Abstract
Regulated timing of cell division cycles, and geometrical precision in the planar orientation of cell division, are critical during organismal development and remain important for the maintenance of polarized structures in adults. Mounting evidence suggests that these processes are coordinated at the centrosome through the action of proteins that mediate both cell cycle and cell attachment. Our recent work identifying HEF1 as an activator of the Aurora A kinase suggests a novel hub for such integrated signaling. We suggest that defects in components of the machinery specifying the temporal and spatial integration of cell division may induce cancer and other diseases through pleiotropic effects on cell migration, proliferation, apoptosis, and genomic stability.
Keywords: centrosome, polarity, mitosis, attachment, cilia, Aurora-A, HEF1, Cas
Introduction
During metazoan development, cell division is regulated by diffusible and localized extracellular factors that promote or inhibit proliferation, specify mitotic division orientation and symmetry, regulate differentiation into distinct cell types, and in some cases promote directed migration or apoptosis of dividing cells. These cues are essential during the conversion of a single fertilized egg into a complex multicellular organism. They remain important in adults, coordinating the limited cell division required for maintenance of organs. Because of these critical regulatory roles, mutated forms of the proteins comprising the machinery to transmit extracellular information to the cell division apparatus are frequently identified as oncogenes and tumor suppressors, or as cancer-predisposing factors. As summarized below, work by many groups has begun to outline a network of signaling proteins that operate to connect these processes, many of which utilize the centrosome as a central communication point for transmission of information. Aurora-A (AurA) kinase1,2 is now appreciated as an important transducer of signals at centrosomes; our recent studies describing interactions between HEF1 and AurA required for AurA activation3 illuminate a new branch of this signaling network. In this article, we will first summarize the diverse signaling functions that have been identified for centrosomes, then describe how the association of AurA and HEF1 may impact these functions.
Roles of the centrosome
The centrosome is composed of two paired orthogonal centrioles surrounded by “pericentriolar material” (PCM) that varies in abundance and content during cell cycle, and comprises hundreds of structural and signaling proteins. The centrosome has its own duplication cycle (reviewed in4–7), and was for a long time thought of predominantly as an organizing structure for cellular microtubules (a microtubule organizing center, MTOC). As such, its actions in physically nucleating the two ends of the mitotic spindle were a major focus of study. Through studies over the past decade, this view of the centrosome has been significantly revised. It has now been shown that the centrosome provides a contained platform to coordinate signaling related to polarity and cell cycle coordination. Several excellent reviews summarize centrosomal biology at length7–10. In brief (see also Figure 1), important centrosome-associated functions to consider include:
Figure 1. The centrosome coordinates diverse cellular processes.
These include (A) orientation of the mitotic spindle in asymmetric cell divisions, (B) specification of the site of neurite process extension (neurites), (C) nucleation of cilia and flagella, (D) orientation of cell migration, (E) and regulation of cell cycle progression through G1 (checkpoints), (F) mitotic entry, and (G) mitotic exit. See text for details.
1] Orientation of the mitotic spindle in asymmetric cell divisions
Cells growing in a plane (for example, as a sheet of epithelial cells) may divide in different directions. Symmetric planar division can extend the size of the sheet, with two daughter cells assuming the same fate as their mother. Asymmetric cell divisions orthogonal to the direction of the plane allow a mother cell to spawn two daughter cells with different cell fates, and can cause cell propagation into a new dimension. Drosophila has been a productive model system for demonstrating the importance of the centrosome in these processes. Recent studies have addressed asymmetrically dividing neuroepithelial cells giving rise to neuroblasts11,12, male germline stem cells producing gonialblasts after an apical-basal division away from a germline stem cell “hub”13 (Fig 1A), and the syncitial divisions of early embryos14. In these works, centrosomes have been shown to be the target for proteins that directly orient the mitotic spindle by forming physical bridges with polarity cues associated with the cell surface and cortical actin. Planar (lateral) divisions are specified based on signals from the adematous polyposis coli (APC) tumor suppressor protein, and Armadillo/beta-catenin. In the absence of these dominant signals, basal signals provided by Bazooka/Par3, a component of the cell polarity machinery15,16, can direct cell divisions along the apical-basal axis11.
There is mounting evidence that this signaling machinery is conserved through evolution. APC is distantly related to Kar9p, an S. cerevisiae protein that acts as a cue for orientation of the mitotic spindle to the bud, and associates with both the spindle pole body (the yeast “centrosome”) and the actin cortex within the tip of a forming bud 17,18. Excitingly, recent studies by Lechler and Fuchs have provided evidence for a similar process occurring during the stratification and differentiation steps of epidermal development in mammals19. In this case, both integrins and cadherins provided essential signals regulating the polarity complex (Par3 /Pins/aPKC), and additionally influence NuMA and dynactin activity, thus controlling the orientation of the centrosome and spindle.
2] Specification of the site of process extension (neurites and cilia)
Centrosomes also influence the polarization of external cell processes. In a recent study of neurite formation that utilized both mammalian hippocampal neurons and Drosophila third instar neurons20, it was shown that formation of a neurite projection from an apparently undifferentiated, rounded cell body occurred at the site where the centrosome and associated Golgi apparatus abut the cell cortex (Fig 1B). This site is specified based on the prior mitotic division, such that the new neurite forms on the opposite side of the cell from the previous cleavage plane. After cytokinesis, the cortex proximal to the centrosome undergoes transient lamellipodial extension. This is followed by formation of what becomes the dominant neurite at the same site. The observation that an actin polymerization inhibitor (cytochalasin D) can suppress neurite extension implies initial reorganization of the actin cytoskeleton precedes the organization of microtubules and secretory machinery at the time of neurite extension.
As a separate example of centrosome-based polarization of non-mitotic structures, in non-proliferating (G0, stationary) eukaryotic cells, one of the centrioles within a centrosome undergoes a differentiation to form the ciliary basal body (21 and refs therein), which then recruits microtubules and the vesicular trafficking machinery to create a cilium (Fig 1C). These cilia are non-motile, share many (although not all) proteins with centrosomes, and are typically reabsorbed if cells return to proliferation. Mutations in a number of the proteins associated with basal body (for example, inversin and others) are associated with diseases involving abnormal planar cell polarity, and in some cases, abnormal cell growth (10,22 and others). Simons et al. have shown that inversin, a cilia-basal body-centrosome protein, directly interacts with Disheveled, which associates in turn with beta-catenin23, previously shown to orient the spindle in Drosophila studies13. Increasing evidence suggests that in stationary cells, receptors for external signals are specifically localized to the cilia24. It has been proposed that the cilia may also coordinate signals determining whether cells remain in or emerge from stationary phase, through communication with the cell cycle25. In this context, the Nek kinase family may play an important role, as many of the members of this family of kinases are distributed between basal body and centrosome, and some (e.g. Nek2) are known to regulate centrosome dynamics and possibly affect spindle checkpoints, through influencing centriolar cohesion21,26.
3] Centrosomes and cell migration
The studies of centrosomes in neurites suggesting a role for centrosome in regulation of both actin and tubulin cytoskeletons is of additional interest because of reports suggesting a role for the centrosome in orienting cell migration (Fig 1D). An initial study in Dictyostelium observed that positioning of the centrosome in front of the nucleus, behind the leading edge of a migrating cell, was important for the stabilization of the direction of cell migration, perhaps by orienting the microtubule network in support of the actin-based motility machinery27. Subsequent work by others in some cases supported28, and in others contradicted this observation29, while additional work has suggested that the contribution of the centrosome may be to increase the efficiency rather than directionality of migration, through regulation of microtubule dynamics30. In one model, of fibroblast migration, it has been demonstrated that a signal dependent on the Cdc42 GTPase is required to orient the centrosome to face the direction of migration, while microtubules emanating from the centrosome interact at their plus ends with EB1 and APC, making contact with the cell cortex at sites involving Discs-large (Dlg), and once again specified by the polarity complex (aPKC and other proteins)31–33. At present, it seems likely that the phenomenon of centrosomal contribution to migration is cell type specific34, which may reflect the abundance of differing polarization-associated proteins in diverse cell types. It is only now becoming widely appreciated that cells migrate by a variety of different strategies, and that as cancer cells become metastatic, they can serially adapt different strategies 35,36: hence, the importance of these observations in human disease remains to be established.
4] Requirement for progression through G1 (checkpoints)
Centrosomal integrity is important for a number of different cell cycle transitions. Cells with centrosomes ablated by multiple approaches 9, and references therein) undergo G1 arrest (Fig 1E). One mechanism proposed for this arrest is based on the observation that the cyclin E/Cdk2 and cyclin A/Cdk2 complexes that promote entry into S-phase have an obligate association with centrosomes37,38, such that cyclin E mutated to eliminate a centrosome localization domain is unable to promote entry into S phase37. Conversely, Cdk2/cyclin E also is required for the centrosome duplication cycle38,39, and overexpression of cyclin E can promote centrosome overduplication37. A second mechanism of centrosome control of cell cycle may involve the activation at the centrosome of a p53-dependent cell cycle checkpoint, as cells with defective centrosomes (due to depletion of components by siRNA) do not undergo G1 arrest in p53-deficient cells (40, and discussed in 9).
5] Roles in entering and exiting mitosis
The best-studied aspects of centrosomes are their roles in relation to G2/M processes (Fig 1F: reviewed in depth in5,7–9,41). Prior to G2/M transition, a series of interactions between Cdk1 and inhibitors such as Chk1 and Cdc25B at the centrosome restrain Cdk1 activity. At mitotic entry, the AurA and Plk1 kinases act at the centrosome to activate Cdk1/cyclin B and perform other actions necessary to initiate the intracellular organization accompanying karyo- and cyto-kinesis42,43. Gamma-tubulin and other proteins associate with PCM components such as pericentrin, promoting formation of astral microtubules44. Later in mitosis (Fig 1G), centrosomes are centers for ubiquitination activity, governing the action of the anaphase promoting complex/cyclosome (APC/C) in causing the degradation of substrates such as cyclin B45–47. Separate studies indicate that the centrosome may also nucleate cellular degradation and proteasome activation at other phases in the cell cycle as well48. In 2001, Piel et al. made the intriguing observation that the mother centriole must undergo an excursion to the region of the midbody to allow completion of cytokinesis, suggesting delivery of some final signal to promote excision49. Although the nature of this signal remains to be established in detail, Cep55, which interacts with ERK kinases as well as Cdk1 and Plk1, has recently been shown to migrate from centrosome to midbody at cytokinesis, and play an important role in abscission and return to G150.
Introduction to AurA
The AurA kinase is also known as STK15, STK6, BTAK, ARK1, HsAirk1, and Aik; it is a member of the Ipl family of kinases (reviewed in1). AurA is abundant at the centrosome in G2 to M phase, degraded upon completion of cytokinesis, and present at very low levels in G1 and S phases, in part because of efficient post-translational degradation by the ubiquitination machinery51. Although present at the centrosome from early G2, AurA only becomes active around the time of prophase. This activation process is not completely understood, but requires AurA interactions with the proteins Ajuba42, TPX252,53, and (as we have recently described3) HEF1. Upon activation, AurA phosphorylates substrates that promote progression through the stages of mitosis: these include Cdc25B, TPX2, Eg5, Lats2, histone H3, D-Tacc, Brca1 and others, with the list continuing to expand (reviewed in1). Among its defined activities, one of the most important is in promoting the activation of cyclin B/Cdk142, which occurs physically at the centrosome, and may be mediated through phosphorylation and inactivation of Cdc25B54. Failure of AurA activation results in G2 arrest or a defective entry into mitosis, marked by failure of centrosomes to separate and associated monopolar mitotic spindles, and consequent defects in chromosome alignment: failure to complete cytokinesis may arise from this, or also involve additional defects55,56.
In the past several years, AurA has attracted increasing attention because it has been found to be overexpressed in many tumors arising from breast, colon, ovary, and other tissues57–61, and because it has been shown to function as an oncogene when exogenously expressed in various cell line models62–65. AurA overexpression, whether in naturally occurring tumors or following deliberate overexpression, is associated with increased numbers of centrosomes and multipolar spindles, which arise as a consequence of failed cytokinesis. As the overexpressed AurA is not limited to expression in G2 and M phases at the centrosome, but is also detecting throughout the cytoplasm in cells in all cell cycle compartments, it is not clear at present whether the transforming activity of AurA arises from hyperactivated AurA targeting its normal substrates, or through anomalous targeting by AurA of additional substrates (as in the references 61,66). Unexpectedly, even overexpression of a kinase-inactive form of AurA can induce supernumerary centrosomes (although it cannot transform cells62), supporting the idea that the protein has at least two different functions in regulating centrosome numbers. At least one set of important functions of the overexpressed active AurA is to override the spindle checkpoint, which causes resistance to spindle targeting agents such as taxol63 and may arise in part through abrogation of the function of the Chfr mitotic checkpoint protein67. Separately, numerous reports have now documented a physical association between AurA and p53, most likely occurring directly at the centrosome (e.g., 68–70). Although the functional consequences of these interactions are currently controversial, based on conflicting studies using varying assay conditions62–64,68,69, it appears that AurA is able to influence and in some cases override the post-mitotic checkpoint. Based on these various properties, AurA is now being actively exploited as a target for development of new anti-cancer agents (reviewed in 2).
Introduction to HEF1
The newcomer to the discussion of AurA and centrosome functions is HEF1 (71 also known as Nedd9 and Cas-L72). HEF1 and two related proteins, Efs/Sin73,74, and p130Cas/Bcar175, comprise the Cas protein family76,77. These proteins are multidomain scaffolding proteins, with an amino-terminal SH3 domain followed by a large number of potential SH2 binding sites in a “substrate domain”; the carboxy-termini of the proteins, although well conserved within the family, are less well functionally characterized, lacking significant sequence homology outside the group. The first established and best-studied role for this group of proteins is as components of the integrin-dependent attachment signaling cascade, localized to focal complexes and focal adhesions on the basal cell surface. Upon receipt of attachment signals from the extracellular matrix through integrins at the focal adhesion, Cas proteins associate with focal adhesion kinase (FAK) and a Src family kinase. As a result of these interactions, the activity of Src is elevated78,79, and Src phosphorylates Cas extensively in the Cas substrate domain, creating active SH2 binding sites80. These sites bind the adaptor protein Crk/CrkII, subsequently recruiting DOCK180 and C3G; these associations cause signals to propagate further, through DOCK180 to Rac and Pak, and through C3G to the Ras-related GTPase Rap1, in each case promoting lamellipodia formation and cell migration (81,82). HEF1, p130Cas, and Efs each increase cell migration when overexpressed76,77.
Extending out from this set of functions, members of the Cas family have also been shown to influence additional cell processes. Through the C3G-Rac signaling axis, p130Cas was shown to be important for phagocytosis83,84. In normal epithelial cells, detachment of a cell from external supports triggers a suicide program termed “anoikis”, which acts as a surveillance mechanism against cancer 85,86. Cas proteins are components of the attachment-dependent cell survival signaling cascade, with both HEF1 and p130Cas influencing cell viability under different attachment conditions87,88. Elevated Cas levels activate Ras-dependent pathways89, enhancing Raf>MEK>ERK proliferation signaling, and also stimulating PI-3-K90. P130Cas overexpression has been shown to confer tamoxifen resistance on cells, and elevated expression of Cas proteins has been shown to associate with poor prognosis in breast cancer, although the mechanism for Cas action in these cases is not well defined91–95.
Although the Cas proteins have many overlapping functions, some features distinguish HEF1. The most well-studied member of the Cas family, p130Cas, is near ubiquitously expressed. In contrast, HEF1 expression varies considerably between different cell types and tissues71,72,96,97. It is most abundant in vivo in tissues with polarized cell populations, including epithelial cells, neuronal and glial cells, and lymphoid cells, and its signaling action may be particularly important in these cell lineages97,98. p130Cas is abundant at all phases of cell cycle. In contrast, HEF1 is very low in G0/G1 phase cells, with abundance peaking in G2 and M phase3,99. HEF1 expression is induced by various pro-growth or pro-migratory stimuli, including all-trans retinoic acid, which induces polarized neurite extension in brain development100, and TGF-beta101–104, which induces epithelial-mesenchymal transition in development and metastasis105,106: recently, HEF1(Nedd9) elevation was described as part of the lung metastasis transcriptional signature107. Besides being transcriptionally regulated by TGF-beta, HEF1 physically associates with downstream effectors of TGF-beta, the SMAD proteins: this causes post-translational regulation of the protein via the ubiquitination-proteasome machinery102–104, and raises the possibility that interaction with HEF1 may target other proteins for proteasomal degradation. Finally, we have now shown that HEF1 localizes to the centrosome, where it associates with and positively regulates the activity of AurA kinase, through a mechanism yet to be defined3.
AurA, HEF1, and expanded roles in centrosome-associated signaling
In our recent study3, we demonstrated that like AurA, HEF1 accumulated at the centrosome predominantly between G2 and M phase in normal and cancerous breast cell lines. Depletion of HEF1 by siRNA did not affect AurA accumulation at the centrosome, but blocked the activation of AurA at mitotic entry, and led to accumulation of cells with monopolar spindles. Conversely, overexpression of HEF1 induced AurA hyperactivation, and produced cells with multipolar spindles and supernumerary centrosomes. HEF1 also activated AurA kinase activity with both proteins in a purified in vitro system, indicating a direct mode of action. Further, in vitro and in vivo domain mapping experiments demonstrated that the sequences of HEF1 required for AurA activation differed from those required for HEF1-dependent regulation of cell spreading, ruling out the possibility that the HEF1-dependent effects at the centrosome seen were secondary consequences of changes in cell attachment. Finally, the phenotype of HEF1 differed in one important way from that of AurA depletion: in HEF1-depleted cells, premature splitting of the centrosomal pairs was observed, such that in an asynchronous population with similar profiles ~70% of cells had separated centrosomes, rather than ~27% in control siRNA-depleted cells3. This implied that HEF1 might have a second action at centrosomes, in regulation of centrosomal cohesion. Indeed, we showed that HEF1 negatively regulated the action of Nek2, such that this kinase, which promotes centrosome splitting26,108–110, had enhanced activity in HEF1-depleted cells. At present, it is not clear whether this reflects a direct or indirect consequence of loss of HEF1.
Upon initial inspection, the association of HEF1 and AurA, and the implicit potential for cross-signaling between the focal adhesion attachment machinery and centrosome-based cell division machinery, may seem surprising. However, returning to the list of centrosomal signaling roles summarized above, there are a number of reasons why the establishment of HEF1-AurA association is relevant to current models for development and cancer (Figure 2).
Figure 2. Demonstrated and speculative models for HEF1 and AurA interactions.
We have shown (1) that HEF1 promotes AurA activation at the centrosome. Based on the biology summarized herein, and demonstrated protein-protein interactions placing AurA and HEF1 or p130Cas at specific intracellular locales, we speculate that HEF1 and/or p130Cas (2) may be a component of the integrin-dependent machinery orienting the mitotic spindle. We hypothesize that hosphorylation of HEF1 by AurA (3) may promote HEF1 localization to re-establishing focal adhesions at the end of mitosis, contributing to cell spreading: impairment of the HEF1-AurA interaction, or defective post-mitotic spreading, may contribute to activation of p53 and a post-mitotic checkpoint (4). Separately, HEF1 or p130Cas at cilia may coordinate signaling complexes in G0 cells (5), or in response to external signals (growth reentry, or shearing force, given the hypothetical role of Cas proteins as stretch sensors, may trigger AurA activity, leading to ciliary disassembly (6). Proteins with which Cas or AurA have been shown to functionally associate relevant to these models are noted in blue.
First, as noted above, the orientation of cell division plane depends in part on both planar (cadherin-associated) and basal (integrin-associated) external adhesion cues. Focusing on basal signals, HEF1 and Pak, both of which are downstream integrin effectors in cell migration, are now known to associate with and activate AurA at the centrosome3,111, discussed in112. Pak centrosomal localization requires association with another centrosomal protein, GIT1, which also binds the focal adhesion protein paxillin113. Thess are not isolated instances of focal adhesion proteins finding a new use in mitosis. For example, the mitotic kinase WARTS/Lats has been shown to interact with the focal adhesion protein zyxin, with both proteins proximal to the centrosome at the astral microtubules in early mitosis, and collaborating in mitotic initiation (e.g., 114). In their recent work describing extracellular matrix control of cell division axis, Thery et al have proposed that one factor contributing to spindle orientation is cell shape anisotropy arising from greater membrane retraction on non-adhesive surfaces115. Interestingly, Cas and associated proteins CrkII and C3G have been implicated as an integrin-associated stretch-sensing machinery, with application of mechanical force activating downstream signaling116. The numerous connections between focal adhesions and centrosomes now being identified make it plausible that re-use of the existing basal attachment machinery in the G2 and M phase of cell cycle may offer an economical means to coordinate mitotic division polarity.
Reciprocally, the plane and symmetry of mitotic division leads to the segregation of proteins that reinforce and extend polarity signals. AurA has been shown to be required for this latter process. In C. elegans, depletion of AurA (air-1) causes defective segregation of P-granules and the protein Pie-1, indicating loss of mitotic asymmetry117. In Drosophila, flies with mutated AurA are unable to properly segregate the cell fate determinant Numb, with Numb distributed around the cell cortex instead of polarized in one daughter cell118. The polarity machinery (Dlg, Pins, Bazooka/Par3, and aPKC) also specifies asymmetric Numb localization; how AurA signals might interact with this machinery is not clear. A possible role for AurA in governing localization of proteins to focal adhesions or adherens junctions in higher eukaryotes has never been investigated to our knowledge, although intriguingly, our data suggest that mutation of the AurA phosphorylation site on HEF1 influences the ability of HEF1 to return to focal adhesions at the end of mitosis (results not shown).
As noted above, centrosomes give rise to basal bodies in non-proliferating cells, and a number of proteins are shared between centrosome, basal body, and cilia (or flagella, in lower eukaryotes). Bolstering the idea of focal adhesion/centrosome cross-signaling, some recent studies have established relationships between Aurora, Cas proteins, Pak and these additional structures. In the algae Chlamydomonas, the AurA ortholog (CALK) is essential for the regulation of flagellar disassembly119. CALK itself is phosphorylated (presumably affecting its activity) in response to an array of stimuli normally promoting flagellar resorption. The identity of the proteins transmitting resorption signals to CALK is not known119. Nephrocystin and polycystins are proteins that are evolutionarily conserved, cilia-associated proteins that are abundant in renal cells in mammals. Mutations in these proteins are associated with a variety of polycystic kidney disorders120,121; studies of their orthologs in lower eukaryotes such as C. elegans indicate the defects may involve sensing or response of external chemical or physical signals121,122. p130Cas has been identified as an interactor for cilia-associated proteins, including nephrocystin121,122 and polycystin-1123, providing functional coupling between the cilium and proteins including FAK, paxillin, and other focal adhesion components122,123. HEF1 mRNA is particularly abundant in kidney tissue71: specific localization of HEF1 to cilia, and in renal cells, is currently under investigation. An exciting recent study has also implicated specific Pak kinase activity at the cilium in quiescent cells, where it has been proposed to contribute to environmental sensing and tissue homeostasis24. The fact that these recent studies have established relationships between Aurora, Cas proteins, and Pak and these additional centrosome-related structures further buttresses the idea of focal adhesion to centrosome (to cilia?) cross-signaling,
It has long been known that loss of cell attachment induces defective cytokinesis, and arrest in early G1124,125. As described above, AurA hyperactivation or overexpression promotes defective cytokinesis, and influences the activity of p53, with some studies finding that inactivation of the p53 checkpoint is necessary to promote AurA-dependent cell transformation. Like AurA, HEF1 overexpression and depletion induce M phase defects3. We have begun to investigate interactions between HEF1 and the post-mitotic checkpoint machinery. As shown in Figure 3, elutriation of populations of p53-positive MCF7 cells with HEF1 depleted for 48 hours initially reveals a significant fraction of cells have >4N DNA content. However, when these cells are collected, replated, and cell cycle compartmentalization reassayed after 24 hours, the majority of the >4N cells are lost. This implies loss of HEF1 is not able to overcome the post-mitotic checkpoint, and places HEF1 on a signaling pathway relevant to detachment-induced cell cycle arrest or apoptosis. Intriguingly, the HEF1-interacting protein FAK has been shown both to localize to the centrosome 126, and to interact directly with p53127. Both HEF13,99 and FAK128 are subject to substantial changes in phosphorylation during mitosis. These phosphorylations influence the ability of these proteins to associate with different partners.
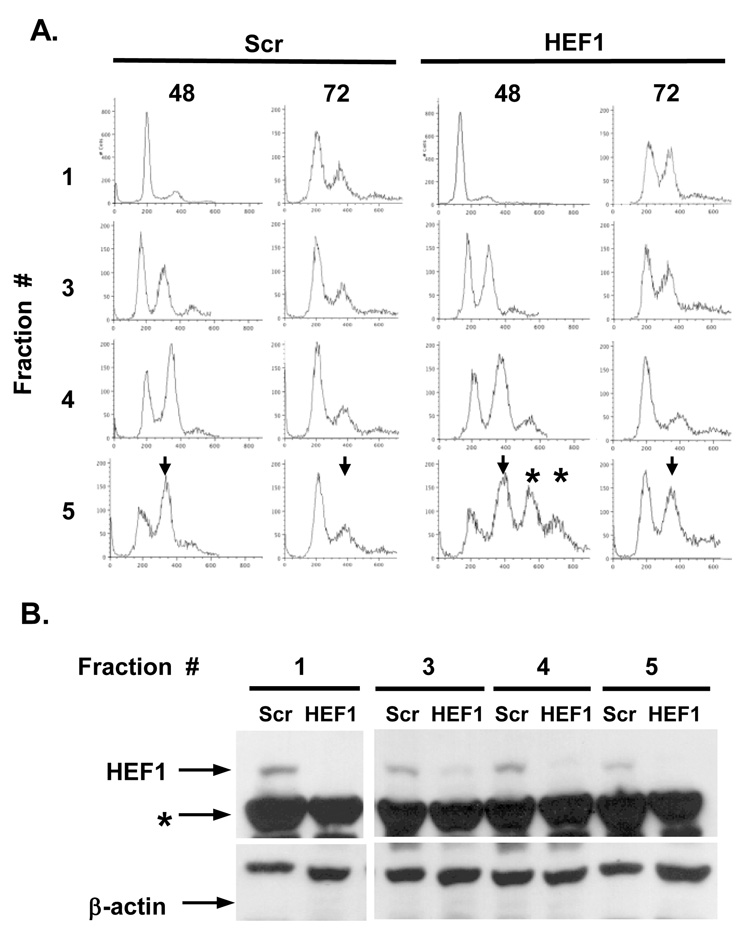
Figure 3. Depletion of HEF1 causes transient aneuploidy.
A. 109 MCF7 cells treated with control non-specific siRNA duplex (Scr) or HEF1-specific siRNA for 48h were elutriated to separate cell cycle fractions using a Beckman J elutriating centrifuge. The FACS profiles of representative fractions across the gradient are shown (48 hours): in parallel, an aliquot of each fraction of the elutriated cells was replated, grown for an additional 24 hours, and then reassayed by FACS (72 hours). Arrows in fraction 5 indicate 4N DNA peaks; asterisks represent peaks of >4N DNA content, present at 48 hours but absent at 72 hours after siRNA treatment. B. Western blot analysis of fractions shown in A after elutriation (time 48 hours). siRNA to HEF1 depletes HEF1 by 75–90% versus Scr control (based on NIH Image analysis of scanned films). Asterisk marks a non-specific cross-reacting band, which serves as one loading control: additionally, blots were stripped and reprobed with antibody to β-actin.
Speculatively, HEF1 and associated proteins such as FAK and Pak may act in part as attachment-sensing checkpoint proteins at mitotic entry and exit. Movement of these proteins from the basal cell surface to the centrosome at G2/M may provide a signal that cells have successfully disassembled focal adhesions, and are ready for mitotic rounding. Later in M phase, the destruction or phosphorylation of these proteins to remove them from the mitotic machinery, and their reinstatement at focal adhesions at cytokinesis may be a licensing event for cell reattachment and progression through G1 phase. On the other hand, it is also well-established that Cas proteins and FAK influence G1 progression by other means, exclusive of dialog with the checkpoint machinery: for example, FAK regulates cyclin D1 expression129, as do small GTPases and Cas effectors such as Rac130,131; while Cas proteins positively regulate serum response proliferation signals89. Separating the various threads connecting AurA and Cas proteins to the control of cell division will take some time.
Conclusions and future questions
Characterization of proteins at the intersection of attachment, mitotic, and checkpoint signaling might be expected to offer important insights into cancer development, given that the deregulation of such proteins might simultaneously promote not only metastasis and tumor cell survival, but also genomic instability. Intriguingly, a recent study mutating genes associated with asymmetric cell division in Drosophila neuroblasts demonstrated that loss of Pins, Numb, and others resulted in the creation of tumors with some properties of stem cells, characterized by genome instability and centrosome alterations132. In higher eukaryotes, it is difficult to track the genetic and epigenetic changes associated with tumor cell initiation, because by the time tumors have become large enough to detect, additional changes may have occurred. At present, the relationship of the status of AurA and human cancer initiation is complicated, with some studies identifying overexpression of AurA in large tumors, and others showing it as an event in early tumors, subsequently selected against (also see discussion in133): for AurA and other proteins such as p130Cas, HEF1, and associated factors, more investigation is required.
A fundamental question arising from these many converging studies is the relationship between the cell asymmetry control machinery and the etiology of most human cancers. Suggestively, a significant number of the asymmetry control proteins are almost by definition exclusively or predominantly expressed in polarized cell types, such as epithelial or neuronal cells. This may contribute to the predisposition of such cells (rather than non-polarized fibroblast or stromal cells) to form solid tumors, based on their possession of an apparatus that connects more vital cell processes. In the past several years, studies of the growth of cultured cells in more natural three-dimensional matrix environments has begun to reveal unexpected convergence between polarization cues and many cancer-related signaling processes, that differ from previous findings made in cells grown by traditional culture in two-dimensions134–136. Extension of these studies to include analysis of mitotic processes is likely to tie together the sequence and interdependence of events leading to tumorigenesis.
Acknowledgments
This work was supported by research grant NIH CA63366, the Susan Komen Breast Cancer Foundation, the Department of Defense, and Tobacco Settlement funding from the State of Pennsylvania (to EAG); and by NIH core grant CA-06927 to Fox Chase Cancer Center. ENP was supported by the US Department of Defense Breast Cancer Training grant DAMD17-00-1-0249. We thank our colleagues Lisa Henske, Jon Chernoff, Fabrice Roegiers, and Eti Cukierman for helpful discussions contributing to some of the ideas in this work, and Eugene Izumchenko and Jon Chernoff for useful comments on the manuscript.
References
- 1.Marumoto T, Zhang D, Saya H. Aurora-A - a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 2.Andrews PD. Aurora kinases: shining lights on the therapeutic horizon? Oncogene. 2005;24:5005–5015. doi: 10.1038/sj.onc.1208752. [DOI] [PubMed] [Google Scholar]
- 3.Pugacheva EN, Golemis EA. The focal adhesion scaffolding protein HEF1 regulates activation of the Aurora-A and Nek2 kinases at the centrosome. Nat Cell Biol. 2005;7:937–946. doi: 10.1038/ncb1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bornens M. Centrosome composition and microtubule anchoring mechanisms. Curr Opin Cell Biol. 2002;14:25–34. doi: 10.1016/s0955-0674(01)00290-3. [DOI] [PubMed] [Google Scholar]
- 5.Meraldi P, Nigg EA. The centrosome cycle. FEBS Lett. 2002;521:9–13. doi: 10.1016/s0014-5793(02)02865-x. [DOI] [PubMed] [Google Scholar]
- 6.Palazzo RE, Vogel JM, Schnackenberg BJ, Hull DR, Wu X. Centrosome maturation. Curr Top Dev Biol. 2000;49:449–470. doi: 10.1016/s0070-2153(99)49021-0. [DOI] [PubMed] [Google Scholar]
- 7.Rieder CL, Faruki S, Khodjakov A. The centrosome in vertebrates: more than a microtubule-organizing center. Trends Cell Biol. 2001;11:413–419. doi: 10.1016/s0962-8924(01)02085-2. [DOI] [PubMed] [Google Scholar]
- 8.Doxsey S, McCollum D, Theurkauf W. Centrosomes in cellular regulation. Annu Rev Cell Dev Biol. 2005;21:411–434. doi: 10.1146/annurev.cellbio.21.122303.120418. [DOI] [PubMed] [Google Scholar]
- 9.Doxsey S, Zimmerman W, Mikule K. Centrosome control of the cell cycle. Trends Cell Biol. 2005;15:303–311. doi: 10.1016/j.tcb.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Badano JL, Teslovich TM, Katsanis N. The centrosome in human genetic disease. Nat Rev Genet. 2005;6:194–205. doi: 10.1038/nrg1557. [DOI] [PubMed] [Google Scholar]
- 11.Lu B, Roegiers F, Jan LY, Jan YN. Adherens junctions inhibit asymmetric division in the Drosophila epithelium. Nature. 2001;409:522–525. doi: 10.1038/35054077. [DOI] [PubMed] [Google Scholar]
- 12.Roegiers F, Younger-Shepherd S, Jan LY, Jan YN. Bazooka is required for localization of determinants and controlling proliferation in the sensory organ precursor cell lineage in Drosophila. Proc Natl Acad Sci U S A. 2001;98:14469–14474. doi: 10.1073/pnas.261555598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- 14.McCartney BM, McEwen DG, Grevengoed E, Maddox P, Bejsovec A, Peifer M. Drosophila APC2 and Armadillo participate in tethering mitotic spindles to cortical actin. Nat Cell Biol. 2001;3:933–938. doi: 10.1038/ncb1001-933. [DOI] [PubMed] [Google Scholar]
- 15.Macara IG. Parsing the polarity code. Nat Rev Mol Cell Biol. 2004;5:220–231. doi: 10.1038/nrm1332. [DOI] [PubMed] [Google Scholar]
- 16.Schneider SQ, Bowerman B. Cell polarity and the cytoskeleton in the Caenorhabditis elegans zygote. Annu Rev Genet. 2003;37:221–249. doi: 10.1146/annurev.genet.37.110801.142443. [DOI] [PubMed] [Google Scholar]
- 17.Miller RK, Rose MD. Kar9p is a novel cortical protein required for cytoplasmic microtubule orientation in yeast. J Cell Biol. 1998;140:377–390. doi: 10.1083/jcb.140.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segal M, Bloom K. Control of spindle polarity and orientation in Saccharomyces cerevisiae. Trends Cell Biol. 2001;11:160–166. doi: 10.1016/s0962-8924(01)01954-7. [DOI] [PubMed] [Google Scholar]
- 19.Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Anda FC, Pollarolo G, Da Silva JS, Camoletto PG, Feiguin F, Dotti CG. Centrosome localization determines neuronal polarity. Nature. 2005;436:704–708. doi: 10.1038/nature03811. [DOI] [PubMed] [Google Scholar]
- 21.Quarmby LM, Mahjoub MR. Caught Nek-ing: cilia and centrioles. J Cell Sci. 2005;118:5161–5169. doi: 10.1242/jcs.02681. [DOI] [PubMed] [Google Scholar]
- 22.Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, Tan PL, Phillips HM, Leroux MR, Henderson DJ, Murdoch JN, Copp AJ, Eliot MM, Lupski JR, Kemp DT, Dollfus H, Tada M, Katsanis N, Forge A, Beales PL. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 23.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Quarmby LM, Parker JD. Cilia and the cell cycle? J Cell Biol. 2005;169:707–710. doi: 10.1083/jcb.200503053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fry AM. The Nek2 protein kinase: a novel regulator of centrosome structure. Oncogene. 2002;21:6184–6194. doi: 10.1038/sj.onc.1205711. [DOI] [PubMed] [Google Scholar]
- 27.Ueda M, Graf R, MacWilliams HK, Schliwa M, Euteneuer U. Centrosome positioning and directionality of cell movements. Proc Natl Acad Sci U S A. 1997;94:9674–9678. doi: 10.1073/pnas.94.18.9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danowski BA, Khodjakov A, Wadsworth P. Centrosome behavior in motile HGF-treated PtK2 cells expressing GFP-gamma tubulin. Cell Motil Cytoskeleton. 2001;50:59–68. doi: 10.1002/cm.1041. [DOI] [PubMed] [Google Scholar]
- 30.Abal M, Piel M, Bouckson-Castaing V, Mogensen M, Sibarita JB, Bornens M. Microtubule release from the centrosome in migrating cells. J Cell Biol. 2002;159:731–737. doi: 10.1083/jcb.200207076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Etienne-Manneville S, Manneville JB, Nicholls S, Ferenczi MA, Hall A. Cdc42 and Par6-PKCzeta regulate the spatially localized association of Dlg1 and APC to control cell polarization. J Cell Biol. 2005;170:895–901. doi: 10.1083/jcb.200412172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cau J, Hall A. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J Cell Sci. 2005;118:2579–2587. doi: 10.1242/jcs.02385. [DOI] [PubMed] [Google Scholar]
- 33.Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- 34.Yvon AM, Walker JW, Danowski B, Fagerstrom C, Khodjakov A, Wadsworth P. Centrosome reorientation in wound-edge cells is cell type specific. Mol Biol Cell. 2002;13:1871–1880. doi: 10.1091/mbc.01-11-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 36.Friedl P. Prespecification and plasticity: shifting mechanisms of cell migration. Curr Opin Cell Biol. 2004;16:14–23. doi: 10.1016/j.ceb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto Y, Maller JL. A centrosomal localization signal in cyclin E required for Cdk2-independent S phase entry. Science. 2004;306:885–888. doi: 10.1126/science.1103544. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto Y, Hayashi K, Nishida E. Cyclin-dependent kinase 2 (Cdk2) is required for centrosome duplication in mammalian cells. Curr Biol. 1999;9:429–432. doi: 10.1016/s0960-9822(99)80191-2. [DOI] [PubMed] [Google Scholar]
- 39.Lacey KR, Jackson PK, Stearns T. Cyclin-dependent kinase control of centrosome duplication. Proc Natl Acad Sci U S A. 1999;96:2817–2822. doi: 10.1073/pnas.96.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bobinnec Y, Khodjakov A, Mir LM, Rieder CL, Edde B, Bornens M. Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J Cell Biol. 1998;143:1575–1589. doi: 10.1083/jcb.143.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dutertre S, Descamps S, Prigent C. On the role of aurora-A in centrosome function. Oncogene. 2002;21:6175–6183. doi: 10.1038/sj.onc.1205775. [DOI] [PubMed] [Google Scholar]
- 42.Hirota T, Kunitoku N, Sasayama T, Marumoto T, Zhang D, Nitta M, Hatakeyama K, Saya H. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell. 2003;114:585–598. doi: 10.1016/s0092-8674(03)00642-1. [DOI] [PubMed] [Google Scholar]
- 43.Jackman M, Lindon C, Nigg EA, Pines J. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat Cell Biol. 2003;5:143–148. doi: 10.1038/ncb918. [DOI] [PubMed] [Google Scholar]
- 44.Dictenberg JB, Zimmerman W, Sparks CA, Young A, Vidair C, Zheng Y, Carrington W, Fay FS, Doxsey SJ. Pericentrin and gamma-tubulin form a protein complex and are organized into a novel lattice at the centrosome. J Cell Biol. 1998;141:163–174. doi: 10.1083/jcb.141.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang J, Raff JW. The disappearance of cyclin B at the end of mitosis is regulated spatially in Drosophila cells. Embo J. 1999;18:2184–2195. doi: 10.1093/emboj/18.8.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wakefield JG, Huang JY, Raff JW. Centrosomes have a role in regulating the destruction of cyclin B in early Drosophila embryos. Curr Biol. 2000;10:1367–1370. doi: 10.1016/s0960-9822(00)00776-4. [DOI] [PubMed] [Google Scholar]
- 47.Raff JW, Jeffers K, Huang JY. The roles of Fzy/Cdc20 and Fzr/Cdh1 in regulating the destruction of cyclin B in space and time. J Cell Biol. 2002;157:1139–1149. doi: 10.1083/jcb.200203035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wigley WC, Fabunmi RP, Lee MG, Marino CR, Muallem S, DeMartino GN, Thomas PJ. Dynamic association of proteasomal machinery with the centrosome. J Cell Biol. 1999;145:481–490. doi: 10.1083/jcb.145.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piel M, Nordberg J, Euteneuer U, Bornens M. Centrosome-dependent exit of cytokinesis in animal cells. Science. 2001;291:1550–1553. doi: 10.1126/science.1057330. [DOI] [PubMed] [Google Scholar]
- 50.Fabbro M, Zhou BB, Takahashi M, Sarcevic B, Lal P, Graham ME, Gabrielli BG, Robinson PJ, Nigg EA, Ono Y, Khanna KK. Cdk1/Erk2- and plk1-dependent phosphorylation of a centrosome protein, cep55, is required for its recruitment to midbody and cytokinesis. Dev Cell. 2005;9:477–488. doi: 10.1016/j.devcel.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Fukuda T, Mishina Y, Walker MP, DiAugustine RP. Conditional transgenic system for mouse aurora a kinase: degradation by the ubiquitin proteasome pathway controls the level of the transgenic protein. Mol Cell Biol. 2005;25:5270–5281. doi: 10.1128/MCB.25.12.5270-5281.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eyers PA, Erikson E, Chen LG, Maller JL. A novel mechanism for activation of the protein kinase Aurora A. Curr Biol. 2003;13:691–697. doi: 10.1016/s0960-9822(03)00166-0. [DOI] [PubMed] [Google Scholar]
- 53.Tsai MY, Wiese C, Cao K, Martin O, Donovan P, Ruderman J, Prigent C, Zheng Y. A Ran signalling pathway mediated by the mitotic kinase Aurora A in spindle assembly. Nat Cell Biol. 2003;5:242–248. doi: 10.1038/ncb936. [DOI] [PubMed] [Google Scholar]
- 54.Dutertre S, Cazales M, Quaranta M, Froment C, Trabut V, Dozier C, Mirey G, Bouche JP, Theis-Febvre N, Schmitt E, Monsarrat B, Prigent C, Ducommun B. Phosphorylation of CDC25B by Aurora-A at the centrosome contributes to the G2-M transition. J Cell Sci. 2004;117:2523–2531. doi: 10.1242/jcs.01108. [DOI] [PubMed] [Google Scholar]
- 55.Glover DM, Leibowitz MH, McLean DA, Parry H. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 1995;81:95–105. doi: 10.1016/0092-8674(95)90374-7. [DOI] [PubMed] [Google Scholar]
- 56.Marumoto T, Honda S, Hara T, Nitta M, Hirota T, Kohmura E, Saya H. Aurora-a kinase maintains the fidelity of early and late mitotic events in HeLa cells. J Biol Chem. 2003 doi: 10.1074/jbc.M306275200. [DOI] [PubMed] [Google Scholar]
- 57.Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C, Chan CS, Novotny M, Slamon DJ, Plowman GD. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. Embo J. 1998;17:3052–3065. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW, Sahin A, Brinkley BR, Sen S. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–193. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]
- 59.Goepfert TM, Adigun YE, Zhong L, Gay J, Medina D, Brinkley WR. Centrosome amplification and overexpression of aurora A are early events in rat mammary carcinogenesis. Cancer Res. 2002;62:4115–4122. [PubMed] [Google Scholar]
- 60.Tanner MM, Grenman S, Koul A, Johannsson O, Meltzer P, Pejovic T, Borg A, Isola JJ. Frequent amplification of chromosomal region 20q12-q13 in ovarian cancer. Clin Cancer Res. 2000;6:1833–1839. [PubMed] [Google Scholar]
- 61.Tanaka T, Kimura M, Matsunaga K, Fukada D, Mori H, Okano Y. Centrosomal kinase AIK1 is overexpressed in invasive ductal carcinoma of the breast. Cancer Res. 1999;59:2041–2044. [PubMed] [Google Scholar]
- 62.Meraldi P, Honda R, Nigg EA. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53−/− cells. Embo J. 2002;21:483–492. doi: 10.1093/emboj/21.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anand S, Penrhyn-Lowe S, Venkitaraman AR. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell. 2003;3:51–62. doi: 10.1016/s1535-6108(02)00235-0. [DOI] [PubMed] [Google Scholar]
- 64.Zhang D, Hirota T, Marumoto T, Shimizu M, Kunitoku N, Sasayama T, Arima Y, Feng L, Suzuki M, Takeya M, Saya H. Cre-loxP-controlled periodic Aurora-A overexpression induces mitotic abnormalities and hyperplasia in mammary glands of mouse models. Oncogene. 2004;23:8720–8730. doi: 10.1038/sj.onc.1208153. [DOI] [PubMed] [Google Scholar]
- 65.Tatsuka M, Katayama H, Ota T, Tanaka T, Odashima S, Suzuki F, Terada Y. Multinuclearity and increased ploidy caused by overexpression of the aurora- and Ipl1-like midbody-associated protein mitotic kinase in human cancer cells. Cancer Res. 1998;58:4811–4816. [PubMed] [Google Scholar]
- 66.Gritsko TM, Coppola D, Paciga JE, Yang L, Sun M, Shelley SA, Fiorica JV, Nicosia SV, Cheng JQ. Activation and overexpression of centrosome kinase BTAK/Aurora-A in human ovarian cancer. Clin Cancer Res. 2003;9:1420–1426. [PubMed] [Google Scholar]
- 67.Yu X, Minter-Dykhouse K, Malureanu L, Zhao WM, Zhang D, Merkle CJ, Ward IM, Saya H, Fang G, van Deursen J, Chen J. Chfr is required for tumor suppression and Aurora A regulation. Nat Genet. 2005;37:401–406. doi: 10.1038/ng1538. [DOI] [PubMed] [Google Scholar]
- 68.Chen SS, Chang PC, Cheng YW, Tang FM, Lin YS. Suppression of the STK15 oncogenic activity requires a transactivation-independent p53 function. Embo J. 2002;21:4491–4499. doi: 10.1093/emboj/cdf409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Katayama H, Sasai K, Kawai H, Yuan ZM, Bondaruk J, Suzuki F, Fujii S, Arlinghaus RB, Czerniak BA, Sen S. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat Genet. 2004;36:55–62. doi: 10.1038/ng1279. [DOI] [PubMed] [Google Scholar]
- 70.Zantema A, Fransen JA, Davis-Olivier A, Ramaekers FC, Vooijs GP, DeLeys S, Van der Eb AJ. Localization of the E1B proteins of adenovirus 5 in transformed Sells, as revealed by interaction with monoclonal antibodies. Virology. 1985;142:44–58. doi: 10.1016/0042-6822(85)90421-0. [DOI] [PubMed] [Google Scholar]
- 71.Law SF, Estojak J, Wang B, Mysliwiec T, Kruh GD, Golemis EA. Human Enhancer of Filamentation 1 (HEF1), a novel p130Cas-like docking protein, associates with FAK and induces pseudohyphal growth in yeast. Mol. Cell. Biol. 1996;16:3327–3337. doi: 10.1128/mcb.16.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Minegishi M, Tachibana K, Sato T, Iwata S, Nojima Y, Morimoto C. Structure and function of Cas-L, a 105-kD Crk-associated substrate-related protein that is involved in beta-1 integrin-mediated signaling in lymphocytes. J. Exp. Med. 1996;184:1365–1375. doi: 10.1084/jem.184.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ishino M, Ohba T, Sasaki H, Sasaki T. Molecular cloning of a cDNA encoding a phosphoprotein, Efs, which contains a Src homology 3 domain and associates with Fyn. Oncogene. 1995;11:2331–2338. [PubMed] [Google Scholar]
- 74.Alexandropoulos K, Cheng G, Baltimore D. Proline-rich sequences that bind to Src homology 3 domains with individual specificities. Proc. Nat. Acad. Sci. USA. 1995;92:3110–3114. doi: 10.1073/pnas.92.8.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sakai R, Iwamatsu A, Hirano N, Ogawa S, Tanaka T, Mano H, Yazaki Y, Hirai H. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk snd v-Src in a tyrosine phosphorylation-dependent manner. EMBO J. 1994;13:3748–3756. doi: 10.1002/j.1460-2075.1994.tb06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O'Neill GM, Fashena SJ, Golemis EA. Integrin signaling: a new Cas(t) of characters enters the stage. Trends Cell Biol. 2000;10:111–119. doi: 10.1016/s0962-8924(99)01714-6. [DOI] [PubMed] [Google Scholar]
- 77.Bouton AH, Riggins RB, Bruce-Staskal PJ. Functions of the adapter protein Cas: signal convergence and the determination of cellular responses. Oncogene. 2001;20:6448–6458. doi: 10.1038/sj.onc.1204785. [DOI] [PubMed] [Google Scholar]
- 78.Alexandropoulos K, Baltimore D. Coordinate activation of c-Src by SH3- and SH2-binding sites on a novel, p130Cas-related protein, Sin. Genes Dev. 1996;10:1341–1355. doi: 10.1101/gad.10.11.1341. [DOI] [PubMed] [Google Scholar]
- 79.Burnham MR, Bruce-Staskal PJ, Harte MT, Weidow CL, Ma A, Weed SA, Bouton AH. Regulation of c-SRC activity and function by the adapter protein CAS. Mol Cell Biol. 2000;20:5865–5878. doi: 10.1128/mcb.20.16.5865-5878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brabek J, Constancio SS, Siesser PF, Shin NY, Pozzi A, Hanks SK. Crk-associated substrate tyrosine phosphorylation sites are critical for invasion and metastasis of SRC-transformed cells. Mol Cancer Res. 2005;3:307–315. doi: 10.1158/1541-7786.MCR-05-0015. [DOI] [PubMed] [Google Scholar]
- 81.Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA. CAS/Crk coupling serves as a "molecular switch" for induction of cell migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fashena SJ, Einarson MB, O'Neill GM, Patriotis CP, Golemis EA. Dissection of HEF1-dependent functions in motility and transcriptional regulation. J. Cell. Sci. 2002;115:99–111. doi: 10.1242/jcs.115.1.99. [DOI] [PubMed] [Google Scholar]
- 83.Albert ML, Kim JI, Birge RB. alphavbeta5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat Cell Biol. 2000;2:899–905. doi: 10.1038/35046549. [DOI] [PubMed] [Google Scholar]
- 84.Bruce-Staskal PJ, Weidow CL, Gibson JJ, Bouton AH. Cas, Fak and Pyk2 function in diverse signaling cascades to promote Yersinia uptake. J Cell Sci. 2002;115:2689–2700. doi: 10.1242/jcs.115.13.2689. [DOI] [PubMed] [Google Scholar]
- 85.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 86.Frisch SM. The epithelial cell default-phenotype hypothesis and its implications for cancer. BioEssays. 1997;19:705–709. doi: 10.1002/bies.950190811. [DOI] [PubMed] [Google Scholar]
- 87.O'Neill GM, Golemis EA. Proteolysis of the docking protein HEF1 and implications for focal adhesion dynamics. Mol. Cell. Biol. 2001;21:5094–5108. doi: 10.1128/MCB.21.15.5094-5108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Almeida EA, Ilic D, Han Q, Hauck CR, Jin F, Kawakatsu H, Schlaepfer DD, Damsky CH. Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase. J Cell Biol. 2000;149:741–754. doi: 10.1083/jcb.149.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hakak Y, Martin GS. Cas Mediates Transcriptional Activation of the Serum Response Element by Src. Mol Cell Biol. 1999;19:6953–6962. doi: 10.1128/mcb.19.10.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Felekkis KN, Narsimhan RP, Near R, Castro AF, Zheng Y, Quilliam LA, Lerner A. AND-34 activates phosphatidylinositol 3-kinase and induces anti-estrogen resistance in a SH2 and GDP exchange factor-like domain-dependent manner. Mol Cancer Res. 2005;3:32–41. [PubMed] [Google Scholar]
- 91.van der Flier S, Brinkman A, Look MP, Kok EM, Meijer-Van Gelder ME, Klijn JG, Dorssers LC, Foekens JA. Bcar1/p130Cas protein and primary breast cancer: prognosis and response to tamoxifen treatment. J Natl Cancer Inst. 2000;92:120–127. doi: 10.1093/jnci/92.2.120. [DOI] [PubMed] [Google Scholar]
- 92.Brinkman A, van Der Flier S, Kok EM, Dorssers LC. BCAR1, a human homologue of the adapter protein p130Cas, and antiestrogen resistance in breast cancer cells. J Natl Cancer Inst. 2000;92:112–120. doi: 10.1093/jnci/92.2.112. [DOI] [PubMed] [Google Scholar]
- 93.van der Flier S, Chan CM, Brinkman A, Smid M, Johnston SR, Dorssers LC, Dowsett M. BCAR1/p130Cas expression in untreated and acquired tamoxifen-resistant human breast carcinomas. Int J Cancer. 2000;89:465–468. doi: 10.1002/1097-0215(20000920)89:5<465::aid-ijc11>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 94.Dorssers LC, Van der Flier S, Brinkman A, van Agthoven T, Veldscholte J, Berns EM, Klijn JG, Beex LV, Foekens JA. Tamoxifen resistance in breast cancer: elucidating mechanisms. Drugs. 2001;61:1721–1733. doi: 10.2165/00003495-200161120-00004. [DOI] [PubMed] [Google Scholar]
- 95.Cai D, Felekkis KN, Near RI, O'Neill GM, van Seventer JM, Golemis EA, Lerner A. The GDP exchange factor AND-34 is expressed in B cells, associates with HEF1, and activates Cdc42. J Immunol. 2003;170:969–978. doi: 10.4049/jimmunol.170.2.969. [DOI] [PubMed] [Google Scholar]
- 96.Manie SN, Astier A, Haghayeghi N, Canty T, Druker BJ, Hirai H, Freedman AS. Regulation of integrin-mediated p130(Cas) tyrosine phosphorylation in human B cells. A role for p59(Fyn) and SHP2. J Biol Chem. 1997;272:15636–15641. doi: 10.1074/jbc.272.25.15636. [DOI] [PubMed] [Google Scholar]
- 97.Natarajan M, Stewart JE, Golemis EA, Pugacheva EN, Alexandropoulos K, Cox BD, Wang W, Grammer JR, Gladson CL. HEF1 is a necessary and specific downstream effector of FAK that promotes the migration of glioblastoma cells. Oncogene. 2005 doi: 10.1038/sj.onc.1209199. [DOI] [PubMed] [Google Scholar]
- 98.Seo S, Asai T, Saito T, Suzuki T, Morishita Y, Nakamoto T, Ichikawa M, Yamamoto G, Kawazu M, Yamagata T, Sakai R, Mitani K, Ogawa S, Kurokawa M, Chiba S, Hirai H. Crk-associated substrate lymphocyte type is required for lymphocyte trafficking and marginal zone B cell maintenance. J Immunol. 2005;175:3492–3501. doi: 10.4049/jimmunol.175.6.3492. [DOI] [PubMed] [Google Scholar]
- 99.Law SF, Zhang Y-Z, Klein-Szanto A, Golemis EA. Cell-cycle regulated processing of HEF1 to multiple protein forms differentially targeted to multiple compartments. Mol. Cell. Biol. 1998;18:3540–3551. doi: 10.1128/mcb.18.6.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Merrill RA, See AW, Wertheim ML, Clagett-Dame M. Crk-associated substrate (Cas) family member, NEDD9, is regulated in human neuroblastoma cells and in the embryonic hindbrain by all-trans retinoic acid. Dev Dyn. 2004;231:564–575. doi: 10.1002/dvdy.20159. [DOI] [PubMed] [Google Scholar]
- 101.Levy L, Hill CS. Smad4 dependency defines two classes of transforming growth factor {beta} (TGF-{beta}) target genes and distinguishes TGF-{beta}-induced epithelial-mesenchymal transition from its antiproliferative and migratory responses. Mol Cell Biol. 2005;25:8108–8125. doi: 10.1128/MCB.25.18.8108-8125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zheng M, McKeown-Longo PJ. Regulation of HEF1 expression and phosphorylation by TGF-beta 1 and cell adhesion. J Biol Chem. 2002;277:39599–39608. doi: 10.1074/jbc.M202263200. [DOI] [PubMed] [Google Scholar]
- 103.Liu X, Elia AEH, Law SF, Golemis EA, Farley J, Wang T. A novel ability of Smad3 to regulate proteasomal degradation of a Cas family member, HEF1. EMBO J. 2000;19:6759–6769. doi: 10.1093/emboj/19.24.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Feng L, Guedes S, Wang T. AIP4/hItch is an Ubiquitin E3 ligase For HEF1 In TGF-{beta} signaling pathways. J Biol Chem. 2004 doi: 10.1074/jbc.M403221200. [DOI] [PubMed] [Google Scholar]
- 105.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 106.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 107.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massague J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Faragher AJ, Fry AM. Nek2A kinase stimulates centrosome disjunction and is required for formation of bipolar mitotic spindles. Mol Biol Cell. 2003;14:2876–2889. doi: 10.1091/mbc.E03-02-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mayor T, Hacker U, Stierhof YD, Nigg EA. The mechanism regulating the dissociation of the centrosomal protein C-Nap1 from mitotic spindle poles. J Cell Sci. 2002;115:3275–3284. doi: 10.1242/jcs.115.16.3275. [DOI] [PubMed] [Google Scholar]
- 110.Fry AM, Meraldi P, Nigg EA. A centrosomal function for the human Nek2 protein kinase, a member of the NIMA family of cell cycle regulators. Embo J. 1998;17:470–481. doi: 10.1093/emboj/17.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao ZS, Lim JP, Ng YW, Lim L, Manser E. The GIT-associated kinase PAK Stargets to the centrosome and regulates Aurora-A. Mol Cell. 2005;20:237–249. doi: 10.1016/j.molcel.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 112.Cotteret S, Chernoff J. Pak GITs to Aurora-A. Dev Cell. 2005;9:573–574. doi: 10.1016/j.devcel.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 113.Turner CE, West KA, Brown MC. Paxillin-ARF GAP signaling and the cytoskeleton. Curr Opin Cell Biol. 2001;13:593–599. doi: 10.1016/s0955-0674(00)00256-8. [DOI] [PubMed] [Google Scholar]
- 114.Hirota T, Morisaki T, Nishiyama Y, Marumoto T, Tada K, Hara T, Masuko N, Inagaki M, Hatakeyama K, Saya H. Zyxin, a regulator of actin filament assembly, targets the mitotic apparatus by interacting with h-warts/LATS1 tumor suppressor. J Cell Biol. 2000;149:1073–1086. doi: 10.1083/jcb.149.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thery M, Racine V, Pepin A, Piel M, Chen Y, Sibarita JB, Bornens M. The extracellular matrix guides the orientation of the cell division axis. Nat Cell Biol. 2005;7:947–953. doi: 10.1038/ncb1307. [DOI] [PubMed] [Google Scholar]
- 116.Tamada M, Sheetz MP, Sawada Y. Activation of a signaling cascade by cytoskeleton stretch. Dev Cell. 2004;7:709–718. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 117.Schumacher JM, Ashcroft N, Donovan PJ, Golden A. A highly conserved centrosomal kinase, AIR-1, is required for accurate cell cycle progression and segregation of developmental factors in Caenorhabditis elegans embryos. Development. 1998;125:4391–4402. doi: 10.1242/dev.125.22.4391. [DOI] [PubMed] [Google Scholar]
- 118.Berdnik D, Knoblich JA. Drosophila Aurora-A is required for centrosome maturation and actin-dependent asymmetric protein localization during mitosis. Curr Biol. 2002;12:640–647. doi: 10.1016/s0960-9822(02)00766-2. [DOI] [PubMed] [Google Scholar]
- 119.Pan J, Wang Q, Snell WJ. An aurora kinase is essential for flagellar disassembly in Chlamydomonas. Dev Cell. 2004;6:445–451. doi: 10.1016/s1534-5807(04)00064-4. [DOI] [PubMed] [Google Scholar]
- 120.Hildebrandt F, Omram H. New insights: nephronophthisis-medullary cystic kidney disease. Pediatr Nephrol. 2001;16:168–176. doi: 10.1007/s004670000518. [DOI] [PubMed] [Google Scholar]
- 121.Donaldson JC, Dempsey PJ, Reddy S, Bouton AH, Coffey RJ, Hanks SK. Crk-associated substrate p130(Cas) interacts with nephrocystin and both proteins localize to cell-cell contacts of polarized epithelial cells. Exp Cell Res. 2000;256:168–178. doi: 10.1006/excr.2000.4822. [DOI] [PubMed] [Google Scholar]
- 122.Benzing T, Gerke P, Hopker K, Hildebrandt F, Kim E, Walz G. Nephrocystin interacts with Pyk2, p130(Cas), and tensin and triggers phosphorylation of Pyk2. Proc Natl Acad Sci U S A. 2001;98:9784–9789. doi: 10.1073/pnas.171269898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Geng L, Burrow CR, Li HP, Wilson PD. Modification of the composition of polycystin-1 multiprotein complexes by calcium and tyrosine phosphorylation. Biochim Biophys Acta. 2000;1535:21–35. doi: 10.1016/s0925-4439(00)00079-x. [DOI] [PubMed] [Google Scholar]
- 124.Ben-Ze'ev A, Raz A. Multinucleation and inhibition of cytokinesis in suspended cells: reversal upon reattachment to a substrate. Cell. 1981;26:107–115. doi: 10.1016/0092-8674(81)90038-6. [DOI] [PubMed] [Google Scholar]
- 125.Orly J, Sato G. Fibronectin mediates cytokinesis and growth of rat follicular cells in serum-free medium. Cell. 1979;17:295–305. doi: 10.1016/0092-8674(79)90155-7. [DOI] [PubMed] [Google Scholar]
- 126.Xie Z, Tsai LH. Cdk5 phosphorylation of FAK regulates centrosome-associated miocrotubules and neuronal migration. Cell Cycle. 2004;3:108–110. [PubMed] [Google Scholar]
- 127.Golubovskaya VM, Finch R, Cance WG. Direct interaction of the N-terminal domain of focal adhesion kinase with the N-terminal transactivation domain of p53. J Biol Chem. 2005;280:25008–25021. doi: 10.1074/jbc.M414172200. [DOI] [PubMed] [Google Scholar]
- 128.Yamakita Y, Totsukawa G, Yamashiro S, Fry D, Zhang X, Hanks SK, Matsumura F. Dissociation of FAK/p130(CAS)/c-Src complex during mitosis: role of mitosis-specific serine phosphorylation of FAK. J Cell Biol. 1999;144:315–324. doi: 10.1083/jcb.144.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhao J, Pestell R, Guan JL. Transcriptional Activation of Cyclin D1 Promoter by FAK Contributes to Cell Cycle Progression. Mol Biol Cell. 2001;12:4066–4077. doi: 10.1091/mbc.12.12.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Welsh CF, Roovers K, Villanueva J, Liu Y, Schwartz MA, Assoian RK. Timing of cyclin D1 expression within G1 phase is controlled by Rho. Nat Cell Biol. 2001;3:950–957. doi: 10.1038/ncb1101-950. [DOI] [PubMed] [Google Scholar]
- 131.Roovers K, Assoian RK. Effects of rho kinase and actin stress fibers on sustained extracellular signal-regulated kinase activity and activation of G(1) phase cyclin-dependent kinases. Mol Cell Biol. 2003;23:4283–4294. doi: 10.1128/MCB.23.12.4283-4294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 132.Caussinus E, Gonzalez C. Induction of tumor growth by altered stem-cell asymmetric division in Drosophila melanogaster. Nat Genet. 2005;37:1125–1129. doi: 10.1038/ng1632. [DOI] [PubMed] [Google Scholar]
- 133.Giet R, Petretti C, Prigent C. Aurora kinases, aneuploidy and cancer, a coincidence or a real link? Trends Cell Biol. 2005;15:241–250. doi: 10.1016/j.tcb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 134.Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, Werb Z, Bissell MJ. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 136.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]