Abstract
Nonviral gene carriers must associate with and become internalized by cells in order to mediate efficient transfection. Methods to quantitatively measure and distinguish between cell association and internalization of delivery vectors are necessary in characterizing the trafficking of vector formulations. Here, we demonstrate the utility of nitro-2,1,3-benzoxadiazol-4-yl (NBD)-labeled oligos for discriminating between bound and internalized gene carriers associated with cells. Brief exposure to dithionite quenches extracellular NBD-labeled material, but is unable to penetrate the cell membrane and quench internalized material. We have verified that dithionite-mediated quenching of extracellular materials occurs in both polymer- and lipid-based gene delivery systems incorporating NBD-labeled oligos. By exploiting this property, the efficiencies of cellular binding and internalization of lipid- and polymer-based vectors were studied and correlated to their transfection efficiencies. Additionally, spatiotemporal information regarding binding and internalization of NBD-labeled gene carriers can be obtained using conventional widefield fluorescence microscopy since dithionite-mediated quenching of extracellular materials reveals the intracellular distribution of gene carriers without the need for optical sectioning. Hence, incorporation of environmentally-sensitive NBD-oligos into gene carriers allows for facile assessment of binding and internalization efficiencies of vectors in live cells.
Introduction
Nonviral gene carriers offer the potential to safely and efficiently mediate the delivery of nucleic acid-based therapeutics into targeted cells (1, 2). Nonviral vectors are typically based on cationic polymers or lipids which, upon self-assembly with plasmid DNA, form complexes termed polyplexes or lipoplexes, respectively. Despite efforts to design more efficient nonviral gene carriers, viral vectors remain the leading candidates for gene therapy due to their significantly higher gene transfer efficiencies compared to synthetic vectors. A number of physical barriers hinder the progress of gene carriers from the cell surface to the nucleus (3). Toward the design of more efficient nonviral gene carriers, it is important to quantitatively compare various vectors and formulations for their abilities to overcome each of the intracellular barriers. The initial barrier encountered upon arrival at targeted cells, cellular internalization, is a prerequisite to achieve transgene expression. A technique to reliably measure the degree of internalization of gene carriers into cells would facilitate the establishment of structure-function relationships in the development of improved nonviral gene carriers.
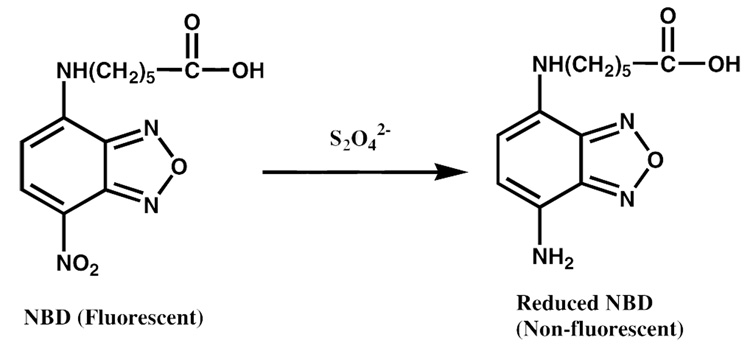
Here, a strategy is described for differentiating between extra- and intracellular gene carriers using 7-nitro-2,1,3-benzoxadiazol-4-yl (NBD)-labeled oligonucleotides as environment-sensitive probes. NBD is a highly fluorescent compound that is irreversibly quenched by exposure to the reducing agent dithionite (Scheme 1). It was previously demonstrated that NBD-labeled lipids are useful tools to monitor lipid asymmetry at the plasma membrane since cell membranes are relatively impermeable to dithionite (4). By the same principles, we demonstrate here that NBD-labeled oligos can be incorporated into polymer- or lipid-based nonviral gene carriers to distinguish between extra- and intracellular materials. Current protocols to quantify vector internalization include cell treatment with polyion wash buffers to remove cell-associated materials or cell exposure to anti-fluorophore antibodies or Trypan blue to quench extracellular fluorescence. The NBD/dithionite approach presented here offers a reliable, cost-effective alternative that is compatible with downstream analysis by flow cytometry, fluorescence microscopy, and fluorimetry in whole cells or lysates.
Scheme 1.
Dithionite-mediated reduction of the fluorophore nitro-2,1,3-benzoxadiazol-4-yl (NBD) to a nonfluorescent derivative.
To demonstrate the utility of NBD-labeled oligos, the internalization efficiencies of a series of lipoplex and polyplex formulations were evaluated in a high-throughput, fluorescence-based assay and compared to overall transfection efficiency. Additionally, we show that dithionite-mediated quenching of extracellular NBD-labeled gene carriers enables discrimination between bound and internalized materials associated with small-diameter neurites using widefield fluorescence microscopy, an application that was previously hindered by technical limitations. Hence, NBD-labeled oligos are a useful tool for investigating the binding and internalization profiles of nonviral gene carriers.
Experimental Procedures
2.1. Incorporation of NBD-labeled oligos into nonviral gene delivery systems
2.1.1. Synthesis of NBD-oligos and TR-oligos
Oligonucleotides with a 5’ amino group (5’–NH2 – TTC TCC GAA CGT GTC ACG TT –3’) were synthesized and desalted by Integrated DNA Technologies (Coralville, IA). Oligos were conjugated to succinimidyl ester derivatives of either 6-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)hexanoate (NBD-X) or Texas Red®-X (TR) (Invitrogen, Carlsbad, CA) following guidelines provided by the manufacturer. Briefly, oligos were purified by chloroform extraction to remove interfering compounds such as tris (hydroxymethyl) aminomethane (Tris). 100 µg of purified oligo was then diluted in 0.1 M sodium tetraborate buffer, pH 8.5, and the dye, freshly dissolved in DMSO, was added to this solution at a 40:1 or 20:1 dye:oligo ratio for NBD and TR, respectively. The conjugation reaction proceeded overnight at room temperature, and then free dye was separated from oligos by ethanol precipitation. Yields and conjugation efficiencies were determined based on absorbance measurements at 260, 465, and 595 nm to measure oligo, NBD, and TR concentrations, respectively. The NBD-labeled oligo was recovered with a 90% yield and the labeling efficiency was 81%. For the TR-labeled oligo, the yield and labeling efficiency were 75% and 85%, respectively.
2.1.2. Formulation of lipoplexes and polyplexes incorporating labeled oligos
Lipofectamine™ 2000 (L2K) was purchased from Invitrogen (Carlsbad, CA) and poly(ethylenimine) (PEI; 25 kDa MW, branched) was obtained from Sigma (St. Louis, MO). Lipoplexes were formulated by condensing labeled oligos with L2K according to the manufacturer’s instructions. Briefly, oligos were diluted in phosphate-buffered saline (PBS) (for fluorimeter-based quenching studies) or in OptiMEM (Invitrogen) (for cell binding and internalization studies) to a concentration of 0.01 µg/µl, and were added to an equal volume of L2K diluted in PBS or OptiMEM. The ratio of DNA (µg) to L2K (µl) was varied from 1:0.5 to 1:3. The mixture was incubated for 20 minutes at room temperature to allow lipoplex self-assembly. To formulate polyplexes, oligos were diluted to a concentration of 0.02 µg/µl in PBS or F12-K medium with no supplements (ATCC; Manassas, VA), and an equal volume of PEI diluted in PBS or F12-K medium was added. This mixture was incubated for 20 minutes at room temperature to allow particle formation. PEI amine to DNA phosphate (N/P) ratios were calculated based on the monomer molecular weights of 43 Da and 330 Da for PEI and DNA, respectively, and polyplexes were formulated at N/P ratios from 3 to 10.
2.1.3. Fluorescence measurements of dithionite-mediated NBD quenching in gene carriers
Lipoplexes and polyplexes were formulated as described in the previous section using NBD-labeled oligos. To evaluate dithionite-mediated quenching of NBD-labeled gene carriers, lipoplexes were formulated at a DNA:L2K ratio of 1:3 and polyplexes were formulated at an N/P ratio of 5. Sodium dithionite was purchased from EMD Chemicals (Gibbstown, NJ), and was prepared as a 1 M stock solution in 1 M Tris, pH 10 immediately prior to use. Fluorescence measurements were obtained using a TECAN Safire II microplate reader (Tecan Systems, Inc., San Jose, CA). NBD was excited at a wavelength of 465 nm, and emission was read at 535 nm. Initial fluorescence measurements were taken in wells containing 100 µl of the lipoplex solution, polyplex solution, or a solution containing an equivalent amount of free oligo. Subsequently, 9.6 µl of either 1 M dithionite in 1 M Tris (for a final dithionite concentration of 88 mM) or 9.6 µl of 1 M Tris alone (as a negative control) was added to the wells containing lipoplexes or polyplexes and incubated for 2 minutes at room temperature prior to repeating NBD fluorescence measurements. The pH sensitivity of NBD emission was tested by measuring the emission intensities of NBD-oligos in PBS (pH 7.4), Tris buffer (pH 10.0), and acetate buffer (pH 4.5). To verify that dithionite-mediated quenching was specific to NBD, lipoplexes formulated using a 90/10 mixture of NBD/TR were also analyzed. TR samples were excited at 595 nm and emission was read at 615 nm.
2.2. Binding and uptake of NBD-labeled lipoplexes and polyplexes in cultured cells
2.2.1. Cell culture
HeLa cells were purchased from ATCC (CCL-2) and cultured in minimum essential medium (MEM) supplemented with 10% fetal bovine serum and antibiotics. Cells were passaged every 3–4 days.
2.2.2. Microscopy-based binding and uptake assay
HeLa cells were seeded on a 24-well plate at 5 × 104 cells/0.5 ml/well and allowed to attach overnight. NBD-labeled polyplexes (N/P = 5) and lipoplexes (L2K:DNA = 3) were formulated as described in section 2.1.2. Cells were rinsed with PBS, and medium was replaced with 500 µl/well OptiMEM. Polyplexes or lipoplexes incorporating 1 µg NBD-oligo were added to each well and incubated for either 1 hour at 4 °C or for 3 hours at 37 °C. Following the specified incubation period, medium was removed and the cells were rinsed once with PBS. Then, dithionite in Tris buffer or an equivalent volume of Tris buffer was diluted to a concentration of 88 mM in PBS and incubated with the cells for 5 minutes. Following incubation, cells were rinsed 3 times with PBS and imaged immediately by brightfield and fluorescence microscopy using a standard FITC filter set.
2.3. Measurement of internalization and transfection efficiencies
2.3.1. Validation of internalization measurements by fluorimetry and flow cytometry
HeLa cells were seeded at 8 × 104 cells/well on a 24-well plate for fluorimetry or at 3 × 105 cells/well on a 6-well plate for flow cytometry and allowed to attach overnight. Cells were rinsed with PBS and pre-incubated in OptiMEM at 4 °C or 37 °C for one hour. NBD-labeled polyplexes (N/P = 5) were formulated as described in section 2.1.2., with the exception that polyplexes were formulated in water. After polyplex addition, cells were incubated at 4 °C or 37 °C for an additional hour. Medium was removed and cells were incubated with cold 10 mM Tris in PBS with or without 10 mM dithionite for 2 minutes. Cells were then rinsed 2 times with PBS. For flow cytometry, cells were harvested by trypsinization and cell populations in each sample were analyzed for mean fluorescence using a BD FACScan analyzer. Cell lysate was prepared for fluorimetry by resuspending cells in 0.1% Triton X-100 in water. After one freeze-thaw cycle, 10× PBS was added to the lysate for a final concentration of 1× prior to fluorescence measurements. Experiments were performed in triplicate and values are reported in relative fluorescence units.
2.3.2. Measurement of internalization and transfection efficiencies for polyplexes and lipoplexes
HeLa cells were seeded at 8 × 104 cells/well on a 24-well plate and allowed to attach overnight. Cells were rinsed with PBS and pre-incubated with OptiMEM at 37 °C for one hour. Polyplexes (N/P ratios of 10, 7, 5, and 3) and lipoplexes (lipid:DNA ratios of 3:1, 1:1, and 0.5:1) were formulated using NBD-labeled oligos or gWiz™ Luciferase plasmid (Aldevron; Fargo, ND) as described previously. Cells were incubated with polyplex or lipoplex solutions at 37 °C for 5 hours. For internalization measurements, dithionite treatment and lysate preparation were performed as described in section 2.3.1. For transfection efficiency measurements, media was replaced after five hours and reporter gene expression was measured 48 hours later. To measure luciferase reporter gene expression, cells were lysed with 1× Reporter Lysis Buffer (Promega; Madison, WI) and luciferase activity was measured using Promega’s Luciferase Assay System. Total protein in lysate samples was measured using a Micro BCA Protein Assay Kit (Pierce; Rockford, IL). Values are reported in relative light units (RLU)/mg protein and are the mean ± standard deviation of triplicate samples.
2.4. Ratiometric imaging to differentiate between intracellular and extracellular material in neuron-like cells
2.4.1. Culture of neuron-like PC-12 cells
PC-12 cells were obtained from ATCC (CRL-1721) and were cultured in F12-K medium supplemented with 15% horse serum, 2.5% fetal bovine serum, and antibiotics in a 37 °C, 5% CO2 environment. Cells were fed by replacing the growth medium every 2–3 days, and were passaged when ~80% confluent. Cells were detached from the flask by exposure to trypsin-EDTA for 2 minutes, and were collected in 1 ml of F12-K medium. To obtain a single-cell suspension, cells were passed through a series of fire-polished glass pipettes of decreasing diameter. For differentiation to a neuron-like phenotype, cells were plated on a poly-D-lysine-coated flask in differentiation medium consisting of F12-K medium supplemented with 1% horse serum, antibiotics, and NGF at a concentration of 100 ng/ml.
2.4.2. Ratiometric imaging to differentiate between intracellular and extracellular material
NBD/TR-double-labeled oligos were complexed with L2K at an L2K:DNA ratio of 3 as described in previous sections. Lipoplexes were adsorbed to the surface of wells of a 96-well plate by incubation for 1 hour at room temperature. Dithionite was added to samples at a final concentration of 88 mM and incubated for 5 minutes at room temperature before rinsing with PBS. As a negative control for NBD quenching, an equivalent volume of Tris buffer was added to lipoplexes in separate wells. Green and red fluorescence images were taken in each sample using identical image acquisition settings. Ratio analysis was performed using MetaMorph software to measure the green/red signal ratio for individual particles in dithionite-treated and untreated wells. Specifically, a particle was selected in the red image and the integrated pixel intensity was measured using the Region Measurements function in MetaMorph. The integrated pixel intensity for the particle was corrected for background fluorescence based on the integrated pixel intensity of a background region near the particle. Background-subtracted integrated pixel intensity values measured in both the green and red channels were used to calculate the green/red signal ratio.
6-day differentiated, neuron-like PC-12 cells were plated sparsely on a poly-L-lysine-coated 24-well plate to allow separation between cells, and were allowed to attach overnight. TR/NBD-double-labeled lipoplexes were formulated using a 90/10 NBD-/TR-oligo mixture. Cells were incubated with lipoplexes for 3 hours at 37 °C. Cells were then rinsed with PBS, incubated with PBS containing 88 mM dithionite for 5 minutes, rinsed 3 times, and imaged by brightfield and fluorescence microscopy. Internalized material was confirmed using the image analysis techniques described above.
Results
3.1. Packaged NBD-labeled oligos are quenched by dithionite
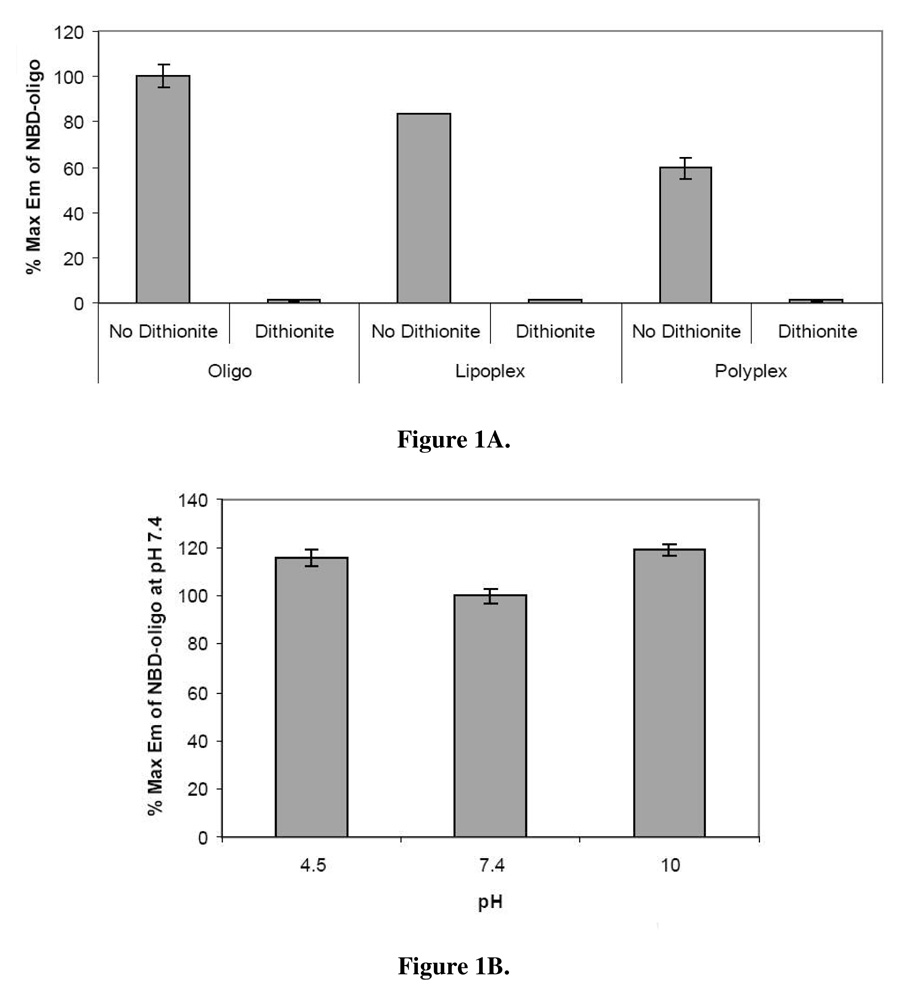
NBD fluorescence is irreversibly quenched upon exposure to dithionite by the reaction illustrated in Scheme 1. It was previously demonstrated that brief exposure to dithionite at concentrations up to 100 mM are nontoxic to cells, and that the NBD nitro group reduction occurs efficiently at a dithionite concentration of 88 mM (4). As shown in Fig. 1A, brief exposure of NBD-labeled oligos to 88 mM dithionite quenched their fluorescence by 99%.
Figure 1. Effect of dithionite treatment and pH on NBD fluorescence emission intensity.
(A) Fluorescence emission of NBD-labeled oligos and lipid- and polymer-based gene carriers incorporating NBD-labeled oligos was measured in the presence and absence of dithionite. Values are presented as the percentage fluorescence emission compared to an equivalent amount of uncomplexed, unquenched NBD-oligo ± standard deviation. (B) Fluorescence emission of NBD-oligos was measured in acidic, neutral, and basic buffers. Values are presented as the percentage fluorescence emission compared to an equivalent amount of NBD-oligo in PBS (pH 7.4) ± standard deviation.
NBD-labeled oligos were also examined for their susceptibility to dithionite-mediated quenching when incorporated into lipid-or polymer-based gene delivery systems, termed “lipoplex” and “polyplex”, respectively (Fig 1A). Condensation of NBD-labeled oligos by cationic lipids or polymers caused slight fluorescence quenching prior to the addition of dithionite. Quenching is commonly observed upon condensation of fluorophore-labeled DNA with polycations, as tight packing leads to fluorophore self-quenching. Still, the remaining NBD fluorescence was readily detectable above background. Dithionite exposure reduced the fluorescence signal by 99% and 98% for lipoplexes and polyplexes, respectively, and the resulting fluorescence levels were only slightly above background.
To serve as a useful probe that discriminates between extra-and intracellular gene carriers, NBD-oligos must be insensitive to pH fluctuations such as the acidification that occurs when gene carriers are transported through the endo-/lysosomal pathway. The fluorescence emission from NBD-oligos was compared at pH values of 4.5, 7.4, and 10.0 to determine the effect of pH on fluorescence emission. Exposure to acidic or basic environments did not significantly influence the NBD emission intensity (Fig. 1B).
3.2. NBD-labeled gene carriers exposed to cells function as an environment-sensitive probe
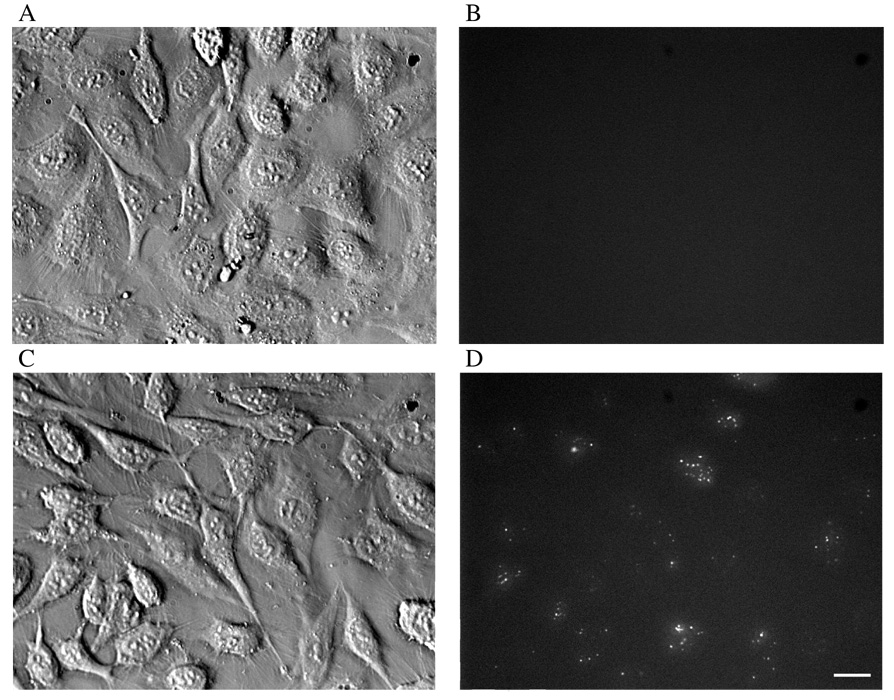
To validate NBD-labeled gene carriers as a tool for distinguishing between bound and internalized material in cells, polyplexes incorporating NBD-labeled oligos were incubated with HeLa cells at either 4 °C or 37 °C, and were subsequently exposed to dithionite to quench extracellular fluorescence. Since endocytosis is inhibited at 4 °C, it was expected that the labeled material would bind to, but remain outside of cells. In contrast, it was expected that the gene carriers would be internalized by cells during incubation at 37 °C. As shown in Fig. 2A and B, the fluorescence signal from NBD-labeled polyplexes that bound to cells at 4 °C was quenched by exposure to dithionite. In contrast, when cells were incubated with NBD-labeled polyplexes at 37 °C, polyplexes underwent internalization into cells, and the signal was not quenched due to the inability of dithionite to penetrate cellular membranes (Fig. 2C and D). Similar results were obtained for NBD-labeled lipoplexes (data not shown).
Figure 2. Selective quenching of cell-associated, uninternalized gene carriers in HeLa cells.
NBD-labeled polyplexes that bound to HeLa cells at 4 °C, but which were not internalized, were quenched by dithionite (A and B), while polyplexes incubated with cells for 3 hours at 37 °C were internalized and protected from dithionite-mediated quenching (C and D). Brightfield and corresponding fluorescence images are shown. (Scale bar = 20 µm)
3.3. Validation of internalization measurements based on quenching of extracellular NBD fluorescence
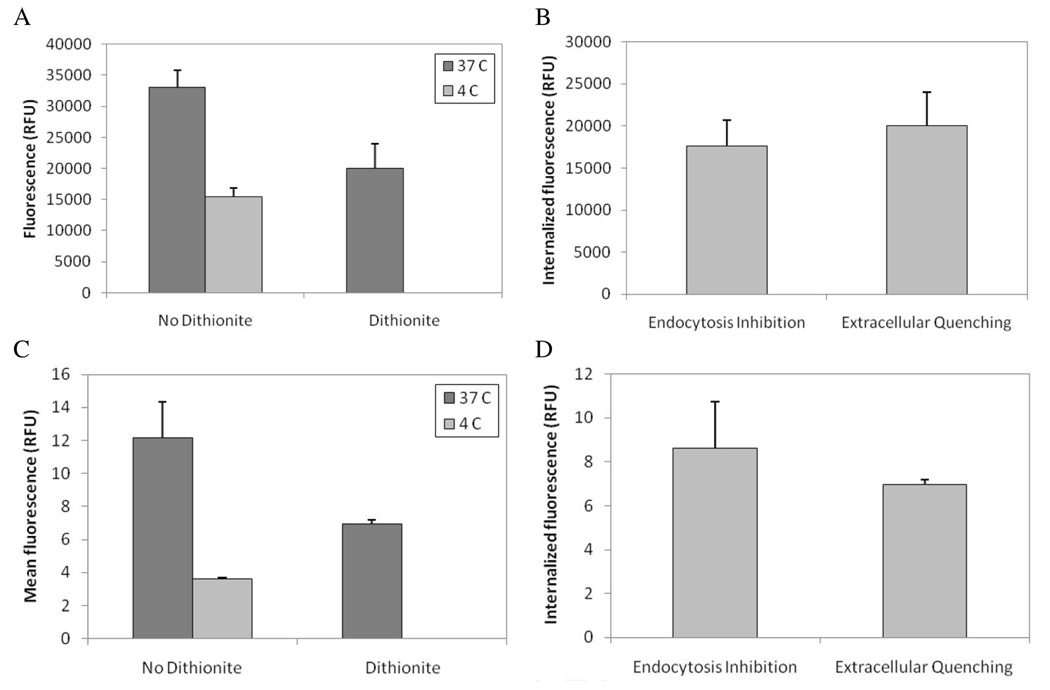
The degree of cellular internalization of gene carriers, determined based on the amount of unquenched NBD-oligo in dithionite-treated cell populations, was measured both by flow cytometry and by fluorescence measurements in cell lysates. To validate internalization measurements based on NBD fluorescence remaining after dithionite treatment, internalization was also measured by inhibiting endocytosis in samples that were not treated with dithionite (Fig 3). In the absence of dithionite treatment, the degree of polyplex internalization can be determined by inhibiting endocytosis by 4 °C. The fluorescence of internalized polyplexes is calculated by subtracting total cell-associated fluorescence of cells incubated with polyplexes at 4 °C from total cell-associated fluorescence of cells incubated with polyplexes at 37 °C. Dithionite treatment of cells incubated with polyplex at 4 °C abolishes all fluorescence (Fig. 3A and 3C). Therefore, it was hypothesized that the amount of internalized polyplex can also be determined quantitatively by simply measuring the total cell fluorescence from dithionite-treated cells. As shown in Fig. 3B and 3D, internalization measurements derived from dithionite-treated cells were in close agreement with those based on a comparison between cell-associated fluorescence in samples incubated with polyplexes at 37 °C versus 4 °C (p > 0.05) with both flow cytometry and fluorescence measurements of cell lystates. Similar data were obtained when cells were treated with NBD-labeled lipoplexes (data not shown). Thus, dithionite treatment of cells exposed to NBD-labeled vectors can be used as a rapid method to quantify internalization. It should be noted that, for studies involving the quantification of internalized vectors, dithionite treatment methods were modified to reduce the extent of exposure of cells to dithionite. Specifically, the dithionite concentration was reduced from 88 mM to 10 mM and the incubation time was reduced from 5 minutes to 2 minutes. Under these conditions, extracellular materials were efficiently quenched, while the quenching of internalized materials due to leakage of dithionite across the cell membrane was minimized.
Figure 3. Quantification of vector internalization by endocytosis inhibition versus extracellular quenching with dithionite treatment.
Cells were incubated with NBD-labeled polyplexes at 37 °C to allow binding and endocytosis of gene carriers. For endocytosis inhibition, a separate cell sample was incubated with polyplexes at 4 °C. For extracellular quenching with dithionite treatment, cells were incubated with polyplexes at 37 °C and then briefly treated with dithionite to quench extracellular fluorescence. NBD fluorescence was measured in cell lysates using a plate reader (A) and in suspensions of whole cells based on flow cytometry analysis (C). The degree of cellular internalization of polyplexes was measured either by subtracting cell-associated fluorescence at 4 °C from cell-associated fluorescence at 37 °C (No Dithonite) or by measuring unquenched fluorescence associated with cells following dithionite treatment (Dithionite). Differences in the degree of polyplex internalization measured based on endocytosis inhibition versus dithionite quenching were not statistically significant using either fluorescence measurements in lysate (B) or flow cytometry data (D).
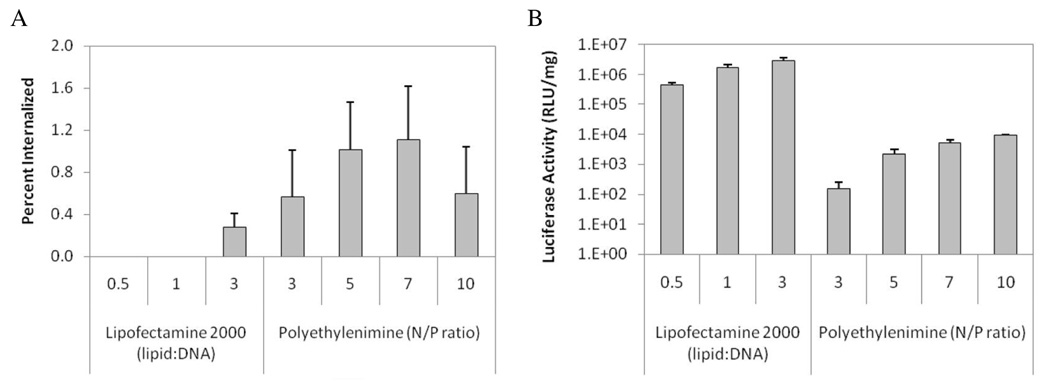
3.4. The degree of internalization of gene carriers varies with formulation parameters
The degree of internalization of various polyplex and lipoplex formulations was compared to the transfection efficiency mediated by the same formulation. As shown in Fig. 4, both internalization and transfection efficiencies differed significantly between lipoplexes and polyplexes. Interestingly, while lipoplex internalization by cells was at least 4-fold less efficient than polyplex internalization, reporter gene expression in the lipoplex-treated samples was two orders of magnitude higher. Although some fluorescence quenching occurs upon condensation of NBD-oligos by cationic polymers and lipids (Fig. 1), treatment of NBD-labeled lipoplexes and polyplexes with lysate containing 150 mM NaCl resulted in full vector unpackaging and fluorescence recovery (data not shown). Hence, the internalization data reported here are not confounded by differences in the degree of NBD-oligo self-quenching.
Figure 4. Correlation between the degree of vehicle internalization and transfection efficiency.
(A) The degree of cellular internalization of various lipoplex and polyplex formulations incorporating NBD-oligos was determined based on the amount of unquenched NBD fluorescence following dithionite treatment, and was normalized by the total NBD fluorescence added to the cells. (B) Transfection efficiencies of identical formulations incorporating luciferase-encoding plasmid DNA are presented as mean RLU/mg protein ± standard deviation.
3.5. NBD/Texas Red double-labeled gene carriers enable discrimination between internalized and cell-associated materials in neuron-like cells
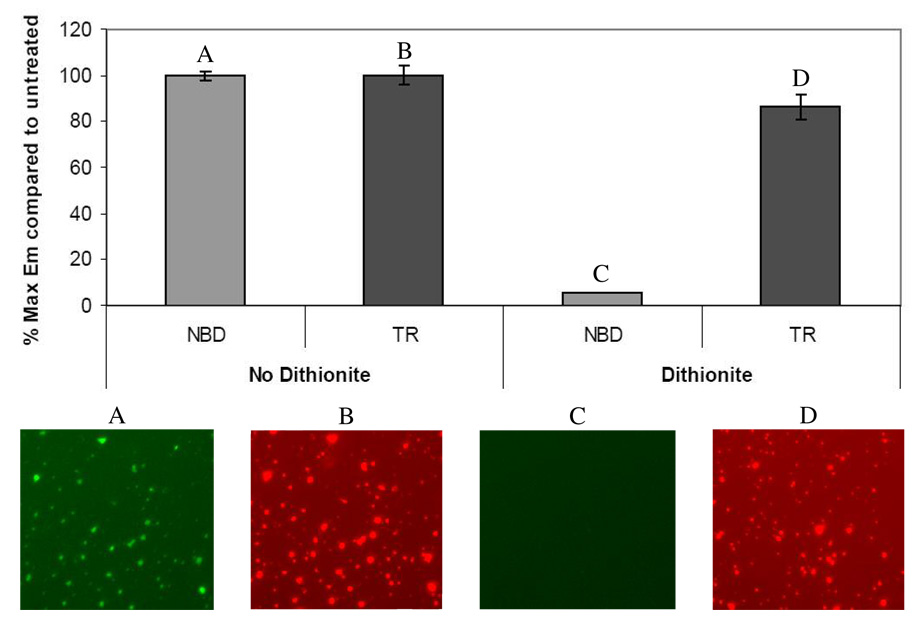
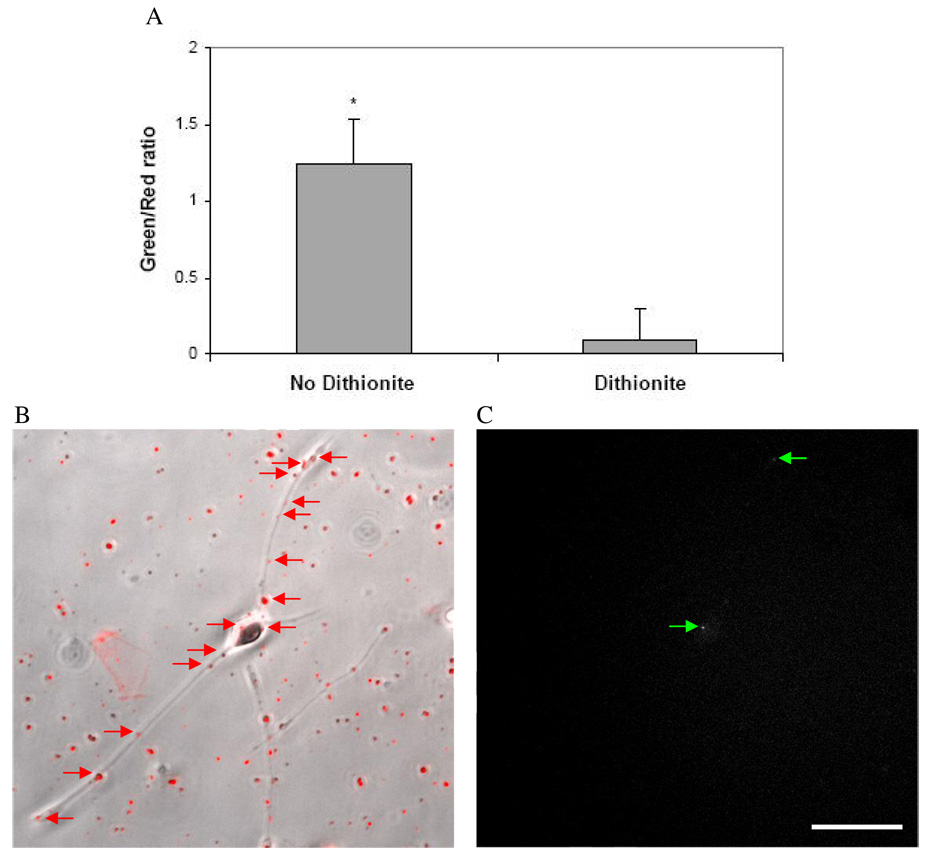
In order to simultaneously image surface-bound and internalized vectors, lipoplexes were double-labeled with the non-quenchable, pH-insensitive dye, Texas Red (TR), along with NBD. As expected, the NBD signal in double-labeled lipoplexes was quenched by dithionite, while the TR signal remained unaffected (Fig. 5). To quantitatively differentiate between uninternalized and internalized particles, ratiometric analysis methods were established using microscopy images of NBD/TR-double-labeled lipoplexes. A comparison of dithionite-treated versus untreated lipoplexes revealed that the green/red fluorescence emission ratios obtained were significantly different between treated and untreated lipoplexes (Fig. 6A). By applying ratiometric analysis to images NBD/TR-lipoplex-exposed, dithionite-treated cells and by comparing green/red fluorescence ratios of individual particles to the values obtained from quenched or unquenched particles in Fig. 6A, it was possible to confirm internalization of a fraction of the lipoplexes that bound to neuron-like PC-12 cells (Fig. 6B and C).
Figure 5. Selective quenching of NBD in double-labeled lipoplexes.
NBD/TR-double-labeled lipoplexes were examined by fluorimetry and fluorescence microscopy.
Figure 6. Ratiometric analysis distinguishes between intracellular and extracellular lipoplexes in neuron-like PC-12 cells.
(A) The ratio of NBD to TR fluorescence signals is significantly different between dithionite-treated and untreated particles (*p < 0.001). NBD/TR-double-labeled lipoplexes that associated with the cell are indicated by red arrows in the brightfield/fluorescence overlay image (B), while lipoplexes that were internalized retained NBD fluorescence and are indicated by green arrows (C). (Scale bar = 50 µm)
Discussion
Efficient intracellular delivery of DNA or RNA is critical for the success of nucleic acid-based therapies. A method to rapidly determine the efficiency of delivery vector uptake in mammalian cells would contribute toward the design and selection of promising delivery vehicles. For example, screening of polymer suites and libraries has yielded new materials with improved delivery efficiencies (5–7). Subsequent determination of the efficiency of specific cellular trafficking steps would provide valuable insights on structure-function relationships. Since most carriers form positively-charged nanoparticles upon complexation with nucleic acids, and since these particles are known to bind robustly to the cell membrane through electrostatic interactions, methods are necessary to distinguish between carriers that are internalized by cells and those which remain associated with the cell surface but fail to internalize. Additionally, the ability to discriminate between extra-and intracellular materials would enable evaluation of the spatiotemporal distribution of gene carriers in cells using standard widefield microscopy.
Various methods have been described to discriminate between surface-bound and internalized materials. Notably, several dye/quencher combinations have been employed to eliminate fluorescence from uninternalized materials associated with cells. For example, antibodies raised against fluorescent dye molecules, such as fluorescein, can selectively quench extracellular fluorescence due to tight binding of the antibody to the dye (8, 9). Additionally, treatment of cells with Trypan Blue eliminates extracellular green fluorescence through an energy transfer mechanism (10, 11). This technique has been employed to quench extracellular fluorescence associated with gene carriers and fluorescent microspheres (12–14). Finally, washing cells with buffers containing high salt concentrations has been utilized to dissociate complexes adsorbed to the cell surface through electrostatic interactions (15, 16). While implementation of these quenching techniques has provided important insight into the internalization efficiencies of various macromolecules, these techniques have limitations for the analysis of gene carrier internalization. First, it is unclear how efficiently antibodies can access fluorophores that are packaged within nanoparticles consisting of cationic lipids or polymers and nucleic acids. Additionally, the efficiency of quenching with Trypan Blue has been shown to depend on the specific brand of Trypan Blue used (17). Finally, since previous methods to quench extracellular fluorescence have employed fluorescein-labeled gene carriers, it is important to consider that the pH-sensitivity of fluorescein can confound measurements of internalization since its fluorescence may also be quenched in acidic intracellular compartments. The NBD/dithionite combination described here offers a reliable and cost-effective alternative.
In this work, NBD-labeled oligos were incorporated into polymer-and lipid-based nonviral gene carriers to differentiate between internalized vehicles and uninternalized vehicles associated with the cell plasma membrane. NBD-labeled gene carriers were quenched by exposure to dithionite, but retained their fluorescence in the intracellular milieu due to the relative impermeability of the cell plasma membrane to dithionite. As a demonstration of the utility of NBD-oligos in formulations screening, the internalization efficiencies of various polyplex and lipoplex formulations were measured. Internalization measurements were then compared to the efficiency of reporter gene expression mediated by the same formulation. Interestingly, while lipoplexes were internalized less efficiently than polyplexes, they mediated significantly higher transfection efficiencies in the cell line tested. This phenomenon was previously observed by Varga et al. (18), and is likely due to the enhanced intracellular trafficking efficiency of lipoplexes compared to polyplexes. Consequently, while internalization measurements may serve as an initial, rapid screen to identify formulations with high binding and internalization efficiencies, transfection screening should also be performed to uncover differences in the intracellular trafficking efficiencies of internalized materials.
It should be noted that slow diffusion of dithionite through cell membranes has been observed, and can result in intracellular fluorescence quenching (4). However, NBD quenching of extracellular material occurs within seconds whereas leakage through the cell membrane takes place on the order of minutes. The timing of fluorescence measurements may require optimization, especially for systems where perturbations to the integrity of the cell membrane can occur. Protocols have been described to account for the permeability of cellular membranes by measuring the initial rate of quenching, rather than the extent of quenching, and could be utilized if a highly precise determination of gene carrier internalization were required (19). Nonetheless, the NBD/dithonite combination described here offers a means for facile evaluation of gene carrier internalization that does not suffer from the limitations of previously described approaches.
NBD-oligos are also useful to distinguish between extracellular and internalized materials by fluorescence microscopy. Confocal laser scanning microscopy is routinely employed to study the intracellular distribution of nonviral gene carriers in cells. By taking optical slices as thin as 0.5 µm, confocal imaging has revealed the intracellular distribution of fluorescently-labeled gene carriers in fibroblasts and other cell types with subcellular detail (20). However, this technique is not reliable for imaging gene carriers in neuronal projections, where the depth of field is similar in size to the typical neurite diameter. This limitation was encountered by Suk et al. (21) in a study of the intracellular transport of PEI/DNA polyplexes in rat primary neurons. Although it is possible to narrow the slice depth in confocal images by applying digital deconvolution algorithms, it would be beneficial to develop an alternative method for differentiating between internalized and surface-bound gene carriers that can be implemented using conventional widefield fluorescence microscopy. By incorporating mixtures of NBD- and TR-labeled oligos into gene carriers and employing ratiometric imaging, it was possible to reliably differentiate between extra- and intracellular lipoplexes in the neurites of NGF-differentiated PC-12 cells. This concept is similar to ratiometric imaging of fluorescein/Cy5-labeled gene vectors to measure the pH environments of gene carriers in the cell, which has been described previously (8, 22), but it interrogates a different step in the delivery pathway.
In future applications, the NBD/dithionite system can potentially be exploited to measure the efficiency and dynamics of cell binding, internalization, and vesicular escape of gene carriers. While brief exposure of cells to dithionite quenches primarily extracellular NBD fluorescence, exposure beyond 30 seconds begins to slowly quench cytoplasmic fluorescence at a rate of approximately 4% per minute due to slow leakage of dithionite across the outer plasma membrane (4). However, NBD enclosed by intracellular vesicles such as endosomes remains largely unquenched by dithionite that leaks into the cytoplasm (23). By exposing cells to NBD-labeled gene carriers and measuring the quenching kinetics of NBD, it may be possible to measure the percentages of surface-bound, vesicle-enclosed, and cytosolic gene carriers. Since it is of interest to compare different gene vectors for their abilities to escape from endosomes, comparative studies using NBD-labeled oligos condensed by various polymers or lipids would elucidate structure-function relationships, while avoiding more time-consuming techniques such as subcellular fractionation.
Supplementary Material
Flow cytometry plots corresponding to the data presented in Figure 3C are provided as Figure S1. This information is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This work was funded by the NIH/NINDS (5R21NS052030-02) and an NSF CAREER Award (BES-0454324). JMB acknowledges the Whitaker Foundation and the University of Washington’s Engineered Biomaterials Training Program for graduate fellowship support. Flow cytometry studies were conducted at the UW Department of Immunology Cell Analysis Facility.
References
- 1.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4:581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 2.Gao X, Kim KS, Liu D. Nonviral gene delivery: what we know and what is next. Aaps J. 2007;9:E92–E104. doi: 10.1208/aapsj0901009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medina-Kauwe LK, Xie J, Hamm-Alvarez S. Intracellular trafficking of nonviral vectors. Gene Ther. 2005;12:1734–1751. doi: 10.1038/sj.gt.3302592. [DOI] [PubMed] [Google Scholar]
- 4.McIntyre JC, Sleight RG. Fluorescence assay for phospholipid membrane asymmetry. Biochemistry. 1991;30:11819–11827. doi: 10.1021/bi00115a012. [DOI] [PubMed] [Google Scholar]
- 5.Akinc A, Lynn DM, Anderson DG, Langer R. Parallel synthesis and biophysical characterization of a degradable polymer library for gene delivery. J Am Chem Soc. 2003;125:5316–5323. doi: 10.1021/ja034429c. [DOI] [PubMed] [Google Scholar]
- 6.Hwang SJ, Bellocq NC, Davis ME. Effects of structure of beta-cyclodextrin-containing polymers on gene delivery. Bioconjug Chem. 2001;12:280–290. doi: 10.1021/bc0001084. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Reineke TM. Poly(glycoamidoamine)s for gene delivery. structural effects on cellular internalization, buffering capacity, and gene expression. Bioconjug Chem. 2007;18:19–30. doi: 10.1021/bc060029d. [DOI] [PubMed] [Google Scholar]
- 8.Forrest ML, Pack DW. On the kinetics of polyplex endocytic trafficking: implications for gene delivery vector design. Mol Ther. 2002;6:57–66. doi: 10.1006/mthe.2002.0631. [DOI] [PubMed] [Google Scholar]
- 9.Mallender WD, Carrero J, Voss EW., Jr Comparative properties of the single chain antibody and Fv derivatives of mAb 4-4-20. Relationship between interdomain interactions and the high affinity for fluorescein ligand. J Biol Chem. 1996;271:5338–5346. doi: 10.1074/jbc.271.10.5338. [DOI] [PubMed] [Google Scholar]
- 10.Hed J, Hallden G, Johansson SG, Larsson P. The use of fluorescence quenching in flow cytofluorometry to measure the attachment and ingestion phases in phagocytosis in peripheral blood without prior cell separation. J Immunol Methods. 1987;101:119–125. doi: 10.1016/0022-1759(87)90224-9. [DOI] [PubMed] [Google Scholar]
- 11.Sun B, Eckhardt ER, Shetty S, van der Westhuyzen DR, Webb NR. Quantitative analysis of SR-BI-dependent HDL retroendocytosis in hepatocytes and fibroblasts. J Lipid Res. 2006;47:1700–1713. doi: 10.1194/jlr.M500450-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Bengali ZJCR, Shea LD. Gene expression and internalization following vector adsorption to immobilized proteins: dependence on protein identity and density. J Gene Med. 2007;9:668–678. doi: 10.1002/jgm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rejman J, Bragonzi A, Conese M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol Ther. 2005;12:468–474. doi: 10.1016/j.ymthe.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 14.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dailey LA, Kleemann E, Merdan T, Petersen H, Schmehl T, Gessler T, Hanze J, Seeger W, Kissel T. Modified polyethylenimines as non viral gene delivery systems for aerosol therapy: effects of nebulization on cellular uptake and transfection efficiency. J Control Release. 2004;100:425–436. doi: 10.1016/j.jconrel.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 16.Ruponen M, Ronkko S, Honkakoski P, Pelkonen J, Tammi M, Urtti A. Extracellular glycosaminoglycans modify cellular trafficking of lipoplexes and polyplexes. J Biol Chem. 2001;276:33875–33880. doi: 10.1074/jbc.M011553200. [DOI] [PubMed] [Google Scholar]
- 17.Van Amersfoort ES, Van Strijp JA. Evaluation of a flow cytometric fluorescence quenching assay of phagocytosis of sensitized sheep erythrocytes by polymorphonuclear leukocytes. Cytometry. 1994;17:294–301. doi: 10.1002/cyto.990170404. [DOI] [PubMed] [Google Scholar]
- 18.Varga CM, Tedford NC, Thomas M, Klibanov AM, Griffith LG, Lauffenburger DA. Quantitative comparison of polyethylenimine formulations and adenoviral vectors in terms of intracellular gene delivery processes. Gene Ther. 2005;12:1023–1032. doi: 10.1038/sj.gt.3302495. [DOI] [PubMed] [Google Scholar]
- 19.Angeletti C, Nichols JW. Dithionite quenching rate measurement of the inside-outside membrane bilayer distribution of 7-nitrobenz-2-oxa-1,3-diazol-4-yllabeled phospholipids. Biochemistry. 1998;37:15114–15119. doi: 10.1021/bi9810104. [DOI] [PubMed] [Google Scholar]
- 20.Akita H, Ito R, Khalil IA, Futaki S, Harashima H. Quantitative three-dimensional analysis of the intracellular trafficking of plasmid DNA transfected by a nonviral gene delivery system using confocal laser scanning microscopy. Mol Ther. 2004;9:443–451. doi: 10.1016/j.ymthe.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Suk JS, Suh J, Lai SK, Hanes J. Quantifying the intracellular transport of viral and nonviral gene vectors in primary neurons. Exp Biol Med (Maywood) 2007;232:461–469. [PubMed] [Google Scholar]
- 22.Akinc A, Langer R. Measuring the pH environment of DNA delivered using nonviral vectors: implications for lysosomal trafficking. Biotechnol Bioeng. 2002;78:503–508. doi: 10.1002/bit.20215. [DOI] [PubMed] [Google Scholar]
- 23.Pomorski T, Herrmann A, Zimmermann B, Zachowski A, Muller P. An improved assay for measuring the transverse redistribution of fluorescent phospholipids in plasma membranes. Chem Phys Lipids. 1995;77:139–146. doi: 10.1016/0009-3084(95)02473-v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow cytometry plots corresponding to the data presented in Figure 3C are provided as Figure S1. This information is available free of charge via the Internet at http://pubs.acs.org.