Abstract
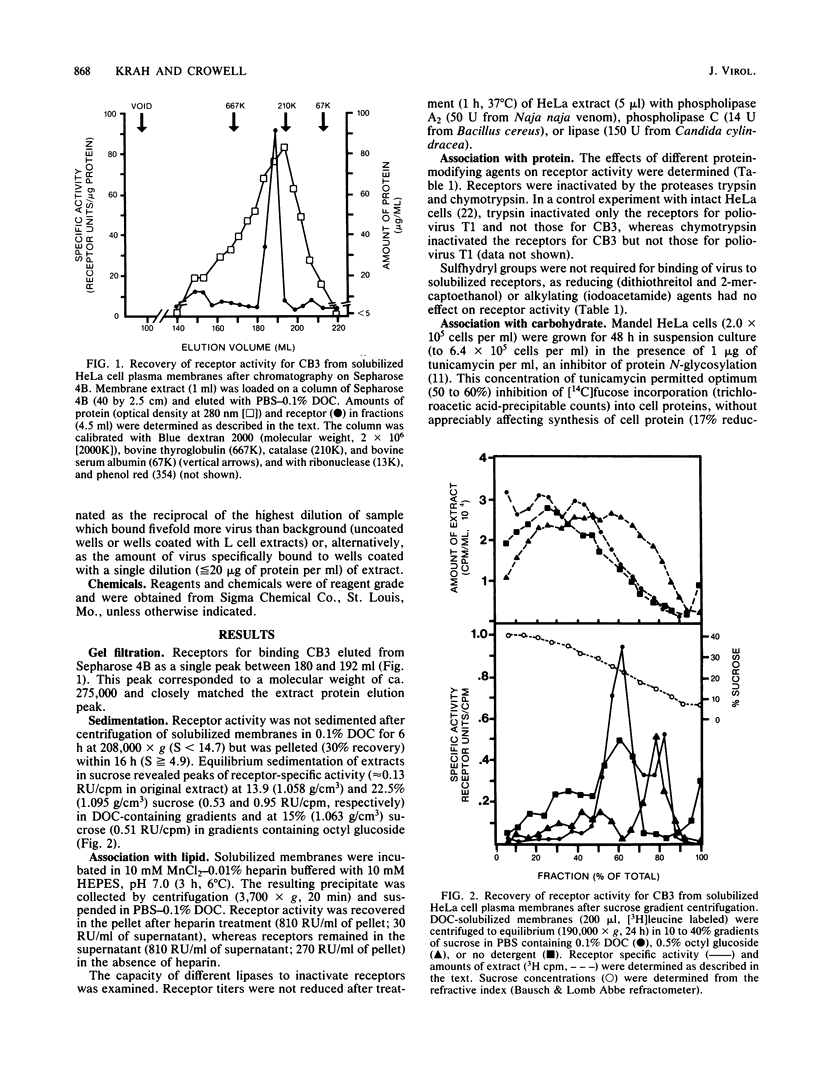
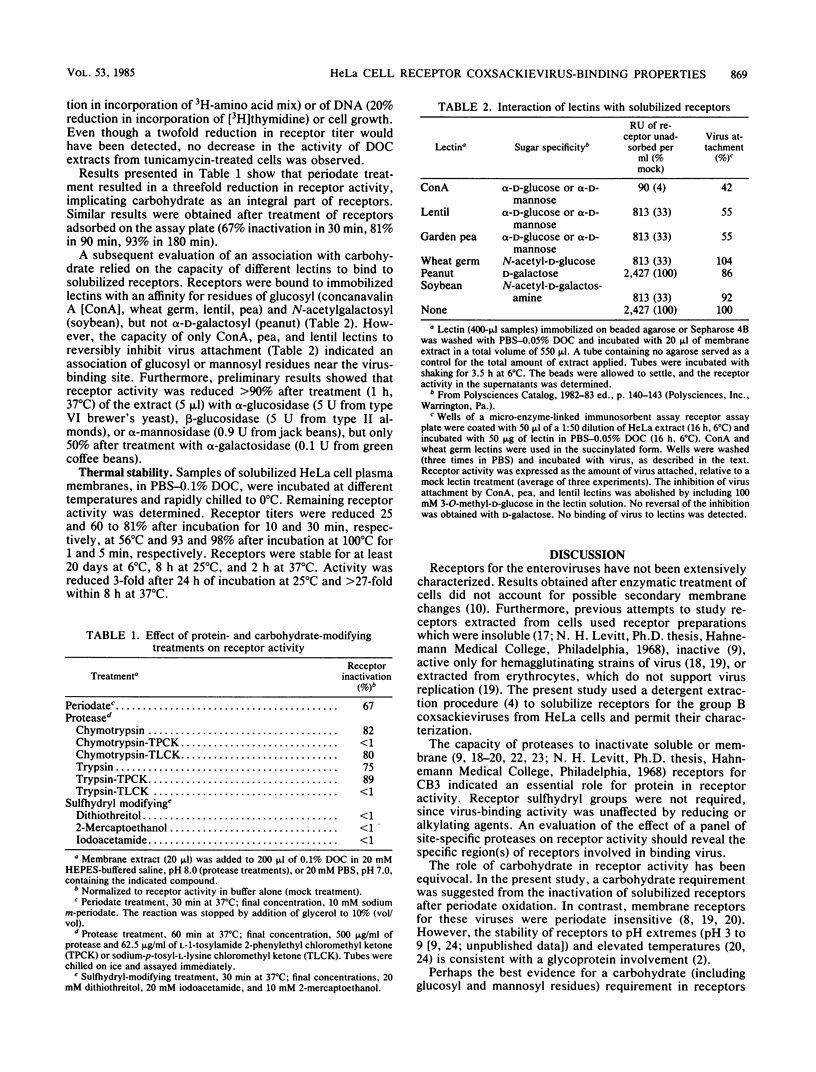
Physical and chemical properties of deoxycholate-solubilized HeLa cell plasma membrane receptors for binding group B coxsackieviruses were determined. Receptors eluted from Sepharose 4B with an apparent molecular weight of 275,000 and sedimented with an S value of between 14.7 and 4.9 and a buoyant density of 1.06 to 1.10 g/cm3. Virus-binding activity was destroyed after treatment with proteases, glycosidases, and periodate but was unaffected by lipases or reducing or alkylating agents. Additionally, lectins, including concanavalin A, adsorbed receptors and inhibited virus attachment. The composite data suggested that glycoprotein is an integral part of the receptors for binding virus.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CROWELL R. L., SYVERTON J. T. The mammalian cell-virus relationship. VI. Sustained infection of HeLa cells by Coxsackie B3 virus and effect on superinfection. J Exp Med. 1961 Feb 1;113:419–435. doi: 10.1084/jem.113.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWELL R. L., SYVERTON J. T. The viral range in vitro of a malignant, human epithelial cell (strain HeLa, Gey). IV. The cytopathogenicity of C viruses. J Immunol. 1955 Feb;74(2):169–177. [PubMed] [Google Scholar]
- Crowell R. L., Philipson L. Specific alterations of coxsackievirus B3 eluted from HeLa cells. J Virol. 1971 Oct;8(4):509–515. doi: 10.1128/jvi.8.4.509-515.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell R. L. Specific cell-surface alteration by enteroviruses as reflected by viral-attachment interference. J Bacteriol. 1966 Jan;91(1):198–204. doi: 10.1128/jb.91.1.198-204.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R. J., Gravell M., Crowell R. L. Evidence for the susceptibility of primary fetal mouse cells in culture to coxsackievirus A13. Proc Soc Exp Biol Med. 1969 Nov;132(2):743–748. doi: 10.3181/00379727-132-34301. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. The location and nature of enterovirus receptors in susceptible cells. J Exp Med. 1961 Aug 1;114:161–171. doi: 10.1084/jem.114.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. The mammalian cell-virus relationship. II. Adsorption, reception, and eclipse of poliovirus by HeLa cells. J Exp Med. 1959 May 1;109(5):487–504. doi: 10.1084/jem.109.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King I. A., Tabiowo A. Effect of tunicamycin on epidermal glycoprotein and glycosaminoglycan synthesis in vitro. Biochem J. 1981 Aug 15;198(2):331–338. doi: 10.1042/bj1980331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krah D. L., Crowell R. L. A solid-phase assay of solubilized HeLa cell membrane receptors for binding group B coxsackieviruses and polioviruses. Virology. 1982 Apr 15;118(1):148–156. doi: 10.1016/0042-6822(82)90328-2. [DOI] [PubMed] [Google Scholar]
- Levitt N. H., Crowell R. L. Comparative studies of the regeneration of HeLa cell receptors for poliovirus T1 and coxsackievirus B3. J Virol. 1967 Aug;1(4):693–700. doi: 10.1128/jvi.1.4.693-700.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Crowell R. L., Philipson L. Unrelated animal viruses share receptors. Nature. 1976 Feb 26;259(5545):679–681. doi: 10.1038/259679a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McLaren L. C., Scaletti J. V., James C. G. Isolation and properties of enterovirus receptors. Wistar Inst Symp Monogr. 1968;8:123–135. [PubMed] [Google Scholar]
- PHILIPSON L., BENGTSSON S., BRISHAMMAR S., SVENNERHOLM L., ZETTERQVIST O. PURIFICATION AND CHEMICAL ANALYSIS OF THE ERYTHROCYTE RECEPTOR FOR HEMAGGLUTINATING ENTEROVIRUSES. Virology. 1964 Apr;22:580–590. doi: 10.1016/0042-6822(64)90080-7. [DOI] [PubMed] [Google Scholar]
- Roesing T. G., Toselli P. A., Crowell R. L. Elution and uncoating of Coxsackievirus B3 by isolated HeLa cell plasma membranes. J Virol. 1975 Mar;15(3):654–657. doi: 10.1128/jvi.15.3.654-657.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAJAC I., CROWELL R. L. EFFECT OF ENZYMES ON THE INTERACTION OF ENTEROVIRUSES WITH LIVING HELA CELLS. J Bacteriol. 1965 Mar;89:574–582. doi: 10.1128/jb.89.3.574-582.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAJAC I., CROWELL R. L. LOCATION AND REGENERATION OF ENTERIOVIRUS RECEPTORS OF HELA CELLS. J Bacteriol. 1965 Apr;89:1097–1100. doi: 10.1128/jb.89.4.1097-1100.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac I., Crowell R. L. Differential inhibition of attachment and eclipse activities of HeLa cells for enteroviruses. J Virol. 1969 Apr;3(4):422–428. doi: 10.1128/jvi.3.4.422-428.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


