Until relatively recently, the choices of stimulant medications for children were limited. Amphetamines and immediate-release methylphenidate (MPH) have been used for several decades, but in their native “immediate release” (IR) formulations, both have rapid onsets and short durations of action, and must therefore be given at least twice daily for optimal clinical efficacy throughout the day. The challenge to the clinician using short-acting preparations was to find the optimum dosing regimen for each child—and dosing requirements could vary as much as sixfold between individual children.
After a morning dose of MPH, peak concentration (Cmax) is reached about 2 hours after dosing, and concentrations have declined by half approximately 2 hours later (T1/2). Higher doses will produce higher Cmax values, but do not affect the time of the peak (Tmax) or onset or T1/2. For several decades, it was assumed that the clinician merely had to find the Cmax that would produce the optimal clinical response 1 to 2 hours after dosing, and keep this constant throughout the day. Smaller doses were therefore used toward the end of the day; these were the “sculpted” dosing regimens of the early 1990s. For example, children in the MTA study who were treated with an average MPH dose received MPH 10 mg in the morning, 10 mg at noon, and 5 mg in the afternoon. However, after the 14-month treatment phase, compliance with drug treatment worsened considerably, in part because medicated children suffered teasing at the hands of their peers when called to the principal’s office to take noontime medication doses. The longer formulations then available included Ritalin SR® and Dexedrine® spansules. Neither medication provided coverage for the whole day, and the reasons for this were a mystery. Several manufacturers therefore embarked on the development of more effective once-daily medications in collaboration with investigators at the University of California at Irvine laboratory school (analogue classroom) using surrogate measures of drug efficacy.
Development of Concerta OROS-MPH
An effective once-daily stimulant formulation should deliver medication in a pattern that produces optimum clinical effects that begin shortly after administration and are maintained for the desired duration. To find this pattern, a “sipping study”1 was conducted among 36 children with ADHD who were already being treated effectively with immediate-release MPH regimens. These children attended a laboratory school (the analog classroom) on several Saturdays, where they were given either active medication or placebo in various patterns every 30 minutes throughout the day. They were also evaluated multiple times throughout the day using the Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) rating scale,2 which had been developed to measure not ADHD symptoms (inattention, overactivity, impulsivity, etc) but the classic behavioural manifestations of ADHD in the classroom (eg, getting started, staying on task, interacting with peers, working neatly, staying seated, or remaining quiet). Academic productivity was measured objectively by means of a 10-minute written math test containing problems of appropriate difficulty for the child—both number of problems attempted and number of correctly worked problems were tracked.
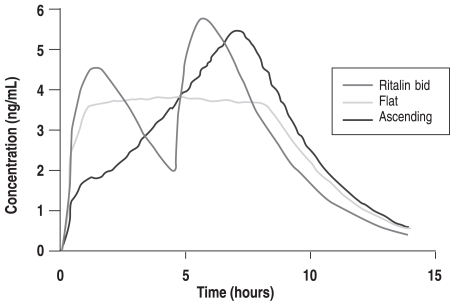
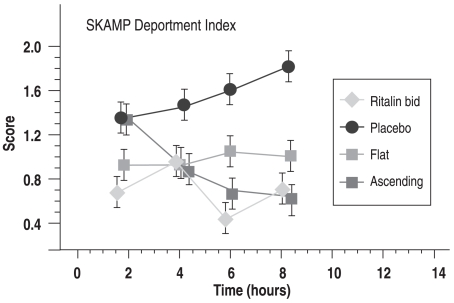
The known pharmacokinetic profiles revealed that twice-daily administration of immediate release (IR) MPH produced a highly variable pattern of plasma MPH concentration (Figure 1A: left side, Ritalin bid), which was associated with a corresponding variability in SKAMP scores (Figure 1B: right side, Ritalin bid). Dosing IR-MPH as an initial bolus at 7:30 am followed by a small but constant dose every 30 minutes from 8:30 am to 3:00 pm (with doses depending on the child’s usual IR-MPH dose) produced a flat plasma profile (zero-order drug delivery; Figure 1A: left side, Flat) and SKAMP scores that showed full efficacy in the morning compared to the child’s standard drug regimen. However, surprisingly, about 40% of the effect was lost later in the day (Figure 1B: right side, Flat). Finally, an experimental condition defined by dosing IR-MPH without a large initial bolus but ascending doses throughout the day (selected to produce high afternoon plasma levels that matched the afternoon peak of the tid regime) showed no or low efficacy during the morning, but full efficacy in the afternoon (Figures 1A and B: Ascending).
Figure 1A.
Acute tolerance: Development of a concept Simulated Plasma Profiles
Swanson J, et al. Clin Pharmacol Ther 1999;66:295.
Copyright © 1999, reprinted with permission from The American Society for Clinical Pharmacology & Therapeutics.
A. Study 1: Simulated plasma methylphenidate concentrations for a 20 mg total daily dose delivered by twice-daily (bid), flat, and ascending dosing regimens.
Figure 1B.
Acute tolerance: Development of a concept Impact on SKAMP
Swanson J, et al. Clin Pharmacol Ther 1999;66:295.
Copyright © 1999, reprinted with permission from The American Society for Clinical Pharmacology & Therapeutics.
B. Study 1: Peak and trough responses for an example efficacy measure (attention subscale of the SKAMP rating scale) for the bid, flat, ascending, and placebo treatments.
This laboratory school study had two important findings. First, the decline in efficacy with the flat plasma profile suggested that acute drug tolerance (tachyphylaxis) was developing in response to exposure to relatively high drug levels over 3 to 4 hours. The hypothesis of acute tolerance would explain why formulations such as Ritalin SR, which used a zero-order drug delivery as a target, did not have the anticipated long-acting efficacy. In the face of this acute tolerance, the traditional dosing protocol (such as the “sculpted” procedure used in the MTA study) should be reversed: once a morning bolus achieves its initial rapid effect, afternoon doses should not be smaller than the morning dose (based on the hypothesis that some carryover would occur, and a smaller dose would thus maintain the initially effective plasma concentration at a constant level across the day), but instead should be equal to or larger than the initial bolus to produce higher concentrations in the afternoon than in the morning to maintain full efficacy. Second, the experimental ascending condition demonstrated that a bolus dose was not necessary to achieve full efficacy, and that an appropriately designed continuous delivery of MPH could reach the same level of efficacy as the afternoon efficacy following multiple bolus doses of equal size (which along with carryover produces an ascending series of peaks across the day). Of course, to achieve rapid onset an initial bolus would be required, but if the decline from the peak of the initial bolus could be avoided, then perhaps it could be slightly smaller than the typical initial bolus of a clinical regime based on the rationale that the first bolus must be sufficient to maintain adequate efficacy of the drug despite its short, 2-hour half-life (for which the plasma concentrations fall by 50% over 2 hours).
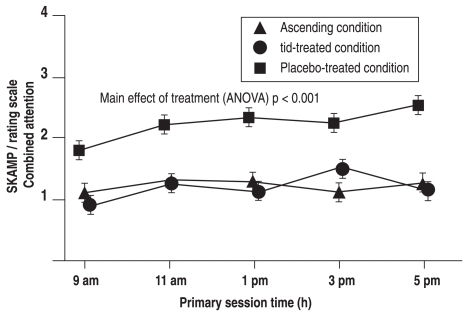
These findings were confirmed in a second proof-of-concept study3 with a randomized, double-blind crossover design. In this trial, 32 confirmed MPH responders received either placebo or IR-MPH three times daily, or an ascending delivery of small doses designed to maintain full efficacy after an initial large bolus selected to achieve rapid onset of the effect; both active treatments produced similar significant reductions in SKAMP scores compared to placebo (Figure 2).
Figure 2.
An ascending profile of MPH delivery maintains SKAMP attention scores
Swanson J, et al. Arch Gen Psychiatry 2003;60:204. Copyright © 2003, American Medical Association. All rights reserved.
Results of the proof-of-concept study: the Attention and Performance subscales of the Swanson, Kotkin, Agler, Mylnn, and Pelham (SKAMP) rating scale and the percentage of errors made on the computerized mathematics test.
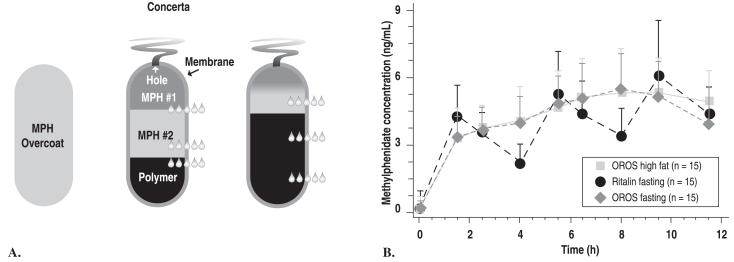
The next phase of development involved engineering a way to deliver MPH in the ascending pattern. The initial plan had been to use the original OROS system, a round pill designed for zero-order drug delivery; instead, because of the results described above, an oblong pill was designed that achieved the desired first-order drug delivery. This pill is coated with IR-MPH in order to produce a bolus with a rapid onset of effect. The rest of the total daily dose is contained in a reservoir, together with a polymer that expands when it absorbs water diffusing into the compartment. This expanding polymer pushes the drug out of a laser-drilled hole into the GI tract, from whence it is absorbed. Prototypes with a single compartment OROS reservoir did not reliably achieve the targeted ascending profile, so a reservoir with two drug compartments was developed, the second containing a higher MPH concentration than the first. This formulation was shown to produce the desired initial rapid rise in MPH plasma level and smooth ascending pharmacokinetic profile produced by a gradient of MPH concentrations that exit the laser-drilled hole as the contents of the two reservoir compartments mix in the water that crosses the membrane3 (Figure 3).
Figure 3.
Proof-of-product study: PK profiles for OROS-MPH (Concerta) and IR-MPH (Ritalin)
(left side) A. Greenhill LL, et al. J Am Acad Child Adolesc Psychiatry 2003;42:1234
(right side) B. Swanson JM, et al. Arch Gen Psychiatry 2003;60:204. Copyright © 2003, American Medical Association. All rights reserved.
3B. The pharmacokinetic profiles from a 3-way crossover study of immediate release OROS-methylphenidate hydrochloride administered with a (high-fat breakfast) and without (fasting) food, and tid (3 times daily)-methylphenidate administered in the fasting state. OROS is a new oral once-a-day formulation to deliver methylphenidate by osmotic pump process based on OROS technology (ALZA Corp, Mountain View, Calif).
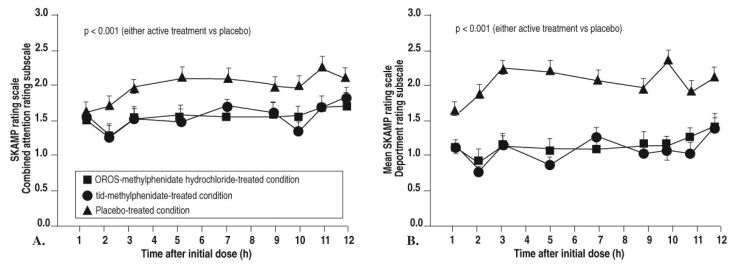
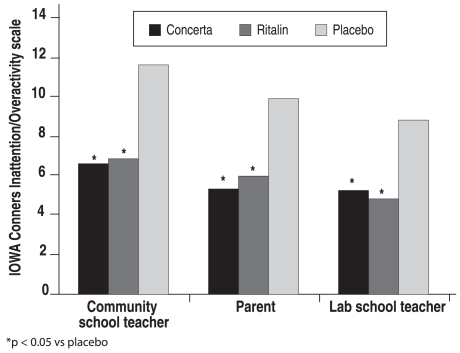
An additional proof-of-product study using this new formulation3 showed that SKAMP scores for both attention and deportment decreased dramatically in the morning after the initial bolus, and these decreases were maintained for 12 hours after dosing (Figure 4). This trial3 of OROS-MPH compared with placebo and IR-MPH three times daily (as well as a parallel study by another team of investigators4) was carried out in both the laboratory school and natural settings; in this study, ratings on the Inattention/Overactivity subscale of the Inattention/Overactivity with Aggression Conners (IOWA-C) rating scale were well correlated with each other and consistent with the results of the previous study (Figure 5).
Figure 4.
Proof-of-product study: SKAMP scores for attention and deportment with OROS-MPH and IR-MPH
Swanson JM, et al. Arch Gen Psychiatry 2003;60:204. Copyright © 2003, American Medical Association. All rights reserved.
Attention ratings using the Swanson, Kotkin, Agler, Mylnn, and Pelham (SKAMP) rating scale by the University of California, Irvine, Laboratory School showing the onset and duration of OROS-methylphenidate hydrochloride-treated condition and tid (3 times daily)-methylphenidate-treated condition in the proof-of-product study. OROS is a new oral once-a-day formulation to deliver methylphenidate by osmotic pump process based on OROS technology (ALZA Corp, Mountain View, Calif).
| A. | |||||||||
| OROS-methylphenidate-treated condition | (n = 60) | 59 | 59 | 60 | 60 | 60 | 59 | 59 | 60 |
| tid-methylphenidate-treated condition | (n = 61) | 62 | 61 | 62 | 62 | 62 | 62 | 60 | 60 |
| Placebo-treated condition | (n = 60) | 59 | 58 | 58 | 59 | 59 | 58 | 58 | 58 |
| B. | |||||||||
| OROS-methyphenidate-treated condition | (n = 60) | 59 | 58 | 60 | 60 | 60 | 59 | 59 | 60 |
| tid-methylphenidate-treated condition | (n = 61) | 62 | 61 | 62 | 62 | 62 | 62 | 60 | 60 |
| Placebo-treated condition | (n = 60) | 59 | 58 | 58 | 59 | 59 | 58 | 59 | 58 |
Figure 5.
Mean I/O ratings from IOWA-C
Adapted from Swanson JM, et al. Arch Gen Psychiatry 2003;60:204. Copyright © 2003, American Medical Association. All rights reserved.
IOWA (Inattention and Overactivity With Aggression) Conners rating scale from 3 sources (parent, University of California, Irvine, laboratory school teacher, and community school teacher) in the proof-of-product study of the OROS-methylphenidate hydrochloride-treated condition, tid (3 times daily)-methylphenidate-treated condition, and placebo-treated condition.
A large longer-term open-label study5 involved 407 children aged 6 to 13 years with documented response to MPH who had participated in previous efficacy or pharmacokinetic studies of OROS-MPH. The effectiveness (as measured by the IOWA-C rating scale, Global Assessment Scale, and teachers’ ratings of peer interactions) and tolerability of OROS-MPH was shown to be maintained throughout the 12 months of the analysis. It is important to note that at the beginning of the study, children were assigned to one of three doses of OROS-MPH to match their clinically effective doses established in the initial clinical trials (28.5% on 18 mg daily, 47.4% on 36 mg daily, and 24.1% on 54 mg daily). Doses could be adjusted upward or downward (or interrupted for weekends or nonschool days) at the discretion of the physician who reviewed the child at the monthly clinic visits. After 12 months of treatment, only 15.0% were still taking 18 mg daily, while 40.0% took 36 mg daily and 45.0% took 54 mg daily. The average dose increased from 35 mg to 41 mg daily, and mean total dose per kg of body weight increased from 1.09 mg/kg at baseline to 1.26 mg/kg at month 12. Thus, in a study in which doses were carefully monitored, almost half the participants required OROS-MPH doses of at least 54 mg daily to achieve optimal effects. The authors noted that the increased dosage over time was consistent with other studies such as the MTA trial,6,7 and might reflect a fine-tuning of the dose after the initial response is manifested, an increase in dose as the child matures and body size increases, or differences in evaluation by parents and teachers of the optimal or maximum response.
The Development of Adderall XR
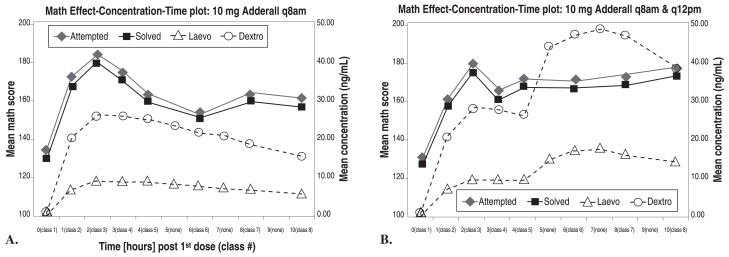
The pharmacokinetic and pharmacodynamic effects of Adderall were tested in the analog classroom in studies similar to those used for the development for Concerta. A double-blind, crossover comparison of 10 mg once-daily with 10 mg twice-daily Adderall (considered the standard dosing regime for IR Adderall at the time of this study) among 12 subjects8 found that with once-daily administration, although high serum concentrations were maintained in the afternoon, clinical efficacy waned, as predicted by the hypothesis of acute tolerance. The twice-daily dosing produced approximately doubled afternoon plasma levels and maintained full efficacy in the afternoon (Figure 6).
Figure 6.
Math problems attempted and solved with Adderall 10 mg q8am (left) or q8am and q12pm (right)
Greenhill LL, et al. A pharmacokinetic/pharmacodynamic study comparing a single morning dose of Adderall to twice-daily dosing in children with ADHD. J Am Acad Child Adolesc Psychiatry 2003;42:1234-1241.
A. SKAMP score: pharmacokinetics and pharmacodynamics: 10 mg of Adderall given at 8 am.
B. SKAMP score: pharmacokinetics and pharmacodynamics 10 mg of Adderall given at 8 am and noon.
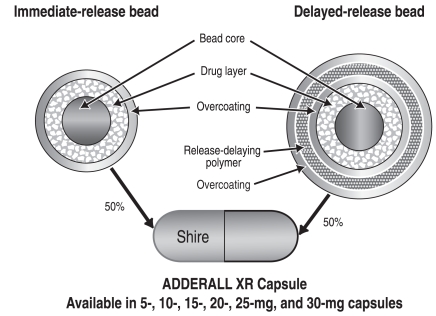
Because amphetamine has a long pharmacokinetic half-life, doubling of serum concentration can be achieved by giving a second bolus dose of amphetamine, as demonstrated in the above study. Also, the technology of polymer-coated beads can be used to delay the release of the second dose after a morning administration that contains an IR component (uncoated beads) and a delayed-release component (coated beads). Adderall XR—an extended-release formulation of mixed amphetamine salts—is composed of 50% immediate-release beads and 50% delayed-release beads designed to release medication after about 4 hours to mimic the effects of two doses of IR Adderall given 4 hours apart (Figure 7).8,9,10 The product is formulated in capsules containing 5 to 30 mg of drug, and can be sprinkled onto food for children who have difficulty swallowing pills. Adderall XR is currently unavailable in Canada.
Figure 7.
ADDERALL XR Pulse Delivery System
Greenhill LL, et al. J Am Acad Child Adolesc Psychiatry 2003;42:1234.
Proposed mechanisms of acute tolerance
It is known that oral clinical doses of MPH act to block the dopamine transporter in the striatum, increasing the availability of dopamine at the synapse. It has been hypothesized that in response to this increased dopamine availability, postsynaptic dopamine receptors are inactivated by a process of internalization (retracting into the cell membrane).11 One way to overcome this mechanism of tolerance is to provide a medication-free interval; when synaptic dopamine concentrations return to baseline levels, the receptors re-emerge from the cell membrane, reverting to their normal sensitive states. A second way is to use a larger dose of MPH to produce a larger increase in synaptic dopamine concentration to compensate for the desensitization of the receptor. Studies to elucidate the mechanisms of acute tolerance to MPH and to amphetamine are underway.
Footnotes
* All trademark rights used under licence.
REFERENCES
- 1.Swanson J, Gupta S, Guinta D, et al. Acute tolerance to methylphenidate in the treatment of attention deficit hyperactivity disorder in children. Clin Pharmacol Ther. 1999;66:295–305. doi: 10.1016/S0009-9236(99)70038-X. [DOI] [PubMed] [Google Scholar]
- 2.Swanson JM, Wigal SB, Udrea D, et al. Evaluation of individual subjects in the analog classroom setting I: examples of graphical and statistical procedures for within-subject ranking of responses to different delivery patterns of methylphenidate. Psychopharmacol Bull. 1998;34:825–832. [PubMed] [Google Scholar]
- 3.Swanson JM, Gupta S, Lam A, et al. Development of a new once-a-day formulation of methylphenidate for the treatment of attention-deficit/hyperactivity disorder: proof-of-concept and proof-of-product studies. Arch Gen Psychiatry. 2003;60:204–211. doi: 10.1001/archpsyc.60.2.204. [DOI] [PubMed] [Google Scholar]
- 4.Pelham WE, Gnagy EM, Burrows-MacLean L, et al. Once-a-day Concerta methylphenidate versus three-times-daily methylphenidate in laboratory and natural settings. Pediatrics. 2001;107:e105. doi: 10.1542/peds.107.6.e105. [DOI] [PubMed] [Google Scholar]
- 5.Wilens T, Pelham W, Stein M, et al. ADHD treatment with once-daily OROS methylphenidate: interim 12-month results from a long-term open-label study. J Am Acad Child Adolesc Psychiatry. 2003;42:424–433. doi: 10.1097/01.CHI.0000046814.95464.7D. [DOI] [PubMed] [Google Scholar]
- 6.The MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- 7.The MTA Cooperative Group. Moderators and mediators of treatment response for children with attention-deficit/hyperacivity disorder. Arch Gen Psychiatry. 1999;56:1088–1096. doi: 10.1001/archpsyc.56.12.1088. [DOI] [PubMed] [Google Scholar]
- 8.Greenhill LL, Swanson JM, Steinhoff K, et al. A Pharmacokinetic/pharmacodynamic study comparing a single morning dose of Adderall to twice-daily dosing in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2003;42:1234–1241. doi: 10.1097/00004583-200310000-00015. [DOI] [PubMed] [Google Scholar]
- 9.McCracken JT, Biederman J, Greenhill LL, et al. Analog classroom assessment of a once-daily mixed amphetamine formulation, SLI381 (Aderall XRP), in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2003;42:673–683. doi: 10.1097/01.CHI.0000046863.56865.FE. [DOI] [PubMed] [Google Scholar]
- 10.McGough JJ, Biederman J, Greenhill LL, et al. Pharmacokinetics of SLI381 (ADDERALL XR), an extended-release formulation of Adderall. J Am Acad Child Adolesc Psychiatry. 2003;42:684–691. doi: 10.1097/01.CHI.0000046850.56865.CB. [DOI] [PubMed] [Google Scholar]
- 11.Swanson JM, Volkow ND. Serum and brain concentrations of methylphenidate: implications for use and abuse. Neurosci Biobehav Rev. 2003;27:615–621. doi: 10.1016/j.neubiorev.2003.08.013. [DOI] [PubMed] [Google Scholar]