Abstract
Hepatocyte growth factor/scatter factor (HGF/SF) stimulates the motility of epithelial cells, initially inducing centrifugal spreading of colonies followed by disruption of cell–cell junctions and subsequent cell scattering. In Madin–Darby canine kidney cells, HGF/SF-induced motility involves actin reorganization mediated by Ras, but whether Ras and downstream signals regulate the breakdown of intercellular adhesions has not been established. Both HGF/SF and V12Ras induced the loss of the adherens junction proteins E-cadherin and β-catenin from intercellular junctions during cell spreading, and the HGF/SF response was blocked by dominant-negative N17Ras. Desmosomes and tight junctions were regulated separately from adherens junctions, because they were not disrupted by V12Ras. MAP kinase, phosphatidylinositide 3-kinase (PI 3-kinase), and Rac were required downstream of Ras, because loss of adherens junctions was blocked by the inhibitors PD098059 and LY294002 or by dominant-inhibitory mutants of MAP kinase kinase 1 or Rac1. All of these inhibitors also prevented HGF/SF-induced cell scattering. Interestingly, activated Raf or the activated p110α subunit of PI 3-kinase alone did not induce disruption of adherens junctions. These results indicate that activation of both MAP kinase and PI 3-kinase by Ras is required for adherens junction disassembly and that this is essential for the motile response to HGF/SF.
INTRODUCTION
Hepatocyte growth factor/scatter factor (HGF/SF)1 is a multifunctional cytokine possessing a wide spectrum of biological activities. It is secreted by cells of mesenchymal origin and acts as a mitogen, dissociation, and motility factor for many epithelial cells in culture (Stoker et al., 1987; Gherardi et al., 1989; Nakamura et al., 1989) through its receptor the Met tyrosine kinase (Bottaro et al., 1991; Weidner et al., 1993). HGF/SF is therefore believed to be a potent epithelial morphogen leading to epithelial–mesenchymal interconversion (Montesano et al., 1991). In addition, HGF/SF is a mitogen for mature hepatocytes in primary culture (Nakamura et al., 1989), and mice lacking HGF/SF show reduced liver size, although they actually die in utero as a result of abnormal development of the placenta (Schmidt et al., 1995; Uehara et al., 1995). HGF/SF plays an active role in liver, kidney, and lung regeneration after tissue damage, contributes to wound repair, and can act as an angiogenic factor (reviewed in Zarnegar and Michalopoulos, 1995). Furthermore, HGF/SF has been shown to be involved in the development of some tumors and in the process of carcinoma cell invasion (Rong et al., 1992; Bellusci et al., 1994).
Madin–Darby canine kidney (MDCK) epithelial cells have been used extensively as an in vitro model for HGF/SF-induced epithelial–mesenchymal conversion. HGF/SF initially induces centrifugal spreading of MDCK cells in colonies and subsequently stimulates cell–cell dissociation, allowing each cell to “scatter” or detach from colonies and migrate independently of other cells (Stoker and Perryman, 1985; Ridley et al., 1995). Cell migration is dependent on the actin cytoskeleton and involves the extension of lamellipodia at the leading edge and the formation of new contacts with the extracellular matrix, followed by retraction and detachment of the trailing edge (Lauffenburger and Horwitz, 1996). The motile response to HGF/SF therefore involves a series of changes to the actin cytoskeleton, including membrane ruffling, lamellipodium formation, and a decrease in stress fibers and cortical actin (Ridley et al., 1995). These changes are mediated by Ras and Rac, two proteins belonging to the Ras superfamily of small GTPases (Hall, 1994; Tapon and Hall, 1997). HGF/SF activates Ras by increasing the level of Ras-GTP (Graziani et al., 1993), and HGF/SF-induced cell motility is dependent on Ras (Hartmann et al., 1994; Ridley et al., 1995). Microinjection of activated Ras protein induces Rac-dependent ruffling, lamellipodium formation, and spreading of MDCK cell colonies but does not induce cell dissociation or scattering. In contrast, MDCK cell lines expressing constitutively active Ras display a scattered phenotype in some cases (Schoenenberger et al., 1991), suggesting that high levels of Ras activity can in the long term induce destabilization of cell–cell interactions presumably by altering patterns of gene expression.
The signaling pathways downstream of Ras that are involved in the HGF/SF response have not been characterized in detail. Ras is known to activate multiple signal transduction pathways, including the p42/p44 MAP kinase (MAPK) cascade, phosphatidylinositide 3-kinase (PI 3-kinase), and Ral-GDS, a guanine nucleotide exchange factor for the Ras-related protein Ral (Marshall, 1996). It has been shown that HGF/SF activates MAPK in A549 cells (Ponzetto et al., 1994), but the role of MAPK activation in the scattering response of MDCK cells is unknown. HGF/SF also activates PI 3-kinase, and wortmannin, an inhibitor of PI 3-kinase, blocks scattering of MDCK cells in response to HGF/SF (Royal and Park, 1995). However, the stage of the scattering response that is inhibited by wortmannin has not been defined. PI 3-kinase may in turn lead to activation of Rac (reviewed in Parker, 1995), and this may be a consequence of the product of PI 3-kinase, phosphatidylinositol-3,4,5-trisphosphate, binding to and activating Rac exchange factors (Han et al., 1998). Because Rac is required for HGF/SF-induced lamellipodium formation (Ridley et al., 1995), PI 3-kinase may therefore provide a link between Ras and Rac.
In addition to changes in actin organization, the migratory response of MDCK cells to HGF/SF also requires the disruption of cell–cell junctions. In epithelial cells, the lateral plasma membranes of adjacent cells interact via adherens junctions, tight junctions, and desmosomes. In adherens junctions, the extracellular domains of the transmembrane cadherins interact homophilically, whereas the intracellular domains are linked to the actin cytoskeleton via α- and β-catenin, vinculin, and α-actinin. In desmosomes, desmosomal cadherins (desmocollins and desmogleins) interact with intermediate filaments (via desmoplakins and plakophilins) (Ben-Ze’ev, 1997). Epithelial cells also require tight junctions to maintain their polarity as they constitute a barrier between the apical and basolateral plasma membranes and prevent the diffusion of macromolecules between cells (Rodriguez-Boulan and Nelson, 1989). Tight junctions contain ZO-1, a peripheral membrane protein that appears to link the integral membrane protein occludin to the actin cytoskeleton (Gumbiner, 1993).
The molecular mechanisms underlying HGF/SF-induced disruption of epithelial cell junctions have not been defined. Our previous work established that Ras acts downstream of the Met receptor and is required for lamellipodium formation and cell spreading in MDCK cells (Ridley et al., 1995), and we have therefore investigated the roles of Ras and signaling pathways activated by Ras in regulating the disruption of intercellular adhesions.
MATERIALS AND METHODS
Materials
The pSG5-5-Myc-p110α-WT-3-K-Ras plasmid was a gift from Jonathan Backer (Albert Einstein College of Medicine, New York, NY); the pEXV3-N17H-Ras-3-Myc and the pCDNA3-V12H-Ras constructs were from Julian Downward (Imperial Cancer Research Fund, London, United Kingdom); the pEXV3-AFG MAP kinase kinase 1 (MAPKK1), pEXV3-Ala-221 MAPKK1, pEXV3-wild-type MAPKK1, and pEXV3-Glu-217/Glu-221 MAPKK1 constructs were from Chris Marshall (Institute of Cancer Research, London, United Kingdom); and the pEFHm-RAFCAAX-Myc construct was from Richard Marais (Institute of Cancer Research, London, United Kingdom). The rabbit polyclonal antibody against the C terminus of bovine p110α (aa 1054–1068) was a gift from Roya Hooshmand-Rad (Ludwig Institute for Cancer Research, Uppsala, Sweden). The rabbit polyclonal antibody recognizing MAPKK1 (Alessi et al., 1994) was a gift from Chris Marshall. The rabbit antiserum to desmoplakin C terminus (DP 145) was kindly provided by Tony Magee (National Institute for Medical Research, London, United Kingdom). The rabbit anti-Ras antibody and the mouse anti-c-Myc antiboby were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The mouse anti-E-cadherin and mouse anti-β-catenin antibodies were obtained from Transduction Laboratories (Lexington, KY). The rabbit anti-ZO-1 and the rabbit anti-occludin antibodies were obtained from Zymed (San Franscisco, CA). Secondary antibodies were purchased from Jackson ImmunoResearch (West Grove, PS) (TRITC-labeled donkey anti-rat antibody and FITC-labeled donkey and TRITC-labeled goat anti-mouse antibodies) and from Southern Biotechnology (Birmingham, AL) (FITC-labeled goat anti-rabbit antibody).
Cell Culture
A subclone of MDCK cells (Ridley et al., 1995) was grown in DMEM containing 10% bovine FCS. Cells for microinjection or stimulation with HGF/SF were seeded in 15-mm wells at 1 × 104 cells/well on 13-mm circular glass coverslips. After 3 d, cells were transferred to DMEM containing 0.5% FCS with or without 50 μM PD098059 (Calbiochem, Nottingham, United Kingdom) or 20 μM LY294002 (Calbiochem), and HGF/SF (10 ng/ml; RD Systems, Abingdon, United Kingdom) was added and/or cell groups were injected at the edge and in the middle of colonies.
Expression and Purification of Recombinant Proteins
The recombinant proteins V12Ras and N17Rac1 were expressed in Escherichia coli from the pGEX-2T vector as glutathione S-transferase fusion proteins and purified as previously described (Ridley et al., 1992). Active protein concentrations for GTP-binding proteins were determined by a filter binding assay using [3H]GDP (Hall and Self, 1986). Total protein concentrations were estimated using a protein assay kit (Bio-Rad, Hercules, CA).
Microinjection
For microinjection proteins were diluted in 50 mM Tris-Cl (pH 7.5), 100 mM NaCl, 5 mM MgCl2, and microinjected into the cytoplasm of MDCK cells maintained in 0.5% FCS for 1 h before injection. To identify injected cells, tetramethylrhodamine dextran (molecular weight 10,000; Molecular Probes, Leiden, The Netherlands) at 5 mg/ml was microinjected together with recombinant proteins.
Cells microinjected with plasmids encoding N17Ras, AFG MAPKK1, Ala-221 MAPKK1, Glu-217/Glu-221 MAPKK1, wild-type MAPKK1, p110α, or RafCAAX were incubated at 37°C for 4–6 h to allow protein expression. Plasmid-injected cells were detected by using an antibody against the expressed protein. Cells microinjected with the protein V12Ras or N17Rac1 were incubated for 1, 2, 4, 6, 8, and 16 h according to the different experiments. In some experiments in which the injected component had an inhibitory effect, cells were treated with HGF/SF (10 ng/ml) for 2, 4, 6, 8, and 16 h as indicated in the figure legends. Cells were then fixed and stained for F-actin and junctional complexes as described below.
Immunofluorescence
MDCK cells were fixed with 3.7% formaldehyde dissolved in PBS (137 mM NaCl, 3 mM KCl, 13 mM Na2HPO4 [7H2O], 2 mM KH2PO4, 0.9 mM CaCl2, 0.7 mM MgCl2 [6H2O], pH 7.4) for 10–20 min at room temperature and permeabilized for 5 min with 0.2% Triton X-100. Primary and secondary antibodies were diluted in PBS containing 0.5% bovine serum albumin, and all incubations were for 1 h at room temperature. The cell junction proteins were visualized by mouse anti-E-cadherin (1:200) or mouse anti-β-catenin (1:400), respectively. Secondary staining was performed using FITC-labeled donkey or TRITC-labeled goat anti-mouse antibodies (1:200). Tight junctions were stained for ZO-1 with a rabbit anti-ZO-1 antibody (1:200) and for occludin with a rabbit anti-occludin antibody (1:100) and desmosomes with a rabbit anti-desmoplakin I/II antiserum (DP145) (1:200). Cells injected with pEXV3-AFG MAPKK1, pEXV3-Ala-221 MAPKK1, pEXV3-wild-type MAPKK1 or pEXV3-Glu-217/Glu-221 MAPKK1 were detected with a rabbit anti-MAPKK1 antibody (1:100); cells injected with pEFHm-RAFCAAX-Myc were visualized with a mouse anti-c-Myc antibody (1:500), pSG5-5-Myc-p110α-WT-3-K-Ras-injected cells with a rabbit anti-p110α antibody (1:200), and pEXV3-N17H-Ras-3-Myc and pCDNA3-V12H-Ras injected cells with a rat or rabbit anti-Ras antibody (1:50). The secondary stain for the rat anti-Ras antibody was a TRITC-labeled donkey anti-rat antibody (1:400). Actin filaments were detected by incubation with 0.8 nM TRITC-phalloidin (Sigma, Poole, United Kingdom) or 20 nM FITC-phalloidin (Molecular Probes, Leiden, The Netherlands). Subsequently, the specimens were mounted in Moviol (La Jolla, CA).
Image Generation
The specimens were analyzed by confocal laser scanning microscopy using Zeiss (Welwyn Garden City, United Kingdom) LSM 310 and LSM 510 microscopes. Data were collected using a 40×, numerical aperture 1.3, oil immersion objective (Zeiss, Jena, Germany). Image files collected with the LSM 310 and the LSM 510 microscopes described a matrix of 1024 × 1024 pixels that represented the average of eight frames scanned at 0.062 Hz. Fluorophores were excited at either 488 or 543 nm and visualized with a 540 ± 25- or 608 ± 32-nm bandpass filter, respectively, where the levels of interchannel crosstalk were insignificant.
Phase-contrast micrographs were imaged with a Coolview 12, 1024 × 1024, 12-bit cooled charge-coupled device camera (Photonic Science, Robertsbridge, United Kingdom) using a 63×, numerical aperture 1.4, oil immersion objective (Zeiss) on an Axiophot microscope (Zeiss) illuminated at intensities that avoided saturation of the pixel content. The data sets were collected using Image Pro Plus, version 3 (Media Cybernetics, Silver Spring, MD). To produce micrographs the 8 bits of data with the highest content of information were transferred to Adobe Photoshop, version 3.0 (Adobe Systems, San Jose, CA).
Western Blotting
MDCK cells were grown in 35-mm dishes in DMEM containing 10% FCS. After 3 d, subconfluent cells were incubated with DMEM containing 0.5% FCS for 1 h with or without 50 μM PD098059 or 20 μM LY294002 before adding HGF/SF at 10 ng/ml for 10 min or 4 or 16 h. Subsequently, cells were washed in ice-cold PBS (137 mM NaCl, 3 mM KCl, 8 mM Na2HPO4, 2 mM KH2PO4, pH 7.4). For detection of activated p42/p44 MAPK, the cells were lysed in 20 mM Tris-HCl (pH 8.0), 40 mM Na4P2O7, 50 mM NaF, 5 mM MgCl2, 100 μM Na3VO4, 10 mM EGTA (pH 8.0), 1% Triton X-100, 0.5% sodium deoxycholate, 20 μg/ml aprotinin, 20 μg/ml leupeptin, 3 mM PMSF for 10 min at 4°C. The lysates were centrifuged at 10,000 × g for 5 min, and equal amounts of protein were electrophoresed on 10% SDS-polyacrylamide gels and then transferred to polyvinylidene difluoride membranes. For detection of activated phosphorylated p42/p44 MAPK, membranes were blocked in 5% nonfat dried milk in PBS containing 0.1% Tween 20 and then incubated with the primary antibody rabbit anti-active MAPK polyclonal antibody (Promega, Madison, WI) diluted 1:20,000 in PBS containing 0.05% Tween 20 and 0.5% BSA for 16 h at 4°C. Membranes were then incubated for 1 h at room temperature with (1:1500) horseradish peroxidase–conjugated donkey anti-rabbit antibody (Amersham, Little Chalfont, United Kingdom) in PBS containing 0.1% Tween 20 and 5% nonfat dried milk.
For detection of total p42/p44 MAPK (extracellular signal-regulated kinase [ERK] 1 and 2), membranes were blocked in 5% nonfat dried milk in Tris-buffered saline (TBS; 20 mM Tris-HCl, pH 7.6, 137 mM NaCl) containing 0.1% Tween 20 and then incubated with a rabbit anti-ERK1 antibody (Santa Cruz Biotechnology) diluted 1:1000 in TBS-Tween containing 5% nonfat dried milk for 1 h at room temperature. Membranes were then incubated with horseradish peroxidase-conjugated donkey anti-rabbit antibody (1:3000) in TBS-Tween containing 5% nonfat dried milk.
Detergent-soluble and -insoluble fractions were obtained by lysing cells in NP-40 buffer (25 mM HEPES/NaOH pH 7.4, 150 mM NaCl, 4 mM EDTA, 25 mM NaF, 1% NP-40, 1 mM Na3VO4, 1 mM PMSF, 10 μg/ml leupeptin, 10 μg/ml aprotinin) for 30 min at 4°C. The lysates were centrifuged at 10,000 × g for 30 min, and the supernatant was collected as the NP-40-soluble fraction. The pellet was resuspended in 100 μl of 25 mM HEPES, pH 7.5, 4 mM EDTA, 25 mM NaF, 1% SDS, and 1 mM Na3VO4 using a Wheaton homogenizer (Jencons, Leighton Buzzard, United Kingdom). After adding 900 μl of the NP-40 buffer, the homogenate was passed 10 times through a 27-gauge needle and left for 30 min on a rotating wheel at 4°C. The lysates were then centrifuged at 10,000 × g for 30 min, and the supernatant was used as the NP-40-insoluble fraction.
Equal volumes of all fractions were electrophoresed on 7.5% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes, which were blocked for 1 h at room temperature with TBS-Tween containing 5% nonfat dried milk. The membranes were incubated for 1 h with a mouse anti-β-catenin (1:500) or a mouse anti-E-cadherin antibody (1:2500) and then further incubated for 1 h with horseradish peroxidase-conjugated sheep anti-mouse antibody (1:3000; Amersham) in blocking solution at room temperature. Immunoreactive bands were visualized using enhanced chemiluminescence as described by the manufacturer (Amersham).
RESULTS
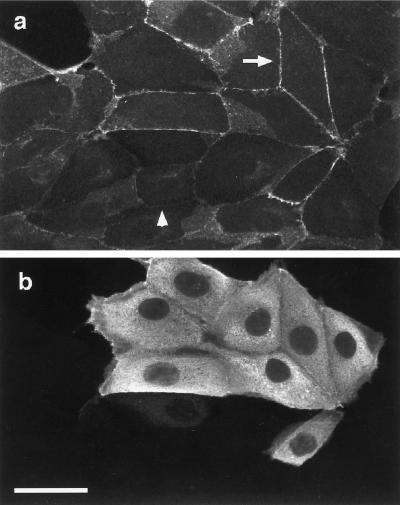
The MAPKK1/2 Inhibitor PD098059 Blocks HGF/SF-induced Scattering
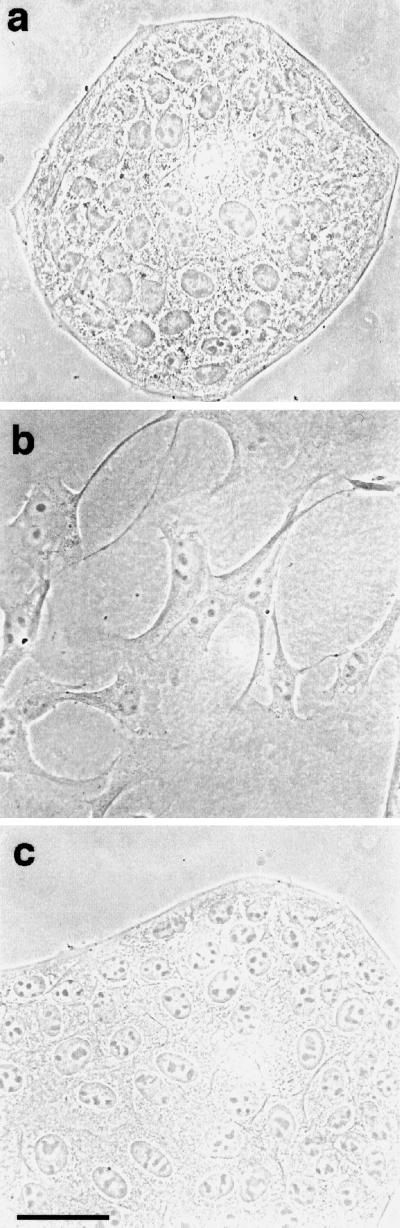
The motile response of MDCK epithelial cells to HGF/SF can be divided into two stages (Stoker and Perryman, 1985; Dowrick et al., 1991; Ridley et al., 1995). First, cells extend lamellipodia and spread for 4–6 h after addition of HGF/SF, and then they start to detach from each other so that by 16 h most cells have assumed a fibroblastic, “scattered” morphology (Figure 1, a and b). During this second stage, the average migration rate of MDCK cells is more than doubled compared with unstimulated cells, from ∼10 μm/h to between 20 and 25 μm/h (Ridley et al., 1995; our unpublished data). HGF/SF was titrated on MDCK cells, and the lowest concentration giving an optimal scattering response was determined to be 10 ng/ml. This concentration was therefore used in all subsequent experiments.
Figure 1.
HGF/SF-induced scattering is blocked by the MAPKK1/2 inhibitor PD098059. Phase-contrast pictures of a colony of MDCK cells are shown before the addition of HGF/SF (a) or at 16 h (b) after addition of HGF/SF. Cells in c were preincubated with 50 μM PD098059 before addition of HGF/SF for 16 h. The bar in c represents 50 μm and applies also to a and b.
Ras is required for HGF/SF-induced motile responses (Hartmann et al., 1994; Ridley et al., 1995) and is known to activate the p42/p44 MAPK cascade (Marshall, 1996). To determine whether inhibiting the p42/p44 MAPK cascade affected the motility response to HGF/SF, cells were treated with the synthetic compound PD098059, which has been shown to inhibit growth factor-induced activation of MAPKK1/2 in various cell types and thus to inhibit p42/p44 MAPK activation (Alessi et al., 1995; Dudley et al., 1995; Pang et al., 1995). Pretreatment of cells with PD098059 at 50 μM (IC50 for MAPKK1/2; Alessi et al., 1995) for 1 h before addition of HGF/SF completely blocked scattering (Figure 1c).
Time-lapse video microscopy of cells treated with PD098059 confirmed that the motile response to HGF/SF was inhibited and that cells did not spread or scatter (our unpublished results). The inhibitor was not toxic to MDCK cells for at least 48 h after addition, and its effects were fully reversible. When cells were washed free of PD098059 and fresh HGF/SF was added, they scattered completely, and scattering was induced earlier than usual (after 2 h) (our unpublished results). These observations suggest that p42/p44 MAPK activation is required for a key step of the motility response to HGF/SF.
HGF/SF-induced Disruption of Adherens Junctions Is Inhibited by PD098059 or by the PI 3-Kinase Inhibitor LY294002
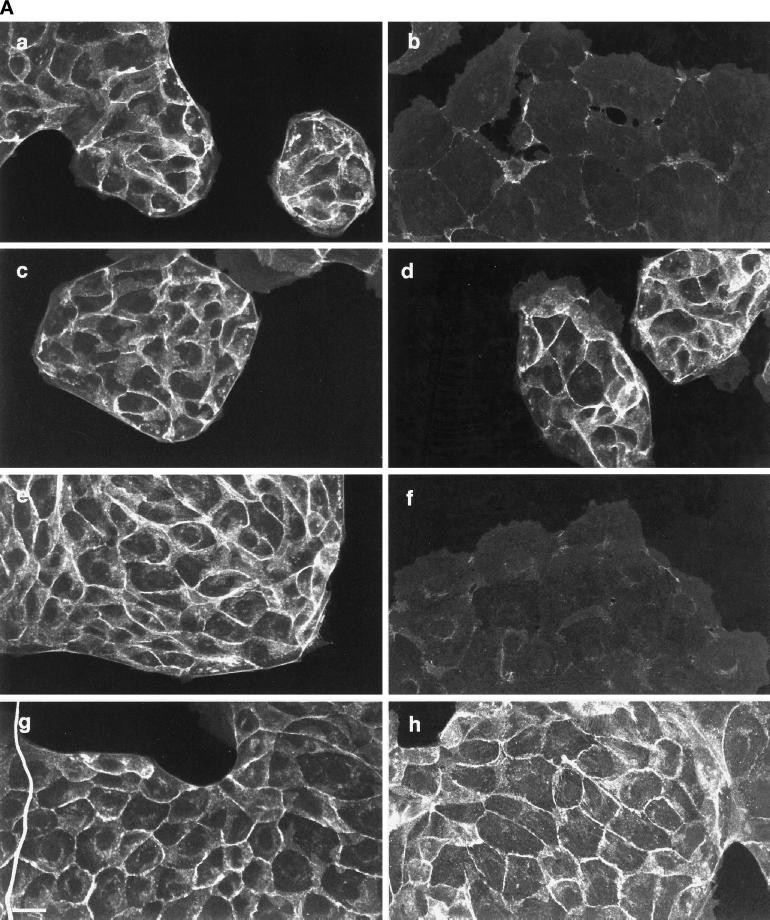
The images in Figure 1 show that loss of cell–cell contact induced by HGF/SF is prevented by PD098059. Cell–cell contact in MDCK cells is maintained via tight junctions, desmosomes, and adherens junctions. To determine whether p42/p44 MAPK activation plays a specific role in the disruption of any of these intercellular junctions, the localization of adherens junction proteins was investigated initially, because these proteins have been implicated in several signal transduction processes (reviewed in Barth et al., 1997). Immunofluorescence staining showed that the adherens junction components β-catenin and E-cadherin localized to intercellular junctions in unstimulated MDCK cells (Figure 2A, a and e). When cells were incubated with HGF/SF, however, β-catenin and E-cadherin started to disperse within 2 h and were completely lost from intercellular contacts by 4 h (Figure 2A, b and f). In many cases cell–cell contact was maintained despite the loss of β-catenin and E-cadherin, suggesting that other junctions were still present (see below). When cells were pretreated for 1 h with 50 μM PD098059 before addition of HGF/SF for 4 h (Figure 2A) or 16 h (our unpublished results), the HGF/SF-induced dispersal of both β-catenin and E-cadherin was prevented (Figure 2A, c and g).
Figure 2.
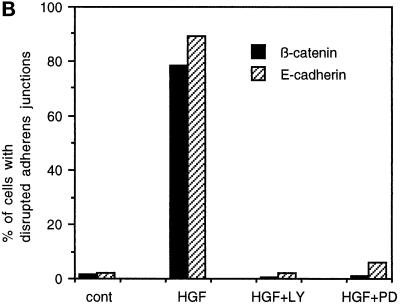
Disruption of adherens junctions by HGF/SF is blocked by the MAPKK1/2 inhibitor PD098059 and the PI 3-kinase inhibitor LY294002. (A) MDCK cells were stimulated with HGF/SF for 4 h (b and f) or were preincubated with 50 μM PD098059 (c and g) or 20 μM LY294002 (d and h) before addition of HGF/SF for 4 h. MDCK cells were then fixed and stained for β-catenin (a–d) and E-cadherin (e–h) localization. Bar, 10 μm. (B) MDCK cells were treated as indicated with 20 μM LY294002 (LY) or 50 μM PD098059 (PD) before addition of HGF/SF (HGF) for 4 h. Disrupted junctions in control cells (cont) and stimulated cells were defined as junctions where no E-cadherin and β-catenin could be detected at the sites of intercellular contact and were subsequently counted. The numbers represent the mean of 300 cells, which were counted in three independent experiments.
Another downstream target of Ras and the Met receptor is PI 3-kinase (Ponzetto et al., 1993; Marshall, 1996). Inhibition of PI 3-kinase using the fungal metabolite wortmannin has previously been shown to reduce HGF/SF-induced scattering (Royal and Park, 1995). We investigated the role of PI 3-kinase in HGF/SF-induced disruption of adherens junctions using LY294002, a synthetic compound that blocks PI 3-kinase specifically (IC50 = 1.4 μM) without affecting the activity of protein serine/threonine kinases, protein tyrosine kinases, and lipid kinases when used at concentrations of up to 50 μM (Vlahos et al., 1994). When cells were preincubated for 1 h with the PI 3-kinase inhibitor LY294002 at 20 μM, β-catenin- and E-cadherin-containing junctions remained intact even 4 h after addition of HGF/SF (Figure 2A, d and h). Actin filament staining revealed that lamellipodium extension was also inhibited by LY294002 (our unpublished results), in agreement with previous studies in other cell types showing that PI 3-kinase is required for lamellipodium formation and membrane ruffling (Parker, 1995; Vanhaesebroeck et al., 1997).
The effects of PD098059 and LY294002 on adherens junctions were quantitated by scoring cells for β-catenin and E-cadherin staining at cell–cell contacts (Figure 2B). Only 2% of control MDCK cells lacked detectable β-catenin or E-cadherin staining at sites of cell interaction. However, when cells were treated with HGF/SF for 4 h, 78% of the cells showed disrupted adherens junctions when stained for β-catenin, and 89% of the cells had lost E-cadherin from intercellular contacts. Preincubation of the cells with LY294002 or PD098059 completely inhibited the loss of β-catenin and E-cadherin from junctions.
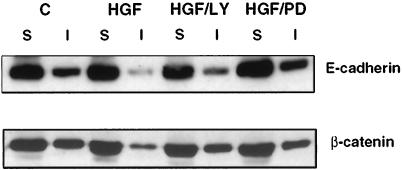
The HGF/SF-induced loss of E-cadherin and β-catenin from intercellular junctions could be a result of degradation of the proteins and/or their relocalization away from the junctions. Fractionation of MDCK cell lysates into NP-40-soluble and -insoluble fractions showed that HGF/SF induced a decrease in the amount of E-cadherin and β-catenin in the insoluble fraction, but that the overall level of each protein was unchanged (Figure 3). These results indicate that the HGF/SF-induced loss of adherens junctions observed by immunocytochemical staining correlates with a decrease in the association of E-cadherin and β-catenin with the detergent-insoluble fraction, which is presumed to contain cytoskeletally associated protein complexes. A small proportion of NP-40-insoluble β-catenin and E-cadherin remained after the addition of HGF/SF, which presumably reflects some association of these proteins with the actin cytoskeleton even when adherens junctions are lost. A similar proportion of Triton X-100-insoluble E-cadherin was observed in MDCK cells maintained in low Ca2+ to prevent adherens junction formation (Shore and Nelson, 1991). PD098059 and LY294002 inhibited the redistribution of E-cadherin and β-catenin to the soluble fraction (Figure 3). From these results we conclude that both p42/p44 MAPK and PI 3-kinase are required for the disassembly of adherens junctions during HGF/SF-induced cell spreading.
Figure 3.
E-cadherin and β-catenin are redistributed from the NP-40-insoluble fraction to the soluble fraction in response to HGF/SF. Cell lysates of unstimulated control cells (C) or cells incubated with HGF/SF for 4 h (HGF) or cells treated with 20 μM LY 294002 for 1 h before addition of HGF/SF (HGF/LY) or 50 μM PD98059 (HGF/PD) were fractionated into a NP-40-soluble (S) and NP-40-insoluble (I) fraction. Equal sample volumes were loaded and probed for E-cadherin (top blot) and β-catenin (bottom blot).
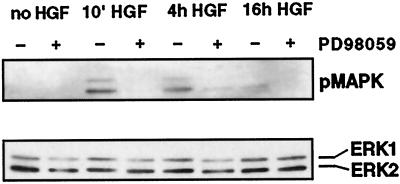
HGF/SF Induces Long-Term Activation of p42/p44 MAPK
It has been shown that HGF/SF rapidly activates the p42/p44 MAPK cascade in A549 cells (Ponzetto et al., 1994), but the response in MDCK cells has not been reported, nor has the length of time for which p42/p44 MAPK remains activated after HGF/SF stimulation been investigated. To determine the time frame within which p42/p44 MAPK is activated and how this relates to the spreading and scattering phases of the motile response induced by HGF/SF, and to confirm that the effects of PD098059 on disruption of adherens junctions and scattering correlate with an inhibition of p42/p44 MAPK activation, MDCK cells were incubated with HGF/SF, with or without preincubation with PD098059, for differing lengths of time.
Western blotting of MDCK cell lysates with an antibody recognizing only the activated, phosphorylated form of p42/p44 MAPK revealed that HGF/SF activates MAPK within 10 min and that this activation is maintained for at least 4 h (Figure 4, top blot). At 16 h there was still a low level of phosphorylated p42/p44 MAPK detectable, but this was not always observed in different experiments. Activation of p42/p44 MAPK was inhibited at all time points by preincubating the cells for 1 h with PD098059 (Figure 4, top blot). The inhibitory effects of PD098059 on HGF/SF-induced responses therefore correlate with its action in preventing p42/p44 MAPK activation. The Western blot was reprobed with an antibody recognizing total p42/p44 MAPK (ERK1/2) to confirm that similar levels of p42/p44 MAPK were present in all lanes (Figure 4, bottom blot). p42/p44 MAPK is therefore active throughout the cell-spreading stage of the HGF/SF-induced motility response, consistent with it playing an active role in inducing adherens junction disassembly. This prolonged activation of p42/p44 MAPK by HGF/SF contrasts with the rapid time course of activation and inactivation induced by many growth factors (Marshall, 1996).
Figure 4.
HGF/SF induces sustained activation of p42/p44 MAPK. Cells were stimulated with HGF/SF (HGF) for 10 min or 4 or 16 h with (+) or without (−) preincubation with 50 μM PD098059. The cells were subsequently lysed, and the lysates were electrophoresed on an SDS-gel. The top panel shows Western blotting for the activated, phosphorylated form of p42/p44 MAPK (pMAPK). The bottom panel shows the blot reprobed with an anti-ERK1 antibody against total p42/p44 MAPK (ERK1/ERK2).
Dominant Negative Mutants of MAPKK1 Inhibit HGF/SF-induced Loss of Adherens Junctions
The data obtained with the MAPKK1/2 inhibitor PD098059 suggest that p42/p44 MAPK is involved in mediating the loss of adherens junctions induced by HGF/SF. Further evidence for this role of p42/p44 MAPK was obtained by expressing two dominant negative versions of MAPKK1 in MDCK cells: a kinase dead mutant, AFG MAPKK1 (in which asparagine is substituted by alanine in the DFG motif to prevent Mg2+ binding), and a mutant that cannot be phosphorylated by Raf (Ala-221 MAPKK1, in which serine 221 is substituted with alanine; see Cowley et al., 1994). Expression vectors encoding these proteins were microinjected into cells, which were then incubated for 6 h to allow expression of the proteins and subsequently treated with HGF/SF for 2 h (Figure 5, a and b) or 16 h (our unpublished results). The AFG MAPKK1 (Figure 5) and the Ala-221 MAPKK1 (our unpublished results) mutants prevented cells from scattering and inhibited the loss of β-catenin (Figure 5a; the arrow indicates β-catenin at intercellular junctions of injected cells, and the arrowhead points to a disrupted junction in uninjected cells) and E-cadherin (our unpublished results) from adherens junctions. Microinjection of an expression vector encoding wild-type MAPKK1 did not inhibit the HGF/SF-induced disruption of junctions, indicating that the inhibitory effect of dominant negative MAPKK1s is not a nonspecific consequence of overexpressing MAPKK1 (our unpublished results). Together, these results indicate that activation of p42/p44 MAPK by HGF/SF contributes to cell motility at least in part by mediating the disruption of adherens junctions.
Figure 5.
Dominant negative mutants of MAPKK1 block disruption of adherens junctions. MDCK cells were microinjected with a construct expressing a dominant negative mutant of MAPKK1, AFG MAPKK1 (100 ng/μl). After microinjection cells were incubated for 6 h before addition of HGF/SF for 2 h. Cells were stained for β-catenin (a) and for expression of the mutant MAPKK1 using the rabbit anti-MAPKK1 antibody (b). The arrow in a shows β-catenin localizing to adherens junctions in injected cells, and the arrowhead indicates disrupted adherens junctions in uninjected cells. Bar, 10 μm.
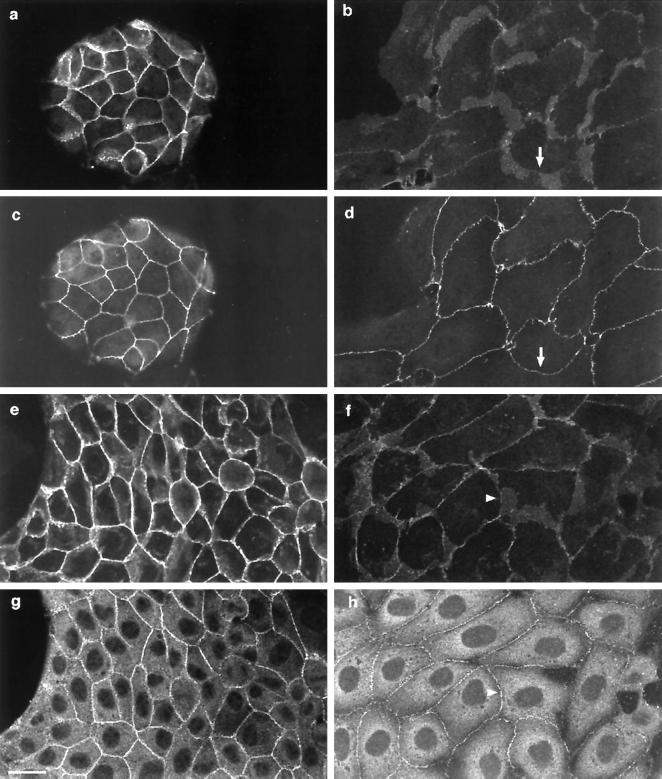
HGF/SF-induced Dispersal of Adherens Junctions Precedes Loss of Tight Junctions and Desmosomes
In addition to adherens junctions containing β-catenin and E-cadherin, tight junctions and desmosomes have to be disrupted for epithelial cells to detach from each other. To determine whether the loss of these three types of junctions is coordinately regulated during the HGF/SF response, we investigated the localization of the tight junction proteins ZO-1 and occludin and the desmosomal component desmoplakin at different time points after addition of HGF/SF.
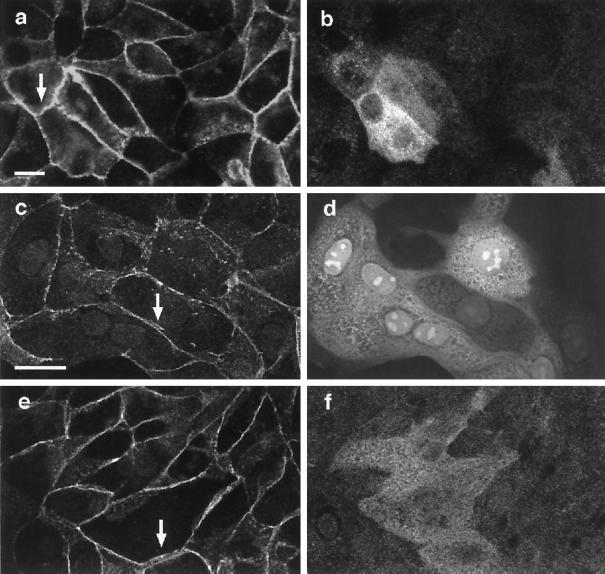
As previously reported, ZO-1 localized to regions of intercellular contact in MDCK cells (Figure 6c) (Anderson et al., 1988; Siliciano and Goodenough, 1988; Balda et al., 1996). Desmoplakin was localized to cell–cell boundaries in a punctate manner as previously described (Figure 6g) (Penn et al., 1987). When cells were treated with HGF/SF for 4 h (Figure 6, b, d, f, and h), ZO-1 (Figure 6d) and desmoplakin (Figure 6h) still localized to areas of cell–cell contact, whereas β-catenin levels were very low or not detectable in these same cell–cell contacts (Figure 6, b and f). Even 16 h after HGF/SF addition, ZO-1 and desmoplakin were still present at many sites of cell–cell contact, but β-catenin was only rarely detected (our unpublished results). Similar results were observed with occludin (our unpublished results). The dispersal of adherens junction proteins therefore occurs considerably earlier than the disruption of desmosomes and tight junctions, suggesting that the stability of these latter junctions is regulated differently than adherens junctions. It could nevertheless be possible that a reorganization of tight junctions and/or desmosomes occurs earlier but is not detectable by immunofluorescence staining and may be required for their final disruption.
Figure 6.
Adherens junctions are disrupted before tight and desmosomal junctions in response to HGF/SF. Unstimulated MDCK cells were costained for β-catenin (a) and ZO-1 (c) or for β-catenin (e) and desmoplakin (g). Costaining of cells treated with HGF/SF for 4 h with β-catenin (b) and ZO-1 (d) or with β-catenin (f) and desmoplakin (h) indicates that β-catenin is lost from intercellular junctions (e.g., arrow in b and arrowhead in f) where ZO-1 (d, arrow) and desmoplakin (h, arrowhead) are still present. Bar, 10 μm.
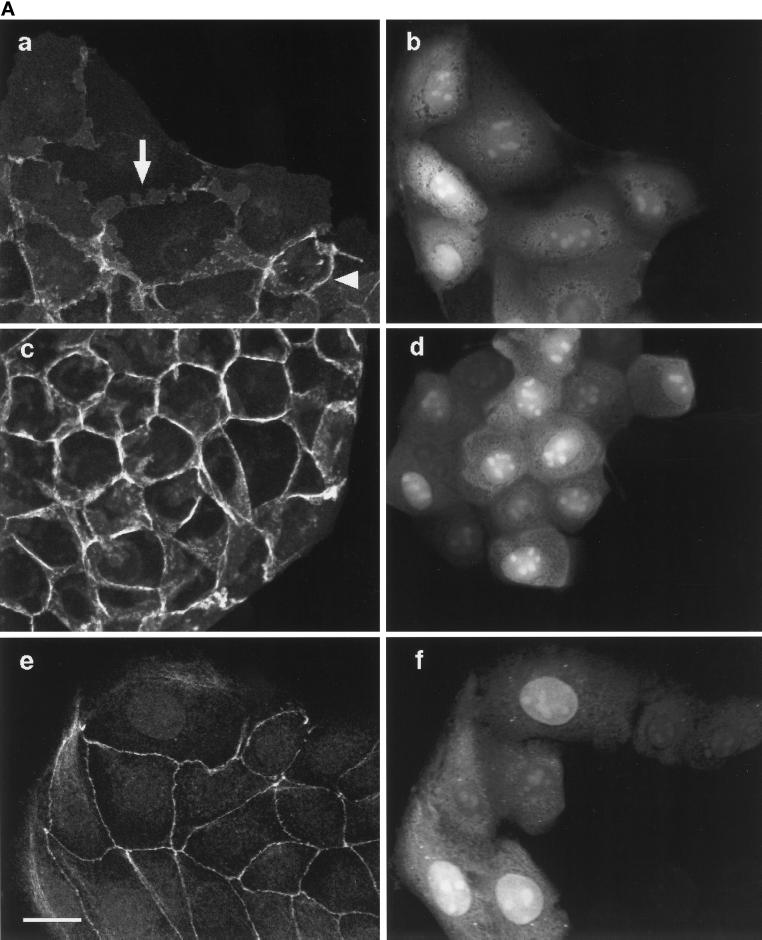
Ras Induces Disruption of Adherens Junctions but Not Tight Junctions or Desmosomes
Our results show that two targets of Ras signaling, the p42/p44 MAPK cascade and PI 3-kinase, are required for adherens junction disassembly. We therefore investigated whether Ras alone could induce changes in intercellular junctions. Constitutively active V12Ras protein disrupted adherens junctions by 1 h after microinjection, as monitored with β-catenin (Figure 7, A, a, arrow, and B) and E-cadherin (Figure 7B) staining. Quantification of these responses showed that V12Ras and HGF/SF disrupted adherens junctions to a similar extent (compare Figures 2B and 7B). The loss of E-cadherin and β-catenin from junctions was blocked when cells were pretreated with 50 μM PD098059 1 h before microinjection (Figure 7, A, c, and B) or 20 μM LY294002 (Figure 7B). Similarly, microinjection of expression vectors encoding the dominant negative MAPKK1 mutants AFG MAPKK1 and Ala-221 MAPKK1 inhibited the Ras-induced disruption of adherens junctions observed after microinjection of an expression vector encoding V12Ras (pCDNA3-V12H-Ras) (our unpublished results). These results show that V12Ras signaling is sufficient to induce adherens junction disassembly and that, as with HGF/SF, this is dependent on both p42/p44 MAPK and PI 3-kinase.
Figure 7.
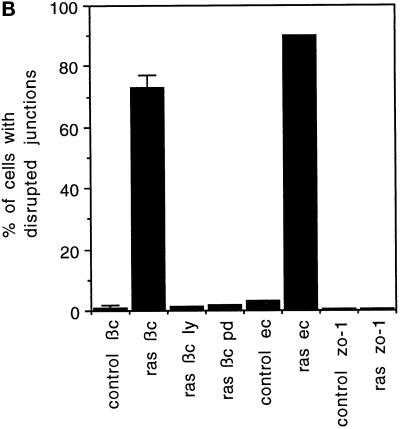
Ras disrupts adherens junctions through p42/p44 MAPK and PI 3-kinase but does not disrupt desmosomes or tight junctions. (A) Cells were microinjected with V12Ras (35 ng/μl), fixed after 1 h, and stained for β-catenin (a and c) or ZO-1 (e). Microinjected cells were detected by coinjection of TRITC–dextran (b, d, and f). The arrow in a shows the dispersion of β-catenin in injected cells, and the arrowhead indicates the localization of β-catenin at adherens junctions in uninjected cells. Cells were preincubated with 50 μM PD098059 in c and d before microinjection. Bar, 10 μm. (B) Cells microinjected with V12Ras (ras) were preincubated with or without LY294002 (LY) or PD098059 (PD) for 1 h before injection, as indicated. Cells were fixed 1 h after injection and stained for β-catenin (bc) and E-cadherin (ec) or incubated for 16 h and stained for ZO-1 (zo-1). The numbers represent the mean of at least three independent experiments (the error bars represent the mean of 5 experiments) where 100 cells were injected in each experiment or where 100 uninjected cells (control) were counted.
In contrast to its effects on adherens junctions, Ras did not detectably disrupt tight junctions or desmosomes. ZO-1 was detected in nearly all cell–cell contacts in uninjected cells, and at different time points after V12Ras injection (2, 4, 6, 8, and 16 h), the localization of ZO-1 was unaltered (Figure 7, shown for 8 h in A, e, and 16 h in B). Similarly, the localization of desmoplakin was unchanged at these time points (our unpublished results). The V12Ras protein was still active up to 16 h after injection, as determined by the presence of lamellipodia on injected cells stained for actin filaments (our unpublished results) (Ridley et al., 1995). We therefore conclude that Ras induces disruption of adherens junctions but not tight junctions and desmosomes, and therefore that the latter junctions are regulated by a different signaling pathway not activated by Ras.
Ras and Rac Are Required for the HGF/SF-induced Breakdown of Adherens Junctions
The above experiments show that constitutively active Ras can activate signaling pathways leading to the disassembly of adherens junctions. To determine whether Ras is normally required for HGF/SF-induced loss of adherens junctions, a dominant negative Ras protein, N17Ras, was expressed in MDCK cells. Dispersal of β-catenin (Figure 8) and E-cadherin (our unpublished results) induced by HGF/SF was inhibited by microinjection of an expression vector encoding N17Ras (Figure 8a, arrowhead indicating β-catenin in injected cells).
Figure 8.
Ras and Rac mediate the disruption of adherens junctions. In a, cells were microinjected with a construct expressing N17Ras (100 ng/μl). After microinjection cells were incubated for 6 h before addition of HGF/SF for 2 h and stained for β-catenin. In c, cells were microinjected with N17Rac1 protein (150 ng/μl) and incubated for 2 h with HGF/SF before staining for β-catenin. Cells were coinjected with V12Ras (100 ng/μl) and N17Rac1 (100 ng/μl), incubated for 2 h, and subsequently stained for β-catenin in e. The arrowheads show β-catenin localization at intercellular junctions in injected cells, and the arrows indicate dispersed adherens junctions in uninjected cells (a and c). Microinjected cells were detected by either staining with a rat anti-Ras antibody (b) or coinjection of TRITC–dextran (d and f). Bar, 10 μm.
Because Ras-induced spreading and actin reorganization is dependent on Rac (Ridley et al., 1995), the possibility that Rac also acts downstream of Ras to mediate adherens junction disassembly was investigated. In cells injected with a dominant negative version of Rac, N17Rac1 (Ridley et al., 1992), the redistribution of β-catenin induced by HGF/SF was prevented (Figure 8c, arrowhead indicating β-catenin in injected cell). The Ras-mediated disruption of adherens junctions was also dependent on Rac, because in cells coinjected with V12Ras and N17Rac1 proteins, Ras-induced dispersal of β-catenin was completely blocked (Figure 8e). These data indicate that the breakdown of adherens junctions induced by HGF/SF is dependent on Ras acting upstream of Rac.
Activation of PI 3-Kinase or p42/p44 MAPK Is Not Sufficient to Induce Disruption of Adherens Junctions
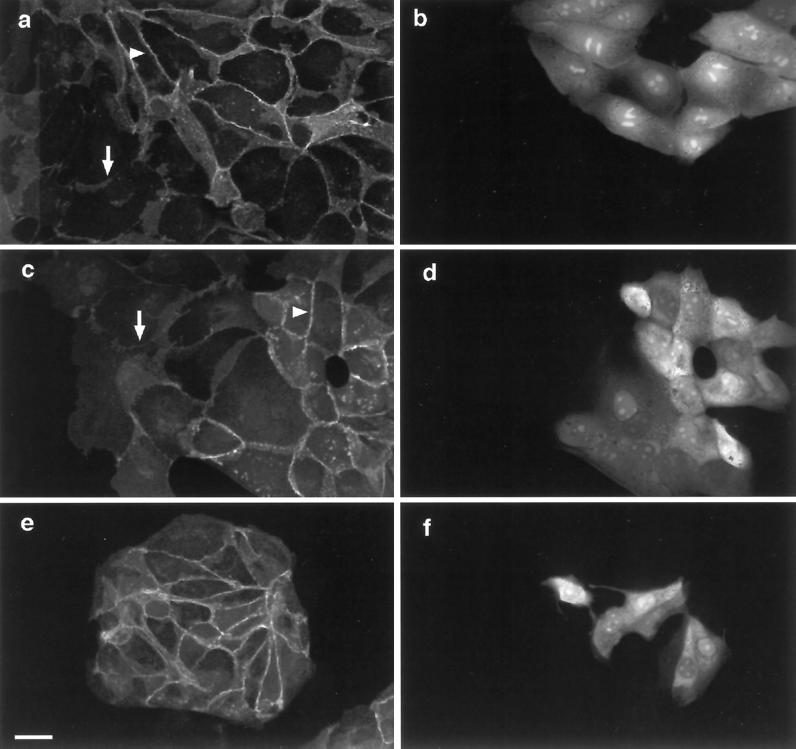
Because inhibition of either PI 3-kinase or p42/p44 MAPK activation prevents HGF/SF-induced disruption of adherens junctions (Figure 2A), the possibility that activation of either PI 3-kinase or p42/p44 MAPK alone or in combination could induce adherens junction disassembly was investigated. To test the effect of PI 3-kinase, MDCK cells were injected with a plasmid encoding the p110α catalytic subunit of PI 3-kinase (pSG5-5-Myc-p110α-WT-3-K-Ras, which is targeted to the plasma membrane by virtue of the addition of a carboxyl-terminal membrane localization sequence from K-Ras). Unlike V12Ras, activated 110α did not induce dispersal of β-catenin (Figure 9a) or E-cadherin (our unpublished results) from intercellular junctions. In fact, β-catenin levels at intercellular junctions appeared slightly increased in injected cells relative to uninjected cells. Similarly, constitutively active V12Rac1 protein enhanced the levels of β-catenin and E-cadherin at intercellular junctions (our unpublished results), as previously reported (Hordijk et al., 1997; Takaishi et al., 1997).
Figure 9.
Expression of membrane-targeted p110α and Raf does not disrupt adherens junctions. Cells were microinjected with plasmids encoding membrane-targeted p110α (pSG5-5-Myc-p110α-WT-3-K-Ras; 100 ng/μl; (a and b), Raf (pEFHm-RAFCAAX-Myc; c and d), or p110α and Raf (e and f), incubated for 4 h, and then stained for β-catenin (a, c, and e) and for expression of p110α with a rabbit anti-p110α antibody (b and f). Cells injected with the Raf plasmid were visualized by coinjection of TRITC–dextran (d). Control experiments showed that in cells coinjected with TRITC–dextran and the Raf plasmid, exogenous Raf (myc tagged) was expressed in 100% of injected cells. The arrows indicate the presence of β-catenin at adherens junctions of injected cells. Bar, 10 μm.
To test the effect of activating p42/p44 MAPK on adherens junctions, expression vectors encoding activated Raf (pEFHm-RAFCAAX-Myc) or activated MAPKK1 (pEXV3-Glu-217/Glu-221 MAPKK1) were injected into MDCK cells. Both of these are known to induce activation of p42/p44 MAPK (Cowley et al., 1994; Leevers et al., 1994). Neither RafCAAX (Figure 9) nor activated MAPKK1 (our unpublished results) induced disruption of adherens junctions, either injected alone (Figure 9, c and d) or together with p110α (Figure 9, e and f). Taken together, our results suggest that PI 3-kinase, Rac, and p42/p44 MAPK activation are necessary but not sufficient to induce adherens junction disassembly, and that there may be still another signal downstream of Ras required for this response. Alternatively, it may be that the precise time course of MAPK and PI 3-kinase activation by HGF/SF is important for their effect on adherens junctions and that this time course is not mimicked by comicroinjection of the expression constructs for RafCAAX and p110α.
DISCUSSION
HGF/SF stimulates the motility of MDCK epithelial cells (Stoker et al., 1987; Gherardi et al., 1989), and this response is dependent on Ras (Hartmann et al., 1994; Ridley et al., 1995). The motile response is biphasic: cells initially extend lamellipodia and spread but remain attached to each other in colonies and then subsequently detach from each other and move independently. We have found that adherens junctions are disassembled during HGF/SF- and Ras-induced cell spreading before the loss of cell–cell interactions. Desmosomal and tight junction components remain associated with intercellular junctions during spreading, and therefore cell–cell contact is maintained through these junctions after adherens junctions have been disassembled. Both HGF/SF and Ras are known to activate p42/p44 MAPK and PI 3-kinase (Graziani et al., 1991; Ponzetto et al., 1993, 1994; Avruch et al., 1994; Rodriguez-Viciana et al., 1994), and we have shown that p42/p44 MAPK activation is required for HGF/SF-induced motility and is specifically involved in the loss of adherens junctions. PI 3-kinase has previously been implicated in HGF/SF-induced scattering, although the stage at which it acts was not established (Royal and Park, 1995). We have now demonstrated that it, like p42/p44 MAPK, is required for adherens junction disassembly. At least two signals downstream of Ras are therefore required for disruption of adherens junctions and cell spreading. However, activated Ras induces loss of adherens junctions and lamellipodium extension, but this is not sufficient for cells to detach from each other and scatter; therefore another signal must be required for the breakdown of desmosomes and tight junctions and/or for increased motility of cells.
HGF/SF- and Ras-induced disruption of adherens junctions was also dependent on Rac, which we have previously shown is required for HGF/SF-induced lamellipodium extension (Ridley et al., 1995). This implies that either the initial Rac-dependent extension of lamellipodia is an essential prerequisite for the loss of adherens junctions, or that Rac acts directly to destabilize these junctions. In contrast to this involvement of Rac in the HGF/SF response, constitutively activated Rac by itself does not induce lamellipodium formation in MDCK cells but in fact induces the accumulation of F-actin at intercellular junctions and enhances the formation of adherens junctions (Ridley et al., 1995; Hordijk et al., 1997; Takaishi et al., 1997). In addition, Rac was shown to be required for the formation of cadherin-dependent junctions in keratinocytes (Braga et al., 1997). However, this effect of Rac can be inhibited by Ras signaling, because coinjection of activated Ras with activated Rac prevented the stabilization of adherens junctions by Rac and led to junctional disassembly (our unpublished data). One possible explanation for these observations is that Ras induces the modification of one or more adherens junction components such that the junctions are no longer stable, and that under these conditions it is not possible for Rac to signal enhanced junctional assembly. Presumably the end result of the opposing actions of Ras and Rac on adherens junctions depends on the relative level of signaling from each protein, because, for example, activated Rac can induce junctional stabilization in Ras-transformed MDCK cell lines (Hordijk et al., 1997). In this model, when the appropriate Ras signal, perhaps p42/p44 MAPK activation, is strong enough, junctions disassemble, and then Rac activity is diverted to stimulate lamellipodium formation.
Unlike Ras, expression of an activated catalytic subunit of PI 3-kinase, p110α, did not induce adherens junction disassembly but instead induced an accumulation of adherens junction components at intercellular junctions similar to that previously reported for V12Rac1 (Hordijk et al., 1997; Takaishi et al., 1997). This is consistent with a model in which PI 3-kinase is an upstream regulator of Rac (Wennström et al., 1994; Hawkins et al., 1995; Rodriguez-Viciana et al., 1997; Han et al., 1998). These results suggest that PI 3-kinase activation downstream of Ras is not by itself sufficient to induce adherens junction disassembly, and imply that the function of PI 3-kinase activation in the motility response to HGF/SF is to activate Rac. Similarly, constitutively active forms of Raf or MAPKK1, which induce p42/p44 MAPK activation, are not able to induce loss of adherens junctions. A combination of activated p110α and Raf is also unable to mimic the effect of activated Ras, suggesting that another signal downstream of Ras is required in addition to PI 3-kinase and Raf. Alternatively, it is possible that the precise time course of HGF/SF-induced PI 3-kinase and Raf activation is important to achieve disruption of adherens junctions, and that this is not mimicked by coinjected the two expression vectors.
How do p42/p44 MAPK and PI 3-kinase act to induce disassembly of adherens junctions? One possibility is that they induce changes in the phosphorylation state of adherens junction components. It has been shown that v-Src induces tyrosine phosphorylation of the adherens junction proteins β-catenin and E-cadherin and that this correlates with disruption of adherens junctions in MDCK cells (Behrens et al., 1993). However, tyrosine phosphorylation of β-catenin is not required for the weakening of cadherin-based cell adhesions induced by v-Src (Takeda et al., 1995), and the authors suggest that other components of intercellular junctions, such as ZO-1 or ezrin/radixin/moesin, may be more relevant targets for v-Src. We did not observe any increase in the tyrosine phosphorylation of either E-cadherin or β-catenin >4 h after HGF/SF addition (our unpublished data), and it is therefore unlikely that the disruption of adherens junctions by HGF/SF and Ras is regulated by the level of tyrosine phosphorylation of these proteins. It is quite possible, however, that p42/p44 MAPK acts by phosphorylating junctional proteins on serine/threonine residues. In addition, PI 3-kinase induces activation of the serine/threonine kinase Akt (Hemmings, 1997), and this or other downstream kinases could similarly modify proteins in adherens junctions. Little is known of the potential serine/threonine phosphorylation of adherens junction components, and it will therefore be important to investigate this in future studies on adherens junction stability.
The breakdown of desmosomal and tight junctions induced by HGF/SF occurs well after the disruption of adherens junctions (this paper; also see Stoker and Perryman, 1985) and is apparently regulated independently, because it is not induced by activated Ras. Previous experiments have suggested that loss of adherens junctions leads to disruption of desmosomes and tight junctions, because MDCK cells dissociate after addition of antibodies to the extracellular domain of E-cadherin (Takeichi, 1991). It is possible that in our experiments a very low level of adherens junctions is retained and that this is sufficient to maintain the integrity of desmosomes and tight junctions. Alternatively, the E-cadherin antibodies could be mimicking E-cadherin ligands and activating signaling pathways that lead to loss of the other junctions, as well as acting passively to prevent E-cadherin homotypic interactions. In this case, our results imply that these signals are not activated by Ras. However, desmosomes and tight junctions clearly have to be disrupted during scattering, and thus in addition to adherens junctions they are likely to be a major target of HGF/SF-initiated signals leading to cell–cell detachment. The time delay of 4–6 h between addition of HGF/SF and loss of cell contacts suggests that new gene expression could be involved. However, use of general inhibitors such as cycloheximide (which blocks protein synthesis) has not been informative, because the effects of cycloheximide on cell morphology suggest that synthesis of a labile protein is continuously required to maintain HGF/SF-induced actin reorganization (Ridley et al., 1995). Identification of specific genes whose expression is altered by HGF/SF is likely to be more informative in analyzing regulation of the scattering response. One candidate gene is Slug, which encodes a zinc finger protein, is up-regulated at the transcriptional level by fibroblast growth factor 1, and is required for fibroblast growth factor-1- and HGF/SF-induced loss of cell junctions in a bladder carcinoma cell line (Savagner et al., 1997). The prolonged activation of p42/p44 MAPK by HGF/SF we observe in MDCK cells could be important in mediating changes in gene transcription (Marshall, 1996), and it will therefore be of interest to investigate whether p42/p44 MAPK regulates the transcription of genes such as Slug, which may be involved in long-term responses to HGF/SF.
In conclusion, we have demonstrated that activation of p42/p44 MAPK, PI 3-kinase and Rac downstream of Ras is required for HGF/SF-induced disruption of adherens junctions. Loss of adherens junctions is essential for the motile response to HGF/SF and occurs during the spreading response before cell–cell detachment. Components of the p42/p44 MAPK cascade, PI 3-kinase, and its downstream targets are therefore candidate targets for the design of therapies aimed at preventing the migration of epithelial cells in vivo, for example during carcinoma invasion. These signaling pathways are also important for cell proliferation, and thus inhibiting them may have a dual impact in preventing both proliferation and migration of cancer cells.
ACKNOWLEDGMENTS
We are grateful to Ritu Garg for excellent cell culture assistance and to Alan Entwistle and Mike O’ Hare for help with image generation. We thank Jonathan Backer, Julian Downward, Richard Marais, and Chris Marshall for gifts of plasmids and Chris Marshall, Roya Hooshmand-Rad, and Tony Magee for providing antibodies. In addition, we thank Sally Leevers and Bart Vanhaesebroeck for helpful discussions.
Abbreviations used:
- ERK
extracellular signal-regulated kinase
- HGF/SF
hepatocyte growth factor/scatter factor
- MAPK
MAP kinase
- MAPKK
MAP kinase kinase
- MDCK
Madin–Darby canine kidney
- PI 3-kinase
phosphatidylinositide 3-kinase
- TBS
Tris-buffered saline
REFERENCES
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Saito Y, Campbell DG, Cohen P, Sithanandam G, Rapp U, Ashworth A, Marshall CJ, Cowley SJ. Identification of the sites of MAP kinase kinase-1 phosphorylated by p74raf-1. EMBO J. 1994;13:1610–1619. doi: 10.1002/j.1460-2075.1994.tb06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JM, Stevenson BR, Jesaitis LA, Goodenough DA, Mooseker MS. Characterization of ZO-1, a protein component of tight junction from mouse liver and Madin-Darby canine kidney cells. J Cell Biol. 1988;106:1141–1149. doi: 10.1083/jcb.106.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avruch J, Zhang X-F, Kyriakis JM. Raf meets Ras: completing the framework of a signal transduction pathway. Trends Biochem Sci. 1994;19:279–283. doi: 10.1016/0968-0004(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Balda MS, Whitney JA, Flores C, Gonzáles S, Cereijdo M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth AIM, Näthke IS, Nelson WJ. Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol. 1997;9:683–690. doi: 10.1016/s0955-0674(97)80122-6. [DOI] [PubMed] [Google Scholar]
- Behrens J, Vakaet L, Friis R, Winterhager E, van Roy F, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellusci S, Moens G, Gaudino G, Comoglio P, Nakamura T, Thierry JP, Jouanneau J. Creation of an hepatocyte growth factor/scatter factor autocrine loop in carcinoma cells induces invasive properties associated with increased tumorgenicity. Oncogene. 1994;9:1091–1099. [PubMed] [Google Scholar]
- Ben-Ze’ev A. Cytoskeletal and adhesion proteins as tumor suppressors. Curr Opin Cell Biol. 1997;9:99–108. doi: 10.1016/s0955-0674(97)80158-5. [DOI] [PubMed] [Google Scholar]
- Bottaro DP, Rubin JS, Faletto DL, Chan AML, Kmiecik TE, Vande Woude GF, Aaronson SA. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- Braga VMM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of the MAP kinase kinase is necessary and sufficient for PC12 differentiation and transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Dowrick PG, Prescott AR, Warn RM. Scatter factor affects major changes in the cytoskeletal organization of epithelial cells. Cytokine. 1991;3:299–310. doi: 10.1016/1043-4666(91)90498-3. [DOI] [PubMed] [Google Scholar]
- Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardi E, Gray J, Stoker M, Perryman M, Furlong R. Purification of scatter factor, a fibroblast-derived basic protein that modulates epithelial interactions and movement. Proc Natl Acad Sci USA. 1989;86:5844–5848. doi: 10.1073/pnas.86.15.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziani A, Gramaglia D, Cantley LC, Comoglio PM. The tyrosine-phosphorylated hepatocyte growth factor/scatter factor receptor associates with phosphatidylinositol 3-kinase. J Biol Chem. 1991;266:22087–22090. [PubMed] [Google Scholar]
- Graziani A, Gramaglia D, dalla Zonca P, Comoglio PM. Hepatocyte growth factor/scatter factor stimulates the ras-guanine nucleotide exchanger. J Biol Chem. 1993;268:9165–9168. [PubMed] [Google Scholar]
- Gumbiner B. Breaking through the tight junction barrier. J Cell Biol. 1993;123:1631–1633. doi: 10.1083/jcb.123.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- Hall A, Self AJ. The effect of Mg2+ on the guanine nucleotide exchange rate of p21N-ras. J Biol Chem. 1986;261:10963–10965. [PubMed] [Google Scholar]
- Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller RD, Krishna UM, Falck JR, White MA, Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- Hartmann G, Weidner KM, Schwarz H, Birchmeier W. The motility signal of scatter factor/hepatocyte growth factor mediated through the receptor tyrosine kinase met requires intracellular action of ras. J Cell Biol. 1994;269:21936–21939. [PubMed] [Google Scholar]
- Hawkins PT, et al. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- Hemmings BA. PtdIns(3,4,5)P3 gets its message across. Science. 1997;277:534. doi: 10.1126/science.277.5325.534. [DOI] [PubMed] [Google Scholar]
- Hordijk PL, ten Kloster JP, van der Kammen RA, Michiels F, Oomen LCJM, Collard JG. Inhibition of invasion of epithelial cells by tiam1-rac signaling. Science. 1997;278:1464–1466. doi: 10.1126/science.278.5342.1464. [DOI] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Marshall CJ. Ras effectors. Curr Opin Cell Biol. 1996;8:197–204. doi: 10.1016/s0955-0674(96)80066-4. [DOI] [PubMed] [Google Scholar]
- Montesano R, Matsumoto K, Nakamura T, Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- Pang L, Sawade T, Decker SJ, Saltiel AR. Inhibition of MAP kinase kinase blocks the differentiation of PC12 cells induced by nerve growth factor. J Biol Chem. 1995;270:13585–13588. doi: 10.1074/jbc.270.23.13585. [DOI] [PubMed] [Google Scholar]
- Parker PJ. Intracellular signalling. PI 3-kinase puts GTP on the rac. Curr Biol. 1995;5:57–59. doi: 10.1016/s0960-9822(95)00113-8. [DOI] [PubMed] [Google Scholar]
- Penn EJ, Hobson C, Rees DA, Magee AI. Structure and assembly of desmosome junctions: biosynthesis, processing, and transport of the major protein and glycoprotein components in cultured epithelial cells. J Cell Biol. 1987;105:57–68. doi: 10.1083/jcb.105.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzetto C, Bardelli A, Maina F, Longati P, Panayotou G, Dhand R, Waterfield MD, Comoglio PM. A novel recognition motif for phosphatidylinositol 3-kinase binding mediates its association with the hepatocyte growth factor/scatter factor receptor. Mol Cell Biol. 1993;13:4600–4608. doi: 10.1128/mcb.13.8.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, Graziani A, Panayotou G, Comoglio PM. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Comoglio PM, Hall A. Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac and Rho in MDCK cells. Mol Cell Biol. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein Rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Nelson WJ. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245:718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Khwaja A, Marte BM, Pappin D, Das P, Waterfield MD, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- Rong S, Bodescot M, Blair D, Dunn J, Nakamura T, Mizuno K, Park M, Chan A, Aaronson S, Vande Woude GF. Tumorigenicity of the met proto-oncogene and the gene for hepatocyte growth factor. Mol Cell Biol. 1992;12:5152–5158. doi: 10.1128/mcb.12.11.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal I, Park M. Hepatocyte growth factor-induced scatter of Madin-darby canine kidney cells requires phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:27780–27787. doi: 10.1074/jbc.270.46.27780. [DOI] [PubMed] [Google Scholar]
- Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol. 1997;137:1403–1419. doi: 10.1083/jcb.137.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- Schoenenberger CA, Zuk A, Kendall D, Matlin KS. Multilayering and loss of apical polarity in MDCK cells transformed with K-ras. J Cell Biol. 1991;112:873–889. doi: 10.1083/jcb.112.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore EM, Nelson WJ. Biosynthesis of the cell adhesion molecule uvomorulin (E-cadherin) in Madin-Darby canine kidney epithelial cells. J Biol Chem. 1991;266:19672–19680. [PubMed] [Google Scholar]
- Siliciano JD, Goodenough DA. Localization of the tight junction protein, ZO-1, is modulated by extracellular calcium and cell-cell contact in Madin-Darby canine kidney epithelial cells. J Cell Biol. 1988;107:2389–2399. doi: 10.1083/jcb.107.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M, Gheradi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell motility. Nature. 1987;327:239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- Stoker M, Perryman M. An epithelial scatter factor released by embryo fibroblasts. J Cell Sci. 1985;77:209–223. doi: 10.1242/jcs.77.1.209. [DOI] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by rac and rho small G proteins in MDCK cells. J Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda H, Nagafuchi A, Yonemura S, Tsukita S, Behrens J, Birchmeier W, Tsukita S. V-src kinase shifts the cadherin-based cell adhesion from the strong to the weak state and β-catenin is not required for the shift. J Cell Biol. 1995;131:1839–1847. doi: 10.1083/jcb.131.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers SJ, Panayotou G, Waterfield MD. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidlyinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Cell Biol. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Weidner KM, Sachs M, Birchmeier W. The met receptor tyrosine kinase transduces motility, proliferation, and morphogenic signals of scatter fcator/hepatocyte growth factor in epithelial cells. J Cell Biol. 1993;121:145–154. doi: 10.1083/jcb.121.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennström S, Hawkins P, Cooke F, Hara K, Yonezawa K, Kasuga M, Jackson T, Claesson-Welsh L, Stephens L. Activation of phosphoinositide 3-kinase is required for PDGF-stimulated membrane ruffling. Curr Biol. 1994;4:385–393. doi: 10.1016/s0960-9822(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Zarnegar R, Michalopoulos GK. The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J Cell Biol. 1995;129:1177–1180. doi: 10.1083/jcb.129.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]