Abstract
The spindle pole body (SPB) in Saccharomyces cerevisiae functions as the microtubule-organizing center. Spc110p is an essential structural component of the SPB and spans between the central and inner plaques of this multilamellar organelle. The amino terminus of Spc110p faces the inner plaque, the substructure from which spindle microtubules radiate. We have undertaken a synthetic lethal screen to identify mutations that enhance the phenotype of the temperature-sensitive spc110–221 allele, which encodes mutations in the amino terminus. The screen identified mutations in SPC97 and SPC98, two genes encoding components of the Tub4p complex in yeast. The spc98–63 allele is synthetic lethal only with spc110 alleles that encode mutations in the N terminus of Spc110p. In contrast, the spc97 alleles are synthetic lethal with spc110 alleles that encode mutations in either the N terminus or the C terminus. Using the two-hybrid assay, we show that the interactions of Spc110p with Spc97p and Spc98p are not equivalent. The N terminus of Spc110p displays a robust interaction with Spc98p in two different two-hybrid assays, while the interaction between Spc97p and Spc110p is not detectable in one strain and gives a weak signal in the other. Extra copies of SPC98 enhance the interaction between Spc97p and Spc110p, while extra copies of SPC97 interfere with the interaction between Spc98p and Spc110p. By testing the interactions between mutant proteins, we show that the lethal phenotype in spc98–63 spc110–221 cells is caused by the failure of Spc98–63p to interact with Spc110–221p. In contrast, the lethal phenotype in spc97–62 spc110–221 cells can be attributed to a decreased interaction between Spc97–62p and Spc98p. Together, these studies provide evidence that Spc110p directly links the Tub4p complex to the SPB. Moreover, an interaction between Spc98p and the amino-terminal region of Spc110p is a critical component of the linkage, whereas the interaction between Spc97p and Spc110p is dependent on Spc98p.
INTRODUCTION
The centrosome organizes microtubules both spatially and temporally within a cell. Although at the ultrastructural level centrosomes may differ across a spectrum of organisms, the essential function of microtubule nucleation remains the same. Accordingly, conserved components are being found in diverse species. A prominent centrosomal component that is highly conserved is γ-tubulin (Oakley and Oakley, 1989; Oakley et al., 1990; Zheng et al., 1991; Raff et al., 1993; Horio and Oakley, 1994). γ-Tubulin localizes to centrosomes and is essential for microtubule nucleation (Horio et al., 1991; Stearns et al., 1991; Zheng et al., 1991). In addition to being at the centrosome, γ-tubulin is present in large cytoplasmic complexes (Stearns and Kirschner, 1994; Moudjou et al., 1996). A soluble 25S complex from Xenopus extracts called the γ-tubulin–containing ring complex (γ-TuRC) is a helical ring structure capable of nucleating microtubules in vitro and capping the minus ends of stabilized microtubules (Moritz et al., 1995; Zheng et al., 1995). One current model postulates that γ-tubulin and associated proteins form a scaffold upon which the α/β tubulin heterodimers assemble into a 13-protofilament microtubule (Oakley et al., 1990; Zheng et al., 1995). How γ-tubulin is anchored at the centrosome remains unknown.
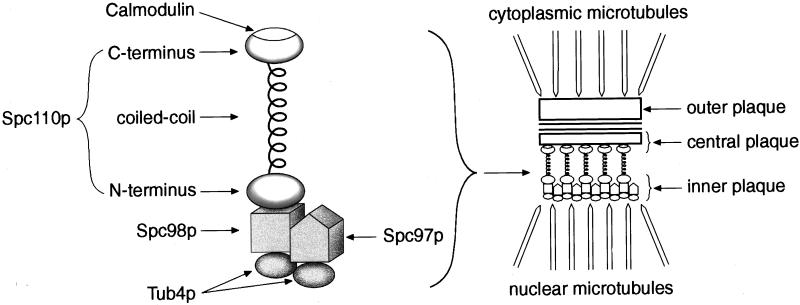
In Saccharomyces cerevisiae, the spindle pole body (SPB) serves as the organism’s microtubule-organizing center (Byers, 1981a, b). The multilayered structure includes an outer plaque radiating cytoplasmic microtubules, a central plaque embedded in the nuclear envelope, and an inner plaque radiating nuclear microtubules (Rout and Kilmartin, 1990; Bullitt et al., 1997). Spc110p is an essential structural component that spans between the central and inner plaques. A central coiled-coil domain (residues 155–798) is flanked by N-terminal and C-terminal regions. The C-terminal region of Spc110p has a calmodulin-binding site and is localized to the central plaque (Sundberg et al., 1996), while its N terminus faces the inner plaque (Spang et al., 1996b). The coiled-coil region is largely dispensable because a strain carrying an SPC110 allele with three quarters of the central α-helical domain deleted still has a nearly normal growth rate (Kilmartin et al., 1993).
Spc110p is regulated throughout the cell cycle. Transcription of SPC110 is under the control of an MluI cell cycle box and is induced in late G1 at the time that the SPB duplicates (Kilmartin et al., 1993). Later in the cell cycle as the spindle forms, Spc110p is phosphorylated. Phosphorylation is removed at the time of anaphase (Friedman et al., 1996).
A mutational analysis of Spc110p revealed three regions with distinct functions (Sundberg and Davis, 1997). Region I consists of residues 1–163, N-terminal to the coiled coil. Region II includes amino acid residues 772–836, and region III is the calmodulin-binding site between amino acid residues 897–917. Temperature-sensitive mutations in region I of SPC110 lead to a mitotic arrest dependent on the MAD1 checkpoint. The spindles appear morphologically normal when analyzed either in electron micrographs of thin sections or by immunofluorescence. Region II in the C terminus of Spc110p is required for stable attachment of Spc110p to the central plaque. Mutations here do not interfere with the initial assembly of Spc110p onto the SPB, but Spc110p separates from the SPB during mitosis. Finally, temperature-sensitive mutations in the calmodulin-binding site cause early misassembly of spindle pole components and broken spindles (Kilmartin and Goh, 1996; Stirling et al., 1996; Sundberg et al., 1996).
To learn more about the function of the N terminus, we performed a synthetic lethal screen for mutations that would enhance the subtle phenotype of the region I mutant spc110–221. The screen identified both SPC97 and SPC98, two genes that encode spindle pole components with many similar properties. Spc97p and Spc98p are similar in size and share short stretches of sequence similarity. Together with the yeast homologue of γ-tubulin, Tub4p, they form a 6S soluble complex (Sobel and Snyder, 1995; Geissler et al., 1996; Marschall et al., 1996; Spang et al., 1996a; Knop et al., 1997). Two-hybrid assays suggest that each one can bind Tub4p. Since the 6S complex contains one molecule each of Spc97p and Spc98p and at least two molecules of Tub4p, one possible arrangement is that Spc97p and Spc98p each bind one molecule of Tub4p (Geissler et al., 1996; Knop et al., 1997). All three proteins are found at the inner plaque of the SPB in the nucleus and at the outer plaque of the SPB in the cytoplasm. Finally, recent results suggest that the N terminus of Spc110p interacts equally well with Spc98p and Spc97p (Knop and Schiebel, 1997). Despite these similarities, Spc97p and Spc98p must have unique functions because each is essential for cell viability. By performing a detailed genetic analysis and two-hybrid analyses in two different systems, we demonstrate that Spc97p and Spc98p can be distinguished on the basis of their interactions with Spc110p. Our results argue that Spc98p is the component of the Tub4p complex primarily responsible for interaction with Spc110p.
MATERIALS AND METHODS
Strains and Media
Strains used in this study are listed in Table 1. Strains TNY2–2B and TNY2–4B were obtained by dissection of the diploid created by mating HSY14 (Sundberg and Davis, 1997) with GZY7–27C (Zhu, 1996). Strains TNY135 and TNY136 contain spc110–250, a null allele encoding a truncated version of Spc110p of 79.4 kDa. Strains TNY160–165 are the original strains isolated in the synthetic lethal screen. Strain TNY161 was backcrossed five times to to give strain TNY137. Strain TNY165 was backcrossed two and three times to give strains TNY75–16D and TNY76–1C, respectively. Strain TNY76–1C was mated to strain TNY75–16D to create the diploid DCY101. Strains TNY163 and TNY162 were backcrossed once to give strains TNY113–10A and TNY114–39C, respectively. Strain TNY150, which has URA3 integrated at the SPC98 locus, was constructed by transformation of strain TNY2–2B with plasmid pTN32. Strain TNY150 was mated to strain TNY76–1C to create the diploid TNY117.
Table 1.
Yeast strains
| Strain | Genotype | Source |
|---|---|---|
| DCY101 | spc98-63/spc98-63 spc110-221/spc110-221 MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ade3Δ his3-11,15/his3-11,15 lys2Δ::HIS3/lys2Δ::HIS3 leu2-3,112/leu2-3,112 ura3-1/ura3-1 pHS26 | This study |
| EMY84-3D | MATa ade2-1oc ade3Δ his3-11,15 lys2Δ::HIS3 leu2-3,112 trp1-1 | E. Muller |
| EMY84-6C | MATα ade2-1oc ade3Δ his3-11,15 lys2Δ::HIS3 leu2-3,112 trp1-1 | E. Muller |
| TNY2-2B | spc110-221 MATa ade2-1oc ade3Δ his3-11,15 lys2Δ::HIS3 trp1-1 ura3-1 pHS26 | This study |
| TNY2-4B | spc110-221 MATα ade2-1oc ade3Δ his3-11,15 lys2Δ::HIS3 trp1-1 ura3-1 pHS26 | This study |
| TNY31 | SPC97/spc97-114 spc110-220/spc110-221 MATaMATα ade2-1oc/ade2-1oc ade3Δ/ade3Δ his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 lys2Δ::HIS3/lys2Δ::HIS3 trp1-1/trp1-1 ura3-1/ura3-1 pHS26 | This study |
| TNY47 | spc97-114/SPC97 spc110-221/spc110-224 MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ade3Δ his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 lys2Δ::HIS3/lys2Δ::HIS3 trp1-1/trp1-1 URA3/ura3-1 pHS26 | This study |
| TNY49 | spc97-114/SPC97 spc110-221/spc110-225 MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ade3Δ his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 lys2Δ::HIS3/lys2Δ::HIS3 trp1-1/trp1-1 URA3/ura3-1 pHS26 | This study |
| TNY51 | spc97-114/SPC97 spc110-221/spc110-222 MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ade3Δ his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 lys2Δ::HIS3/lys2Δ::HIS3 trp1-1/trp1-1 URA3/ura3-1 pHS26 | This study |
| TNY64-5C | spc97-114 SPC110 MATα ade2-1oc ade3Δ his3-11,15 leu2-3,112 lys2Δ::HIS3 trp1-1 pHS26 | This study |
| TNY75-16D | spc98-63 spc110-221 MATα ade2-1oc ade3Δ his3-11,15 lys2Δ::HIS3 leu2-3,112 ura3-1 pHS26 | This study |
| TNY76-1C | spc98-63 spc110-221 MATa ade2-1oc ade3Δ his3-11,15 lys2Δ::HIS3 leu2-3,112 ura3-1 pHS26 | This study |
| TNY82 | spc97-62/SPC97 spc110-221/spc110-220 MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ade3Δ his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 lys2Δ::HIS3/lys2Δ::HIS3 TRP1/trp1-1 ura3-1/ura3-1 pHS26 | This study |
| TNY83 | SPC97/spc97-62 spc110-222/spc110-221 MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ade3Δ his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 lys2Δ::HIS3/lys2Δ::HIS3 trp1-1/TRP1 ura3-1/ura3-1 pHS26 | This study |
| TNY84 | SPC97/spc97-62 spc110-224/spc110-221 MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ade3Δ his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 lys2Δ::HIS3/lys2Δ::HIS3 trp1-1/TRP1 ura3-1/ura3-1 pHS26 | This study |
| TNY86 | SPC97/spc97-62 spc110-226/spc110-221 MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ade3Δ his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 LYS2/lys2Δ::HIS3 trp1-1/TRP1 ura3-1/ura3-1 pHS26 | This study |
| TNY88 | SPC97/spc97-113 spc110-220/spc110-221 MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ade3Δ his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 lys2Δ::HIS3/lys2Δ::HIS3 trp1-1/TRP1 ura3-1/ura3-1 pHS26 | This study |
| TNY89 | SPC97/spc97-113 spc110-222/spc110-221 MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ade3Δ his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 lys2Δ::HIS3/lys2Δ::HIS3 trp1-1/TRP1 ura3-1/ura3-1 pHS26 | This study |
| TNY91 | SPC97/spc97-113 spc110-225/spc110-221 MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ade3Δ his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 lys2Δ::HIS3/lys2Δ::HIS3 trp1-1/TRP1 ura3-1/ura3-1 pHS26 | This study |
| TNY92 | SPC97/spc97-113 spc110-226/spc110-221 MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ade3Δ his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 LYS2/lys2Δ::HIS3 trp1-1/TRP1 ura3-1/ura3-1 pHS26 | This study |
| TNY106 | spc98-63/SPC98 spc110-221/spc110-220 MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ade3Δ his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 lys2Δ::HIS3/lys2Δ::HIS3 trp1-1/trp1-1 ura3-1/ura3-1 pHS26 | This study |
| TNY107 | spc98-63/SPC98 spc110-221/spc110-222 MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ade3Δ his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 lys2Δ::HIS3/lys2Δ::HIS3 trp1-1/trp1-1 ura3-1/ura3-1 pHS26 | This study |
| TNY108 | spc98-63/SPC98 spc110-221/spc110-224 MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ade3Δ his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 lys2Δ::HIS3/lys2Δ::HIS3 trp1-1/trp1-1 ura3-1/ura3-1 pHS26 | This study |
| TNY109 | spc98-63/SPC98 spc110-221/spc110-225 MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ade3Δ his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 lys2Δ::HIS3/lys2Δ::HIS3 trp1-1/trp1-1 ura3-1/ura3-1 pHS26 | This study |
| TNY110 | spc98-63/SPC98 spc110-221/spc110-226 MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ade3Δ his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 lys2Δ::HIS3/LYS2 trp1-1/trp1-1 ura3-1/ura3-1 pHS26 | This study |
| TNY113-10A | spc97-113 spc110-221 MATα ade2-1oc ade3Δ his3-11,15 lys2Δ::HIS3 leu2-3,112 ura3-1, pHS26 | This study |
| TNY114-39C | spc97-62 spc110-221 MATα ade2-1oc ade3Δ his3-11,15 lys2Δ::HIS3 leu2-3,112 trp1-1 ura3-1 pHS26 | This study |
| TNY117 | spc110-221/spc110-221 spc98-63/SPC98::URA3::SPC98 MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ade3Δ his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 trp1-1/trp1-1 ura3-1/ura3-1 pHS26 | This study |
| TNY127 | spc97-114/SPC97 spc110-221/spc110-226 MATa/MATα ade2-1oc/ade2-1oc ade3Δ/ade3Δ his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 lys2Δ::HIS3/LYS2 trp1-1/trp1-1 ura3-1/ura3-1 pHS26 | This study |
| TNY135 | spc110-250 MATa ade2-1oc ade3Δ his3-11,15 lys2Δ::HIS3 trp1-1 ura3-1 pHS26 | This study |
| TNY136 | spc110-250 MATα ade2-1oc ade3Δ his3-11,15 lys2Δ::HIS3 leu2-3,112 ura3-1 pHS26 | This study |
| TNY137 | spc97-114 spc110-221 MATa ade2-1oc ade3Δ his3-11,15 lys2Δ::HIS3 leu2-3,112 ura3-1 pHS26 | This study |
| TNY150 | spc110-221 SPC98::URA3::SPC98 MATa ade2-1oc ade3Δ his3-11,15 lys2Δ::HIS3 trp1-1 ura3-1 | This study |
| TNY160 | nsl2-118 spc110-221 MATα ade2-1oc ade3Δ his3-11,15 lys2Δ::HIS3 leu2-3,112 ura3-1 pHS26 | This study |
| TNY161 | spc97-114 spc110-221 MATα ade2-1oc ade3Δ his3-11,15 lys2Δ::HIS3 leu2-3,112 ura3-1 pHS26 | This study |
| TNY162 | spc97-62 spc110-221 MATα ade2-1oc ade3Δ his3-11,15 lys2Δ::HIS3 leu2-3,112 ura3-1 pHS26 | This study |
| TNY163 | spc97-113 spc110-221 MATα ade2-1oc ade3Δ his3-11,15 lys2Δ::HIS3 leu2-3,112 ura3-1 pHS26 | This study |
| TNY164 | nsl2-85 spc110-221 MATα ade2-1oc ade3Δ his3-11,15 lys2Δ::HIS3 leu2-3,112 ura3-1 pHS26 | This study |
| TNY165 | spc98-63 spc110-221 MATα ade2-1oc ade3Δ his3-11,15 lys2Δ::HIS3 leu2-3,112 ura3-1 pHS26 | This study |
| PJ69-4A | MATa trp1-901 leu2-3,112 ura3-52 his3-200 gal4Δ gal80Δ LYS::GAL1-HIS3 GAL2-ADE2 met2::GAL7-lacZ | (James et al., 1996) |
| Y190 | MATa trp1-901 leu2-3,112 ura3-52 his3-200, ade2-101 gal4Δ gal80Δ URA3::GAL1-lacZ LYS2::GAL1-HIS3 | (Harper et al., 1993) |
YPD, SD-uracil low adenine, and YPD low adenine (Geiser et al., 1991; Muller, 1996) were described previously. SD-lys is SD medium supplemented with 1× amino acid mix without lysine, 50 μg/ml adenine, and 25 μg/ml uracil. The complete 100× amino acid mix was described previously (Geiser et al., 1991).
Plasmids
Plasmids used in this study are listed in Table 2. Plasmid pTN9 was created by cloning the SacII–ClaI fragment containing SPC97 from pTN6 into pMM66, which is pRS316 without the NcoI site in URA3.
Table 2.
Plasmids
| Plasmid | Parent plasmid | Relevant markersa | Source or reference |
|---|---|---|---|
| pACTII | GAL4-activator domain HA epitope LEU2 2μ | (Durfee et al., 1993) | |
| pAS2 | GAL4-DNA-binding domain HA epitope TRP1 2μ | (Harper, et al., 1993) | |
| pBluescriptII SK+ | bla, f1 origin | Stratagene | |
| pDC1 | pTN9 | spc97-114 | This study |
| pDC2 | pTN9 | spc97-62 | This study |
| pDC3 | pTN9 | spc97-113 | This study |
| pDC4 | PCR Blunt | spc98-63 | This study |
| pDC5 | PCR Blunt | spc98-63 | This study |
| pDC7 | pHS90 | spc98-63 (aa 1-846 containing S754F) | This study |
| pDC9 | pHS89 | spc98-63 | This study |
| pDV1A | pACTII | SPC110 (aa 1-150) | This study |
| pDV4 | pACTII | SPC97 (aa 1-823) | This study |
| pDV6 | pACTII | SPC97 (aa 1-548) | This study |
| pDV8 | pAS2 | SPC98 (aa 1-846) | This study |
| pDV9 | pTHS37 | SPC98 (aa 1-361) | This study |
| pDV13 | pAS2 | TUB4 (aa 1-473) | This study |
| pDV16 | pACTII | TUB4 (aa 1-473) | This study |
| pDV17 | pACTII | SPC110 (aa 1-183) | This study |
| pDV18 | pAS2 | SPC97 (aa 1-823) | This study |
| pDV20 | pACTII | spc110-221 (aa 1-183; see Sundberg and Davis, 1997 for description of mutations) | This study |
| pDV21 | pAS2 | spc97-62 (aa 1-823 containing L22F) | This study |
| pDV22 | pAS2 | spc97-114 (aa 1-823 containing E56K) | This study |
| pDV23 | pAS2 | spc98-63 (aa 1-846 containing S754F) | This study |
| pGEMT | Bacterial vector for cloning PCR products | Promega | |
| pLAMIN | pGBT-CYH | GAL4-DNA-binding domain-lamin fusion TRP1 CEN | D. Lockshon |
| pHS26 | pTD29 | SPC110 ADE3 LYS2 2μ origin | (Sundberg et al., 1996) |
| pHS88 | pGF29 | SPC98 URA3 2μ origin | (Sundberg and Davis, 1997) |
| pHS89 | pRS316 | SPC98 URA3 CEN6 | (Sundberg and Davis, 1997) |
| pHS90 | pGEMT | PCR fragment containing SPC98 (1-846) flanked by SmaI (at Start) and XhoI | H. Sundberg |
| pHS91 | pGF29 | TUB4 URA3 2μ origin | (Sundberg and Davis, 1997) |
| pHS92 | pGF29 | SPC97 URA3 2μ origin | (Sundberg and Davis, 1997) |
| pJG109 | YCp50 | SPC110 URA3 CEN | (Geiser, 1993) |
| pMM66 | pRS316 | URA3 lacks the NcoI site CEN6 | M. Moser |
| pRD54 | pRS316 | GAL1-HA | Gift of R. Deshaies |
| pRS306 | URA3 f1 origin | (Sikorski and Hieter, 1989) | |
| pRS316 | URA3 CEN6 ARSH4 f1 origin | (Sikorski and Hieter, 1989) | |
| pTD103 | pRS306 | spc98-63 URA3 | This study |
| pTD104 | pRS306 | SPC98 URA3 | This study |
| pTD105 | pRS306 | BglII–SacII fragment of spc98-63 inserted at BamHI and SacII URA3 | This study |
| pTD106 | pRS306 | BglII–SacII fragment of SPC98 inserted at XhoI and SacII sites URA3 | This study |
| pTHS36 | pACTII | SPC98 (aa 149-846) inserted at NcoI and XhoI | H. Sundberg |
| pTHS37 | pACTII | SPC98 (aa 1-846) inserted at SmaI and XhoI | H. Sundberg |
| pTN1 | YCp50 | SPC97 URA3 CEN | This study |
| pTN2 | pTN1 | 4.8-kb SacI deletion from pTN1 URA3 CEN | This study |
| pTN3 | pRS316 | BglII–MunI fragment containing SPC97 URA3 CEN6 | This study |
| pTN4 | pTN3 | 1.3-kb BamHI deletion from SPC97 URA3 CEN6 | This study |
| pTN5 | pBluescriptII SK+ | ClaI–NotI fragment containing SPC97 | This study |
| pTN6 | pTN5 | SPC97b | This study |
| pTN9 | pMM66 | SPC97b URA3 CEN6 | This study |
| pTN26 | pRD54 | GAL1-HA-SPC97b URA3 CEN6 | This study |
| pTN31 | pGF27 | SPC97b TRP1 2μ origin | This study |
| pTN32 | pRS306 | SPC98 URA3 | This study |
Unless stated otherwise, all markers from the parent plasmid are present in the new construct.
An NcoI site was introduced at the initial ATG of SPC97 and an internal NcoI site was abolished by a silent mutation.
Most two-hybrid constructs were created by cloning PCR products. For construction of pDV1A and pDV17, the template was plasmid pHS31 (Friedman et al., 1996). For construction of pDV20, the template was plasmid pHS42, which contains the spc110–221 allele (Sundberg and Davis, 1997). When necessary, primers were tagged with restriction enzyme sites to facilitate cloning in frame with GAL4 in pACTII.
Plasmid pDV23 was made in several steps. First, a PCR fragment encoding the C-terminal region of Spc98–63p, which includes S754F, was cloned into pHS90 to replace the corresponding fragment of wt SPC98 and generate pDC7. Then, the fragment containing the entire coding sequence of spc98–63 from pDC7 was inserted into pAS2.
For plasmid pDV21, a PCR fragment containing the first 630 base pairs (bp) of spc97–62 was generated from the gap-repaired plasmid pDC2. The PCR fragment was then used to replace the corresponding fragment of SPC97 in plasmid pDV18. pDV22 was similarly created, except the template for PCR was the gap-repaired plasmid pDC1. All constructs made by inserting PCR fragments were sequenced.
Synthetic Lethal Screen
Strains carrying the spc110–221 allele are unable to grow at 37°C but form normal-sized colonies at 30°C. A plasmid shuffle technique was used to screen for mutations that enhance the phenotype conferred by spc110–221 by preventing growth at 30°C (Muller, 1996). These synthetic lethal mutations make spc110–221 cells growing at 30°C dependent on plasmid pHS26 carrying wt SPC110. Plasmid pHS26 contains an additional gene, the ADE3 gene, which allows formation of a red pigment. Therefore, cells that require plasmid pHS26 for viability form solid red colonies. Cells harboring other mutations that are not synthetic lethal with spc110–221 can lose plasmid pHS26 and give rise to colonies that sector white. Therefore, the primary screen was for cells that form solid red colonies at 30°C. The details of the screen are given below.
TNY2–2B (pHS26) and TNY2–4B (pHS26) were mutagenized to 3–15% survival with 1% ethyl methanesulfonate, and cells were plated on YPD low-adenine plates. Solid red colonies were streaked twice on YPD low-adenine to confirm the phenotype. The secondary screen distinguished the mutants that had integrated the plasmid into a chromosome from the mutants that still had the plasmid as a separate entity. Each candidate was crossed with a haploid wt strain (either EMY84–3D or EMY84–6C). In the diploid, the synthetic lethal interaction between nsl and spc110–221 is abolished by the presence of wt NSL and SPC110 genes. Therefore, if the plasmid has not integrated, the colonies sector white. If the plasmid has integrated, the diploid cannot lose the plasmid and the colonies remain solid red. Only mutants that were able to sector white when mated to a wt haploid were taken through the tertiary screen.
The tertiary screen identified cells that retained the pHS26 plasmid because the mutation rendered them dependent upon ADE3, LYS2, or some other region of the plasmid. Another plasmid carrying SPC110 and URA3 (pJG109) was introduced into the solid red candidates. Only strains that were able to sector white when provided with another source of Spc110p were considered further. Of the 75 reproducibly solid red colonies isolated, 59 were eliminated through the secondary and tertiary screens. The 16 synthetic lethal candidates that passed both secondary and tertiary screens were placed in complementation groups.
Complementation Analysis
Both mating types of all synthetic lethal candidates were made. All possible pairwise matings were then done between the mutants. Complementation between two synthetic lethal mutants was assessed by determining whether the resulting diploids could lose pHS26 and sector white. One expected complementation group consisted of mutants having additional mutations in spc110–221. The intragenic lethal mutations were identified by mating each of the synthetic lethal strains to a strain having a nonfunctional truncated spc110 allele (either TNY135 or TNY136). If the resulting diploid was not able to lose pHS26, the mutant was placed in the SPC110 complementation group.
Cloning of NSL1
Approximately 10 genome equivalents of a centromere-based (YCp50) yeast genomic library (Rose et al., 1987) were transformed into the nsl1–114 spc110–221 mutant. The transformed colonies were plated on SD-ura low ade at 30°C for 3–4 d, after which sectoring colonies were picked. The sectoring colonies were restreaked on SD-ura low ade until pure white colonies were obtained. The complementing library plasmid was rescued from a pure white colony as described (Hoffman and Winston, 1987) and transformed into XL1-blue bacteria. The library plasmids recovered from the bacteria were digested with HindIII to ascertain whether the insert contained SPC110. For library plasmids that did not contain SPC110, the ends of the library insert were sequenced with primers specific to YCp50 and then analyzed using the Saccharomyces Genome Database.
Mapping of Mutations in SPC97 and SPC98
The spc97 mutations were mapped by gap repair (Orr-Weaver and Szostak, 1983) combined with a plasmid-shuffle technique (Davis, 1990). Plasmids pDC1, pDC2, and pDC3 were recovered from TNY137 (spc97–114), TNY162 (spc97–62), and TNY163 (spc97–113), respectively. Each was sequenced using the DNA Sequencing Kit (Perkin Elmer-Cetus Applied Biosystems, Norwalk, CT). The spc97–62 allele contained a C>T change at bp 64 (as measured from the initial ATG), leading to the mutation L22F. The spc97–113 allele had a G>A change at bp 512, which gives the mutation R171K. The spc97–114 allele contained a G>A change at bp 166, causing the mutation E56K.
The mutation in spc98–63 responsible for synthetic lethality was identified by sequencing. The final 370 bp of spc98–63 were recovered from two different colonies of TNY76–1C by PCR using Vent polymerase (New England Biolabs, New Haven, CN). The two fragments were cloned into PCR-Blunt plasmids (Invitrogen, San Diego, CA) yielding pDC4 and pDC5. Sequencing of each plasmid revealed a single C>T point mutation at bp 2261 in spc98–63, which results in the mutation S754F. Proof that this mutation conferred synthetic lethality was obtained by testing the ability of the C2261T spc98 to confer synthetic lethality at three different gene dosages. A CEN plasmid was created by replacing the last 359 bp of wt SPC98 on pHS89 with the same region of pDC5 carrying the single mutation C2261T to make plasmid pDC9. The low-copy number vector pDC9 suppressed the synthetic lethality of the spc98–63 spc110–221 mutant. We then tested whether the C2261T mutation was sufficient to confer synthetic lethality when integrated at URA3. The integrating plasmids pTD103 and pTD104 carrying spc98 with C2261T and wt SPC98, respectively, were cut with StuI to allow integration at ura3–1 in strain TNY76–1C carrying spc98–63 and spc110–221. We found that even two copies of the C2261T allele were sufficient to suppress the synthetic lethality. We next integrated a fragment of spc98 carrying the C2261T mutation at the SPC98 locus. Plasmids pTD105 and pTD106 carrying only the 3′-end of C2261T spc98 or SPC98, respectively, were cut with AflII and transformed into strain TNY2–2B carrying spc110–221 and wt SPC98. This results in the replacement of SPC98 by one complete copy of the gene and one copy lacking the promoter and the 5′-coding region of the gene, thus yielding only one expressed full-length copy. If the single C2261T mutation is sufficient to confer synthetic lethality, then some of the colonies transformed with the fragment carrying the single mutation C2261T (pTD105) should display the synthetic lethal phenotype, i.e., be solid red at 30°C. We observed that 45% of the colonies transformed with pTD105 were solid red and 0.3% of the colonies transformed with the wt fragment (pTD106) were solid red. Therefore, the C2261T mutation in spc98–63 is sufficient to confer synthetic lethality in a haploid spc110–221 strain when it is the only expressed copy of the gene.
Test for Allele Specificity
Six different temperature-sensitive alleles of SPC110 were tested for their interaction with the spc97 and spc98 alleles. We obtained the double-mutant strains by crossing each synthetic lethal strain (having either spc97 or spc98 and spc110–221) with a strain carrying another spc110 allele. We concluded that a particular spc97 or spc98 allele exhibited lethality with a spc110 allele if >90% of the tetrads had two viable spores and two inviable spores (or spores that formed solid red colonies) at 30°C. At least 15 tetrads were analyzed for each diploid.
Two-Hybrid Assay
To analyze two-hybrid interactions, we used the ADE2 reporter in the PJ69–4A strain and the lacZ reporter in the Y190 strain. Yeast transformations were done using the high-efficiency lithium acetate method of Gietz and Schiestl (1995). A Gal4p–DNA-binding domain (Gal4DB) fusion plasmid and a Gal4p-activation domain (Gal4AD) fusion plasmid were cotransformed into PJ69–4A, and equal aliquots of transformants were plated onto SD-trp,-leu (−TL), and SD-trp,-leu,-ade (−TLA) plates. Plates were incubated at 30°C, and growth of colonies was scored between day 3 and day 6. Colonies plated on −TL plates were typically fully grown after 3 d, while colonies plated on −TLA plates took between 3 and 6 d to be fully grown. The growth rate of colonies on −TLA plates, as compared with that of colonies on −TL plates, was used as a measure of the strength of the signal generated by a given combination of Gal4AD and Gal4DB plasmids. Once cells were fully grown, the plating efficiency on −TLA plates, as compared with the plating efficiency on −TL plates, was used as another measure of the strength of the signal generated by the two-hybrid interaction. (See footnotes to Table 5 for specific designation of different growth rates.) We have observed that scoring expression of the reporter gene in a population of transformants is superior to analyzing the activity of the reporter in a single transformant, e.g., by measuring β-galactosidase in a liquid culture. Our previous two-hybrid analyses (Geiser et al., 1993) have indicated that the β-galactosidase activity can vary substantially between transformants, perhaps due to variable gene expression. In addition, preparing cultures for measuring enzyme activity can impose a selection on growth that changes the level of gene expression. By examining the growth of a population of initial transformants, this selection is precluded. Finally, our methods of analysis yield results that are consistent from transformation to transformation. All transformations were performed at least in duplicate. Each Gal4p-fusion construct was checked for its ability to activate transcription of the reporter genes nonspecifically. pACTII was used as a negative control to be cotransformed with a Gal4DB-based construct, while pLamin was cotransformed with a Gal4AD-based construct.
Table 5.
Two-hybrid interactions between Spc110p and components of the Tub4p complex
| Gal4p-DNA binding domain fused to | Gal4p-activation domain fused to | Growth on [-trp,-leu,-ade] platesa | Colony colorb |
|---|---|---|---|
| A. wt proteins | |||
| Spc98p | Spc110p (aa 1-183) | ++++ | Bc |
| Spc97p | Spc110p (aa 1-183) | − | Bd |
| Tub4p | Spc110p (aa 1-183) | −/+ | LB (≤1%) |
| Spc98p | Spc110p (aa 1-150) | +++ | B |
| Spc97p | Spc110p (aa 1-150) | − | W (3d); B (5d)e |
| B. Mutant proteins | |||
| Spc98p | Spc110-221p (aa 1-183) | ++ | W |
| Spc97p | Spc110-221p (aa 1-183) | nd | W |
| Spc98-63p | Spc110p (aa 1-183) | ++++ | B |
| Spc98-63p | Spc110p (aa 1-150) | ++ | nd |
| Spc98-63p | Spc110-221p (aa 1-183) | − | nd |
| Spc97-62p | Spc110p (aa 1-183) | − | W |
| Spc97-114p | Spc110p (aa 1-183) | − | W |
| C. Controls | |||
| laminf | Spc110p (aa 1-183) | − | W |
| lamin | Spc110p (aa 1-150) | − | W |
| lamin | Spc110-221p (aa 1-183) | − | W |
Growth of cells on SD-trp, -leu, -ade (−TLA) plates is scored between day 3 and when colonies are fully developed by day 5 or 6. When colonies are fully developed, the plating efficiency on −TLA plates is compared to the plating efficiency on SD-trp, -leu plates (−TL). ++++, plating efficiency on −TLA is ≥90% of −TL; colonies are fully developed on day 3; +++, ≥90% plating of −TL, colonies are small on day 3 and fully developed by day 5; ++, 50-90% plating of −TL; colonies are barely visible on day 3, fully developed by day 5; +, 50% plating of −TL; colonies are barely visible on day 3, fully developed by day 6; +/−, 25% plating of −TL; colonies are not visible on day 3, fully developed by day 6; −/+, no growth on day 3; colonies are barely visible on day 5, but do not get bigger thereafter; −, no growth. Results are tabulated from at least two independent transformations.
Colony-lift filter assays using X-gal are performed to measure β-galactosidase activity. (%) indicates the % of the total number of colonies transferred onto nitrocellulose filters that display color. A (≤5%) is considered background rather than a true interaction that occurs when 50-95% of transferred colonies display color after a 30° incubation overnight. Generally, “blue” colonies are blue within the first 1–3 h after incubation, but the final scoring reported here is for an overnight incubation to include weak interactions. B, blue; LB, light blue; W, white; nd, not determined. Observations are made from at least two independent transformations.
Blue colonies develop within 1 h of incubation at 30°C.
Blue colonies develop after 2 h incubation at 30°C.
Assays were performed using 3- or 5-d-old transformants.
Lamin instead of the vector pAS2 was used as the negative control because pAS2 by itself activates transcription of the reporter genes.
Gal4DB and Gal4AD plasmids were also transformed into Y190. We used Y190 even though PJ69–4A also contains a lacZ reporter because the promoter driving lacZ in PJ69–4A is activated by the cell permeabilization required to measure β-galactosidase by colony lifts (James et al., 1996). Y190 transformants were grown on −TL plates at 30°C for 3 d, and then lacZ activity was measured by the colony lift filter assay using X-gal as the substrate (Bartel and Fields, 1995). In some cases, the filter assays were repeated using 5-d-old transformants.
Expression of the fusion proteins was analyzed in an immunoblot of whole-cell extracts (Friedman et al., 1996) using anti-HA antibodies (monoclonal antibody 12CA5 from Boehringer Mannheim (Indianapolis, IN) or polyclonal antibody from Santa Cruz Biotechnology, Santa Cruz, CA). All fusion proteins to be compared were included on a single blot. At least two transformants were analyzed for each construct. For the comparison of the mutant Spc97p and the mutant Spc98p proteins with their wt counterparts, the levels of expression were normalized to major proteins in yeast cells as detected by staining the blot with Ponceau S.
RESULTS
A Synthetic Lethal Screen with spc110–221 Gives Three Complementation Groups
We performed a synthetic lethal screen with the spc110–221 allele using the red/white colony-sectoring assay combined with the plasmid-shuffle technique described in MATERIALS AND METHODS. The spc110–221 allele encodes mutations in the N-terminal region, region I. Strains carrying spc110–221 grow well at temperatures below 34°C. Out of 2 × 105 colonies screened for each mating type, we isolated 16 strains that contain mutations that lower the permissive temperature of the spc110–221 mutant to 30°C or below. Ten of these strains have additional mutations in the spc110–221 gene. The remaining six strains have mutations that define three complementation groups designated NSL1, NSL2, and NSL3 for N-terminal synthetic lethal (Table 3).
Table 3.
Complementation groups from the synthetic lethal screen with spc110-221
| Group | Alleles | Phenotype of double mutanta
|
Gene | ||
|---|---|---|---|---|---|
| 21°C | 25°C | 30°C | |||
| I | nsl1-62 | S | S | R | NSL1 = SPC97 |
| nsl1-113 | S | S | R | ||
| nsl1-114 | R | R | R | ||
| II | nsl2-85 | R | R | R | NSL2 |
| nsl2-118 | R | R | R | ||
| III | nsl3-63 | R | R | R | NSL3 = SPC98 |
The double mutant refers to an nsl spc110-221 mutant. These results were observed for the original isolates and were confirmed through at least one backcross. The phenotype is based on the red/white colony sectoring assay. S, Colonies sector; the double mutant does not display lethality at the indicated temperature. R, Colonies are solid red; the double mutant displays lethality at the indicated temperature.
NSL1 Is SPC97
When a yeast genomic library was transformed into a member of group I (nsl1–114), a library insert containing a portion of chromosome VIII complemented the synthetic lethality of nsl1–114 and spc110–221. Six potential ORFs flanked by CDC23 and ENO2 are contained in the insert. Subcloning narrowed the complementing region to a 3-kilobase (kb) segment containing YHR172w. This gene was subsequently identified as SPC97 (Knop et al., 1997). A plasmid containing a deletion of a 1.3 kb BamHI fragment from SPC97 does not complement the nsl1 mutation. Therefore, SPC97 is necessary to complement the synthetic lethal interaction of spc110–221 and nsl1–114. This 3-kb segment also complements the other alleles of NSL1 but not the alleles of NSL2 or NSL3.
Gap repair indicated that the mutations in the spc97 alleles clustered in the N-terminal part of the protein (see MATERIALS AND METHODS). The spc97–114 mutation resulted in an amino acid change from glutamate to lysine at position 56 of the protein. This change of a negatively charged amino acid to a positively charged amino acid confers the most severe phenotype of the spc97 alleles such that the spc110–221 spc97–114 double mutant is not viable at any temperature (Table 3). Leucine 22 is changed to phenylalanine in the spc97–62 allele. In the spc97–113 allele, arginine 171 is changed to a lysine. These conservative replacements have a milder effect and only decrease the permissive temperature of the spc110–221 to 30°C (Table 3). None of these alleles confer a phenotype in a strain carrying a single copy of wt SPC110 on the chromosome.
NSL3 Is SPC98
Because we found that spc97 exhibited synthetic lethality with spc110–221, we reasoned that mutations in SPC98 or TUB4, which encode other components in the Tub4p complex, may have been obtained in the screen. We transformed low copy number (CEN) plasmids containing either SPC97, SPC98, or TUB4 into the nsl2 spc110–221 mutants and the nsl3–63 spc110–221 mutant. The nsl2 spc110–221 mutants were not suppressed by SPC97, SPC98, or TUB4 on low-copy number plasmids. Therefore, NSL2 does not encode an identified component of the Tub4p complex. In contrast, SPC98 on a low-copy number plasmid fully suppressed the synthetic lethality conferred by the nsl3–63 allele. To determine whether nsl3–63 is linked to SPC98, we first constructed a strain containing spc110–221 and a copy of SPC98 marked with the URA3 gene (see MATERIALS AND METHODS). This strain was then mated to the nsl3–63 spc110–221 strain, and the resulting diploid was dissected. In the 37 tetrads in which URA3 segregated 2:2, all synthetic lethal spores were uracil auxotrophs, indicating that NSL3 is tightly linked to SPC98.
As final proof that nsl3–63 is an allele of SPC98, we demonstrated that the single mutation S754F in Spc98–63p is necessary and sufficient for synthetic lethality with spc110–221. The C2261T mutation in spc98–63 was fortuitously identified by sequencing the final 370 bases of spc98–63. To determine whether this mutation was sufficient to confer synthetic lethality, we tested three different dosages of spc98 carrying the C2261T mutation: on a CEN plasmid, as a second integrated copy, and finally as a single copy integrated in place of wt SPC98. In a diploid strain (DCY101) homozygous for both spc110–221 and spc98–63, synthetic lethality is observed in the presence of the low-copy number plasmid carrying C2261T spc98 (pDC9). In a haploid spc110–221 spc98–63 strain, even one extra copy of C2261T spc98 is sufficient to suppress the synthetic lethality. However, a single integrated copy of spc98 carrying the C2261T mutation conferred synthetic lethality to a spc110–221 strain as described in MATERIALS AND METHODS.
Further Genetic Interactions of SPC110 with SPC97 and SPC98
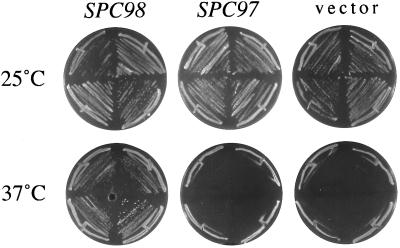
SPC98 is a dosage-dependent suppressor of the temperature sensitivity of a diploid spc110–221 mutant, but not of the temperature sensitivity conferred by any of the other spc110 alleles (Sundberg and Davis, 1997). SPC97 and TUB4 are not dosage-dependent suppressors of any of our spc110 alleles in diploids. Here we show that SPC98 on a centromere plasmid is sufficient for suppression of the temperature sensitivity in a haploid spc110–221 mutant (Figure 1). In contrast, a multicopy plasmid containing SPC97 does not suppress the phenotype of a haploid spc110–221 strain. These results suggest that the spc110–221 allele confers a specific defect in interaction with Spc98p.
Figure 1.
Suppression of the temperature-sensitive spc110–221 allele by SPC98. A haploid spc110–221 mutant (TNY2–2B) is suppressed by SPC98 on a CEN plasmid (pHS89) but not by SPC97 on a multicopy plasmid (pTN31). The vector plasmid was pRS316.
Defects at the N and C termini of Spc110p affect distinct functions (Sundberg and Davis, 1997). We tested whether the effects of the spc97 and spc98 mutations were specific to alleles of spc110 encoding N-terminal mutations in Spc110p. The spc98–63 allele is specific and exhibits synthetic lethality with only N-terminal spc110 alleles and not with any of the C-terminal spc110 alleles. In contrast, the spc97 alleles are lethal with both N-terminal and C-terminal alleles of spc110 (Table 4). Even the mild spc97 alleles, 97–62 and 97–113, affect both N-terminal and C-terminal spc110 alleles.
Table 4.
Synthetic lethality between temperature-sensitive spc110 alleles and spc97 and spc98 alleles
| Location of mutationsa | Allele | spc97-62 | spc97-113 | spc97-114 | spc98-63 | |
|---|---|---|---|---|---|---|
| N terminus | Region I | spc110-221 | SLb | SL | SL | SL |
| Region I | spc110-222 | SL | SL | SL | SL | |
| C terminus | Region II | spc110-226 | NO | NO | SL | NO |
| Region III | spc110-220 | NO | NO | SL | NO | |
| Regions II + III | spc110-225 | NO | SL | SL | NO | |
| Truncation | spc110-224 | SL | NO | SL | NO | |
Mutations in the spc110 alleles fall into three regions: region I is residues 1-163 N-terminal to the coiled-coil domain. Region II is aa 772-836. Region III is aa 897-917 and is the calmodulin-binding site.
SL, Synthetic lethality is detected between the two alleles at 30°C. NO, Double mutants are able to sector at 30°C. Genetic analysis is described in MATERIALS AND METHODS. The diploid strains that were sporulated to obtain these results are listed in Table 1.
Interactions between Spc98p and Spc110p
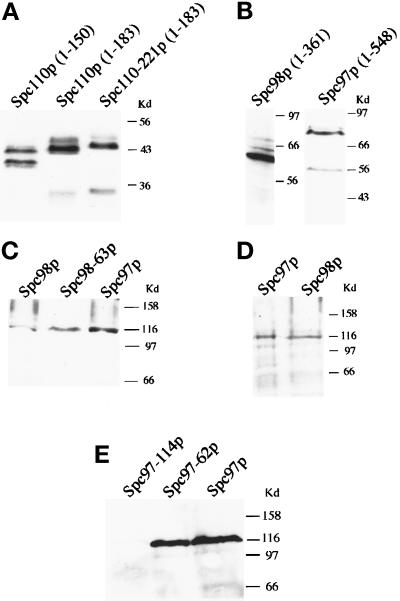
The diverse and allele-specific genetic interactions observed between SPC98 and SPC110 suggest that the two proteins interact. We sought evidence for physical interactions between Spc110p and Spc98p using a two-hybrid assay in which a positive interaction confers adenine prototrophy by allowing expression of the ADE2 gene. Three Gal4p-Spc110p-fusion constructs containing residues localized to the N-terminal region of Spc110p were created. Spc110p1–183 includes all of region I and 33 amino acid residues predicted to be part of the coiled-coil domain. Spc110p1–150 includes the majority of region I and none of the predicted coiled-coil sequence. To examine the effects of mutations in Spc110p on interactions, we also created the Spc110–221p1–183 construct. These three fusion constructs of Spc110p are stably expressed, and their levels of expression are similar as analyzed by Western blotting (Figure 2A). A construct encoding full-length Spc110p fused to the Gal4p-activation domain was not used because it is completely inactive in the two-hybrid assay and fails to interact even with calmodulin (Geiser et al., 1993).
Figure 2.
Immunoblots of Gal4p-fusion proteins in the two-hybrid strains. Panels A, B, C, and E are analyzed in strain PJ69–4A; panel D is analyzed in strain Y190. Each panel indicates a different blot. Whole-cell extracts obtained from an approximately equal amount of cells growing in midlog phase are loaded in each lane. panels A, B, and E are probed with rabbit anti-HA antibodies; panels C and D are probed with mouse anti-HA antibodies. In panel A, all Gal4p-Spc110p constructs migrate as doublets with molecular weights ranging from 40 to 44 kDa; in panel B, Gal4p-Spc98p1–361 migrates as a 64-kDa protein, and Gal4p-Spc97p1–548 migrates as a 84-kDa protein.
All three constructs that contain N-terminal sequences of Spc110p showed an interaction with Spc98p as measured by expression of the ADE2 reporter gene (Table 5A). Spc110p1–183 gives the strongest signal, whereas the shorter construct Spc110p1–150 gives a weaker signal, suggesting that the extra residues in Spc1101–183 are important for binding to Spc98p. Mutations in the spc110–221 allele substantially reduced the interactions between Spc110–221p and Spc98p (Table 5B), indicating that the binding site for Spc98p is disrupted in Spc110–221p. The S754F mutation in Spc98–63p abolishes the interaction with Spc110–221p1–183 consistent with the lethal phenotype of the spc110–221 spc98–63 mutant. Finally, the S754F mutation has no detectable effect on interaction with wt Spc110p1–183 in the two-hybrid assay (Table 5B).
Interactions between Spc110p and Spc97p
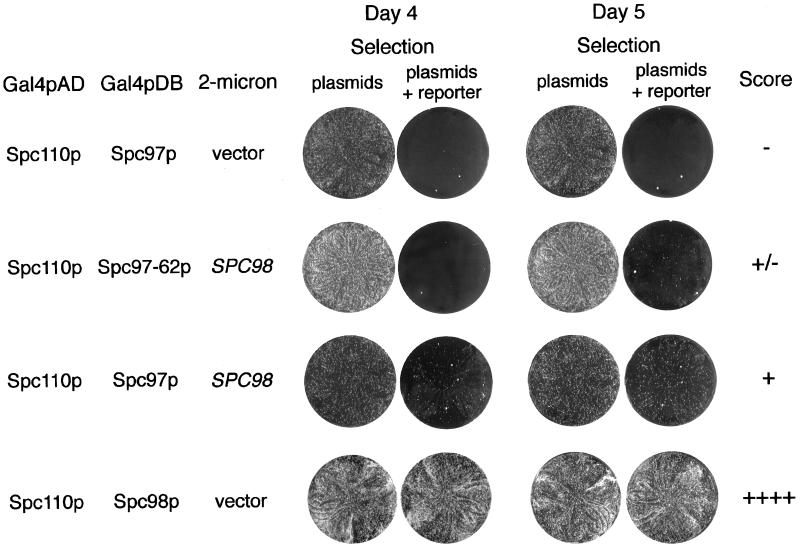
In contrast to Spc98p, Spc97p failed to interact with any of the Spc110p constructs as measured by expression of the ADE2 reporter gene in strain PJ69–4A. However, using a different two-hybrid strain (Y190) and monitoring expression of a different reporter (lacZ) allowed detection of a weak interaction between Spc97p and Spc110p1–183 (Table 5). We looked for conditions that would allow detection of the interaction between Spc97p and Spc110p in the PJ69–4A strain background. We found that increasing the level of wt Spc98p sufficiently enhanced the interaction between Gal4AD-Spc110p1–183 and Gal4DB-Spc97p to allow adenine prototrophy in strain PJ69–4A (Table 6A and Figure 3). The specificity of this three-way interaction is indicated by the fact that overexpressing TUB4 did not enhance the interaction between Spc110p and Spc97p (Table 6A), and overexpressing SPC97 did not enhance the interaction between Spc110–221p and Spc98p (Table 6B). Thus, Spc98p promotes the interaction between Spc110p and Spc97p fusion proteins in strain PJ69–4A. By Western blotting, the level of expression of Gal4-Spc97p and Gal4-Spc98p is similar in strain PJ69–4A and strain Y190 (Figure 2, C and D). Thus, differences in expression of the fusion proteins in the two strains are not the cause of the discrepancy.
Table 6.
Three-way interactions in the two-hybrid assay between Spc110p and Spc97p, Tub4p, or Spc98p
| Gal4p–DNA-binding domain fused to | Gal4p-activation domain fused to | Protein overexpresseda | Growth on [-trp,-leu,-ura,-ade] platesb |
|---|---|---|---|
| A. Interactions with Spc97 or Tub4p | |||
| Spc97p | Spc110p (aa 1-183) | Vector | − |
| Spc97p | Spc110p (aa 1-183) | Spc98p | + |
| Spc97p | Spc110p (aa 1-183) | Tub4p | − |
| Tub4p | Spc110p (aa 1-183) | Vector | −/+ |
| Tub4p | Spc110p (aa 1-183) | Spc98p | +/− |
| Tub4p | Spc110p (aa 1-183) | Spc97 | −/+ |
| Spc97-62p | Spc110p (aa 1-183) | Spc98p | +/− |
| Spc97-114p | Spc110p (aa 1-183) | Spc98p | − |
| Spc97p | Spc110-221p (aa 1-183) | Spc98p | − |
| B. Interactions with Spc98p | |||
| Spc98p | Spc110p (aa 1-183) | Vector | ++++ |
| Spc98p | Spc110p (aa 1-183) | Spc97p | ++++ |
| Spc98p | Spc110p (aa 1-150) | Vector | +++ |
| Spc98p | Spc110p (aa 1-150) | Spc97p | ++ |
| Spc98p | Spc110-221p (aa 1-183) | Vector | ++ |
| Spc98p | Spc110-221p (aa 1-183) | Spc97p | +/− |
| Spc98-63p | Spc110p (aa 1-183) | Vector | ++++ |
| Spc98-63p | Spc110p (aa 1-183) | Spc97p | ++++ |
| Spc98-63p | Spc110p (aa 1-150) | Vector | ++ |
| Spc98-63p | Spc110p (aa 1-150) | Spc97p | ++ |
| Spc98-63p | Spc110-221p (aa 1-183) | Vector | − |
| Spc98-63p | Spc110-221p (aa 1-183) | Spc97p | − |
Overexpression plasmids are 2μ based and contain URA3 as the selectable marker. The indicated gene products are expressed from their own promoters in these plasmids. Plasmids are given in Table 2.
Comparisons of growth rate are made between SD-trp,-leu,-ura plates vs. SD-trp,-leu,-ura,-ade plates in order to select for the third cotransformed plasmid. See footnotes under Table 5 for explanations of the scoring technique.
Figure 3.
Examples of three-way interactions in the two-hybrid system as scored by expression of the ADE2 gene. Each row shows a combination of two Gal4p-fusion proteins plus a third protein produced from the 2μ plasmid. The plasmids are given in Table 2. The two plates on the left indicate growth after 4 d, and the two on the right show growth after 5 d. The medium for selection of the plasmids is SD-trp,-leu,-ura. Interaction between proteins confers adenine prototrophy. The medium for selection of the plasmids and the reporter is SD-trp,-leu,-ura,-ade. The scoring designation for each combination is given in the last column.
We next examined the interactions between mutant Spc97p and Spc110p fusion proteins. No interaction was detected between Spc97p and Spc110–221p1–183 as measured either by expression of ADE2 in the presence of extra copies of SPC98 (Table 6A) or by expression of β-galactosidase in strain Y190 (Table 5B). Since the interaction between Spc97p and Spc110–221p1–183 was not detectable, we could not test whether the synthetic lethal mutation in Spc97–62p had any further effects on interaction with Spc110–221p1–183. However, the mutation in the Spc97–62p fusion protein reduced interactions with the wt Spc110p fusion protein in the absence (Tables 5B) or presence of extra Spc98p (Table 6A and Figure 3). By Western blotting, Gal4-Spc97–62p is expressed at a similar level as wt Gal4-Spc97p (Figure 2E), indicating that decreased interactions are not due to a decreased amount of fusion protein. No interaction is detectable between Spc97–114p and Spc110p, but the low level of the Spc97–114p fusion protein may be the cause (Figure 2E).
Interactions between Spc110p and Tub4p
Although TUB4 was not identified in our synthetic lethal screen, we tested for interactions between Tub4p and Spc110p because it forms a complex with Spc97p and Spc98p. When fused to the Gal4pDB, Tub4p gives a background signal in both two-hybrid systems (−/+). Interaction with Spc110p1–183 was not detectable above this background (Table 5). Extra copies of SPC98 promoted the interaction between Spc110p and Tub4p to a level that was detectable above background (Table 6A). Note that extra copies of SPC97 have no effect on this interaction.
Genetic Evidence That Interaction between Spc97p and Spc110p Is Dependent on Spc98p
Our genetic and two-hybrid results led to a two-part hypothesis. First, Spc98p is primarily responsible for the interaction between the Tub4p complex and Spc110p. Second, the interaction between Spc97p and Spc110p is dependent on Spc98p. We performed additional analyses to test this hypothesis. Consistent with the hypothesis, SPC98 is a dosage-dependent suppressor of the synthetic lethality between spc97 and spc110, but SPC97 is not a suppressor of the synthetic lethality between spc98 and spc110 (Table 7). The ability of SPC98 to suppress correlates with the severity of the spc97 allele. The most severe allele, spc97–114, is only suppressed at 25°C, whereas the milder alleles, spc97–62 and spc97–113, are suppressed at 30°C.
Table 7.
Dosage-dependent suppression of synthetic lethality at 30°Ca
| Plasmid | Synthetic lethal mutant
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
spc110-221 spc97-114
|
spc110-221 spc97-113
|
spc110-221 spc97-62
|
spc110-221 spc98-63
|
|||||
| 25°C | 30°C | 25°Cb | 30°C | 25°C | 30°C | 25°C | 30°C | |
| 2 μ SPC97 | +++ | +++ | NAb | +++ | NA | +++ | − | − |
| 2 μ SPC98 | + | − | NA | ++ | NA | + | +++ | +++ |
| 2 μ TUB4 | − | − | NA | − | NA | − | ++ | + |
Each synthetic lethal strain was transformed with the indicated plasmid, and transformants were restreaked on selective plates at 25°C and 30°C. The plasmids were pHS92 (2μ SPC97), pHS88 (2μ SPC98), and pHS91 (2μ TUB4). The strains were (in order from left to right): TNY137, TNY163, TNY162, and TNY76-1C. Sectoring was assessed after 8 d. The ability of a solid red mutant to sector white at the indicated temperature indicates whether its synthetic lethality is suppressed. +++, the mutant is suppressed; the colonies are almost completely white. ++, the mutant is suppressed; the colonies have some red sectors. +, the mutant is suppressed; the colonies have larger red sectors. −, the mutant is not suppressed; the colonies are as solid red as colonies with empty vector.
Not applicable; these alleles do not confer a synthetic lethal phenotype at 25°C. See Table 3.
Interactions among Components of the Tub4p Complex
Our hypothesis predicts that the synthetic lethality between spc97 and spc110 is caused indirectly by a reduced interaction between the Spc97p mutant proteins and Spc98p. To test this prediction, we characterized the interactions among the components of the Tub4p complex using a two-hybrid assay.
We first analyzed the interactions among the wt proteins. wt Spc97p, Spc98p, and Tub4p all interact with each other in pairwise combinations (Table 8A) as shown previously (Knop et al., 1997). However, our results differ from the previous results in two ways. First, we found that residues 1–361 of Spc98p are sufficient for interaction between Spc98p and Tub4p, and additional sequences C-terminal of residue 551 in Spc98p are not required (Table 8A). Second, interaction between two molecules of Tub4p is readily detected in our two-hybrid system.
Table 8.
Two-hybrid interactions among components of the Tub4p complex
| Gal4p-DNA binding domain fused to | Gal4p-activation domain fused to | Growth on [-trp,-leu,-ade] plates a |
|---|---|---|
| A. wt proteins | ||
| Spc97p | Spc98p | ++++ |
| Spc97p | Spc98p (aa 148-846) | +++ |
| Spc97p | Spc98p (aa 1-361) | + |
| Spc97p | Tub4p | ++++ |
| Spc97p | Spc97p | − |
| Spc98p | Spc97p | ++++ |
| Spc98p | Spc97p (aa 1-548)b | ++ |
| Spc98p | Tub4p | ++++ |
| Spc98p | Spc98p | − |
| Tub4p | Spc98p | ++ |
| Tub4p | Spc98p (aa 148-846) | +++ |
| Tub4p | Spc98p (aa 1-361) | +++ |
| Tub4p | Spc97p | ++++ |
| Tub4p | Spc97p (aa 1-548) | +++ |
| Tub4p | Tub4p | +++ |
| B. Mutant proteins | ||
| Spc97-62p | Spc98p | +++ |
| Spc97-62p | Spc98p (aa 148-846) | ++ |
| Spc97-62p | Spc98p (aa 1-361) | − |
| Spc97-62p | Tub4p | ++++ |
| Spc97-114p | Spc98p | +/− |
| Spc97-114p | Spc98p (aa 148-846) | − |
| Spc97-114p | Spc98p (aa 1-361) | − |
| Spc97-114p | Tub4p | ++++ |
| Spc98-63p | Spc97p | ++ |
| Spc98-63p | Spc97p (aa 1-548) | ++++ |
| Spc98-63p | Tub4p | ++++ |
See Table 5 for explanations of this scoring technique.
Numbers in parentheses indicate amino acid residues of the tested protein that are fused to the Gal4p domain. If no numbers are indicated, the full-length protein is included.
Our interest lay in characterizing the effects of the synthetic lethal mutations isolated in this study on interactions among the components of the Tub4p complex. In agreement with our prediction, the L22F mutation in Spc97–62p reduced interaction with all of the Spc98p fusion proteins (Table 8). The E56K mutation in Spc97–114p abolished these interactions. The effects of the S754F mutation on the interaction of Spc98–63p with Spc97p is complex. The mutation actually enhanced interaction with the N-terminal (1–548) sequences of Spc97p but reduced the interaction with full-length Spc97p (Table 8B). Finally, the mutations in Spc97–62p, Spc97–114p, and Spc98–63p did not affect their interactions with Tub4p (Table 8B).
Evidence That Spc97p Sequesters Spc98p from Spc110p
One distinction between SPC97 and SPC98 is that SPC98 is a dosage-dependent suppressor of spc110–221 but SPC97 is not (see Figure 1). We explored whether this lack of suppression was merely due to insufficient SPC97 being expressed from the high-copy number vector by testing whether even higher expression levels could suppress the temperature sensitivity conferred by any of the spc110 alleles. Even overexpression of SPC97 using a GAL-inducible promoter did not suppress the temperature sensitivity of the spc110 mutants. Instead, the GAL overexpression of SPC97 from a CEN plasmid lowered the permissive temperature of all the spc110 mutants but had little effect on a wt strain (Table 9).
Table 9.
GAL overexpression of SPC97 in spc110 diploid strains
| Straina | Permissive temperature
|
|
|---|---|---|
| GAL1 vectorb | GAL1-SPC97 | |
| spc110-220/spc110-220 | 32°C | 30°C |
| spc110-221/spc110-221 | 34°C | 21°C |
| spc110-224/spc110-224 | 30°C | Dead |
| spc110-225/spc110-225 | 30°C | 25°C |
| spc110-226/spc110-226 | 32°C | 21°C |
| SPC110/SPC110 | 37°C | 37°Cc |
The strains used were (in order of appearance) HSY20, HSY21, HSY46, HSY48, HSY83 (Sundberg and Davis, 1997) and JGY46 (Geiser et al., 1991).
The GAL1 vector is pRD54 and the GAL1-SPC97 plasmid is pTN26.
The colonies are healthy but smaller than ones that just have the GAL vector.
To further explore the toxic effects of overexpression of SPC97, we analyzed the effects of extra copies of SPC97 on the interaction between Spc98p and Spc110p by the two-hybrid assay. While extra copies of SPC97 had no effect on the interaction between Spc98p and Spc110p1–183, it reduced the interaction of Spc98p with either Spc110p1–150 or Spc110–221p1–183 (Table 6B). Since the mutation in Spc98–63p reduces its ability to interact with full- length Spc97p (Table 8B), extra copies of SPC97 had no effect on the interaction of the mutant Spc98–63p with Spc110p1–150 or with Spc110–221p1–183 (Table 6B).
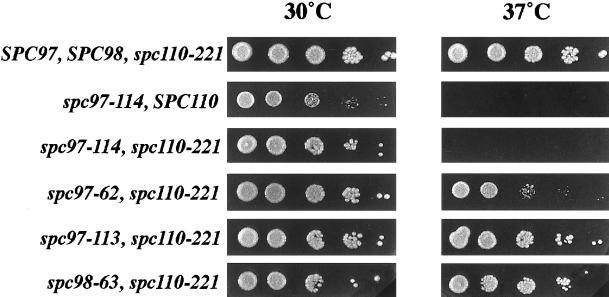
Overexpression of SPC110 Is Toxic to the spc97 Mutants
The toxic effect of overexpression was not confined to SPC97. Overexpression of SPC110 confers a temperature-sensitive growth defect to two of the spc97 mutant strains, spc97–114 and spc97–62 (Figure 4). The toxic effect is strongest with the spc97–114 mutant, which is killed at 37°C by overexpression of SPC110 and is independent of the spc110–221 allele. The spc97 alleles, by themselves, do not have a phenotype at 37°C, and a strain containing only the spc110–221 allele grows well with extra copies of SPC110 at 37°C (Figure 4). Furthermore, overexpression of SPC110 does not cause any growth defects in the spc98–63 spc110–221 mutant. Thus, overexpression of SPC110 causes temperature sensitivity only in the presence of the spc97 mutations.
Figure 4.
Overexpression of Spc110p is toxic to spc97 mutants. Each strain contains SPC110 on a multicopy plasmid (pHS26). The relevant genotype is given beside each row. The strains were (in order from top to bottom): TNY2–4B, TNY64–5C, TNY137, TNY114–39C, TNY113–10A, and TNY76–1C. Serial dilutions containing 1 × 105, 1 × 104, 1 × 103, 1 × 102, and 10 cells were plated for each strain on YPD and incubated at 30°C and 37°C for 52 h.
DISCUSSION
Previous analyses of SPC110 suggest a role for Spc110p in anchoring the inner plaque to the central plaque at the SPB (Kilmartin and Goh, 1996; Sundberg et al., 1996; Sundberg and Davis, 1997; Knop and Schiebel, 1997). A screen for mutations that enhance the temperature-sensitive phenotype conferred by an spc110 allele encoding mutations in the N terminus of Spc110p identified SPC97 and SPC98. Spc97p, Spc98p, and Tub4p form a soluble 6S complex in yeast cells and are found at the inner and outer plaques of the SPB (Rout and Kilmartin, 1990; Spang et al., 1996a; Knop et al., 1997). All three proteins are required for proper spindle formation, but it remains to be shown how the Tub4p complex assembles into the inner and outer plaques of the SPB (Geissler et al., 1996; Marschall et al., 1996; Spang et al., 1996a; Knop et al., 1997). Although they share many properties, Spc97p and Spc98p are not similar in their interactions with Spc110p. The genetic and two-hybrid results together strongly argue that Spc98p is primarily responsible for attaching the Tub4p complex to the N terminus of Spc110p (Figure 5). The evidence is summarized in Table 10.
Figure 5.
Model of the linkage of the Tub4p complex to Spc110p at the SPB.
Table 10.
Characteristics of Spc97p and Spc98p
| Properties | Spc97p | Spc98p |
|---|---|---|
| Similarities | ||
| Binds to Tub4p | Yesa | Yesb |
| Present in Tub4p-complex | Yesa | Yesa |
| Primary sequence similarity | Yes | Yes |
| Mutants show synthetic lethality with spc110-221 (contains mutations in the N-terminal region I) | Yes | Yes |
| Differences | ||
| Overexpression suppresses spc110-221 | No | Yes |
| Interaction with wt Spc110p in two-hybrid assays | Weak | Robust |
| Overexpression influences interactions with Spc110p in two-hybrid assay | Weakens Spc98p-Spc110p interaction | Enhances Spc97p-Spc110p interaction |
| Mutants show synthetic lethality with spc110 alleles encoding mutations in C-terminal regions | Yes | No |
| Interaction with wt Spc110p reduced by synthetic lethal mutations | Yes | No |
| Overexpression toxic to spc110 mutants | Yes | No |
| Overexpression of SPC110 is toxic to mutants | Yes | No |
Interaction between the N terminus of Spc110p and Spc98p generated a robust signal in two different two-hybrid systems. Interaction of Spc110p with Spc97p and Tub4p did not. Overexpression of SPC98 enhanced the interaction between Spc110p and Spc97p and between Spc110p and Tub4p. Thus, Spc98p mediates or stabilizes the interactions between Spc110p and the other two components of the Tub4p complex.
At the nonpermissive temperature, the spc110–221 allele causes cells to arrest at the MAD1 checkpoint with spindles that appear morphologically normal (Sundberg and Davis, 1997). SPC98 is the only gene in the Tub4p complex that can suppress the temperature-sensitive growth of spc110–221 mutants ([Sundberg and Davis, 1997] and further characterized herein). Thus, the interaction between Spc98p and Spc110p is specifically impaired in these cells.
Mutations in either SPC98 or SPC97 worsen the phenotype conferred by spc110–221, but they do so through different mechanisms. The spc98–63 allele further compromises the impaired interaction between Spc98p and Spc110–221p. As measured in a two-hybrid assay, the S754F mutation encoded by spc98–63 completely abolishes the interaction of Spc98–63p with Spc110–221p. The specificity of this effect was shown in two ways. First, the S754F mutation has no effect on the interaction of Spc98–63p with wt Spc110p. Second, spc98–63 enhances the phenotypes conferred only by alleles of spc110 encoding N-terminal mutations and not by alleles encoding C-terminal mutations even though the latter group of alleles confers more severe phenotypes. This type of allele specificity is strong genetic evidence for a direct interaction between Spc98p and Spc110p.
In contrast to the spc98 allele, the spc97 alleles indirectly affect interaction with Spc110–221p by decreasing interaction with Spc98p. The evidence for this indirect effect is twofold. First, overexpression of SPC110 is toxic to spc97–114 spc110–221 and spc97–62 spc110–221 mutants at 37°C. This toxic effect is the opposite of that expected if the spc97 alleles directly decreased the binding of mutant Spc97p to Spc110–221p (also see further discussion below). Second, as expected for an effect mediated through a third protein (in this case Spc98p), the effects of the spc97 alleles are not specific to the spc110–221 allele. The lack of specificity is shown in three ways. The spc97 alleles decrease the permissive temperature of the C-terminal Spc110p mutants, which have a normal N-terminal region. Moreover, in addition to decreasing interaction with Spc110–221p, the L22F mutation decreases the interaction of Spc97–62p with wt Spc110p as measured in the two-hybrid assay. Finally, the reduction in binding to Spc110p occurs because the mutation in Spc97–62p reduces its binding to Spc98p.
The distinction between Spc98p and Spc97p is further demonstrated by the effects of overexpressing each of the genes. Overexpression of SPC98 enhances the interaction between Spc97p and Spc110p as measured by the two-hybrid assay. In vivo, overexpression of SPC98 suppresses the synthetic lethality of all spc97 spc110–221 mutants. In contrast, overexpression of SPC97 interferes with the ability of wt Spc98p to interact with Spc110p1–150 or Spc110–221p and lowers the permissive temperatures of spc110 mutants. Because the decreased interaction between Spc98–63p and Spc97p prevents the toxic effect of overproduced Spc97p, we believe the toxicity is due to the sequestration of Spc98p by Spc97p.
The diverse dosage-dependent effects described here suggest that the stoichiometry of Spc97p and Spc98p is delicately balanced. Spc98p is responsible for targeting the Tub4p complex to the nucleus and is the only protein in the complex that contains a nuclear localization signal (Geissler et al., 1996; Pereira et al., 1998). Moreover, overexpressing SPC98 causes the accumulation of Spc98p in the nucleus, whereas overexpressing SPC97 leads to the accumulation of Spc97p in the cytoplasm (Knop et al., 1997; Knop and Schiebel, 1997). It is tempting to speculate that the appropriate ratio of Tub4p complex in the cytoplasm and in the nucleoplasm must exist to ensure a balance in the nucleation of nuclear and cytoplasmic microtubules.
The stoichiometry of Spc110p to the components of the Tub4p complex is also balanced. High expression of SPC97 from a GAL1 promoter is toxic to all spc110 mutants. The overproduction of wild-type Spc97p may sequester Spc98p in the cytoplasm and limit the amount of Spc98p that can incorporate into the inner plaque. This deficit of Spc98p in the nucleus is exacerbated in the spc110 mutants by the weakened attachment of the inner plaque to the SPB, whether by disruption of the C terminus or N terminus of Spc110p (Sundberg and Davis, 1997). Conversely, a high level of SPC110 is toxic to spc97 mutants at 37°C. Overproducing Spc110p may sequester Spc98p in the nucleus, resulting in a deficit of Spc98p in the cytoplasm. This deficit is worsened by mutations in Spc97p that impair binding to Spc98p and therefore interfere with the ability of Spc97p to keep Spc98p in the cytoplasm.
Recently, Tassin et al. (1997) reported the identification of human and Xenopus proteins, of 100 and 116 kDa, respectively, that cross-react with monoclonal antibodies raised against yeast Spc110p. The vertebrate Spc110p-related proteins localize to centrosomes and are phosphorylated during mitosis. Interestingly, a portion of the Spc110p-related protein identified in Xenopus extract copurifies with a γ-TuRC–like complex of 25S. Furthermore, antibodies against Spc110p inhibit microtubule nucleation on isolated centrosomes. We and others find no evidence for a soluble complex containing Spc110p and the proteins of the Tub4p complex in yeast (Vinh, unpublished results; Knop and Schiebel, 1997). However, the evidence presented here and that reported previously (Knop and Schiebel, 1997) demonstrate that Spc110p interacts with the Tub4p complex in yeast. The association of Spc110p with the microtubule nucleation machinery has been detected cytologically. Mutant cells containing Spc110p defective in binding calmodulin exhibit an aberrant intranuclear structure that emanates microtubules and contains Spc110p, Tub4p, and Spc98p (Sundberg et al., 1996; Sundberg and Davis, 1997). Strong overexpression of wt Spc110p results in the formation of a polymeric structure that anchors microtubules (Kilmartin and Goh, 1996). Therefore, Spc110p is physically associated with structures that polymerize microtubules in both the SPB and the centrosome, two organelles that are structurally diverse. These observations suggest that the underlying mechanism of microtubule nucleation is conserved between yeast and higher eukaryotes.
ACKNOWLEDGMENTS
We thank P. James for providing strain PJ69–4A before publication and S. Fields for gifts of plasmids and discussions. We thank M. Flory, S. Francis, E. Muller, and H. Sundberg for careful reading of the manuscript and helpful discussions. This work was supported by National Institutes of Health grant GM-40506, National Institute General Medical Sciences (T.N.D). T. Nguyen was supported by Public Health Service National Research Service Award T32 GM-07270, National Institute of General Medical Sciences. D.V. was supported by a Public Health Service National Research Service Award F32 GM-17946, National Institute of General Medical Sciences.
REFERENCES
- Bartel PL, Fields S. Analyzing protein-protein interactions using two-hybrid system. Methods Enzymol. 1995;254:241–263. doi: 10.1016/0076-6879(95)54018-0. [DOI] [PubMed] [Google Scholar]
- Bullitt E, Rout MP, Kilmartin JV, Akey CW. The yeast spindle pole body is assembled around a central crystal of Spc42p. Cell. 1997;89:1077–1086. doi: 10.1016/s0092-8674(00)80295-0. [DOI] [PubMed] [Google Scholar]
- Byers B. Cytology of the yeast life cycle. In: Strathern JN, Jones EW, Broach JR, editors. The Molecular Biology of the Yeast Saccharomyces: Life Cycle and Inheritance. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1981a. pp. 59–96. [Google Scholar]
- Byers B. Multiple roles of the spindle pole bodies in the life cycle of Saccharomyces cerevisiae. In: von Wettstein D, Friis J, Kielland-Brandt M, Stenderup A, editors. Molecular Genetics in Yeast, Alfred Benzon Symposium 16. Copenhagen: Munksgaard; 1981b. pp. 119–134. [Google Scholar]
- Davis T. Genetic analysis of calcium-binding proteins in yeast. In: Smith VL, Dedman JR, editors. Stimulus Response Coupling: The Role of Intracellular Calcium-binding Proteins. Boston: CRC Press; 1990. pp. 237–249. [Google Scholar]
- Durfee T, Becherer K, Chen P-L, Yeh S-H, Yang Y, Kilburn AE, Lee W-H, Elledge SJ. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 1993;7:555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- Friedman DB, Sundberg HA, Huang E, Davis TN. The 110-kD spindle pole body component of Saccharomyces cerevisiae is a phosphoprotein that is modified in a cell cycle-dependent manner. J Cell Biol. 1996;132:903–914. doi: 10.1083/jcb.132.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser JR. Genetic analysis of the essential calmodulin functions in Saccharomyces cerevisiae. Thesis. Seattle, WA: University of Washington; 1993. [Google Scholar]
- Geiser JR, Sundberg HA, Chang BH, Muller EGD, Davis TN. The essential mitotic target of calmodulin is the 110-kilodalton component of the spindle pole body in Saccharomyces cerevisiae. Mol Cell Biol, 1993;13:7913–7924. doi: 10.1128/mcb.13.12.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser JR, van-Tuinen D, Brockerhoff SE, Neff MM, Davis TN. Can calmodulin function without binding calcium? Cell. 1991;65:949–959. doi: 10.1016/0092-8674(91)90547-c. [DOI] [PubMed] [Google Scholar]
- Geissler S, Pereira G, Spang A, Knop M, Souès S, Kilmartin J, Schiebel E. The spindle pole body component Spc98p interacts with the Saccharomyces cerevisiae at the sites of microtubule attachment. EMBO J. 1996;15:3899–3911. [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Schiestl RH. Transforming yeast with DNA. Methods Mol Cell Biol. 1995;5:255–269. [Google Scholar]
- Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Hoffman CS, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene, 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Horio T, Oakley BR. Human γ-tubulin functions in fission yeast. J Cell Biol. 1994;126:1465–1473. doi: 10.1083/jcb.126.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio T, Uzawa S, Jung MK, Oakley BR, Tanaka K, Yanagida M. The fission yeast gamma-tubulin is essential for mitosis and is localized at microtubule organizing centers. J Cell Sci. 1991;99:693–700. doi: 10.1242/jcs.99.4.693. [DOI] [PubMed] [Google Scholar]
- James P, Halladay J, Craig EA. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin JV, Dyos SL, Kershaw D, Finch JT. A spacer protein in the Saccharomyces cerevisiae spindle pole body whose transcript is cell cycle-regulated. J Cell Biol. 1993;123:1175–1184. doi: 10.1083/jcb.123.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin JV, Goh P-Y. Spc110p: assembly properties and role in the connection of nuclear microtubules to the yeast spindle pole body. EMBO J. 1996;15:4592–4602. [PMC free article] [PubMed] [Google Scholar]
- Knop M, Pereira G, Geissler S, Grein K, Schiebel E. The spindle pole body component Spc97p interacts with the γ-tubulin of Saccharomyces cerevisiae and functions in microtubule organization and spindle pole body duplication. EMBO J. 1997;16:1550–1564. doi: 10.1093/emboj/16.7.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop M, Schiebel E. Spc98p and Spc97p of the yeast γ-tubulin complex mediate binding to the spindle pole body via their interaction with Spc110p. EMBO J. 1997;16:6985–6995. doi: 10.1093/emboj/16.23.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschall LG, Jeng RL, Mulholland J, Stearns T. Analysis of Tub4p, a yeast γ-tubulin-like protein: implications for microtubule-organizing center function. J Cell Biol. 1996;134:443–454. doi: 10.1083/jcb.134.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M, Braunfeld MB, Sedat JW, Alberts B, Agard DA. Microtubule nucleation by γ-tubulin-containing rings in the centrosome. Nature. 1995;378:638–640. doi: 10.1038/378638a0. [DOI] [PubMed] [Google Scholar]
- Moudjou M, Bordes N, Paintrand M, Bornens M. γ-Tubulin in mammalian cells: the centrosomal and the cytosolic forms. J Cell Sci. 1996;109:875–887. doi: 10.1242/jcs.109.4.875. [DOI] [PubMed] [Google Scholar]
- Muller EGD. A glutathione reductase mutant of yeast accumulates high levels of oxidized glutathione and requires thioredoxin for growth. Mol Biol Cell. 1996;7:1805–1813. doi: 10.1091/mbc.7.11.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley BR, Oakley CE, Yoon Y, Jung MK. γ-Tubulin is a component of the spindle pole body that is essential for microtubule function in Aspergillus nidulans. Cell. 1990;61:1289–1301. doi: 10.1016/0092-8674(90)90693-9. [DOI] [PubMed] [Google Scholar]
- Oakley CE, Oakley BR. Identification of γ-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans. Nature. 1989;338:662–664. doi: 10.1038/338662a0. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver TL, Szostak JW. Yeast recombination: the association between double-strand gap repair and crossing-over. Proc Natl Acad Sci USA. 1983;80:4417–4421. doi: 10.1073/pnas.80.14.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira G, Knop M, Schiebel E. Spc98p directs the yeast γ-tubulin complex into the nucleus and is subject to cell cycle-dependent phosphorylation on the nuclear side of the spindle pole body. Mol Biol Cell. 1998;9:775–793. doi: 10.1091/mbc.9.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff JW, Kellogg DR, Alberts BM. Drosophila γ-tubulin is part of a complex containing two previously identified centrosomal MAPs. J Cell Biol. 1993;121:823–835. doi: 10.1083/jcb.121.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MD, Novick P, Thomas JH, Botstein D, Fink GR. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60:237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Rout MP, Kilmartin JV. Components of the yeast spindle and spindle pole body. J Cell Biol. 1990;111:1913–1927. doi: 10.1083/jcb.111.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel SG, Snyder M. A highly divergent γ-tubulin gene is essential for cell growth and proper microtubule organization in Saccharomyces cerevisiae. J Cell Biol. 1995;131:1775–1788. doi: 10.1083/jcb.131.6.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A, Geissler S, Grein K, Schiebel E. γ-Tubulin-like Tub4p of Saccharomyces cerevisiae is associated with the spindle pole body substructures that organize microtubules and is required for mitotic spindle formation. J Cell Biol. 1996a;134:429–441. doi: 10.1083/jcb.134.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A, Grein K, Schiebel E. The spacer protein Spc110p targets calmodulin to the central plaque of the yeast spindle pole body. J Cell Sci. 1996b;109:2229–2237. doi: 10.1242/jcs.109.9.2229. [DOI] [PubMed] [Google Scholar]
- Stearns T, Evans L, Kirschner M. γ-Tubulin is a highly conserved component of the centrosome. Cell. 1991;65:825–836. doi: 10.1016/0092-8674(91)90390-k. [DOI] [PubMed] [Google Scholar]
- Stearns T, Kirschner M. In vitro reconstitution of centrosome assembly and function: the central role of γ-tubulin. Cell. 1994;76:623–637. doi: 10.1016/0092-8674(94)90503-7. [DOI] [PubMed] [Google Scholar]
- Stirling DA, Rayner TF, Prescott AR, Stark MJR. Mutations which block the binding of calmodulin to Spc110p cause multiple mitotic defects. J Cell Sci. 1996;109:1297–1310. doi: 10.1242/jcs.109.6.1297. [DOI] [PubMed] [Google Scholar]
- Sundberg HA, Davis TN. A mutational analysis identifies three functional regions of the spindle pole component Spc110p in Saccharomyces cerevisiae. Mol Biol Cell. 1997;8:2575–2590. doi: 10.1091/mbc.8.12.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg HA, Goetsch L, Byers B, Davis TN. Role of calmodulin and Spc110p interaction in the proper assembly of spindle pole body components. J Cell Biol. 1996;133:111–124. doi: 10.1083/jcb.133.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassin AM, Celati C, Paintrand M, Bornens M. Identification of an Spc110p-related protein in vertebrates. J Cell Sci. 1997;110:2533–2545. doi: 10.1242/jcs.110.20.2533. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Jung MK, Oakley BR. γ-Tubulin is present in Drosophila melanogaster and Homo sapiens and is associated with the centrosome. Cell. 1991;65:817–823. doi: 10.1016/0092-8674(91)90389-g. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Wong M-L, Alberts B, Mitchison T. Nucleation of microtubule assembly by a γ-tubulin-containing ring complex. Nature. 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]
- Zhu G. Characterization of three fork head proteins in Saccharomyces cerevisiae. Thesis. Seattle, WA: University of Washington; 1996. , 134. [Google Scholar]