Summary
The formation of locomotor circuits depends on the spatially organized generation of motor columns that innervate distinct muscle and autonomic nervous system targets along the body axis. Within each spinal segment, multiple motor neuron classes arise from a common progenitor population, however, the mechanisms underlying their diversification remain poorly understood. Here, we show that the Forkhead domain transcription factor Foxp1 plays a critical role in defining the columnar identity of motor neurons at each axial position. Using genetic manipulations, we demonstrate that Foxp1 establishes the pattern of LIM-HD protein expression, and accordingly organizes motor axon projections, their connectivity with peripheral targets, and the establishment of motor pools. These functions of Foxp1 act in accordance with the rostrocaudal pattern provided by Hox proteins along the length of the spinal cord, suggesting a model by which motor neuron diversity is achieved through the coordinated actions of Foxp1 and Hox proteins.
Keywords: Foxp1, motor neurons, neuronal fate, transcription factors, neural circuits
Introduction
The formation of neural networks depends upon the spatially organized generation of neurons with specialized functions and distinct synaptic specificities. Central to this process are the actions of inductive growth factors, which establish the patterned expression of transcription factors within neural progenitors and postmitotic neurons to provide these cells with a coordinate position within the nervous system related to their function (Jessell, 2000; Lupo et al., 2006; O'Leary et al., 2007). The complement of transcription factors expressed by a neuron also determines its migration, axon guidance and target recognition behaviors (McEvilly et al., 2002; Butler and Tear, 2007; Polleux et al., 2007), and can further influence its neurotransmitter status and pre-synaptic inputs (Goridis and Brunet, 1999; Vrieseling and Arber, 2006). However, it remains unresolved how patterning information across multiple axes is consolidated into the region-specific transcription factor “codes” that direct particular programs of neuronal differentiation and neural circuit assembly.
Considerable progress towards understanding this process has been made by studying the development of motor neurons (MNs) in the spinal cord. Spinal MNs are generated when two inductive signals, Sonic hedgehog and retinoic acid (RA), induce the expression of the essential MN determinant Olig2 in neural progenitors (Briscoe and Novitch, 2008). As MNs arise from Olig2+ cells, they subsequently diversify into distinct functional subtypes based on their position along the rostrocaudal axis and within each body segment (Jessell, 2000; Landmesser, 2001). While the rostrocaudal patterning of MNs has been well described (Liu et al., 2001; Dasen et al., 2003; Dasen et al., 2005), the mechanisms that establish the intrasegmental diversification of MNs have not been defined. Moreover, it remains unclear how these positional cues are integrated to allow MNs to segregate into different classes that innervate distinct muscle and autonomic nervous system targets throughout the body.
MNs first organize into longitudinal columns that extend along the rostrocaudal axis of the embryo to facilitate the matching of MNs with their synaptic targets (Landmesser, 1978; Jessell, 2000). At limb levels, newly born MNs separate to form a median motor column (MMC) that innervates trunk muscles, and a lateral motor column (LMC) that innervates the developing limbs (Jessell, 2000; Shirasaki and Pfaff, 2002). A similar bifurcation occurs in the thoracic spinal cord, leading to the formation of an MMC and a different group of lateral MNs termed the preganglionic motor column (PGC; referred to as the Column of Terni in chickens), which innervates the sympathetic nervous system (Jessell, 2000; Shirasaki and Pfaff, 2002). MMC and LMC MNs then separate further to form medial and lateral subcolumns (MMCm, MMCl, LMCm, and LMCl) that respectively innervate the dorsal and ventral halves of the trunk and limbs (Jessell, 2000; Shirasaki and Pfaff, 2002). Once this columnar organization has been established, MNs subdivide into even smaller groups, termed motor pools, which innervate the individual muscles within each target region (Romanes, 1964; Jessell, 2000; Dasen et al., 2005).
The rostrocaudal position of the motor columns is established by the functions of specific Hox transcription factors expressed along the body axis. The cross-repressive actions of Hox6 and Hox9 proteins play a critical role in specifying the formation of LMC versus PGC motor columns at brachial and thoracic levels, respectively (Dasen et al., 2003), while Hox10 proteins regulate LMC formation at lumbar levels (Carpenter et al., 1997; Lin and Carpenter, 2003; Shah et al., 2004). At later times, the combinatorial expression of different Hox proteins further subdivides the columns into individual motor pools, indicating that Hox proteins can contribute to the intrasegmental organization of MNs (Dasen et al., 2005). However, the same pattern of Hox protein expression is often observed within multiple motor columns present at the same rostrocaudal position (Figure S1; Liu et al., 2001; Dasen et al., 2005), suggesting that additional mechanisms exist to provide MNs with their intrasegmental identity.
To date, the best candidates for regulating the intrasegmental identity of MNs are members of the LIM-Homeodomain (LIM-HD) transcription factor family. The specific profile of LIM-HD proteins expressed by a MN correlates with its columnar status (Tsuchida et al., 1994; Jessell, 2000; Shirasaki and Pfaff, 2002), and experimental alterations of the code of LIM-HD proteins expressed by a MN can alter its cell body settling position, axonal projections, and target specificities (Sharma et al., 1998; Kania et al., 2000; Sharma et al., 2000; Kania and Jessell, 2003; Thaler et al., 2004). However, most LIM-HD proteins are broadly expressed by MNs as they are formed (Sharma et al., 1998; Tanabe et al., 1998), leaving it unresolved how the intrasegmental identity of the motor columns is initially assigned.
To identify novel regulators of MN diversification, we recently performed an analysis of the genes that are differentially expressed in control versus Olig2 mutant spinal cord progenitors, which lack the ability to form MNs (B.G.N., unpublished; Mukouyama et al., 2006; Briscoe and Novitch, 2008). Through this approach, we identified the Forkhead domain transcription factor Foxp1 as a protein prominently expressed by subsets of MNs at limb and thoracic levels of the spinal cord, suggesting that Foxp1 might contribute to the generation of different populations of MNs within these body segments (Figures 1 and S2). Foxp1 has previously been shown to play an essential role in B cell development as well as the pathogenesis of lymphoma (Haralambieva et al., 2006; Hu et al., 2006), and is also required for heart, lung, and esophagus development (Wang et al., 2004; Shu et al., 2007). Although Foxp1 expression has been observed in multiple regions of the central nervous system (Tamura et al., 2003), its function in neural development has not previously been examined.
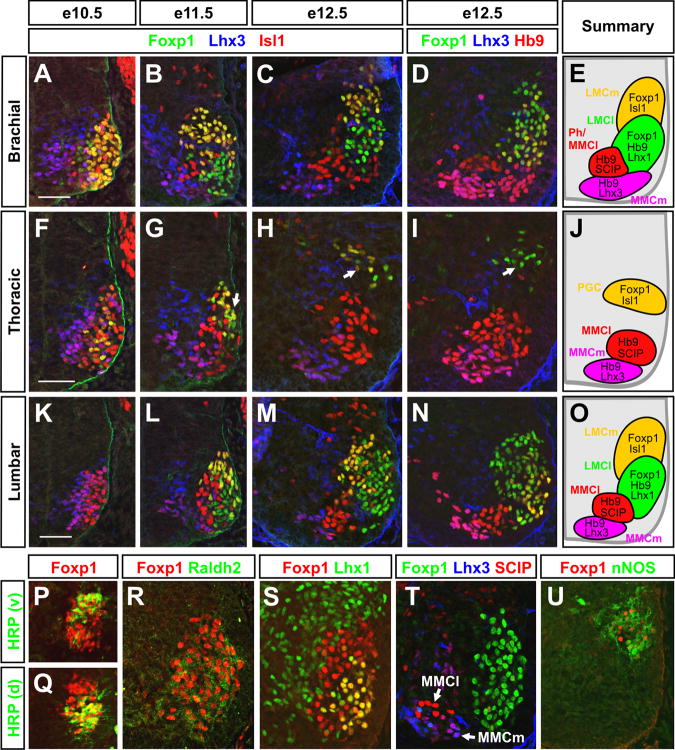
Figure 1. Foxp1 is selectively expressed by developing LMC and PGC MNs and distinguishes these cells from MMCm and MMCl MNs.
(A-O) Antibody costaining analysis of Foxp1 and LIM-HD protein expression in the developing mouse spinal cord.
(P-Q) HRP injections into ventral (P) or dorsal (Q) limb muscles at e13.5 confirms that Foxp1 is present in both LMC MN populations.
(R-T) Foxp1 expression in the e12.5 rostral brachial spinal cord coincides with the expression of the LMC markers Raldh2 and Lhx1 but not SCIP, which is expressed by MMCm and MMCl MNs.
(U) Foxp1 expression in the e12.5 thoracic spinal cord coincides with the PGC MN marker nNOS. Ventrolateral quadrants of the spinal cord are shown in all images. Scale bars = 50 μm.
In this study, we demonstrate that Foxp1 plays a critical role in providing the intrasegmental identity of MNs by distinguishing both LMC and PGC MNs from MMC MNs along the body axis. When misexpressed, Foxp1 expands the formation of LMC and PGC MNs at the expense of MMC MNs. Conversely, in Foxp1 mutant mice, LMC and PGC MNs are transformed into MNs with MMC characteristics exhibited by changes in their LIM-HD transcription factor expression profile, aberrant expression of axon guidance receptors, altered axonal projections to peripheral targets, and inability to form LMC-specific motor pools. Lastly, we provide evidence that the pattern of Foxp1 expression in the spinal cord is shaped by the actions of Hox proteins and that their combined activities are required for the segment-appropriate generation of motor columns and pools.
Results
Foxp1 expression distinguishes LMC and PGC MNs from MMC MNs at each segmental level
To assess the function of Foxp1 in MN development, we mapped its expression in brachial and thoracic motor columns. Foxp1 was first detectable in the mouse spinal cord at e9.5, and by e10.5 was confined to laterally positioned MNs identified by Isl1 and Hb9 expression (Figures 1A and 1F; data not shown). Foxp1 was also present in the lumbar spinal cord, though its expression did not begin until ∼e10.5-e11.0 (data not shown). At e11.5-e12.5, the time at which the mature arrangement of motor columns first becomes apparent in the brachial spinal cord, Foxp1 was expressed by both LMCm and LMCl MNs, distinguished by their differential expression of Isl1, Hb9, Lhx1, and the pan-LMC marker Raldh2 (Figures 1A-E, 1K-1O, 1R, 1S, and Table 1; Tsuchida et al., 1994; Sockanathan and Jessell, 1998; Kania et al., 2000; Dasen et al., 2003). The LMC identity of these Foxp1+ cells was also confirmed by horseradish peroxidase (HRP) retrograde labeling from dorsal and ventral forelimb muscles (Figures 1P and 1Q). At all stages and levels examined, Foxp1 was absent from MMCm MNs identified by their expression of Lhx3 and Hb9 (Figures 1A-1E, 1L-1O, and Table 1; Tsuchida et al., 1994). The selective pattern of Foxp1 expression in LMC MNs was also observed in the chick spinal cord, where Foxp1 preceded the onset of Raldh2 expression, as well as the subdivision of the LMC into LMCl and LMCm (Figures S3A, S3C-E, S3H-S3N).
Table 1. Molecular markers used to distinguish MN columnar identities.
| Motor Neuron Subclass | Molecular Markers |
|---|---|
| Brachial and lumbar levels | |
| MMCm/Rhomboideus MNs | Lhx3, Hb9, Isl1, Isl2, SCIPlow* |
| MMCl/Phrenic MNs | Hb9+, Isl1high, Isl2, SCIPhigh* |
| LMCm | Foxp1, Hb9low, Isl1, Isl2, Raldh2 |
| LMCl | Foxp1, Lhx1, Hb9, Isl2, Raldh2 |
| Thoracic level | |
| MMCm | Lhx3, Hb9, Isl1, Isl2, SCIPlow |
| MMCl | Hb9+, Isl1high, Isl2, SCIPhigh |
| PGC | Foxp1, Hb9low, Isl1, Isl2low, nNOS |
SCIP has also been observed to label LMCm motor pools in the caudal brachial and caudal lumbar spinal cord (Dasen et al., 2005; Luria and Laufer, 2007). To avoid confusion with these LMCm MNs, our analyses examine SCIP expression only in the rostral portion of these spinal cord segments.
In the thoracic spinal cord of e10.5 mouse embryos, where limb-innervating LMC MNs are not formed, low levels of Foxp1 were present in a laterally positioned population of Isl1+ MNs (Figure 1F). At e11.5 and later stages, these Foxp1+ MNs were positioned more dorsally (Figures 1G-1J), and expressed neuronal nitric oxide synthase (nNOS; Figure 1U), consistent with the characteristics of PGC MNs (Markham and Vaughn, 1991; Wetts and Vaughn, 1994; Thaler et al., 2004). As observed at limb levels, Foxp1 was not expressed by MMCm MNs, and was further absent from MMCl MNs, identified by their high expression of Isl1, Hb9, and SCIP, and their lack of Lhx3 expression (Figures 1F-1J, 1T, Table 1). The selective expression of Foxp1 in PGC MNs was also evident in the chick Column of Terni (Figures S3B, S3F, and S3G). Foxp1 further labeled parasympathetic MNs in the sacral spinal cord (data not shown), but it was not detectable in MNs at upper cervical levels where only MMC MNs are formed (Tsuchida et al., 1994).
Through this analysis, we unexpectedly found that MNs with an MMCl transcription factor profile (Foxp1- Lhx3- Isl1high Hb9+ SCIPhigh) exist at almost every axial level (Figures 1A-1O, 1T, and Table 1). This intriguing result suggests that MMCl MNs are not confined to the thoracic spinal cord as previously thought (Tsuchida et al., 1994), but rather extend along the body axis. The settling position of the MMCl MNs at upper cervical levels was consistent with the location of phrenic MNs that innervate the diaphragm (Goshgarian and Rafols, 1981; Lindsay et al., 1991), but the specific identities of MMCl MNs at other levels remains to be determined. Together, these findings demonstrate that Foxp1 expression defines the earliest known step in the separation of the LMC and PGC MNs from MMC MNs, which arise from a common set of neural progenitors (Figure S2).
Foxp1 promotes the formation of LMC and PGC MNs
The reciprocal pattern of Lhx3 and Foxp1 expression within the motor columns suggested that these transcription factors might regulate each other's expression to specify LMC, PGC, or MMCm MN identities. To test this possibility, we first performed misexpression experiments in the developing chick spinal cord using CMV-based plasmid expression vectors. The ectopic expression of Lhx3 within brachial MNs potently suppressed the expression of Foxp1 (Figures 2A and 2B), and reduced the expression of the LMC markers Raldh2 and Lhx1 (data not shown). Lhx3 misexpression in the mouse has similarly been shown to promote MMCm characteristics at the expense of LMC and PGC MNs (Sharma et al., 2000), which taken together with these findings suggests that one of the ways in which Lhx3 may promote MMCm MN development is by repressing Foxp1 expression and thereby preventing LMC and PGC MN formation.
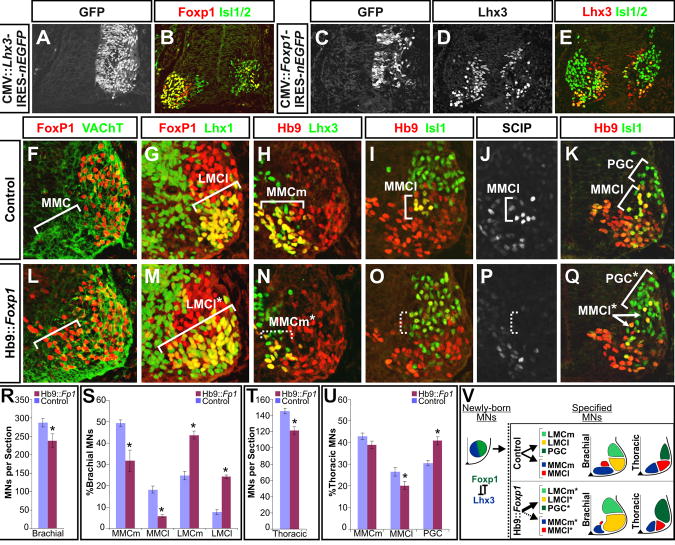
Figure 2. Foxp1 misexpression is sufficient to repress Lhx3 and MMC MN fates, and promotes the early formation of LMC and PGC MNs.
(A-E) Effects of misexpression of Lhx3 or Foxp1 in the brachial spinal cord of chick embryos. Images representative of 5-10 embryos for each experiment.
(F, L) Costaining analysis of Foxp1 and the general MN marker VAChT demonstrates that Foxp1 is expressed by most MNs in e11.5 Hb9::Foxp1 transgenic animals. Arrows indicate the normal position of MMC MNs in control and transgenic animals.
(G-J; M-P) Analysis of the rostral forelimbs reveals an expansion in the production of Lhx1+ LMCl MNs, and a reduction in both Lhx3+ MMCm (H, N), and Isl1high Hb9+ SCIPhigh MMCl MNs (I, J, O, P).
(K,Q) The transgenic misexpression of Foxp1 at thoracic levels increases the appearance of dorsally migrating Isl1+ Hb9low PGC MNs, and decreases the number of Isl1high Hb9+ MMCl MNs.
(R-U) Quantification of MN numbers in Hb9::Foxp1 and littermate control embryos. Mean ± SEM were calculated by pooling multiple sections collected from at least two embryos of each genotype. Results are representative of 12 embryos analyzed. (R, T) Hb9::Foxp1 animals show a small reduction in total MNs at brachial and thoracic levels, p < 0.001 and p < 0.01 respectively. (S) MMCm and MMCl MNs were reduced in Hb9::Foxp1 embryos (p < 0.001 in both cases), while LMCm and LMCl MNs were increased (p < 0.05 and p < 0.001, respectively). (U) Foxp1 misexpression at thoracic levels led to a small decrease in MMCm formation (p = 0.16), a more significant decrease in MMCl formation (p < 0.05), and an increase in PGC MN formation (p < 0.001).
(V) Schematic summary of the misexpression experiments.
To examine whether Foxp1 could offset Lhx3 expression and direct LMC and PGC MN formation, we similarly misexpressed Foxp1 in the chick spinal cord. Foxp1 misexpression strongly inhibited Lhx3 expression, but also reduced the total number of MNs formed (Figures 2C-2E), making it difficult to assess the specificity of its effects on Lhx3 and MN development in this system. We therefore generated transgenic mice in which Foxp1 was expressed under the control of the mouse Hb9 promoter, which drives expression in most spinal MNs (Wichterle et al., 2002). Although Hb9::Foxp1 expression still led to a ∼15% decrease in total MN numbers (Figure 2R), it more significantly changed the composition of the motor columns. In the brachial spinal cord, Foxp1 misexpression resulted in a ∼35% decrease in the generation of MMCm MNs and a ∼70% decrease in the generation of MMCl MNs relative to littermate controls (Figures 2H-2J, 2N-2P, and 2S). The loss of MMC MNs was reciprocated by a two to three-fold increase in the generation of LMCm and LMCl MNs, and an inappropriate scattering of these cells throughout ventral horns (Figures 2G, 2M, and 2S).
In the thoracic spinal cord, the Hb9::Foxp1 transgene was partially silenced between e10.5-e11.5 (data not shown). Nonetheless, a similar albeit less pronounced effect on MN development was observed compared to that seen at brachial levels. Foxp1 misexpression here led to a ∼10% decrease in total MN numbers, but it again disproportionately reduced the formation of MMCm and MMCl MNs by ∼5% and ∼25% respectively (Figures 2T and 2U). These changes coincided with a ∼30% increase in immature PGC MNs clustered in a dorsolateral position in the ventral spinal cord (Figures 1G-J, 2K, 2Q, and 2U). However, we did not observe a significantly increased number of nNOS+ cells at later times in development (data not shown), suggesting that sustained expression of Foxp1 may be required for the maturation and/or survival of PGC MNs. Collectively, these findings provide evidence that the ectopic expression of Foxp1 is sufficient to suppress Lhx3 and redirect MMC MNs towards LMC and PGC fates (Figure 2V).
Foxp1 is required for LMC and PGC MN development
To assess the endogenous function of Foxp1, we next analyzed Foxp1 mutant mice for defects in MN formation. While the Foxp1 mutation is embryonic lethal at e14.5 due to cardiac failure (Wang et al., 2004), the development of the spinal cord was grossly intact. No significant changes in the total number of Olig2+ MN progenitors or differentiated MNs were seen at e9.5-e12.5 (Figures 3M and data not shown). However, we observed striking differences in the settling position of brachial MNs in the Foxp1 mutants, as most cells failed to assume the dorsolateral position characteristic of LMC MNs (arrows in Figures 3A and 3G). The disruption in motor column organization was further evident after examining the differential expression of Hb9 and Isl1 within MNs. Whereas staining for these proteins readily distinguished both LMCm and LMCl MNs in control animals (Figures 1E, 3B, and Table 1; Kania et al., 2000), we observed a ∼60-90% reduction in these MNs in the Foxp1 mutants and a corresponding increase in MMC MNs (Figures 3H, 3N, S5E, and S5F). Cells expressing the general LMC marker Raldh2 and the LMCl marker Lhx1 were similarly reduced (Figures 3C, 3D, 3I, 3J, and 3N). Intriguingly, the remaining Lhx1+ MNs in the Foxp1 mutants expressed very low levels of Lhx1 and Raldh2, and ∼40% of these cells aberrantly coexpressed Isl1 (Figures S4A, S4B, and data not shown), suggesting that these persistent LMC-like cells have a mixed columnar identity.
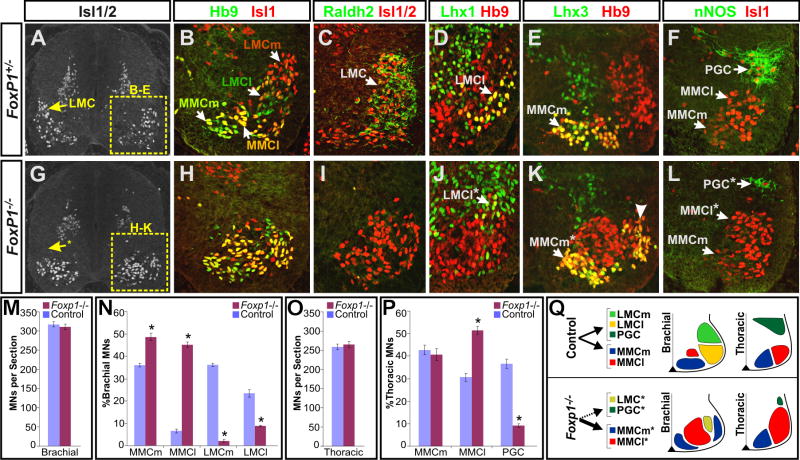
Figure 3. LMC and PGC MNs are transformed into MMC MNs in the absence of Foxp1.
(A-L) Antibody costaining analysis of transverse sections of e12.5 FoxP1+/- heterozygous and FoxP1-/- homozygous mutant littermates indicates an alteration in spinal motor column identities.
(B, C, D, H, I, J) Analysis of LIM-HD protein and Raldh2 expression at brachial levels indicates a considerable loss of LMC motor neurons in Foxp1-null animals, and an increased genesis of Hb9+ Isl1high MMCl MNs.
(E, K) Foxp1 mutants show an excessive production of laterally positioned MMCm-like cells that resemble rhomboideus MNs.
(F,L) nNOS staining at thoracic levels indicates a dramatic loss of sympathetic MNs in the Foxp1 mutant mice, and a corresponding increase in the generation of Isl1+ MMCl MNs.
(M-O) Quantification of MN numbers in e12.5 Foxp1-/- mutants and littermate controls. Mean ± SEM were calculated by pooling multiple sections collected from at least two embryos of each genotype. Motor column identities were designated by the following antibody costaining combinations: MMCm, Lhx3+ Isl1+; MMCl, Lhx3- Hb9+ Isl1high; LMCm, Hb9low Isl1+; LMCl, Lhx1+ Hb9+; PGC, nNOS+ Isl1+.
(M) Total MN numbers are not significantly changed in the Foxp1 mutants. (N) Analysis in the mid-forelimb level (C5-C7) shows a significant increase in the generation of MMCm and MMCl MNs and loss of LMCm and LMCl MNs (p < 0.0001 in all cases). (O) Analysis of motor column distribution at thoracic levels shows no change in MMCm MN formation, but an increased generation of MMCl MNs and concomitant loss of PGC MNs in the Foxp1 mutants (p < 0.0001 in both cases).
(Q) Schematic summary of the Foxp1 mutant phenotype.
Coincident with the loss of LMC MNs, we observed that Lhx3+ MNs at mid forelimb levels were increased in Foxp1 mutant spinal cord by ∼35%, and that these ectopic cells coalesced to form a cluster of cells at the lateral edge of the ventral spinal cord that was separated from the medially positioned MMCm (Figures 3E, 3K, 3N, S5C, and S5D). The settling position of these MNs is reminiscent of rhomboideus MNs, a population of laterally positioned MMCm-like cells present in the brachial spinal cord that innervates axial muscles (Tsuchida et al., 1994). While these ectopic cells accounted for a portion of the Foxp1 mutant MNs, most cells lacked Lhx3 and instead expressed high levels of both Hb9 and Isl1, thus resembling phrenic MNs and other MMCl populations that normally form at this axial level (Figures 1B-1E, 3H, 3N, S5C-S5F).
In the thoracic spinal cord, the formation of MMCm MNs did not appear to be significantly changed (Figures 3O, S5I, S5J). However, we observed a ∼70% increase in the production of MMCl MNs in the Foxp1 mutants (Figures 3O, S5K, S5L) and a comparable reduction in nNOS+ PGC MNs (Figures 3F, 3L, and 3O), indicating that Foxp1 is required to suppress the MMCl fate and promote PGC MN formation. Lastly, we examined the lumbar phenotype of the Foxp1 mutants. Here, we again did not find an expansion in MMCm MNs, but rather the overproduction of MMCl MNs at the expense of the LMC (Figures S5O-S5R; data not shown).
Since most Foxp1 mutant MNs appeared to transform into a seemingly uniform group of MMCl MNs irrespective of their segmental position, we examined the pattern of Hox protein expression within MNs to assess whether the loss of Foxp1 also affected their rostrocaudal identities. Despite the clear changes in motor column organization within each body segment, the MN expression of Hoxa5, Hoxc6, Hoxc8, Hoxc9, and Hoxa10 was preserved (Figure S5). We further observed that aspects of the Foxp1 mutant phenotype were associated with the presence of Hox proteins within specific spinal cord segments. For example, whereas an expansion of MMCl cells was seen throughout the brachial to lumbar spinal cord (Figure S5E, S5F, S5K, S5L, S5Q, and S5R), the production of ectopic rhomboideus MNs was confined to the regions that expressed Hoxc6, but not Hoxc9 or Hoxa10 (Figures S5C, S5D, S5I, S5J, S5O, and S5P). Importantly, rhomboideus MNs normally arise solely from this portion of the spinal cord (Tsuchida et al., 1994; Ensini et al., 1998), indicating that the disruptions in motor column formation seen in the Foxp1 mutants is consistent with the intact rostrocaudal pattern specified by Hox proteins. These findings thus provide evidence that Foxp1 plays a critical role in directing both LMC and PGC MN fates, and further suggest that the diversification of MN subtypes throughout the spinal cord may be attributed to the combinatorial actions of Foxp1 and different Hox proteins expressed along the body axis (Figures 3Q and S5AA).
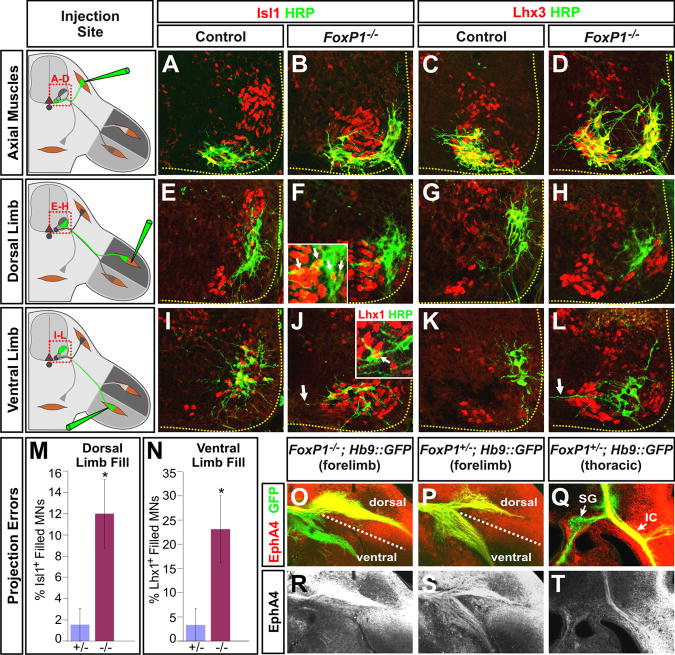
Foxp1 function is critical for the projections of LMC and PGC motor axons
The apparent transformation of all MNs into MMC MNs in the Foxp1 mutants led us to next consider how this defect alters the pattern of motor axon projections and their connectivity with synaptic targets, given that MMC MNs do not normally innervate the limbs or the sympathetic ganglia. We monitored MN projections using neurofilament antibodies, which label both motor and sensory axons, and the MN-restricted expression of GFP driven by a Hb9::GFP reporter transgene (Wichterle et al., 2002). Despite their MMC identity, Foxp1 mutant motor axons nevertheless projected into both the dorsal and ventral halves of the forelimb (Figures 4A-4D). The dorsal nerves, however, were significantly reduced in both their caliber and length, and lacked several branches seen in the controls (Figures 4G and 4H). Nevertheless, the ventral forelimb nerves appeared to be intact (Figures 4G and 4H). A comparable phenotype was also seen in the hindlimbs of the Foxp1 mutants (Figures 4K and 4L; data not shown), further indicating that the loss of Foxp1 affects dorsal but not ventral limb innervation.
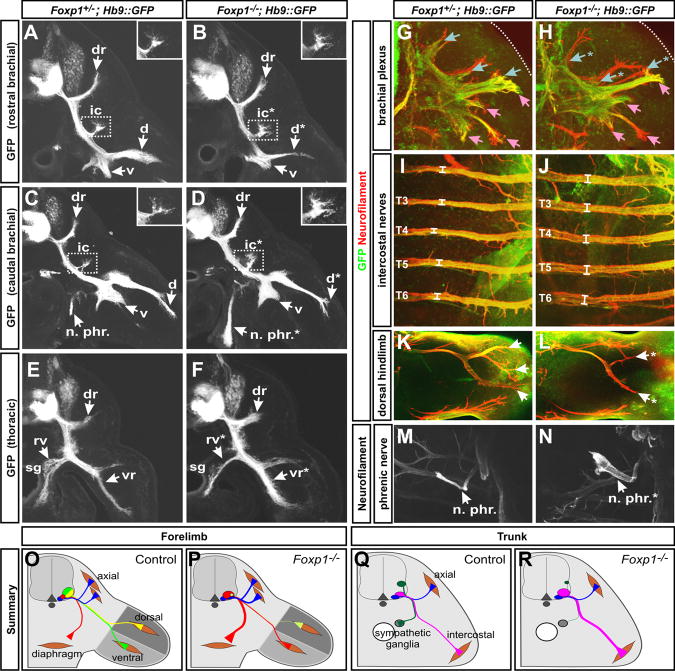
Figure 4. Redirection of LMC and PGC motor fibers toward MMC muscle targets in Foxp1 mutants.
(A-F) Analysis of motor fibers at e11.5 in vibratome sections of Hb9::GFP; Foxp1-null and littermate control embryos. Sections represent the following positions: (A, B) rostral brachial plexus, (C, D) caudal brachial plexus, and (E, F) rostral thoracic level. dr, dorsal ramus; ic, ramus intercostalis externus; d, dorsal plexus; n. phr., phrenic nerve; v, ventral plexus; rv, ramus visceralis; vr, ventral ramus; sg, sympathetic ganglia.
(G-N). Whole mount immunohistochemistry of motor (Hb9::GFP, green) and sensory plus motor fibers (neurofilament, red) in (G, H) the e11.5 brachial plexus, (I, J) e12.5 rostral intercostal nerves, (K, L) e12.5 dorsal hindlimb, and (M, N) e12.5 phrenic nerve (M, N). Blue and pink arrows in (G-H) designates dorsally and ventrally projecting nerves, respectively. Intercostal nerves in (I, J) were found to have an average diameter of 28.3±1.0 μm in control embryos and 40.3±1.9 μm in Foxp1 mutants, p < 0.01. * in all panels designates locations with reproducible changes in axon projections. Images are representative of > 3 embryos of each genotype analyzed.
(O-R) Schematic summary of axon misprojections.
Given the expansion of MMC MNs in the Foxp1 mutants, it seemed plausible that the loss of dorsal limb innervation might result from a redirection of the mutant motor axons towards MMC peripheral targets. We indeed observed that the phrenic nerves at forelimb levels were enlarged (Figures 4C, 4D, 4M, and 4N), and more motor fibers projected into the ramus intercostalis externus (boxed regions in Figures 4A-4D), a branch populated by rhomboideus motor axons (Nakao and Ishizawa, 1994). Likewise, thoracic motor fibers innervating the sympathetic ganglia were greatly reduced, while axons extending to the intercostal muscles were increased (Figures 4E-4H). Thus, at both limb and trunk levels of the spinal cord, Foxp1 function is required to promote the appropriate motor projection patterns of LMC and PGC MNs, and suppress projections associated with MMC MNs (Figures 4O-4R).
Altered motor axon topography in Foxp1 mutants
The unexpected ability of Foxp1 mutant MNs to innervate the limbs in spite of their MMC molecular identity raised the question of whether their projections occurred randomly or were organized in a topographic manner consistent with their altered LIM-HD expression profile. To distinguish between these possibilities, we injected HRP into different muscle groups to retrogradely label MNs in the brachial spinal cord, and analyzed their cell body position and LIM-HD status. Whereas HRP injections into axial muscles labeled a single cluster of MMCm MNs that expressed both Isl1 and Lhx3 in control embryos, the same procedure in the Foxp1 mutants labeled both the medial and laterally positioned groups of MMCm cells, though no labeling was seen in the MMCl MNs (Figures 5A-5D). These results suggest that only the excess MMCm MNs formed in the Foxp1 mutant embryos contribute to the innervation of axial muscles, and this phenotype correlates with the expression of Lhx3.
Figure 5. Topographic misprojections of motor axons in Foxp1 mutant embryos.
(A-D). MN projections to axial muscles were traced using HRP injections into axial muscles in e13.5 control and Foxp1 mutant embryos, and subjected to costaining analysis with the indicted antibodies.
(E-H) MN projections to the dorsal limbs were similarly traced using HRP injections. In both control and Foxp1 mutants, most dorsal projecting MNs lacked Isl1 staining and instead expressed Lhx1 (data not shown). However, in the Foxp1 mutants, some of the dorsally projecting neurons aberrantly expressed Isl1 (inset in panel F).
(I-L) Injections of HRP into the ventral limbs labels a dorsolaterally positioned group of Isl1+ cells in the controls, and a ventromedially positioned group of Isl1+ MN in Foxp1 mutants. Some ventrally projecting MNs in the Foxp1 mutants express both Lhx1 and Isl1 (inset in panel J). Arrows in panels J and L indicate the unusual horizontal morphology of dendrites labeled by retrograde labeling from the ventral limbs in the Foxp1 mutants.
(M) Quantification of retrograde labeling of MNs following HRP injections into dorsal and ventral limb muscles. The percentage of HRP labeled MNs that are Isl1+ following injections into the dorsal limb or Lhx1+ following injections into the ventral limbs are shown (p < 0.05 in both cases).
(N, O, Q, R) Distribution of EphA4 receptor in vibratome sections of the rostral brachial plexus from control or Foxp1-null littermates.
(P, S) Equivalent analysis of EphA4 expression in thoracic sections of wild-type embryos. SG, sympathetic ganglia; IC, intercostal nerves.
We next analyzed the topographic organization of limb-projecting MNs by HRP injections into dorsal and ventral forelimb muscles. In control embryos, dorsal muscle injections consistently labeled a ventrolaterally positioned population of LMCl MNs that expressed both Foxp1 and Lhx1, but not Isl1 (Figures 1Q, 5E, 5G, 5M; Table 1). In the Foxp1 mutants, HRP injections into the dorsal limb labeled the small number of LMCl-like MNs that persist in the absence of Foxp1. The majority of these labeled cells were clustered in a position similar to that seen in the control embryos and expressed Lhx1 and little to no Isl1 or Lhx3 (Figures 3J and 5F, 5H), much like normal LMCl MNs. However, ∼12% of MNs innervating the dorsal limb in the Foxp1 mutants inappropriately expressed Isl1 (inset in Figure 5F and Figure 5M), which is normally associated with LMCm projections to the ventral limbs (Kania et al., 2000; Kania and Jessell, 2003).
Injections of HRP into ventral forelimb muscles similarly labeled discrete populations of MNs in both control and Foxp1 mutant embryos. In the controls, these MNs displayed the expected LMCm LIM-HD profile, Lhx1- Isl1+ Lhx3-, appropriate for their innervation of ventral muscles (Figures 5I, 5K; Table 1). However, in the Foxp1 mutants, both the MMCl MNs and ∼20% of the Lhx1+ MNs were labeled by these HRP injections (Figure 5J, 5L, and 5M), indicating that some of the persistant LMCl-like MNs in the Foxp1 mutants had inappropriately innervated the ventral limbs. These misprojecting MNs typically expressed both Lhx1 and Isl1 (data not shown), suggesting that this phenotype may be a consequence of their mixed MN identity.
While the excess MMCl MNs formed in the Foxp1 mutants exhibited a LIM-HD profile indistinguishable from LMCm MNs in control animals and were able to effectively innervate the ventral limbs, these mutant MNs nonetheless assumed an aberrant ventromedial location in the spinal cord that resembled the normal settling position of MMCl MNs (Figures 1C-1E, 1H-1I, 5J, 5L, S6). These results support the conclusion that in the Foxp1 mutants the ventral limbs are innervated by MMCl MNs rather than bone fide LMCm MNs. We further observed that the dendrites of control MNs that were labeled by ventral limb HRP injections displayed a characteristic radial morphology (Figures 5E, 5G, S6A, S6C, and S6E; Vrieseling and Arber, 2006), while similarly labeled Foxp1 mutant MNs dendrites had long lateral processes that extended towards the ventral midline of the spinal cord (arrows in Figures 5J, 5L, S6D-S6F), reminiscent of the dendritic morphology of both phrenic and intercostal MNs (Figure S6C, S6G, S6K; Lindsay et al., 1991). Together, these results indicate that in the absence of Foxp1, MNs extend axons into the limbs in a topographic manner that is consistent with their altered LIM-HD profile. However, a portion of these MNs make axon guidance errors which may be attributed to either their inappropriate coexpression of Lhx1 and Isl1, or their adopting the cellular features of MMCl rather than LMC MNs.
Altered distribution of EphA4 on Foxp1 mutant motor axons
To define the basis of the disorganized MN projections to the limbs, we next examined the distribution of the tyrosine kinase receptor EphA4, which is normally expressed selectively expressed on LMCl motor axons to guide their projections away from ephrin A ligands produced in the ventral limb (Helmbacher et al., 2000; Eberhart et al., 2002; Kania and Jessell, 2003). In the Foxp1 mutants, EphA4 was present on both the dorsal and ventral branches of the forelimb nerves (Figures 5N, 5O, 5Q, and 5R), demonstrating that the distribution of this critical axon guidance receptor was altered in these animals. Intriguingly, EphA4 antibody staining robustly labeled MMCl MNs and intercostal projections in the thoracic spinal cord of control animals (Figures 5P, 5S, S7E, and S7F), suggesting that the altered distribution of EphA4 on Foxp1 mutant motor axons is likely another consequence of the transformation of LMC and PGC MNs towards an MMCl fate.
While EphA4+ expression normally directs the growth of LMC motor axons away from the ventral limb, many EphA4+ motor axons in the Foxp1 mutants nevertheless projected ventrally (Figures 5O and 5R). Thus, we infer that despite their expression of EphA4, Foxp1 mutant MNs may have a reduced capacity to respond to ephrins produced by the limb mesenchyme. Alternatively, their expression of Isl1 may lead to changes in the expression of other signaling factors that override the repulsive effects of EphA4 and ephrin As and direct motor projections towards the ventral limb, as has been observed with Isl1 misexpression in LMCl MNs (Kania and Jessell, 2003; Huber et al., 2005).
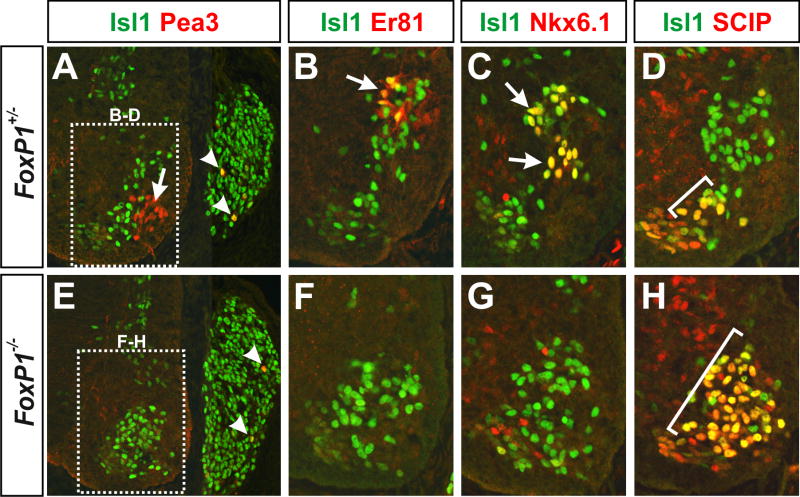
Defects in limb-level motor pool formation in Foxp1 mutants
One of the final steps in the assembly of motor circuits is the establishment of motor pools, a process in which MNs respond to target derived signals by turning on the expression of a series of transcription factors that are important for MN clustering, axonal branching, dendritic morphology, and connectivity with proprioceptive sensory afferents (Ladle et al., 2007). Although the columnar identity of Foxp1 mutant MNs was markedly disrupted, their axons were nevertheless able to innervate limb muscles, raising the question of whether the loss of LMC identity also affects the formation of limb-associaed motor pools. To address this issue, we monitored the expression of several transcription factors which are involved in motor pool formation at limb levels including Pea3, Er81, Runx1, Nkx6.1, and SCIP (Arber et al., 2000; Haase et al., 2002; Livet et al., 2002; Dasen et al., 2005; De Marco Garcia and Jessell, 2008). Whereas Pea3, Er81, Runx1, and Nkx6.1 labeled distinct LMCm and LMCl motor pools in control embryos, each of these markers were dramatically reduced in both brachial and lumbar MNs in Foxp1 mutants (Figures 6A-6C, 6E-6G, and data not shown). Importantly, the expression of Pea3 and Er81 in adjacent sensory neurons in the dorsal root ganglia was not changed (Figures 6A, 6E), indicating that the observed defects in motor pool formation are specific to MNs. Although most motor pool markers were reduced in the Foxp1 mutants, the expression of SCIP was significantly increased (Figures 6D and 6H). This phenotype most likely reflects the expanded production of SCIP+ MMCl MNs seen throughout the Foxp1 mutant spinal cord (Figures 1T, S5U, S5V, S5Y, S5Z, S6K). Together, these findings indicate that while Foxp1 mutant MNs are capable of innervating limb muscles, they ultimately fail to express several transcription factors critical for the formation of limb-level motor pools and sensory-motor connectivity.
Figure 6. Foxp1 is required for the appropriate formation of LMC-associated motor pools.
(A-H) Antibody costaining analysis of motor pool markers in the e13.0-e13.5 rostral hindlimb. Images representative of > 4 embryos of each genotype analyzed.
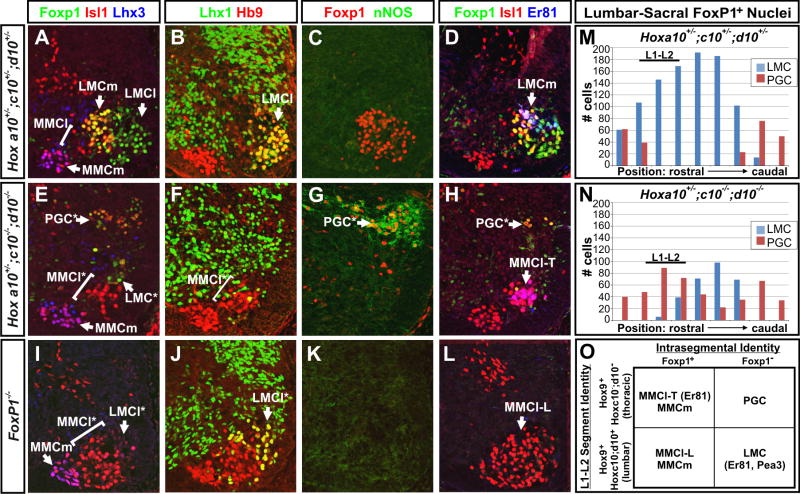
The formation of lumbar LMC MNs and motor pools requires the combined activities of Foxp1 and Hox10 proteins
Previous studies have found that the formation of LMC MNs and hindlimb motor pools also depends on the function of Hox10 proteins (Carpenter et al., 1997; Lin and Carpenter, 2003; Wu et al., 2008), raising the question of whether these defects can be attributed to a change in the pattern of Foxp1 expression or function. We therefore examined e13.5 Hoxa10+/-; c10+/-; d10+/- “3-allele” controls and Hoxa10+/-; c10-/-; d10-/- “5-allele” mutant spinal cords (Wellik and Capecchi, 2003) for changes in Foxp1 expression and hindlimb MN development. In the Hox10 5-allele mutants, Foxp1+ cells were reduced by 25-75%, with the most severe deficits seen at the L1 and L2 levels (Figure 7M and 7N). In addition, the level of Foxp1 expression within each cell was reduced, and the Foxp1+ cells were strikingly mispositioned (Figures 7A, 7C, 7D, 7E, 7G, and 7H). Whereas Foxp1+ cells normally settled in the ventrolateral quadrant of the lumbar spinal cord and expressed the LMC markers Raldh2 and Lhx1 (Figures 7B and data not shown), Foxp1+ cells in the Hox10 5-allele mutants assumed an aberrant dorsolateral position and expressed the PGC marker nNOS (Figures 7E, 7F, 7G, 7M, 7N, and data not shown), indicating that their fate had transformed from LMC to PGC. MMCl cells were also ∼5-fold increased in the Hox10 5 allele mutants, but MMCm MN numbers were not changed, (Figures 7A, 7D, 7E, 7H).
Figure 7. Cooperative functions of Foxp1 and Hox proteins are required for the formation of LMC MNs and motor pools.
(A-H) Antibody costaining analysis of lumbar (L1-L2) sections of e13.5 Hoxa10+/-; Hoxc10+/-; Hoxd10+/- 3-allele control and Hoxa10+/-; Hoxc10-/-; Hoxd10-/- 5-allele mutant embryos reveals changes in the pattern of Foxp1 expression and its abnormal association with the PGC marker nNOS rather than the LMCl marker Lhx1 and the LMCm motor pool marker Er81. Hox10 5-allele mutants also show an expansion of thoracic MMCl MNs, which express Er81 (MMCl-T), into the lumbar spinal cord.
(I-J) Analysis of motor column formation in the lumbar spinal cord (L1-L2) of age-matched Foxp1 mutant embryos. Foxp1 mutants show a reduced formation of LMCl MNs and an increased formation of MMCl that lack Er81 expression (MMCl-L).
(M-N) Distribution of Foxp1+ MNs as LMC-associated (nNOS-) and PGC-associated (nNOS+) along the rostrocaudal extent of the lumbar spinal cord of Hox10 3-allele control and Hox10 5-allele mutant litermates. Counts are representative of 3 embryos examined per genotype.
(O) Summary of the coordinate functions of Hox10 and Foxp1 in the determination of thoracic and lumbar motor columns and motor pools. In Hox10 5-allele mutants LMC MNs are transformed to a PGC fate, and lumbar MMCl MNs express the thoracic motor pool marker Er81 (MMCl-T). In Foxp1 mutants, LMC MNs are transformed into lumbar MMCl MNs that lack Er81 expression (MMCl-L). LMC MNs and LMC-associated motor pools only form in the presence of both Hoxc10/d10 and Foxp1.
In several ways, the Hox10 5 allele mutant phenotype resembled that seen in the Foxp1 mutants, as both showed an expansion in the generation of MMCl at lumbar levels and a loss of LMC MNs (Figures 7E, 7F, 7H, 7I, 7J, 7L). Moreover, in both mutant strains, limb-associated motor pool markers failed to be expressed (Figures 6A-6C, 6E-6G, 7D, 7H, 7L, and data not shown; Wu et al., 2008). There was, however, a key difference: Er81, a marker of thoracic motor pools (Figure S6L; Cohen et al., 2005), was present in the expanded population of MMCl MNs in the Hox10 5-allele mutants whereas it was absent from MMCl MNs in the Foxp1 mutants (Figures 7D, 7H, and 7L). The expression of Er81 in Hox10 5-allele mutants is consistent with the fate of lumbar MNs having been transformed anteriorly, leading to the formation of thoracic-associated motor columns and motor pools (MMCl-T) in the lumbar spinal cord. In contrast, the absence of Er81 from lumbar MMCl MNs in the Foxp1 mutants, strongly suggests that the segmental identity of these MNs is intact, and that the expanded MMCl MNs here have adopted the properties of MMCl MNs that normally form in the lumbar spinal cord (MMCl-L), and accordingly lack Er81 expression.
Together, these data demonstrate that Hox10 functions are critical for both the appropriate pattern of Foxp1 expression in the lumbar spinal cord and the ability of Foxp1-expressing cells to generate LMC instead of PGC MNs. These findings further suggest that the diversification of MNs results from the combined actions of Hox proteins acting to pattern MNs along the rostrocaudal axis and Foxp1 acting within each segment to determine distinct columnar and pool identities (Figure 7O).
Discussion
The formation of motor circuits requires the function of diverse MN subtypes that are dedicated to the innervation of specific muscle groups and the autonomic nervous system. To achieve this outcome, MNs are first organized into longitudinal columns that help to pair MNs with their peripheral targets. While a great deal of progress has been made in understanding the role of Hox proteins in the rostrocaudal patterning of MNs, the means by which different motor columns emerge from a common progenitor domain within each body segment has remained unclear. Our findings shed light on this problem by demonstrating that the Forkhead domain protein Foxp1 plays a critical role in promoting the formation of LMC MNs at limb levels, and PGC MNs in the trunk, while suppressing MMC MN fates. These results support a model by which MN diversity is achieved through the combined actions of Foxp1 and Hox proteins in specifying the profile of LIM-HD, ETS domain, and other transcription factors that control motor axon projections, connectivity, and ultimately motor pool formation.
Foxp1 and the suppression of MMC MN fates
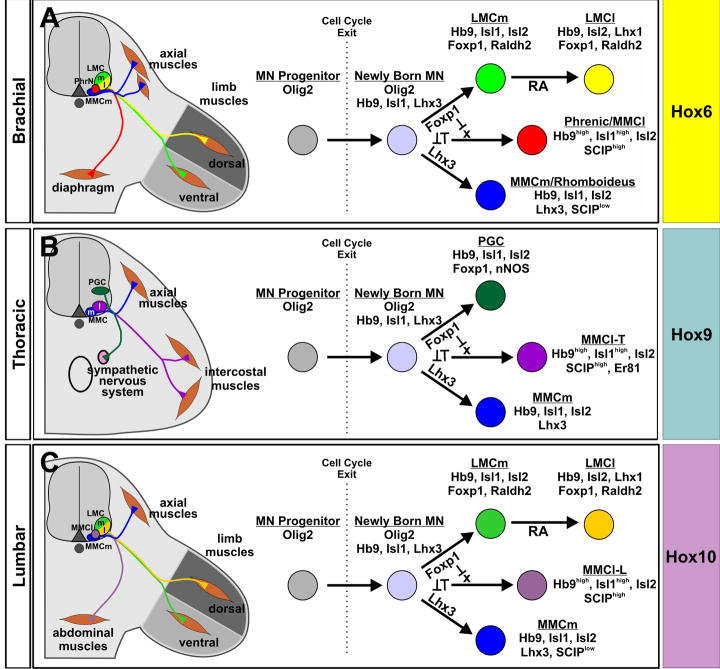
Foxp1 is selectively expressed in both LMC and PGC MN lineages from the earliest stages in their formation, and its function plays an essential role in separating these cells from MMCm and MMCl MNs that form at each segmental level (Figure 8). One of the ways in which Foxp1 achieves this outcome is by suppressing the expression of Lhx3, which itself promotes MMCm characteristics (Sharma et al., 2000), and potently suppress Foxp1 expression. However, the absence of Foxp1 from MMCl MNs, which do not express Lhx3, indicates that other factors may participate in offsetting Foxp1 expression to allow this MN class to be formed. The existence of an MMCl determinant is also suggested by the phenotype of Foxp1 mutant mice, which show a more significant increase in the generation of MMCl rather than MMCm MNs. Alternatively, MMCl development could serve as a “default” state that newborn MNs revert to when they fail to express either Lhx3 or Foxp1.
Figure 8. The integrated functions of Foxp1 and Hox proteins determine the columnar fate of MNs throughout the body.
(A-C) Proposed models for how different classes of MNs are formed at distinct rostrocaudal positions. At each axial level, MNs arise from a common population of Olig2+ neural progenitors. Soon after cell cycle exit, newly born MNs adopt one of three potential fates due to cross-repressive interactions between Lhx3 and Foxp1, and the ability of Foxp1 to block MMCl MN development. MMCl MN formation may further depend upon the function of an additional determinant (X). The establishment of segment-specific motor columns and motor pools then proceeds in accordance to the Hox protein profile expressed by the MNs. Hox proteins may further participate in the Foxp1-dependent intrasegmental patterning of MNs by regulating the level of its expression. LMC MN diversification is further driven by the actions of retinoid signaling (Sockanathan and Jessell, 1998; Sockanathan et al., 2003).
Previously, MMCl MNs were thought to exist solely at thoracic levels (Tsuchida et al., 1994), but our results indicate that a population of MMCl MNs exists along the rostrocaudal extent of the spinal cord. It is not surprising that these MNs have been overlooked at limb levels, since they normally exist in small numbers, and their LIM-HD expression profile is identical to that of LMCm MNs. MMCl motor axons at thoracic levels have been shown to innervate intercostal and other ventral body wall muscles (Tsuchida et al., 1994; Sharma et al., 2000; Thaler et al., 2004), and our data suggest that MMCl MNs in the cervical spinal cord also contribute to the phrenic nerves that innervate the diaphragm. Thus, a shared feature of MMCl MNs is their ability to innervate ventral hypaxial muscles. Retrograde labeling studies have observed that the settling position and size of abdominal projecting MNs is very similar to phrenic MNs, and abdominal and intercostal motor pools are often intermingled (Miller, 1987), consistent with all of these MN groups having a common columnar identity.
Foxp1 and the development of LMC and PGC MNs
Foxp1 also plays a critical role as the earliest known determinant for the LMC and PGC MN fates. One of the ways in which Foxp1 may contribute to LMC development is through its ability to regulate components of the retinoid signaling pathway, such as Raldh2, that promote Lhx1 expression and LMCl differentiation (Sockanathan and Jessell, 1998). It is thus fitting that Foxp1 and Raldh2-deficient mice have similar defects in LMC MN development (Vermot et al., 2005; Ji et al., 2006), though the absence of Foxp1 has a much more severe effect, consistent with Foxp1 playing a role upstream of Raldh2 and retinoid signaling in the assignment of LMC MN identity.
Given the broad defects in LMC MN formation in the Foxp1 mutants, it remains unclear why any Lhx1+ MNs persist in these animals. One possibility is that Foxp1 could act in a redundant manner with other members of the Foxp family or other factors that promote LMC MN development. While we cannot rule out the contributions of the latter, we have observed that both Foxp2 and Foxp4 are normally expressed in the spinal cord in a pattern that is non-overlapping with Foxp1, but their expression does not expand in the Foxp1 mutants (D.L.R and B.G.N, unpublished data). An alternative possibility is that the MMCl MNs that form at limb levels in the Foxp1 mutants are responsive to retinoids produced by the neighboring mesenchyme, as thoracic spinal cord explants have been shown to be capable of forming Lhx1+ LMCl MNs in response to retinoid administration in vitro, and the misexpression of Raldh2 at thoracic levels can achieve similar results in vivo (Sockanathan and Jessell, 1998).
Compared to LMC MNs, relatively little is known about the origins of PGC MNs. Some of the critical factors in their genesis include members of the Hox9 protein family, which promote PGC formation at brachial levels when misexpressed in chick (Dasen et al., 2003), presumably by changing the pattern of Foxp1 expression and the context in which it functions. High levels of Isl protein activity are also required for the generation of PGC MNs, as mice lacking Isl2 display a striking loss of nNOS+ MNs in the thoracic spinal cord (Thaler et al., 2004), similar to the Foxp1 mutant phenotype. In the Isl2 mutants, Lhx3 expression appears to expand (Thaler et al., 2004), and the repressive actions of Lhx3 on Foxp1 could thus underlie the loss of PGC MNs in these animals.
One of the most prominent features of PGC MNs is their characteristic migration in the spinal cord, first laterally and then dorsally (Markham and Vaughn, 1991). This initial lateral movement bears a striking resemblance to the migratory behavior of LMC MNs (Sockanathan and Jessell, 1998; Kania and Jessell, 2003), which seems fitting given that both cells depend upon Foxp1 for their formation. Since all MNs settle in a ventromedial position in the Foxp1 mutant spinal cord, it thus seems likely that Foxp1 serves an additional function in directing these MN migrations.
Combinatorial actions of Foxp1 and Hox proteins in the specification of MN identity
Our findings that the assignment of different MN fates along the spinal cord requires the function of both Foxp1 and Hox proteins raises the question of how might these transcription factor classes functionally intersect? While the expression of Hox proteins is preserved in the Foxp1 mutant spinal cord, the loss of Hox10 function was associated with a reduction in the number of Foxp1+ MNs formed, suggesting that Hox proteins can regulate the pattern of Foxp1 expression. In both mouse and chick, we also found that Foxp1 protein levels are much lower in PGC MNs than in LMC MNs (Figures 1, S3, and data not shown), raising the possibility that Hox proteins may further gate the function of Foxp1 by regulating the level of its expression. While we have no evidence that differential levels of Foxp1 expression can alone account for the formation of LMC versus PGC MNs, we have found that the production of early PGC MNs in Hb9::Foxp1 transgenic mice was primarily associated with the cells that expressed low to moderate levels of Foxp1 (data not shown), suggesting that the level of Foxp1 expression may be important for the development of PGC MNs.
An alternative possibility is that Foxp1 and Hox proteins serve as transcriptional coregulators, with the specificity of MN fates determined by the actions of Foxp1 and the different Hox proteins expressed at each body level. For example, in the brachial and lumbar spinal cord, where Hox6 or Hox10 proteins are highly expressed, Foxp1 directs newly born MNs to develop into LMC MNs and further establish limb-associated motor pools (Figures 8A and 8C). In contrast, in the thoracic spinal cord where Hox9 proteins are expressed, cells expressing Foxp1 differentiate into PGC MNs (Figure 8B). Studies in Drosophila have shown that the transcriptional repressor functions of a Forkhead domain protein, Sloppy paired, can augment the function of Hox proteins in the anteroposterior patterning of the abdomen (Gebelein et al., 2004). Sloppy paired and a HD protein, Engrailed, demarcate spatially distinct compartments within each abdominal segment, and modulate the function of Hox proteins in directing specific cell fates within each region (Gebelein et al., 2004). By this analogy, proteins such as Foxp1 and Lhx3 may serve a similar function in the spinal cord, producing MN compartments in which Hox proteins work to specify different MN fates. Cooperative interactions between Hox proteins and the Forkhead proteins Foxa1 and Foxa2 have also been recently implicated in the development of Clara cells in the lung (Yoshimi et al., 2005), suggesting that the functional intersections between Hox and Fox proteins may play a general role in tissue patterning and cell fate determination.
The importance of MN columnar fates in the assembly of locomotor circuits
In the absence of Foxp1, MNs with an MMCl columnar identity assume the role of LMC MNs in innervating the limbs, suggesting that environmental factors can override the genetic program within the MNs and allow them to extend axons towards inappropriate targets. Similar conclusions have been drawn from experiments in which Lhx3 has been misexpressed, producing MMCm MNs that innervate the limbs (Sharma et al., 2000). Despite this epigenetic control of motor axon growth, the MMCl MNs in the Foxp1 mutants do not go on to form limb-associated motor pools, and their dendritic structure retains features associated with MMCl MNs rather than LMC MNs. Thus, the genetic makeup of the MNs plays a critical role in the later stages of sensory-motor circuit assembly.
In many ways, the limb-projecting MNs in the Foxp1 mutants recapitulate the phenotype of heterotopic transplantation of thoracic spinal cord segments into lumbar levels in the chick (O'Brien et al., 1990; O'Brien and Oppenheim, 1990). In these experiments, thoracic-derived MNs successfully innervated limb muscles. Later in development, however, these transplanted MNs and the muscles that they innervated degenerated and hindlimb motor activity was accordingly lost (O'Brien et al., 1990; O'Brien and Oppenheim, 1990). In the early stages of limb innervation by thoracic-derived MNs, these cells maintained electrophysiological characteristics of MMCl MNs (O'Brien et al., 1990), which our findings show should be unable to form limb-associated motor pools. Thus, the degeneration of MNs in these transplantation experiments most likely results from the failure to complete this critical step in the assembly of motor circuits.
Together, these observations point to the importance of the columnar identity of MNs in the assembly of motor networks. While these findings provide novel insights into the hierarchical nature of MN development and functional diversification, they also present important considerations for studies looking to repair damaged or diseased motor circuits using stem-cell derived MNs (Hedlund et al., 2007). While MNs generated from either mouse or human embryonic stem cells (ESCs) have the capacity to broadly innervate muscles when transplanted into the chick spinal cord, the methods used to produce these cells primarily direct the formation of MMCm MNs and not other MN classes (Wichterle et al., 2002; Soundararajan et al., 2006; Lee et al., 2007). Most ESC-derived MNs do not express Foxp1 (D.L.R. and B.G.N., unpublished data), which may account for their lack of LMC and PGC characteristics. Given that the columnar identity of MNs is critical for the subsequent development of motor pools and circuit assembly, a better understanding of how Foxp1 and Hox proteins contribute to MN development should yield valuable insights into how a therapeutically beneficial panoply of MN subtypes may be created.
Experimental Procedures
Animal preparation
Olig2GFP/+ and Foxp1+/- heterozygous mice were maintained as previously described (Wang et al., 2004; Mukouyama et al., 2006). For tracing of motor axons, an Hb9::GFP reporter transgene (Wichterle et al., 2002) was bred into the Foxp1 mutant background. Hox10 mutant embryos were as previously described (Wellik and Capecchi, 2003). Hb9::Foxp1 transgenic animals were generated by inserting a cDNA corresponding to the murine Foxp1A isoform, an isoform found to be expressed in MNs (D.L.R. unpublished data), behind the -9 kb mouse Hb9 promoter (Arber et al., 1999; Wichterle et al., 2002). Purified DNA was microinjected into fertilized eggs obtained by mating (C57BL/6 X SJL)F1 female mice with (C57BL/6 X SJL)F1 male mice. Fertilized chicken eggs (Michigan State University Poultry Farm; AA Lab Eggs, Inc.) were incubated at 38°C staged, and electroporated as previously described (Novitch et al., 2001).
Immunohistochemistry and in situ hybridization
Antibody staining and in situ hybridization histochemistry was performed on cryosectioned tissues as previously described (Novitch et al., 2001; Novitch et al., 2003). Antibodies and probes used are described in the supplemental methods. Whole mount and vibratome antibody staining was performed as previously described (Eberhart et al., 2002; Huber et al., 2005). Fluorescence and DIC images were collected using a Zeiss Axioskop Imager or Axioobserver microscope equipped with the Apotome optical imaging system, or a Zeiss LSM5 Exciter confocal imaging system. Images were processed using the Zeiss Axiovision and LSM Exciter software suites, and Adobe Photoshop CS2.
Motor Neuron Quantification
The total number of labeled MNs per section was quantified from 12 μm cryosections sampled at 100 μm or 200 μm intervals along the rostrocaudal axis of the indicated regions. The percentage of labeled MNs per section was determined by dividing the indicated MN subtype values by the total number of MNs present based on the counts of all Hb9+ or Isl1+ cells on serial sections. Summarized counts were taken by averaging across several sections from multiple embryos. Motor fibers were analyzed in 3D confocal stacks taken from whole mount stains or vibratome sections and quantified for diameter and length. In all cases, the students t-test was applied to determine the statistical significance between experimental and control groups.
Retrograde Labeling of Motor Neurons
HRP was prepared, injected into e13.0-13.5 limb and axial muscle targets at forelimb levels, and quantified as previously described (Kania et al., 2000; Kania and Jessell, 2003; Huber et al., 2005).
Supplementary Material
Acknowledgments
We thank J. Briscoe, S. Butler, J. Feldman, D. Geshwind, A. Kania, C. Krull, S. Price, S. Sockanathan, M. Sofroniew, and L. Yang for reagents, experimental instruction, helpful discussions, and comments on the manuscript; S. Rakshit and A. Yallowitz for help in the collection of Hox10 mutant embryos; D. Meijer and S. Pfaff for generously providing reagents; J. Dasen and T. Jessell for generously providing reagents and communicating results prior to publication. We also acknowledge W. Filipiak, T. Sauders and the Transgenic Animal Model Core of the University of Michigan's Biomedical Research Core Facilities for the preparation of transgenic mice. Core support was provided by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor (Grant 085P1000815). D.W. was supported by a grant from the NIH (DK071929). E.E.M. was supported by a grant from the NIH (HL071589). B.G.N. was supported by grants from the Whitehall Foundation (2004-05-90-APL), the March of Dimes Foundation (5-FY2006-281), the Muscular Dystrophy Association (92901), and the NINDS (NS053976).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. doi: 10.1016/s0896-6273(01)80026-x. [DOI] [PubMed] [Google Scholar]
- Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2000;101:485–498. doi: 10.1016/s0092-8674(00)80859-4. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Novitch BG. Regulatory pathways linking progenitor patterning, cell fates and neurogenesis in the ventral neural tube. Philos Trans R Soc Lond B Biol Sci. 2008;363:57–70. doi: 10.1098/rstb.2006.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SJ, Tear G. Getting axons onto the right path: the role of transcription factors in axon guidance. Development. 2007;134:439–448. doi: 10.1242/dev.02762. [DOI] [PubMed] [Google Scholar]
- Carpenter EM, Goddard JM, Davis AP, Nguyen TP, Capecchi MR. Targeted disruption of Hoxd-10 affects mouse hindlimb development. Development. 1997;124:4505–4514. doi: 10.1242/dev.124.22.4505. [DOI] [PubMed] [Google Scholar]
- Cohen S, Funkelstein L, Livet J, Rougon G, Henderson CE, Castellani V, Mann F. A semaphorin code defines subpopulations of spinal motor neurons during mouse development. Eur J Neurosci. 2005;21:1767–1776. doi: 10.1111/j.1460-9568.2005.04021.x. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Liu JP, Jessell TM. Motor neuron columnar fate imposed by sequential phases of Hox-c activity. Nature. 2003;425:926–933. doi: 10.1038/nature02051. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell. 2005;123:477–491. doi: 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- De Marco Garcia NV, Jessell TM. Early Motor Neuron Pool Identity and Muscle Nerve Trajectory Defined by Postmitotic Restrictions in Nkx6.1 Activity. Neuron. 2008;57:217–231. doi: 10.1016/j.neuron.2007.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart J, Swartz ME, Koblar SA, Pasquale EB, Krull CE. EphA4 constitutes a population-specific guidance cue for motor neurons. Dev Biol. 2002;247:89–101. doi: 10.1006/dbio.2002.0695. [DOI] [PubMed] [Google Scholar]
- Ensini M, Tsuchida TN, Belting HG, Jessell TM. The control of rostrocaudal pattern in the developing spinal cord: specification of motor neuron subtype identity is initiated by signals from paraxial mesoderm. Development. 1998;125:969–982. doi: 10.1242/dev.125.6.969. [DOI] [PubMed] [Google Scholar]
- Gebelein B, McKay DJ, Mann RS. Direct integration of Hox and segmentation gene inputs during Drosophila development. Nature. 2004;431:653–659. doi: 10.1038/nature02946. [DOI] [PubMed] [Google Scholar]
- Goridis C, Brunet JF. Transcriptional control of neurotransmitter phenotype. Curr Opin Neurobiol. 1999;9:47–53. doi: 10.1016/s0959-4388(99)80006-3. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Rafols JA. The phrenic nucleus of th albino rat: a correlative HRP and Golgi study. J Comp Neurol. 1981;201:441–456. doi: 10.1002/cne.902010309. [DOI] [PubMed] [Google Scholar]
- Haase G, Dessaud E, Garces A, de Bovis B, Birling M, Filippi P, Schmalbruch H, Arber S, deLapeyriere O. GDNF acts through PEA3 to regulate cell body positioning and muscle innervation of specific motor neuron pools. Neuron. 2002;35:893–905. doi: 10.1016/s0896-6273(02)00864-4. [DOI] [PubMed] [Google Scholar]
- Haralambieva E, Adam P, Ventura R, Katzenberger T, Kalla J, Holler S, Hartmann M, Rosenwald A, Greiner A, Muller-Hermelink HK, et al. Genetic rearrangement of FOXP1 is predominantly detected in a subset of diffuse large B-cell lymphomas with extranodal presentation. Leukemia. 2006;20:1300–1303. doi: 10.1038/sj.leu.2404244. [DOI] [PubMed] [Google Scholar]
- Hedlund E, Hefferan MP, Marsala M, Isacson O. Cell therapy and stem cells in animal models of motor neuron disorders. Eur J Neurosci. 2007;26:1721–1737. doi: 10.1111/j.1460-9568.2007.05780.x. [DOI] [PubMed] [Google Scholar]
- Helmbacher F, Schneider-Maunoury S, Topilko P, Tiret L, Charnay P. Targeting of the EphA4 tyrosine kinase receptor affects dorsal/ventral pathfinding of limb motor axons. Development. 2000;127:3313–3324. doi: 10.1242/dev.127.15.3313. [DOI] [PubMed] [Google Scholar]
- Hu H, Wang B, Borde M, Nardone J, Maika S, Allred L, Tucker PW, Rao A. Foxp1 is an essential transcriptional regulator of B cell development. Nat Immunol. 2006;7:819–826. doi: 10.1038/ni1358. [DOI] [PubMed] [Google Scholar]
- Huber AB, Kania A, Tran TS, Gu C, De Marco Garcia N, Lieberam I, Johnson D, Jessell TM, Ginty DD, Kolodkin AL. Distinct roles for secreted semaphorin signaling in spinal motor axon guidance. Neuron. 2005;48:949–964. doi: 10.1016/j.neuron.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Ji SJ, Zhuang B, Falco C, Schneider A, Schuster-Gossler K, Gossler A, Sockanathan S. Mesodermal and neuronal retinoids regulate the induction and maintenance of limb innervating spinal motor neurons. Dev Biol. 2006;297:249–261. doi: 10.1016/j.ydbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Kania A, Jessell TM. Topographic motor projections in the limb imposed by LIM homeodomain protein regulation of ephrin-A:EphA interactions. Neuron. 2003;38:581–596. doi: 10.1016/s0896-6273(03)00292-7. [DOI] [PubMed] [Google Scholar]
- Kania A, Johnson RL, Jessell TM. Coordinate roles for LIM homeobox genes in directing the dorsoventral trajectory of motor axons in the vertebrate limb. Cell. 2000;102:161–173. doi: 10.1016/s0092-8674(00)00022-2. [DOI] [PubMed] [Google Scholar]
- Ladle DR, Pecho-Vrieseling E, Arber S. Assembly of motor circuits in the spinal cord: driven to function by genetic and experience-dependent mechanisms. Neuron. 2007;56:270–283. doi: 10.1016/j.neuron.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Landmesser L. The distribution of motoneurones supplying chick hind limb muscles. J Physiol. 1978;284:371–389. doi: 10.1113/jphysiol.1978.sp012545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser LT. The acquisition of motoneuron subtype identity and motor circuit formation. Int J Dev Neurosci. 2001;19:175–182. doi: 10.1016/s0736-5748(00)00090-3. [DOI] [PubMed] [Google Scholar]
- Lee H, Shamy GA, Elkabetz Y, Schofield CM, Harrsion NL, Panagiotakos G, Socci ND, Tabar V, Studer L. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells. 2007;25:1931–1939. doi: 10.1634/stemcells.2007-0097. [DOI] [PubMed] [Google Scholar]
- Lin AW, Carpenter EM. Hoxa10 and Hoxd10 coordinately regulate lumbar motor neuron patterning. J Neurobiol. 2003;56:328–337. doi: 10.1002/neu.10239. [DOI] [PubMed] [Google Scholar]
- Lindsay AD, Greer JJ, Feldman JL. Phrenic motoneuron morphology in the neonatal rat. J Comp Neurol. 1991;308:169–179. doi: 10.1002/cne.903080204. [DOI] [PubMed] [Google Scholar]
- Liu JP, Laufer E, Jessell TM. Assigning the positional identity of spinal motor neurons: rostrocaudal patterning of Hox-c expression by FGFs, Gdf11, and retinoids. Neuron. 2001;32:997–1012. doi: 10.1016/s0896-6273(01)00544-x. [DOI] [PubMed] [Google Scholar]
- Livet J, Sigrist M, Stroebel S, De Paola V, Price SR, Henderson CE, Jessell TM, Arber S. ETS gene Pea3 controls the central position and terminal arborization of specific motor neuron pools. Neuron. 2002;35:877–892. doi: 10.1016/s0896-6273(02)00863-2. [DOI] [PubMed] [Google Scholar]
- Lupo G, Harris WA, Lewis KE. Mechanisms of ventral patterning in the vertebrate nervous system. Nature reviews. 2006;7:103–114. doi: 10.1038/nrn1843. [DOI] [PubMed] [Google Scholar]
- Luria V, Laufer E. Lateral motor column axons execute a ternary trajectory choice between limb and body tissues. Neural Develop. 2007;2:13. doi: 10.1186/1749-8104-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Vaughn JE. Migration patterns of sympathetic preganglionic neurons in embryonic rat spinal cord. J Neurobiol. 1991;22:811–822. doi: 10.1002/neu.480220803. [DOI] [PubMed] [Google Scholar]
- McEvilly RJ, de Diaz MO, Schonemann MD, Hooshmand F, Rosenfeld MG. Transcriptional regulation of cortical neuron migration by POU domain factors. Science. 2002;295:1528–1532. doi: 10.1126/science.1067132. [DOI] [PubMed] [Google Scholar]
- Miller AD. Localization of motoneurons innervating individual abdominal muscles of the cat. J Comp Neurol. 1987;256:600–606. doi: 10.1002/cne.902560412. [DOI] [PubMed] [Google Scholar]
- Mukouyama YS, Deneen B, Lukaszewicz A, Novitch BG, Wichterle H, Jessell TM, Anderson DJ. Olig2+ neuroepithelial motoneuron progenitors are not multipotent stem cells in vivo. Proc Natl Acad Sci U S A. 2006;103:1551–1556. doi: 10.1073/pnas.0510658103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Ishizawa A. Development of the spinal nerves in the mouse with special reference to innervation of the axial musculature. Anatomy and embryology. 1994;189:115–138. doi: 10.1007/BF00185771. [DOI] [PubMed] [Google Scholar]
- Novitch BG, Chen AI, Jessell TM. Coordinate regulation of motor neuron subtype identity and pan-neuronal properties by the bHLH repressor Olig2. Neuron. 2001;31:773–789. doi: 10.1016/s0896-6273(01)00407-x. [DOI] [PubMed] [Google Scholar]
- Novitch BG, Wichterle H, Jessell TM, Sockanathan S. A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron. 2003;40:81–95. doi: 10.1016/j.neuron.2003.08.006. [DOI] [PubMed] [Google Scholar]
- O'Brien MK, Landmesser L, Oppenheim RW. Development and survival of thoracic motoneurons and hindlimb musculature following transplantation of the thoracic neural tube to the lumbar region in the chick embryo: functional aspects. J Neurobiol. 1990;21:341–355. doi: 10.1002/neu.480210208. [DOI] [PubMed] [Google Scholar]
- O'Brien MK, Oppenheim RW. Development and survival of thoracic motoneurons and hindlimb musculature following transplantation of the thoracic neural tube to the lumbar region in the chick embryo: anatomical aspects. J Neurobiol. 1990;21:313–340. doi: 10.1002/neu.480210207. [DOI] [PubMed] [Google Scholar]
- O'Leary DD, Chou SJ, Sahara S. Area patterning of the mammalian cortex. Neuron. 2007;56:252–269. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Polleux F, Ince-Dunn G, Ghosh A. Transcriptional regulation of vertebrate axon guidance and synapse formation. Nature reviews. 2007;8:331–340. doi: 10.1038/nrn2118. [DOI] [PubMed] [Google Scholar]
- Romanes GJ. The Motor Pools of the Spinal Cord. Prog Brain Res. 1964;11:93–119. doi: 10.1016/s0079-6123(08)64045-5. [DOI] [PubMed] [Google Scholar]
- Shah V, Drill E, Lance-Jones C. Ectopic expression of Hoxd10 in thoracic spinal segments induces motoneurons with a lumbosacral molecular profile and axon projections to the limb. Dev Dyn. 2004;231:43–56. doi: 10.1002/dvdy.20103. [DOI] [PubMed] [Google Scholar]
- Sharma K, Leonard AE, Lettieri K, Pfaff SL. Genetic and epigenetic mechanisms contribute to motor neuron pathfinding. Nature. 2000;406:515–519. doi: 10.1038/35020078. [DOI] [PubMed] [Google Scholar]
- Sharma K, Sheng HZ, Lettieri K, Li H, Karavanov A, Potter S, Westphal H, Pfaff SL. LIM homeodomain factors Lhx3 and Lhx4 assign subtype identities for motor neurons. Cell. 1998;95:817–828. doi: 10.1016/s0092-8674(00)81704-3. [DOI] [PubMed] [Google Scholar]
- Shirasaki R, Pfaff SL. Transcriptional codes and the control of neuronal identity. Annu Rev Neurosci. 2002;25:251–281. doi: 10.1146/annurev.neuro.25.112701.142916. [DOI] [PubMed] [Google Scholar]
- Shu W, Lu MM, Zhang Y, Tucker PW, Zhou D, Morrisey EE. Foxp2 and Foxp1 cooperatively regulate lung and esophagus development. Development. 2007;134:1991–2000. doi: 10.1242/dev.02846. [DOI] [PubMed] [Google Scholar]
- Sockanathan S, Jessell TM. Motor neuron-derived retinoid signaling specifies the subtype identity of spinal motor neurons. Cell. 1998;94:503–514. doi: 10.1016/s0092-8674(00)81591-3. [DOI] [PubMed] [Google Scholar]
- Sockanathan S, Perlmann T, Jessell TM. Retinoid Receptor Signaling in Postmitotic Motor Neurons Regulates Rostrocaudal Positional Identity and Axonal Projection Pattern. Neuron. 2003;40:97–111. doi: 10.1016/s0896-6273(03)00532-4. [DOI] [PubMed] [Google Scholar]
- Soundararajan P, Miles GB, Rubin LL, Brownstone RM, Rafuse VF. Motoneurons derived from embryonic stem cells express transcription factors and develop phenotypes characteristic of medial motor column neurons. J Neurosci. 2006;26:3256–3268. doi: 10.1523/JNEUROSCI.5537-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura S, Morikawa Y, Iwanishi H, Hisaoka T, Senba E. Expression pattern of the winged-helix/forkhead transcription factor Foxp1 in the developing central nervous system. Gene Expr Patterns. 2003;3:193–197. doi: 10.1016/s1567-133x(03)00003-6. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, William C, Jessell TM. Specification of motor neuron identity by the MNR2 homeodomain protein. Cell. 1998;95:67–80. doi: 10.1016/s0092-8674(00)81783-3. [DOI] [PubMed] [Google Scholar]
- Thaler JP, Koo SJ, Kania A, Lettieri K, Andrews S, Cox C, Jessell TM, Pfaff SL. A postmitotic role for Isl-class LIM homeodomain proteins in the assignment of visceral spinal motor neuron identity. Neuron. 2004;41:337–350. doi: 10.1016/s0896-6273(04)00011-x. [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Vermot J, Schuhbaur B, Le Mouellic H, McCaffery P, Garnier JM, Hentsch D, Brulet P, Niederreither K, Chambon P, Dolle P, Le Roux I. Retinaldehyde dehydrogenase 2 and Hoxc8 are required in the murine brachial spinal cord for the specification of Lim1+ motoneurons and the correct distribution of Islet1+ motoneurons. Development. 2005;132:1611–1621. doi: 10.1242/dev.01718. [DOI] [PubMed] [Google Scholar]
- Vrieseling E, Arber S. Target-induced transcriptional control of dendritic patterning and connectivity in motor neurons by the ETS gene Pea3. Cell. 2006;127:1439–1452. doi: 10.1016/j.cell.2006.10.042. [DOI] [PubMed] [Google Scholar]
- Wang B, Weidenfeld J, Lu MM, Maika S, Kuziel WA, Morrisey EE, Tucker PW. Foxp1 regulates cardiac outflow tract, endocardial cushion morphogenesis and myocyte proliferation and maturation. Development. 2004;131:4477–4487. doi: 10.1242/dev.01287. [DOI] [PubMed] [Google Scholar]
- Wellik DM, Capecchi MR. Hox10 and Hox11 genes are required to globally pattern the mammalian skeleton. Science. 2003;301:363–367. doi: 10.1126/science.1085672. [DOI] [PubMed] [Google Scholar]
- Wetts R, Vaughn JE. Choline acetyltransferase and NADPH diaphorase are co-expressed in rat spinal cord neurons. Neuroscience. 1994;63:1117–1124. doi: 10.1016/0306-4522(94)90577-0. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang G, Scott SA, Capecchi MR. Hoxc10 and Hoxd10 regulate mouse columnar, divisional and motor pool identity of lumbar motoneurons. Development. 2008;135:171–182. doi: 10.1242/dev.009225. [DOI] [PubMed] [Google Scholar]
- Yoshimi T, Nakamura N, Shimada S, Iguchi K, Hashimoto F, Mochitate K, Takahashi Y, Miura T. Homeobox B3, FoxA1 and FoxA2 interactions in epithelial lung cell differentiation of the multipotent M3E3/C3 cell line. Eur J Cell Biol. 2005;84:555–566. doi: 10.1016/j.ejcb.2004.12.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.