Abstract
Disruptions in the normal program of tissue repair can result in poor wound healing, which perturbs the integrity of barrier tissues such as the skin. Such defects in wound repair occur in transplant recipients treated with the immunosuppressant drug rapamycin (sirolimus). Intraepithelial lymphocytes, such as γδT cells in the skin, mediate tissue repair through the production of cytokines and growth factors. The capacity of skin-resident T cells to function during rapamycin treatment was analyzed in a mouse model of wound repair. Rapamycin treatment renders skin γδ T cells unable to proliferate, migrate and produce normal levels of growth factors. The observed impairment of skin γδ T cell function is directly related to the inhibitory action of rapamycin on mammalian target of rapamycin (mTOR). Skin γδ T cells treated with rapamycin are refractory to IL-2 stimulation and attempt to survive in the absence of cytokine and growth factor signaling by undergoing autophagy. Normal wound closure can be restored in rapamycin-treated mice by addition of the skin γδ T cell-produced factor, insulin-like growth factor-1. These studies not only reveal that mTOR is a master regulator of γδ T cell function but also provide a novel mechanism for the increased susceptibility to nonhealing wounds that occurs during rapamycin administration. This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
Keywords: T cells, skin, cell surface molecules, cell activation
INTRODUCTION
The ability of epithelial tissues to rapidly repair and regenerate in the face of damage is fundamental to barrier maintenance. During normal wound healing, a delicate balance of factors and cell types orchestrates the process in a precisely timed manner. Disruptions can result in the debilitating condition of a chronic, non-healing wound. Much effort has been directed toward determining which factors impact wound repair processes in chronic wounds, but there is less known about what may regulate the cellular sources of the secreted factors important in proper wound healing (1–5).
Nonhealing wounds are exhibited by patients administered the immunosuppressant rapamycin (also called sirolimus) (6–10), however the mechanisms contributing to this wound healing defect are unclear. Rapamycin is commonly administered for the prophylaxis of acute rejection of transplanted solid organs, used to coat arterial stents, and it is currently being examined in clinical trials for the treatment of hematological cancers (11–14). Rapamycin is known to be a potent inhibitor of certain effector αβ T lymphocyte populations (15–19), while other populations such as regulatory T cells exhibit a selective survival (20). In other studies cytokine-driven responses by peripheral naïve T cells are not impacted by rapamycin, suggesting a reliance on other signaling molecules such as Pim-1 and 2 (21). However, the effects of rapamycin on intraepithelial lymphocytes (IELs) such as skin γδ T cells have not been evaluated.
Closely associated with the epithelia is an interdigitating population of resident lymphocytes expressing the γδ TCR (22). Skin γδ T cells express a canonical Vγ3Vδ1 TCR which recognizes an unidentified self antigen expressed on damaged or stressed keratinocytes (23, 24). Intraepithelial γδ T cells are thought to act as primary responders to damage or disease due to their ability to sense and respond to epithelial damage or disruption (25–27). Specialized roles have been attributed to skin γδ T cells in the regulation of wound repair, epithelial homeostasis, cutaneous malignancy, and contact hypersensitivity (27–30).
Skin γδ T cells produce cytokines, chemokines, and growth factors with both autocrine and paracrine functions. Homeostatic production of growth factors such as insulin-like growth factor 1 (IGF-1) by skin γδ T cells maintains skin homeostasis (30). IGF-1 is a peptide hormone known to regulate both keratinocyte and skin γδ T cell migration and survival (30–33). In the context of wound healing, production of factors such as TNF-α, IGF-1, and keratinocyte growth factor (KGF-1) by skin γδ T cells promotes wound closure, re-epithelialization and inflammatory cell recruitment to the wound site (27, 30, 34, 35). To perform these functions the normally dendritic skin γδ T cells retract their dendrites, adopt a rounded morphology, and migrate to the site of trauma where they proliferate locally (27). Unlike naïve αβ T lymphocytes, skin γδ T cells reside in the epidermis in a pre-activated state. They exhibit constitutive IL-2 promoter activation (36) and expression of activation markers CD25 (IL-2 receptor α) and CD69, suggesting that they are primed for a rapid response. However, little is known about the signaling pathways that regulate this response.
On the molecular level, rapamycin inhibits the serine/threonine kinase mammalian target of rapamycin (mTOR). mTOR is a central protein in a complex network that regulates amino acid and nutrient sensing in particular cell types through the formation of two separate protein complexes, mTORC1 (mTOR complex 1) and mTORC2 (37–39). Each multi-protein complex regulates distinct pathways of the signaling network. Rapamycin binds to the cellular protein FKBP12 and subsequently inhibits the disassociation of mTORC1, which consists of mTOR, raptor, and mLST8 (37, 40). This complex has downstream effects that include the regulation of cell cycle, translation machinery, and autophagy (41). The second complex, mTORC2, is involved in cytoskeletal rearrangement (42). mTORC2 is comprised of mLST8, SIN1 and rictor, and is not immediately disrupted following rapamycin treatment in many types of cells (42). However, prolonged treatment of some cell lines with rapamycin can result in reduced levels of mTORC2 through reduction of rictor-bound mTOR, and decreased phosphorylation of AKT Ser473, the downstream target of mTORC2 (43). It has been controversial whether rapamycin results in anergy or apoptosis of transplant-specific effector T cells (19, 44, 45). Some groups suggest that mTOR regulates certain types of αβ T cells by impairing their ability to receive costimulation and cytokine signals (46, 47).
Here we establish a murine model for the examination of wound healing defects mediated by rapamycin. Data presented here indicate that rapamycin negatively impacts skin γδ T cell function during wound repair. Rapamycin arrests the IEL in G1 phase and blocks proliferative responses to cytokines such as IL-2, rendering them anergic. Our data show that skin γδ T cells do not undergo apoptosis when cytokine signals are suppressed by rapamycin. Instead, skin γδ T cells treated with rapamycin enter autophagy in an attempt to survive in the absence of cytokine signaling. As the skin IEL become anergic and autophagic, they exhibit impaired homeostatic and activation-induced functions. This dysfunction is indicated by a diminished ability to produce soluble factors such as IGF-1 and TNF-α and delayed activation-induced cell migration. These effects are mediated through mTOR as we observe reduced phosphorylation of both mTORC1 and mTORC2 downstream targets in skin γδ T cells treated with rapamycin. Finally, the addition of the skin γδ T cell-produced factor, IGF-1, is able to successfully restore normal wound closure in rapamycin-treated mice. These studies demonstrate the selective inhibition of γδ T cell function in the skin of rapamycin-treated animals resulting in defective wound repair and represent a novel pathway in the regulation of skin γδ T lymphocytes.
MATERIALS AND METHODS
Cell culture
The skin γδ T cell line 7–17 was maintained in complete RPMI 1640 (Mediatech, Inc.) supplemented with 10% heat-inactivated FBS (Omega Scientific) and 20U/ml recombinant IL-2. The keratinocyte line PAM 2–12 was maintained in complete DMEM (Mediatech, Inc.) supplemented with 10% heat-inactivated FBS. Epidermal cells and skin γδ T cells were isolated from trypsin-digested epidermal sheets (27, 34) and maintained in complete RPMI 1640 with 10% FBS and 10U/ml recombinant IL-2. For short-term cell lines, skin γδ T cells were initially cultured with 2µg/ml Concanavalin A (Sigma-Aldrich) and 1µg/ml indomethacin (Sigma-Aldrich). All cells were maintained at 37°C under 5% CO2.
Animals and wounding procedure
TCRδ−/− mice on the C57Bl/6 background were purchased from Jackson ImmunoResearch Laboratories. C57Bl/6 and TCRδ−/− mice were bred at The Scripps Research Institute and housed in specific pathogen-free conditions according to The Scripps Research Institute Institutional Animal Care and Use guidelines. Mice were used between 10 and 16 weeks of age. For rapamycin administration, mice were injected i.p. with 200µl containing 1% rapamycin (Sigma-Aldrich) in 0.2% carboxymethlycellulose (Sigma-Aldrich) and 0.25% Tween-80 (Sigma-Aldrich) in distilled H2O or with vehicle control daily. Since rapamycin is originally diluted in ethanol, the vehicle control contains equal amounts of diluent. For wound healing experiments, mice were administered rapamycin or vehicle control for three days prior to wounding and daily administration was continued. Wounding was performed on mice anesthetized with isofluorane as previously described (27, 34). Briefly, the dorsal surface of the mouse was shaved, back skin and panniculus carnosus was pulled up, and one or two sets of sterile full-thickness wounds were generated using a sterile 2-mm punch tool. Wounds were left uncovered, and mice were housed individually with sterile paper bedding. In some experiments 100 ng of recombinant IGF-1 (Sigma-Aldrich) or buffer alone was applied to each wound site immediately post-wounding and daily thereafter. Wounds on at least 6 mice were examined per condition in at least three independent experiments. For wound closure kinetics, images were acquired with a Nikon Coolpix S4 and wound size monitored using Image J software (NIH). To examine rounding of skin γδ T cells at the wound site, full-thickness wounds were generated in mouse ears using a 1-mm punch tool and wounded tissue was harvested 2 hours later.
Antibodies and Flow cytometry
FITC-, PE-, or allophycocyanin-conjugated monoclonal antibodies specific for γδ TCR (GL3), CD25 (PC61), and Thy 1.2 (53-2.1) were purchased from BD Biosciences. Other antibodies used for flow cytometry include goat anti-IGF-1 (G-17) (Santa Cruz), rat anti-CD69 (H1.2F3) (eBiosciences), BrdU flow kit (BD Biosciences), and Annexin-V Apoptosis kit (BD Biosciences). Rabbit antibodies specific for S6kinase, p-S6kinase (Thr389), Akt, and p-Akt (Ser473) were purchased from Cell Signaling Technology. Rat anti-Ki-67 antigen (DakoCytomation) was used for immunohistochemistry with biotin-conjugated mouse anti-rat secondary antibody (Jackson ImmunoResearch). Antibodies specific for CD3ε (500A2) (1µg/ml) were used for stimulation of 7–17 cells and skin γδ T cells in epidermal sheets. Other secondary antibodies used include HRP-conjugated goat anti-rabbit (Southern Biotechnology) and FITC-conjugated donkey anti-goat (Jackson ImmunoResearch). For flow cytometry, Cytofix/Cytoperm kit (BD Biosciences) was used for intracellular cytokine/growth factor staining. Cells were acquired with CellQuestPro on a BD FACSCalibur HTS and analyzed with FlowJo software (TreeStar).
Skin Organ Culture
Skin organ cultures from C57Bl/6 and TCRδ−/− mice were established as previously described (27, 34). Briefly, gel foam (Pfizer) was soaked in media. 2-mm full-thickness biopsy wounds were generated and placed dermis-side down on gel foam in 10% DMEM supplemented with rapamycin or ethanol control in 24-well plates. In some cases 7–17 skin γδ T cells were incubated in the presence of 20ng/ml rapamycin or ethanol control for 15 hours, stimulated for 2 hours with anti-CD3ε, washed thoroughly, and plated at a density of 3 × 105 cells per well. Recombinant IGF-1 was added at a concentration of 100ng/ml to some wells. Images of wounds were acquired and kinetics of closure quantified using Image J software (NIH).
Western Blot Analysis
7–17 cells were incubated in starvation media for 2 hours followed by culture in the presence of 20ng/ml rapamycin or ethanol control for 2 hours (p-S6kinase) or 24 hours (p-Akt). Next, cells were stimulated for various time-points with 40U/ml IL-2, and harvested in lysis buffer containing 62.5mM Tris HCL (pH 6.8), 2% (v/v) SDS, 50 mM DTT, 10% glycerol, and .01% bromphenol blue. After lysis, insoluble material was removed by centrifugation at 12,000 g for 10 minutes. Samples were separated on SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked for 1 hour with 1 × TBS, .02% sodium azide, 3% BSA, and 10% goat serum. Primary antibody diluted in 1 × TBS, 1% BSA, .2% Tween-20, .02% sodium azide, and 3% goat serum was incubated with the membrane overnight at 4°C. HRP-conjugated secondary antibodies and enhanced chemiluminescence (Pierce Chemical Co.) were used to detect primary antibody.
Epidermal sheet immunofluorescence
Wounded or nonwounded ears from rapamycin or vehicle-treated mice were excised, peeled into halves, and digested in 3% ammonium isothiocyanate (Sigma-Aldrich). Epidermal sheets were peeled and stained with PE-conjugated anti-γδ TCR, Lysotracker Green, and/or DAPI (Sigma-Aldrich). Digital images were acquired (Zeiss AxioCam HRc). To examine migration of skin γδ T cells, ear skin was cultured in complete DMEM supplemented with 10% FBS and 20ng/ml rapamycin in 24-well plates. For these experiments 1µg/ml anti-CD3ε (500A2) antibody or 100ng/ml IGF-1 were added to culture. After culture, epidermal sheets were washed with PBS and processed as described above. Quantification of dendrite number and autophagosomes were performed using Photoshop CS2 software (Adobe). Over 200 cells were counted per mouse and at least 3 mice were examined per condition.
Proliferation assays
Cells were cultured between 5 and 8 hours in 10% complete DMEM supplemented with rapamycin or ethanol before addition to wells coated with stimulatory anti-CD3ε or γδ TCR antibodies. In some cases, 100ng/ml IGF-1, 40U/ml IL-2, or 5µg/ml Concanavalin A (Sigma-Aldrich) were added. Cells were cultured for 14–16 hours before addition of 1µCi/well 3H thymidine (MP Biomedicals) for 8–10 hours. Samples were harvested and 3H incorporation was determined by a β-counter. To examine cell cycle, the keratinocyte line PAM 2–12 or the skin γδ T cell line 7–17 was seeded at 2×105 cells/ well in a 6-well plate in complete DMEM supplemented with 10% FBS overnight prior to addition of 20ng/ml rapamycin (Sigma-Aldrich) or ethanol. Fresh rapamycin or ethanol was supplemented daily. After 24 hours, 100µg/ml BrdU was added and proliferation monitored using flow cytometry.
Immunohistochemistry
Wounded skin was excised from rapamycin or vehicle-treated mice, fixed in ethanol, and embedded in paraffin. Sections were prepared and stained with biotinylated antibodies to Ki-67 followed by peroxidase-conjugated streptavidin (Jackson ImmunoResearch). The presence of positive cells was revealed by incubation in metal-enhanced DAB (Pierce Chemical Co.) and counterstained with hematoxylin (Sigma-Aldrich). Control staining was done without primary antibody. At least two wounds from each of three mice per condition were examined.
Acridine Orange Staining
7–17 skin γδ T cells were seeded on coverslips in the presence or absence of 20ng/ml rapamycin for 15 hours in complete RPMI supplemented with 10% FBS. Coverslips were washed in PBS supplemented with 10% FBS and 10U/ml IL-2, incubated with 1µg/ml Acridine Orange (Sigma-Aldrich) for 15 minutes at 25°C, and washed thoroughly prior to examination with a fluorescence microscope. Starvation was used as a positive control, while Bafilomycin treatment was used as a negative control. Digital images were acquired and number of autophagic cells quantified using Photoshop software (Adobe). Cells with greater than 2 positively staining vesicles were defined as autophagic.
RESULTS
Rapamycin impairs the ability of skin γδ T cells to mediate wound closure
To examine how the mTOR inhibitor, rapamycin, impacts wound repair, we established a murine model of wound healing in which mice were administered rapamycin daily. The defect in wound repair has been described in humans (6–8) and rats (9, 48), however this has not been investigated in the murine system and no mechanism has been ascribed to this defect. Wild-type C57BL/6J mice were administered daily with rapamycin or vehicle control for three days prior to wounding and treatment was continued as wound closure was monitored. Wound size was measured over a period of 14 days (Figure 1A, B). Similar to findings in humans, rapamycin-treated mice exhibited delays in the rate of wound closure as compared to those treated with vehicle control. This defect was especially evident on days 4, 7 and 10 post-wounding. Further, significantly fewer wounds had completely closed on day 10 in rapamycin-treated animals as compared to wounds from vehicle control-treated animals (Figure 1C). A delay in complete wound closure of approximately three days was observed, similar to the delay observed in mice lacking γδ T cells (TCRδ−/− mice) (27). These data confirm that rapamycin has a negative impact on wound closure.
Figure 1. Rapamycin treatment delays wound closure and impairs skin γδ T cell wound healing functions.
Wound closure was monitored on mice administered rapamycin as compared to vehicle control (A). Rapamycin-treated mice exhibited a delay in wound closure as compared to those treated with vehicle. Data are mean ± SEM and representative of three experiments. At least six mice per condition have been examined in each experiment. (B) Representative images from wounds acquired four days post-wounding. (C) Percent of observed wounds remaining open 10 days post-wounding. Data are mean ± SD. (D) Rapamycin impairs wound closure in skin from wildtype mice to a similar degree as skin from untreated TCRδ−/− mice using a skin organ culture system. (E). Rapamycin inhibits the ability of 7–17 skin γδ T cells to restore normal wound closure to TCRδ−/− skin in organ culture. Data in skin organ cultures are mean ± SEM and representative of at least three experiments. *P < 0.05, **P < 0.005 versus vehicle control (2-tailed, unpaired Student’s t test).
Given the potent immunosuppressive nature of rapamycin and the similarity of wound closure kinetics to TCRδ−/− mice, the contribution of skin γδ T cell dysfunction to impaired wound healing in rapamycin-treated mice was examined. Using a well-established system of skin organ culture (27, 49, 50), the early stages of wound healing were monitored in vitro. In cultures supplemented with rapamycin, wound closure in this skin organ culture model was inhibited, similar to our findings in vivo (Figure 1D). The kinetics of closure observed in wounded skin treated with rapamycin were similar to those observed in skin from TCRδ−/− mice (Fig. 1D). In addition, there was no further defect in wound repair in TCRδ−/− skin upon rapamycin treatment (Fig. 1D). In order to identify whether rapamycin impairs the ability of skin γδ T cells to mediate wound repair, we utilized a model we have previously established to assess skin γδ T cell wound healing functions. In this model, the addition of activated skin γδ T cells to ex vivo cultures of wounded skin from TCR δ−/− mice restores normal wound closure kinetics (27). Here we examined whether rapamycin treatment impairs the ability of skin γδ T cells to affect early wound closure. When skin from TCR δ−/− mice was cultured with activated skin γδ T cells, early wound closure was restored as we previously reported (27) (Figure 1E). However, rapamycin pretreatment of activated skin γδ T cells prior to their addition to TCR δ−/− skin organ culture impaired the ability of skin γδ T cells to restore early wound closure (Figure 1E). These results indicate that rapamycin impairs skin γδ T cell function, diminishing their capacity to mediate wound closure. This implicates the mTOR signaling cascade in wound healing functions mediated by skin γδ T cells.
Rapamycin treatment inhibits phosphorylation of the mTOR targets p70 S6 kinase and Akt in skin γδ T cells
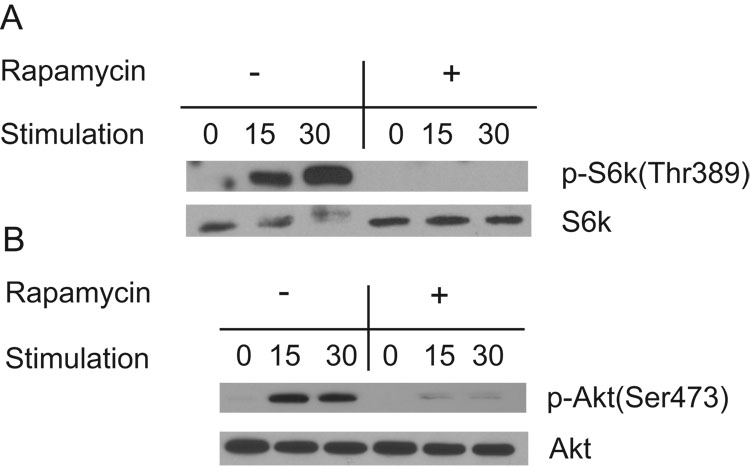
Little is known about the signaling cascades activated in intra-epithelial γδ T cells. In an effort to determine whether T cell trophic factors such as the cytokine IL-2 signal via mTOR in skin γδ T cells, we examined the molecular targets of the two mTOR complexes. Signaling through mTORC1 induces the phosphorylation of p70 S6 kinase at many sites, including threonine 389 (37). To evaluate mTOR activity, the skin γδ T cell line, 7–17, was stimulated with IL-2 in the presence and absence of rapamycin. Phosphorylation at Thr389 was evident in IL-2-stimulated skin γδ T cells within 15 minutes and observed at higher levels by 30 minutes. This activation-induced phosphorylation is repressed in the presence of rapamycin (Figure 2A) demonstrating that IL-2-mediated phosphorylation of S6kinase is dependent on mTOR.
Figure 2. Rapamycin treatment of skin γδ T cells directly inhibits IL-2 signaling through mTOR.
(A) IL-2 (40U/ml) stimulates phosphorylation of pS6kinase at Thr389 (a marker of mTOR activation) within 30 minutes, while rapamycin inhibits phosphorylation of this site. 7–17 skin γδ T cells were treated with rapamycin or ethanol control for two hours following two hours of starvation and were then stimulated with IL-2 for various time-points. (B) Akt is phosphorylated at Ser473 in skin γδ T cells stimulated with IL-2. Prolonged (24 hr) rapamycin-treatment inhibits this response suggesting that activity of mTORC2 is inhibited. 7–17 skin γδ T cells were treated with rapamycin for 24 hours. During the last two hours, cells were also starved prior to stimulation with IL-2. Lysates were prepared for Western blot analysis.
mTOR is a component of another complex, mTORC2, that mediates cytoskeletal rearrangement (42). Signaling through mTORC2 induces phosphorylation of Akt at serine 473 (43, 51). Although the mTORC2 complex was originally reported to be rapamycin resistant (38, 42), the phosphorylation of Akt Ser473 by mTORC2 is diminished in particular cell types after 24 hours of rapamycin treatment (43). In an effort to determine whether mTORC2 activity is present in skin γδ T cells and whether this activation is rapamycin-sensitive, we treated the skin γδ T cell line with and without rapamycin, stimulated the cells with IL-2 or anti-CD3ε antibodies, and evaluated Akt phosphorylation. Upon IL-2 treatment or TCR stimulation of skin γδ T cells, Akt is phosphorylated at Ser473 within 15 minutes. After 24 hours of rapamycin treatment this phosphorylation is severely impaired (Fig. 2B). Similar to previous reports, rapamycin was unable to inhibit Akt Ser473 phosphorylation at timepoints earlier than 24 hours of treatment (data not shown).
Skin γδ T cells are unresponsive to IL-2 or mitogen stimulation in the presence of rapamycin
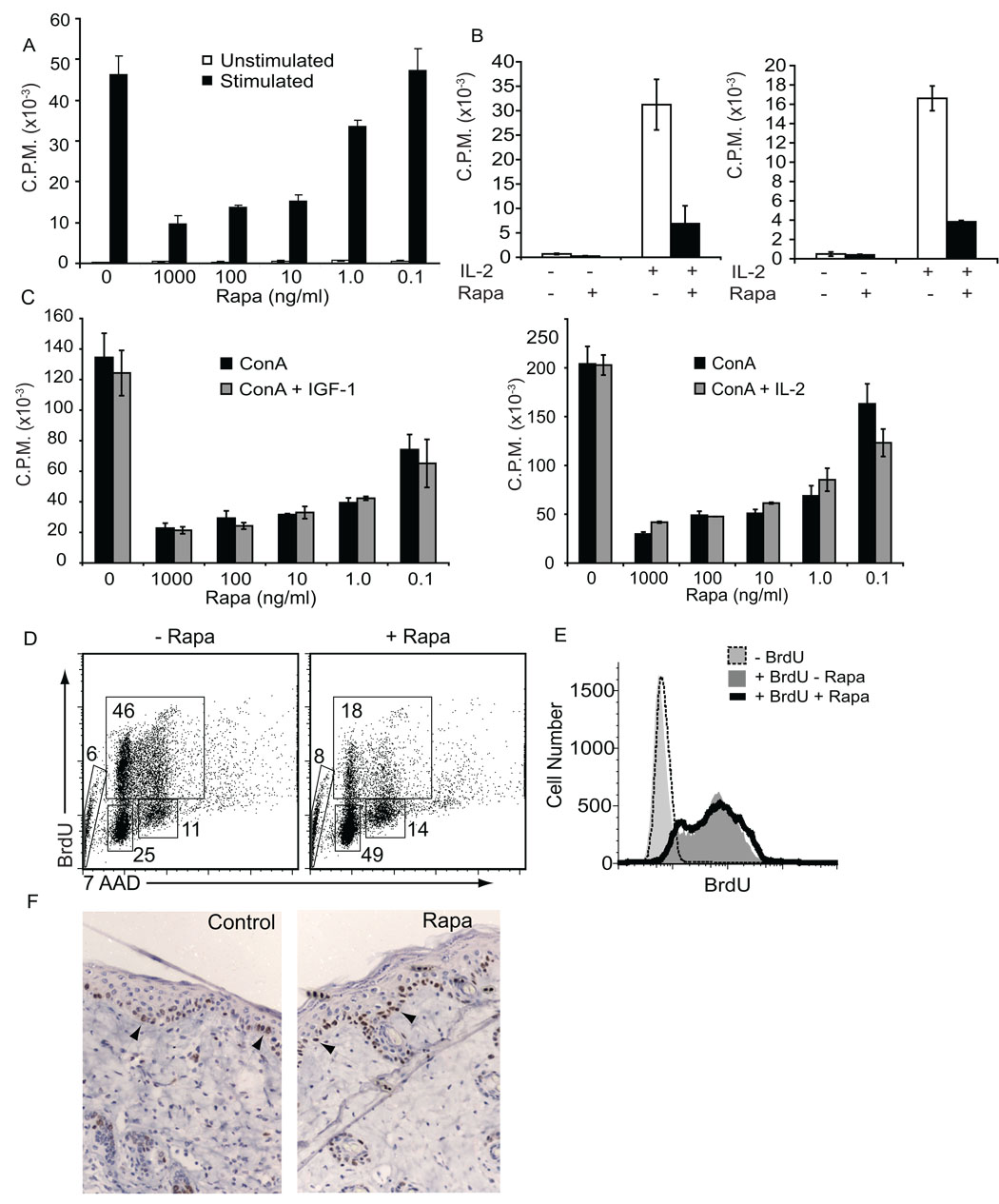
IL-2-stimulated proliferation of certain αβ T lymphocyte subsets induces the phosphorylation of p70 S6 kinase in a rapamycin-sensitive manner (52). However, peripheral regulatory T cells are resistant to rapamycin treatment, suggesting they rely on alternate pathways for proliferation (20). To examine whether skin γδ T cell proliferation is dependent on mTOR, skin γδ T cells were treated with various concentrations of rapamycin prior to stimulation with antibodies specific for CD3ε (Figure 3A, C) or γδ TCR (data not shown), or addition of IL-2 (Figure 3B, left panel). Rapamycin treatment inhibited proliferation of the skin γδ T cell line, 7–17, to either anti-CD3ε or cytokine stimulation at rapamycin concentrations as low as 10ng/ml. Similar results were obtained with γδ T cells isolated from the skin of C57Bl/6 mice (Figure 3B, right panel). This proliferative defect could not be restored in the presence of rapamycin by supplementing the stimulus with IL-2 or IGF-1 (Figure 3C). Removal of the inhibitor from the culture for several days restores normal skin γδ T cell proliferation (data not shown). To identify at which stage of the cell cycle rapamycin-treated skin γδ T cells arrest, BrdU incorporation and DNA content analyses were performed. Rapamycin treatment blocks skin γδ T cells from exiting the G1 phase of the cell cycle, preventing entry into synthesis (S) phase (Figure 3D). These results suggest that skin γδ T cells depend on mTOR signaling as a major pathway for exit from G1 phase.
Figure 3. Inhibition of mTOR suppresses skin γδ T cell but not keratinocyte proliferation.
Skin γδ T cell proliferative responses to anti-CD3ε (A) and IL-2 (B) stimulation are impaired upon mTOR inhibition by rapamycin. 7–17 skin γδ T cells (A and B, left panel) or DETC from murine skin (B, right panel) were incubated for 4 hours with 20ng/ml rapamycin prior to stimulation with 1ug/ml anti-CD3ε (A) or 40U/ml IL-2 (B) as assessed by 3H thymidine incorporation. (C) Neither IGF-1 nor IL-2 addition can rescue the defect. Either 100ng/ml IGF-1 or 40U/ml IL-2 was added to the cultures simultaneous with Concanavalin A stimulation. Data represents the mean ± SD. (D) When mTOR is inhibited, skin γδ T cells are blocked at the G1 stage of the cell cycle. 7–17 skin γδ T cells were treated with 20ng/ml rapamycin for 17 hours and pulsed with 100ug/ml BrdU for 12 hours, prior to staining for BrdU incorporation. Numbers indicate percentage of cells within indicated gates. (E) Keratinocyte proliferation is not impaired upon rapamycin treatment. The PAM 212 cell line was treated with 20ng/ml rapamycin or vehicle control for 48 hours with 100ug/ml BrdU and analyzed by flow cytometry. (F) Five days post-wounding, keratinocytes proliferate at the wound site in mice treated with or without rapamycin. Arrows indicate Ki-67 positive cells in the epidermis.
The bulk of the epidermis consists of keratinocytes. To examine whether rapamycin impacts the ability of keratinocytes to proliferate, the PAM 212 cell line was incubated with BrdU for 48 hours in the presence of rapamycin (Figure 3E). In contrast to skin γδ T cells, keratinocytes retain the ability to proliferate during treatment with rapamycin. Similar results were obtained with keratinocytes pulsed with BrdU for 16 or 24 hours (data not shown). Since previous studies have identified defects in keratinocyte stem cell proliferation in the presence of rapamycin (53), we monitored keratinoycte proliferation in vivo. We performed biopsy punch wounds on the skin of rapamycin or vehicle-treated mice and stained skin sections for Ki-67 expression to identify proliferating cells (Figure 3F). Keratinocyte proliferation at the wound site was comparable between rapamycin and vehicle-treated mice. Using either BrdU to monitor keratinocyte turn-over or Ki-67 staining to identify proliferating cells, no defect in keratinocyte proliferation was found in the presence of rapamycin. Thus, at clinically relevant doses, rapamycin specifically inhibits skin γδ T cell proliferation in the epidermal compartment. These results demonstrate cell-specific regulation of proliferation via mTOR signaling within the epidermis.
Rapamycin induces autophagy, not apoptosis, of skin γδ T cells
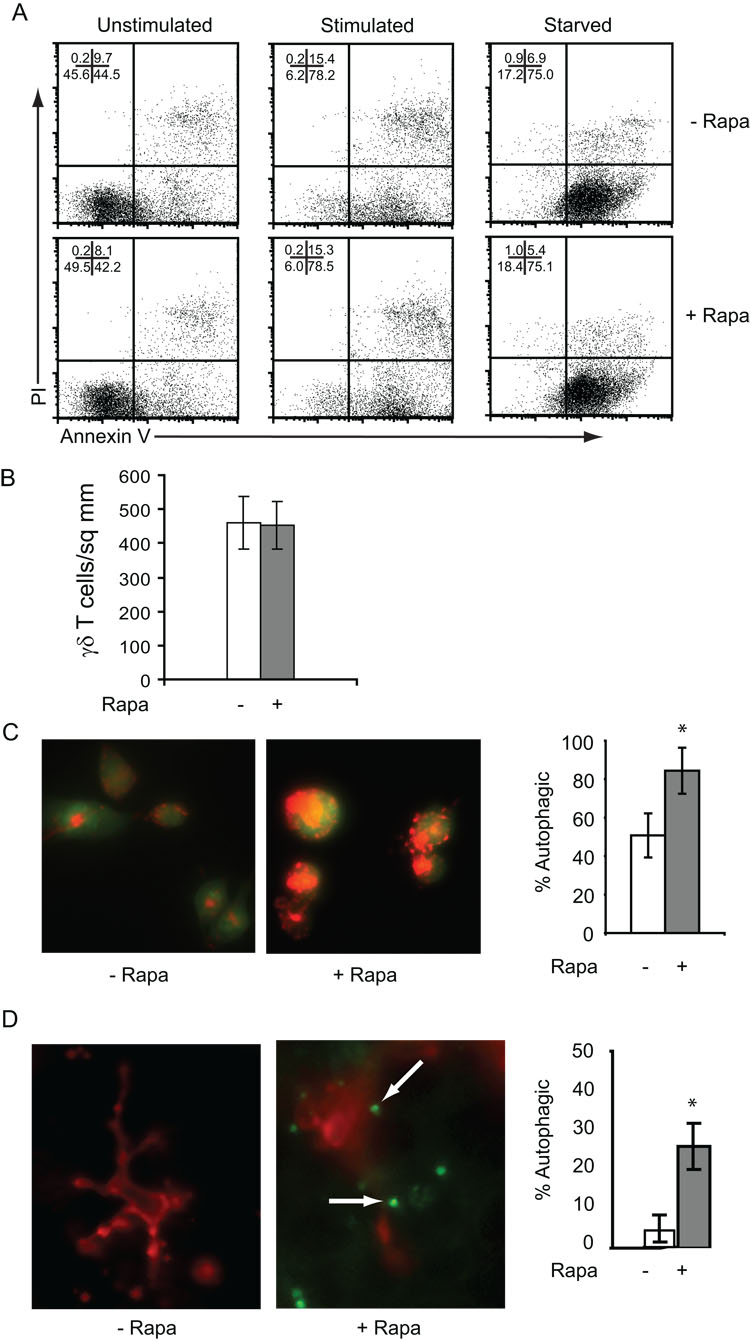
In several T cell populations, such as double-positive thymocytes or effector T cells, rapamycin has been shown to inhibit proliferation and increase the susceptibility to apoptosis (18, 54). In other cases, lymphocyte populations undergo macroautophagy, a process in which the cell catabolizes proteins and organelles as a survival mechanism (55). Having observed the diminished proliferative capacity of rapamycin-treated skin γδ T cells, we examined whether these cells were undergoing increased levels of apoptosis. For these experiments, skin γδ T cells were treated with rapamycin and apoptosis was examined by Annexin V binding and PI staining. No increase in apoptosis was evident in skin γδ T cells treated with rapamycin (Figure 4A). Further, apoptosis is not exacerbated when rapamycin-treated skin γδ T cells are stimulated to undergo activation-induced cell death or are cytokine-starved (Figure 4A). Additionally, rapamycin does not induce skin γδ T cells to undergo apoptosis when administered for several days in skin organ culture (data not shown). In fact, rapamycin treatment does not seem to affect the viability of skin γδ T cells either in vitro or in vivo. γδ TCR-bearing cells were quantified in epidermal sheets via immunofluorescent staining, and no measurable decrease in the number of cells per square millimeter was detected after up to 14 days of rapamycin treatment (Figure 4B). Taken together these results suggest that rapamycin treatment does not induce or exacerbate apoptosis in skin γδ T cells.
Figure 4. Rapamycin promotes autophagy rather than apoptosis in skin γδ T cells.
(A) Inhibition of mTOR with rapamycin does not induce or augment apoptosis. 7–17 skin γδ T cells were left untreated, stimulated with antibodies specific for CD3ε, or starved for six hours in the presence of rapamycin or ethanol control. Cells were then stained with annexin-V and propidium iodide (PI) to assess apoptosis and analyzed by flow cytometry. (B) Similar numbers of skin γδ T cells remain in the epidermis of mice treated with rapamycin for 14 days. Epidermal sheets were prepared from mice administered rapamycin or vehicle control and stained with antibodies specific for the γδ TCR. The number of γδ T cells was quantified per mm2. (C) Rapamycin induces skin γδ T cells to undergo autophagy. 7–17 skin γδ T cells were incubated in the presence or absence of rapamycin for 15 hours prior to staining with acridine orange to identify autophagosomes. An increased number of cells treated with rapamycin exhibit more than two autophagosomes as compared to ethanol control-treated cells. (D) An increased number of skin γδ T cells undergo autophagy in vivo after rapamycin administration. Epidermal sheets were prepared from mice administered rapamycin as compared to vehicle control. Sheets were stained with fluorescent antibodies to the γδ TCR to identify skin γδ T cells and Lysotracker Green to identify autophagosomes. The number of autophagosomes was quantified per γδ T cell and cells with greater than two autophagosomes considered autophagic. Arrows indicate autophagosomes within skin γδ T cells. Data represents mean ± SD. *P < 0.05 versus vehicle control (2-tailed, unpaired Student’s t test).
Although inhibiting mTOR does not affect skin γδ T cell survival, it is possible that this inhibition causes the cells to undergo other cellular changes that affect activation-induced functions, such as the lysosomal degradation process called autophagy. mTOR can act as a sensor to regulate the autophagy pathway (56, 57). CD4+ T cells treated with rapamycin undergo autophagy in vitro, implicating the process in T cell homeostasis (55). Autophagy is indicated by the presence of large autophagosomes in the cytoplasm. To determine whether rapamycin-mediated mTOR inhibition induces autophagy in skin γδ T cells, we used several common methods to detect autophagosomes in skin γδ T cells. First, cells were treated with rapamycin or vehicle control and stained with acridine orange to monitor the number of autophagic vesicles. Rapamycin treatment significantly increased the incidence of skin γδ T cells undergoing autophagy as compared to the basal level observed in vehicle control-treated cells (Figure 4C). To identify whether skin γδ T cells also undergo autophagy during rapamycin treatment in vivo, we used Lysotracker Green staining to identify the number of cells with acidic vesicles in the epidermis. In mice administered rapamycin, there were increased numbers of skin γδ T cells exhibiting large numbers of acidic vesicles (Figure 4D). These data indicate that mTOR inhibition of skin γδ T cells not only inhibits proliferation, but induces autophagy.
Activation-induced morphology changes in skin γδ T cells are inhibited by rapamycin
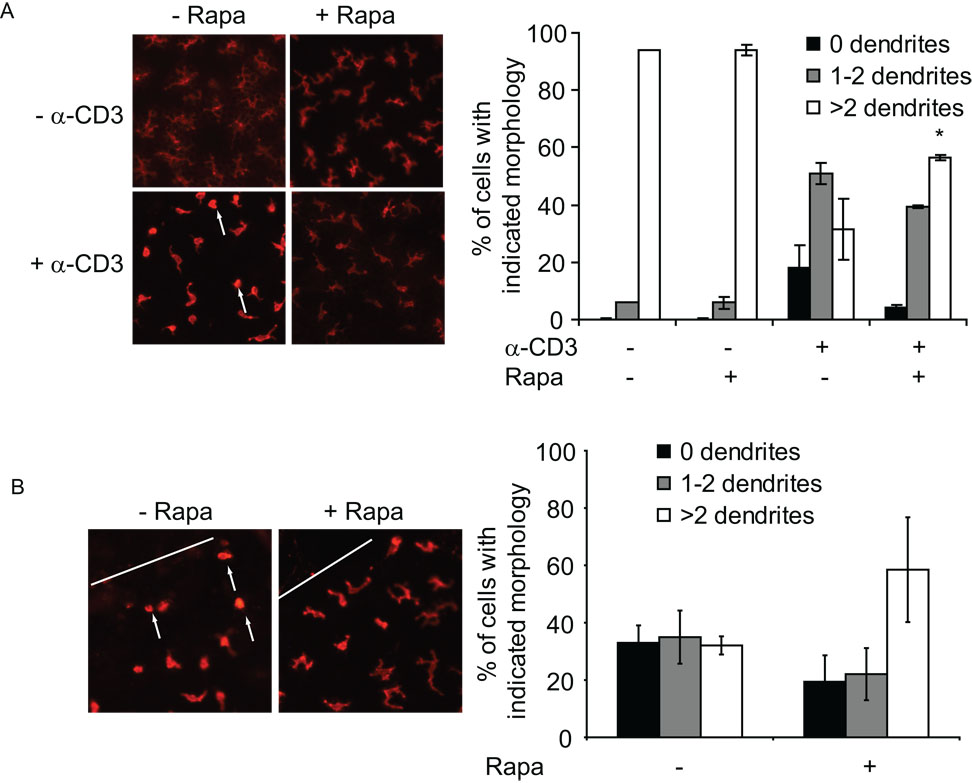
The process of autophagy preserves the cytoskeleton, preventing its degradation while recycling cellular macromolecules in an effort to stave off cell death (57). To identify whether blocking mTORC2 function with rapamycin impacts the capacity of skin γδ T cells to alter their cellular morphology upon activation, skin from rapamycin-treated mice was subsequently cultured with stimulating antibodies specific for CD3ε. Skin γδ T cells normally exhibit a highly dendritic morphology. Upon TCR ligation, the skin γδ T cells normally become rounded within several hours and initiate migration (Fig 5A).
Figure 5. Rapamycin treatment inhibits activation-induced morphology changes.
(A) Skin γδ T cells from mice administered rapamycin exhibit a decreased ability to “round-up” in response to stimulation. Epidermal sheets were isolated from mice treated with rapamycin or vehicle control and stimulated with antibodies to CD3ε prior to immunofluorescent staining. The number of dendrites per skin γδ T cell were quantified in at least 300 cells per mouse and is representative of at least 6 mice per condition. Data represents the mean ± SD. *P < 0.05 versus vehicle control (2-tailed, unpaired Student’s t test). (B) Skin γδ T cells exhibit a delay in morphology changes at the wound site in rapamycin-treated mice. Epidermal sheets were prepared two hours post-wounding in mice treated with rapamycin and stained with fluorescent antibodies specific for the γδ TCR. The white line indicates the wound edge. At least 150–200 cells were quantified from wounds in mice from three separate experiments.
However, skin γδ T cells in the epidermis of mice treated with rapamycin did not exhibit this characteristic morphology change (Fig 5A). These results were replicated using rapamycin treatment in vitro and once again neither IL-2 nor IGF-1 were able to restore normal cell rounding and migration (data not shown).
One of the primary responses of skin γδ T cells to wounding is activation-induced rounding of these characteristically dendritic cells prior to migration to the wound edge and proliferation at the wound site (27). To identify whether rapamycin treatment inhibits this response in vivo, skin γδ T cells were examined from wounds isolated from rapamycin and vehicle-treated mice. In rapamycin-treated mice, skin γδ T cells adjacent to the wound edge maintained a dendritic morphology, while cells from control-treated mice exhibited a rounded appearance (Fig 5 B). Further, skin γδ T cells isolated from the wound site have a reduced capacity to produce factors such as TNF-α (data not shown). These results provide further evidence that activation-induced functions of skin γδ T cells are negatively regulated by rapamycin. It is possible that normal homeostatic functions are similarly altered.
Rapamycin negatively impacts skin γδ T cell homeostatic functions
Since mTOR has the capacity to regulate transcription and translation through p70 S6 kinase and eIF-4E binding proteins (58) and to regulate skin γδ T cell autophagy, there may be consequences of rapamycin treatment on normal cellular homeostasis. Although long-term treatment with rapamycin does not impair surface expression of activation markers such as CD25 or CD69 (Figure 6A), skin γδ T cells isolated from mice treated with rapamycin express a reproducible reduction in levels of TCR as compared to mice administered vehicle alone (Figure 6A). Down-regulation of TCR could have negative implications for T cell activation. In addition, skin γδ T cells isolated from rapamycin-treated mice have reduced levels of homeostatic IGF-1 production (Figure 6B). Constitutive IGF-1 production by skin γδ T cells has been shown to play an important role in skin homeostasis (30), by promoting keratinocyte migration and survival of both γδ T cells and keratinocytes.
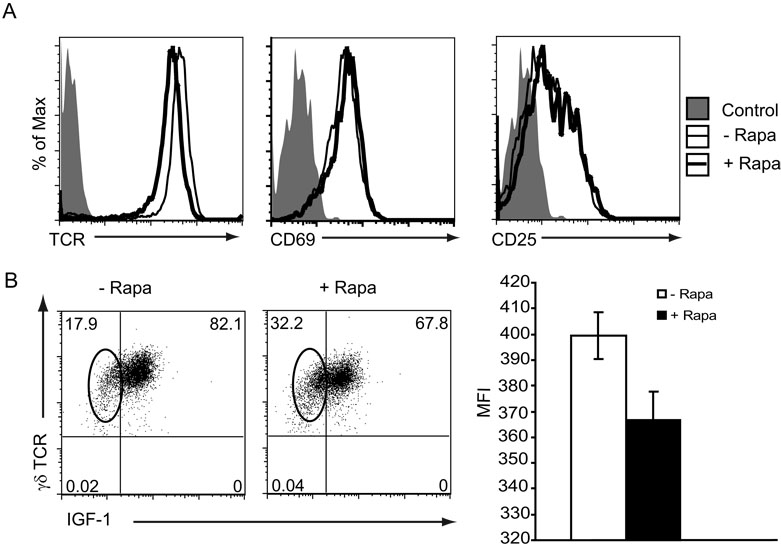
Figure 6. Constitutive TCR and IGF-1 expression are reduced in γδ T cells from rapamycin-treated mice.
(A) γδ TCR expression is reproducibly reduced in mice treated with rapamycin, while the activation markers CD69 and CD25 are unaffected. Epidermal cells were isolated from mice administered rapamycin or vehicle control for 14 days and stained for various markers with fluorescent antibodies. (B) Skin γδ T cells isolated from rapamycin-treated mice exhibited decreased levels of IGF-1 production. Epidermal cells were isolated from mice administered rapamycin or vehicle control and stained with fluorescent antibodies for intracellular IGF-1 production. Gates are set on live, Thy1.2 positive cells. Oval highlights skin γδ T cells without IGF-1 production. These results are representative of at least three mice per condition performed in three experiments. The mean fluorescence intensity (MFI) of IGF-1 expression by γδ TCR+ cells in these experiments is presented in the right panel.
Addition of skin γδ T cell-produced growth factors such as IGF-1 restores wound closure in rapamycin-treated skin
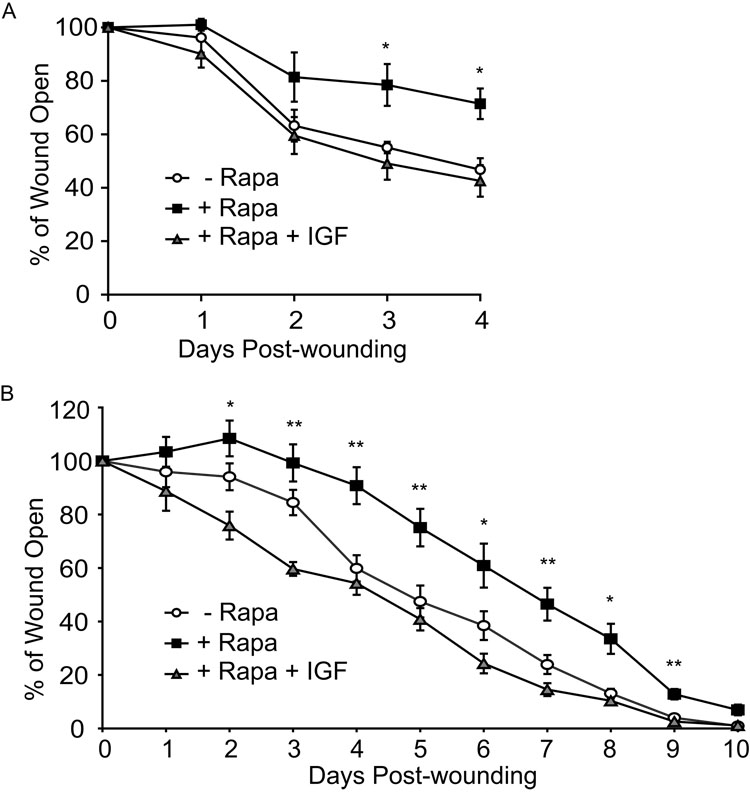
Since our data demonstrate that rapamycin has a negative impact on skin γδ T cell function in vivo, we examined the possibility that the addition of growth factors produced exclusively by skin γδ T cells in the epidermal compartment would restore rapid wound closure in rapamycin-treated mice. To examine this, recombinant IGF-1 was added to wounded skin in organ culture and wound closure assessed. Addition of IGF-1 to skin treated with rapamycin did indeed restore wound closure (Figure 7A). To assess whether addition of IGF-1 would have the same effect in vivo, mice were administered rapamycin for three days prior to wounding and daily thereafter. Upon wounding, either recombinant IGF-1 or buffer alone was applied directly to the wound. Treatment of wounds in rapamycin-treated mice with IGF-1 restored normal rates of wound healing (Figure 7B). Notably at days 4, 7, and 10 when the greatest difference between rapamycin-treated and vehicle-treated mice was evident (Figure 1A, 7B) addition of IGF-1 to rapamycin-treated wounds clearly restored wound healing kinetics to those of untreated animals (Figure 7B). These studies indicate that dysfunctional wound repair caused by rapamycin can be rescued by the addition of factors normally produced by skin γδ T cells.
Figure 7. Application of IGF-1 to wounds rescues rapamycin-induced wound healing defect.
(A) IGF-1 supplemented to rapamycin-treated skin in culture rescues wound closure. Skin from wildtype mice was wounded and cultured in the presence or absence of rapamycin. Digital images were acquired to monitor wound closure. 100ng/ml IGF-1 was supplemented in various wells. (B) IGF-1 rescues wound repair in rapamycin-treated mice. Mice were treated with rapamycin or vehicle control, wounded on the dorsal surface, administered IGF-1 or buffer control, and wound closure measured over time. Data represents mean ± SEM. *P < 0.05, **P < 0.005 versus vehicle control (2-tailed, unpaired Student’s t test).
DISCUSSION
Rapamycin is a powerful clinical therapeutic administered for the prophylaxis of allograft rejection, coated on arterial stents, and currently in clinical trials for the treatment of cancer. Several studies have described the potent inhibitory action of rapamycin on various αβ T lymphocyte populations, as the mechanism employed to prevent rejection of transplanted solid organs (59). Here we show that similar to anti-allograft αβ T lymphocytes, intra-epithelial γδ T lymphocytes are suppressed by rapamycin treatment. Due to the inhibitory effects of rapamycin on cell cycle and function, there has been speculation that rapamycin renders effector T cells anergic (19, 45). Anergy has been described as a mechanism by which lymphocytes are functionally inactive following stimulation, but survive in a nonresponsive state (60). Skin γδ T cells are present but with diminished functional capacity in rapamycin-treated mice. Our results show that γδ TCR expression is reduced, cytokine and growth factor production is decreased, and proliferation and cell cycle initiation are inhibited. This may be caused by a block in mTOR-mediated costimulatory responses (signal 2) or a defect in IL-2 signals (signal 3). IL-2 receptor signaling is key for the proliferation and cell cycle progression of skin γδ T lymphocytes (61). We show that IL-2 responsiveness is diminished in rapamycin-treated skin γδ T cells, which may be exacerbated by the reduced levels of TCR expression. Upon removal of rapamycin normal skin γδ T cell function is restored suggesting rapamycin inhibition is reversible.
In the presence of rapamycin, skin γδ T cells survive in the hyporesponsive state for a prolonged time. Instead of undergoing apoptosis, skin γδ T cells appear to undergo autophagy as they attempt to survive with diminished mTOR signaling. This mechanism of survival may be vital for cell types such as skin γδ T cells which are only seeded in the murine skin from the fetal thymus and can not be repopulated by the adult thymus (62). Since treatment with rapamycin impairs the ability of skin γδ T cells to proliferate or migrate in response to T cell growth factors such as IL-2 or IGF-1, the skin γδ T cell may become autophagic in an attempt to survive in the absence of cytokine/growth factor signaling. Although most cells undergo a basal level of autophagy, this process is induced during nutrient deprivation in an attempt to break down macromolecules and recycle the components (57, 63). With skin γδ T cells undergoing autophagy and unable to proliferate, the number of skin γδ T cells in the epidermal compartment may eventually diminish. In our studies a decrease was not observed after two weeks of rapamycin treatment, but long-term, sustained rapamycin administration, as would occur in transplant recipients, may impact skin T cell numbers. The increased level of autophagy may also impair the cellular machinery involved in activation, leading to inhibition of downstream effector functions. Rapamycin treatment directly affects pathways involved in nutrient sensing and cellular growth through inhibition of mTORC1 and mTORC2, but increased levels of autophagy may also induce degradation of signaling molecules involved in other independent pathways. It is still unclear whether particular proteins are specifically targeted for degradation during autophagy.
Skin-resident γδ T cells are known to play roles in the early stages of wound repair (27, 34). Similar to TCRδ−/− mice, the delay in wound healing observed in rapamycin-treated mice occurs within the first three days of wound healing. The precise timing of wound repair is critical as key growth factors, cytokines, and chemokines participate in the entry, exit, and function of resident and inflammatory cells at the wound site. Though rapamycin has been shown to potently inhibit αβ T lymphocytes, peripheral αβ T lymphocytes do not infiltrate the wound site until 7 days post-wounding and play roles in the later stages of wound repair (64). Thus the early timing of the wound healing defect observed during rapamycin treatment makes it unlikely that αβ T cell inhibition by rapamycin is responsible for the delay in wound closure.
The cell-specific nature of rapamycin inhibition is a testament to the complex and sometimes redundant nature of the mTOR pathway. In contrast to allograft-specific αβ T cells which undergo anergy or apoptosis (19, 45, 65), rapamycin treatment leads to an accumulation of CD25+ regulatory T lymphocytes in mice (66) and humans (17). In addition, some tumor cell lines are susceptible to apoptosis upon rapamycin treatment (67, 68). Since keratinocytes compose the bulk of the epidermis, their proliferative capacity is essential to wound repair. However, during wound repair, we show that keratinocytes retain their ability to proliferate in the presence of rapamycin. Although signaling through PI3-K is important for keratinocyte proliferation (69), it must not depend on mTOR. In addition, IGF-1 application rescues normal wound closure rates suggesting that keratinocytes can respond to growth factors normally in the presence of the inhibitor. In the epidermis, IGF-1 is exclusively produced by skin γδ T cells (30), but its receptor is widely expressed among cells in the epidermal compartment including keratinocytes. IGF-1 plays key roles in both keratinocyte and skin γδ T cell migration and survival (30–33). Addition of this growth factor restores wound closure upon rapamycin treatment, presumably by overcoming the impaired function of the skin γδ T cells and acting upon neighboring keratinocytes to promote migration. Thus supplementing the wound environment with exogenous IGF-1 restores the paracrine effects of skin γδ T cell-derived IGF-1 on other epidermal cells, re-establishing wound closure.
Rapamycin acts by binding FKBP12 and inhibiting the serine/threonine kinase mTOR. Although there appears to be an ever expanding list of downstream targets for mTOR, the best studied readout of mTOR function in mTORC1 is phosphorylation of p70 S6kinase (70). S6kinase is involved in proliferative responses of αβ T lymphocytes and our results indicate that IL-2 signaling induces mTOR phosphorylation of S6kinase in skin γδ T cells as well and this phosphorylation is inhibited by rapamycin. The second complex formed with mTOR has been shown to regulate spatial aspects of cell growth. mTORC2 affects cytoskeletal reorganization in yeast and is implicated as an upstream regulator of Rho GTPases in mammalian cells (42). Although originally reported as a rapamycin-resistant complex (42), recent evidence shows that in particular cell types mTORC2 is sensitive to prolonged rapamycin treatment (43). Skin γδ T cells exhibit rapamycin-sensitive phosphorylation of Akt Ser473 suggesting that mTORC2 is not functional during rapamycin treatment in these cells. The defect we observe in the capacity of skin γδ T cells to undergo rapid activation-induced morphology changes, both in vitro and in vivo, in the presence of rapamycin highlights the capacity for the drug to inhibit cytoskeletal functions. To our knowledge, this is the first time that a T cell has been shown to exhibit mTORC2 function and cytoskeletal changes that are rapamycin-sensitive.
Both αβ and γδ TCR-expressing cells are found in human skin. Similar to the mouse, human skin T cells exhibit a restricted TCR repertoire specific to the cutaneous environment (71, 72) and are able to produce TNF-α and IFN-γ upon stimulation (73). Skin γδ T cells in the human epidermis are also able to produce IGF-1 (our unpublished data). In addition, human skin γδ T cells have tumoricidal capabilities much like the mouse γδ T cell (73). Our studies suggest that prolonged rapamycin treatment would result in skin γδ T cell dysfunction and an inability to respond to injury. Here we have identified a key signaling pathway vital for skin γδ T cell function and a novel mechanism that may contribute to wound healing defects observed during treatment with rapamycin (6–10).
ACKNOWLEDGEMENTS
We would like to thank Dr. Deborah Witherden for technical advice and manuscript review. We thank Alexandre Webster for technical assistance. We are grateful to Dr. Wendy Havran for generously providing advice and support.
Footnotes
This work was supported in part by grants from National Institutes of Health DK073098, AI07244, and the Leukemia and Lymphoma Society. This is manuscript number 19281.
Abbreviations used in this paper: mammalian target of rapamycin (mTOR), intraepithelial lymphocytes (IELs), insulin-like growth factor-1 (IGF-1), keratinocyte growth factor-1 (KGF-1)
REFERENCES
- 1.Beer HD, Florence C, Dammeier J, McGuire L, Werner S, Duan DR. Mouse fibroblast growth factor 10: cDNA cloning, protein characterization, and regulation of mRNA expression. Oncogene. 1997;15:2211–2218. doi: 10.1038/sj.onc.1201383. [DOI] [PubMed] [Google Scholar]
- 2.Brown DL, Kane CD, Chernausek SD, Greenhalgh DG. Differential expression and localization of insulin-like growth factors I and II in cutaneous wounds of diabetic and nondiabetic mice. Am J Pathol. 1997;151:715–724. [PMC free article] [PubMed] [Google Scholar]
- 3.Danilenko DM, Ring BD, Tarpley JE, Morris B, Van GY, Morawiecki A, Callahan W, Goldenberg M, Hershenson S, Pierce GF. Growth factors in porcine full and partial thickness burn repair. Differing targets and effects of keratinocyte growth factor, platelet-derived growth factor-BB, epidermal growth factor, and neu differentiation factor. Am J Pathol. 1995;147:1261–1277. [PMC free article] [PubMed] [Google Scholar]
- 4.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 5.Werner S. Keratinocyte growth factor: a unique player in epithelial repair processes. Cytokine Growth Factor Rev. 1998;9:153–165. doi: 10.1016/s1359-6101(98)00010-0. [DOI] [PubMed] [Google Scholar]
- 6.Dean PG, Lund WJ, Larson TS, Prieto M, Nyberg SL, Ishitani MB, Kremers WK, Stegall MD. Wound-healing complications after kidney transplantation: a prospective, randomized comparison of sirolimus and tacrolimus. Transplantation. 2004;77:1555–1561. doi: 10.1097/01.tp.0000123082.31092.53. [DOI] [PubMed] [Google Scholar]
- 7.Hymes LC, Warshaw BL. Sirolimus in pediatric patients: results in the first 6 months post-renal transplant. Pediatr Transplant. 2005;9:520–522. doi: 10.1111/j.1399-3046.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- 8.Kuppahally S, Al-Khaldi A, Weisshaar D, Valantine HA, Oyer P, Robbins RC, Hunt SA. Wound healing complications with de novo sirolimus versus mycophenolate mofetil-based regimen in cardiac transplant recipients. Am J Transplant. 2006;6:986–992. doi: 10.1111/j.1600-6143.2006.01282.x. [DOI] [PubMed] [Google Scholar]
- 9.Schaffer M, Schier R, Napirei M, Michalski S, Traska T, Viebahn R. Sirolimus impairs wound healing. Langenbeck's archives of surgery / Deutsche Gesellschaft fur Chirurgie. 2007;392:297–303. doi: 10.1007/s00423-007-0174-5. [DOI] [PubMed] [Google Scholar]
- 10.Valente JF, Hricik D, Weigel K, Seaman D, Knauss T, Siegel CT, Bodziak K, Schulak JA. Comparison of sirolimus vs. mycophenolate mofetil on surgical complications and wound healing in adult kidney transplantation. Am J Transplant. 2003;3:1128–1134. doi: 10.1034/j.1600-6143.2003.00185.x. [DOI] [PubMed] [Google Scholar]
- 11.Augustine JJ, Bodziak KA, Hricik DE. Use of sirolimus in solid organ transplantation. Drugs. 2007;67:369–391. doi: 10.2165/00003495-200767030-00004. [DOI] [PubMed] [Google Scholar]
- 12.MacDonald AS. Rapamycin in combination with cyclosporine or tacrolimus in liver, pancreas, and kidney transplantation. Transplant Proc. 2003;35:201S–208S. doi: 10.1016/s0041-1345(03)00231-8. [DOI] [PubMed] [Google Scholar]
- 13.Ruchin PE, Muller DW, Faddy SC, Baron DW, Roy PR, Wilson SH. Long-Term Clinical Follow-up of Sirolimus-Eluting (CYPHERtrade mark) Coronary Stents in the Treatment of InStent Restenosis in an Unselected Population. Heart Lung Circ. 2007 doi: 10.1016/j.hlc.2007.02.090. [DOI] [PubMed] [Google Scholar]
- 14.Witzig TE, Kaufmann SH. Inhibition of the phosphatidylinositol 3-kinase/mammalian target of rapamycin pathway in hematologic malignancies. Current treatment options in oncology. 2006;7:285–294. doi: 10.1007/s11864-006-0038-1. [DOI] [PubMed] [Google Scholar]
- 15.Dumont FJ, Melino MR, Staruch MJ, Koprak SL, Fischer PA, Sigal NH. The immunosuppressive macrolides FK-506 and rapamycin act as reciprocal antagonists in murine T cells. J Immunol. 1990;144:1418–1424. [PubMed] [Google Scholar]
- 16.Makrigiannis AP, Hoskin DW. Inhibition of CTL induction by rapamycin: IL-2 rescues granzyme B and perforin expression but only partially restores cytotoxic activity. J Immunol. 1997;159:4700–4707. [PubMed] [Google Scholar]
- 17.Nikolaeva N, Bemelman FJ, Yong SL, van Lier RA, ten Berge IJ. Rapamycin does not induce anergy but inhibits expansion and differentiation of alloreactive human T cells. Transplantation. 2006;81:445–454. doi: 10.1097/01.tp.0000194860.21533.b9. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K, Reynolds M, Ogawa N, Longo DL, Burdick J. Augmentation of T-cell apoptosis by immunosuppressive agents. Clinical transplantation. 2004;18 Suppl 12:72–75. doi: 10.1111/j.1399-0012.2004.00222.x. [DOI] [PubMed] [Google Scholar]
- 19.Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, Powell JD. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J Immunol. 2007;178:2163–2170. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- 20.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 21.Fox CJ, Hammerman PS, Thompson CB. The Pim kinases control rapamycin-resistant T cell survival and activation. J Exp Med. 2005;201:259–266. doi: 10.1084/jem.20042020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asarnow DM, Kuziel WA, Bonyhadi M, Tigelaar RE, Tucker PW, Allison JP. Limited diversity of γδ antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988;55:837–847. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- 23.Havran WL, Chien Y-H, Allison JP. Recognition of self antigens by skin-derived T cells with invariant γδ antigen receptors. Science. 1991;252:1430–1432. doi: 10.1126/science.1828619. [DOI] [PubMed] [Google Scholar]
- 24.Lewis JM, Tigelaar RE. Recognition of an epidermal stress antigen by murine γδ dendritic epidermal T cells (DETC) J.Invest.Dermatol. 1991;96:538A. [Google Scholar]
- 25.Born WK, Lahn M, Takeda K, Kanehiro A, O'Brien RL, Gelfand EW. Role of γδ T cells in protecting normal airway function. Respir Res. 2000;1:151–158. doi: 10.1186/rr26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial γδ T cells. Proc Natl Acad Sci U S A. 2002;99:14338–14343. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, Havran WL. A role for skin γδ T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 28.Girardi M, Lewis J, Glusac E, Filler RB, Geng L, Hayday AC, Tigelaar RE. Resident skin-specific γδ T cells provide local, nonredundant regulation of cutaneous inflammation. J Exp Med. 2002;195:855–867. doi: 10.1084/jem.20012000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC. Regulation of cutaneous malignancy by γδ T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 30.Sharp LL, Jameson JM, Cauvi G, Havran WL. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat Immunol. 2005;6:73–79. doi: 10.1038/ni1152. [DOI] [PubMed] [Google Scholar]
- 31.Haase I, Evans R, Pofahl R, Watt FM. Regulation of keratinocyte shape, migration and wound epithelialization by IGF-1- and EGF-dependent signalling pathways. J Cell Sci. 2003;116:3227–3238. doi: 10.1242/jcs.00610. [DOI] [PubMed] [Google Scholar]
- 32.Sadagurski M, Yakar S, Weingarten G, Holzenberger M, Rhodes CJ, Breitkreutz D, Leroith D, Wertheimer E. Insulin-like growth factor 1 receptor signaling regulates skin development and inhibits skin keratinocyte differentiation. Mol Cell Biol. 2006;26:2675–2687. doi: 10.1128/MCB.26.7.2675-2687.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tavakkol A, Elder JT, Griffiths CE, Cooper KD, Talwar H, Fisher GJ, Keane KM, Foltin SK, Voorhees JJ. Expression of growth hormone receptor, insulin-like growth factor 1 (IGF-1) and IGF-1 receptor mRNA and proteins in human skin. J Invest Dermatol. 1992;99:343–349. doi: 10.1111/1523-1747.ep12616668. [DOI] [PubMed] [Google Scholar]
- 34.Jameson JM, Cauvi G, Sharp LL, Witherden DA, Havran WL. γδ T cell-induced hyaluronan production by epithelial cells regulates inflammation. J Exp Med. 2005;201:1269–1279. doi: 10.1084/jem.20042057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jameson JM, Cauvi G, Witherden DA, Havran WL. A keratinocyte-responsive γδ TCR is necessary for dendritic epidermal T cell activation by damaged keratinocytes and maintenance in the epidermis. J Immunol. 2004;172:3573–3579. doi: 10.4049/jimmunol.172.6.3573. [DOI] [PubMed] [Google Scholar]
- 36.Yui MA, Sharp LL, Havran WL, Rothenberg EV. Preferential activation of an IL-2 regulatory sequence transgene in TCR γδ and NKT cells: subset-specific differences in IL-2 regulation. J Immunol. 2004;172:4691–4699. doi: 10.4049/jimmunol.172.8.4691. [DOI] [PubMed] [Google Scholar]
- 37.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 38.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 39.Tokunaga C, Yoshino K, Yonezawa K. mTOR integrates amino acid- and energy-sensing pathways. Biochem Biophys Res Commun. 2004;313:443–446. doi: 10.1016/j.bbrc.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 40.Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, Erdjument-Bromage H, Tempst P, Sabatini DM. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 41.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 42.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 43.Sarbassov dos D, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5:1298–1302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 45.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162:2775–2784. [PubMed] [Google Scholar]
- 46.Feuerstein N, Huang D, Prystowsky MB. Rapamycin selectively blocks interleukin-2-induced proliferating cell nuclear antigen gene expression in T lymphocyte. Evidence for inhibition of CREB/ATF binding activities. J Biol Chem. 1995;270:9454–9458. doi: 10.1074/jbc.270.16.9454. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez J, Harris T, Childs G, Prystowsky MB. Rapamycin blocks IL-2-driven T cell cycle progression while preserving T cell survival. Blood cells, molecules & diseases. 2001;27:572–585. doi: 10.1006/bcmd.2001.0420. [DOI] [PubMed] [Google Scholar]
- 48.Ekici Y, Emiroglu R, Ozdemir H, Aldemir D, Karakayali H, Haberal M. Effect of rapamycin on wound healing: an experimental study. Transplant Proc. 2007;39:1201–1203. doi: 10.1016/j.transproceed.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura K, Saitoh A, Yasaka N, Furue M, Tamaki K. Molecular mechanisms involved in the migration of epidermal dendritic cells in the skin. J Investig Dermatol Symp Proc. 1999;4:169–172. doi: 10.1038/sj.jidsp.5640203. [DOI] [PubMed] [Google Scholar]
- 50.Stoitzner P, Zanella M, Ortner U, Lukas M, Tagwerker A, Janke K, Lutz MB, Schuler G, Echtenacher B, Ryffel B, Koch F, Romani N. Migration of langerhans cells and dermal dendritic cells in skin organ cultures: augmentation by TNF-alpha and IL-1beta. J Leukoc Biol. 1999;66:462–470. [PubMed] [Google Scholar]
- 51.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 52.Calvo V, Crews CM, Vik TA, Bierer BE. Interleukin 2 stimulation of p70 S6 kinase activity is inhibited by the immunosuppressant rapamycin. Proc Natl Acad Sci U S A. 1992;89:7571–7575. doi: 10.1073/pnas.89.16.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Javier AF, Bata-Csorgo Z, Ellis CN, Kang S, Voorhees JJ, Cooper KD. Rapamycin (sirolimus) inhibits proliferating cell nuclear antigen expression and blocks cell cycle in the G1 phase in human keratinocyte stem cells. J Clin Invest. 1997;99:2094–2099. doi: 10.1172/JCI119382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian L, Lu L, Yuan Z, Lamb JR, Tam PK. Acceleration of apoptosis in CD4+CD8+ thymocytes by rapamycin accompanied by increased CD4+CD25+ T cells in the periphery. Transplantation. 2004;77:183–189. doi: 10.1097/01.TP.0000101005.44661.3E. [DOI] [PubMed] [Google Scholar]
- 55.Li C, Capan E, Zhao Y, Zhao J, Stolz D, Watkins SC, Jin S, Lu B. Autophagy is induced in CD4+ T cells and important for the growth factor-withdrawal cell death. J Immunol. 2006;177:5163–5168. doi: 10.4049/jimmunol.177.8.5163. [DOI] [PubMed] [Google Scholar]
- 56.Castedo M, Ferri KF, Kroemer G. Mammalian target of rapamycin (mTOR): pro-and anti-apoptotic. Cell Death Differ. 2002;9:99–100. doi: 10.1038/sj.cdd.4400978. [DOI] [PubMed] [Google Scholar]
- 57.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- 59.Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003;35:7S–14S. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 60.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 61.Ye SK, Maki K, Lee HC, Ito A, Kawai K, Suzuki H, Mak TW, Chien Y, Honjo T, Ikuta K. Differential roles of cytokine receptors in the development of epidermal γδ T cells. J Immunol. 2001;167:1929–1934. doi: 10.4049/jimmunol.167.4.1929. [DOI] [PubMed] [Google Scholar]
- 62.Havran WL, Allison JP. Origin of Thy-1+ dendritic epidermal cells of adult mice from fetal thymic precursors. Nature. 1990;344:68–70. doi: 10.1038/344068a0. [DOI] [PubMed] [Google Scholar]
- 63.Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–448. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- 64.Engelhardt E, Toksoy A, Goebeler M, Debus S, Brocker EB, Gillitzer R. Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol. 1998;153:1849–1860. doi: 10.1016/s0002-9440(10)65699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li J, DeFea K, Roth RA. Modulation of insulin receptor substrate-1 tyrosine phosphorylation by an Akt/phosphatidylinositol 3-kinase pathway. J Biol Chem. 1999;274:9351–9356. doi: 10.1074/jbc.274.14.9351. [DOI] [PubMed] [Google Scholar]
- 66.Shibutani S, Inoue F, Aramaki O, Akiyama Y, Matsumoto K, Shimazu M, Kitajima M, Ikeda Y, Shirasugi N, Niimi M. Effects of immunosuppressants on induction of regulatory cells after intratracheal delivery of alloantigen. Transplantation. 2005;79:904–913. doi: 10.1097/01.tp.0000158023.21233.de. [DOI] [PubMed] [Google Scholar]
- 67.Hosoi H, Dilling MB, Shikata T, Liu LN, Shu L, Ashmun RA, Germain GS, Abraham RT, Houghton PJ. Rapamycin causes poorly reversible inhibition of mTOR and induces p53-independent apoptosis in human rhabdomyosarcoma cells. Cancer Res. 1999;59:886–894. [PubMed] [Google Scholar]
- 68.Stromberg T, Dimberg A, Hammarberg A, Carlson K, Osterborg A, Nilsson K, Jernberg-Wiklund H. Rapamycin sensitizes multiple myeloma cells to apoptosis induced by dexamethasone. Blood. 2004;103:3138–3147. doi: 10.1182/blood-2003-05-1543. [DOI] [PubMed] [Google Scholar]
- 69.Pankow S, Bamberger C, Klippel A, Werner S. Regulation of epidermal homeostasis and repair by phosphoinositide 3-kinase. J Cell Sci. 2006;119:4033–4046. doi: 10.1242/jcs.03175. [DOI] [PubMed] [Google Scholar]
- 70.Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol. 2004;24:200–216. doi: 10.1128/MCB.24.1.200-216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holtmeier W, Pfander M, Hennemann A, Zollner TM, Kaufmann R, Caspary WF. The TCR-delta repertoire in normal human skin is restricted and distinct from the TCR-delta repertoire in the peripheral blood. J Invest Dermatol. 2001;116:275–280. doi: 10.1046/j.1523-1747.2001.01250.x. [DOI] [PubMed] [Google Scholar]
- 72.Holtmeier W, Pfander M, Zollner TM, Kaufmann R, Caspary WF. Distinct TCR delta repertoires are present in the cutaneous lesions and inflamed duodenum of patients with dermatitis herpetiformis. Exp Dermatol. 2002;11:527–531. doi: 10.1034/j.1600-0625.2002.110605.x. [DOI] [PubMed] [Google Scholar]
- 73.Ebert LM, Meuter S, Moser B. Homing and function of human skin γδ T cells and NK cells: relevance for tumor surveillance. J Immunol. 2006;176:4331–4336. doi: 10.4049/jimmunol.176.7.4331. [DOI] [PubMed] [Google Scholar]