Abstract
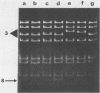
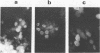
Polyomavirus mutants were isolated from PCC4 embryonal carcinoma cells infected with a variant strain of polyomavirus (ev 1001h) and were found to contain a tandem duplication overlapping the enhancers and the origin of replication. These mutants were able to infect several lines of embryonal carcinoma cells, including PCC4, F9, and LT1. The sequence and structure of one of these mutants are presented and compared with those of other PyEC PCC4 mutants previously described.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berstine E. G., Hooper M. L., Grandchamp S., Ephrussi B. Alkaline phosphatase activity in mouse teratoma. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3899–3903. doi: 10.1073/pnas.70.12.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Covarrubias L., Cervantes L., Covarrubias A., Soberón X., Vichido I., Blanco A., Kupersztoch-Portnoy Y. M., Bolivar F. Construction and characterization of new cloning vehicles. V. Mobilization and coding properties of pBR322 and several deletion derivatives including pBR327 and pBR328. Gene. 1981 Jan-Feb;13(1):25–35. doi: 10.1016/0378-1119(81)90040-8. [DOI] [PubMed] [Google Scholar]
- Fujimura F. K., Deininger P. L., Friedmann T., Linney E. Mutation near the polyoma DNA replication origin permits productive infection of F9 embryonal carcinoma cells. Cell. 1981 Mar;23(3):809–814. doi: 10.1016/0092-8674(81)90445-1. [DOI] [PubMed] [Google Scholar]
- Gaudray P., Tyndall C., Kamen R., Cuzin F. The high affinity binding site on polyoma virus DNA for the viral large-T protein. Nucleic Acids Res. 1981 Nov 11;9(21):5697–5710. doi: 10.1093/nar/9.21.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges E., Vasseur M., Blangy D. Polyoma virus mutants as probes of variety among mouse embryonal carcinoma cell lines. Differentiation. 1982;22(1):62–65. doi: 10.1111/j.1432-0436.1982.tb01225.x. [DOI] [PubMed] [Google Scholar]
- Katinka M., Vasseur M., Montreau N., Yaniv M., Blangy D. Polyoma DNA sequences involved in control of viral gene expression in murine embryonal carcinoma cells. Nature. 1981 Apr 23;290(5808):720–722. doi: 10.1038/290720a0. [DOI] [PubMed] [Google Scholar]
- Katinka M., Yaniv M., Vasseur M., Blangy D. Expression of polyoma early functions in mouse embryonal carcinoma cells depends on sequence rearrangements in the beginning of the late region. Cell. 1980 Jun;20(2):393–399. doi: 10.1016/0092-8674(80)90625-x. [DOI] [PubMed] [Google Scholar]
- Kelly F., Condamine H. Tumor viruses and early mouse embryos. Biochim Biophys Acta. 1982 Apr 29;651(2-3):105–141. doi: 10.1016/0304-419X(82)90009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linney E., Donerly S. DNA fragments from F9 PyEC mutants increase expression of heterologous genes in transfected F9 cells. Cell. 1983 Dec;35(3 Pt 2):693–699. doi: 10.1016/0092-8674(83)90102-2. [DOI] [PubMed] [Google Scholar]
- Magnusson G., Nilsson M. G. Viable polyoma virus variant with two origins of DNA replication. Virology. 1982 May;119(1):12–21. doi: 10.1016/0042-6822(82)90060-5. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nicolas J. F., Avner P., Gaillard J., Guenet J. L., Jakob H., Jacob F. Cell lines derived from teratocarcinomas. Cancer Res. 1976 Nov;36(11 Pt 2):4224–4231. [PubMed] [Google Scholar]
- Ruley H. E., Fried M. Sequence repeats in a polyoma virus DNA region important for gene expression. J Virol. 1983 Jul;47(1):233–237. doi: 10.1128/jvi.47.1.233-237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART S. E., EDDY B. E., BORGESE N. Neoplasms in mice inoculated with a tumor agent carried in tissue culture. J Natl Cancer Inst. 1958 Jun;20(6):1223–1243. doi: 10.1093/jnci/20.6.1223. [DOI] [PubMed] [Google Scholar]
- Sekikawa K., Levine A. J. Isolation and characterization of polyoma host range mutants that replicate in nullipotential embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1100–1104. doi: 10.1073/pnas.78.2.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens L. C., Varnum D. S. The development of teratomas from parthenogenetically activated ovarian mouse eggs. Dev Biol. 1974 Apr;37(2):369–380. doi: 10.1016/0012-1606(74)90155-9. [DOI] [PubMed] [Google Scholar]
- Swartzendruber D. E., Lehman J. M. Neoplastic differentiation: interaction of simian virus 40 and polyoma virus with murine teratocarcinoma cells in vitro. J Cell Physiol. 1975 Apr;85(2 Pt 1):179–187. doi: 10.1002/jcp.1040850204. [DOI] [PubMed] [Google Scholar]
- Vasseur M., Katinka M., Herbomel P., Yaniv M., Blangy D. Physical and biological features of polyoma virus mutants able to infect embryonal carcinoma cell lines. J Virol. 1982 Sep;43(3):800–808. doi: 10.1128/jvi.43.3.800-808.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasseur M., Kress C., Montreau N., Blangy D. Isolation and characterization of polyoma virus mutants able to develop in embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1068–1072. doi: 10.1073/pnas.77.2.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv M. Regulation of eukaryotic gene expression by transactivating proteins and cis acting DNA elements. Biol Cell. 1984;50(3):203–216. doi: 10.1111/j.1768-322x.1984.tb00268.x. [DOI] [PubMed] [Google Scholar]