Abstract
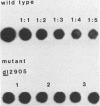
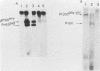
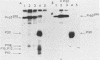
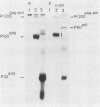
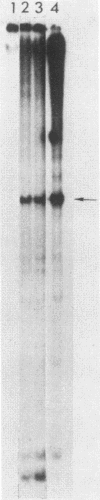
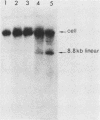
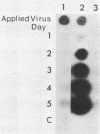
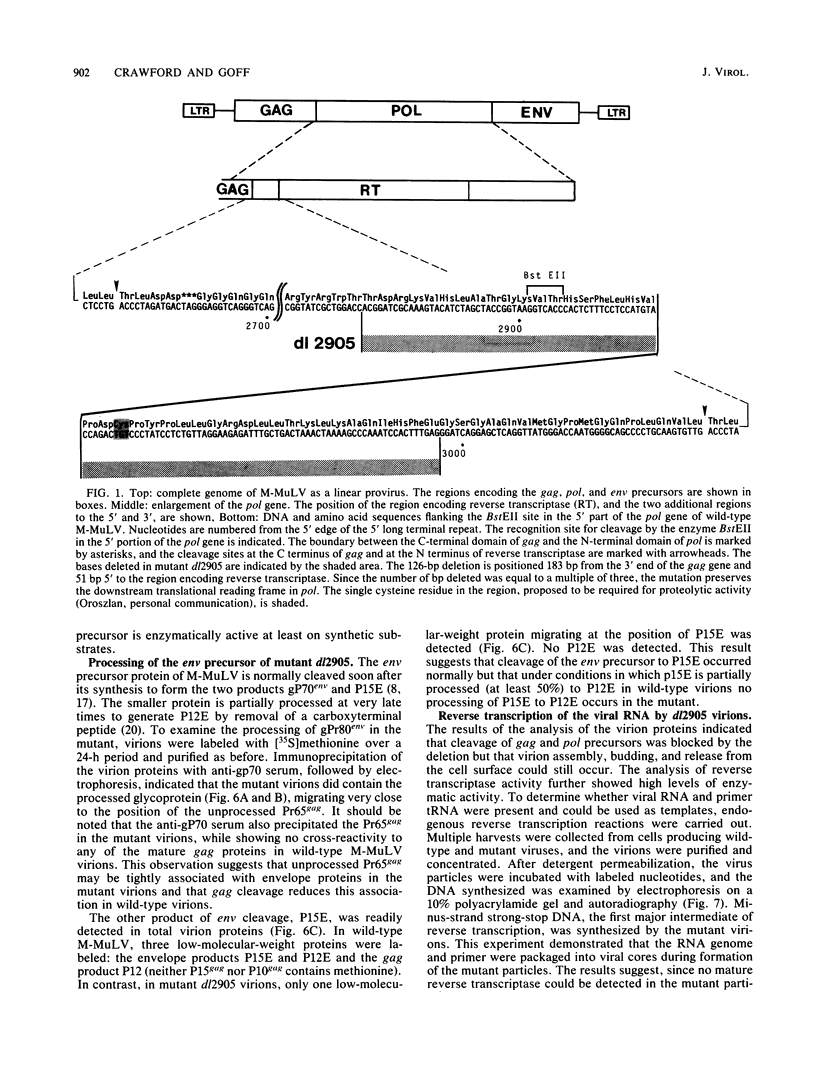
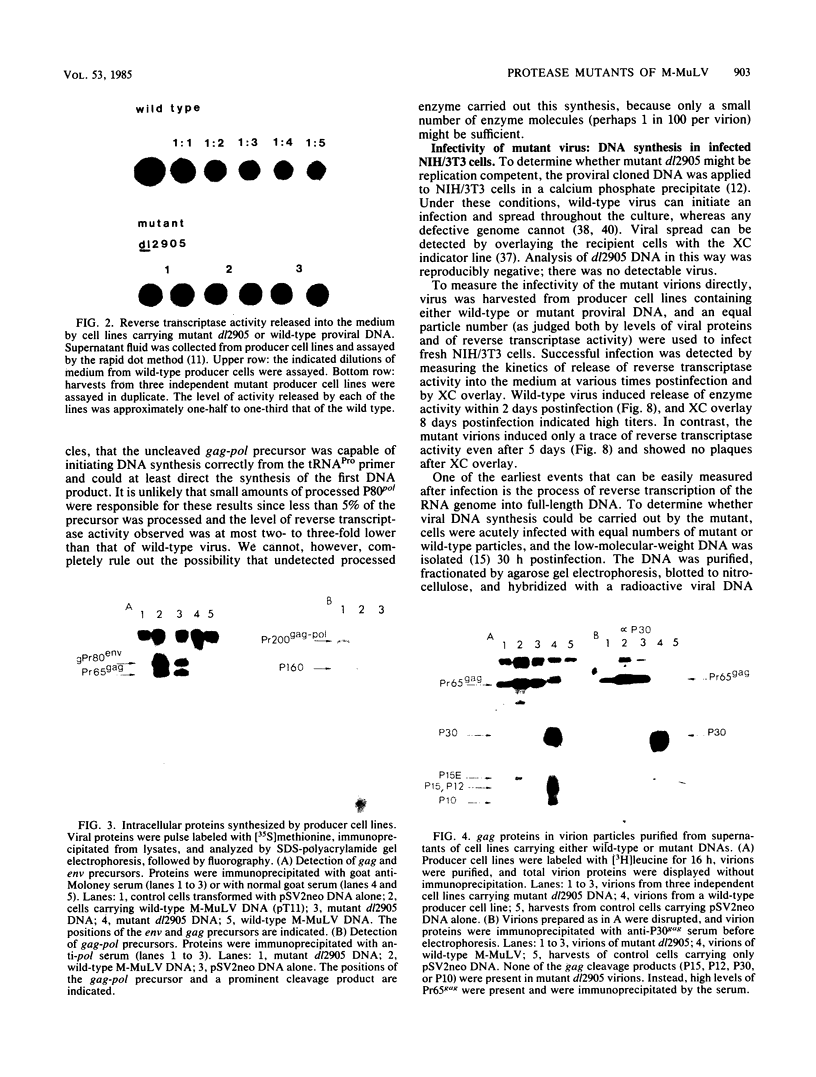
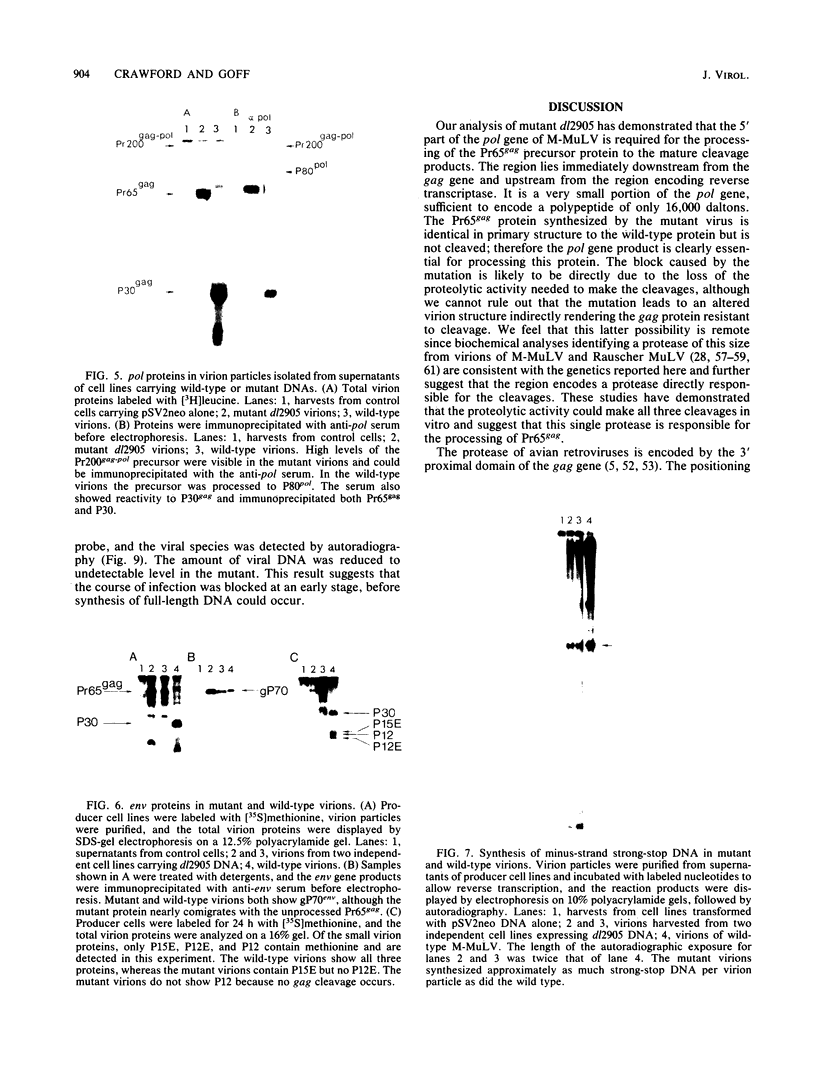
Deletion mutations in the 5' part of the pol gene of Moloney murine leukemia virus were generated by restriction enzyme site-directed mutagenesis of cloned proviral DNA. DNA sequence analysis indicated that one such deletion was localized entirely within the 5' part of the pol gene, did not affect the region encoding reverse transcriptase, and preserved the translational reading frame downstream of the mutation. The major viral precursor polyproteins (Pr65gag, Pr200gag-pol, and gPr80env) were synthesized at wild-type levels in cell lines carrying the mutant genome. These cell lines assembled and released wild-type levels of virion particles into the medium. Cleavage of both Pr65gag and Pr200gag-pol precursors to the mature proteins was completely blocked in the mutant virions. Surprisingly, these virions contained high levels of active reverse transcriptase; examination of the endogenous reverse transcription products synthesized by the mutant virions revealed normal amounts of minus-strand strong-stop DNA, indicating that the RNA genome was packaged and that reverse transcription in detergent-permeabilized virions was not significantly impaired. Processing of gPr80env to gP70env and P15E was not affected by the mutation, but cleavage of P15E to P12E was not observed. The mutant particles were poorly infectious; analysis indicated that infection was blocked at an early stage. The data are consistent with the idea that the 5' part of the pol gene encodes a protease directly responsible for processing Pr65gag, and possibly Pr200gag-pol, to the structural virion proteins. It appears that cleavage of the gag gene product is not required for budding and release of virions and that complete processing of the pol gene product to the mature form of reverse transcriptase is not required for its functional activation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcement L. J., Karshin W. L., Naso R. B., Arlinghaus R. B. "gag" polyprotein precursors of Rauscher murine leukemia virus. Virology. 1977 Sep;81(2):284–297. doi: 10.1016/0042-6822(77)90145-3. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Retroviruses. Annu Rev Biochem. 1978;47:35–88. doi: 10.1146/annurev.bi.47.070178.000343. [DOI] [PubMed] [Google Scholar]
- Chumakov I., Stuhlmann H., Harbers K., Jaenisch R. Cloning of two genetically transmitted Moloney leukemia proviral genomes: correlation between biological activity of the cloned DNA and viral genome activation in the animal. J Virol. 1982 Jun;42(3):1088–1098. doi: 10.1128/jvi.42.3.1088-1098.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford S., Goff S. P. Mutations in gag proteins P12 and P15 of Moloney murine leukemia virus block early stages of infection. J Virol. 1984 Mar;49(3):909–917. doi: 10.1128/jvi.49.3.909-917.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar K. J., Moelling K. Biochemical properties of p15-associated protease in an avian RNA tumor virus. J Virol. 1978 Oct;28(1):106–118. doi: 10.1128/jvi.28.1.106-118.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin R. K., Manning J. S. Coordination of cleavage of gag and env gene products of murine leukemia virus: implications regarding the mechanism of processing. Virology. 1984 Apr 30;134(2):368–374. doi: 10.1016/0042-6822(84)90304-0. [DOI] [PubMed] [Google Scholar]
- Eisenman R. N., Vogt V. M. The biosynthesis of oncovirus proteins. Biochim Biophys Acta. 1978 Apr 6;473(3-4):187–239. doi: 10.1016/0304-419x(78)90014-8. [DOI] [PubMed] [Google Scholar]
- Famulari N. G., Buchhagen D. L., Klenk H. D., Fleissner E. Presence of murine leukemia virus envelope proteins gp70 and p15(E) in a common polyprotein of infected cells. J Virol. 1976 Nov;20(2):501–508. doi: 10.1128/jvi.20.2.501-508.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar A. F., Phillips L. A., Sarma P. S., Peebles P. T., Chopra H. C. Presence of sarcoma genome in a "non-infectious" mammalian virus. Nat New Biol. 1971 Nov 17;234(46):69–72. doi: 10.1038/newbio234069a0. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Gilboa E., Witte O. N., Baltimore D. Structure of the Abelson murine leukemia virus genome and the homologous cellular gene: studies with cloned viral DNA. Cell. 1980 Dec;22(3):777–785. doi: 10.1016/0092-8674(80)90554-1. [DOI] [PubMed] [Google Scholar]
- Goff S., Traktman P., Baltimore D. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981 Apr;38(1):239–248. doi: 10.1128/jvi.38.1.239-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Kleid D. G., Panet A., Rothenberg E., Baltimore D. Ordered transcription of RNA tumor virus genomes. J Mol Biol. 1976 Sep 5;106(1):109–131. doi: 10.1016/0022-2836(76)90303-x. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W., Casjens S. R. Assembly of bacteriophage lambda heads: protein processing and its genetic control in petit lambda assembly. J Mol Biol. 1975 Jan 15;91(2):187–199. doi: 10.1016/0022-2836(75)90159-x. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Hardy W., Jr, Tress E., Fleissner E. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. V. Identification of a new murine viral protein, p15(E). J Virol. 1975 Jul;16(1):53–61. doi: 10.1128/jvi.16.1.53-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamjoom G. A., Naso R. B., Arlinghaus R. B. Further characterization of intracellular precursor polyproteins of Rauscher leukemia virus. Virology. 1977 May 1;78(1):11–34. doi: 10.1016/0042-6822(77)90075-7. [DOI] [PubMed] [Google Scholar]
- Karshin W. L., Arcement L. J., Naso R. B., Arlinghaus R. B. Common precursor for Rauscher leukemia virus gp69/71, p15(E), and p12(E). J Virol. 1977 Sep;23(3):787–798. doi: 10.1128/jvi.23.3.787-798.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lobel L. I., Goff S. P. Construction of mutants of Moloney murine leukemia virus by suppressor-linker insertional mutagenesis: positions of viable insertion mutations. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4149–4153. doi: 10.1073/pnas.81.13.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A. H., Soong M. M., Wong P. K. Maturation of Moloney murine leukemia virus. Virology. 1979 Feb;93(1):269–274. doi: 10.1016/0042-6822(79)90297-6. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Maxwell S., Arlinghaus R. B. In vitro proteolytic cleavage of Gazdar murine sarcoma virus p65gag. J Virol. 1981 Sep;39(3):963–967. doi: 10.1128/jvi.39.3.963-967.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Verma I. M. Two base changes restore infectivity to a noninfectious molecular clone of Moloney murine leukemia virus (pMLV-1). J Virol. 1984 Jan;49(1):214–222. doi: 10.1128/jvi.49.1.214-222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelling K., Scott A., Dittmar K. E., Owada M. Effect of p15-associated protease from an avian RNA tumor virus on avian virus-specific polyprotein precursors. J Virol. 1980 Feb;33(2):680–688. doi: 10.1128/jvi.33.2.680-688.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E. C., Jr, Kopchick J. J., Watson K. F., Arlinghaus R. B. Cell-free synthesis of a precursor polyprotein containing both gag and pol gene products by Rauscher murine leukemia virus 35S RNA. Cell. 1978 Feb;13(2):359–369. doi: 10.1016/0092-8674(78)90204-0. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Henderson L. E., Stephenson J. R., Copeland T. D., Long C. W., Ihle J. N., Gilden R. V. Amino- and carboxyl-terminal amino acid sequences of proteins coded by gag gene of murine leukemia virus. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1404–1408. doi: 10.1073/pnas.75.3.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Andersson P., Olshevsky U., Weinberg R., Baltimore D., Gesteland R. Translation of MuLV and MSV RNAs in nuclease-treated reticulocyte extracts: enhancement of the gag-pol polypeptide with yeast suppressor tRNA. Cell. 1978 Jan;13(1):189–199. doi: 10.1016/0092-8674(78)90149-6. [DOI] [PubMed] [Google Scholar]
- Pinter A., deHarven E. Protein composition of a defective murine sarcoma virus particle possessing the enveloped type-A morphology. Virology. 1979 Nov;99(1):103–110. doi: 10.1016/0042-6822(79)90041-2. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Schwartzberg P., Colicelli J., Goff S. P. Construction and analysis of deletion mutations in the pol gene of Moloney murine leukemia virus: a new viral function required for productive infection. Cell. 1984 Jul;37(3):1043–1052. doi: 10.1016/0092-8674(84)90439-2. [DOI] [PubMed] [Google Scholar]
- Schwartzberg P., Colicelli J., Goff S. P. Deletion mutants of Moloney murine leukemia virus which lack glycosylated gag protein are replication competent. J Virol. 1983 May;46(2):538–546. doi: 10.1128/jvi.46.2.538-546.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg P., Colicelli J., Gordon M. L., Goff S. P. Mutations in the gag gene of Moloney murine leukemia virus: effects on production of virions and reverse transcriptase. J Virol. 1984 Mar;49(3):918–924. doi: 10.1128/jvi.49.3.918-924.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A., Witte W. N., Rothenberg E., Baltimore D. High frequency of aberrant expression of Moloney murine leukemia virus in clonal infections. Cell. 1978 Jul;14(3):601–609. doi: 10.1016/0092-8674(78)90245-3. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Shoemaker C., Goff S., Gilboa E., Paskind M., Mitra S. W., Baltimore D. Structure of a cloned circular Moloney murine leukemia virus DNA molecule containing an inverted segment: implications for retrovirus integration. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3932–3936. doi: 10.1073/pnas.77.7.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Characterization of temperature-sensitive mutants of murine leukemia virus. Virology. 1973 Jul;54(1):53–59. doi: 10.1016/0042-6822(73)90113-x. [DOI] [PubMed] [Google Scholar]
- Traktman P., Baltimore D. Protease bypass of temperature-sensitive murine leukemia virus maturation mutants. J Virol. 1982 Dec;44(3):1039–1046. doi: 10.1128/jvi.44.3.1039-1046.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zaane D., Dekker-Michielsen J. A., Bloemers H. P. Virus-specific precursor polypeptides in cells infected with Rauscher leukemia virus: synthesis, identification, and processing. Virology. 1976 Nov;75(1):113–129. doi: 10.1016/0042-6822(76)90011-8. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Wight A., Eisenman R. In vitro cleavage of avian retrovirus gag proteins by viral protease p15. Virology. 1979 Oct 15;98(1):154–167. doi: 10.1016/0042-6822(79)90534-8. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Baltimore D. Relationship of retrovirus polyprotein cleavages to virion maturation studied with temperature-sensitive murine leukemia virus mutants. J Virol. 1978 Jun;26(3):750–761. doi: 10.1128/jvi.26.3.750-761.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. K., McCarter J. A. Studies of two temperature-sensitive mutants of Moloney murine leukemia virus. Virology. 1974 Apr;58(2):396–408. doi: 10.1016/0042-6822(74)90075-0. [DOI] [PubMed] [Google Scholar]
- Wong P. K., Russ L. J., McCarter J. A. Rapid, selective procedure for isolation of spontaneous temperature-sensitive mutants of Moloney leukemia virus. Virology. 1973 Feb;51(2):424–431. doi: 10.1016/0042-6822(73)90441-8. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. A comparison of avian and murine retrovirus polyprotein cleavage activities. Virology. 1981 May;111(1):239–250. doi: 10.1016/0042-6822(81)90668-1. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. Partial characterization of a P70 proteolytic factor that is present in purified virions of Rauscher leukemia virus (RLV). Biochem Biophys Res Commun. 1977 May 9;76(1):54–63. doi: 10.1016/0006-291x(77)91667-9. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. Physicochemical characterization and specificity of the murine leukaemia virus Pr65gag proteolytic factor. J Gen Virol. 1980 Jun;48(Pt 2):329–340. doi: 10.1099/0022-1317-48-2-329. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. Properties of a P70 proteolytic factor of murine leukemia viruses. Cell. 1977 Nov;12(3):709–719. doi: 10.1016/0092-8674(77)90271-9. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. p65 of Gazdar murine sarcoma viruses contains antigenic determinants from all four of the murine leukemia virus (MuLV) gag polypeptides (p15, p12, p30, and p10) and can be cleaved in vitro by the MuLV proteolytic activity. Virology. 1982 Apr 30;118(2):380–388. doi: 10.1016/0042-6822(82)90357-9. [DOI] [PubMed] [Google Scholar]
- van de Ven W. J., van Zaane D., Onnekink C., Bloemers H. P. Impaired processing of precursor polypeptides of temperature-sensitive mutants of Rauscher murine leukemia virus. J Virol. 1978 Feb;25(2):553–561. doi: 10.1128/jvi.25.2.553-561.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Helm K. Cleavage of Rous sarcoma viral polypeptide precursor into internal structural proteins in vitro involves viral protein p15. Proc Natl Acad Sci U S A. 1977 Mar;74(3):911–915. doi: 10.1073/pnas.74.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]