Abstract
There is much interest in the development of nanoscale drug delivery system with MRI visibility to optimize the delivery efficiency and therapeutic efficacy under image guidance. Here we report on the successful fabrication of nanoscale micelles based on biodegradable poly(L-glutamic acid)-b-polylactide (PG-b-PLA) block copolymer with paramagnetic Gd3+ ions chelated to their shell. (PG-b-PLA) was synthesized by sequential polymerization reactions: anionic polymerization of L-lactide followed by ring opening polymerization of benzyl glutamate N-carboxylic anhydride. The metal chelator p-aminobenzyldiethylenetriaminepenta(acetic acid) (DTPA) was readily conjugated to the side chain carboxylic acids of poly(L-glutamic acid). The resulting copolymer formed spherical micelles in aqueous solution with an average diameter of 230 nm at pH 7.4. The size of PG(DTPA)-b-PLA micelles decreased with increasing pH value. DTPA-Gd chelated to the shell layer of the micelles exhibited significantly higher spin-lattice relaxivity (r1) than a small-molecular-weight MRI contrast agent, indicating that water molecules could readily access the Gd ions in the micelles. Because of the presence of multiple carboxylic acid functional groups in the shell layer, polymeric micelles based on biodegradable PG(DTPA-Gd)-b-PLA may be a suitable platform for the development of MRI-visible, targeted nanoscale drug delivery systems.
Keywords: Polymeric micelle, Poly(L-glutamic acid), Block copolymer, Nanoparticle, Magnetic resonance imaging
INTRODUCTION
Recent advances in molecular imaging as well as nanotechnology have led to the natural merger of these two evolving fields and provide us an unprecedented opportunity to invent molecular therapeutic carriers that their delivery can be optimized under image guidance (termed theragnostic). The key ingredients of a successful theragnostic approach are imaging the molecular features of cancer and noninvasive visualization and monitoring of delivery of therapeutic agents. The latter is a pivotal element to guide and optimize the design of drug delivery efficiency. Magnetic resonance imaging (MRI) is an ideal imaging modality for theragnostic approach owing to its high spatial and temporal resolution 1, 2.
A variety of nanoscale carrier systems, including dendrimers 3, 4, linear polymers 5-7, proteins 8, and micelles 9-11, has been proposed and evaluated as MRI contrast agents. Among these nanocarriers, micellar systems complexed with paramagnetic Gd(III) ions are very promising because of their ability to provide positive contrast (i.e. T1-weighted images), robust structural features, and simple fabrication. Most Gd-containing micellar carriers are formed from low-molecular-weight surfactants composed of a hydrophobic chain and a hydrophilic head group, such as the p-aminobenzyldiethylenetriaminepenta(acetic acid)-Gd (DTPA-Gd) complex. In such a micellar assembly, the rate of water exchange is similar to that observed in DTPA-Gd because the Gd complex is exposed in the exterior shell layer of the micelle 9. Gd-containing micelles not only have a long rotational correlation time, thus exhibiting a high proton T1 relaxivity, but also can be conjugated with tumor-targeting ligands for molecular MRI. For example, mixed micellar aggregates containing C-terminal cholecystokinin octapeptide (CCK) and Gd3+ complexes have been synthesized as tumor-specific MRI contrast agents 12. However, micelles composed of low-molecular-weight surfactants usually exhibit a relatively high critical micelle concentration (CMC). Limited in vivo stability of this type of micelle can be an obstacle to its clinical application.
Polymeric micelles consisting of amphiphilic block copolymers usually exhibit very low CMC and are more stable than micelles formed from surfactants 13-15. In addition, they possess a high loading capacity, are capable of delivering multiple agents 16, and allow the introduction of targeting moieties to the surface, which is crucial for multivalent recognition and high target avidity. Moreover, tailoring the structure of the hydrophilic segment of the copolymer can readily modulate their pharmacological properties. For example, micelles with copolymers having a poly(ethylene glycol) (PEG) segment usually demonstrate prolonged blood circulation time, which is an appealing feature for contrast agents used for blood pool imaging 17. Several polymerization methods, including atom transfer radical polymerization (ATRP) 18, 19, reversible addition fragmentation transfer (RAFT) polymerization 20, and anionic ring-opening polymerization 21, have been used to create amphiphilic block copolymers with well-defined structures. These synthetic methods offer excellent control in the composition, size, and morphology of polymeric micelles.
Up to date, few studies of polymeric micelles with paramagnetic Gd3+ ions stably chelated to the shell layer have been reported. Turner et al. 22 conjugated DTPA-Gd to preformed polymeric micelles to create micelles containing 2.7% Gd by weight. We hypothesize that conjugation of the metal chelator DTPA to the hydrophilic block before the formation of micelles, rather than after the formation of micelles, should increase the loading of Gd3+ ions in the polymeric micelles due to reduced steric hindrance in introducing Gd3+ to the micelles as compared to introducing bulkier DTPA. Importantly, an ideal micellar MRI contrast agent would be those that can be readily degraded and cleared from the body after their functions are over. Toward this end, PG-b-PLA was selected as the polymeric backbone in our study. Both PG and PLA are well-documented to be biodegradable and biocompatible 23, 24. To the best of our knowledge, this is the first report on successful fabrication of nanoscale micelles comprised of biodegradable block copolymer with paramagnetic Gd3+ ions chelated to their shells.
EXPERIMENTAL PROCEDURES
Materials
L-Lactide was purchased from Aldrich-Sigma (St. Louis, MO) and was recrystallized twice from toluene before use. Naphthalene, N-(tert-butoxycarbonyl) 2-aminoethanol (t-Boc-aminoethanol), pyridine, 4-dimethylaminopyridine, 1, 3-diisopylcarbodiimide (DIC), 4-(2-pyridylazo)resorcinol (PAR), and L-glutamic acid γ-benzyl ester were also obtained from Aldrich-Sigma. Triphosgene was obtained from Acros Chemicals (Somerville, NJ). Pyrene (analytic grade) was purchased from Fluka (Buchs, Switzerland). All anhydrous solvents except tetrahydrofuran (THF) were purchased from Acros and used without further purification. THF was freshly distilled over sodium with benzophenone. Spectra/Por (molecular weight cut-off (MWCO) = 3000) dialysis membrane was purchased from Spectrum Laboratories (Rancho Dominguez, CA). p-(s-2-[4-Aminobenzyl]-diethylenetriamine penta-tert-butyl acetate) (NH2-Bz-DTPA[t-butyl ester]) was synthesized according to previously reported procedures 25, 26 with some modifications (see Supporting Information). Magnevist (gadolinium complex of DTPA) was obtained from Berlex Laboratories, Inc. (Wayne, NJ).
General Methods
1H nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance 300 spectrometer (Billerica, MA) using tetramethylsilane as an internal standard. Infrared (IR) spectra were recorded on a Perkin Elmer Spectrum 2000 FTIR system (Norwalk, CT). Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) spectra were measured on a Voyager-DE-STR MALDI-TOF (Applied Biosystems, Framingham, MA) mass spectrometer equipped with a nitrogen laser emitting at 337 nm. Spectra were acquired in linear-positive mode with delayed extraction. Samples were prepared by mixing 10 μL (1 mg/mL) of the sample solution with 10 μL (10 mg/mL) of 2, 5-dihydroxybenzoic acid (DHBA) in THF. The number-average (Mn) and weight-average (Mw) molecular weight were calculated from the MALDI-TOF spectra by using the Data Explorer software (Applied Biosystems). Gel permeation chromatography (GPC) was performed in DMF containing 10 mM LiCl on a Waters system (Millford, MA) equipped with TOSOHAAS GMHHR-M column and a Waters 2410 refractive index detector. Fluorescence intensities were measured by a Fluorolog fluorescence spectrophotometer (Horiba Jobin Yvon, Inc., Edison, NJ) at room temperature. The Gd(III) content of the micelle was determined by inductively coupled plasma optical emission spectroscopy (ICP-OES), performed by Galbraith Laboratories, Inc. (Knoxville, TN).
Synthesis of Amine-Terminated Polylactide (NH2-PLA)
N-(tert-Butoxycarbonyl) 2-aminoethanolate-PLA was synthesized by an anionic ring-opening polymerization of L-lactide initiated by potassium t-Boc-aminoethanolate at room temperature under argon. The initiator was prepared in situ by treating t-Boc-aminoethanol (1 mmol) with potassium naphthalene (1 mmol) in 30 mL freshly distilled THF for 10 min at room temperature under argon. L-lactide (35 mmol) in THF (30 mL) was then introduced into the initiator solution using a syringe. After 2 h of polymerization, the polymer was recovered by precipitation into a 10-fold excess of cold methanol and stirred for 30 min, filtered, and dried in a vacuum to produce the t-Boc-aminoethanolate-PLA as a white solid. The yield was ca. 90%. The molecular weight of the polymer as determined by MALDI-TOF mass spectroscopy was Mn= 5610 (Mw∕Mn=1.26).
To remove the t-Boc protection group, 1 g of t-Boc-aminoethanolate-PLA was dissolved in 12 mL of anhydrous methylene chloride under argon, and then 12 mL of anhydrous trifluoroacetic acid was added. The reaction solution was stirred at room temperature for 4 h, and solvents were removed under a vacuum. The polymer was redissolved in methylene chloride, and the solution was washed sequentially with 5% NaHCO3 aqueous solution and deionized water and dried over MgSO4. After filtration and evaporation of the solvent, the resulting NH2-terminated aminoethyl-PLA was dried in a vacuum (yield: 86%).
Preparation of Poly(L-Glutamic Acid)-b-Polylactide (PG-b-PLA)
To synthesize γ-benzyl-L-glutamate-N-carboxylic anhydride (Bz-Glu-NCA), 10 g of triphosgene (33.7 mmol) in 20 mL dry ethyl acetate was gradually added through an addition funnel into a solution of 20 g of γ-benzyl-L-glutamate (76.0 mmol) in 600 mL of anhydrous ethyl acetate. The reaction mixture was L allowed to reflux under argon for 3 h. The resulting clear solution was cooled to -5°C and then washed with 200 mL of deionized water and 200 mL of 0.5% NaHCO3 solution. The organic layer was dried with anhydrous MgSO4. After filtration and evaporation of the solvent, the crude NCA product was recrystallized three times from THF/hexane solution to yield 7.2 g (81.1%) of Bz-Glu-NCA (m.p. 95-96°C).
Polymerization of Bz-Glu-NCA was performed by mixing a solution of 0.4 g of NH2-PLA (0.0713 mmol) in 2 mL anhydrous dimethylformamide (DMF) with a solution of 1.32 g (0.005 mol) freshly prepared Bz-Glu-NCA in 15 mL anhydrous DMF. The reaction mixture was stirred under argon at 40°C for 48 h. The polymerization reaction was monitored by FTIR until the peak spectrum at 1854 and 1786 cm-1 characteristic of cyclic N-carboxylic anhydride disappeared. The reaction mixture was then poured into a large excess of diethyl ether. The precipitate was collected by centrifugation, washed with diethyl ether, and dried in a vacuum to yield 1.28 g (85%) of product. 1H NMR [DMSO-d6], γ [ppm]: 7.26, C6H5CH2-; 5.20-5.22, -CHCH3; 5.03, -OCH2-; 3.95, -NHCH-; 2.27, 2.08,-CH2CH2-; 1.45, -CHCH3. On the basis of integral ratios of characteristic peaks of PG and PLA in the 1H NMR spectra, the degree of polymerization (DP) of the PG was estimated to be 50.
To remove the benzyl protecting group, poly(benzyl-L-glutamate)-b-PLA (1 g) was dissolved in 12 mL trifluoroacetic acid (TFA) under argon at 0°C, followed by the addition of 1.2 mL of trifluoromethanesulfonic acid (TFMSA) and 1.4 mL of thioanisole. The reaction mixture was gently stirred under argon at 0°C for 1 h and then at room temperature for 30 min. The reaction mixture was poured into cooled diethyl ether. The resulting white precipitate was collected by filtration and dried in a vacuum to yield 0.6 g (85.7%) of product. 1H NMR [DMSO-d6/CF3COOD], γ [ppm]: 5.14-5.10, -CHCH3; 3.98, -NHCH-; 2.30, 1.96,-CH2CH2-; 1.43, -C HCH3. 1H NMR confirmed complete removal of the benzyl group from poly(benzyl-L-glutamate)-b-PLA.
Synthesis of PG(DTPA)-b-PLA
PG-b-PLA (440 mg) and NH2-Bz-DTPA(t-butyl ester) (710 mg, 0.91 mmol) were dissolved in 8 mL of anhydrous DMF. 1,3-Diisopropylcarbodiimide (159 mg,1.26 mmol), 0.43 mL of pyridine, and a catalytic amount of 4-dimethylaminopyridine were added to the reaction solution. The reaction mixture was stirred at 4 °C overnight. The polymer solution was concentrated and precipitated to ethyl ether in an ice bath. The solid was collected by filtration and dried in a vacuum to yield 0.95 g of product. 1H NMR [DMSO-d6], γ [ppm]: 7.47, 7.12, -C6H4-; 5.17-5.22, -CHCH3; 4.26, 4.03, 3.70, 3.42-3.48, 2.89, 2.73, -NHCH- and other H of DTPA; 2.45-2.27,-CHCH2CH2-; 1.47, -CHCH3; 1.40, -C(CH3)3.
To remove the protection groups, 200 mg of purified PG(DTPA[penta-t-butyl ester])-b-PLA was combined with 2 mL trifluroacetic acid (TFA) at room temperature for 4 hr, and then the TFA was removed under a vacuum. The solid was redissolved in DMF and the solution precipitated to cold ethyl ether. The product was collected and dried in a vacuum to yield a white powder (138 mg, 69%). 1H NMR [DMSO-d6], γ [ppm]: 7.49, 7.12, -C6H4-; 5.15-5.22, -CHCH3; 4.28, 4.00, 3.70, 3.40-3.48, 2.89, 2.73, -NHCH- and other H of DTPA; 2.45-2.27, -CHCH2CH2-; 1.46, -CHCH3.
Formation of Micelles and Chelation with Gd3+ Ions
PG(DTPA)-b-PLA (10 mg) was dissolved in 10 mL of DMF, and the resulting copolymer solution was transferred into a pre-swollen membrane (Spectra/Por, MWCO=3,000) and dialyzed sequentially against 10 mM phosphate-buffered saline (PBS) (pH 7.4), deionized water, and 10 mM sodium acetate buffer (pH 5.5), each for 24 h. To chelate Gd3+ ions, 0.01 M Gd(Ac)3 in 10 mM sodium acetate buffer (pH = 5.5) was added in small fractions to the resulting micelle solution in 10 mL of 10 mM sodium acetate buffer. After extensive dialysis against 1 mM ethylenediamine tetraacetic acid (EDTA) solution and water (MWCO=3,000), the resulting Gd-loaded micelle solution was lyophilized to yield 8.5 mg of white powder. The absence of free Gd3+ ions was confirmed using PAR color reaction.
Determination of Critical Micelle Concentration
The CMC of micelles in water was determined fluorospectrometrically using pyrene as a hydrophobic fluorescence probe 27, 28. Polymeric micelles with concentrations ranging from 5×10-4 to 1 g/L were mixed with a saturated aqueous solution of pyrene (12 × 10-7 mol/L). After incubation at room temperature for 24 h, the fluorescence signal was quantified. The CMC was determined from the signal intensity ratio at 334 nm and 331 nm (I334/I331)-versus-concentration curve. The experiment was performed with emission wavelengths of 393 nm.
Measurement of Particle Size
Particle sizes was measured by using dynamic light scattering at a scatter angle of 90° on a ZetaPLUS (Brookhaven Instruments Corp., Holtsville, NY). Correlation data were analyzed using a BI-9000AT Digital Autocorrelator (Brookhaven) and a non-negatively constrained least squares (CONTIN) approximation was used to calculate the particle size distribution.
Transmission Electron Microscopy (TEM)
A drop of the sample solution was placed on a 400 mesh copper grid coated with a 0.5 wt % poly(vinyl formal) aqueous solution. About 5 min after deposition, the sample solution was removed by using filter paper. Negative staining was performed using a droplet of 1% uranyl acetate solution. The sample was air dried and examined with a JEM 1010 transmission electron microscope (JEOL USA, Inc., Peabody, MA) at an accelerating voltage of 80 kV. Digital images were obtained using the AMT Imaging System (Advanced Microscopy Techniques Corp, Danvers, MS).
Evaluation of Cytotoxicity
The cytotoxicity of PG(DTPA)-b-PLA micelles and Gd-loading micelles was evaluated in vitro using the (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) (MTT) assay (Sigma-Aldrich). Mouse embryonic fibroblast NIH/3T3 cells were seeded in a 96-well plate at 5×105 cells/well and allowed to adhere overnight. The DMEM medium was replaced and the micelles diluted with culture medium were added in concentrations ranging from 1 μg/mL to 500 μg/mL. The cells were incubated continuously for 72 h before cell viability was analyzed using the MTT assay. The cell viability of the micelle-treated cells was determined relative to untreated cells.
Measurement of MR Relaxivity
Solutions of micelles were prepared in deionized water at Gd concentrations of 0.005, 0.01, 0.02, 0.04, 0.08, 0.16, and 0.32 mM. Spin lattice (T1) was measured at 4.7 tesla on a Bruker Biospec small animal MRI system (Bruker Biospin Corp., Billerica, MA) using inversion recovery and multiecho pulse sequences. Relaxivities (r1 in mM-1 S-1) were obtained using linear least-square regression analysis of the slopes of an 1/T1 versus [Gd] plot.
RESULTS AND DISCUSSION
Synthesis of PG(DTPA)-b-PLA Block Copolymer
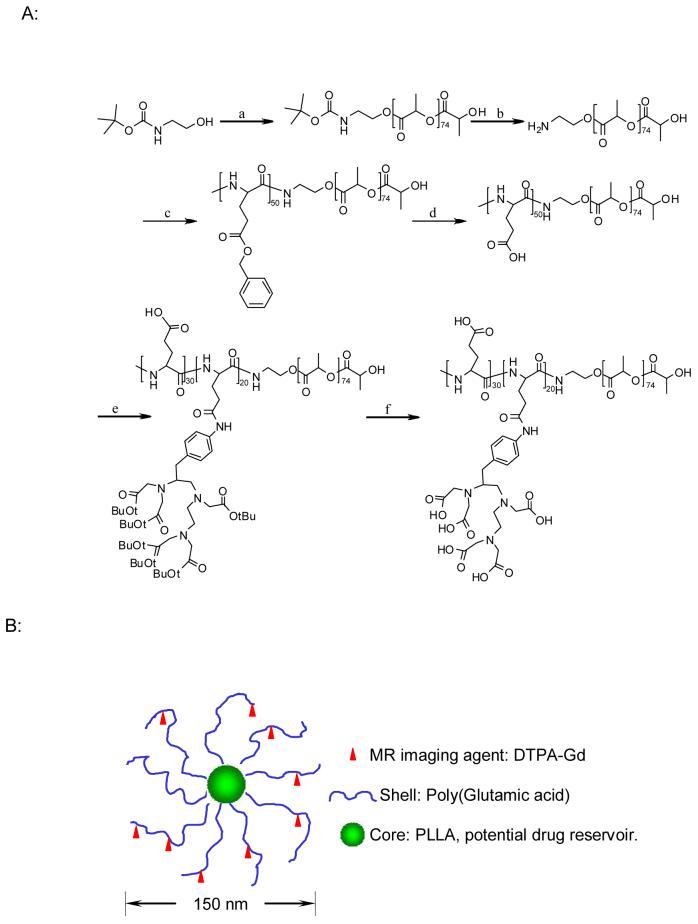
A novel metal-containing initiator 21 and ammonium-mediated polymerization of Bz-L-Glu-NCA 29 have been reported for the synthesis of poly(amino acids) with narrow molecular weight distribution, but these methods are not suitable for the preparation of PLA-containing block copolymers. Previously, Arimura et al. 30 reported the synthesis of block copolymer poly(aspartic acid)-b-PLA. In this study, we have adapted a similar approach for the synthesis of PG-b-PLA, with some modifications. Instead of 25% HBr/AcOH, TFA was used to remove the t-Boc protection group from t-Boc-aminoethanolate-PLA. Fig. 1 shows the reaction scheme for the synthesis of PG(DTPA)-b-PLA block copolymer. We first synthesized amine-terminated PLA (NH2-PLA) by anionic polymerization of lactide. t-Boc-aminoethanol was used as an initiator in the presence of K/naphthalene. The absolute molecular weight of t-Boc-aminoethanolate PLA was established by MALDI-TOF mass spectroscopy: t-Boc-NH-ethyl-PLA had a number-average molecular weight (Mn) of 5,610 Da (degree of polymerization = 74) and a polydispersity (Mw/Mn) of 1.26. Next, after removal of the t-Boc protection group, the resulting amine-terminated PLA, NH2-PLA, was used to initiate the ring-opening polymerization of Bz-L-Glu-NCA to produce block copolymer poly(benzyl-L-glutamate)-b-PLA. It should be noted that the purity of the monomer Bz-L-Glu-NCA is crucial to obtaining poly(benzyl-L-glutamate) with the designated molecular weight and with narrow molecular weight distribution. In our hands, the procedures adapted from Poche et al.31 afforded Bz-L-Glu-NCA with high purity (m.p. = 95-96°C; literature data32: m.p. = 90-91°C). Purification steps for the monomer included washing the crude product twice in a cold aqueous solution of sodium bicarbonate and water and recrystallization from dry THF/hexane at least three times. The structure of the resulting block copolymers was determined by 1H NMR. On the basis of the ratio of peak intensities between the methyl protons in PLA (-CHCH3, γ = 1.49 ppm) and the benzyl protons in poly(benzyl-L-glutamate) (-CH2C6H5, γ= 7.26 ppm), the degree of polymerization for poly(benzyl-L-glutamate) was estimated to be 50.
Fig. 1.
Synthesis of PG(DTPA)-b-PLA (A) and schematic model of the micellar structure with DTPA-Gd chelated to the shell layer (B). a: Naphthalene/K, L-lactide; b: TFA; c: Benzyl glutamate NCA; d: 1 M trifluoromethane sulfonic acid (TFMSA)/thioanisole/trifluoroacetic acid; e: DIC, pyridine, DMAP, p-aminobenzyldiethylenetriaminepenta(acetic acid tert butyl ester); and f: TFA.
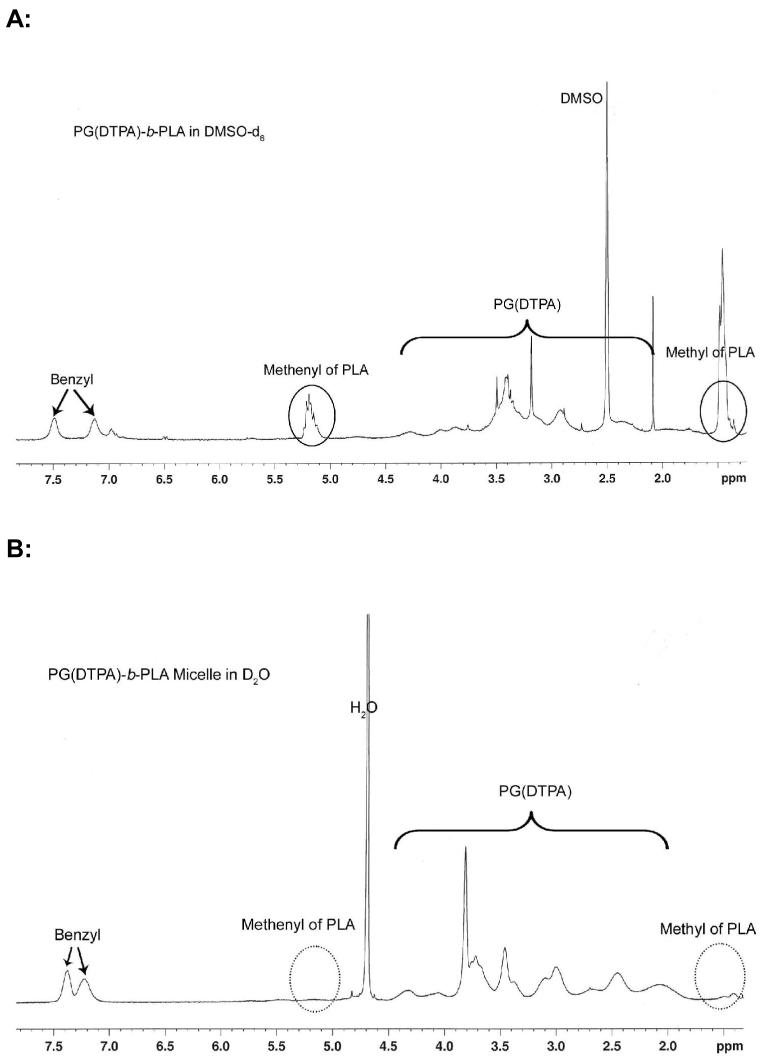
The protection group on the side chains of the poly(benzyl-L-glutamate) block was removed under mild conditions, i.e., 1M trifluoromethanesulfonic acid and 1 M thioanisole in TFA 30. Finally, p-NH2-Bz-DTPA(t-butyl ester) was conjugated to the side-chain carboxylic acid groups of poly(L-glutamic acid) in anhydrous DMF via a carbodiimide-mediated coupling reaction. The 1H-NMR spectrum of PG[NH-Bz-DTPA(t-butyl ester)]-b-PLA revealed that about 40% of the carboxylic acid groups in PG were conjugated with NH2-Bz-DTPA(t-butyl ester) (data not shown). Removal of the t-butyl protection groups with TFA yielded the target copolymer PG(DTPA)-b-PLA (Fig. 2). The removal of benzyl groups under mild and anhydrous conditions prevented the PLA chains from hydrolytic degradation. This observation is supported by GPC data, which showed a similar molecular weight distribution pattern before and after deblocking reaction (data not shown). It has been previously shown that PLA is stable in anhydrous TFA 33, a condition used in our study to remove t-butyl protecting groups.
Fig. 2.
1H NMR spectra of PG(DTPA)-b-PLA in DMSO-d6 (A) and its corresponding micelles in aqueous D2O solution (2 mg/mL) (B). The NMR signals from the PLA segment (circles) disappeared in the micelles.
The DTPA analogue p-NH2-Bz-DTPA(t-butyl ester) used in this study was synthesized in five steps with the combination of previously reported procedures25, 26. Instead of using hydrogen chloride gas in the first step in the preparation of methyl 4-nitrophenylalaninate hydrochloride25, we used thionyl chloride, which as a liquid is much easier to handle.
Preparation and Characterization of Gd-Loaded Polymeric Micelles
Direct dialysis of PG(DTPA)-b-PLA solution in DMF against PBS at pH 7.4 afforded the corresponding polymeric micelle. Chelation of Gd3+ to DTPA in the micelles was accomplished in sodium acetate buffer at pH 5.5. To ensure that no Gd3+ ions were chelated to the carboxylic acid groups in the PG block of the copolymer, a dilute EDTA solution was used as a receiving medium during dialysis. PAR color assay confirmed the absence of free Gd3+ ions after extensive dialysis. The content of Gd3+ in the micelles was 5% by weight, which was twofold higher than that obtained in shell-crosslinked knedel-like nanoparticles through post-micelle modification with DTPA/Gd to the preformed micelles22.
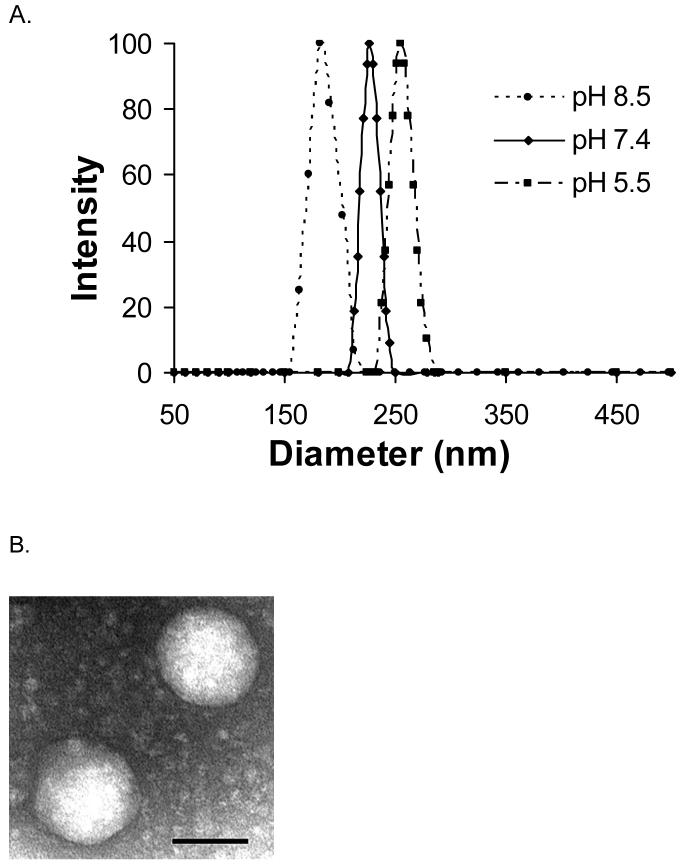
Dynamic light scattering revealed an average hydrodynamic diameter of 230 nm for PG(DTPA)-b-PLA micelles in 10 mM PBS (pH 7.4) before chelating with Gd (Fig. 3A). The size of the micelles was affected by the pH of the solution, for example, the diameter of micelles prepared from PG(DTPA)-b-PLA was reduced from 256 nm in sodium acetate buffer (pH 5.5) to 185 nm in sodium bicarbonate buffer (pH 8.5). Due to charge repulsion between the negatively charged COO-1 groups, the hydrodynamic volume of each PG polymer chain will increase. We observed decreased micelle size with increasing pH. Such a phenomenon was also reported recently in the literature 34, 35. Tian and Hammond 35 suggest that reduced micelle size upon increasing the pH of the solutions may be attributed to the change in the shape of the hydrophilic chains owing to charge repulsion between head groups. After the DTPA of the micelle was chelated with Gd3+ ion, the hydrodynamic diameter of Gd-loading micelles in PBS at pH 7.4 was reduced from 230 nm to 210 nm, which may be ascribed to the decreased anionic repulsion at pH 7.4 after Gd3+ chelation. TEM observation confirmed the spherical shape of the Gd-loading micelles (Fig. 3B). The micelles had a diameter of about 150 nm, as measured by TEM, which was smaller than that measured with dynamic light scattering because of shrinkage of the micelles in a dry state. The micelle structure was further confirmed by 1H-NMR, which showed the disappearance of proton peaks (-CH3: γ=1.5 ppm and -CH-: γ=5.1 ppm) from the PLA segment because the mobility of the PLA chain is reduced in the hydrophobic micellar core (Fig. 2B).
Fig. 3.
Micelle size characterized by dynamic light scattering and TEM. (A): The mean diameters of PG(DTPA)-b-PLA micelles at pH 8.5, pH 7.4, and pH 5.5 were 184.9 nm, 229.6 nm, and 256.1 nm, respectively. (B): TEM of Gd-loading PG(DTPA)-b-PLA micelle in pH 7.4 PBS. Scale bar is 100 nm.
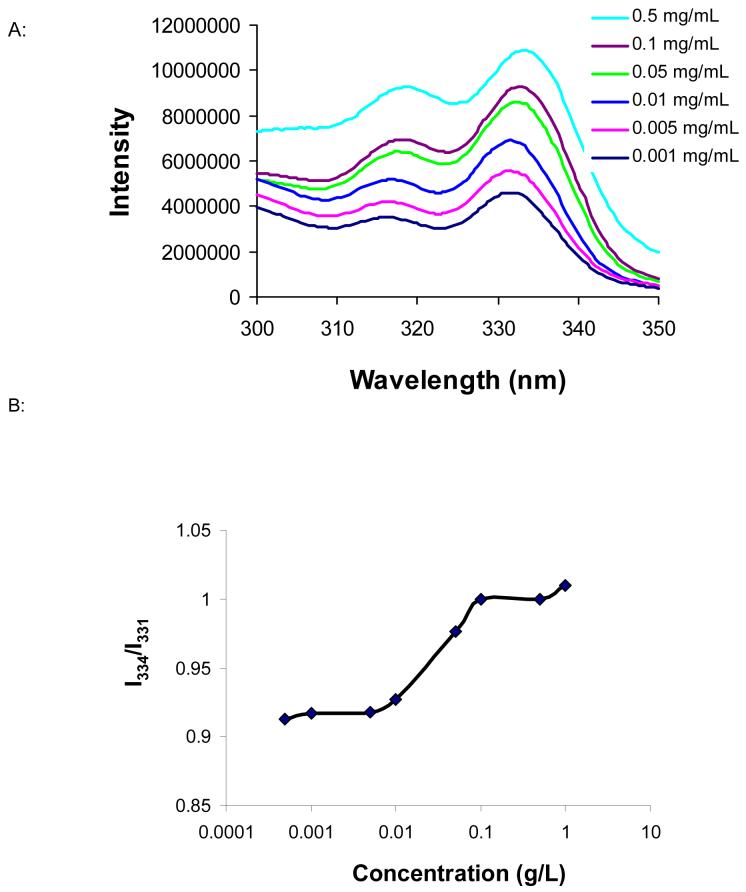
The CMC is directly related to the stability of the micelle in vivo 36, since the micelle solution will be diluted upon entering the bloodstream. On the basis of the fluorescence measurement with pyrene as a hydrophobic probe 28, the CMC of the Gd3+ complex-containing micelle was ca. 12 mg/L (Fig. 4). This value is comparable to that of other polymeric micelles (e.g., the CMC of acetal-PEG-b-PLA was 3-12 mg/L 27).
Fig. 4.
(A): Excitation spectra of pyrene as a function of copolymer concentration in water. For reasons of clarity, spectra at copolymer concentrations of 0.0005 and 1.0 mg/mL were omitted from the plot. (B): Plot of excitation intensity ratio at I334/I331 against logarithm of copolymer concentration. The experiment was performed with emission wavelengths of 393 nm. The CMC measured was 12 mg/L.
Relaxivity and Cytotoxicity
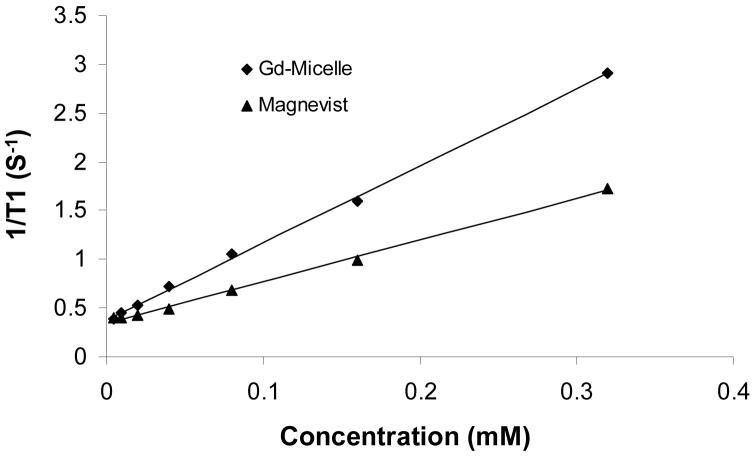
The relaxivity of the Gd complex depends on the water-exchange rate (kex) and the rotational correlation lifetime (τR). It has been found that when Gd3+ ions are chelated to the shell layer of micelles, the water-exchange rate is identical to that of a small molecular equivalent, suggesting that the access of water molecules from the bulk to the paramagnetic center in the shell layer of the micelle is not limited 9. In contrast, Gd3+ complex entrapped in the hydrophobic core of a micelle has a low water-exchange rate, resulting in decreased relaxivity. For example, Gd3+ complex entrapped in the core of micelles formed from a cationic polymer and an anionic block copolymer PEG-b-poly(aspartic acid) exhibited a lower relaxivity than that of the corresponding small molecular Gd3+ complex 37. For micelles formed from PG(DTPA-Gd)-b-PLA, we observed a higher relaxivity at 4.7T (r1, 7.90 mM-1S-1), which was approximately two times higher than that of the small-molecular-weight DTPA-Gd (r1, 4.26 mM-1S-1) (Fig.5). These data suggest that Gd3+ complex in PG(DTPA-Gd)-b-PLA micelles had an increased rotational correlation lifetime.
Fig. 5.
Results of the T1 relaxivity (r1) measurements of Gd-micelle and DTPA-Gd measured at 4.7 T and room temperature.
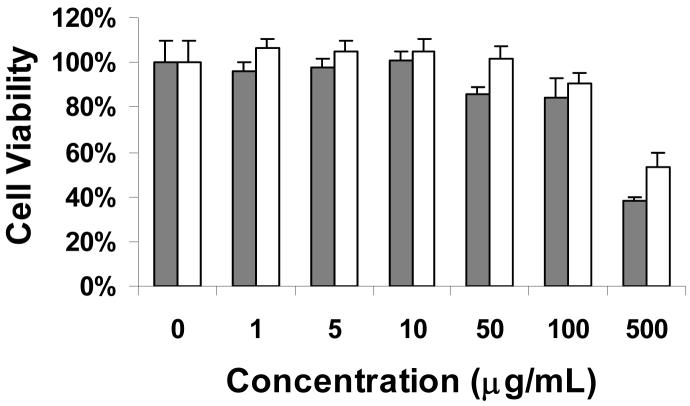
The viability of the cells cultured in the media treated with varying concentrations of test micelles, based on the MTT assay, is shown in Fig. 6 Both the PG(DTPA)-b-PLA micelles and the Gd-loading micelles exhibited no cytotoxicity at concentrations up to 100 μg/mL; at micelle concentrations of 500 μg/mL, cell viability decreased to about 50%. Of note, we observed that Gd3+-loaded micelles were significantly less cytotoxic than the plain unloaded micelles (p = 0.0027, student’s t-test) at 500 μg/mL, suggesting that the cytotoxic effect observed at a high micelle concentration may be caused by an osmotic effect of highly charged species.
Fig. 6.
Cell viability of micelles formed from PG(DTPA)-b-PLA and Gd-loading PG(DTPA-Gd)-b-PLA evaluated by MTT. The NIH/3T3 cell line was used. PG(DTPA)-b-PLA: grey box; PG(DTPA-Gd)-b-PLA: white box.
CONCLUSIONS
In this study, we have demonstrated that it is feasible to fabricate nanoscale polymeric micelles comprised of poly(L-glutamic acid)(DTPA)-b-polylactide copolymer with paramagnetic Gd3+ ions chelated to their shells by chelating Gd3+ ion to the preformed PG(DTPA)-b-PLA micelle, and the resulting Gd-containing micelle exhibited approximately two times higher than T1 relaxivity that of the small-molecular-weight DTPA-Gd. These micelles can become multifunctional when drugs are entrapped in the hydrophobic core and targeting moieties are conjugated to the shell layer. Future studies are necessary to determine the pharmacokinetics of PG(DTPA-Gd)-b-PLA micelles and MR imaging properties in animal model in order to fully assess the potential application of this class of material in targeted drug delivery and in theragnostic application.
Supplementary Material
ACKNOWLEDGMENTS
This work is supported by the National Cancer Institute (grant R01 CA119387) and the John S. Dunn Foundation. We thank Douglas Webb and Jim Bankson of the Small Animal Cancer Imaging Research Facility (SACIRF) at M. D. Anderson Cancer Center for obtaining the relaxivity data. We also thank Dawn Chalaire for expert editorial assistance.
REFERENCES AND NOTES
- 1.Mulder WJ, Strijkers GJ, van Tilborg GA, Griffioen AW, Nicolay K. NMR Biomed. 2006;19:142–64. doi: 10.1002/nbm.1011. [DOI] [PubMed] [Google Scholar]
- 2.Neuberger T, Schopf B, Hofmann M, Rechenberg B. J. Magn. Magn. Mater. 2005;293:483–496. [Google Scholar]
- 3.Kobayashi H, Sato N, Kawamoto S, Saga T, Hiraga A, Haque TL, Ishimori T, Konishi J, Togashi K, Brechbiel MW. Bioconjugate Chem. 2001;12:100–7. doi: 10.1021/bc000075s. [DOI] [PubMed] [Google Scholar]
- 4.Wiener EC, Brechbiei MW, briothers H, Magin RL, Gansow OA, Tomalia DA, Lauterbur PC. Magn. Reson. Med. 1999;31:1–8. doi: 10.1002/mrm.1910310102. [DOI] [PubMed] [Google Scholar]
- 5.Lu ZR, Wang X, Parker DL, Goodrich KC, Buswell HR. Bioconjugate Chem. 2003;14:715–9. doi: 10.1021/bc0340464. [DOI] [PubMed] [Google Scholar]
- 6.Schuhmann-Giampieri G, Schmitt-Willich H, Frenzel T, Press WR, Weinmann HJ. Invest. Radiol. 1991;26:969–74. doi: 10.1097/00004424-199111000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Wen X, Jackson EF, Price RE, Kim EE, Wu Q, Wallace S, Charnsangavej C, Gelovani JG, Li C. Bioconjugate Chem. 2004;15:1408–15. doi: 10.1021/bc049910m. [DOI] [PubMed] [Google Scholar]
- 8.Schmiedl U, Ogan M, Paajanen H, Marotti M, Crooks LE, Brito AC, Brasch RC. Radiology. 1987;162:205–10. doi: 10.1148/radiology.162.1.3786763. [DOI] [PubMed] [Google Scholar]
- 9.Andre JP, Toth E, Fischer H, Seelig A, Macke HR, Merbach AE. Chem. Eur. J. 1999;5:2977–2983. [Google Scholar]
- 10.Nicolle GM, Toth E, Eisenwiener KP, Macke HR, Merbach AE. J. Biol. Inorg. Chem. 2002;7:757–69. doi: 10.1007/s00775-002-0353-3. [DOI] [PubMed] [Google Scholar]
- 11.Terreno E, Cabella C, Carrera C, Delli Castelli D, Mazzon R, Rollet S, Stancanello J, Visigalli M, Aime S. Angew. Chem., Int. Ed. 2007;46:966–8. doi: 10.1002/anie.200604027. [DOI] [PubMed] [Google Scholar]
- 12.Accardo A, Tesauro D, Roscigno P, Gianolio E, Paduano L, D’Errico G, Pedone C, Morelli G. J. Am. Chem. Soc. 2004;126:3097–107. doi: 10.1021/ja039195b. [DOI] [PubMed] [Google Scholar]
- 13.Forrest ML, Won CY, Malick AW, Kwon GS. J Controlled Release. 2006;110:370–7. doi: 10.1016/j.jconrel.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Kataoka K, Harada A, Nagasaki Y. Adv. Drug Deliv. Rev. 2001;47:113–31. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama M, Okano T, Sakurai Y, Ekimoto H, Shibazaki C, Kataoka K. Cancer Res. 1991;51:3229–36. [PubMed] [Google Scholar]
- 16.Nasongkla N, Bey E, Ren J, Ai H, Khemtong C, Guthi JS, Chin SF, Sherry AD, Boothman DA, Gao J. Nano Lett. 2006;6:2427–30. doi: 10.1021/nl061412u. [DOI] [PubMed] [Google Scholar]
- 17.Torchilin VP. Adv. Drug Deliv. Rev. 2002;54:235–52. doi: 10.1016/s0169-409x(02)00019-4. [DOI] [PubMed] [Google Scholar]
- 18.Robinson KL, de Paz-Banez MV, Wang XS, Armes SP. Macromolecules. 2001;34:5799–5805. [Google Scholar]
- 19.Zhang Q, Remsen EE, Wolley KL. J. Am. Chem. Soc. 2000;122:3642–3651. [Google Scholar]
- 20.ten Cate MGJ, Rettig H, Bernhardt K, Borner HG. Macromolecules. 2005;38:10643–10649. [Google Scholar]
- 21.Deming TJ. Nature. 1997;390:386–9. doi: 10.1038/37084. [DOI] [PubMed] [Google Scholar]
- 22.Turner JL, Pan D, Plummer R, Chen ZY, Whittaker AK, Wooley KL. Adv. Funct. Mater. 2005;15:1248–1254. [Google Scholar]
- 23.Li C. Adv. Drug Deliv. Rev. 2002;54:695–713. doi: 10.1016/s0169-409x(02)00045-5. [DOI] [PubMed] [Google Scholar]
- 24.Ignatius AA, Claes LE. Biomaterials. 1996;17:831–9. doi: 10.1016/0142-9612(96)81421-9. [DOI] [PubMed] [Google Scholar]
- 25.Corson DT, Meares CF. Bioconjugate Chem. 2000;11:292–9. doi: 10.1021/bc990125x. [DOI] [PubMed] [Google Scholar]
- 26.Cummins CH, Rutter EW, Jr., Fordyce WA. Bioconjugate Chem. 1991;2:180–6. doi: 10.1021/bc00009a007. [DOI] [PubMed] [Google Scholar]
- 27.Nagasaki Y, Okada T, Scolz C, Iijima M, Kato M, Kataoka K. Macromolecules. 1998;31:1473–1479. [Google Scholar]
- 28.Wilhelm M, Zhao CL, Wang Y, Xu R, Winnk MA. Macromolecules. 1991;24:1033–1040. [Google Scholar]
- 29.Dimitrov I, Schlaad H. Chem. Commun. (Camb) 2003:2944–5. doi: 10.1039/b308990h. [DOI] [PubMed] [Google Scholar]
- 30.Arimura H, Ohya Y, Ouchi T. Biomacromolecules. 2005;6:720–5. doi: 10.1021/bm0494491. [DOI] [PubMed] [Google Scholar]
- 31.Poche DS, Moore MJ, Bowles JL. Synth. Commun. 1999;29:843–854. [Google Scholar]
- 32.Caillol S, Lecommandoux S, Mingotaud AF, Schappacher M, Soum A, Bryson N, Meyrueix R. Macromolecules. 2003;36:1118–1124. [Google Scholar]
- 33.Caillol S, Lecommandoux S, Mingotaud A-F, Schappacher M, Soum A, Bryson N, Meyrueix R. Macromolecules. 2003;36:1118–1124. [Google Scholar]
- 34.Shen X, Zhang L, Jiang X, Hu Y, Guo J. Angew. Chem., Int. Ed. 2007;46:7104–7107. doi: 10.1002/anie.200701368. [DOI] [PubMed] [Google Scholar]
- 35.Tian L, Hammond PT. Chem. Mater. 2006;18:3976–3984. [Google Scholar]
- 36.Allen C, Maysinger D, Eisenberg A. Colloids Surf., B. 1999;16:3–27. [Google Scholar]
- 37.Nakamura E, Makino K, Okano T, Yamamoto T, Yokoyama M. J. Controlled Release. 2006;114:325–33. doi: 10.1016/j.jconrel.2006.05.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.