Abstract
Human females are more sensitive than males to brief nociceptive stimuli such as heat and cold. However, a more pronounced peripheral vasoconstriction by females than by males during prolonged nociceptive stimulation predicts that females would be more sensitive to prolonged cold but not heat stimulation. We tested this possibility with reflex (lick/guard) and operant escape and preference tests of sensitivity to prolonged stimulation of Long-Evans and Sprague-Dawley rats.
Escape responses to cold stimulation revealed a greater sensitivity of females. In contrast, males were more sensitive to nociceptive heat stimulation. An operant preference test of relative sensitivity to cold or heat stimulation confirmed these results. Cold was more aversive than heat for females, but heat was more aversive than cold for males. Recordings of skin temperature during nociceptive heat stimulation were consistent with the results of operant testing. A reduction in skin temperature (peripheral vasoconstriction) during nociceptive stimulation should increase cold sensitivity as observed for females relative to males.
Lick/guard testing did not confirm the results of operant testing. Lick/guard (L/G) responding to nociceptive heat stimulation was greater for females than for males. Female escape responses to heat were more variable than males, but L/G responding of males to the same stimulus was more variable than for females.
Perspective
A variety of chronic pain conditions are more prevalent for females, and psychological stress (with attendant sympathetic activation) is implicated in development and maintenance of these conditions. Therefore, understanding relationships between gender differences in pain sensitivity and sympathetic activation could shed light on mechanisms for some varieties of chronic pain.
Keywords: Sex differences, sympathetic activation, operant escape, thermal sensitivity, pain sensitivity
Introduction
Females are more vulnerable than males to development of numerous chronic pain conditions – e.g., fibromyalgia, temporomandibular joint pain, back pain, irritable bowel syndrome, headache and arthritis4;39. Such differences in susceptibility have generated considerable interest in the relative nociceptive sensitivity of males and females. Presumably, the idea has been that individuals with high pain sensitivity when healthy are most likely to express chronic pain in response to pathology. Overall, studies of elicited pain in normal human subjects have reported a greater sensitivity of females – usually documented as lower thresholds and less pain tolerance39. However, differences in psychological state of human males and females, some of which are inherent to experimental testing situations, have contributed to variable results of human studies of pain sensitivity13;16;22;24;26;31;40. Also, different methods of experimental stimulation have produced inconsistencies between studies in the relative pain sensitivity of males and females39. Given these sources of variability, it has been difficult to identify physiological characteristics of each sex which could account for a greater pain sensitivity of human females across all psychological states and stimulus conditions.
Numerous investigations of humans have revealed sex differences in autonomic tone and reactivity. At rest, measures of heart rate variability indicate that the sympathetic nervous system is dominant for tonic central regulation of blood pressure and heart rate of males but not females6;9;14;20;21;30;42;52. However, cutaneous blood flow and skin temperature are lower for females at rest5;12;15;25;32;34, suggesting greater tonic sympathetic activation of females’ peripheral vasculature. Differences in autonomic expression are evidenced also during experimental conditions that phasically activate the sympathetic nervous system (SNS). This is important, because the SNS is organized to deal with threatening external events. In response to stressful experiences or sensations such as pain, heart rate and blood pressure are increased for males more than females9;16–18;21;41;44. In contrast, particularly in warm environmental conditions that attenuate tonic sympathetic tone, phasic cutaneous vasoconstriction is observed for females more than males3;8;9;12;18;32;33.
The patterns of autonomic tone and responsivity for males and females indicate that uniform sex differences in thermal pain sensitivity across all stimulus conditions might not be observed. Rather, sex differences could depend on stimulus temperature. At neutral environmental temperatures, baseline skin temperature of females is typically is low, due to cutaneous vasoconstriction, which can be exacerbated by stressful (e.g., painful) situations. A prediction from these effects on cutaneous vasculature and skin temperature is that females would be especially sensitive to cold1 and less sensitive to heat. Also, because of estrus influences on autonomic reactivity and pain sensitivity3;7;10;19;21;23;28;38;41;43, female responses to nociceptive stimulation should be more variable. However, sex differences have not been revealed by reflex tests that are commonly used for evaluation of the nociceptive sensitivity of rodents35,36. Reflex responses are subserved by spinal and brainstem levels of the neuraxis and cannot assess potential sex differences in cerebral processing of pain27,48–52. Therefore, the present study evaluated nociceptive sensitivity with operant escape tests and compared the results with reflex tests of female and male rats, using nociceptive heat and cold stimulation. Psychophysical testing of lab animals bypasses the factor of psychosocial influences on pain sensitivity.
Materials and Methods
Male and female rats of two strains (Long-Evens hooded and Sprague-Dawley hairless) were maintained in groups of 3 or 4 in large enclosures (32 in high, 18 in wide, 24 in deep) with enrichments. Hairless rats are well adapted to facial testing, which requires depilitation to ensure optimal thermal stimulation. In order to determine the generality of the sex differences with stimulation of facial and glabrous skin, and to compare hairless and furred strains with possible differences in thermoregulation, the sensitivity of the paws to thermal stimulation was evaluated for both hairless and hooded rats. Food and water were provided ad libitum to the hooded rats in the enclosures. Hairless rats were food deprived for 13–15 hours before facial testing sessions but had ad libitum access at all other times; Ad libitum food was provided on days not involving facial testing. Weights were recorded weekly to monitor general health. All experimental procedures were approved by the University of Florida Institutional Animal Care and Utilization committee and conformed to National Institutes of Health guidelines for care and use of experimental animals.
Behavioral testing: Thermal stimulation of plantar skin
Escape testing
The apparatus for escape from thermal stimulation of the paws consisted of a dark (0.5 foot candles) area (6 in. wide, 8 in. long) with a thermally regulated floor (plate compartment) and a brightly lit (3200 foot candles) area (6 in wide, 6 in long) with a thermally neutral escape platform (escape compartment). The animals could pass freely from compartment to compartment, choosing between thermal stimulation in the dark platform area and bright light over the escape platform. Occupancy of the escape platform was detected by microswitches and was timed by proprietary software. The apparatus was ventilated with room air to minimize differences in ambient temperature between compartments.
After a period of acclimation to the testing apparatus, 23 male and 20 female Long-Evans (hooded) rats and 6 male and 6 female hairless Sprague-Dawley rats were trained to escape from nociceptive thermal stimulation according to methods previously reported49–51. Training progressed gradually with exposure to nociceptive stimulation. First, the animals were adapted to the testing apparatus with the plate at neutral temperatures and the light off in the escape compartment. Then, on alternate days without light in the escape compartment, heat or cold stimulation was changed from 36°C to 44°C in 2°C increments or from 36°C to 20°C, 15°C, 10°C and then 5°C. The same sequence of temperatures was then presented over days with the light on in the escape compartment. Three weeks of testing on a fixed schedule (see below) were conducted to stabilize performance before data collection.
Lick/guard testing
Reflexive lick/guard (L/G) responding of the hooded rats was evaluated in a ventilated 6 in. by 8 in. enclosure with a heated floor and no escape option. Training of L/G reflex responding is not required, but the duration of responding can change over time with repeated testing. Therefore, L/G testing was conducted for two weeks before data collection began. Durations of licking and guarding were entered into a spreadsheet by a technician-observer, using proprietary software. Licking of either hindpaw is a stereotyped response involving extension of one leg, holding the hindpaw with the forepaws and fanning the toes. Guarding was scored when one hindleg was flexed and elevated in a posture distinct from normal ambulation.
Pretest and test trials
Trial times for escape testing of hooded rats were 10 minutes and for escape testing of hairless rats were 15 minutes. Lick/guard trial times were 10 minutes. Daily test sessions consisted of two trials in adjacent test enclosures. The purposes of the first daily trial (pretest) were to acclimate the animals to the testing environment and compare effects of different histories of thermal stimulation on behavior in the second trial. For escape and L/G testing of hooded rats, the plate temperatures in the two apparatuses were as follows for the sequential daily trials: 36°C then 44.5°C or 0.3°C then 44.5°C. The 36°C pretest is neutral (non-stressfull and non-nociciceptive) and stabilizes paw temperatures, and the 0.3°C is stressful and nociceptive. Escape testing of hairless rats presented sequential trials of 10°C and then 44.5°C or 44.5°C and then 10°C, with measurement of behavior in the second trial. These pretests simulated the alternation of cold and hot temperatures experienced in thermal preference tests.
Thermal preference testing
Preferences for hot or cold stimulation of the paws were evaluated in an enclosure with two 6 in. by 8 in. compartments. The floor of one compartment was cold and the other was hot. In preliminary training sessions, temperatures were varied to produce equal occupancy of the hot and cold sides for either males or females. For hooded females, the temperatures used for data collection were 10°C and 45°C. The hooded males’ aversion for heat was such that a range of 10°C and 43°C was utilized. Hairless males and females were tested with temperatures of 10°C and 45°C. After selection of the testing temperatures, two weeks of testing preceded data collection for this study. With unrestricted access to the compartments through a door in a dividing wall, the animals alternately heated and cooled their paws. The time spent in each compartment was recorded by proprietary software that detected light beam interruptions on each side of the dividing wall. Eight male and 17 female hooded rats and 6 male and 6 female hairless rats were tested for thermal preference. The animals were placed in an enclosure for 15 min. pretest trials with a floor temperature of 36°C, to acclimate the animals to the testing environment and establish desired paw temperatures. They then were placed on the cold side of the thermal preference enclosure to begin a 15 min. test trial.
Behavioral testing: Thermal stimulation of the face
For operant testing of facial sensitivity, 6 male and 6 female hairless rats were trained to drink sweetened condensed milk while making facial contact with a 37°C thermode37. The skin that contacted the thermode during drinking was depilitated under light isofluorane anesthesia (2.5%) once a week to maximize thermal transfer. The timing of each contact with the lick tube was detected by an electronic circuit and recorded with DATAQ hardware and software. Training sessions continued until liquid intake during a test trial was 10 g or greater. The thermode temperature was set to 45°C, 48°C or 52°C in separate testing sessions, in order to evaluate heat sensitivity over a range of suprathreshold nociceptive stimulus intensities. Trials of facial sensitivity were continued until the animals became satiated and ceased drinking, but the data utilized for analysis were generated before the rate of drinking declined because of satiation. Events recorded during testing were transformed into numerical data using custom written subroutines for Lab View (Texas Instruments, v. 7.1). Consistent with the operant test of sensitivity to paw stimulation, escape duration was measured as pauses in licking. Licking was a secure indication that an animal’s face was in contact with the thermode, and licking interference was the most reliable indicant of escape.
Recording of skin temperature responses to thermal stimulation
Recordings of skin and body temperature were obtained from 10 male and 11 female hooded rats and from 6 male and 6 female hairless rats. Each animal received a subcutaneous injection of diazepam (10 mg/kg) and then was placed into an induction box where 3% isoflurane was delivered. After induction of anesthesia, animals were placed on a 37°C thermal blanket and covered. Anesthesia was maintained with 1.5% isoflurane via a nose cone. Rectal core temperature was monitored continuously. A thermocouple was placed on the plantar skin of the left forepaw for recording of skin temperature. The thermocouple tip was contained within 20 mm2 wells of thermoconductive paste in adhesive foam pads. Skin temperature and rectal core temperature were observed until stable (usually 15 minutes). Then a 2.5 cm by 2.5 cm square thermode, preheated with circulating water, was strapped in contact with the plantar surface of the left hindpaw. Skin and core temperatures were recorded during 10 min of 44.5°C stimulation and for an additional 10 min after removal of the thermode from the left hindpaw. All temperature recordings were obtained and stored using a precision thermocouple system (GEC Instruments, Gainesville, FL). After anesthesia was discontinued, animals were observed until alert and fully recovered and then were returned to their communal enclosures.
Data acquisition and statistical analysis
For analysis of behavioral performance during stimulation of the paws, the duration of responding to thermal stimulation was recorded. The overall duration of escape platform occupancy and the total duration of lick/guard responding to 44.5°C were utilized to compare differences between male and female hooded rats for the operant and reflex tests. The cumulative duration of escape platform occupancy was plotted to reveal sex differences in operant responding of hairless rats to 10°C and 44.5°C. Thermal preference of hooded and hairless rats was quantified by subtracting successive durations of occupancy on the cold plate from successive durations on the hot plate during a trial. Accordingly, a cold aversion (heat preference) was evident as a progressive accumulation of positive durations and a heat aversion (cold preference) by accumulation of negative values, response by response.
The frequency of licking is extremely regular and stereotyped for rats, permitting detection of pauses (escape) by setting a criterion for inter-lick intervals. In the present study, the average inter-lick interval was 0.19 sec for females (0.17 sec standard deviation) and 0.17 sec for males (0.19 sec standard deviation), calculated over thousands of lick intervals less than 5 sec. Based on these observations, the criterion for a pause in licking was conservatively set at 1 sec.
Skin and core temperature responses to nociceptive heat stimulation were expressed relative to the baseline temperature recorded immediately before contact of the thermode with the left hindpaw. Skin and core temperatures were sampled at 0.1 sec intervals and averaged over 2.5 min. periods within 20 min. recording sessions.
Statistical tests utilized Statistica software (StatSoft, Inc., Tulsa OK). T-tests compared overall escape or L/G durations for male vs. female hooded rats (Figure 1), coefficients of variability for escape of hooded rats and resting core and skin temperatures (Table 1). Repeated measures analysis of variance (ANOVA) was utilized for all other statistical tests. A probability level of 0.05 was applied throughout for significance.
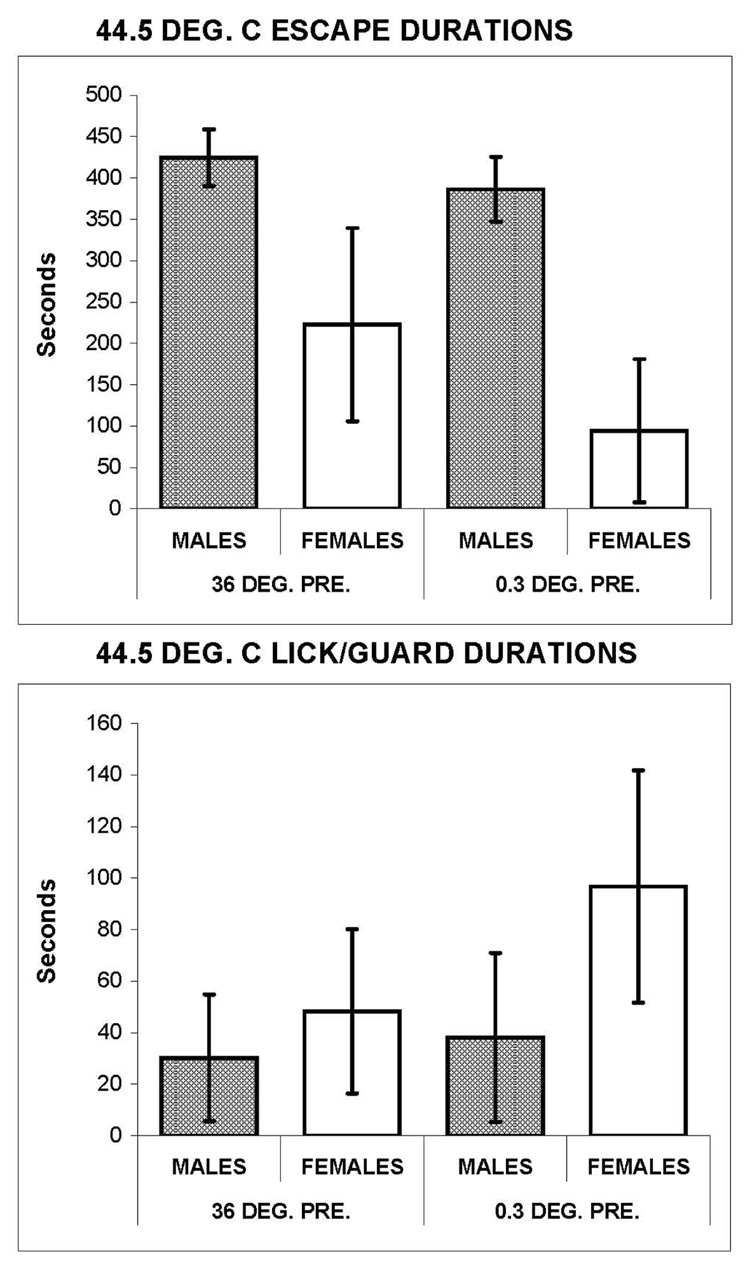
Figure 1.
Total operant escape (top panel) and lick-guard (bottom panel) durations during trials with 44.5°C stimulation of the paws of 23 male and 20 female hooded rats. Each testing session consisted of a 10 min. pretest exposure to 36°C or 0.3°C prior to a 10 min. test trial of 44.5°C stimulation. Escape durations on test trials were significantly lower for females following both pretest conditions. Female L/G durations were significantly higher following both pretest conditions.
Table 1. Baseline core and skin temperatures (Degrees C).
Baseline core and skin temperatures (degrees C) recorded immediately before application of thermal stimulation to the left hindpaw of hooded and hairless rats. Skin temperatures were recorded from the left forepaw (LFP).
| Hooded | Hairless | |||
|---|---|---|---|---|
| Females | Males | Females | Males | |
| Core | 37.4 (0.2) | 37.8 (1.0) | 37.0 (0.2) | 36.8 (0.4) |
| LFP | 35.3 (0.7) | 36.7 (0.9) | 34.8 (0.7) | 34.6 (0.5) |
Means (Standard Deviations)
Results
Figure 1 shows that male and female hooded rats differed in their sensitivity to a low level of nociceptive heat stimulation. The total duration of escape responses within 44.5°C test trials is shown for males and females. The duration of operant escape from 44.5°C was significantly greater for males (top panel) when preceded by a pretest trial of 36°C (t=7.91, df=41, p<0.001) and especially when preceded by 0.3°C (t=14.56, df=41, p<0.001).
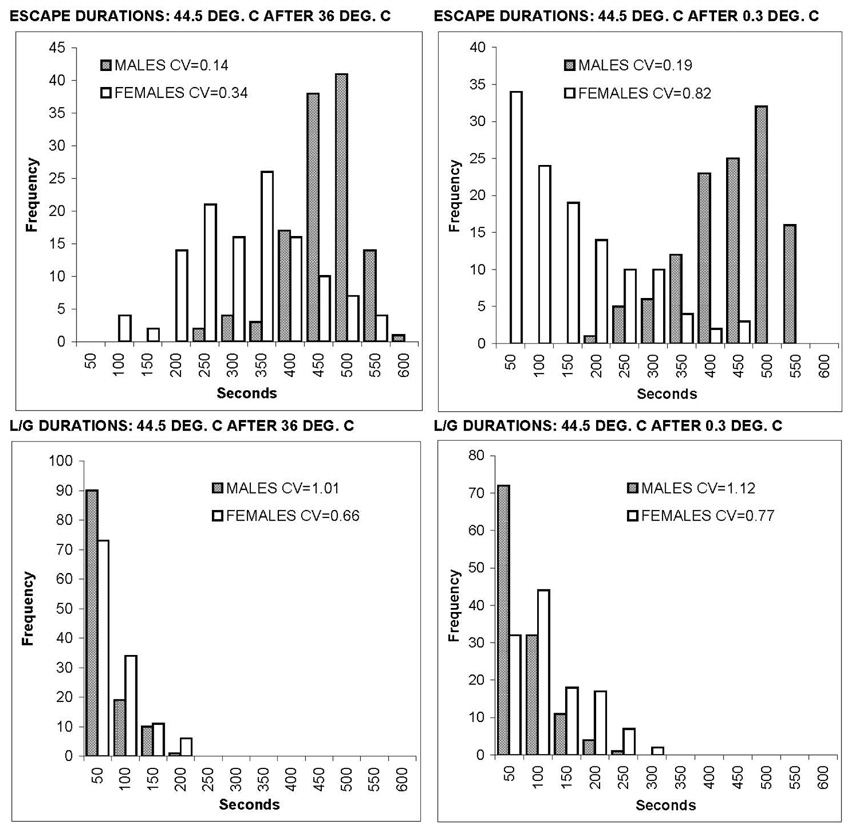
The variability in operant responses by males and females to 44.5°C stimulation is depicted in Figure 2 (top panels) with frequency histograms and associated coefficients of variability (CV = standard deviation / mean). The histograms show the frequency of total escape durations that fell within 50 sec bins of all the 600 sec test trials presented to males and females. The statistical significance of sex differences in variability of operant responding was evaluated by t-tests for independent groups, comparing CVs of individual animals across test trials. Coefficients of variability for escape responding to 44.5°C were significantly greater for females than for males when preceded by a pretest of 36°C (t=7.91, df=41, p<0.001) and especially when preceded by 0.3°C (t=14.56, df=41, p<0.001).
Figure 2.
Frequency histograms of total escape durations (upper panels) and L/G durations (lower panels) of 23 male and 20 female hooded rats during 44.5°C stimulation of the paws in test trials. Coefficients of variability (CVs) for each distribution are listed with the legends. The coefficients of variability for escape duration were higher for females, especially after a pretest trial of 0.3°C stimulation, which skewed the distribution of female responses and shifted the peak to the left. The variability of male L/G durations exceeded that of females. Male escape and L/G durations were uninfluenced by the pretest temperature.
The variability of female escape durations was significantly increased by the 0.3°C pretest (t-test for dependent means; t=2.94, df=19, p=0.008). The frequency histograms of response durations shown in Figure 2 reveal a substantial transformation from a normal distribution of female escape durations after a 36°C pretest, to a highly skewed distribution after a 0.3°C pretest. The peak of female response durations shifted from the 300–350 sec. bin for test trials after 36°C to the 0–50 msec bin after 0.3°C. Thus, the altered distribution of female escape durations after cold preconditioning was associated with reduced heat pain sensitivity. The mean escape duration for 44.5°C was significantly less after the 0.3°C pretest, compared to the 36°C pretest (t=3.94, df=38, p<0.001; Figure 1, top panel). An effect of cold preconditioning on escape responding to 44.5°C was not observed for males.
Reflex responding to 44.5°C (Figure 1, bottom panel) was greater for females than for males when preceded by a 36°C trial (t=2.09, df=41, p=0.04) or a 0.3°C trial (t=4.92, df=41, p<0.001), in contrast to escape durations, which were higher for males (Figure 1, top panel). Also, cold preconditioning of females significantly increased L/G responding to 44.5°C, compared to the 36°C pretest (t=3.92, df=19, p<0.001), in contrast to escape from 44.5°C which was decreased by the 0.3°C pretest. Thus, sex differences in reflex responding to 44.5°C were opposite to greater escape responding by males, and a sensitizing effect of cold preconditioning on L/G responding of females was opposite to a desensitizing effect on escape.
Substantial differences in escape and L/G responding are revealed by the frequency histograms in Figure 2. Because L/G testing did not offer an escape option, considerably more nociceptive stimulation was received during L/G trials than during escape trials. Despite this difference, the total duration of L/G responding to 44.5°C rarely exceeded 150 sec. In contrast, total escape duration usually exceeded 150 sec (ranging from 100 to 550 sec for females and 250 to 550 sec for males) when preceded by 36°C. The L/G reflexes removed a hindpaw from the thermally regulated plate, but hindlimb flexion was not used by the animals to escape 44.5°C stimulation during most of the 44.5°C test trials.
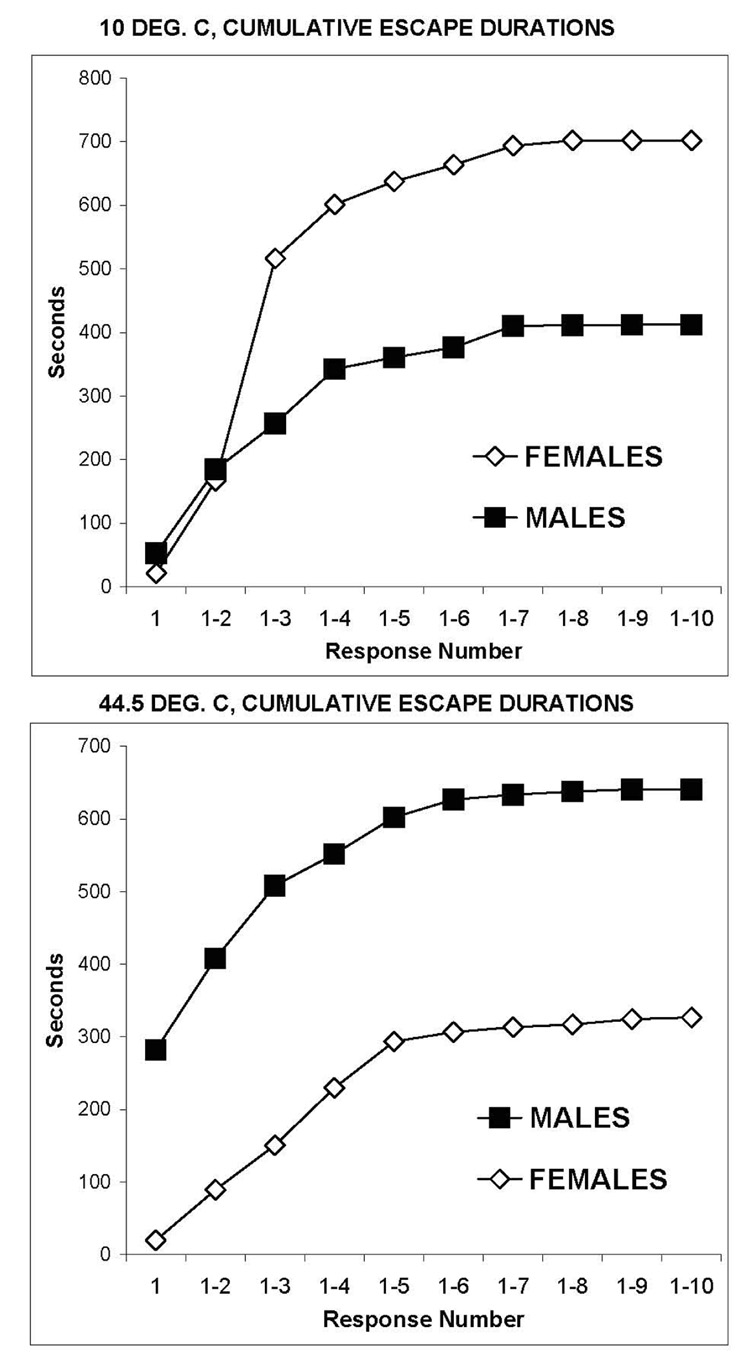
Figure 3 presents the accumulated duration of escape, response by response, during test trials of hairless rats trained to escape from 10°C after a pretest trial at 44.5°C and from 44.5°C after a pretest trial at 10°C. The accumulated escape duration plateaued when the maximum number of responses for the group was reached. Consistent with tests of hooded rats, escape durations of male rats were significantly greater than females when tested at 44.5°C (F=36.41, df between=1, df within= 9, p<0.001). In contrast, the escape durations of female rats were significantly higher than males when tested at 10°C (interaction: F=7.96, between df=1, within df=9, p<0.001).
Figure 3.
Escape durations of 6 male and 6 female hairless rats during 15 min. test trials at 10°C after a 15 min. pretest trial at 44.5°C (upper panel) and during 44.5°C trials after a pretest at 10°C (lower panel). The cumulative durations of escape, response by response, show a progressively greater sensitivity of females to 10°C, but males were more sensitive to 44.5°C throughout the trial.
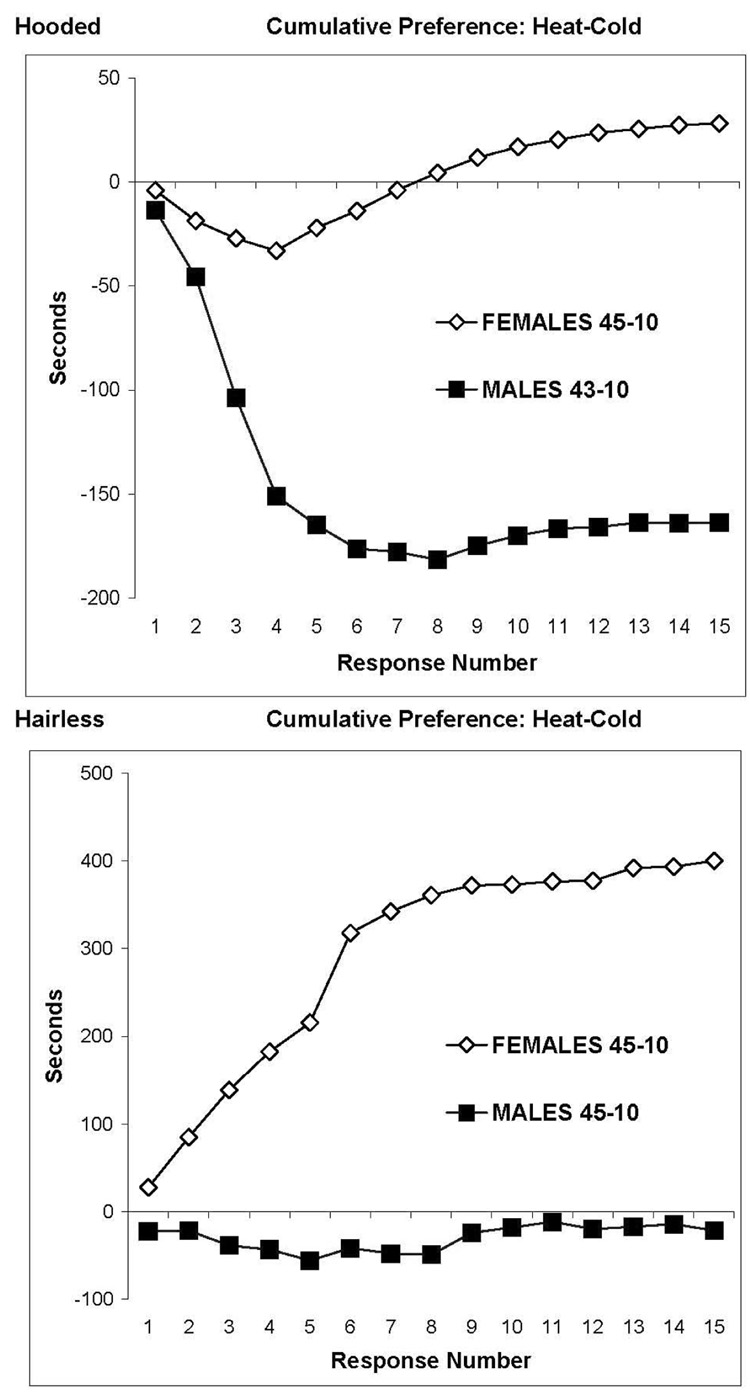
Given that escape testing revealed a greater sensitivity of males to heat but a greater sensitivity of females to cold, it follows that a similar sex difference should be observed for thermal preference testing. Figure 4 shows this to be true for both hooded rats (top panel) and hairless rats (bottom panel). Thermal preference is shown, response by response, as difference scores (heat – cold duration for the first occupancy within each side of the test chamber, then heat - cold duration for the second occupancy, etc.). Thermal preferences differed between the two strains, but for each strain females spent more time on the heated side than males, and males spent more time on the cold side than females (the heat – cold values were higher for females than males). Hairless females exhibited a strong cold aversion (positive values, bottom panel) when tested with 10°C vs. 45°C, but the hairless males did not exhibit a cold aversion for the same temperatures (interaction: F=6.23, between df=1, within df=14, p<0.001). The hooded males were tested at 10°C vs. 43°C because of a strong heat aversion (negative values) they demonstrated on the task. Female hooded rats did not demonstrate the heat aversion, even when paired with a higher level of heat (45°C) than the males (43°C). The difference in preference for hooded males and females was statistically significant (interaction: F=5.77, between df=1, within df=14, p<0.001). Thus, the same relative aversions were observed for hairless and hooded rats (heat aversion for males and cold aversion for females).
Figure 4.
Thermal preferences for stimulation of the paws of 8 male and 17 female hooded rats (upper panel) and 6 male and 6 female hairless rats (lower panel). Each plot was constructed by subtracting the first time of occupancy on the cold plate from the first duration on the hot plate and so forth, response by response, up to 15. The hooded females spent nearly equal time on the hot (45°C) and cold (10°C) plates, but the males strongly preferred the cold side (relative heat aversion), even though the hot plate temperature was set 2°C lower than for females. The hairless males spent approximately equal time on the hot and cold plates, but the females strongly preferred heat (relative cold aversion).
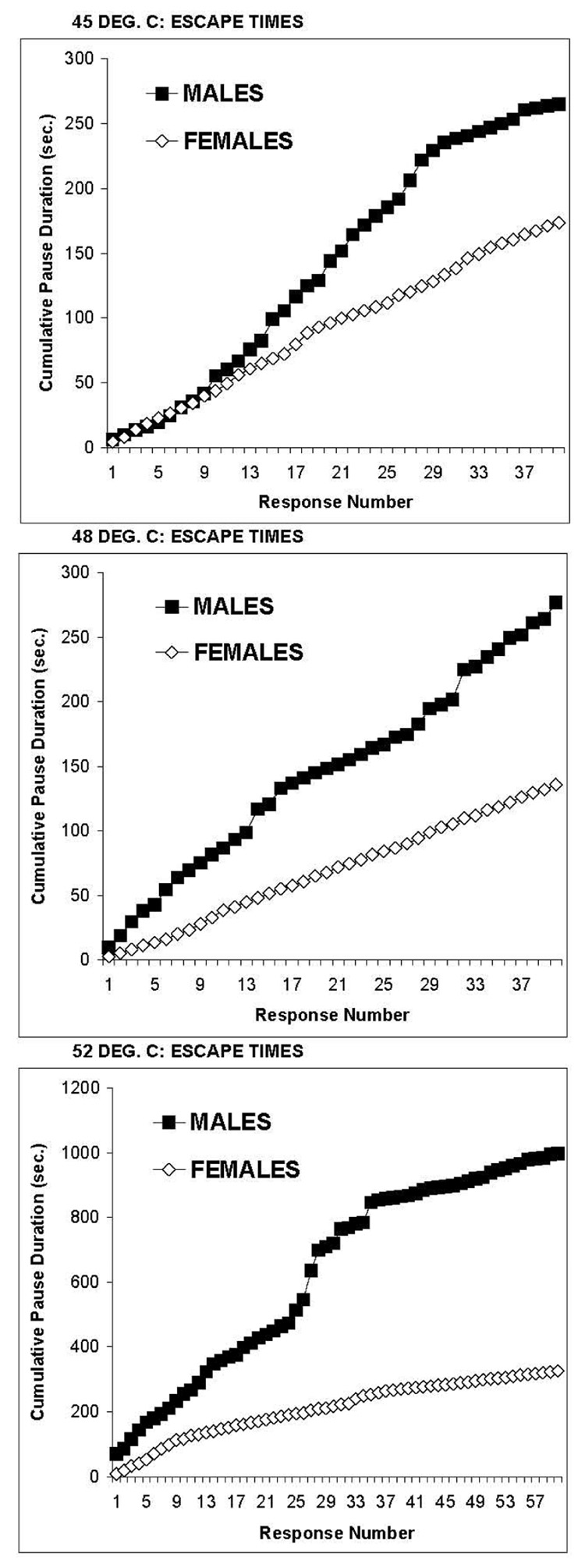
The hairless rats were tested for facial pain sensitivity to evaluate sex differences for stimulation of non-glabrous skin. The principal measure for facial sensitivity testing was the duration of interruptions in drinking, which is comparable to the duration of platform occupancy to escape from stimulation of the paws. For paw stimulation, bright light provides motivation to return to the thermal plate. For facial stimulation, hunger and thirst motivate the animals to return to thermode contact. Consistent with paw stimulation, males were hypersensitive to nociceptive heat stimulation of the face, relative to females. Figure 5 reveals significantly greater escape times (pauses in drinking) for males when the thermode temperature was 45°C (interaction: F=3.58, between df=1, within df=39, p<0.001), 48°C (interaction: F=3.14, between df=1, between df=39, P<0.001) or 52°C (interaction: F=8.98, between df=1, within df=39, P<0.001).
Figure 5.
Cumulative durations of pauses in feeding are shown for 6 male and 6 female hairless rats stimulated on the face by 45°C (top panel), 48°C (middle panel) or 52°C (bottom panel). All three temperatures interfered with the feeding of males more than females. The pauses were long and numerous for stimulation of the males with 52°C, which forced a strategy of numerous short bouts of feeding to reduce the duration of each high intensity heat stimulus.
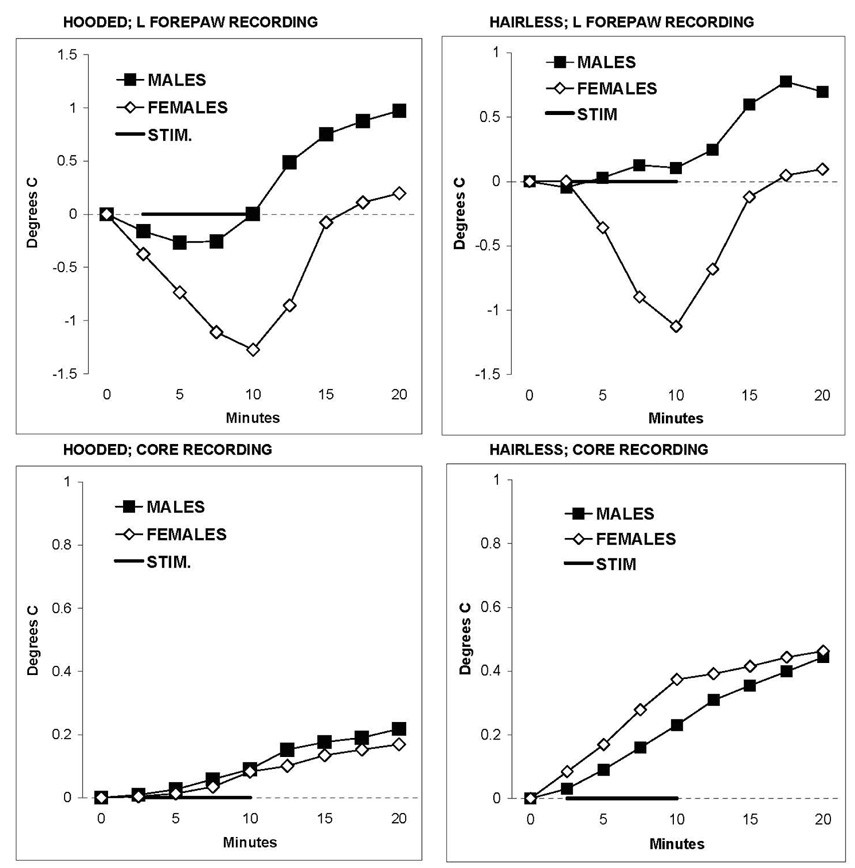
A final test for the hooded and hairless rats was conducted to determine whether the behavioral performance of males and females in response to nociceptive heat stimulation was associated with different levels of sympathetic responsivity to the same stimulus. Figure 6 plots changes in left forepaw skin temperature (relative to a stable pre-stimulation baseline) during and after stimulation of the left hindpaw with 44.5°C for 10 min (top panels). Female hooded and hairless rats both responded with a drop in forepaw skin temperature that developed gradually during stimulation and then returned to baseline. In contrast, forepaw skin temperatures of male rats remained near baseline during heat stimulation and then increased during the last 10 min of recording. The differences in skin temperature response profiles for males and females were statistically significant (hooded: interaction: F=5.98, between df=1, within df=8, p<0.001) (hairless: interaction: F=3.43, between df=1, within df=8, p=0.003). These effects of nociceptive stimulation are presumed to result from sympathetic activation, cutaneous vasoconstriction and skin cooling by females that was absent or minimal for males. Core temperatures increased slightly for all animals throughout the recording sessions and did not differ between sexes (bottom panels).
Figure 6.
Skin and core body temperatures, relative to baseline, for 10 male and 11 female hooded rats and 6 male and 6 female hairless rats during and after stimulation of the left hindpaw with 44.5°C for 10 min. For both strains, sustained nociceptive heat stimulation progressively reduced the temperature of the left forepaw of females, but this response was minimal or absent for males. Core temperatures increased slightly and comparably for males and females during and after stimulation.
Table 1 presents stabilized baseline core and left forepaw skin temperatures for the hooded and hairless rats, recorded immediately prior to thermal stimulation of the left hindpaw. Core temperatures were similar and not significantly different for males and females of each strain. Similarly, skin temperatures were insignificantly different for male and female hairless rats. Baseline skin temperatures were higher for hooded male rats than for females (t=3.76, df=19, p=0.001). Lower baseline skin temperatures for hooded females do not account for their responsivity to nociceptive stimulation, which should be greater for high resting temperatures. Considering both strains, baseline core and skin temperatures appeared not to be a factor in the greater responsivity of females to nociceptive stimulation.
Discussion
Investigations of sex differences in pain sensitivity of humans have concluded that females are generally more sensitive39, which is consistent with a relative prevalence of chronic pain conditions among women4;46. A separate literature has described sex differences in resting sympathetic tone and reactivity to experimental conditions that are stressful (see introduction). The studies of autonomic balance predict that females should be more sensitive to cold and less sensitive to heat. Accordingly, escape from nociceptive cold stimulation was greater for female rats relative to males, and escape from nociceptive heat was greater for males relative to females.
Sex differences in sensitivity of rats to nociceptive heat stimulation were demonstrated for stimulation of facial skin and glabrous skin of the paws, as revealed by operant testing paradigms. Males were more sensitive to nociceptive heat stimulation of the glabrous skin of the paws or hairy skin of the face. The facial testing paradigm utilized hunger as a counteracting motivation to escape from thermal stimulation, and escape from stimulation of the paws was discouraged by aversion for bright light. Thus, the greater sensitivity of males to heat generalized across glabrous and hairy skin, strains, and positive (non-stressful food reward) vs. negative (stressful bright light) motivation to receive nociceptive stimulation. Stimulation of the paws revealed a greater sensitivity of females to nociceptive cold stimulation, with light as the conflicting motivation. The opposite performance of males and females for escape from cold and heat stimulation of the paws controls for possible sex differences in sensitivity to bright light or stress. The thermal preference test confirmed these results with stimulation of the paws but without food or bright light as motivators for repeated exposure to heat and cold. Also, greater aversion of females for cold (heat preference) and males for heat (cold preference) was observed for two strains of rats with different baseline sensitivities (hooded rats were more sensitive to heat, and hairless rats were more sensitive to cold; Figure 4). The test of escape from stimulation of the face demonstrated that sex differences for paw stimulation did not represent a differential thermoregulation of the distal extremities. The greater aversion for cold by females and for heat by males in the escape and preference tests controlled for potential differences in activity levels or exploratory tendencies of males and females.
An important procedural factor that complicates interpretation of human studies is that psychological state and experimenter/subject interactions can determine or influence sex differences in sensitivity to brief presentations of nociceptive stimuli (see introduction). Psychological state is not an issue for laboratory animal studies, and an advantage of automated escape testing is that there is no interaction between the experimenter and the animals during thermal stimulation. Without these psychological influences, the differential sensitivity of female and male rats to cold and hot thermal stimulation was substantial, raising questions as to why this has not been observed with quantitative sensory testing of humans. The answer may have to do with the temporal progression of sympathetic activation, which is dependent upon sluggish hormonal release. Figure 6 shows that at least 5 minutes was required to differentiate between the skin temperature responses of males and females to nociceptive stimulation. Therefore, continuous or repeated thermal stimulation within trials longer than 5 minutes may be required to reveal effects of autonomic regulation on pain sensitivity. These conditions have rarely been met in human studies of pain sensitivity, but continuous ratings of capsaicin pain reveal a greater sensitivity of females that develops after 5 minutes2;29.
The sex differences in sympathetic reactivity (skin temperature responses to nociceptive stimulation) were consistent with the thermal pain sensitivity of males and females. The enhanced peripheral vasoconstriction of females during nociceptive stimulation cools the skin. After nociceptive cold stimulation, recovery to baseline skin temperatures would be opposed by the vasoconstriction, and female escape durations were longer than for males. The opposite would occur after nociceptive heat stimulation (faster recovery to baseline temperatures), and escape durations of females were shorter than for males. However, L/G durations for females were greater than for males during prolonged 44.5°C stimulation. Clearly, sex differences in operant and reflex sensitivity to heat do not share the same mechanism.
Reflex measures do not substitute for operant tests of pain sensitivity, as demonstrated for a variety of experimental manipulations27;48–51. In contrast to human studies, reflex measures have not consistently revealed sex differences in sensitivity to nociceptive stimulation, using short duration stimuli36. Also, reflex testing of many rodent strains has led to the conclusion that female responding is not more variable than male responding35, and L/G responding in the present study did not reveal a greater variability in responding by females that should result from hormonal variations during the estrus cycle. In fact, males’ L/G responses were more variable than females’ (Figure 2). However, consistent with expected influences of ovarian hormones on autonomic reactivity and nociceptive sensations3;7;10;19;21;23;28;38;41;43, operant testing revealed a greater variability of female escape durations. This was particularly the case following preconditioning cold stimulation, which increased the variability of female responses to heat during operant testing but not during reflex testing.
Comparison of cold and heat sensitivity with operant methods of testing revealed sex differences in nociceptive sensitivity that have implications for investigating mechanisms of chronic pain. Some chronic pain conditions are associated with a differential sensitivity to heat and cold. For example, heightened sensitivity of rats to cold but not heat is produced by chronic constriction injury to peripheral nerves49. Cold allodynia is a frequent consequence of nerve injury in humans11; autonomic dysregulation is commonly associated with nerve injury pain45, and neuropathic pain is more prevalent among females than males4. In addition, tonic sympathetic activation with cutaneous vasoconstriction has been associated with development of fibromyalgia, which is prevalent among females47. Therefore, a combination of operant testing for cutaneous cold and heat sensitivity and assessment of autonomic reactivity has the potential to assess potential therapies or mechanisms for chronic pain conditions.
Acknowledgments
Supported by NIH grants NS-07261 (to C.J.V.) and DE-16704 (to J.K.N.). Technical assistance of Jean Kaufman, Karen Murphy, Rich Cannon and Anwarul Azam is gratefully acknowledged. We thank Dr. Charles Widmer for the Labview subroutines.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abramson DI, Tuck S, Lee SW, Richardson G, Chu LSW. Vascular basis for pain due to cold. Arch Physiol Med Rehabil. 1966;47:300–305. [PubMed] [Google Scholar]
- 2.Baad-Hansen L, Poulsen H, Jensen HM, Svensson P. Lack of sex differences in modulation of experimental intraoral pain by diffuse noxious inhibitory controls (DNIC) Pain. 2005;116:359–365. doi: 10.1016/j.pain.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Bartelink M, De Wit A, Wollersheim H, Theeuwes A, Thien T. Skin vascular reactivity in healthy subjects: influence of hormonal status. J Appl Physiol. 1993;74:727–732. doi: 10.1152/jappl.1993.74.2.727. [DOI] [PubMed] [Google Scholar]
- 4.Berkley K. Sex differences in pain. Behav Brain Scs. 1997;20:371–380. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- 5.Bowyer L, Brown M, Jones M. Vascular reactivity in men and women of reproductive age. Am J Obstet Gynecol. 2001;185:88–96. doi: 10.1067/mob.2001.114502. [DOI] [PubMed] [Google Scholar]
- 6.Britton A, Shipley M, Malik M, Hynatkova K, Hemingway H, Marmot M. Changes in heart rate and heart rate variability over time in middle-aged men and women in the general population (from the Whitehall II cohort study) Am J Cardiol. 2007;100:524–527. doi: 10.1016/j.amjcard.2007.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cankar K, Finderle Z, Struci M. Gender differences in cutaneous laser doppler flow response to local direct and contralateral cooling. J Vasc Res. 2000;37:183–188. doi: 10.1159/000025729. [DOI] [PubMed] [Google Scholar]
- 8.Cankar K, Finderle Z, Struci M. The role of alpha1- and alpha2-adrenoceptors in gender differences in cutaneous LD flux response to local cooling. Microvasc Res. 2004;68:126–131. doi: 10.1016/j.mvr.2001.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Cankar K, Finderle Ž. Gender differences in cutaneous vascular and autonomic nervous response to local cooling. Clin Auton Res. 2003;13:214–220. doi: 10.1007/s10286-003-0095-5. [DOI] [PubMed] [Google Scholar]
- 10.Charkoudian N, Stephens DP, Pirkle KC, Kosiba WA, Johnson JM. Influence of female reproductive hormones on local thermal control of skin blood flow. J Appl Physiol. 1999;87:1719–1723. doi: 10.1152/jappl.1999.87.5.1719. [DOI] [PubMed] [Google Scholar]
- 11.Collins E, Novak C, Mackinnon SE, Weisenborn SA. Long-term follow-up evaluation of cold sensitivity following nerve injury. J Hand Surg (Am) 1996;21:1078–1085. doi: 10.1016/S0363-5023(96)80319-4. [DOI] [PubMed] [Google Scholar]
- 12.Cooke J, Creager M, Osmundson PJ, Shepherd JT. Sex differences in control of cutaneous blood flow. Circulat. 1990;82:1607–1615. doi: 10.1161/01.cir.82.5.1607. [DOI] [PubMed] [Google Scholar]
- 13.Emery D, Keefe F, France CR, Affleck G, Waters S, Fondow MD, McKee DC, France JL, Hackshaw KV, Caldwell DS, Stainbrook D. Effects of a brief coping skills training intervention on nociceptive flexion reflex threshold in patients having osteoarthritic knee pain: A preliminary laboratory study of sex differences. J Pain Sympt Manag. 2006;31:262–269. doi: 10.1016/j.jpainsymman.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Evans J, Ziegler M, Patwardhan AR, Ott JB, Kim CS, Leonelli FM, Knap CF. Gender differences in autonomic cardiovascular regulation: spectral, hormonal and hemodynamic indexes. J Appl Physiol. 2001;91:2611–2618. doi: 10.1152/jappl.2001.91.6.2611. [DOI] [PubMed] [Google Scholar]
- 15.Ferrell W, Wong B, Lockhart JC, Ramsay JE. Gender differences in regional cutaneous microcirculatory responses to capsaicin. Fund Clin Pharmacol. 2004;18:195–200. doi: 10.1111/j.1472-8206.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 16.Fillingim R, Browning A, Powell T, Wright RA. Sex differences in perceptual and cardiovascular responses to pain: The influence of a perceived ability manipulation. J Pain. 2002;3:439–445. doi: 10.1054/jpai.2002.128067. [DOI] [PubMed] [Google Scholar]
- 17.Garofalo J, Lawler C, Robinson R, Morgan M, Kenworthy-Heinige T. The role of mood states underlying sex differences in the perception and tolerance of pain. Pain Practice. 2006;6:186–196. doi: 10.1111/j.1533-2500.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- 18.Graham T. Thermal, metabolic and cardiovascular changes in men and women during cold stress. Med Sci Sports Exerc. 1988;20:S185–S192. doi: 10.1249/00005768-198810001-00017. [DOI] [PubMed] [Google Scholar]
- 19.Hassan A, Carter G, Tooke J. Postural vasoconstriction in women during the normal menstrual cycle. Clin Sci. 1990;78:39–47. doi: 10.1042/cs0780039. [DOI] [PubMed] [Google Scholar]
- 20.Holgaard H, Hermansen K, Bjerragaard P. Spectral components of short-term RR interval variability in healthy subjects and effects of risk factors. Eur Heart J. 1994;15:1174–1183. doi: 10.1093/oxfordjournals.eurheartj.a060650. [DOI] [PubMed] [Google Scholar]
- 21.Huikuri H, Pikkujämsä S, Airaksinen KE, Ikäheimo MJ, Rantala AO, Kauma H, Lilja M, Kesäniemi YA. Sex-related differences in autonomic modulation of heart rate in middle-aged subjects. Circulat. 1996;94:122–126. doi: 10.1161/01.cir.94.2.122. [DOI] [PubMed] [Google Scholar]
- 22.Jackson T, Lezzi TCH, Ebnet S, Eglitis K. Gender, interpersonal transactions, and the perception of pain: An experimental analysis. J Pain. 2005;6:228–236. doi: 10.1016/j.jpain.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Kaciuba-Uscilko H, Grucza R. Gender differences in thermoregulation. Curr Opin Clin Nutr Metab Care. 2001;4:533–536. doi: 10.1097/00075197-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Kàllai I, Barke A, Voss U. The effects of experimenter characteristics on pain reports in women and men. Pain. 2004;112:142–147. doi: 10.1016/j.pain.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Kellogg DJ, Liu Y, Pérgola P. Selected contribution: Gender differences in the endothelin-B receptor contribution to basal cutaneous vascular tone in humans. J Appl Physiol. 2001;91:2407–2411. doi: 10.1152/jappl.2001.91.5.2407. [DOI] [PubMed] [Google Scholar]
- 26.Keogh E, Bond FW, Hanmer R, Tilston J. Comparing acceptance- and control-based coping instructions on the cold-pressor experiences of healthy men and women. Europ J Pain. 2005;9:591–598. doi: 10.1016/j.ejpain.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 27.King C, Devine D, Vierck CJ, Mauderli AP, Yezierski RP. Opioid modulation of reflex versus operant responses following stress in the rat. Neurosci. 2007;147:174–182. doi: 10.1016/j.neuroscience.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Kowalczyk W, Evans S, Bisaga AM, Sullivan MA, Comer SD. Sex differences and hormonal influences on response to cold pressor pain in humans. J Pain. 2006;7:151–160. doi: 10.1016/j.jpain.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Lariviere W, McBurney D, Frot M, Balaban CD. Tonic, phasic, and integrator components of psychophysical responses to topical capsaicin account for differences of location and sex. J Pain. 2005;6:777–781. doi: 10.1016/j.jpain.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Liao D, Barnes R, Chambless LE, Simpson RJ, Jr, Sorlie P, Heiss G. Age, race and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability--the ARIC study. Atherosclerosis Risk in Communities. Am J Cardiol. 1995;76:906–912. doi: 10.1016/s0002-9149(99)80260-4. [DOI] [PubMed] [Google Scholar]
- 31.Lowery D, Fillingim R, Wright R. Sex differences and incentive effects on perceptual and cardiovascular resonses to cold pressor pain. Psychosomat Med. 2003;65:284–291. doi: 10.1097/01.psy.0000033127.11561.78. [DOI] [PubMed] [Google Scholar]
- 32.Lubli A, Wolfenson D, Berman A. Sex differences in blood flow distribution of normothermic and heat-stressed rabbits. Am J Physiol Regul Integr Comp Physiol. 1995;268:R66–R71. doi: 10.1152/ajpregu.1995.268.1.R66. [DOI] [PubMed] [Google Scholar]
- 33.Marchand I, Johnson D, Montgomery D, Brisson GR, Perrault H. Gender differences in temperature and vascular characteristics during exercise recovery. Canad J Appl Physiol. 2001;26:425–441. doi: 10.1139/h01-026. [DOI] [PubMed] [Google Scholar]
- 34.Mayrovitz H, Regan M. Gender differences in facial skin blood perfusion during basal and heated conditions determined by laser doppler flowmetry. Microvasc Res. 1993;45:211–218. doi: 10.1006/mvre.1993.1019. [DOI] [PubMed] [Google Scholar]
- 35.Mogil J, Chanda M. The case for the inclusion of female subjects in basic science studies of pain. Pain. 2005;117:1–5. doi: 10.1016/j.pain.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 36.Mogil J, Chesler E, Wilson SG, Juraska JM, Sternberg WF. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci Biobehav Rev. 2000;24:375–389. doi: 10.1016/s0149-7634(00)00015-4. [DOI] [PubMed] [Google Scholar]
- 37.Neubert J, Widmer C, Malphurs W, Rossi H, Vierck CJ, Caudle RM. Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain. 2005;116:386–395. doi: 10.1016/j.pain.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 38.Ogilvie K, Rivier C. Gender difference in hypothalamic-pituitary-adrenal axis response to alcohol in the rat: activational role of gonadal steroids. Brain Res. 1997;766:19–28. doi: 10.1016/s0006-8993(97)00525-8. [DOI] [PubMed] [Google Scholar]
- 39.Riley J, III, Robinson M, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;181 doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 40.Robinson M, Wise E, Gagnon C, Fillingim RB, Price DD. Influences of gender role and anxiety on sex differences in temporal summation of pain. J Pain. 2004;5:77–82. doi: 10.1016/j.jpain.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Sato N, Miyake S. Cardiovascular reactivity to mental stress: relationship with menstrual cycle and gender. J Physiol Anthropol Appl Human Sci. 2004;23:215–223. doi: 10.2114/jpa.23.215. [DOI] [PubMed] [Google Scholar]
- 42.Sinnreich R, Kark J, Friedlander Y, Sapoznikov D, Luria MH. Five minute recordings of heart rate variability for population studies: repeatability and age-sex characteristics. Heart. 1998;80:156–162. doi: 10.1136/hrt.80.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stening K, Eriksson O, Wahren L, Berg G, Hammar M, Blomqvist A. Pain sensations to the cold pressor test in normally mentruating women: comparison with men and relation to menstrual phase and serum sex steroid levels. Am J Physiol Regul Integr Comp Physiol. 2007 doi: 10.1152/ajpregu.00127.2007. Epub: [DOI] [PubMed] [Google Scholar]
- 44.Tousignant-Laflamme Y, Marchand S. Sex differences in cardiac and autonomic response to clinical and experimental pain in LBP patients. Europ J Pain. 2006;10:603–614. doi: 10.1016/j.ejpain.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Treede R, Davis K, Campbell JN, Raja SN. The plasticity of cutaneous hyperalgesia during sympathetic ganglion blockade in patients with neuropathic pain. Brain. 1992;115:607–621. doi: 10.1093/brain/115.2.607. [DOI] [PubMed] [Google Scholar]
- 46.Unruh A. Gender variations in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 47.Vierck C. Mechanisms underlying development of spatially distributed chronic pain (fibromyalgia) Pain. 2006;124:242–263. doi: 10.1016/j.pain.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Vierck CJ, Hansson PT, Yezierski RP. Clinical and pre-clinical pain assessment: Are we measuring the same thing? Pain. 2008;135:7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 49.Vierck C, Acosta-Rua A, Johnson R. Bilateral chronic constriction of the sciatic nerve: A model of long-term cold hyperalgesia. J Pain. 2005;6:507–517. doi: 10.1016/j.jpain.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Vierck C, Acosta-Rua A, Nelligan R, Tester N, Mauderli AP. Low dose systemic morphine attenuates operant escape but facilitates innate reflex responses to thermal stimulation. J Pain. 2002;3:309–319. doi: 10.1054/jpai.2002.125186. [DOI] [PubMed] [Google Scholar]
- 51.Vierck CJ, Kline R, III, Wiley R. Comparison of operant escape and innate reflex responses to nociceptive skin temperatures produced by heat and cold stimulation of rats. Behav Neurosci. 2004;118:627–635. doi: 10.1037/0735-7044.118.3.627. [DOI] [PubMed] [Google Scholar]
- 52.Zhang J. Effect of age and sex on heart rate variability in healthy subjects. J Manip Physiol Ther. 2007;30:374379. doi: 10.1016/j.jmpt.2007.04.001. [DOI] [PubMed] [Google Scholar]