Abstract
The sodium dicarboxylate cotransporter, NaDC1, is a low affinity transporter for citric acid cycle intermediates such as succinate and citrate. The sequence of NaDC1 contains a number of conserved proline residues in predicted transmembrane helices (TM) 7 and 10. These transmembrane domains are of particular importance as they may be involved in determining substrate or cation binding affinity in NaDC1. Four conserved proline residues in TM 7 and 10 of rabbit NaDC1 were replaced with alanine to promote ideal α-helix or glycine to promote free conformation and the mutant transporters were expressed in the HRPE cell line. Mutations of prolines in TM 10 produced decreased protein expression and activity, whereas mutations of prolines in TM 7 completely abolished protein expression and activity. The chemical chaperone glycerol was found to improve expression of the Pro-351 mutants in TM 7, suggesting that these mutants had defects in trafficking. The inactive mutant transporters at position 351 could also be rescued by addition of a proline at a second site. For example, the P351A-F347P mutant had restored activity, although its substrate specificity was altered. We conclude that in TM 7, Pro-327 may be of particular importance in the function of the transporter whereas Pro-351 may affect protein targeting. The prolines in TM 10, at positions 523 and 524, may not be directly involved in transporter function but may be necessary for maintaining structure.
The absorption of tricarboxylic acid cycle intermediates, such as succinate and citrate, across the apical membrane of the kidney proximal tubule and the small intestine is mediated by the Na+/dicarboxylate cotransporter, NaDC1 (1). NaDC1 belongs to the SLC13 gene family that includes transporters for di- and tricarboxylates as well as sulfate (2;3). NaDC1 seems to play a crucial role in the regulation of urinary citrate concentrations and low urinary citrate concentrations or hypocitraturia are usually associated with an increased risk of kidney stone formation (4). NaDC1 may also affect longevity or metabolic status since decreased expression of a related protein, the Indy transporter from Drosophila, produces a doubling of lifespan (5). There is little information about the protein structure of NaDC1 or any member of the SLC13 family. There is experimental evidence that the amino-terminus is located intracellularly and the carboxy-terminus, containing the N-glycosylation site at Asn-578, is located extracellularly (6;7). Furthermore, the carboxy-terminal half of NaDC1 contains determinants of substrate recognition and cation selectivity (8).
Prolines play important structural as well as functional roles in membrane proteins. Normally, hydrogen bonds between backbone amino acids contribute to the structure and stability of transmembrane α-helices. Proline lacks an amide hydrogen which prevents it from forming backbone hydrogen bonds in the helix. This can potentially introduce a kink in the helix, depending on the local environment. Prolines contribute to the structure of membrane proteins by facilitating helix packing (9) or by stabilizing the conformation of α-helices and loops (10). In bacteriorhodopsin, prolines in transmembrane helices are important in determining the rate of protein folding and assembly (11). Prolines also exhibit various functional roles in membrane transporters and ion channels, including acting as ligand binding sites for cations (12) and as molecular switches or hinges involved in conformational changes (13;14).
NaDC1 contains a number of conserved prolines within or at the boundaries of transmembrane helices (TM) although their functional role is still unknown. In this study we focus on prolines in TM 7 and 10 as previous experiments by our group suggest that these TM may be part of the substrate binding domain and at least one cation binding site (15). The conserved prolines in TM 10 of NaDC1 may be important in protein structure or targeting since replacement of these prolines with cysteine resulted in little or no protein on the plasma membrane (16). To understand the possible role of the conserved proline residues in NaDC1, prolines were replaced with alanine or glycine. Alanine is a strong α-helix former and is less flexible whereas glycine, with no side chain, is a strong helix breaker and is more flexible.
The results of this study show that Pro-327 in TM 7 appears to be critical for protein structure as the glycine mutant at this position had no protein expression and could not be rescued by chemical chaperones or second site mutations. Pro-351 in TM 7 produces misfolded or mistargeted proteins when mutated to alanine or glycine. These mutant transporters were unable to insert into the plasma membrane and were accumulated intracellularly. However, these mutants could be rescued by chemical chaperones or insertion of a proline at a second site. Therefore, this residue may be important in targeting and stability of the transporter protein. Interestingly, the only mutant to exhibit a functional change was the double mutant, P351A-F347P, which had a change in Transport Specificity Ratio and decreased Km for succinate. The prolines found in TM 10 at positions 523 and 524 do not appear to have functional roles but might be important for protein stability.
EXPERIMENTAL PROCEDURES
Site-Directed Mutagenesis
Site directed mutagenesis was performed using the QuikChange site-directed mutagenesis kit (Stratagene) according to the manufacturer’s instructions. Rabbit (rb) NaDC1 in pcDNA3.1 vector was used as a template (17). We were unable to make the P327A mutant. Double mutants were prepared using single mutants, P351A, P351G and P327G, as templates. Mutants were verified by sequencing.
Expression of rbNaDC1 Mutants in HRPE Cells
Human retinal pigment epithelial (HRPE) cells transformed with SV40 (AG 06096; Coriell Institute), were cultured in Modified Eagle’s Medium (MEM) containing Glutamax, 25 mM HEPES (Invitrogen) along with 10% heat-inactivated fetal calf serum, 100 units/ml penicillin and 100 μg/ml of streptomycin. Cells were incubated at 37 °C in 5% CO2. Since most of the proline mutants showed significantly lower succinate transport activity compared with wild-type, transport assays were performed in 6 well plates. Those mutants with high activity were further characterized by using dual-label competitive uptake experiments in 24 well plates. For 6 well plates, 3 × 105 cells were plated per well whereas for 24 well plates 1.2 × 105 cells were plated per well. The next day cells were transiently transfected with 3 μl of FuGENE 6 (Roche Applied Science) and 1 μg of plasmid DNA (ratio of 3:1) for 6 well plates. For 24 well plates, cells were transfected with 1.8 μl of FuGENE 6 and 0.6 μg of plasmid DNA (9:3 ratio) (18). For experiments with chemical chaperones, the culture medium was replaced with medium supplemented with 0.5 M glycerol or 250 mM dimethyl sulfoxide (DMSO) 6 hours after transfection, and the cells were cultured in this medium a further 42 hours before transport and protein expression were measured. For all experiments, uptakes in vector-transfected cells were subtracted from uptakes in cells transfected with plasmids containing NaDC1 and mutants.
Transport Assays
Transport assays were carried out 48 hours after transfections. Sodium buffer containing 120 mM NaCl, 5 mM KCl, 1.2 mM MgSO4, 1.2 mM CaCl2, 5 mM D-glucose, 25 mM HEPES, pH adjusted to 7.4 with 1 M Tris was used for all experiments. Each well was washed twice with sodium buffer and then incubated for 30 min with 1 ml sodium buffer containing 100 μM of 3H-succinate (ViTrax). Uptakes were stopped and radioactivity was washed away with four washes of 3 ml sodium buffer. Cells were dissolved in 1% SDS, transferred to scintillation vials and counted with a liquid scintillation counter (Packard Tri-Carb 2100 TR). Kinetic parameters for the wild-type and double mutant were calculated by fitting the transport rates to the Michaelis-Menten equation using nonlinear regression analysis (SigmaPlot 8.0).
Dual-label Competitive Transport Experiments
For dual-label transport assays sodium buffer containing both 10 μM of 3H-succinate (ViTrax) and 20 μM of 14C-citrate (Moravek Biochemicals Inc.) was added to the cells in 24 well plates and competitive transport of these substrates was measured using a dual-label counting protocol on the scintillation counter. The uptake volume used was 250 μl/well. Transport specificity ratios (TSR) for wild type rbNaDC1 and mutants were calculated using: TSR = (νsuccinate/νcitrate) × ([citrate]/[succinate]), where νsuccinate and νcitrate are the initial rates of transport of 3H-succinate and 14C-citrate, [citrate] and [succinate] are the concentrations of citrate and succinate used (19;20). Statistical analysis was performed using Student’s t-test (SigmaStat program).
Cell Surface Biotinylation and Total Protein Expression
Cell surface biotinylations of NaDC1 mutants expressed in HRPE cells were performed using the impermeant reagent, Sulfo-NHS-LC-biotin (Pierce), as described previously (16;18). HRPE cells were grown in 6 well plates and transfected as described above for transport assays. Forty-eight hours after transfections, each well was washed three times with 3 ml phosphate-buffered saline containing 1 mM of Ca2+ and Mg2+ (PBS/CM, pH 9). Then 500 μl of 1.5 mg/ml Sulfo-NHS-LC-biotin (Pierce) freshly prepared in PBS/CM was added to each well. Cells were allowed to incubate for 30 min at room temperature. Cells were washed once and then incubated on ice for 20 min with 3 ml of cold quench buffer (PBS/CM with 100 mM glycine). Sulfo-NHS-LC-biotin labeled cells were then lysed with 0.5 ml of lysis buffer (20 mM Tris (pH 7.5), 1% Triton X-100, 150 mM NaCl, 5 mM EDTA, 10 μg/ml pepstatin, 10 μg/ml leupeptin and 0.5 mM phenylmethylsulfonyl fluoride (PMSF)) on ice with gentle rocking for 30 min. The contents of 2 wells were combined in microcentrifuge tube and spun down for 15 min. Supernatants were transferred to new tubes. Samples were then incubated with 50 μl of Immunopure Immobilized Streptavidin (Pierce) overnight at 4 °C with end-over-end rotation. The next day beads were washed three times with lysis buffer, twice with high salt wash buffer (same as lysis buffer but containing 0.1% Triton X-100 and 500 mM NaCl) and once with no salt wash buffer (50 mM Tris base pH 7.5). Biotinylated proteins were eluted with 50 μl of 3X tricine gel loading buffer (300 mM Tris-HCl pH 7, 30 % glycerol, 12 % SDS, 6% β-mercaptoethanol, 0.005% Commassie blue R-250) for 10 min at 85 °C. The procedure used to measure total biotinylated proteins was almost identical to that of the cell-surface biotinylations except that the lysis buffer was added before the Sulfo-NHS-LC-biotin, and this was followed by the streptavidin beads (7). Samples were applied to 7.5% Tricine gel and transferred for Western blotting as described (21). Blots were incubated with 1:1000 dilutions of anti-NaDC1 antibodies (22) or with 1:2000 dilution of anti-calnexin antibodies (Stressgen). Supersignal West Pico chemiluminescent substrate kit (Pierce) was used to detect antibody binding. Images were captured by using Kodak Image Station 440CF. Image 1D analysis software (Eastman Kodak Co.) was used to analyze quantities of protein expressed.
RESULTS
Proline to Alanine and Glycine Mutations in rbNaDC1
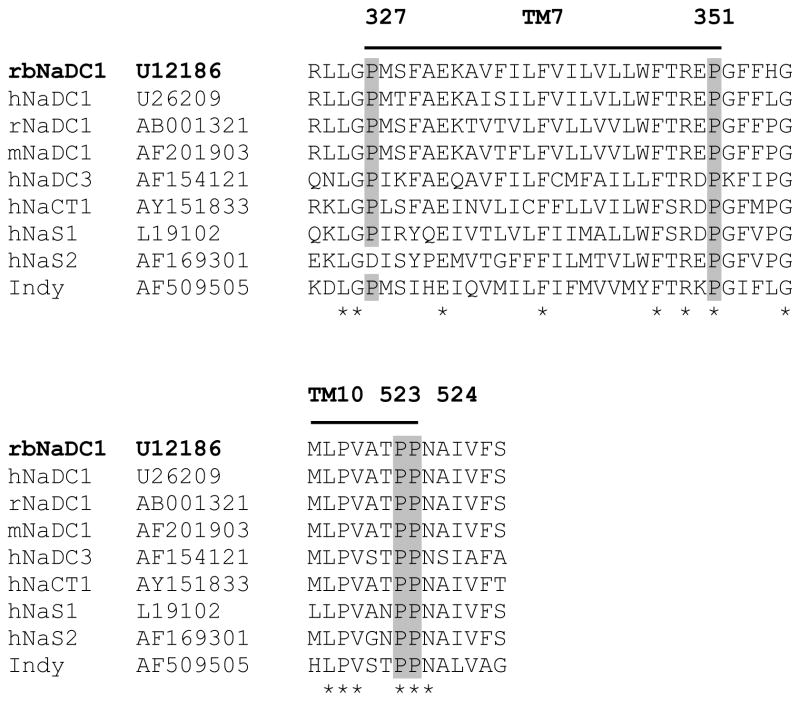
The sequence alignment of rbNaDC1 with other members of SLC13 gene family shows conserved proline residues in TM 7 and 10, based on the predicted secondary structure model (Figure 1) (6). The conserved prolines at positions 327, 351, 523 and 524 were mutated to alanine and glycine to determine the involvement of these residues in the conformational stability of the transporter as well as the transport function. Alanine was substituted for proline because it has a methyl group side chain and tends to promote and maintain α-helical structure. Glycine lacks the methyl group side chain, is less hydrophobic than alanine and prefers a flexible conformation. If a proline kink or distortion of the α-helix is necessary for the stability and function of NaDC1, mutating the proline to glycine will have less of an effect compared with alanine.
Figure 1.
Multiple sequence alignments of transmembrane helices 7 and 10 from rbNaDC1 with some members of SLC13 gene family, including the sodium-independent dicarboxylate transporter from Drosophila, Indy. Positions of conserved prolines mutated in this study are highlighted. The GenBank™ accession numbers are written next to the transporter names. The sequence alignment was performed with ClustalW (1.82) with default parameters using Gonnet matrix. Locations of transmembrane helices 7 and 10, according to the 11 TM helix model based on Rao-Argos buried helix parameter scale (6;35) are shown by lines above the sequence. The asterisks indicate conserved amino acid residues in the proteins.
Protein expression and Transport Activity of Proline Mutants
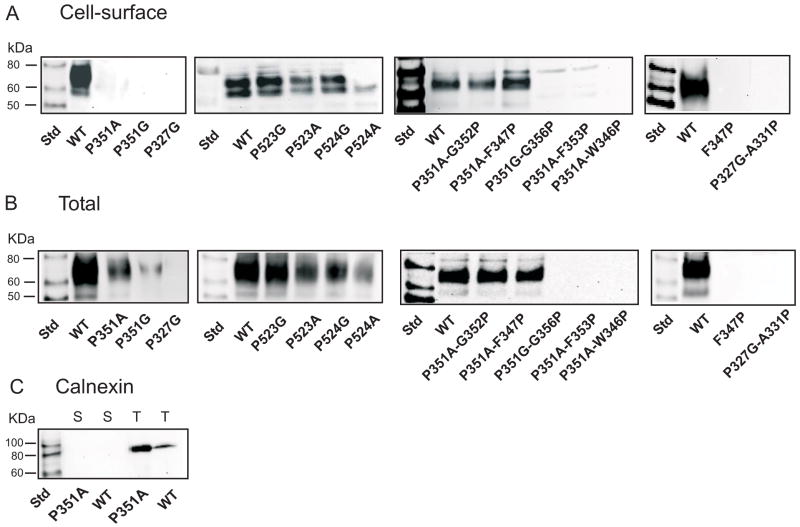
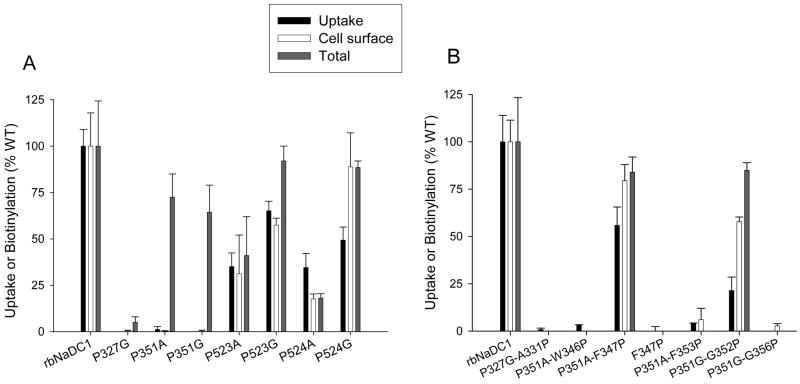
Cell surface expression of mutants was monitored by cell surface biotinylation with the membrane impermeant reagent, Sulfo-NHS-LC-biotin, followed by Western blotting. Total protein expression was measured by lysing the cells before addition of the Sulfo-NHS-LC-biotin. Figure 2 shows single representative blots of cell surface and total protein expression for each proline mutant compared with the wild-type control. The two bands represent differently glycosylated forms of the transporter (6). The blots were scanned to quantitate the protein expression and each mutant was expressed as a percentage of the wild-type rbNaDC1 from the same blot. The transport activity and protein expression of the single proline mutants are compared in Figure 3A. No cell surface expression was seen for Pro-327 and Pro-351 mutants. However, the Pro-351 mutants appeared to be located intracellularly (Figures 2 and 3A) indicating possible defects in protein trafficking. In general, the cell surface expression of most of the mutants correlated with the uptake activity, although the P524G mutant had higher cell surface and total protein expression compared with activity (Figure 3A). The P524A mutant appeared to have lower protein expression compared with activity.
Figure 2.
Western blots of (A) cell surface and (B) total biotinylated protein expression. HRPE cells were transiently transfected with wild-type and mutant rbNaDC1 and then treated with Sulfo-NHS-LC-biotin. For total protein expression, cell lysis buffer was added before addition of Sulfo-NHS-LC-biotin. Western blots were probed with 1:1000 dilution of anti-NaDC1 antibodies, followed by 1:5000 dilution of horseradish peroxidase-linked anti-rabbit Ig. The two bands represent differently glycosylated forms of rbNaDC1 (6;22). (C) Western blots of cell surface (S) and total (T) biotinylated proteins probed with anti-calnexin antibodies (1:2000 dilution), as a marker of endoplasmic reticulum. Chemiluminescent molecular weight standards (Std) are shown in first lane of each blot.
Figure 3.
Activity and expression of single (A) and double (B) mutants of rbNaDC1. The activity, cell surface and total protein expression of the mutants are shown as a percentage of wild-type rbNaDC1 (WT) as control. Transport activity of 100 μM 3H-succinate was measured with 30 minutes incubation in sodium containing buffer. Transport results for the mutants are means ± range or SEM (n = 2 or 3). Protein expression was determined by quantitating the intensities of NaDC1 protein bands from Western blots (such as Figure 2) using Image 1D analysis software. Bars represent mean ± range or SEM (n = 2 to 4 blots). Error bars on the wild-type groups represent the variation between experiments expressed as a percentage of the mean from each panel, n=11 (activity, Panel A) or 18 (activity, B), n=4–6 (blots, A and B). Background uptakes in vector-transfected cells were: (A) 0.8 ± 0.06 and (B) 1.4 ± 0.07 pmol/min-well. Background-corrected rates in wildtype NaDC1 were: (A) 3.2 ± 0.3 or (B) 7.7 ± 1.0 pmol/min-well.
Double mutants were constructed to determine whether the addition of a proline residue at a second site could reverse the effect of removing a proline. Since an α-helix contains 3.6 residues per turn, we constructed the double mutant P327G-A331P but this mutant was not expressed on the plasma membrane or intracellularly (Figures 2 and 3B). Five second site mutations were made in transporters lacking Pro-351, three based on P351A and two based on P351G. The double mutants based on P351G introduced proline in place of Gly-352 or Gly-356. In the other double mutants based on P351A, P351A-F347P, P351A-F353P and P351A-W346P, we tried to introduce rigidity and establish helical conformation by introducing alanine at position 351 and then adding proline at various positions in the helical turn to try to restore the activity. Only two of these mutants had increased protein expression and transport activity relative to the single mutants: P351A-F347P, which was restored to almost wild-type levels, and P351A-G352P, which had low transport activity relative to protein expression (Figure 3B). The double mutants P351A-F353P, P351A-W346P and P351G-G356P, and the single mutant F347P, had no protein expression, measured from total and cell surface biotinylations (Figure 2 and 3B). Overall, the majority of Pro-351 mutants appeared to have problems with misfolding or mistargeting since their cell surface protein expression was reduced.
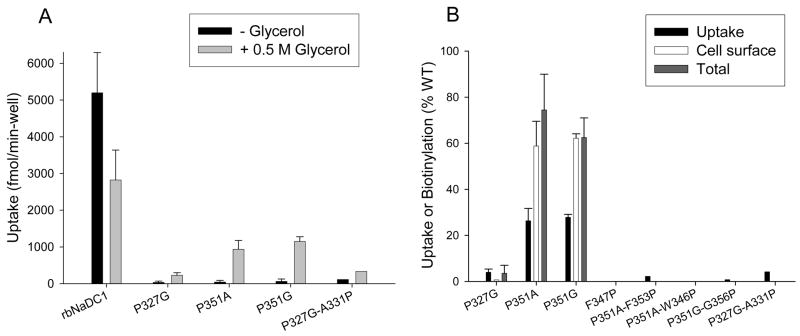
Effect of Chemical Chaperones on Inactive TM 7 Mutants
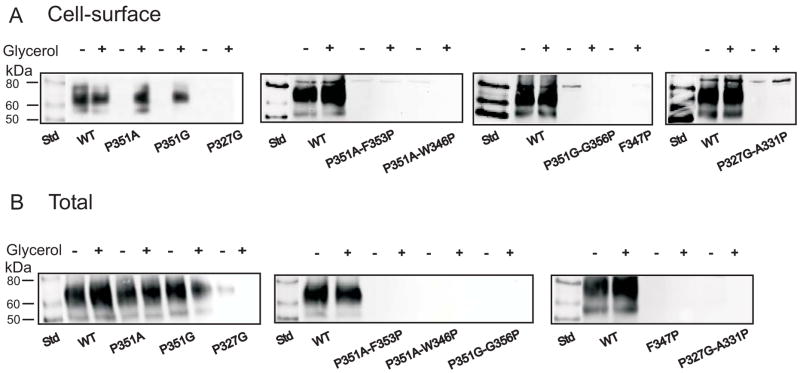
Chemical chaperones, such as glycerol or dimethyl sulfoxide (DMSO), have proven effective in correcting trafficking defects in mutants of Aquaporin-2 (23). Therefore, we tested whether these chemical chaperones could improve the expression of the non-expressing NaDC1 mutants. DMSO (250 mM) had less of an effect in improving cell surface expression of NaDC1 mutants compared with glycerol (results not shown). Glycerol concentrations from 0.2 to 4 M were tested but the most effective was 0.5 or 1 M (results not shown). Figure 4 shows cell surface and total protein expression of wild type and various TM 7 single and double mutants. The addition of 0.5 M glycerol to the wild type NaDC1 decreased succinate transport (Figure 4 and 5A) but the same treatment produced increased activity and cell surface expression of the P351A and P351G mutants (Figure 4 and 5B). The transport activity for both P351A and P351G was lower than the amount of transporter protein found on the cell surface (Figure 5B). Glycerol treatment did not measurably improve the expression of any of the other mutants (Figure 4 and 5).
Figure 4.
Treatment of non-expressing mutants with glycerol. (A) Representative Western blots of cell-surface biotinylated protein expression and (B) total biotinylated protein expression without (−) and with (+) addition of 0.5 M glycerol to the medium, as described in Experimental Procedures. Blots were probed with 1:1000 dilution of anti-NaDC1 antibodies, followed by 1:5000 dilution of horseradish peroxidase-linked anti-rabbit antibodies. Chemiluminescent molecular weight markers (Std) are shown in the first lane of each blot.
Figure 5.
Effect of glycerol on TM 7 mutants (A) Transport of 100 μM 3H-succinate in wild-type and mutant rbNaDC1 with and without addition of 0.5 M glycerol. Results are means ± range or S.E.M (n = 2 or 3). The P327G-A331P result is from a single experiment. (B) Comparison between transport activity and cell surface expression of TM 7 mutants after addition of glycerol. Transport activity, cell surface and total protein biotinylations were measured. Intensities of the protein bands were quantitated using Image 1D software. The data are expressed as a percentage of wild-type control in each experiment. Results are presented as mean ± range (n = 2) for mutants P327G, P351A and P351G and only mean is indicated for all other mutants (n =1).
Functional Characteristics of Proline Mutants
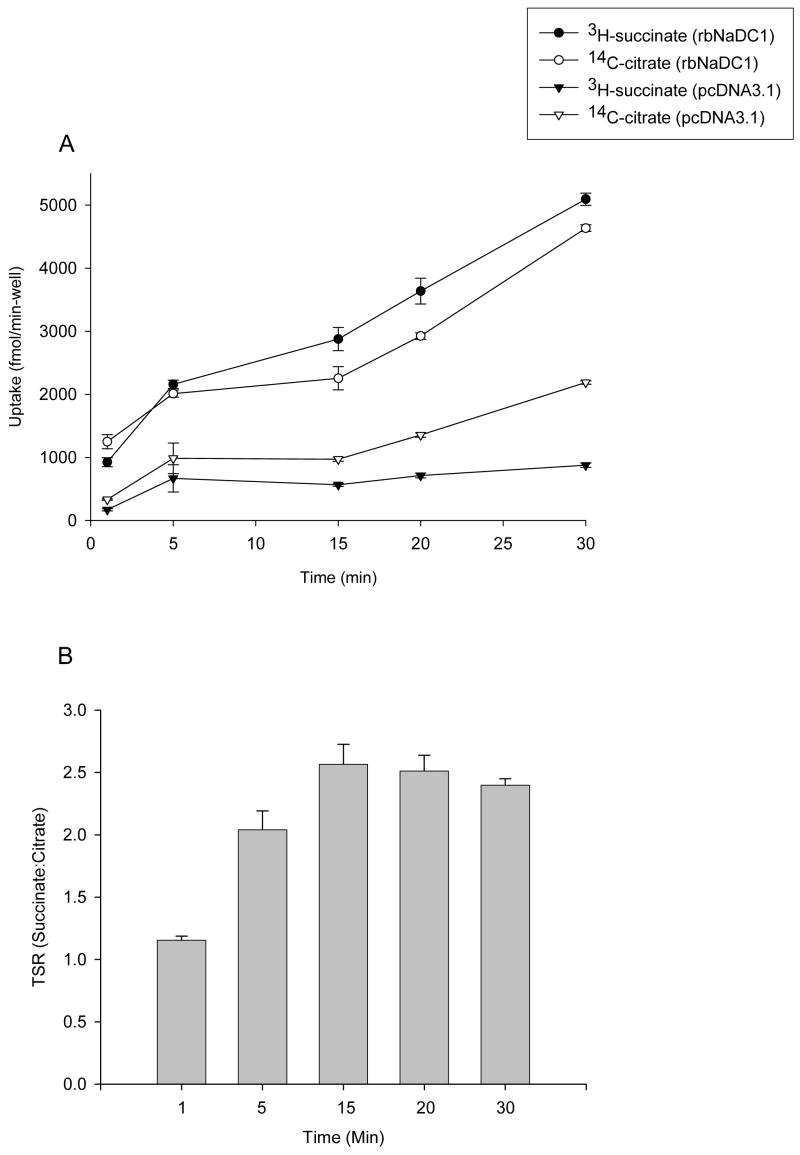
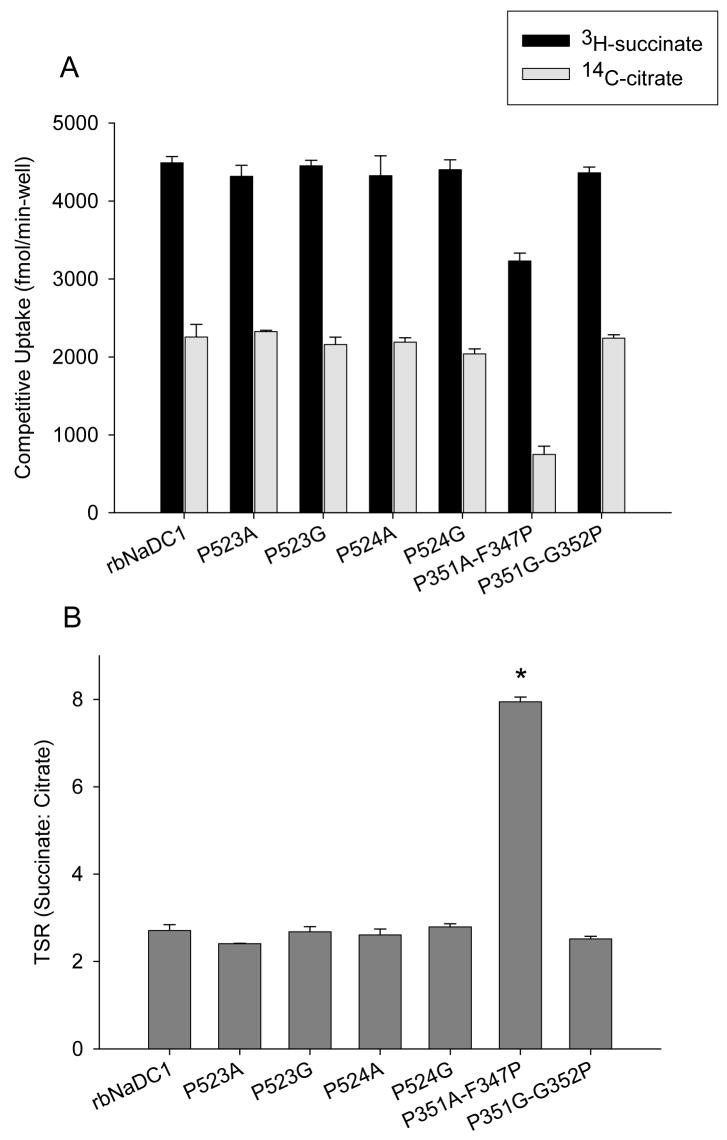
The transport specificity ratio (TSR) is a novel approach to compare the effects of site-directed mutagenesis on function by monitoring relative changes in catalytic specificity (kcat/Km) (20). Previous studies showed that TSR analysis is independent of protein expression and is valid over a wide range of substrate concentrations (19). The TSR is calculated from the initial rates of transport of two substrates in a dual-label competitive uptake experiment. The time course of uptakes of 10 μM 3H-succinate and 20 μM 14C-citrate in HRPE cells expressing the wild-type NaDC1 is shown in Fig. 6A. The time course is linear through 30 min and we selected 20 min as the time point for future experiments. TSR values were found to be similar between 5 to 30 minutes (Figure 6B). The succinate:citrate transport specificity ratio (TSR) for rbNaDC1 and most of the mutants was found to be approximately 2.5, independent of expression and uptake activity of single substrates. Figure 7A shows competitive uptake between radioactive succinate and citrate in each mutant. The only mutant that showed a change in TSR was the P351A-F347P double mutant, which had a mean TSR value of about 8, significantly higher than the wild type (Figure 7B). A different combination of substrate concentrations (0.01 μM 3H-succinate and 10 μM 14C-citrate) was also tested and the TSR values were similar (results not shown). We did kinetic analysis of the double mutant, P351A-F347P. The apparent Km for succinate was found to be approximately 14 μM compared with a Km of about 500 μM in wild type rbNaDC1 (Table 1) (17). Citrate transport by the P351A-F347P mutant was too low for kinetic analysis.
Figure 6.
(A) Time course of competitive uptake of succinate and citrate in wild type rbNaDC1. HRPE cells were transiently transfected with the wild type rbNaDC1 or vector only (pcDNA3.1) as described in Experimental Procedures. Dual-label competitive uptake experiment was performed with 10 μM 3H-succinate and 20 μM 14C-citrate at time points between 1 and 30 min. Results are mean ± SEM (n = 4 wells from a single transfection). (B) Transport specificity ratios (succinate: citrate) calculated from data in (A) are shown for each time point.
Figure 7.
Transport specificity ratio (TSR) of proline mutants compared with wild type rbNaDC1. (A) Competitive transport with 10 μM 3H-succinate and 20 μM 14C-citrate was performed for 20 min in 24-well plates. Bars represent mean ± range, n = 2 separate experiments. (B) TSR (succinate:citrate) of wild type and mutants using data from (A). The TSR for F347P-P351 mutant is significantly greater than wild type and other mutants, P < 0.05.
Table 1.
Succinate kinetics of rbNaDC1 and double mutant P351A-F347Pa
| Protein | Km (μM) | Vmax (fmol/min-well) | n |
|---|---|---|---|
| rbNaDC1 | 590 | 42300 | 1 |
| P51A-F347P | 13.6 ± 0.8 | 1445 ± 48 | 2 |
Wild type rbNaDC1 and the P351A-F347P mutant were transiently expressed in HRPE cells. Succinate uptakes were measured at concentrations up to 2 mM for WT and 0.5 mM for mutant as described in Experimental Procedures. The kinetic values for the mutant represent mean ± range of two independent experiments. The rbNaDC1 kinetic parameters compare well with the Km of 0.5 mM measured in our previous studies (17).
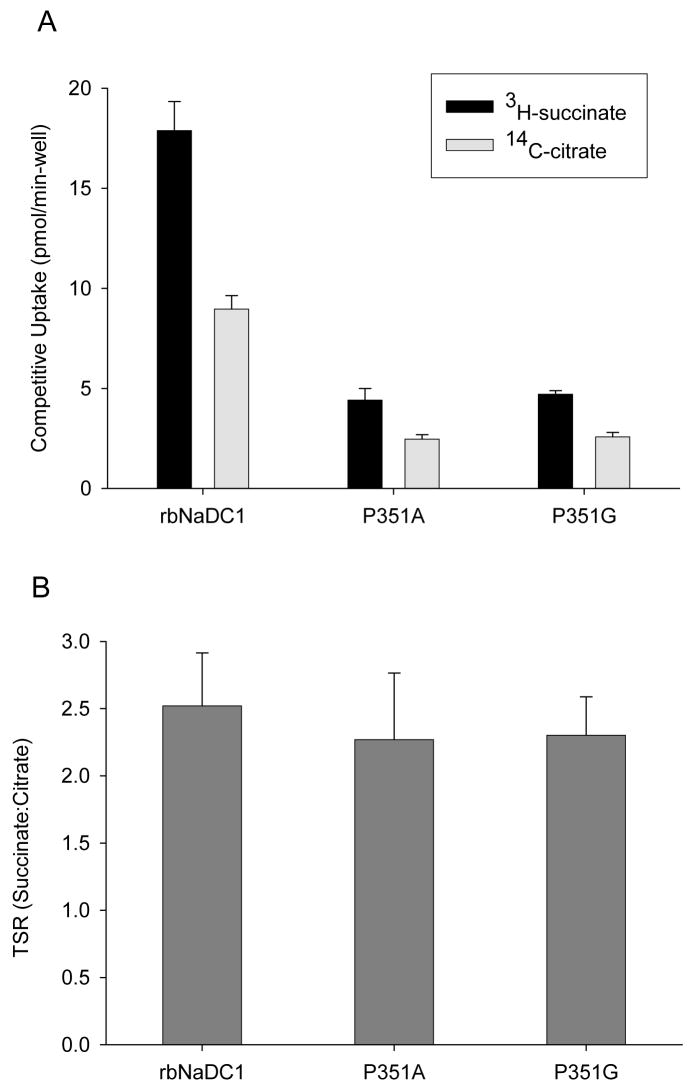
The succinate:citrate TSR values for P351A and P351G were also calculated after treating these mutants with 0.5 M glycerol. Glycerol treatment did not affect the wild-type TSR value, and no significant difference was seen in TSR values between wild-type and the Pro-351 mutants (Figure 8). Therefore, although many of the mutants had differences in expression and activity, there was no change in catalytic efficiency of succinate transport relative to citrate in any mutant except P351A-F347P.
Figure 8.
Transport specificity ratio (TSR) of mutants treated with glycerol to improve expression. (A) Competitive dual-label uptake experiment with 10 μM 3H-succinate and 20 μM 14C-citrate was performed for 20 min. HRPE cells in 6 well plates were transfected with wild type and mutant cDNAs. Medium containing 0.5 M glycerol was added 6 hr after transfection. (B) TSR of wild type and mutants using data from (A). There were no significant differences between wild type and mutants. Results are shown as mean ± range (n = 2 separate transfection experiments).
DISCUSSION
In this study we examined the role of four conserved prolines, at positions 327, 351, 523 and 524, associated with transmembrane helices 7 and 10 in the Na+/dicarboxylate cotransporter, NaDC1. These prolines are conserved in all members of the SLC13 family, including the Indy dicarboxylate transporter from Drosophila, suggesting that these amino acids may be important for the structure or function of these transporters. TM 7 and 10 of NaDC1 have important functional roles since they contain amino acids that determine substrate and cation specificity (15). The results demonstrate that Pro-327 is probably critical for the activity of the transporter and Pro-351, at end of the TM 7, may contribute to protein stability and targeting to the plasma membrane. Although Pro-523 and Pro-524 probably contribute to the stability of TM 10, these prolines do not appear to be involved in the function of the transporter.
Prolines may play important functional roles in transporters and channels because the presence of proline in a transmembrane helix leads to changes in the structure or flexibility of the helix. Since proline is an imino acid it lacks an amino side chain and hence cannot take part in hydrogen bonding, leaving free backbone carbonyls. Proline residues in a transmembrane helix typically introduce a kink in the helix, depending on the local environment, which may allow flexibility of the helix or helix packing (24–27). Experiments with the Shaker K+ channel from Drosophila showed that prolines are necessary for the flexibility of the S6 transmembrane helix, which is important in the gating mechanism (28). Recent studies with mammalian dopamine transporters suggested structural as well as functional importance of TM prolines in plasma membrane targeting, assembly, membrane insertion and contribution to dopamine recognition (29). Prolines in the human noradrenaline transporter are involved in assembly of the transporter, inhibitor binding as well as in conformational changes associated with substrate translocation (30). Prolines are also required for cell surface expression and targeting in various membrane proteins (31). The prolines in transmembrane helices of the lactose permease contribute to helix packing and tight closure of hydrophilic cavity (32).
The conserved proline at position 327 in TM 7 appears to be a critical residue in NaDC1. The protein does not tolerate substitutions at this position. Proteins with the P327G mutation were not found either in total or cell surface biotinylated samples, indicating that a protein trafficking problem is not the reason for a lack of expression. Furthermore, we were unable to rescue the P327G mutant by addition of chemical chaperones or with second site mutations. Pro-351 may be important in determining the structure or stability of NaDC1. Our current secondary structure model places Pro-351 at the extracellular end of TM 7 (Figure 9). Alanine and glycine substitutions at position 351 produced proteins that were found in total protein lysates but were not expressed on the cell surface, indicating problems in protein trafficking or an alteration in stability at the plasma membrane. We tested both these hypothesis by using chemical chaperones and by constructing double mutants. Chemical chaperones such as glycerol, dimethyl sulfoxide, trimethylamine N-oxide, and 4-phenylbutyric acid have been used to prevent mislocalization of CFTRΔF508 (33), mutants of the aquaporin-2 channel (23) and NaCl cotransporter mutants (34). In the present study, the expression and activity of the P351A and P351G mutants could be restored after treatment with 0.5 M glycerol. Since the TSR values of these mutants did not change, the results indicate that Pro-351 may be involved in protein trafficking and membrane targeting rather than in transport function. To determine whether a proline is absolutely required at position 351 or whether the proline could be substituted at a different location in the helix, we constructed five double mutants around the TM 7 helical turn and in extracellular loop 4. The P351G-G352P mutant exhibited a partial increase in activity and protein expression. The replacement of F347 with proline, four amino acids away from the original P351A mutation, almost completely restored activity. Interestingly, this was the only mutant in our study to exhibit changes in catalytic efficiency. The Transport Specificity Ratio (TSR) was almost fourfold higher in P351A-F347P compared with the wild-type, indicating changes in the transport of succinate or citrate (or both). In kinetic experiments we found that the P351A-F347P mutant had an increased affinity for succinate (Km 14 μM), although the Vmax was greatly reduced. The single mutant, F347P, was not expressed and could not be rescued with glycerol. This indicates that the addition of the second proline at Phe-347 to wild-type NaDC1 alters the conformation of the helix and leads to possible misfolding of the protein and alterations in function or stability. Consequently only one proline may be necessary to maintain a stable conformation of TM 7.
Figure 9.
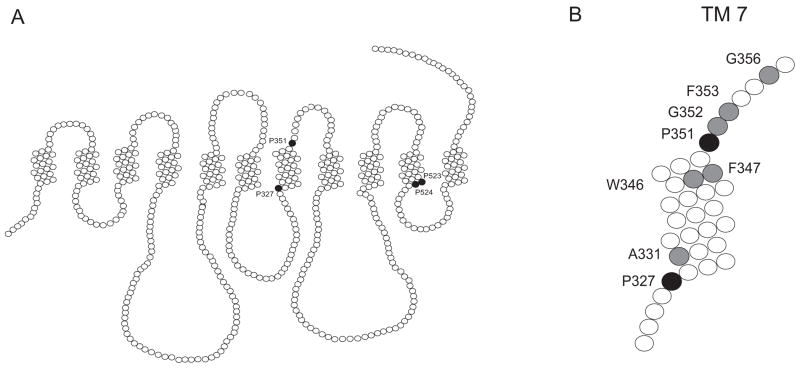
(A) Secondary structure model of NaDC1 showing the location of prolines (filled circles) in TM 7 and 10 that were mutated in this study. The outside of the cell is at the top of the figure. (B) TM 7 showing locations of prolines (black filled circles) and other amino acids (grey filled circles) mutated in this study.
The prolines in TM10 do not appear to have functional roles. Both Pro-523 and Pro-524 could be replaced with alanine or glycine without much change in activity or expression, and the mutants had no change in catalytic efficiency measured by TSR. It is possible that the mutants have equal alterations in the transport of both succinate and citrate, which would not be reflected in the TSR since it is a ratio. Our previous study showed that cysteine substitution of Pro-523 and Pro-524 produced a much greater decrease in expression and activity than the alanine or glycine substitutions in the present study (16). Cysteines do not have any helical preference and provide no flexibility compared with alanine or glycine. Replacement of Pro-524 with alanine appeared to result in lower protein expression than replacement with glycine, which could indicate a preference for flexibility or a kink in TM10. The difference between the alanine and glycine mutants was less pronounced at position 523, but the total protein expression was lower in the P523A mutant. Therefore it is possible that the prolines in TM10 are involved in the structural stability of the transporter, and may introduce bends in the helix, but they do not contribute to transporter function.
In conclusion, the results of this study suggest various roles played by conserved proline residues in NaDC1. The most important prolines are those associated with TM7. Pro-327 is a critical residue as the glycine mutant at this position had no protein expression and could not be rescued by chemical chaperones or second site mutations. Pro-351 appears to be necessary for cell surface expression and regulation of protein trafficking. In contrast, the two conserved prolines in TM10, Pro-523 and 524, may produce bends in the helix, but these prolines do not have functional effects in NaDC1.
Acknowledgments
We thank Dr. Steven King for discussions on the TSR measurements.
Abbreviations
- NaDC1
Sodium dicarboxylate cotransporter 1
- TM
Transmembrane
- helix SLC13
Solute carrier family 13
- TSR
Transport specificity ratio
Footnotes
This study was supported by National Institutes of Health grant DK 46269.
References
- 1.Pajor AM. Molecular properties of sodium/dicarboxylate cotransporters. J Membr Biol. 2000;175:1–8. doi: 10.1007/s002320001049. [DOI] [PubMed] [Google Scholar]
- 2.Girard JP, Baekkevold ES, Feliu J, Brandtzaeg P, Amalric F. Molecular cloning and functional analysis of SUT-1, a sulfate transporter from human high endothelial venules. Proc Natl Acad Sci U S A. 1999;96:12772–12777. doi: 10.1073/pnas.96.22.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markovich D, Forgo J, Stange G, Biber J, Murer H. Expression cloning of rat renal Na+/SO42− cotransport. Proc Natl Acad Sci U S A. 1993;90:8073–8077. doi: 10.1073/pnas.90.17.8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pajor AM. Citrate transport by the kidney and intestine. Seminars in Nephrology. 1999;19:195–200. [PubMed] [Google Scholar]
- 5.Rogina B, Reenan RA, Nilsen SP, Helfand SL. Extended life-span conferred by cotransporter gene mutations in Drosophila. Science. 2000;290:2137–2140. doi: 10.1126/science.290.5499.2137. [DOI] [PubMed] [Google Scholar]
- 6.Pajor AM, Sun N. Characterization of the rabbit renal Na+-dicarboxylate cotransporter using antifusion protein antibodies. Am J Physiol. 1996;271:C1808–C1816. doi: 10.1152/ajpcell.1996.271.6.C1808. [DOI] [PubMed] [Google Scholar]
- 7.Zhang FF, Pajor AM. Topology of the Na+/dicarboxylate cotransporter: the N-terminus and hydrophilic loop 4 are located intracellularly. Biochim Biophys Acta. 2001;1511:80–89. doi: 10.1016/s0005-2736(00)00385-0. [DOI] [PubMed] [Google Scholar]
- 8.Pajor AM, Sun N, Bai L, Markovich D, Sule P. The substrate recognition domain in the Na+/dicarboxylate and Na+/sulfate cotransporters is located in the carboxy-terminal portion of the protein. Biochim Biophys Acta. 1998;1370:98–106. doi: 10.1016/s0005-2736(97)00249-6. [DOI] [PubMed] [Google Scholar]
- 9.Woolfson DN, Williams DH. The influence of proline residues on alpha-helical structure. FEBS Lett. 1990;277:185–188. doi: 10.1016/0014-5793(90)80839-b. [DOI] [PubMed] [Google Scholar]
- 10.Shelden MC, Loughlin P, Tierney ML, Howitt SM. Proline residues in two tightly coupled helices of the sulphate transporter, SHST1, are important for sulphate transport. Biochem J. 2001;356:589–594. doi: 10.1042/0264-6021:3560589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu H, Marti T, Booth PJ. Proline residues in transmembrane alpha helices affect the folding of bacteriorhodopsin. J Mol Biol. 2001;308:437–446. doi: 10.1006/jmbi.2001.4605. [DOI] [PubMed] [Google Scholar]
- 12.Sansom MS. Proline residues in transmembrane helices of channel and transport proteins: a molecular modelling study. Protein Eng. 1992;5:53–60. doi: 10.1093/protein/5.1.53. [DOI] [PubMed] [Google Scholar]
- 13.Brandl CJ, Deber CM. Hypothesis about the function of membrane-buried proline residues in transport proteins. Proc Natl Acad Sci U S A. 1986;83:917–921. doi: 10.1073/pnas.83.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sansom MS, Weinstein H. Hinges, swivels and switches: the role of prolines in signalling via transmembrane alpha-helices. Trends Pharmacol Sci. 2000;21:445–451. doi: 10.1016/s0165-6147(00)01553-4. [DOI] [PubMed] [Google Scholar]
- 15.Kahn ES, Pajor AM. Determinants of substrate and cation affinities in the Na+/dicarboxylate cotransporter. Biochemistry. 1999;38:6151–6156. doi: 10.1021/bi9827722. [DOI] [PubMed] [Google Scholar]
- 16.Pajor AM, Randolph KM. Conformationally sensitive residues in extracellular loop 5 of the Na+/dicarboxylate co-transporter. J Biol Chem. 2005;280:18728–18735. doi: 10.1074/jbc.M501265200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pajor AM. Sequence and functional characterization of a renal sodium/dicarboxylate cotransporter. J Biol Chem. 1995;270:5779–5785. doi: 10.1074/jbc.270.11.5779. [DOI] [PubMed] [Google Scholar]
- 18.Pajor AM. Conformationally-sensitivity residues in transmembrane domain 9 of the Na+/dicarboxylate cotransporter. J Biol Chem. 2001;276:29961–29968. doi: 10.1074/jbc.M011387200. [DOI] [PubMed] [Google Scholar]
- 19.King SC. The “Transport Specificity Ratio”: a structure-function tool to search the protein fold for loci that control transition state stability in membrane transport catalysis. BMC Biochem. 2004;5:16. doi: 10.1186/1471-2091-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King SC, Brown-Istvan L. Use of the transport specificity ratio and cysteine-scanning mutagenesis to detect multiple substrate specificity determinants in the consensus amphipathic region of the Escherichia coli GABA (gamma-aminobutyric acid) transporter encoded by gabP. Biochem J. 2003;376:633–644. doi: 10.1042/BJ20030594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Pajor AM. Mutagenesis of the N-glycosylation site of hNaSi-1 reduces transport activity. Am J Physiol Cell Physiol. 2003;285:C1188–C1196. doi: 10.1152/ajpcell.00162.2003. [DOI] [PubMed] [Google Scholar]
- 22.Pajor AM, Sun N, Valmonte HG. Mutational analysis of histidines in the Na+/dicarboxylate cotransporter, NaDC-1. Biochem J. 1998;331:257–264. doi: 10.1042/bj3310257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamarappoo BK, Verkman AS. Defective aquaporin-2 trafficking in nephrogenic diabetes insipidus and correction by chemical chaperones. J Clin Invest. 1998;101:2257–2267. doi: 10.1172/JCI2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barlow DJ, Thornton JM. Helix geometry in proteins. J Mol Biol. 1988;201:601–619. doi: 10.1016/0022-2836(88)90641-9. [DOI] [PubMed] [Google Scholar]
- 25.Cordes FS, Bright JN, Sansom MS. Proline-induced distortions of transmembrane helices. J Mol Biol. 2002;323:951–960. doi: 10.1016/s0022-2836(02)01006-9. [DOI] [PubMed] [Google Scholar]
- 26.Richardson JS, Richardson DC. Amino acid preferences for specific locations at the ends of alpha helices. Science. 1988;240:1648–1652. doi: 10.1126/science.3381086. [DOI] [PubMed] [Google Scholar]
- 27.Sankararamakrishnan R, Vishveshwara S. Conformational studies on peptides with proline in the right-handed alpha-helical region. Biopolymers. 1990;30:287–298. doi: 10.1002/bip.360300307. [DOI] [PubMed] [Google Scholar]
- 28.Labro AJ, Raes AL, Bellens I, Ottschytsch N, Snyders DJ. Gating of shaker-type channels requires the flexibility of S6 caused by prolines. J Biol Chem. 2003;278:50724–50731. doi: 10.1074/jbc.M306097200. [DOI] [PubMed] [Google Scholar]
- 29.Lin Z, Itokawa M, Uhl GR. Dopamine transporter proline mutations influence dopamine uptake, cocaine analog recognition, and expression. FASEB J. 2000;14:715–728. doi: 10.1096/fasebj.14.5.715. [DOI] [PubMed] [Google Scholar]
- 30.Paczkowski FA, Bryan-Lluka LJ. Role of proline residues in the expression and function of the human noradrenaline transporter. J Neurochem. 2004;88:203–211. doi: 10.1111/j.1471-4159.2004.02149.x. [DOI] [PubMed] [Google Scholar]
- 31.Slepkov ER, Chow S, Lemieux MJ, Fliegel L. Proline residues in transmembrane segment IV are critical for activity, expression and targeting of the Na+/H+ exchanger isoform 1. Biochem J. 2004;379:31–38. doi: 10.1042/BJ20030884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 33.Sato S, Ward CL, Krouse ME, Wine JJ, Kopito RR. Glycerol reverses the misfolding phenotype of the most common cystic fibrosis mutation. J Biol Chem. 1996;271:635–638. doi: 10.1074/jbc.271.2.635. [DOI] [PubMed] [Google Scholar]
- 34.de Jong JC, Willems PH, Goossens M, Vandewalle A, van den Heuvel LP, Knoers NV, Bindels RJ. Effects of chemical chaperones on partially retarded NaCl cotransporter mutants associated with Gitelman’s syndrome in a mouse cortical collecting duct cell line. Nephrol Dial Transplant. 2004;19:1069–1076. doi: 10.1093/ndt/gfg474. [DOI] [PubMed] [Google Scholar]
- 35.Pajor AM, Sun NN. Molecular cloning, chromosomal organization, and functional characterization of a sodium-dicarboxylate cotransporter from mouse kidney. Am J Physiol Renal Physiol. 2000;279:F482–F490. doi: 10.1152/ajprenal.2000.279.3.F482. [DOI] [PubMed] [Google Scholar]