclic cells with hydroxyurea (6). The procyclic cells expressing TbPLK-EYFP at the endogenous level were cultured overnight in the presence of 0.2 mM hydroxyurea and washed, and the culturing was resumed in the absence of hydroxyurea. Cell samples were collected once every 2 hours and subjected to FACScan analysis as previously described. The cells were found synchronized in the late S phase when hydroxyurea was first removed at 0 h (Fig. 5A). After 2 h, most of the cells were shifted to the G2/M phase in a neatly synchronized manner. From 4 to 6 h, there was a steady shift of cells from the G2/M phase to the G1 phase, until most of the cells ended up in the G1 phase (Fig. 5A, left). The EYFP fluorescence detected by the FL1 channel had the highest intensity from 0 to 2 h after the release from hydroxyurea but diminished steadily from 2 to 6 h (Fig. 5A, right). The cells were then gated and sorted according to DNA content as described for Fig. 4 (Fig. 5A, left). The EYFP fluorescence was detected in the S-phase cells at 0 h and in the G2/M-phase cells from 2 to 4 h but disappeared from the G1 cells that began to appear after 4 h (Fig. 5A, left). The data thus provided a clear indication that TbPLK is present in abundance during the S and G2/M phases but vanishes in the G1 phase.
FIG. 5.
Hydroxyurea (HU) synchronization of procyclic-form cells expressing endogenous TbPLK-EYFP. Procyclic-form 29-13 cells with pcBla-PLK-EYFP were treated with 0.2 mM HU overnight, washed twice with growth medium, and collected at the times indicated (0, 2, 4, and 6 h). The collected cells were fixed with ethanol, stained with propidium iodide, and analyzed by flow cytometry. (A) Histograms on the left indicate results from cell sorting for DNA contents (FL2 channel [solid line]). Cells showing fluorescence from TbPLK-EYFP (gray area) were processed in a way similar to that described for Fig. 4. Histograms on the right show results from cell sorting by TbPLK-EYFP fluorescence (FL1 channel). Dashed lines a and b indicate the peak locations in the histograms from the cells collected at 6 h and 2 h after release from HU treatment, respectively. (B) Western blot showing the changing TbPLK-EYFP levels after release from HU treatment. Total proteins from 106 cells stained with anti-GFP antibody (top) or Coomassie brilliant blue G-250 as a loading control (bottom) were applied to the Western analysis. The relative amount of TbPLK-EYFP, determined using ImageJ software, is indicated under each lane. The population of cells expressing PLK-EYFP on the dorsal side (n > 120) is also indicated under each lane. (C) Merged phase-contrast (gray) and PLK-EYFP fluorescence (white, arrowheads) images from the cells at 2 h (predominantly G2/M cells) and 6 h (predominantly G1 cells) after their release from HU treatment. Bars, 10 μm.
To further confirm this conclusion, lysates of the cells released from hydroxyurea treatment for 0, 2, 4, and 6 h were examined by Western blotting using the anti-GFP antibody for quantification of TbPLK-EYFP. The expression level of the protein was given a relative amount of 1 for the 0-h sample (Fig. 5B). It increased to 1.22 after 2 h but decreased to 0.76 and 0.59 after 4 and 6 h, respectively (Fig. 5B). The Western data thus agree well with those from the FACScan analysis.
Cells released from hydroxyurea treatment for different lengths of time were also examined with a fluorescence microscope for the presence of fluorescent spots in the dorsal midportions of the cells. The results from examining ∼120 cells in each sample indicated the presence of fluorescent dorsal spots among 45%, 52%, 15%, and 2% of the 0-h, 2-h, 4-h, and 6-h cell samples, respectively (Fig. 5B). These data once again agree with those from the FACScan and Western blot analyses. Two microscopic photos from the 2-h and 6-h samples are presented in Fig. 5C to demonstrate the difference in the numbers of TbPLK-EYFP-expressing cells. There is little doubt that TbPLK localizes along the FAZ and is expressed in S and G2/M phases but vanishes in the G1 phase of the trypanosome in both forms.
DISCUSSION
In the current investigation, we employed different experimental approaches to demonstrate the discrete presence of TbPLK at the midportion of the dorsal line in both procyclic and bloodstream forms of trypanosomes. The close association of TbPLK with the FAZ in the cytoskeleton preparations further suggests that the protein could be a part of the FAZ. A selective expression of TbPLK during only the S and G2/M phases of the trypanosome cell cycle was also clearly presented in the current study. All these unique features of TbPLK differ significantly from what is known about PLKs in other eukaryotes.
The PLK of S. cerevisiae, Cdc5, is known to localize to the nucleus, the spindle pole body, and the bud neck in accordance with the progression of the cell cycle from S to G2/M phase and eventually to cytokinesis (43, 45). Similarly, in mammalian cells, the Plk1 level is known to peak in late G2 and early M phase, accompanied by dramatic changes in subcellular localization from the cytoplasm and the nucleus during G2 phase to the centrosomes and kinetochores during early mitosis. A fraction of Plk1 then translocalizes to the spindle midzone/midbody late in mitosis, where the contractile cleavage furrow will eventually be fused (11). Thus, in the yeast, which has a closed mitosis like that of trypanosomes, and in mammalian cells, with an open mitosis, extensive translocalizations of the PLKs are apparently necessary during the cell cycle (3, 9, 25, 49), a fact which could be attributed to the multiple essential functions of PLK in regulating the progression of both mitosis and cytokinesis. The single localization of TbPLK to the dorsal spots of cells thus distinguishes it from all the other known PLKs, which could be due to the sole function of TbPLK in regulating only the cytokinesis in trypanosomes (16, 24). This limited function of TbPLK could be due to the fact that none of the Cdc5 substrates that appear to play a role in controlling the mitosis of yeast (33, 44) or the proteins involving PLKs in mitosis in metazoans (12, 20, 35, 36, 41, 46) can find a structural homologue in the T. brucei genome database (4).
An Aurora B homologue (TbAUK1) in the trypanosome plays important roles in controlling both mitosis and cytokinesis (27, 47). The chromosomal passenger complex (CPC) in the trypanosome, composed of TbAUK1 and two other novel proteins, TbCPC1 and TbCPC2 (26), has a typical pattern of CPC translocalization during mitosis but moves all the way to a specific midpoint on the dorsal line of the cell, where the anterior tip of the daughter cell is tethered to the FAZ, where cytokinesis is apparently initiated (22, 26, 38). It has not yet been experimentally demonstrated whether the position of CPC ever overlaps with that of TbPLK during the cell cycle. However, if the positioning of CPC is aimed at separating the two closely parallel FAZs, an interaction between CPC and TbPLK would be anticipated. Since TbPLK is expressed in the late S phase and located at the FAZ along the dorsal line of the cell, it is not unlikely that TbPLK may be expressed in this specific position at an earlier time to recruit the CPC, which moves to the mid-dorsal position late in telophase (26). An examination of the proteins complexed with TbPLK at the dorsal location would be thus a logical step in the next phase of this study.
Recently, a RNAi knockdown of FAZ1, a component of the FAZ structure, resulted in a compromised flagellar attachment and generation of 0N1K cells (zoids) and multinucleated cells, suggesting that destabilization of the FAZ structure causes defective cytokinesis (50). The tip of the daughter FAZ tethering to the existing FAZ could be the site where the initiation of cytokinesis occurs (22, 38). The TbPLK localization close to that tethering point suggests a role for TbPLK in initiating cytokinesis, as suggested by the outcome from its RNAi knockdown (16, 24). It is, however, unclear whether the presence or the timed absence of TbPLK from this location would trigger cytokinesis. In S. cerevisiae, the activation of a ubiquitin ligase known as the anaphase-promoting complex triggers the degradation of PLK Cdc5 (43) to repress the Cdc14 early anaphase release network and to initiate the mitotic exit network (51). Cdc5 is thus degraded prior to anaphase release. In T. brucei, cytokinesis starts when the basal body, kinetoplast, flagellum, and nucleus have all been duplicated and properly localized in a 2N2K cell (53). However, in the current study, TbPLK-EYFP was not found at the mid-dorsal point among any of the 2N2K cells of either form (data not shown), suggesting that TbPLK might have been degraded at that very time point to trigger cytokinesis. Western blot analysis of the lysates from synchronized cells also indicated a rather abrupt disappearance of TbPLK during the transition from G2/M to G1 phase (Fig. 5). Ironically, that is also the time when CPC was found to translocalize from the spindle midpoint to the dorsal midpoint (26). It is thus highly likely that the function of TbPLK is to recruit CPC to the initiating point of cytokinesis and then promptly disappear when cytokinesis is under way. The first visual sign of cytokinetic initiation in the procyclic form is a detachment of the tip of daughter flagellum from the mother flagellum (5). TbPLK RNAi was found to cause flagellum detachment from the cells (16, 24). Taken together, these data indicate that TbPLK at the anterior tip of the new FAZ could also assume the function of stabilizing the junction between the old FAZ and the new FAZ, which would be detached from each other upon the disappearance of TbPLK. TbPLK could thus be performing two crucial functions: recruiting the CPC to initiate cytokinesis and stabilizing the FAZ junction until then.
Acknowledgments
We thank Keith Gull of Oxford University for the L3B2 antibody he so kindly sent us and Arthur Gunzl of the University of Connecticut for his gift of plasmids pC-PTP-Neo and pRPA31-HA-BLA. We are also grateful to Ziyin Li for his assistance in preparing the manuscript and W. Zacheus Cande of UC Berkeley for his valuable discussion during the progression of this work. Gratitude also goes to Jack Taunton of UCSF for the use of his fluorescence microscope during this study.
The work was supported by NIH grant AI-21768 to C.C.W.
Footnotes
Published ahead of print on 11 July 2008.
REFERENCES
- 1.Abrieu, A., T. Brassac, S. Galas, D. Fisher, J. C. Labbe, and M. Doree. 1998. The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J. Cell Sci. 1111751-1757. [DOI] [PubMed] [Google Scholar]
- 2.Alexandru, G., F. Uhlmann, K. Mechtler, M. A. Poupart, and K. Nasmyth. 2001. Phosphorylation of the cohesin subunit Scc1 by Polo/Cdc5 kinase regulates sister chromatid separation in yeast. Cell 105459-472. [DOI] [PubMed] [Google Scholar]
- 3.Barr, F. A., H. H. Sillje, and E. A. Nigg. 2004. Polo-like kinases and the orchestration of cell division. Nat. Rev. Mol. Cell Biol. 5429-440. [DOI] [PubMed] [Google Scholar]
- 4.Berriman, M., E. Ghedin, C. Hertz-Fowler, G. Blandin, H. Renauld, D. C. Bartholomeu, N. J. Lennard, E. Caler, N. E. Hamlin, B. Haas, U. Bohme, L. Hannick, M. A. Aslett, J. Shallom, L. Marcello, L. Hou, B. Wickstead, U. C. Alsmark, C. Arrowsmith, R. J. Atkin, A. J. Barron, F. Bringaud, K. Brooks, M. Carrington, I. Cherevach, T. J. Chillingworth, C. Churcher, L. N. Clark, C. H. Corton, A. Cronin, R. M. Davies, J. Doggett, A. Djikeng, T. Feldblyum, M. C. Field, A. Fraser, I. Goodhead, Z. Hance, D. Harper, B. R. Harris, H. Hauser, J. Hostetler, A. Ivens, K. Jagels, D. Johnson, J. Johnson, K. Jones, A. X. Kerhornou, H. Koo, N. Larke, S. Landfear, C. Larkin, V. Leech, A. Line, A. Lord, A. Macleod, P. J. Mooney, S. Moule, D. M. Martin, G. W. Morgan, K. Mungall, H. Norbertczak, D. Ormond, G. Pai, C. S. Peacock, J. Peterson, M. A. Quail, E. Rabbinowitsch, M. A. Rajandream, C. Reitter, S. L. Salzberg, M. Sanders, S. Schobel, S. Sharp, M. Simmonds, A. J. Simpson, L. Tallon, C. M. Turner, A. Tait, A. R. Tivey, S. Van Aken, D. Walker, D. Wanless, S. Wang, B. White, O. White, S. Whitehead, J. Woodward, J. Wortman, M. D. Adams, T. M. Embley, K. Gull, E. Ullu, J. D. Barry, A. H. Fairlamb, F. Opperdoes, B. G. Barrell, J. E. Donelson, N. Hall, C. M. Fraser, S. E. Melville, and N. M. El-Sayed. 2005. The genome of the African trypanosome Trypanosoma brucei. Science 309416-422. [DOI] [PubMed] [Google Scholar]
- 5.Briggs, L. J., P. G. McKean, A. Baines, F. Moreira-Leite, J. Davidge, S. Vaughan, and K. Gull. 2004. The flagella connector of Trypanosoma brucei: an unusual mobile transmembrane junction. J. Cell Sci. 1171641-1651. [DOI] [PubMed] [Google Scholar]
- 6.Chowdhury, A. R., Z. Zhao, and P. T. Englund. 2008. Effect of hydroxyurea on procyclic Trypanosoma brucei: an unconventional mechanism for achieving synchronous growth. Eukaryot. Cell 7425-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai, W. 2005. Polo-like kinases, an introduction. Oncogene 24214-216. [DOI] [PubMed] [Google Scholar]
- 8.de Graffenried, C. L., H. H. Ho, and G. Warren. 2008. Polo-like kinase is required for Golgi and bilobe biogenesis in Trypanosoma brucei. J. Cell Biol. 181431-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glover, D. M. 2005. Polo kinase and progression through M phase in Drosophila: a perspective from the spindle poles. Oncogene 24230-237. [DOI] [PubMed] [Google Scholar]
- 10.Glover, D. M., I. M. Hagan, and A. A. Tavares. 1998. Polo-like kinases: a team that plays throughout mitosis. Genes Dev. 123777-3787. [DOI] [PubMed] [Google Scholar]
- 11.Golsteyn, R. M., K. E. Mundt, A. M. Fry, and E. A. Nigg. 1995. Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J. Cell Biol. 1291617-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goto, H., T. Kiyono, Y. Tomono, A. Kawajiri, T. Urano, K. Furukawa, E. A. Nigg, and M. Inagaki. 2006. Complex formation of Plk1 and INCENP required for metaphase-anaphase transition. Nat. Cell Biol. 8180-187. [DOI] [PubMed] [Google Scholar]
- 13.Graham, T. M., A. Tait, and G. Hide. 1998. Characterisation of a polo-like protein kinase gene homologue from an evolutionary divergent eukaryote, Trypanosoma brucei. Gene 20771-77. [DOI] [PubMed] [Google Scholar]
- 14.Gruneberg, U., and E. A. Nigg. 2003. Regulation of cell division: stop the SIN! Trends Cell Biol. 13159-162. [DOI] [PubMed] [Google Scholar]
- 15.Hammarton, T. C., J. Clark, F. Douglas, M. Boshart, and J. C. Mottram. 2003. Stage-specific differences in cell cycle control in Trypanosoma brucei revealed by RNA interference of a mitotic cyclin. J. Biol. Chem. 27822877-22886. [DOI] [PubMed] [Google Scholar]
- 16.Hammarton, T. C., S. Kramer, L. Tetley, M. Boshart, and J. C. Mottram. 2007. Trypanosoma brucei Polo-like kinase is essential for basal body duplication, kDNA segregation and cytokinesis. Mol. Microbiol. 651229-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu, F., Y. Wang, D. Liu, Y. Li, J. Qin, and S. J. Elledge. 2001. Regulation of the Bub2/Bfa1 GAP complex by Cdc5 and cell cycle checkpoints. Cell 107655-665. [DOI] [PubMed] [Google Scholar]
- 18.Hudson, J. W., A. Kozarova, P. Cheung, J. C. Macmillan, C. J. Swallow, J. C. Cross, and J. W. Dennis. 2001. Late mitotic failure in mice lacking Sak, a polo-like kinase. Curr. Biol. 11441-446. [DOI] [PubMed] [Google Scholar]
- 19.Jensen, S., M. Segal, D. J. Clarke, and S. I. Reed. 2001. A novel role of the budding yeast separin Esp1 in anaphase spindle elongation: evidence that proper spindle association of Esp1 is regulated by Pds1. J. Cell Biol. 15227-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson, V. L., M. I. Scott, S. V. Holt, D. Hussein, and S. S. Taylor. 2004. Bub1 is required for kinetochore localization of BubR1, Cenp-E, Cenp-F and Mad2, and chromosome congression. J. Cell Sci. 1171577-1589. [DOI] [PubMed] [Google Scholar]
- 21.Kitada, K., A. L. Johnson, L. H. Johnston, and A. Sugino. 1993. A multicopy suppressor gene of the Saccharomyces cerevisiae G1 cell cycle mutant gene dbf4 encodes a protein kinase and is identified as CDC5. Mol. Cell. Biol. 134445-4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohl, L., D. Robinson, and P. Bastin. 2003. Novel roles for the flagellum in cell morphogenesis and cytokinesis of trypanosomes. EMBO J. 225336-5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohl, L., T. Sherwin, and K. Gull. 1999. Assembly of the paraflagellar rod and the flagellum attachment zone complex during the Trypanosoma brucei cell cycle. J. Eukaryot. Microbiol. 46105-109. [DOI] [PubMed] [Google Scholar]
- 24.Kumar, P., and C. C. Wang. 2006. Dissociation of cytokinesis initiation from mitotic control in a eukaryote. Eukaryot. Cell 592-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee, K. S., J. E. Park, S. Asano, and C. J. Park. 2005. Yeast polo-like kinases: functionally conserved multitask mitotic regulators. Oncogene 24217-229. [DOI] [PubMed] [Google Scholar]
- 26.Li, Z., J. H. Lee, F. Chu, A. L. Burlingame, A. Gunzl, and C. C. Wang. 2008. Identification of a novel chromosomal passenger complex and its unique localization during cytokinesis in Trypanosoma brucei. PLoS ONE 3e2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, Z., and C. C. Wang. 2006. Changing roles of aurora-B kinase in two life cycle stages of Trypanosoma brucei. Eukaryot. Cell 51026-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthews, K. R., J. R. Ellis, and A. Paterou. 2004. Molecular regulation of the life cycle of African trypanosomes. Trends Parasitol. 2040-47. [DOI] [PubMed] [Google Scholar]
- 29.McKean, P. G. 2003. Coordination of cell cycle and cytokinesis in Trypanosoma brucei. Curr. Opin. Microbiol. 6600-607. [DOI] [PubMed] [Google Scholar]
- 30.Mutomba, M. C., W. Y. To, W. C. Hyun, and C. C. Wang. 1997. Inhibition of proteasome activity blocks cell cycle progression at specific phase boundaries in African trypanosomes. Mol. Biochem. Parasitol. 90491-504. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen, T. N., B. Schimanski, and A. Gunzl. 2007. Active RNA polymerase I of Trypanosoma brucei harbors a novel subunit essential for transcription. Mol. Cell. Biol. 276254-6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohkura, H., I. M. Hagan, and D. M. Glover. 1995. The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes Dev. 91059-1073. [DOI] [PubMed] [Google Scholar]
- 33.Park, C. J., J. E. Park, T. S. Karpova, N. K. Soung, L. R. Yu, S. Song, K. H. Lee, X. Xia, E. Kang, I. Dabanoglu, D. Y. Oh, J. Y. Zhang, Y. H. Kang, S. Wincovitch, T. C. Huffaker, T. D. Veenstra, J. G. McNally, and K. S. Lee. 2008. Requirement for the budding yeast polo kinase Cdc5 in proper microtubule growth and dynamics. Eukaryot. Cell 7444-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ploubidou, A., D. R. Robinson, R. C. Docherty, E. O. Ogbadoyi, and K. Gull. 1999. Evidence for novel cell cycle checkpoints in trypanosomes: kinetoplast segregation and cytokinesis in the absence of mitosis. J. Cell Sci. 1124641-4650. [DOI] [PubMed] [Google Scholar]
- 35.Pouwels, J., A. M. Kukkonen, W. Lan, J. R. Daum, G. J. Gorbsky, T. Stukenberg, and M. J. Kallio. 2007. Shugoshin 1 plays a central role in kinetochore assembly and is required for kinetochore targeting of Plk1. Cell Cycle 61579-1585. [DOI] [PubMed] [Google Scholar]
- 36.Qi, W., Z. Tang, and H. Yu. 2006. Phosphorylation- and polo-box-dependent binding of Plk1 to Bub1 is required for the kinetochore localization of Plk1. Mol. Biol. Cell 173705-3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson, D. R., and K. Gull. 1991. Basal body movements as a mechanism for mitochondrial genome segregation in the trypanosome cell cycle. Nature 352731-733. [DOI] [PubMed] [Google Scholar]
- 38.Robinson, D. R., T. Sherwin, A. Ploubidou, E. H. Byard, and K. Gull. 1995. Microtubule polarity and dynamics in the control of organelle positioning, segregation, and cytokinesis in the trypanosome cell cycle. J. Cell Biol. 1281163-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schimanski, B., T. N. Nguyen, and A. Gunzl. 2005. Highly efficient tandem affinity purification of trypanosome protein complexes based on a novel epitope combination. Eukaryot. Cell 41942-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seong, Y. S., K. Kamijo, J. S. Lee, E. Fernandez, R. Kuriyama, T. Miki, and K. S. Lee. 2002. A spindle checkpoint arrest and a cytokinesis failure by the dominant-negative polo-box domain of Plk1 in U-2 OS cells. J. Biol. Chem. 27732282-32293. [DOI] [PubMed] [Google Scholar]
- 41.Sharp-Baker, H., and R. H. Chen. 2001. Spindle checkpoint protein Bub1 is required for kinetochore localization of Mad1, Mad2, Bub3, and CENP-E, independently of its kinase activity. J. Cell Biol. 1531239-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sherwin, T., and K. Gull. 1989. The cell division cycle of Trypanosoma brucei brucei: timing of event markers and cytoskeletal modulations. Philos. Trans. R. Soc. Lond. B 323573-588. [DOI] [PubMed] [Google Scholar]
- 43.Shirayama, M., W. Zachariae, R. Ciosk, and K. Nasmyth. 1998. The Polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J. 171336-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snead, J. L., M. Sullivan, D. M. Lowery, M. S. Cohen, C. Zhang, D. H. Randle, J. Taunton, M. B. Yaffe, D. O. Morgan, and K. M. Shokat. 2007. A coupled chemical-genetic and bioinformatic approach to Polo-like kinase pathway exploration. Chem. Biol. 141261-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song, S., T. Z. Grenfell, S. Garfield, R. L. Erikson, and K. S. Lee. 2000. Essential function of the polo box of Cdc5 in subcellular localization and induction of cytokinetic structures. Mol. Cell. Biol. 20286-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor, S. S., and F. McKeon. 1997. Kinetochore localization of murine Bub1 is required for normal mitotic timing and checkpoint response to spindle damage. Cell 89727-735. [DOI] [PubMed] [Google Scholar]
- 47.Tu, X., P. Kumar, Z. Li, and C. C. Wang. 2006. An aurora kinase homologue is involved in regulating both mitosis and cytokinesis in Trypanosoma brucei. J. Biol. Chem. 2819677-9687. [DOI] [PubMed] [Google Scholar]
- 48.Tu, X., and C. C. Wang. 2004. The involvement of two cdc2-related kinases (CRKs) in Trypanosoma brucei cell cycle regulation and the distinctive stage-specific phenotypes caused by CRK3 depletion. J. Biol. Chem. 27920519-20528. [DOI] [PubMed] [Google Scholar]
- 49.van de Weerdt, B. C., and R. H. Medema. 2006. Polo-like kinases: a team in control of the division. Cell Cycle 5853-864. [DOI] [PubMed] [Google Scholar]
- 50.Vaughan, S., L. Kohl, I. Ngai, R. J. Wheeler, and K. Gull. 2008. A repetitive protein essential for the flagellum attachment zone filament structure and function in Trypanosoma brucei. Protist 159127-136. [DOI] [PubMed] [Google Scholar]
- 51.Visintin, C., B. N. Tomson, R. Rahal, J. Paulson, M. Cohen, J. Taunton, A. Amon, and R. Visintin. 2008. APC/C-Cdh1-mediated degradation of the Polo kinase Cdc5 promotes the return of Cdc14 into the nucleolus. Genes Dev. 2279-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wirtz, E., S. Leal, C. Ochatt, and G. A. Cross. 1999. A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol. Biochem. Parasitol. 9989-101. [DOI] [PubMed] [Google Scholar]
- 53.Woodward, R., and K. Gull. 1990. Timing of nuclear and kinetoplast DNA replication and early morphological events in the cell cycle of Trypanosoma brucei. J. Cell Sci. 9549-57. [DOI] [PubMed] [Google Scholar]