Abstract
Germ cells have a critical role in mediating the generation of genetic diversity and transmitting this information across generations. Furthermore, gametogenesis is unique as a developmental process in that it generates highly-specialized haploid gametes from diploid precursor stem cells through meiosis. Despite the importance of this process, progress in elucidating the molecular mechanisms underpinning mammalian germ cell development has been retarded by the lack of an efficient and reproducible system of in vitro culture for the expansion and trans-meiotic differentiation of germline cells. The dearth of such a culture system has rendered the study of germ cell biology refractory to the application of new high-throughput technologies such as RNA interference, leaving in vivo gene-targeting approaches as the only option to determine the function of genes believed to be involved in gametogenesis. Recent reports detailing the derivation of gametes in vitro from stem cells may provide the first steps in developing new tools to solve this problem. This review considers the developments made in modelling germ cell development using stem cells, and some of the challenges that need to be overcome to make this a useful tool for studying gametogenesis and to realize any future clinical application.
Keywords: germ cell, stem cell, spermatogenesis, oogenesis, infertility
Introduction
The germ cells of sexually reproducing organisms have three crucial functions, namely the preservation of genetic integrity, the generation of genetic diversity and the transmission of this information to the next generation through the production of haploid gametes from diploid precursors. Perturbations at any stage of the gametogenic process can result in subfertility, which within human populations is a major public health issue affecting ∼0–15% of couples (De Kretser and Baker, 1999). A better understanding of gametogenesis could aid the development of new therapeutic approaches for subfertility and provide targets for novel contraceptives. Despite these potential benefits, our understanding of the molecular mechanisms that regulate the development of the mammalian germ lineage is poor when compared with that of most somatic cells.
A major reason for this is the lack of a robust in vitro culture system allowing the expansion of germline progenitor cells and their differentiation into mature gametes. This has rendered the study of gametogenesis, and in particular the role of germ cell endogenous factors in the regulation of this process, broadly resistant to recent technological developments in high-throughout analysis of gene function, such as the use of RNA interference to knockdown gene expression.
Previous efforts to establish in vitro models of germ cell development have focused primarily on attempting to immortalize post-natal spermatogenic cells. By introducing the SV40 large T antigen and a temperature-sensitive mutant form of p53 into mouse testicular germ cells, Hofmann et al. (1994) reported the establishment of an immortalized germ cell line capable of undergoing meiosis in vitro when grown at a permissive temperature. However, further characterization of this line failed to detect the presence of transcripts expressed by post-meiotic cells, nor the presence of cells with a haploid DNA content, leading to the conclusion that this cell line was incapable of undergoing trans-meiotic differentiation in vitro (Wolkowicz et al., 1996). Feng et al. (2002) used the TERT subunit of telomerase to immortalize mouse spermatogonia capable of undergoing meiosis and forming acrosome-like structures characteristic of haploid round spermatids at high efficiency when treated with stem cell factor (SCF, the ligand for the c-Kit receptor). Again this result has yet to be replicated by other groups, and despite the potential utility of this cell line to germ cell biologists, its use has not been widely reported. Although methods to maintain and expand mouse spermatogonial stem cells (SSCs) in culture have recently been developed, none appear to support full trans-meiotic differentiation of these cells in vitro (Kanatsu-Shinohara et al., 2003).
Indeed, the range of tools available to reproductive biologists to determine the function of genes with a suspected role in mammalian germ cell development is essentially limited to the study of homologous genes in lower model organisms and the production of transgenic mice with targeted disruptions of genes of interest (Cooke and Saunders, 2002; Matzuk and Lamb, 2002; Lee et al., 2007). While the success of this approach is undeniable, it is laborious, time consuming and costly in comparison to in vitro cell-based assays of gene function, compounded by the difficulty of maintaining mouse lines with reproductive defects through breeding. Furthermore, targeted gene disruptions in transgenic mice classically result in a complete loss-of-function phenotype, and this may not accurately reflect some instances of currently unexplained subfertility seen in human populations that may stem from more subtle hypomorphic alleles.
Alternative animal-based strategies to investigate germ cell development have included the use of grafting gonadal fragments onto immunocompromised mice (Paris and Schlatt, 2007), a technique used elegantly by Naughton et al. (2006) to allow the study of the effects of disrupting the glial cell line-derived neurotrophic factor (GDNF) system on early spermatogenesis, overcoming the perinatal mortality seen in GDNF-null mice. Isolation and genetic modification of SSCs, followed by transplantation back into a host testis to restore spermatogenesis is another approach (Nagano et al., 2001, 2002; Kanatsu-Shinohara et al., 2006), however, both of these techniques require sufficient time for grafts or transplants to become established.
The ability to generate ‘artificial’ (i.e. in vitro-generated) gametes would also have potential therapeutic use where couples currently require gamete donation through absence of their own (Nagy and Chang, 2005). These include individuals suffering from a diverse range of conditions including genetic causes both known (e.g. Y chromosome deletions) and unknown, chromosomal abnormalities (such as Turner's and Klinefelter's syndromes), and iatrogenically following chemotherapy for cancer or inflammatory conditions. Additionally, there is a need for oocytes for the refinement of somatic cell nuclear transfer for stem cell derivation, progress in which is difficult with the restricted numbers of oocytes available through current donation programmes (Hall and Stojkovic, 2006).
What then are the alternative approaches available to study germ cell development? Recent advances in the derivation of germ cells from stem cells may hold some promise. The fields of research on pluripotent stem cells (i.e. those capable of indefinite self-renewal and differentiating into all three germ layers in vitro or in vivo when introduced into a blastocyst to form a chimera) and early germ cells are historically deeply interlinked (Zwaka and Thomson, 2005). Embryonal carcinoma cells (ECCs), the first mammalian pluripotent cells to be isolated and cultured, were derived from rare testicular germ cell tumours known as teratocarcinomas (Solter, 2006). Work establishing the optimal culture conditions for maintaining ECCs in the undifferentiated state laid the foundations for the isolation and propagation of embryonic stem cells (ESCs) from the pluripotent inner cell mass of the mouse, and much later primate and human blastocysts (Chambers and Smith, 2004; Solter, 2006).
Although themselves unipotent (capable only of giving rise to the cell types of one lineage), germ cells share many transcriptional and phenotypic similarities with ESCs (Zwaka and Thomson, 2005). Primordial germ cells (PGCs) are the last cells of the mammalian fetus to retain expression of transcription factors Oct-3/4 (encoded by the gene Pou5F1) and Nanog, involved in the maintenance of the undifferentiated state (Pesce et al., 1998; Chambers et al., 2003; Chambers et al., 2007), and they express similar cell surface markers to pluripotent stem cells such as alkaline phosphatase (Chiquoine, 1954). The concept that a close relationship exists between germ cells and stem cells is reinforced by the finding that under appropriate conditions of in vitro culture, unipotent PGCs, and even post-natal and adult spermatogonia, are capable of undergoing reprogramming into a pluripotent state (Matsui et al., 1992; Resnick et al., 1992; Kanatsu-Shinohara et al., 2004a; Guan et al., 2006).
This review focuses on recent developments in the in vitro modelling of germ cell development using stem cells with particular reference to the implications of these for the understanding of human gametogenesis and defects therein. We address the major approaches taken by stem cell biologists to study germ cell development, and consider the challenges that need to be overcome to make these technological developments useful research tools. Finally, we consider how new work on the derivation of pluripotent stem cells from terminally differentiated adult cells through the introduction of reprogramming factors has the potential to impact on the in vitro modelling of germ cell development.
Germ cell development in rodents and humans
Producing accurate in vitro models of germ cell development is dependent on understanding the normal process of establishing the germ cell lineage in vivo. In lower organisms, such as Drosophila and Caenorhabtidis elegans the germ cells are segregated from the soma at the earliest stage of development through the asymmetric distribution of proteins and mRNAs encoding factors required for germ cell development (Lehmann and Ephrussi, 1994; Seydoux and Schedl, 2001). In contrast, the formation of the first germ cells in mammals occurs comparatively late in development, through an inductive process known as epigenesis (Saitou et al., 2002; McLaren, 2003). This process has been most extensively studied in the mouse, where PGCs arise in the proximal epiblast of the embryo through the inductive action of extraembryonic ectoderm-derived bone morphogenetic proteins (BMPs) (Lawson and Hage, 1994; Lawson et al., 1999; Saitou et al., 2002). In mice, the earliest detectable precursors of the germ cell lineage are a group of six to eight cells expressing the transcriptional repressor Blimp1 in the epiblast cell layer adjacent to the extraembryonic ectoderm (McLaren and Lawson, 2005; Ohinata et al., 2005). Although this small group of Blimp1-positive cells is likely to constitute the first lineage-restricted germ cells in the embryo, surrounding cells may also be recruited to form the population of 40 or so alkaline phosphatase-positive ‘founder’ PGCs detectable at the base of the allantois at e7.5 (McLaren and Lawson, 2005). Blimp1 appears to be essential for the appropriate repression of the somatic Hox gene programme, which would otherwise direct them into same fate as the cells that surround them (Ohinata et al., 2005). Repression of the somatic programme in PGC precursors occurs concomitantly with the up-regulation of Stella (Dppa3), the earliest definitive germ cell marker (Saitou et al., 2002).
From e8.5 in mice, the founder PGCs migrate through the embryo, passing through the hindgut and the dorsal mesentery and arriving at the genital ridges around e10.5, wherein they become known as gonocytes (McLaren, 2003). Germ cell proliferation occurs throughout the migratory period, and continues for two to three days after colonizing the gonads, forming a population of around 25 000 gonocytes at e13.5 when proliferation ceases (Tam and Snow, 1981). Subsequently, these germ cells either cease mitosis and enter meiosis in the female, or progressively arrest in G0/G1 over several days in the male (Adams and McLaren, 2002; McLaren, 2003; Western et al., 2008).
The precise events and timings surrounding the earliest events in the establishment of the germ cell lineage in human embryos remain unknown. Migratory human PGCs have been reported in the endoderm of the human embryo as early as the fourth week of development, following a similar migratory path to that seen in mice through the hindgut and dorsal mesentery arriving at the gonads around the fifth week of development (Witschi, 1948). Colonization of the gonadal primordium is thought to be complete around 10 days later (Wartenburg, 1981).
The first overt signs of sexual dimorphism between the male and female gonads are detectable at around 6 weeks of development. Sex determination and the onset of expression of the testis-determining factor SRY in human fetal gonads occurs between 41 and 44 days post-ovulation in the gonads of male human fetuses, and the subsequent organization of the somatic and germ cells of the developing testis into the testicular cords is first visible at 44 days post-ovulation (Wartenburg, 1981; Hanley et al., 2000).
Interestingly, at this stage of development the human fetal ovary is reported to contain around 30 000 germ cells; ten times as many as are present in the testis of a fetus of the same age (Bendsen et al., 2003, 2006). The gonocytes of both sexes continue to expand by mitosis for a further 3 weeks, increasing 10-fold in number in both the testis and the ovary (resulting in 30 000 and 250 000–300 000 germ cells in the testis and ovary by 9 weeks post-fertilization, respectively) (Bendsen et al., 2003, 2006). However, despite these radical differences in germ cell number, we have been unable to detect significant differences in the levels of expression of key germ cell-associated genes such as deleted in azoospermia-like (DAZL) and the Dead-box RNA helicase (VASA) between human fetal testis and ovaries, although we do observe a significant sexual dimorphism in the expression of OCT-3/4 [A.J.C. and R.A.A. unpublished observations, Anderson et al. (2007)]. Interestingly, no such sex-specific differences in germ cell number have been reported in the mouse, in which both male and female fetal gonads contain roughly equal numbers of germ cells (Tam and Snow, 1981).
Key mouse/human differences in germ cell biology
Due to the regulatory, ethical and logistical difficulties in obtaining sufficient human fetal gonadal tissue for research, most studies into mammalian germ cell development have focused on the mouse, in the hope that these findings can be extrapolated to humans. However, work from our laboratory and others has begun to demonstrate that the differences between the two species in the sequence of events surrounding germ cell development extend beyond merely differences in timing.
Mouse PGCs express Oct-3/4 throughout their migratory period, and in addition up-regulate expression of the cytoplasmic RNA-binding proteins Dazl and Mvh (mouse vasa homologue, also known as Ddx4) as they colonize the gonadal ridges and become gonocytes (Fujiwara et al., 1994; Pesce et al., 1998; Seligman and Page, 1998; Toyooka et al., 2000; Kehler et al., 2004). Expression of these markers continues in male germ cells throughout the period of mitotic arrest and beyond the resumption of spermatogenesis after birth. Human gonocytes, in contrast, express only OCT-3/4 and DAZL proteins. Consistent with previous reports studying older specimens, DAZL protein localizes to the nuclei of gonocytes at this stage, the functional significance of which remains unknown (Rajpert-De Meyts et al., 2004; Anderson et al., 2007). VASA protein is undetectable in proliferating human gonocytes, although they do produce readily detectable amounts of VASA mRNA (Anderson et al., 2007), indicating that the transcripts of this gene may be subjected to similar translational regulation as occurs in the mouse (Reynolds et al., 2005).
In the human fetal ovary, the entry of gonocytes into meiosis is coincident with the loss of OCT-3/4 expression, the concomitant translocation of DAZL protein to the cytoplasm and the onset of detectable VASA protein expression (Stoop et al., 2005; Anderson et al., 2007). Whether these events are linked is unknown, but it is tempting to speculate that the translocation of DAZL protein to the cytoplasm might be necessary to allow the onset of translation of VASA mRNA. In the testis, germ cells undergo a similar maturational process, up-regulating DAZL and VASA, and down-regulating OCT-3/4, although this appears not to be linked to meiotic entry (Rajpert-De Meyts et al., 2004; Anderson et al., 2007).
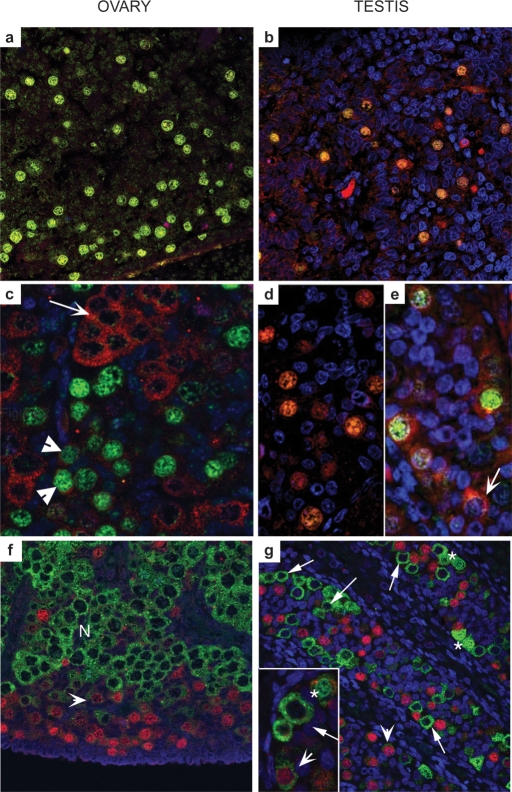
Spatial variations also exist in the expression of key germ cell markers between mouse and human during fetal germ cell development. In the human fetal ovary, proliferating OCT-3/4-positive germ cells are found at the periphery of the organ, with progressively more mature meiotic germ cells found in a gradient towards the centre (Fig. 1a–g) (Stoop et al., 2005; Anderson et al., 2007). This is in contrast with the mouse ovary, in which germ cells enter meiosis in a rostro-caudal wave along the long axis of the gonad (Menke et al., 2003; Bullejos and Koopman, 2004). In mice, the switch from mitotic proliferation to meiotic differentiation has been reported to be initiated by the diffusion of retinoic acid (RA) from the mesonephric duct into the most rostral part of the ovary, and its subsequent diffusion along the gonad (Bowles et al., 2006; Koubova et al., 2006), although this claim remains controversial and other mechanisms for regulating meiotic entry may exist (Best et al., 2008). Given the arrangement of germ cells in the human fetal ovary, it is difficult to envisage how such an analogous system could work, and the mechanisms regulating meiotic entry in human germ cells remain to be determined.
Figure 1:
Distinct subcellular localization of OCT-3/4, DAZL and VASA in human fetal gonads.
In contrast to mouse gonocytes, which express Oct-3/4, Dazl and Mvh proteins, first trimester ovaries (a, 61 day), and testes (b, 64 day) express only OCT-3/4 (green) and DAZL (red) both of which co-localize to germ cell nuclei. In ovaries from the second trimester (c, 14 week) DAZL protein is almost exclusively cytoplasmic and largely localizes to OCT-3/4-negative groups of cells (arrow); a few OCT-3/4-positive cells display low levels of DAZL expression in their cytoplasm (arrowheads). In second trimester testes (d, 16 week; e, 19 week), DAZL is still expressed in the nuclei of some OCT-3/4-positive germ cells but this pattern of expression is variable, with DAZL protein present in the cytoplasm of OCT-3/4-positive and OCT-3/4-negative (arrow, e) cells. (f) Mutually exclusive expression of OCT-3/4 and VASA in the human fetal ovary; cells with intense immuno-positive staining for OCT-3/4 are found at the periphery of the organ (red nuclei), while cytoplasmic VASA protein is most intense in cells located in nests (N) closer to the centre of the ovary. An intermediate population of cells with low intensity nuclear staining for OCT-3/4 and low intensity staining for VASA (arrowheads) was also present. (g) In second trimester human fetal testis, OCT-3/4-positive and VASA-positive germ cells are found within the same seminiferous cords; germ cells with intense nuclear OCT-3/4 expression (red nuclei) are VASA-negative and those with intense cytoplasmic expression of VASA (e.g. arrowed in inset g) were OCT-3/4-negative. Two other populations of male germ cells can be identified; a population with low intensity expression of both OCT-3/4 and VASA (arrowheads) and cells with nuclear VASA expression (asterisks) which were typically found in pairs (adapted from Anderson et al. (2007).
Another recently identified species-specific difference relates to the expression of the transcription factor SOX2, which is expressed in mouse, but not human germ cells (Perrett et al., 2008). SOX2 is a component of the core transcriptional machinery involved in the maintenance of pluripotency in stem cells, and works through binding the promoters of many pluripotency-associtated genes in concert with OCT-3/4 (Niwa, 2007). The functional significance of the lack of SOX2 expression in human germ cells is presently unclear, as they continue to express many of the genes considered dual OCT-3/4–SOX2 targets (Perrett et al., 2008). A screen to identify other germ cell-expressed SoxB-class transcription factors (the subfamily to which SOX2 belongs) that might substitute for SOX2 found none, suggesting that no functional redundancy exists within the SoxB subfamily in germ cells (Perrett et al., 2008). Looijenga and colleagues have suggested that SOX17 may fulfil this role instead (de Jong et al., 2008). These data are interesting given the suggestion that OCT-3/4 may fulfil different functions in germ cells and stem cells through modulating different sets of target genes (Hubbard and Pera, 2003; Kehler et al., 2004).
The differences in localization and onset of expression of these marker genes relative to the stage of germ cell maturation suggest significant differences may exist in the control of germ cell development between mouse and human. Differences between the two species are not limited to spatiotemporal variations in marker gene expression however. Rodents also lack some of the candidate fertility factors known to be clinically relevant in humans. For example, the human Y-linked DAZ genes, which are candidate azoospermia factors in humans, have only autosomal homologues in mice (Reijo et al., 1995; Ruggiu et al., 1997). Therefore, while the study of such homologues can provide critical insight into the biology of the DAZ family proteins (Ruggiu et al., 1997), the clinically relevant condition of Y chromosome microdeletions encompassing the DAZ gene cluster cannot be replicated directly in rodents. There are, therefore, substantial limitations in using rodent models to understand human fertility and the aetiology of human infertility.
Strategies for in vitro culture of PGCs
A significant research effort by numerous labs worldwide has established roles for kit ligand/SCF, leukaemia inhibitory factor (LIF), basic fibroblast growth factor (bFGF), BMPs, forskolin and RA in the maintenance or expansion of isolated mouse PGCs and proliferating gonocytes in vitro (reviewed in De Felici and Pesce, 1994; Donovan, 1994; De Felici et al., 2004). While the study of the effects of some of these factors (especially SCF and RA) on germ cell development in vitro has provided significant insight into their role in vivo, the relevance of data acquired in vitro on others is unclear. For example, mice carrying a germ cell-specific deletion of gp130, the common receptor for interleukin-6 family ligands (which includes LIF), develop a normal complement of PGCs (Molyneaux et al., 2003), whereas blocking the gp130 receptor with an antibody results in the death of isolated PGCs cultured in vitro (Koshimizu et al., 1996), or suppression of meiotic entry (Chuma and Nakatsuji, 2001).
Despite the inclusion of these factors in culture media, PGCs or gonocytes have a limited proliferative lifespan in vitro, before undergoing either death or differentiation (Donovan, 1994). Furthermore, in the absence of appropriate programming from the somatic cells of the gonad, isolated male PGCs will enter meiosis in vitro at the same time as those isolated from female gonads, thus making the investigation of sex-specific differences in germ cell biology difficult to study ex vivo (Nakatsuji and Chuma, 2001).
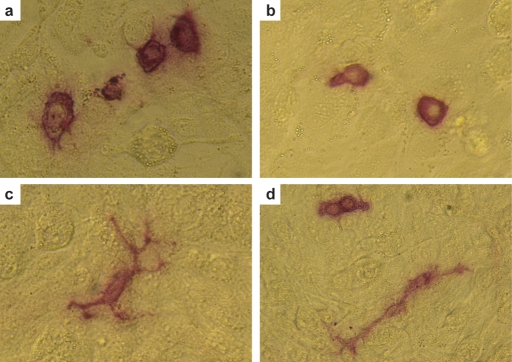
Little is known about the behaviour of isolated human PGCs and gonocytes in culture. Shamblott et al. (1998) reported two populations of human gonocytes in culture, namely ‘stationary’ (round, non-migratory) and ‘migratory’ (motile cells with long processes) germ cells. Turnpenny et al. (2003) described populations of poorly-proliferating and vigorously-proliferating human germ cells, though whether these populations are linked to the populations reported by Shamblott et al. is unclear. More recently, Tu et al. (2007) have also identified that human gonocytes divide into two distinct morphological populations (‘round’ and ‘spindly’), and that treatment with SCF increased the numbers of ‘round’ germ cells in culture compared with those with the ‘spindly’ morphology'. We have identified similar phenomena while trying to culture human gonocytes isolated from fetuses of ∼60 days gestational age on feeder layers of mitotically inactivated STO cells (Fig. 2a–d). How these populations relate to differences in the germ cells in vivo is unknown, but warrants further investigation.
Figure 2:
Morphology of human fetal gonocytes in vitro.
When cultured in vitro on feeder layers of mitotically inactivated STO cells, gonocytes isolated from first trimester testes or ovaries (∼60–65 days gestational age) adopt one of two morphologies, namely round (a, b and d) or migratory (c and d). Both populations can be found in close proximity (d). Germ cells, but not feeders, display strong alkaline phosphatase activity (red).
EGCs: stem cells from PGCs
Matsui et al. (1992) and Resnick et al. (1992) reported the establishment of long term exponentially expanding cultures of mouse PGCs. When cultured on a mitotically inactivated mouse fibroblast feeder layer in the presence of LIF, SCF and bFGF, small numbers of PGCs escaped the in vitro block on proliferation and acquired the ability to divide indefinitely (Matsui et al., 1992; Resnick et al., 1992). These proliferating germ cells could also contribute to a chimera when introduced to a blastocyst (Labosky et al., 1994). Germ cells that have undergone this ‘reprogramming’ event in culture cells are known as embryonic germ cells (EGCs).
Derivation of EGCs from human PGCs/gonocytes was first reported shortly after the derivation of human ESCs (hESCs), using culture conditions similar to those used to derive mouse EG cells (Shamblott et al., 1998; Turnpenny et al., 2003; Liu et al., 2004). Unlike hESCs however, hEGCs do not appear to be fully pluripotent (Aflatoonian and Moore, 2005; Turnpenny et al., 2006). Although capable of giving rise to representative tissues of all three germ layers in vitro, it has not yet been demonstrated that hEGCs are capable of forming teratomas when introduced into immunocompromised mice (the standard test of pluripotency for hESCs in the absence of a chimera-forming assay) (Shamblott et al., 1998; Turnpenny et al., 2003; Turnpenny et al., 2005).
The exact status of hEGCs remains unclear as the repertoire of cell surface antigens they express differs from that expressed by hESCs. Undifferentiated hESCs express the stage-specific embryonic antigens SSEA3 and SSEA4, and up-regulate the SSEA1 antigen on differentiation (Thomson and Odorico, 2000). hEGCs appear to express all three simultaneously, although this too differs from the in vivo situation where human gonocytes express SSEA1, and the expression of SSEA4 is not restricted to the germ cells in the gonad (Shamblott et al., 1998; Kerr et al., 2008). The recent finding that both human gonocytes and hEGCs do not express SOX2 indicates that the latter are at least in part representative in vitro derivatives of the former, and the lack of SOX2 expression in hEGCs may in part explain their lack of total pluripotency (Perrett et al., 2008).
Surprisingly, given their germ cell origin, the possibility of using EGCs as an in vitro model for studying germ cell behaviour has not been extensively explored. Toyooka et al. (2000) reported that culturing mEGCs in aggregates with somatic gonadal cells resulted in the up-regulation of Mvh expression (and by extension germ cell formation in vitro), a response not seen when mESCs were used in similar aggregate cultures. Although this overlooked result perhaps represents the first demonstration of germ cell derivation from a pluripotent stem cell, it more importantly indicates that unlike mESCs, mEGCs may inherit the ability to respond to signals from the gonadal microenvironment from the germ cells from which they were derived (Toyooka et al., 2000). EGCs may therefore represent a potential source of reproducible in vitro-derived germ cells for the study of germ-soma gonadal interactions.
There are difficulties however in the use of hEGCs. They are difficult to maintain in culture compared with hESCs (Aflatoonian and Moore, 2005; Turnpenny et al., 2005), they appear not to retain their pluripotency for longer than 15–20 passages and cyropreservation has proved difficult (Shamblott et al., 1998; Turnpenny et al., 2005). Despite this, EGCs may still provide a valuable model for some studies of germ cell development.
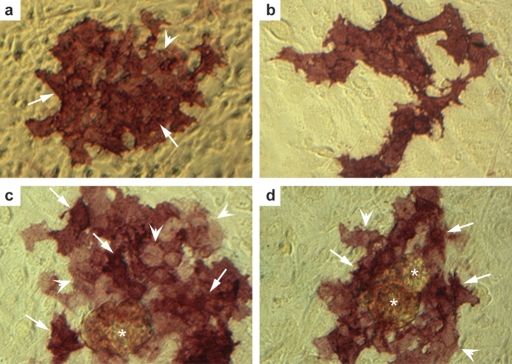
Within our own laboratory we have recently investigated the potential for hEGCs to act as a useful model system for investigating early human germ cell development. As with previous reports, we have found these cells difficult to identify in culture by light microscopy without fixing and staining for alkaline phosphatase activity, and the efficiency of derivation in our hands is significantly lower than that previously reported (Shamblott et al., 1998). We have however, succeeded in establishing proliferative cultures of putative hEGCs from fetal gonads lasting several weeks (Fig. 3a–d) and efforts to determine which, if any, characteristics of PGCs are retained following their conversion to EGCs are underway.
Figure 3:
Colonies of putative human EGCs derived from cultured human gonocytes.
Proliferative cultures of putative human EGCs at late passage 2 (3 weeks in culture), stained for alkaline phosphatase activity (red). Note the variable staining intensity within colonies from regions of strong (arrows, a, c and d) to weak (arrowheads, a, c and d) staining. Cultures were often found in association with aggregations of feeders carried over during subculture (asterixes).
Pluripotent stem cell-derived gametes: making germ cells from stem cells
Although pluripotent ESCs are capable of contributing to the germline of chimaeric embryos and forming fully functional gametes in vivo (Bradley et al., 1984), Toyooka et al. (2000) were unable to detect the induction of Mvh expression in co-cultures of mESCs and gonadal somatic cells, suggesting that mESCs were either unresponsive to the gonadal signals inducing germ cell development, or that the signals produced by somatic cells at this stage were insufficient to induce germ cell development. Subsequent successful derivation of germ cells from ESCs in vitro in 2003 was a significant breakthrough, and one likely to have significant impact on the study of germ cell development (Hubner et al., 2003). Table I gives the major findings of the publications in this field to date.
Table I.
Major developments in pluripotent stem cell-derived gametes.
| Source material | Method | Endpoint | Reference |
|---|---|---|---|
| Mouse ES cells | Monolayer differentiation, followed by sorting on cell surface markers | Formation of follicle-like structures, progressing to blastocyst-like structures by possible parthenogenesis | Hubner et al. (2003) |
| Mouse ES cells | Formation of EBs, isolation of SSEA1-positive cells | EG-like cells isolated when rare residual SSEA1-positive cells isolated from EBs and cultured in presence of RA | Geijsen et al. (2004) |
| Isolation of cells with haploid DNA content expressing post-meiotic male germ cell markers after extended EB differentiation | |||
| Fertilization of oocytes with sperm-like cells, progression to morula stage embryo | |||
| Mouse ES cells | Mvh-LacZ ES cells cultured in aggregates with BMP4-expressing somatic cells | Aggregates formed new tubule structures de novo under testis capsule. All stages of spermatogenic cells detectable | Toyooka et al. (2003) |
| LacZ-positive ES cells isolated and aggregated with fetal gonadal somatic cells, transplanted into testis capsule | Functionality of sperm not tested | ||
| Human ES cells | ES cells differentiated into EBs | Transcripts for PGC, pre-meiotic and post-meiotic germ cells expressed in time-dependent fashion during course of differentiation. Detection of VASA protein in cells at periphery of EBs | Clark et al. (2004) |
| Failure of meiosis, simultaneous detection of male and female germ cell transcriptional programme irrespective of ES cell karyotype | |||
| Porcine skin-derived stem cells | Differentiation into sphere-like structures, oocyte-like cells cultured in presence of porcine follicular fluid | Follicle-like structures containing oocytes with zona pellucida-like casings | Dyce et al. (2005) |
| Progression to blastocyst stage when fertilized | |||
| Mouse ES cells | Differentiation into EBs in the presence of testicular cell-conditioned medium | Ovary-like structures containing oocyte-like cells produced at high efficiency | Lacham-Kaplan et al. (2006) |
| Mouse ES cells | Stimulation with RA, isolation and culture of Stra8-GFP-positive cells, subsequent differentiation into ‘haploid’ cells expressing post-meiotic Prm1-DsRed reporter | Haploid sperm like-cells isolated, used in ICSI producing live mice, although these died shortly after birth due to suspected imprinting defects | Nayernia et al. (2006b) |
EB, embryoid bodies; EG, embryonic germ; BMP, bone morphogenic protein; ES, embryonic stem; RA, retinoic acid, PGC, primordial germ cell; SSEA, stage-specific embryonic antigen.
Hubner et al. (2003) first reported the derivation of germ cells from mESCs. They harnessed a green fluorescent protein (GFP) reporter construct fused to part of the mouse Oct-3/4 promoter gene that restricted expression of the reporter gene to early germ cells and were thus able to detect increasing numbers of GFP-positive cells when mESCs were bulk differentiated in a monolayer. Analysis of the GFP-positive cells after sorting revealed that they had differentiated into early germ cells on the basis of the expression of germ cell markers c-Kit and Mvh. Extended culture of these cells resulted in the formation of aggregates that detached from the culture surface and expanded in suspension, forming structures resembling ovarian follicles, comprising an oocyte with associated somatic cells. Consistent with these structures being follicle-like, they were found to release estradiol into the culture medium. Eventually, the follicle-like structures extruded the putative oocytes, and in some instances the formation of blastocyst-like structures was detected, apparently through a parthenogenetic mechanism (Hubner et al., 2003).
Shortly after the report of mESC-derived ‘oocytes’, two other groups reported the derivation of male germ cells from mESCs. Geijsen et al. (2004) differentiated mESCs into three-dimensional cellular aggregates known as embryoid bodies (EBs), a system that promotes differentiation in the absence of LIF. Due to the lack of surface markers that can be used to sort PGCs from ESCs, Geijsen et al. sorted rare SSEA1-positive cells from EBs that may represent early germ cells or remaining undifferentiated mESCs, and cultured them in the presence of RA, which promotes PGC proliferation but mESC differentiation. Under these conditions, colonies of alkaline phosphatase-positive cells formed that could be cultured indefinitely under mESC culture conditions. These cells progressively underwent imprint erasure, a hallmark of germ cell development in vivo. This, coupled with the unlimited proliferative potential of these cells led the authors to suggest that these cells were probably equivalent to mEGCs—pluripotent derivatives of the EB-derived PGCs (Geijsen et al., 2004). Extending the culture period of the EBs for a further 3–4 weeks, followed by sorting of cells based on the expression of a marker of the acrosome, a structure found only in post-meiotic spermatids—revealed the existence of a haploid cell population. Importantly, isolation of these haploid sperm-like cells and their use in intracytoplasmic sperm injection (ICSI) resulted in oocyte fertilization and embryonic progression to a morula-like stage but no further, probably due to imprinting defects in the mESC-derived sperm-like cells (Geijsen et al., 2004). Transcripts for anti-Müllerian hormone (Amh) and the luteinizing hormone receptor (Lhr) were found in EBs, suggesting that gonadal somatic-like cells were arising in tandem with germ cells (Geijsen et al., 2004). If indeed germ cells and their supporting gonadal somatic cells are closely associated within EBs, this may lead to new insights into the molecular architecture of the germ cell niche.
Toyooka et al. (2003) found that when co-cultured with somatic cells expressing Bmp4, small numbers of mESCs up-regulated GFP- or LacZ-reporter genes under the control of the Mvh promoter, reflecting commitment to the germ cell lineage. Mixing the GFP- or LacZ-positive cells with fetal gonadal somatic cells and implantation of the aggregates beneath the testis capsule resulted in the formation of seminiferous tubule-like structures distinct from those of the host testis. These tubules appeared to contain normal spermatogenesis, and morphologically normal spermatozoa with condensed nuclei and tails. Sperm isolated from the transplant-derived tubules were found to contain the LacZ transgene, demonstrating that they were indeed derived from mESCs, although the fertilizing ability of the sperm was not reported (Toyooka et al., 2003).
Subsequently, Lacham-Kaplan et al. (2006) reported that EBs cultured in media conditioned by cultures of neonatal testicular cells gave rise to ovary-like structures containing cells expressing oocyte-specific markers with remarkably high frequency (83%), although replication of this result has yet to be reported. Novak et al. (2006) derived follicle-like structures similar to those seen by Hubner et al. (2003) but found that they were unable to correctly progress through meiosis, indicating that although in vitro haploid germ cell formation has been reported, synapsis, recombination and appropriate segregation of chromosomes may not occur.
Building on earlier work in teratocarcinoma and bone-marrow-derived stem cells, Nayernia et al. (2006b) reported the production of live offspring from sperm produced in vitro. Using two fluorescent reporter genes (GFP and DsRed) under the control of spermatogonia- (Stra8) and post-meiotic spermatid-specific (Prm1) promoters respectively, they were able to track the progression of mESC-derived germ cells through the spermatogenic process and isolate cells with a sperm-like morphology. Use of these cells for ISCI resulted in a number of pregnancies, although the majority died in utero and the remainer died within a few months of being born, probably as a result of imprinting defects (Nayernia et al., 2006b). This development, if able to be replicated, would represent a significant step forward, and suggest that differentiation of mESCs into germ cells has the potential to produce fully functional gametes.
Progress in producing germ cells from human ESCs has been less successful. To date, Clark et al. (2004) are the only group to have reported the successful formation of germ cells from hESCs differentiated into EBs. Differentiation of hESCs over a period of 2 weeks resulted in a steady decline in the levels of pluripotency markers NANOG and OCT-3/4, and an up-regulation of germ cell-specific transcripts including VASA, SYCP1 and PUMILIO-2. Of particular interest is the finding that differentiating hESCs expressed both the oocyte-specific gene GDF9 and the spermatid-specific gene TEKT1 irrespective of whether the cells were karyotypically female (XX) or male (XY) (Clark et al., 2004). Consistent with the report that mESC-derived oocytes were unable to correctly undergo meiosis (Novak et al., 2006), Clark et al. (2004) were unable to detect hESC-derived germ cells undergoing normal meiosis as judged by the formation of synaptonemal complexes. The simultaneous activation of both the male and female germ cell transcriptional programmes in hESC-derived germ cells may explain the meiotic failure seen in hESC-derived germ cells.
Uses and limitations of stem cell-derived gametes
Despite initial promise there has been little progress using this system as a model for gametogenesis. Germ cell derivation remains very inefficient with less than one of one million starting cells becoming a germ cell (Geijsen et al., 2004). Why the efficiency of this process is so low is unclear, though it may be related to the failure of meiosis seen by Novak et al. and/or the simultaneous activation of both male and female germ cell programmes in ESC-derived gametes resulting in meiotic catastrophe (Clark et al., 2004; Novak et al., 2006). Methodologically, culturing cells in high glucose media appears to be beneficial for deriving germ cells from mESCs (Mizuno et al., 2006), and supplementing culture media with a cocktail of recombinant BMP4, 7 and 8b has been reported to enhance the efficiency of germ cell derivation from hESCs (Kee et al., 2006).
The recent finding that some hESC lines display greater propensities to differentiate along certain lineages than others is of particular interest (Osafune et al., 2008) and suggests that some ESC lines could produce gametes more efficiently that others. Although Clark et al. (2004) were unable to find significant differences in the expression levels of multiple germ cell-associated genes in three independent undifferentiated hESC lines, the quantitative RT–PCR method used in their experiment could only provide a global overview of the transcriptional landscape of the entire hESC population analysed. It is likely that stochastic differences in gene expression between individual ESCs within a differentiating population will have some influence on cell fate decisions. Indeed, immunostaining of undifferentiated mESCs and hESCs reveals a non-uniform pattern of markers including Nanog, Stella, and Dazl within individual colonies (Clark et al., 2004; Payer et al., 2006; Chambers et al., 2007).
A major potential benefit of using stem cells to model germ cell development is the ability to carry out genetic modifications in the starting cell population. Although the genetic modification of PGCs has been demonstrated using retroviruses (De Miguel et al., 2002), low survival rates and poor differentiation of isolated PGCs in vitro makes this a laborious system. Transgenes on the other hand can be readily introduced to ESCs, and clonal lines in which the construct has integrated can be isolated using drug selection. Furthermore, rather than having to use homologous recombination to target a gene for disruption, it should be possible to introduce siRNA constructs that target-specific genes of interest into the undifferentiated ESC population prior to differentiating them into germ cells. Knocking down specific genes in ESCs then assaying their ability to form germ cells when differentiated in vitro could provide a more rapid and efficient method for identifying new candidate fertility genes. The apparent failure of stem cell-derived gametes to undergo meiosis efficiently could also provide opportunities to study the molecular mechanisms regulating meiotic progression and provide insight into the causes of meiotic failure.
Stage-specific approaches to modelling germ cell development
Rather than attempting to recapitulate the entire germ cell differentiation pathway from ESCs in vitro, which appears to happen only with very low efficiency, recent developments in stem cell research may offer the opportunity to study specific stages of the process in vitro using cell lines derived from different stages.
In vivo the germ cell population arises from the proximal epiblast of the post-implantation embryo (McLaren, 2003). Whether the cells within an EB that give rise to germ cells in vitro go through a similar epiblast-like stage early in their differentiation is yet to be established, although the finding that treatment with BMPs increases the efficiency of ESC-derived germ cell formation might suggest that this is the case (Kee et al., 2006). Two groups have recently reported the isolation and culture of pluripotent stem cells from the mouse epiblast (Brons et al., 2007; Tesar et al., 2007). These cells can be expanded and maintained in the undifferentiated state under the same culture conditions as hESCs (in the presence of activin/nodal and FGF, rather than LIF) and display a morphology and transcriptome more akin to that of hESCs than mESCs. Treatment of epiblast stem cells with BMP4 resulted in the up-regulation of Blimp1 within 24 h, followed by later Stella, thus recapitulating the sequence of changes in gene expression seen during the earliest stages of germ cell formation (Tesar et al., 2007). The efficiency of germ cell induction from epiblast stem cells has not yet been reported, but as undifferentiated epiblast stem cells do not express germ cell markers such as Dazl and Stella (Tesar et al., 2007), they may provide a cleaner model for the study of early germ cell development than ESCs, which express many supposedly ‘germ cell-specific’ transcripts.
New developments in the study of germline stem cells may also present opportunities to study the later stages of gametogenesis in vitro. Several groups have reported the isolation of SSCs and their expansion in culture (Kanatsu-Shinohara et al., 2003; Kubota et al., 2004). Although no reports of in vitro differentiation of SSCs to haploid sperm have been forthcoming, these cells can be transplanted back into recipient testes and re-establish spermatogenesis (Kanatsu-Shinohara et al., 2003). The genetic modification of SSCs in vitro has been demonstrated (Nagano et al., 2001, 2002; Kanatsu-Shinohara et al., 2004b, 2006). Combining the introduction of overexpression or knockdown constructs into SSCs with their subsequent transplantation back into recipient testes may offer a viable alternative approach for investigating the effect of disrupting candidate fertility genes on spermatogenesis.
Comparable developments may not be possible in the female as there is believed to be no analogous germ stem cell in the ovary. Work by Johnson et al. (2004, 2005) has challenged this with a report claiming the identification of germline stem cells in the post-natal mouse ovary, possibly deriving from bone marrow, which can replenish the primordial follicle pool. Although this work remains highly controversial (Telfer et al., 2005), it does reopen the prospect that a rare germline stem cell population exits within the post-natal ovary. The finding that bone marrow-derived stem cells from both mouse and human can form cells with spermatogenic characteristics may support this (Nayernia et al., 2006a).
Other strategies for manipulating cell differentiation
A major breakthrough likely to impact on the field of germ cell research is the recent discovery that differentiated adult cells can be reprogrammed into pluripotent stem cells. By introducing a cocktail of transgenes (Oct-3/4, Sox2, Klf4 and c-Myc), mouse fibroblasts could be reprogrammed to ESC-like ‘induced pluripotent cells’ (iPS cells) capable of differentiating into all three germ layers in vitro and in vivo (Takahashi and Yamanaka, 2006). The same group and others have since demonstrated that the same effect is achievable in terminally differentiated human dermal fibroblasts (Takahashi et al., 2007). It has since been reported that the use of c-Myc, the inclusion of which resulted in tumours in chimaeras mice produced using iPS cells, is dispensable for the derivation of both human and mouse iPS cells, although the use of viral vectors to introduce transgenes still poses a risk of insertional mutagenesis (Yu et al., 2007; Nakagawa et al., 2008). Whether human iPS cells are capable of generating germ cells in vitro has yet to be demonstrated but this approach has the potential to greatly increase access to pluripotent stem cells, especially for researchers in countries where hESC derivation or research is limited or banned by law.
Therapeutic potential
The use of ESC-derived gametes in assisted reproduction treatments remains a distant prospect. At present it would require the cloning of an individual, derivation of stem cells from the resultant blastocyst and the subsequent derivation of gametes from them. The recent discovery of iPS technology may offer a shortcut by allowing the creation of pluripotent stem cells directly from a patient's tissues that could then be used as a source of cells from which gametes could be derived in vitro. Even if these challenges could be overcome, major obstacles to the use of in vitro-derived gametes will still exist. The greatest of these is safety. Although cells with a haploid DNA content have been created in vitro whether they consistently form with the correct chromosome complement has yet to be demonstrated. hESCs have been shown to show chromosome gain during extended periods of culture (Draper et al., 2004), so any future use in therapy would require tight quality control procedures and frequent assaying for chromosomal aberrations.
Perhaps most importantly however, the appropriate erasure and re-establishment of sex-specific patterns of genomic imprints will need to be demonstrated. The mEGC-like cells generated by culturing mESC-derived PGCs were found to erase imprints, but no data were presented as to whether the correct androgenetic imprinting pattern was installed when EBs were allowed to differentiate for longer and produce haploid sperm-like cells (Geijsen et al., 2004). The inability of mESC-derived sperm to support development beyond the morula stage would suggest that, at least in part, it was not (Geijsen et al., 2004). Inconsistent erasure and re-establishment of imprints in mESC-derived sperm has been reported and is likely to underpin the premature death and phenotypic abnormalities seen in the offspring produced using in vitro-produced sperm (Nayernia et al., 2006b).
However, quite what level of proof would be required before in vitro-derived gametes could be used therapeutically remains unclear. Indeed, it could be argued that existing reproductive technologies (most notably ICSI) have been adopted without extensive safety analysis, and there remains a concern that assisted reproductive technologies may result in a higher frequency of imprinting defect-associated conditions such as Beckwith–Wiedeman syndrome (Cox et al., 2002; Maher et al., 2003; Allen and Reardon, 2005). Certainly, robust molecular and biochemical documentation of the appropriate resetting of imprints in hESC-derived gametes would be a prerequisite, as too would large-scale karyotyping analyses demonstrating that in vitro-derived gametes were repeatedly produced with the appropriate chromosome number. Determining whether meiosis and recombination occur appropriately during the process of deriving germ cells from pluripotent stem cells is also of some importance, given the suggestion that ESC-derived gametes are unable to complete meiosis (Clark et al., 2004; Novak et al., 2006). Should the meiotic process proceed in vitro without recombination, it would produce gametes genetically identical to the donor cells from which they were derived, albeit with half the number of chromosomes, which arguably has ethical implications for the identity of the offspring.
In the shorter term, ESC-derived artificial gametes have the potential to make a significant clinical impact in two ways. First, a major practical application for all stem cell-derived tissues is in toxicity screening for drugs in development (Rubin, 2008). The effects of candidate drugs on stem cell-derived gametes could provide useful pre-clinical insight into the potential effects of any drug on reproductive function both in screening for toxicity and for application in contraception, and could be extended to investigate the impact of environmental chemicals on germ cell development. Secondly, the short supply of good-quality human oocytes for use in somatic cell nuclear transfer experiments is a major factor limiting research into the generation of so-called ‘patient-specific’ hESC lines with great potential therapeutic benefit (Hall and Stojkovic, 2006). Should the derivation of oocytes from mESCs be repeated using hESCs, it may be that this route could provide a potentially limitless source of in vitro-derived oocytes that can be used as recipients for somatic cell nuclear transfer (Hubner et al., 2003; Nagy and Chang, 2007).
Conclusions
The use of stem cells to model germ cell development in vitro remains an avenue of research which is still very much in its infancy. Confirmation of the reports of Hubner et al. (2003), Toyooka et al. (2003) and Geijsen et al. (2004) at least in part by other groups (Novak et al., 2006; Kerkis et al., 2007), demonstrates that this approach will be of scientific use, and it is likely that issues of efficiency and robustness will be resolved. Information obtained from in vitro germ cell models will reciprocally inform the study of human germ cell development, complimenting data derived from fetal material. As the progression of in vitro-produced human germ cells has yet to pass the premeiotic stage (Clark et al., 2004), the prospect of this technology being used for therapeutic benefit remains a distant but exciting possibility. The rapid advancement of stem cell research, particularly with respect to iPS technology, suggests that useful in vitro approaches to modelling germ cell development are unlikely to be a long way off.
Funding
This work is supported by the UK Medical Research Council (WBS U.1276.00.002.00001.01).
Acknowledgements
We thank Joan Creiger, Anne Saunderson and the staff of the Bruntsfield Suite of the Royal Infirmary of Edinburgh for help with collection of tissue samples; Maxime Mioulane, Dr Joe Mee and Stem Cell Sciences plc for assistance in deriving the human Embryonic Germ Cells; Dr Rod Mitchell and Ms Gillian Cowan for critical reading of the manuscript, and Mr Ronnie Grant for assistance with figures.
References
- Adams IR, McLaren A. Sexually dimorphic development of mouse primordial germ cells: switching from oogenesis to spermatogenesis. Development. 2002;129:1155–1164. doi: 10.1242/dev.129.5.1155. [DOI] [PubMed] [Google Scholar]
- Aflatoonian B, Moore H. Human primordial germ cells and embryonic germ cells, and their use in cell therapy. Curr Opin Biotechnol. 2005;16:530–535. doi: 10.1016/j.copbio.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Allen C, Reardon W. Assisted reproduction technology and defects of genomic imprinting. Bjog. 2005;112:1589–1594. doi: 10.1111/j.1471-0528.2005.00784.x. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Fulton N, Cowan G, Coutts S, Saunders PT. Conserved and divergent patterns of expression of DAZL, VASA and OCT4 in the germ cells of the human fetal ovary and testis. BMC Dev Biol. 2007;7:136. doi: 10.1186/1471-213X-7-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendsen E, Byskov AG, Laursen SB, Larsen HP, Andersen CY, Westergaard LG. Number of germ cells and somatic cells in human fetal testes during the first weeks after sex differentiation. Hum Reprod. 2003;18:13–18. doi: 10.1093/humrep/deg057. [DOI] [PubMed] [Google Scholar]
- Bendsen E, Byskov AG, Andersen CY, Westergaard LG. Number of germ cells and somatic cells in human fetal ovaries during the first weeks after sex differentiation. Hum Reprod. 2006;21:30–35. doi: 10.1093/humrep/dei280. [DOI] [PubMed] [Google Scholar]
- Best D, Sahlender DA, Walther N, Peden AA, Adams IR. Sdmg1 is a conserved transmembrane protein associated with germ cell sex determination and germline-soma interactions in mice. Development. 2008;135:1415–1425. doi: 10.1242/dev.019497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, et al. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- Bradley A, Evans M, Kaufman MH, Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- Bullejos M, Koopman P. Germ cells enter meiosis in a rostro-caudal wave during development of the mouse ovary. Mol Reprod Dev. 2004;68:422–428. doi: 10.1002/mrd.20105. [DOI] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cells. 2003;133:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Chambers I, Smith A. Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene. 2004;23:7150–7160. doi: 10.1038/sj.onc.1207930. [DOI] [PubMed] [Google Scholar]
- Chambers I, Silva J, Colby D, Nichols J, Nijmeijer B, Robertson M, Vrana J, Jones K, Grotewold L, Smith A. Nanog safeguards pluripotency and mediates germline development. Nature. 2007;450:1230–1234. doi: 10.1038/nature06403. [DOI] [PubMed] [Google Scholar]
- Chiquoine AD. The identification, origin, and migration of the primordial germ cells in the mouse embryo. Anat Rec. 1954;118:135–146. doi: 10.1002/ar.1091180202. [DOI] [PubMed] [Google Scholar]
- Chuma S, Nakatsuji N. Autonomous transition into meiosis of mouse fetal germ cells in vitro and its inhibition by gp130-mediated signaling. Dev Biol. 2001;229:468–479. doi: 10.1006/dbio.2000.9989. [DOI] [PubMed] [Google Scholar]
- Clark AT, Bodnar MS, Fox M, Rodriquez RT, Abeyta MJ, Firpo MT, Pera RA. Spontaneous differentiation of germ cells from human embryonic stem cells in vitro. Hum Mol Genet. 2004;13:727–739. doi: 10.1093/hmg/ddh088. [DOI] [PubMed] [Google Scholar]
- Cooke HJ, Saunders PT. Mouse models of male infertility. Nat Rev Genet. 2002;3:790–801. doi: 10.1038/nrg911. [DOI] [PubMed] [Google Scholar]
- Cox GF, Burger J, Lip V, Mau UA, Sperling K, Wu BL, Horsthemke B. Intracytoplasmic sperm injection may increase the risk of imprinting defects. Am J Hum Genet. 2002;71:162–164. doi: 10.1086/341096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felici M, Pesce M. Growth factors in mouse primordial germ cell migration and proliferation. Prog Growth Factor Res. 1994;5:135–143. doi: 10.1016/0955-2235(94)90001-9. [DOI] [PubMed] [Google Scholar]
- De Felici M, Scaldaferri ML, Lobascio M, Iona S, Nazzicone V, Klinger FG, Farini D. Experimental approaches to the study of primordial germ cell lineage and proliferation. Hum Reprod Update. 2004;10:197–206. doi: 10.1093/humupd/dmh020. [DOI] [PubMed] [Google Scholar]
- de Jong J, Stoop H, Gillis A, van Gurp R, van de Geijn GJ, Boer MD, Hersmus R, Saunders P, Anderson R, Oosterhuis J, et al. Differential expression of SOX17 and SOX2 in germ cells and stem cells has biological and clinical implications. J Pathol. 2008;215:21–30. doi: 10.1002/path.2332. [DOI] [PubMed] [Google Scholar]
- De Kretser DM, Baker HW. Infertility in men: recent advances and continuing controversies. J Clin Endocrinol Metab. 1999;84:3443–3450. doi: 10.1210/jcem.84.10.6101. [DOI] [PubMed] [Google Scholar]
- De Miguel MP, Cheng L, Holland EC, Federspiel MJ, Donovan PJ. Dissection of the c-Kit signaling pathway in mouse primordial germ cells by retroviral-mediated gene transfer. Proc Natl Acad Sci USA. 2002;99:10458–10463. doi: 10.1073/pnas.122249399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan PJ. Growth factor regulation of mouse primordial germ cell development. Curr Top Dev Biol. 1994;29:189–225. doi: 10.1016/s0070-2153(08)60551-7. [DOI] [PubMed] [Google Scholar]
- Draper JS, Smith K, Gokhale P, Moore HD, Maltby E, Johnson J, Meisner L, Zwaka TP, Thomson JA, Andrews PW. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol. 2004;22:53–54. doi: 10.1038/nbt922. [DOI] [PubMed] [Google Scholar]
- Dyce PW, Wen L, Li J. In vitro germline potential of stem cells derived from fetal porcine skin. Nat Cell Biol. 2006;8:313–314. doi: 10.1038/ncb1388. [DOI] [PubMed] [Google Scholar]
- Feng LX, Chen Y, Dettin L, Pera RA, Herr JC, Goldberg E, Dym M. Generation and in vitro differentiation of a spermatogonial cell line. Science. 2002;297:392–395. doi: 10.1126/science.1073162. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Komiya T, Kawabata H, Sato M, Fujimoto H, Furusawa M, Noce T. Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression in germ cell lineage. Proc Natl Acad Sci USA. 1994;91:12258–12262. doi: 10.1073/pnas.91.25.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijsen N, Horoschak M, Kim K, Gribnau J, Eggan K, Daley GQ. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature. 2004;427:148–154. doi: 10.1038/nature02247. [DOI] [PubMed] [Google Scholar]
- Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- Hall VJ, Stojkovic M. The status of human nuclear transfer. Stem Cell Rev. 2006;2:301–308. doi: 10.1007/BF02698057. [DOI] [PubMed] [Google Scholar]
- Hanley NA, Hagan DM, Clement-Jones M, Ball SG, Strachan T, Salas-Cortés L, McElreavey K, Lindsay S, Robson S, Bullen P, et al. SRY, SOX9, and DAX1 expression patterns during human sex determination and gonadal development. Mech Dev. 2000;91:403–407. doi: 10.1016/s0925-4773(99)00307-x. [DOI] [PubMed] [Google Scholar]
- Hofmann MC, Hess RA, Goldberg E, Millan JL. Immortalized germ cells undergo meiosis in vitro. Proc Natl Acad Sci USA. 1994;91:5533–5537. doi: 10.1073/pnas.91.12.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard EJ, Pera RA. A germ-cell odyssey: fate, survival, migration, stem cells and differentiation. Meeting on germ cells. EMBO Rep. 2003;4:352–357. doi: 10.1038/sj.embor.embor807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, De La Fuente R, Wood J, Strauss JF, 3rd, Boiani M, Scholer HR. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300:1251–1256. doi: 10.1126/science.1083452. [DOI] [PubMed] [Google Scholar]
- Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, Tschudy KS, Tilly JC, Cortes ML, Forkert R, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–315. doi: 10.1016/j.cell.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S, et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;a 119:1001–1012. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Toyokuni S, Shinohara T. Transgenic mice produced by retroviral transduction of male germ line stem cells in vivo. Biol Reprod. 2004;b 71:1202–1207. doi: 10.1095/biolreprod.104.031294. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ikawa M, Takehashi M, Ogonuki N, Miki H, Inoue K, Kazuki Y, Lee J, Toyokuni S, Oshimura M, et al. Production of knockout mice by random or targeted mutagenesis in spermatogonial stem cells. Proc Natl Acad Sci USA. 2006;103:8018–8023. doi: 10.1073/pnas.0601139103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee K, Gonsalves JM, Clark AT, Pera RA. Bone morphogenetic proteins induce germ cell differentiation from human embryonic stem cells. Stem Cells Dev. 2006;15:831–837. doi: 10.1089/scd.2006.15.831. [DOI] [PubMed] [Google Scholar]
- Kehler J, Tolkunova E, Koschorz B, Pesce M, Gentile L, Boiani M, Lomeli H, Nagy A, McLaughlin KJ, Scholer HR, et al. Oct4 is required for primordial germ cell survival. EMBO Rep. 2004;5:1078–1083. doi: 10.1038/sj.embor.7400279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkis A, Fonseca SA, Serafim RC, Lavagnolli TM, Abdelmassih S, Abdelmassih R, Kerkis I. In vitro differentiation of male mouse embryonic stem cells into both presumptive sperm cells and oocytes. Cloning Stem Cells. 2007;9:535–548. doi: 10.1089/clo.2007.0031. [DOI] [PubMed] [Google Scholar]
- Kerr CL, Hill CM, Blumenthal PD, Gearhart JD. Expression of pluripotent stem cell markers in the human fetal testis. Stem Cells. 2008;26:412–421. doi: 10.1634/stemcells.2007-0605. [DOI] [PubMed] [Google Scholar]
- Koshimizu U, Taga T, Watanabe M, Saito M, Shirayoshi Y, Kishimoto T, Nakatsuji N. Functional requirement of gp130-mediated signaling for growth and survival of mouse primordial germ cells in vitro and derivation of embryonic germ (EG) cells. Development. 1996;122:1235–1242. doi: 10.1242/dev.122.4.1235. [DOI] [PubMed] [Google Scholar]
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci USA. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labosky PA, Barlow DP, Hogan BL. Embryonic germ cell lines and their derivation from mouse primordial germ cells. Ciba Found Symp. 1994;182:157–168. doi: 10.1002/9780470514573.ch9. discussion 168–178. [DOI] [PubMed] [Google Scholar]
- Lacham-Kaplan O, Chy H, Trounson A. Testicular cell conditioned medium supports differentiation of embryonic stem cells into ovarian structures containing oocytes. Stem Cells. 2006;24:266–273. doi: 10.1634/stemcells.2005-0204. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Hage WJ. Clonal analysis of the origin of primordial germ cells in the mouse. Ciba Found Symp. 1994;182:68–84. doi: 10.1002/9780470514573.ch5. discussion 84–91. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, Hogan BL. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999;13:424–436. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Shah C, Xu EY. Gene trap mutagenesis: a functional genomics approach towards reproductive research. Mol Hum Reprod. 2007;13:771–779. doi: 10.1093/molehr/gam069. [DOI] [PubMed] [Google Scholar]
- Lehmann R, Ephrussi A. Germ plasm formation and germ cell determination in Drosophila. Ciba Found Symp. 1994;182:282–296. doi: 10.1002/9780470514573.ch16. discussion 296–300. [DOI] [PubMed] [Google Scholar]
- Liu S, Liu H, Pan Y, Tang S, Xiong J, Hui N, Wang S, Qi Z, Li L. Human embryonic germ cells isolation from early stages of post-implantation embryos. Cell Tissue Res. 2004;318:525–531. doi: 10.1007/s00441-004-0990-7. [DOI] [PubMed] [Google Scholar]
- Maher ER, Brueton LA, Bowdin SC, Luharia A, Cooper W, Cole TR, Macdonald F, Sampson JR, Barratt CL, Reik W, et al. Beckwith-Wiedemann syndrome and assisted reproduction technology (ART) J Med Genet. 2003;40:62–64. doi: 10.1136/jmg.40.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y, Zsebo K, Hogan BL. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992;70:841–847. doi: 10.1016/0092-8674(92)90317-6. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ. Genetic dissection of mammalian fertility pathways. Nat Cell Biol. 2002;4(Suppl):s41–s49. doi: 10.1038/ncb-nm-fertilityS41. [DOI] [PubMed] [Google Scholar]
- McLaren A. Primordial germ cells in the mouse. Dev Biol. 2003;262:1–15. doi: 10.1016/s0012-1606(03)00214-8. [DOI] [PubMed] [Google Scholar]
- McLaren A, Lawson KA. How is the mouse germ-cell lineage established? Differentiation. 2005;73:435–437. doi: 10.1111/j.1432-0436.2005.00049.x. [DOI] [PubMed] [Google Scholar]
- Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol. 2003;262:303–312. doi: 10.1016/s0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Tokumasu A, Nakamura A, Hayashi Y, Kojima Y, Kohri K, Noce T. Genes associated with the formation of germ cells from embryonic stem cells in cultures containing different glucose concentrations. Mol Reprod Dev. 2006;73:437–445. doi: 10.1002/mrd.20395. [DOI] [PubMed] [Google Scholar]
- Molyneaux KA, Schaible K, Wylie C. GP130, the shared receptor for the LIF/IL6 cytokine family in the mouse, is not required for early germ cell differentiation, but is required cell-autonomously in oocytes for ovulation. Development. 2003;130:4287–4294. doi: 10.1242/dev.00650. [DOI] [PubMed] [Google Scholar]
- Nagano M, Brinster CJ, Orwig KE, Ryu BY, Avarbock MR, Brinster RL. Transgenic mice produced by retroviral transduction of male germ-line stem cells. Proc Natl Acad Sci USA. 2001;98:13090–13095. doi: 10.1073/pnas.231473498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M, Watson DJ, Ryu BY, Wolfe JH, Brinster RL. Lentiviral vector transduction of male germ line stem cells in mice. FEBS Lett. 2002;524:111–115. doi: 10.1016/s0014-5793(02)03010-7. [DOI] [PubMed] [Google Scholar]
- Nagy ZP, Chang CC. Current advances in artificial gametes. Reprod Biomed Online. 2005;11:332–339. doi: 10.1016/s1472-6483(10)60841-3. [DOI] [PubMed] [Google Scholar]
- Nagy ZP, Chang CC. Artificial gametes. Theriogenology. 2007;67:99–104. doi: 10.1016/j.theriogenology.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Nakatsuji N, Chuma S. Differentiation of mouse primordial germ cells into female or male germ cells. Int J Dev Biol. 2001;45:541–548. [PubMed] [Google Scholar]
- Naughton CK, Jain S, Strickland AM, Gupta A, Milbrandt J. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol Reprod. 2006;74:314–321. doi: 10.1095/biolreprod.105.047365. [DOI] [PubMed] [Google Scholar]
- Nayernia K, Lee JH, Drusenheimer N, Nolte J, Wulf G, Dressel R, Gromoll J, Engel W. Derivation of male germ cells from bone marrow stem cells. Lab Invest. 2006;a 86:654–663. doi: 10.1038/labinvest.3700429. [DOI] [PubMed] [Google Scholar]
- Nayernia K, Nolte J, Michelmann HW, Lee JH, Rathsack K, Drusenheimer N, Dev A, Wulf G, Ehrmann IE, Elliott DJ, et al. In vitro-differentiated embryonic stem cells give rise to male gametes that can generate offspring mice. Dev Cell. 2006;b 11:125–132. doi: 10.1016/j.devcel.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Niwa H. How is pluripotency determined and maintained? Development. 2007;134:635–646. doi: 10.1242/dev.02787. [DOI] [PubMed] [Google Scholar]
- Novak I, Lightfoot DA, Wang H, Eriksson A, Mahdy E, Hoog C. Mouse embryonic stem cells form follicle-like ovarian structures but do not progress through meiosis. Stem Cells. 2006;24:1931–1936. doi: 10.1634/stemcells.2005-0520. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Payer B, O'Carroll D, Ancelin K, Ono Y, Sano M, Barton SC, Obukhanych T, Nussenzweig M, Tarakhovsky A, et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature. 2005;436:207–213. doi: 10.1038/nature03813. [DOI] [PubMed] [Google Scholar]
- Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y, Cowan CA, Chien KR, Melton DA. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. 2008;26:313–315. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- Paris MC, Schlatt S. Ovarian and testicular tissue xenografting: its potential for germline preservation of companion animals, non-domestic and endangered species. Reprod Fertil Dev. 2007;19:771–782. doi: 10.1071/rd07038. [DOI] [PubMed] [Google Scholar]
- Payer B, Chuva de Sousa Lopes SM, Barton SC, Lee C, Saitou M, Surani MA. Generation of stella-GFP transgenic mice: a novel tool to study germ cell development. Genesis. 2006;44:75–83. doi: 10.1002/gene.20187. [DOI] [PubMed] [Google Scholar]
- Perrett RM, Turnpenny L, Eckert JJ, O'Shea M, Sonne SB, Cameron IT, Wilson DI, Rajpert-De Meyts E, Hanley NA. The early human germ cell lineage does not express SOX2 during i development or upon in vitro culture. Biol Reprod. 2008;78:852–858. doi: 10.1095/biolreprod.107.066175. [DOI] [PubMed] [Google Scholar]
- Pesce M, Wang X, Wolgemuth DJ, Scholer H. Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech Dev. 1998;71:89–98. doi: 10.1016/s0925-4773(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Rajpert-De Meyts E, Hanstein R, Jorgensen N, Graem N, Vogt PH, Skakkebaek NE. Developmental expression of POU5F1 (OCT-3/4) in normal and dysgenetic human gonads. Hum Reprod. 2004;19:1338–1344. doi: 10.1093/humrep/deh265. [DOI] [PubMed] [Google Scholar]
- Reijo R, Lee TY, Salo P, Alagappan R, Brown LG, Rosenberg M, Rozen S, Jaffe T, Straus D, Hovatta O, et al. Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene. Nat Genet. 1995;10:383–393. doi: 10.1038/ng0895-383. [DOI] [PubMed] [Google Scholar]
- Resnick JL, Bixler LS, Cheng L, Donovan PJ. Long-term proliferation of mouse primordial germ cells in culture. Nature. 1992;359:550–551. doi: 10.1038/359550a0. [DOI] [PubMed] [Google Scholar]
- Reynolds N, Collier B, Maratou K, Bingham V, Speed RM, Taggart M, Semple CA, Gray NK, Cooke HJ. Dazl binds in vivo to specific transcripts and can regulate the pre-meiotic translation of Mvh in germ cells. Hum Mol Genet. 2005;14:3899–3909. doi: 10.1093/hmg/ddi414. [DOI] [PubMed] [Google Scholar]
- Rubin LL. Stem cells and drug discovery: the beginning of a new era? Cell. 2008;132:549–552. doi: 10.1016/j.cell.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Ruggiu M, Speed R, Taggart M, McKay SJ, Kilanowski F, Saunders P, Dorin J, Cooke HJ. The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature. 1997;389:73–77. doi: 10.1038/37987. [DOI] [PubMed] [Google Scholar]
- Saitou M, Barton SC, Surani MA. A molecular programme for the specification of germ cell fate in mice. Nature. 2002;418:293–300. doi: 10.1038/nature00927. [DOI] [PubMed] [Google Scholar]
- Seligman J, Page DC. The Dazh gene is expressed in male and female embryonic gonads before germ cell sex differentiation. Biochem Biophys Res Commun. 1998;245:878–882. doi: 10.1006/bbrc.1998.8530. [DOI] [PubMed] [Google Scholar]
- Seydoux G, Schedl T. The germline in C. elegans: origins, proliferation, and silencing. Int Rev Cytol. 2001;203:139–185. doi: 10.1016/s0074-7696(01)03006-6. [DOI] [PubMed] [Google Scholar]
- Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, Donovan PJ, Blumenthal PD, Huggins GR, Gearhart JD. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci USA. 1998;95:13726–13731. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solter D. From teratocarcinomas to embryonic stem cells and beyond: a history of embryonic stem cell research. Nat Rev Genet. 2006;7:319–327. doi: 10.1038/nrg1827. [DOI] [PubMed] [Google Scholar]
- Stoop H, Honecker F, Cools M, de Krijger R, Bokemeyer C, Looijenga LH. Differentiation and development of human female germ cells during prenatal gonadogenesis: an immunohistochemical study. Hum Reprod. 2005;20:1466–1476. doi: 10.1093/humrep/deh800. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tam PP, Snow MH. Proliferation and migration of primordial germ cells during compensatory growth in mouse embryos. J Embryol Exp Morphol. 1981;64:133–147. [PubMed] [Google Scholar]
- Telfer EE, Gosden RG, Byskov AG, Spears N, Albertini D, Andersen CY, Anderson R, Braw-Tal R, Clarke H, Gougeon A, et al. On regenerating the ovary and generating controversy. Cell. 2005;122:821–822. doi: 10.1016/j.cell.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Odorico JS. Human embryonic stem cell and embryonic germ cell lines. Trends Biotechnol. 2000;18:53–57. doi: 10.1016/s0167-7799(99)01410-9. [DOI] [PubMed] [Google Scholar]
- Toyooka Y, Tsunekawa N, Takahashi Y, Matsui Y, Satoh M, Noce T. Expression and intracellular localization of mouse Vasa-homologue protein during germ cell development. Mech Dev. 2000;93:139–149. doi: 10.1016/s0925-4773(00)00283-5. [DOI] [PubMed] [Google Scholar]
- Toyooka Y, Tsunekawa N, Akasu R, Noce T. Embryonic stem cells can form germ cells in vitro. Proc Natl Acad Sci USA. 2003;100:11457–11462. doi: 10.1073/pnas.1932826100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu J, Fan L, Tao K, Zhu W, Li J, Lu G. Stem cell factor affects fate determination of human gonocytes in vitro. Reproduction. 2007;134:757–765. doi: 10.1530/REP-07-0161. [DOI] [PubMed] [Google Scholar]
- Turnpenny L, Brickwood S, Spalluto CM, Piper K, Cameron IT, Wilson DI, Hanley NA. Derivation of human embryonic germ cells: an alternative source of pluripotent stem cells. Stem Cells. 2003;21:598–609. doi: 10.1634/stemcells.21-5-598. [DOI] [PubMed] [Google Scholar]
- Turnpenny L, Cameron IT, Spalluto CM, Hanley KP, Wilson DI, Hanley NA. Human embryonic germ cells for future neuronal replacement therapy. Brain Res Bull. 2005;68:76–82. doi: 10.1016/j.brainresbull.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Turnpenny L, Spalluto CM, Perrett RM, O'Shea M, Hanley KP, Cameron IT, Wilson DI, Hanley NA. Evaluating human embryonic germ cells: concord and conflict as pluripotent stem cells. Stem Cells. 2006;24:212–220. doi: 10.1634/stemcells.2005-0255. [DOI] [PubMed] [Google Scholar]
- Wartenburg H. Differentiation and development of the testes. In: Burger H, de Kretser DM, editors. The Testis. 2nd edn. New York: Raven Press; 1981. pp. 39–81. [Google Scholar]
- Western PS, Miles DC, van den Bergen JA, Burton M, Sinclair AH. Dynamic regulation of mitotic arrest in fetal male germ cells. Stem Cells. 2008;26:339–347. doi: 10.1634/stemcells.2007-0622. [DOI] [PubMed] [Google Scholar]
- Witschi E. Migration of the germ cells of human embryos from the yolk sac to the primitive gonadal folds. Contrib Embryol. 1948;32:67–80. [Google Scholar]
- Wolkowicz MJ, Coonrod SA, Reddi PP, Millan JL, Hofmann MC, Herr JC. Refinement of the differentiated phenotype of the spermatogenic cell line GC-2spd(ts) Biol Reprod. 1996;55:923–932. doi: 10.1095/biolreprod55.4.923. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zwaka TP, Thomson JA. A germ cell origin of embryonic stem cells? Development. 2005;132:227–233. doi: 10.1242/dev.01586. [DOI] [PubMed] [Google Scholar]