Abstract
Retinogeniculate connections are one of the most striking examples of connection specificity within the visual pathway. In almost every connection there is one dominant afferent per geniculate cell, and both afferent and geniculate cell have very similar receptive fields. The remarkable specificity and strength of retinogeniculate connections have inspired comparisons of the lateral geniculate nucleus (LGN) with a simple relay that connects the retina with visual cortex. However, because each retinal ganglion cell diverges to innervate multiple cells in LGN, most geniculate cells must receive additional inputs from other retinal afferents that are not the dominant one. These additional afferents make weaker connections and their receptive fields are not as perfectly matched with the geniculate target as the dominant afferent. We argue that these ‘match imperfections’ are important to create receptive field diversity among the cells that represent each point of visual space in LGN. We propose that the convergence of dominant and weak retinal afferents in LGN multiplexes the array of retinal ganglion cells by creating receptive fields that have a richer range of positions, sizes and response time-courses than those available at the ganglion cell layer of the retina.
Keywords: Thalamus, thalamocortical, visual cortex, V1, Y cell, X cell, response latency, simultaneous recording
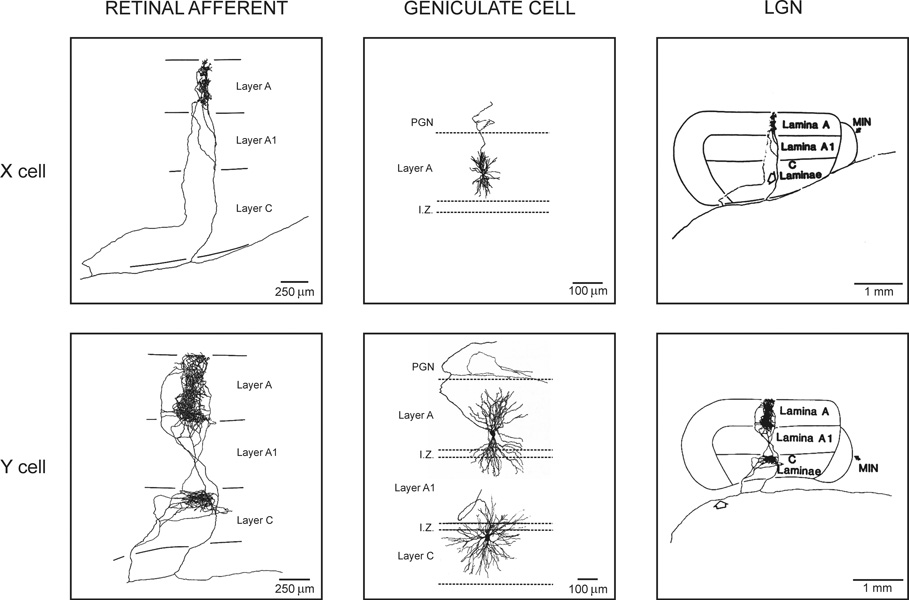
The cat eye has 160,800 retinal ganglion cells that fit within a retinal area of 450 mm2 (Illing & Wassle, 1981). Half of these cells (53–57%) have small receptive fields and are classified as X and a much smaller proportion (2–4%) have larger receptive fields and are classified as Y (Enroth-Cugell & Robson, 1966; Friedlander et al., 1979; Illing & Wassle, 1981). X and Y retinal ganglion cells are the origin of two major functional channels within the cat visual pathway that remain relatively well segregated within the LGN (Cleland et al., 1971b, a; Mastronarde, 1992; Usrey et al., 1999). These two major channels have pronounced anatomical differences. For example, the X retinal afferents have very restricted axon terminals (~100 microns diameter) that are confined to a single layer of LGN and connect small geniculate cells. In contrast, the Y axon terminals are twice as large, usually diverge into two different LGN layers (Sur & Sherman, 1982; Sur et al., 1987) and connect geniculate cells with large dendritic trees that tend to cross layer boundaries ((Friedlander et al., 1979), Fig. 1).
Figure 1. Retinal afferents and geniculate cells.
Left. Axon terminals from X and Y retinal afferents in LGN. X retinal axons project into a single LGN layer and they are very restricted. Y retinal axons can project into two different LGN layers and are wider. Middle. X and Y geniculate cells. X cells have small dendritic trees that are restricted to a single LGN layer. Y cells have larger dendritic trees that frequently cross layers. Right. The same axon terminals from the left of the figure shown at a different scale. Reprinted with permission from (Sur & Sherman, 1982, Copyright 1982 AAAS; Sur, 1988) (left and right) and (Friedlander et al., 1981) (middle).
X and Y retinal ganglion cells diverge at the level of the LGN to connect up to 20 geniculate cells per retinal afferent (Hamos et al., 1987). This divergence could do much more than just copying the properties of each retinal ganglion cell into the geniculate neurons; it could diversify the spatial and temporal properties of the receptive fields that represent each point of visual space. This receptive field diversity could then be used at the cortical level to maximize the spatiotemporal resolution needed to process visual stimuli. In this review, we illustrate this idea with two different examples. In the first example, we show evidence that a single class of Y retinal afferent can be used to build two different types of Y receptive fields within LGN. In the second example, we show that geniculate neurons representing the same point of visual space have a rich variety of receptive field sizes and response latencies that emerges as a consequence of retinogeniculate divergence/convergence.
Retinogeniculate divergence in the Y visual pathway of the cat
Y retinal ganglion cells are a conspicuous minority within the cat retina (2–4%), which is greatly amplified at subsequent stages of the visual pathway. While X retinal ganglion cells diverge, on average, into 1.5 geniculate cells, Y retinal ganglion cells diverge into 9 geniculate cells [X geniculate cells/retinal cells: 120,000/89,000; Y geniculate cells/retinal cells: 60,000/6,700; the Y cells from layer C are not included in this estimate (LeVay & Ferster, 1979; Illing & Wassle, 1981; Peters & Payne, 1993)].
The huge amplification of the Y pathway in the cat is reminiscent of the magnocellular pathway in the primate. In the rhesus monkey, there is little retinogeniculate divergence probably because there is a limit on how many retinogeniculate connections can be accommodated within LGN (the primate retina has 1,120,000 parvocellular cells and 128,000 magnocellular cells (see Masland, 2001 for review)). However, like in the cat, magnocellular cells are a minority within the primate retina (~8% of all retinal ganglion cells) and, by connecting to magnocellular geniculate cells, they are able to reach a remarkably large number of cortical neurons –at the cortical representation of the fovea in layer 4C, magnocellular geniculate cells connect about 29 times more cortical cells than parvocellular geniculate cells (Connolly & Van Essen, 1984). Interestingly, neuronal divergence seems to be delayed one synapse in primate with respect to the cat as is also the case for the construction of simple receptive fields (Hubel & Wiesel, 2005).
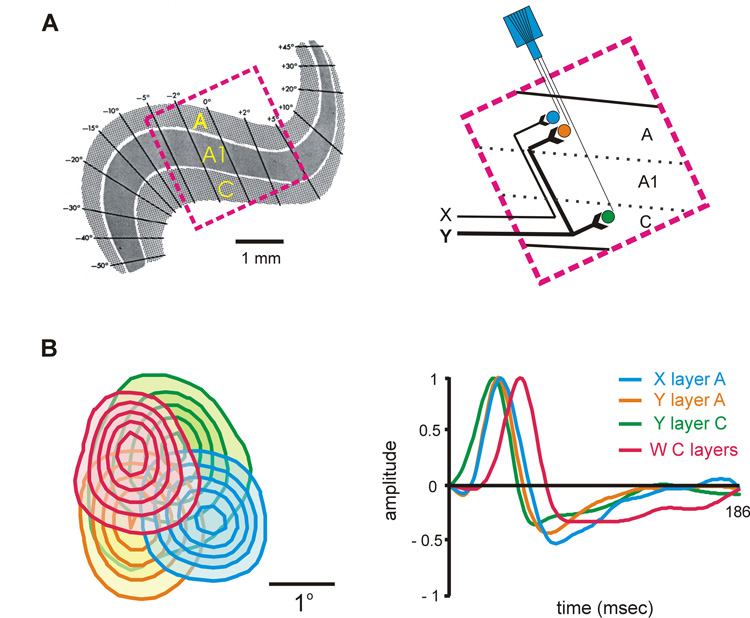
The cat LGN is an excellent model to study the functional consequences of the Y pathway divergence. Unlike the primate, the cat LGN has two main layers that receive Y contralateral input (A and C; A1 receives ipsilateral input) and the retinotopic map of each layer is not excessively folded, making it easier to record from multiple cells with overlapping receptive fields across different LGN layers. Figure 2 illustrates the retinotopic map of cat LGN (Fig. 2A) and the response properties of 4 cells that were simultaneously recorded from different layers. The 4 cells had on-center receptive fields with slightly different positions and receptive field sizes (Fig. 2B, left). Their response time-courses, represented as impulse responses, were also different [receptive fields and impulse responses were obtained with white noise stimuli by reverse correlation (Reid et al., 1997; Yeh et al., 2003)].
Figure 2. Simultaneous recordings from 4 geniculate cells recorded at different layers in the cat LGN.
A. Left. Retinotopic map of cat LGN adapted from (Sanderson, 1971). Right. Schematic representation of the simultaneous recordings. B. Left. Receptive fields of the 4 simultaneously recorded geniculate cells mapped with white noise by reverse correlation. The contour lines show responses at 20%–100% of the maximum response. Right. Impulse responses of the 4 cells obtained by reverse correlation; the impulse response represents the time-course of the receptive field pixel that generated the strongest response. The different cell types are represented in different colors (X cell from layer A, XA, in blue; Y cell from layer A, YA, in orange; Y cell from layer C, YC, in green and W cell from the deep C layers in pink). Throughout this review, on-center receptive fields are represented as continuous lines and off-center receptive fields as discontinuous lines. The figure was reprinted with permission from (Yeh et al., 2003).
As shown in this figure, the Y cells had the largest receptive fields and fastest response time-courses within the group. Moreover, the receptive field was larger and the response latency faster for the Y cell from layer C (YC, shown in green) than the Y cell from layer A (YA, shown in orange). Simultaneous recordings like the one shown in figure 2 allowed us to compare the response properties from neighboring YA and YC cells that had overlapping receptive fields. These measurements demonstrated that, on average, the receptive fields from YC cells are 1.8 times larger than those from YA cells and the response latencies are 2.5 ms faster (p< 0.001, Wilcoxon test).
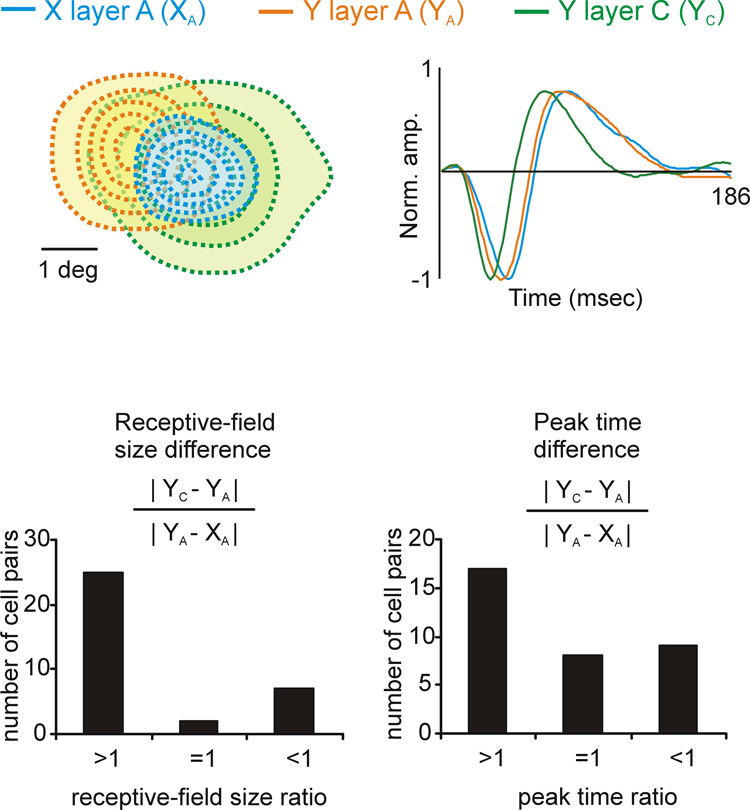
The differences in receptive field size and response latency between Y cells located in different layers were sometimes as pronounced as the differences between X and Y cells located within the same layer. To quantify these differences, we did simultaneous triplet recordings from neighboring YA, YC and XA cells11. The top of figure 3 shows an example of a triplet recording from three off-center geniculate cells of different type (XY, YA, YC). The YC cell had the largest receptive field and fastest response latency and the X cell the smallest receptive field and slowest response latency. For each cell triplet recorded, we calculated a similarity ratio to compare the differences between the YA-YC cells with the differences between the YA-XA cells. A ratio larger than 1 indicates that the YC cell differed from the YA cell more than the YA cell differed from the XA cell. As shown in the histograms at the bottom of figure 3, in many cell triplets the similarity ratio for receptive field size and response latency was larger than 1. Moreover, the mean difference in receptive field size was significantly larger for YC-YA than YA-XA (p<0.001, Wilconxon test). YC and YA cells also differed significantly in other properties that are not illustrated here such as spatial linearity, response transience and contrast sensitivity (Frascella & Lehmkuhle, 1984; Lee et al., 1992; Yeh et al., 2003). These results indicate that Y retinal afferents connect to two types of Y geniculate cells with significantly different response properties, YC and YA.
Figure 3. Comparisons of receptive field size and response latency obtained from triplet recordings of YA, YC and XA cells.
Top. An example of a triplet recording from 3 cells with off-center receptive fields. Receptive fields are shown on the left and impulse responses on the right. Bottom. Comparisons of receptive field size (left) and response latency (right). An index larger than 1 indicates that the differences between YC-YA were larger than the differences between YA-XA. An index smaller than one indicate the opposite. Note that the differences between YC-YA were frequently larger than the differences between YA-XA. The figure was reprinted with permission from (Yeh et al., 2003).
At first sight, this conclusion seems at odds with the idea that retinogeniculate connections are highly specific. If the receptive field of each geniculate neuron resembles very closely the receptive field of the dominant retinal afferent (Cleland et al., 1971b, a; Mastronarde, 1983; Cleland & Lee, 1985; Usrey et al., 1999), it should not be possible to construct two types of Y receptive fields with one type of Y retinal afferent. Certainly, there is no evidence for two types of Y retinal afferents that could match the properties of YA and YC geniculate receptive fields and almost every Y retinal afferent has been found to diverge in the two layers of LGN (Sur & Sherman, 1982; Sur et al., 1987).
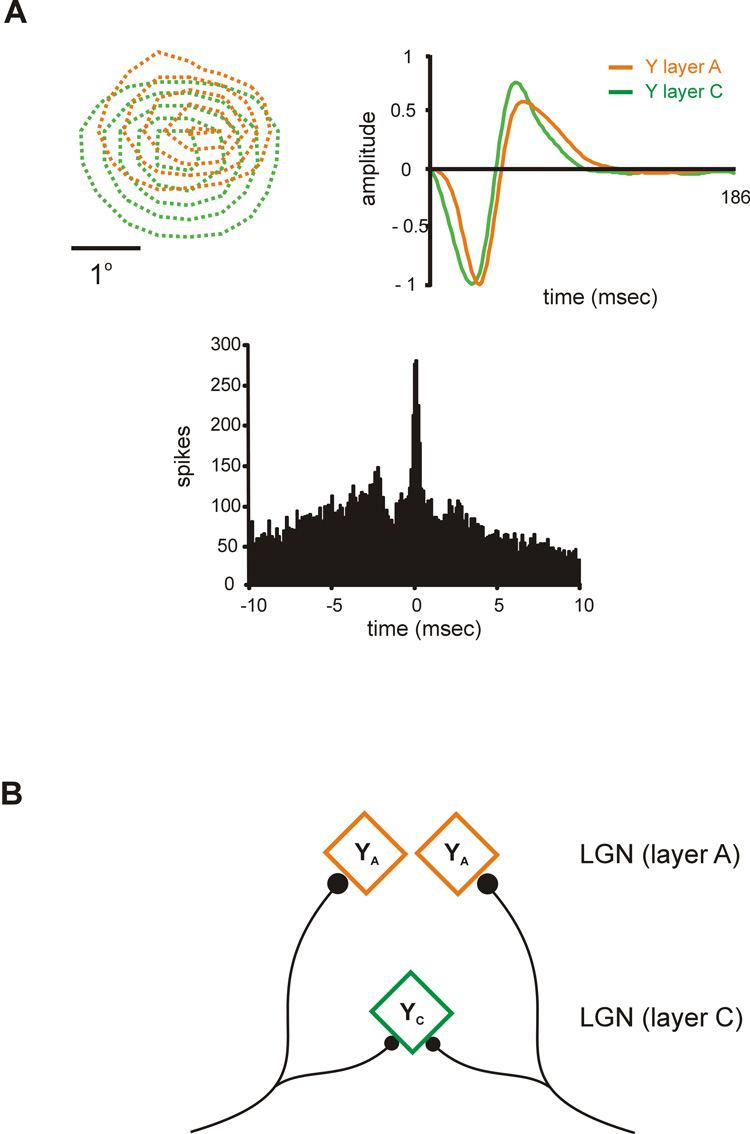
A better understanding of how YA and YC receptive fields are generated requires a precise comparison of the response properties from YA and YC cells that share input from the same retinal afferent. Geniculate neurons that share a common retinal input can be readily identified with cross-correlation analysis because they fire in precise synchrony –their correlogram has a narrow peak of less than 1 ms width centered at zero (Alonso et al., 1996; Usrey et al., 1998; Yeh et al., 2003). Figure 4 shows an example of a pair recording from a YC cell and a YA cell that were tightly correlated (see narrow peak centered at zero in the correlogram, Fig 4A bottom). As expected from cells that share a retinal afferent, the receptive fields of the YA and YC cells were similar in many respects. They were both off-center and they had similar positions in visual space (Fig. 4A left). On the other hand, the receptive fields showed substantial differences that were reminiscent of the differences between YA and YC cells described above. For example, the receptive field was larger and the response latency faster for the YC cell than the YA cell (Fig. 4A top). A similar finding was obtained in recordings from other YC-YA cell pairs. YA and YC cells sharing a retinal afferent always had receptive fields of the same sign (e.g. off-center superimposed with off-center) that were highly overlapped (> 80%). However, they frequently differed in receptive field size and response latency, probably due to the inputs from other retinal afferents that were not shared.
Figure 4. Different types of Y receptive fields (YA and YC) are constructed in LGN with one type of Y retinal afferent.
A. Example of a pair of YA and YC cells that shared input from the same retinal afferent. The two cells had off-center receptive fields that were well overlapped (top, left). However, the YC cell had a slightly larger receptive field and faster response latency (top, right) than the YA cell. The correlograms show a narrow peak centered at zero indicating that both cells fired in precise synchrony, as is characteristic of cells that share a retinal afferent. B. Cartoon of a possible neural mechanism to construct two different types of Y receptive field with a single type of Y retinal afferent. The figure was reprinted with permission from (Yeh et al., 2003).
Interestingly, cell synchrony across layers was weaker and more frequently found than cell synchrony within the same layer (when considering only cell pairs with >80% receptive field overlap). These findings point to a possible mechanism that could allow two types of Y geniculate receptive fields to be constructed with one type of Y retinal afferent. The weaker a more frequent synchrony across layers could be due to a higher divergence of Y retinal afferents within layer C than layer A. As a consequence of this higher divergence, YC geniculate cells would receive input from more retinal afferents than YA cells (Fig. 4B), and due to this higher convergence, YC cells would have larger receptive fields and faster response latencies than YA cells (Fig. 4B, (Yeh et al., 2003)).
Receptive field properties of geniculate neurons representing the same point of visual space
The differences in the response properties of YA and YC cells could be an extreme of a common phenomenon in LGN: geniculate cells that share input from a common retinal afferent may have substantially different receptive fields due to weak retinal inputs that are not shared.
The ganglion cell layer of the retina is a thin structure (< 100 micron thickness) that can only accommodate a limited number of retinal ganglion cells to cover each point of visual space (~30 X cells and 5 Y cells in central retina, Peichl & Wassle, 1979). Reaching such coverage factors is particularly challenging at the area centralis where receptive fields are smallest and therefore, cell density has to be highest [6500 X cells/mm2 and 200 Y cells/mm2 at the area centralis compared with 80 X cells/mm2 and 3 Y cells/mm2 at the far periphery (Peichl & Wassle, 1979)]. The limited space to fit all these retinal ganglion cells has functional consequences: the receptive fields of neighboring cells of a given type (e.g. X or Y) have to be separated by at least half a receptive field center within most of the retina (Wassle et al., 1981a; Wassle et al., 1981b; Mastronarde, 1983; Meister et al., 1995).
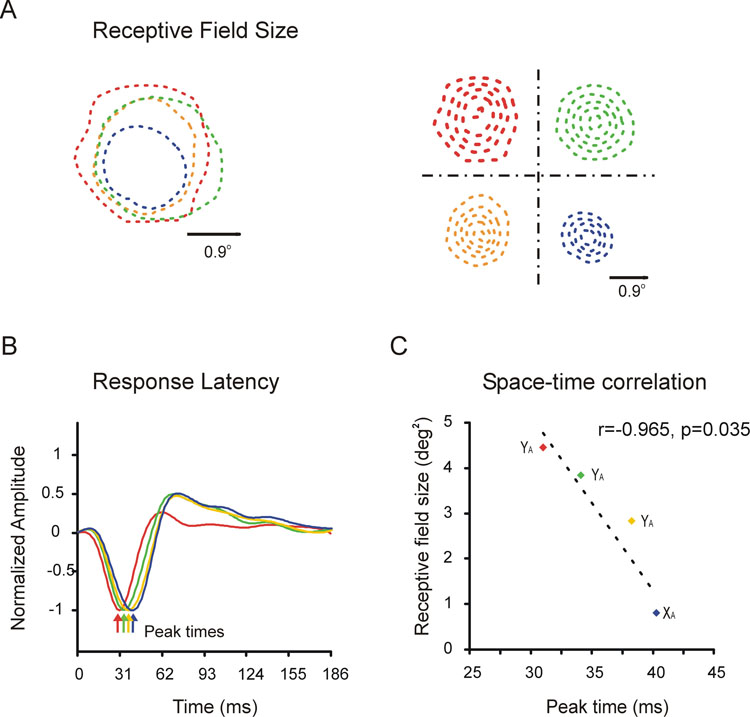
In the cat, the limitation in physical space is somewhat alleviated once the retinal ganglion cells leave the eye. Figure 5A shows the receptive fields of 4 neighboring geniculate cells that were simultaneously recorded within layer A of LGN. The 4 cells had well-overlapped receptive fields of the same sign (off-center). And unlike the retina, the receptive field overlap was almost complete among three cells of the same type (Y cell). Moreover, although the three Y cells showed precise synchronous firing indicating that they shared input from the same retinal afferent, their receptive field sizes (Fig. 5A) and response latencies (Fig. 5B) were substantially different. Interestingly, receptive field size and response latency were correlated (Fig. 5C) suggesting that both properties may be generated by a common mechanism. Receptive field size and response latency could both be determined by the number of retinal afferents that converge onto a given geniculate cell –more convergent afferents will lead to larger receptive fields and faster responses.
Figure 5. Receptive field properties of neighboring geniculate neurons that represent the same point of visual space.
A. Receptive fields from 4 off-center geniculate cells that were simultaneously recorded. The receptive fields of the 4 neurons have very similar positions but they differed substantially in size. The receptive fields are shown as contour plots on the right and superimposed on the left (only the 20% contour is shown on the left for clarity). B. The 4 neurons also differed in their response latencies, as illustrated by the by impulse responses obtained by reverse correlation. C. There was a strong correlation between receptive field size and response latency: the larger the receptive field the faster the response to visual stimuli. The figure was reprinted with permission from (Weng et al., 2005).
Recordings like the one shown in figure 5 demonstrate a surprising diversity of receptive field positions, sizes and response latencies among neighboring neurons within LGN. This receptive field diversity could provide the cortex with a richer representation of space and time that the one available at the retina.
Multiplexing the receptive field properties of the retinal ganglion cells
The connections from the retina to LGN are among the strongest and most specific connections within the visual pathway. One retinal axon can provide more than 100 synapses to the same geniculate cell (Hamos et al., 1987; Chen & Regehr, 2000), a number which is at least 10 times larger than the number of synapses provided by a geniculate axon to a cortical cell (Freund et al., 1985). Moreover, each geniculate cell receives highly specific input from one dominant afferent, whose receptive field is very similar to the geniculate receptive field (Cleland et al., 1971b, a; Cleland & Lee, 1985; Mastronarde, 1992; Usrey et al., 1999).
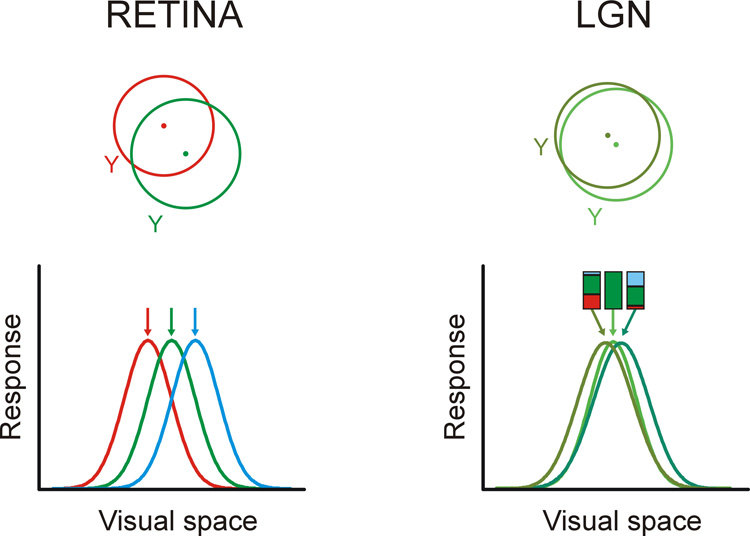
In addition to the dominant afferent, there are other weak retinal inputs that converge at the same geniculate cell but whose receptive fields are not a ‘near-perfect match’ as is the case with the dominant afferent (Cleland et al., 1971a; Mastronarde, 1992; Usrey et al., 1999). The functional significance of these weaker inputs remains unclear. A reasonable possibility is that the weak inputs are remnants of the pruning process during development (Sur et al., 1984; Hamos et al., 1987; Chen & Regehr, 2000). These developmental mistakes (Garraghty et al., 1985) could explain the existence of a few geniculate cells that receive mixed X and Y inputs and have intermediate X/Y properties (Cleland et al., 1971b; Mastronarde, 1992; Usrey et al., 1999). The idea of a developmental error is attractive since most retinogeniculate cells are known to be highly specific of cell type: most X retinal ganglion cells connect to X geniculate cells and most Y retinal ganglion cells connect to Y geniculate cells (Cleland et al., 1971b, a; Cleland & Lee, 1985; Mastronarde, 1992; Usrey et al., 1999). However, our simultaneous recordings from neighboring geniculate cells suggest an alternative interpretation. The weak retinal inputs could be important to interpolate the spatiotemporal receptive fields of the retinal ganglion cells into a more continuous representation of visual space and time (see also Mastronarde, 1992 for a similar idea). Figure 6 illustrates this idea with a cartoon. Representative examples of receptive fields from neighboring neurons recorded within the cat retina (taken from Mastronarde, 1983) and cat LGN (taken from Weng et al., 2005) are shown at the top, and a possible mechanism for the coverage transformation at the bottom. The bottom-left of the cartoon shows the receptive fields of three retinal ganglion cells, illustrated as gaussian curves (shown in red, green and blue) and the bottom-right, the geniculate receptive fields that result from combining the retinal inputs. The LGN gaussian at the center is an exact copy of the green retinal gaussian; it represents a geniculate cell that receives only one retinal input. The LGN gaussians on the sides are obtained from a weighted sum of the green retinal afferent (that contribute 52% of the total input) and the weaker red and blue afferents (that contribute 40% and 8%). The input percentages used in the cartoon are consistent with synaptic weights estimated from counts of retinogeniculate synapses (Hamos et al., 1987) and retinogeniculate correlations measured in pair recordings from retinal and geniculate cells (Cleland et al., 1971b, a; Mastronarde, 1992; Usrey et al., 1999). The weaker the additional retinal inputs, the closer the receptive field positions within the LGN.
Figure 6. Multiplexing the receptive field positions of retinal inputs in LGN.
Top. The cartoon illustrates the receptive fields of two neighboring Y cells in the cat retina (based on Mastronarde, 1983) and two neighboring Y cells in cat LGN (based on Weng et al., 2005). Bottom left. The receptive fields of three Y cells in the retina are represented as gaussian curves in three different colors. Bottom right. The combination of the three retinal inputs yields LGN receptive fields that can sample stimuli at a finer spatial resolution than in the retina. The bar graphs at the top illustrate the relative weights of the retinal inputs that were used to generate the LGN gaussians.
It is estimated that 8–50% of geniculate cells receive input from just one retinal afferent (Cleland et al., 1971a; Cleland & Lee, 1985; Hamos et al., 1987; Mastronarde, 1992). These one-input geniculate cells could be the carriers of a nearly exact copy of the retinal receptive field array (position, size, response time-course) to the cortex. The rest of the geniculate cells are dominated by one retinal input but they also receive input from additional afferents (Cleland et al., 1971b, a; Hamos et al., 1987; Mastronarde, 1992; Usrey et al., 1999). These multiple-input geniculate cells could carry spatiotemporal interpolations that are heavily based on the receptive field of each dominant afferent. Notice that although the cartoon shows retinal and geniculate gaussians with identical widths, the geniculate gaussians should be narrower (Cleland et al., 1971a; Cleland & Lee, 1985) because center-surround interactions are stronger in LGN than in the retina (Hubel & Wiesel, 1961; Singer & Creutzfeldt, 1970; Levick et al., 1972; Singer et al., 1972; Usrey et al., 1999). This increase in surround strength in LGN could be important to reduce the overlap among the geniculate gaussians shown in Figure 6.
Multiplexing the retinal inputs not only could increase the range of receptive field positions but also the receptive field sizes and response latencies within LGN. A continuous representation of response latencies in LGN could be obtained by a weighted sum of the impulse responses from the retinal afferents equivalent to the one illustrated in Figure 6 for visual space. Impulse responses are slower at the borders than at the middle of the retinal receptive field center. Therefore, the combined inputs from dominant afferents (retinal center superimposed with geniculate center) and weak afferents (retinal border superimposed with geniculate center) could provide the basis to generate a continuous range of response latencies within LGN (see Mastronarde, 1992 for beautiful examples of the receptive field relation of a geniculate cell with their multiple retinal inputs).
The idea of using interpolation to improve spatial acuity has been proposed decades ago (Barlow, 1979; Crick et al., 1981; Fahle & Poggio, 1981) and is usually associated with some type of cortical computation (although see Barlow, 1979). However, the properties of retinogeniculate divergence (Sur & Sherman, 1982; Hamos et al., 1987; Sur et al., 1987; Alonso et al., 1996; Yeh et al., 2003), strongly suggest that spatiotemporal interpolation could already be taken place at the level of the LGN, at least in the cat. In that sense, the LGN could serve an important function: to multiplex the receptive field array of retinal ganglion cells and create, by interpolation, a diverse representation of space and time that can be used by the cortex to process visual stimuli more precisely.
ACKNOWLEDGEMENTS
The research was supported by NIH (EY 05253) and The Research Foundation at the University of Connecticut and State University of New York.
Footnotes
The precise retinotopy of LGN and the interelectrode distances used in our experiments strongly suggests that all our recordings came from cells (and not axons) that were located within a cylinder of less than 300 microns of diameter (Sanderson, 1971). Recordings from axons, which were extremely rare in our experiments, had a characteristic spike waveform (Bishop et al., 1962), and could not be maintained for the long periods of time needed for our measurements.
REFERENCES
- Alonso JM, Usrey WM, Reid RC. Precisely correlated firing in cells of the lateral geniculate nucleus. Nature. 1996;383:815–819. doi: 10.1038/383815a0. [DOI] [PubMed] [Google Scholar]
- Barlow HB. Reconstructing the visual image in space and time. Nature. 1979;279:189–190. doi: 10.1038/279189a0. [DOI] [PubMed] [Google Scholar]
- Bishop PO, Burke W, Davis R. The interpretation of the extracellular response of single lateral geniculate cells. J Physiol (Paris) 1962;162:451–472. doi: 10.1113/jphysiol.1962.sp006944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Regehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron. 2000;28:955–966. doi: 10.1016/s0896-6273(00)00166-5. [DOI] [PubMed] [Google Scholar]
- Cleland BG, Dubin MW, Levick WR. Simultaneous recording of input and output of lateral geniculate neurones. Nat New Biol. 1971a;231:191–192. doi: 10.1038/newbio231191a0. [DOI] [PubMed] [Google Scholar]
- Cleland BG, Dubin MW, Levick WR. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971b;217:473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland BG, Lee BB. A comparison of visual responses of cat lateral geniculate nucleus neurones with those of ganglion cells afferent to them. J Physiol. 1985;369:249–268. doi: 10.1113/jphysiol.1985.sp015899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly M, Van Essen D. The representation of the visual field in parvicellular and magnocellular layers of the lateral geniculate nucleus in the macaque monkey. J Comp Neurol. 1984;226:544–564. doi: 10.1002/cne.902260408. [DOI] [PubMed] [Google Scholar]
- Crick FHC, Marr DC, Poggio T. An information-processing approach to understanding the visual cortex. In: FO S, editor. The Organization of the Cerebral Cortex. Cambridge, MA: MIT Press; 1981. [Google Scholar]
- Enroth-Cugell C, Robson JG. The contrast sencitivity of retinal ganglion cells of the cat. J Physiol London. 1966;187:517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahle M, Poggio T. Visual hyperacuity:spatiotemporal interpolation in human vision. Proc R Soc Lond B Biol Sci. 1981;213:451–477. doi: 10.1098/rspb.1981.0075. [DOI] [PubMed] [Google Scholar]
- Frascella J, Lehmkuhle S. A comparison between Y-cells in A-laminae and lamina C of cat dorsal lateral geniculate nucleus. J Neurophysiol. 1984;52:911–920. doi: 10.1152/jn.1984.52.5.911. [DOI] [PubMed] [Google Scholar]
- Freund TF, Martin KA, Somogyi P, Whitteridge D. Innervation of cat visual areas 17 and 18 by physiologically identified X- and Y- type thalamic afferents. II. Identification of postsynaptic targets by GABA immunocytochemistry and Golgi impregnation. J Comp Neurol. 1985;242:275–291. doi: 10.1002/cne.902420209. [DOI] [PubMed] [Google Scholar]
- Friedlander MJ, Lin CS, Sherman SM. Structure of physiologically identified X and Y cells in the cat's lateral geniculate nucleus. Science. 1979;204:1114–1117. doi: 10.1126/science.451559. [DOI] [PubMed] [Google Scholar]
- Friedlander MJ, Lin CS, Stanford LR, Sherman SM. Morphology of functionally identified neurons in lateral geniculate nucleus of the cat. J Neurophysiol. 1981;46:80–129. doi: 10.1152/jn.1981.46.1.80. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Salinger WL, Macavoy MG. The development of cell size in the dorsal lateral geniculate nucleus of monocularly paralyzed cats. Brain Res. 1985;353:99–106. doi: 10.1016/0165-3806(85)90027-6. [DOI] [PubMed] [Google Scholar]
- Hamos JE, Van Horn SC, Raczkowski D, Sherman SM. Synaptic circuits involving an individual retinogeniculate axon in the cat. J Comp Neurol. 1987;259:165–192. doi: 10.1002/cne.902590202. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Integrative action in the cat's lateral geniculate body. J Physiol. 1961;155:385–398. doi: 10.1113/jphysiol.1961.sp006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Brain and Visual Perception. New York: Oxford University Press; 2005. [Google Scholar]
- Illing RB, Wassle H. The retinal projection to the thalamus in the cat: a quantitative investigation and a comparison with the retinotectal pathway. J Comp Neurol. 1981;202:265–285. doi: 10.1002/cne.902020211. [DOI] [PubMed] [Google Scholar]
- Lee D, Lee C, Malpeli JG. Acuity-sensitivity trade-offs of X and Y cells in the cat lateral geniculate complex: role of the medial interlaminar nucleus in scotopic vision. J Neurophysiol. 1992;68:1235–1247. doi: 10.1152/jn.1992.68.4.1235. [DOI] [PubMed] [Google Scholar]
- LeVay S, Ferster D. Proportion of interneurons in the cat's lateral geniculate nucleus. Brain Res. 1979;164:304–308. doi: 10.1016/0006-8993(79)90026-x. [DOI] [PubMed] [Google Scholar]
- Levick WR, Cleland BG, Dubin MW. Lateral geniculate neurons of cat: retinal inputs and physiology. Invest Ophthalmol. 1972;11:302–311. [PubMed] [Google Scholar]
- Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- Mastronarde DN. Correlated firing of cat retinal ganglion cells. I. Spontaneously active inputs to X- and Y-cells. J Neurophysiol. 1983;49:303–324. doi: 10.1152/jn.1983.49.2.303. [DOI] [PubMed] [Google Scholar]
- Mastronarde DN. Nonlagged relay cells and interneurons in the cat lateral geniculate nucleus: receptive-field properties and retinal inputs. Vis Neurosci. 1992;8:407–441. doi: 10.1017/s0952523800004934. [DOI] [PubMed] [Google Scholar]
- Meister M, Lagnado L, Baylor DA. Concerted signaling by retinal ganglion cells. Science. 1995;270:1207–1210. doi: 10.1126/science.270.5239.1207. [DOI] [PubMed] [Google Scholar]
- Peichl L, Wassle H. Size, scatter and coverage of ganglion cell receptive field centres in the cat retina. J Physiol. 1979;291:117–141. doi: 10.1113/jphysiol.1979.sp012803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Payne BR. Numerical relationships between geniculocortical afferents and pyramidal cell modules in cat primary visual cortex. Cereb Cortex. 1993;3:69–78. doi: 10.1093/cercor/3.1.69. [DOI] [PubMed] [Google Scholar]
- Reid RC, Victor JD, Shapley RM. The use of m-sequences in the analysis of visual neurons: linear receptive field properties. Vis Neurosci. 1997;14:1015–1027. doi: 10.1017/s0952523800011743. [DOI] [PubMed] [Google Scholar]
- Sanderson KJ. The projection of the visual field to the lateral geniculate and medial interlaminar nuclei in the cat. J Comp Neurol. 1971;143:101–108. doi: 10.1002/cne.901430107. [DOI] [PubMed] [Google Scholar]
- Singer W, Creutzfeldt OD. Reciprocal lateral inhibition of on- and off-center neurones in the lateral geniculate body of the cat. Exp Brain Res. 1970;10:311–330. doi: 10.1007/BF00235054. [DOI] [PubMed] [Google Scholar]
- Singer W, Poppel E, Creutzfeldt O. Inhibitory interaction in the cat's lateral geniculate nucleus. Exp Brain Res. 1972;14:210–226. doi: 10.1007/BF00234800. [DOI] [PubMed] [Google Scholar]
- Sur M. Development and plasticity of retinal X and Y axon terminations in the cat's lateral geniculate nucleus. Brain Behav Evol. 1988;31:243–251. doi: 10.1159/000116592. [DOI] [PubMed] [Google Scholar]
- Sur M, Esguerra M, Garraghty PE, Kritzer MF, Sherman SM. Morphology of physiologically identified retinogeniculate X- and Yaxons in the cat. J Neurophysiol. 1987;58:1–32. doi: 10.1152/jn.1987.58.1.1. [DOI] [PubMed] [Google Scholar]
- Sur M, Sherman SM. Retinogeniculate terminations in cats: morphological differences between X and Y cell axons. Science. 1982;218:389. doi: 10.1126/science.7123239. [DOI] [PubMed] [Google Scholar]
- Sur M, Weller RE, Sherman SM. Development of X- and Y-cell retinogeniculate terminations in kittens. Nature. 1984;310:246–249. doi: 10.1038/310246a0. [DOI] [PubMed] [Google Scholar]
- Usrey WM, Reppas JB, Reid RC. Paired-spike interactions and synaptic efficacy of retinal inputs to the thalamus. Nature. 1998;395:384–387. doi: 10.1038/26487. [DOI] [PubMed] [Google Scholar]
- Usrey WM, Reppas JB, Reid RC. Specificity and strength of retinogeniculate connections. J Neurophysiol. 1999;82:3527–3540. doi: 10.1152/jn.1999.82.6.3527. [DOI] [PubMed] [Google Scholar]
- Wassle H, Boycott BB, Illing RB. Morphology and mosaic of on- and off-beta cells in the cat retina and some functional considerations. Proc R Soc Lond B Biol Sci. 1981a;212:177–195. doi: 10.1098/rspb.1981.0033. [DOI] [PubMed] [Google Scholar]
- Wassle H, Peichl L, Boycott BB. Morphology and topography of on- and off-alpha cells in the cat retina. Proc R Soc Lond B Biol Sci. 1981b;212:157–175. doi: 10.1098/rspb.1981.0032. [DOI] [PubMed] [Google Scholar]
- Weng C, Yeh CI, Stoelzel CR, Alonso JM. Receptive field size and response latency are correlated within the cat visual thalamus. J Neurophysiol. 2005;93:3537–3547. doi: 10.1152/jn.00847.2004. [DOI] [PubMed] [Google Scholar]
- Yeh CI, Stoelzel CR, Alonso JM. Two different types of Y cells in the cat lateral geniculate nucleus. J Neurophysiol. 2003;90:1852–1864. doi: 10.1152/jn.00417.2003. [DOI] [PubMed] [Google Scholar]