Abstract
Clathrin-coated vesicles (CCV) mediate protein sorting and vesicular trafficking from the plasma membrane and the trans-Golgi network. Before delivery of the vesicle contents to the target organelles, the coat components, clathrin and adaptor protein complexes (APs), must be released. Previous work has established that hsc70/the uncoating ATPase mediates clathrin release in vitro without the release of APs. AP release has not been reconstituted in vitro, and nothing is known about the requirements for this reaction. We report a novel quantitative assay for the ATP- and cytosol- dependent release of APs from CCV. As expected, hsc70 is not sufficient for AP release; however, immunodepletion and reconstitution experiments establish that it is necessary. Interestingly, complete clathrin release is not a prerequisite for AP release, suggesting that hsc70 plays a dual role in recycling the constituents of the clathrin coat. This assay provides a functional basis for identification of the additional cytosolic factor(s) required for AP release.
INTRODUCTION
Most, if not all, intracellular transport vesicles are encased in a proteinaceous coat, one class of which is clathrin-coated vesicles (CCV).1 CCV mediate the transport of lysosomal hydrolases from the trans-Golgi network, as well as the efficient internalization of extracellular solutes such as nutrients, hormones, growth factors, and immunoglobulins at the plasma membrane. Their coat contains two components: clathrin (heavy and light chains) and adaptor protein complexes (AP). There are two distinct classes of CCV-associated APs. The AP1 complex is found on trans-Golgi network–derived CCV, and AP2 is found on plasma membrane-derived CCV (Robinson, 1987; Ahle et al., 1988). More recently, a new AP complex AP3 has been identified that is partially associated with clathrin-coated structures in vivo (Simpson et al., 1997; Dell’ Angelica et al., 1998). Both clathrin-associated AP complexes are heterotetramers consisting of two different subunits of ∼100 kDa (γ and β1 in AP1, α and β2 in AP2), one medium chain subunit (μ1 or μ2) of ∼50 kDa, and a small chain subunit (ς1 or ς2) of ∼17 kDa (reviewed by Robinson, 1994; Kirchhausen et al., 1997; Schmid, 1997).
A fully assembled clathrin coat, viewed by electron microscopy, appears as a polygonal lattice of clathrin on the outer surface of the vesicle, with APs located at each vertex (Vigers et al., 1986a, 1986b; Heuser and Keen, 1988). The APs form the innermost shell of the coat and associate with the plasma membrane via saturable, high-affinity, and protease-sensitive receptors (Mahaffey et al., 1990). In low-ionic strength, low-pH buffers, clathrin can spontaneously self-assemble into a closed polygonal lattice (Kartenbeck, 1978; Woodward and Roth, 1978; Crowther and Pearse, 1981). APs coassemble with clathrin and promote coat assembly under physiological conditions that do not otherwise support clathrin self-assembly (Keen et al., 1979; Pearse and Robinson, 1984; Ahle and Ungewickell, 1989). Given the ability of APs to stimulate the assembly of clathrin in vitro, as well as their ability to interact with the membrane, it is likely that they mediate the assembly of clathrin onto membranes in vivo (Robinson, 1994; Schmid, 1997). APs also serve an important function in recruiting receptors to newly forming coated pits. This recruiting activity has been postulated based on the ability of APs to interact directly with tyrosine containing sorting motifs of constitutively internalized receptors (Pearse, 1988; Sosa et al., 1993; Ohno et al., 1995; Kirchhausen et al., 1997).
After the formation and budding of a CCV, the coat constituents are released to allow for heterotypic membrane fusion with the endosomal compartment. Additionally, the cytosolic pool of coat components is regenerated allowing for repeated rounds of endocytosis. The mechanism for release of clathrin by the ATP-dependent action of hsc70 (uncoating ATPase) has been extensively characterized in vitro (Rothman and Schmid, 1986; Greene and Eisenberg, 1990; Buxbaum and Woodman, 1995). It was recently shown that the CCV-associated DnaJ domain-containing protein, auxilin, is required for hsc70-dependent clathrin release from brain CCV (Ungewickell et al., 1995). This suggests that hsc70 plays a role in vivo for uncoating clathrin from CCV. Consistent with this, clathrin release is inhibited in vivo after microinjection of an inhibitory anti-hsc70 monoclonal antibody (Höning et al., 1994), as reflected by the accumulation of endocytic ligands in punctate structures that colocalize with clathrin.
Whereas hsc70 releases clathrin from CCV, several laboratories have confirmed the initial observation (Schlossman et al., 1984) that APs remain associated with the clathrin-depleted vesicles (Heuser and Keen, 1988; Greene and Eisenberg, 1990; Buxbaum and Woodman, 1995). Because the AP shell is likely to remain a barrier to fusion and given that nearly half of all cellular clathrin and APs are found in the cytosol (Goud et al., 1985), it seems reasonable to assume that APs must also be released from the vesicle in an uncoating reaction. However, AP release has not been reconstituted in vitro; therefore, nothing is known about the mechanism of this reaction. Here we report a novel quantitative assay for the ATP- and cytosol-dependent release of APs from isolated bovine brain CCV. As expected, we show that hsc70 is not sufficient for AP release. However, we demonstate that, together with other unidentified cytosolic factor(s) and ATP, hsc70 is necessary for AP release. Surprisingly, clathrin release does not appear to be a prerequisite for efficient AP release. Together, our findings suggest that hsc70 plays a dual role in recycling both constituents of the clathrin coat.
MATERIALS AND METHODS
Materials
Antibodies.
The mouse monoclonal antibody (AP.6) against 100-kDa α adaptin was the generous gift of Dr. F.M. Brodsky (Chin et al., 1989). Hybridomas are now available through American Type Culture Collection (Rockville, MD). The rabbit polyclonal antibody 0927 was generated from a GST-fusion protein encoding the hinge region (amino acids 619–656) of the bovine α adaptin (Ball et al., 1995). This construct was the gift of Dr. M.S. Robinson. Monoclonal antibody 1B5 was obtained from Stressgen (Victoria, British Columbia, Canada), and 3C5 was the gift of Dr. B.M. Jockusch (Höning et al., 1994). AP1-specific antibody 100.3 (Ahle et al., 1988) was obtained from Sigma Chemical (St. Louis, MO). Species-purified donkey anti-rabbit antibody conjugated to HRP was purchased from Pierce Chemical (Rockford, IL).
Buffers.
Buffer AK: 10 mM (NH4)2SO4, 20 mM HEPES, pH 7.0, 2 mM magnesium acetate, 25 mM KCl, 1 mM PMSF. Buffer AT: 10 mM (NH4)2SO4, 20 mM HEPES pH 8.2, 2 mM magnesium acetate, 100 mM potassium tartrate, 1 mM PMSF, 2% ovalbumin. 2-(N-morpholino)ethanesulfonic acid (MES) buffer: 100 mM MES, pH 6.5, 0.5 mM MgCl2, 1 mM EGTA, 1 mM PMSF, 0.8 mM DTT, 0.02% NaN3. Breaking buffer: 40 mM HEPES, pH 7.0, 4.5 mM magnesium acetate, 1 mM PMSF, 0.8 mM DTT. Blocking buffer: 1% Triton X-100, 0.1% SDS, 2% BSA, 50 mM NaCl, 1 mM Tris, pH 7.4. Unless otherwise stated, all chemicals were reagent grade.
Methods
CCV Isolation.
Bovine brains were harvested immediately after slaughter and either frozen on dry ice for later use or kept at 4°C for CCV isolation as soon as possible after slaughter. Frozen brains were defrosted rapidly using room temperature MES buffer. CCV were isolated as previously described (Pearse, 1982) with minor modifications. Brain tissue (200 g) was rinsed in MES buffer and homogenized with an equal volume of buffer in a Waring Blender. The homogenate was centrifuged at 15,000 × g for 30 min at 4°C. This low-speed supernatant was collected and centrifuged at 100,000 × g for 1 h at 4°C in a Ti45 rotor (Beckman, Fullerton, CA). The crude microsomal pellet was resuspended in 20 ml MES buffer, layered onto two 10% 2H2O/0% Ficoll–90% 2H20/5% Ficoll gradients made in MES buffer, and centrifuged at 28,000 × g for 30 min in a Beckman SW28 rotor. The total supernatants were pooled and diluted at least fourfold in MES buffer, and the crude CCVs were pelleted at 100,000 × g for 1 h. The pellets were resuspended in 20 ml MES buffer, layered onto two 10% 2H2O/0% Ficoll–90% 2H2O/20% Ficoll gradients made in MES buffer and centrifuged to equilibrium at 53,000 × g for 16 h at 4°C. CCV appeared as a slightly bluish band in the lower third of the gradient. These CCVs were harvested, diluted at least fourfold in MES buffer, and concentrated by centrifugation at 100,000 × g for 1 h at 4°C. CCV were collected and resuspended in MES buffer. CCVs were stored at 4°C for no longer than 2–3 wk. Vesicles deteriorated upon prolonged storage (see RESULTS).
Hsc70 isolation.
Hsc70 was purified as previously described (Greene and Eisenberg, 1990; Schlossman et al., 1984). The first high-speed supernatant generated in the CCV preparation was dialyzed into 25 mM HEPES, pH 7.0, 25 mM KCl, 1 mM DTT, and loaded onto a DE52 column (Pharmacia, Uppsala, Sweden). The column was washed with 50 mM KCl and hsc70 was eluted with 150 mM KCl. Hsc70-containing fractions were pooled and loaded onto an ATP-agarose column (Sigma). This column was washed with 1 M KCl, and hsc70 was eluted with 1 mM ATP. Hsc70 was concentrated by 70% ammonium sulfate precipitation and dialyzed into 10 mM HEPES, pH 7.0, 75 mM KCl, and 1 mM magnesium acetate and stored in frozen aliquots. The preparations were >95% pure as assessed by Coomassie-stained SDS-PAGE.
Bovine Brain Cytosol Preparation.
Frozen bovine brain (200 g) was rapidly defrosted in breaking buffer, and Dounce homogenized with equal volumes of breaking buffer. The homogenate was centrifuged at 15,000 × g for 15 min, and the supernatant was collected and centrifuged at 100,000 × g for 1 h. The supernatant of this high-speed centrifugation was collected and stored in frozen aliquots.
Hsc70 depletion of Bovine Brain Cytosol.
Mouse anti-hsc70 monoclonal antibody 1B5 (0.8 mg) was bound to 50 μl of Gamma Bind (Pharmacia), washed, and cross-linked with dimethylpimelimidate (Pierce) as described by Harlow and Lane (1988). These beads were incubated with 100 μl of bovine brain cytosol for 4 h at 4°C, and the unbound material was collected as the hsc70-depleted bovine brain cytosol.
Coat Release Assay.
CCV (3.75 μg) were incubated with or without 0.5 μg of hsc70, the indicated amount of cytosol, and an ATP-regenerating system (containing 800 μM ATP, creatine phosphokinase, and 5 mM creatine phosphate). The final reaction volume was brought to 50 μl with buffer AK and incubated at 25°C for 5 min unless otherwise noted. The reaction mixture was centrifuged in a Beckman TLA100 rotor at 100,000 × g for 10 min at 4°C. Immediately after centrifugation, the top 40 μl of the supernatant containing released coat components was removed without disturbing the pellet. Standard curves were generated by stripping CCVs with 0.5 M Tris, pH 7.0, at 25°C for 5 min before centrifugation as described above.
Western Blot.
The AP reaction supernatant (25 μl) was subjected to standard SDS-PAGE and Western blotting protocols. AP2 was detected using the rabbit polyclonal antibody 0927 (diluted 1:10,000) and HRP-conjugated donkey anti-rabbit antibody (diluted 1:10,000). The blot was developed using the ECL detection kit (Amersham, Arlington Heights, IL).
ELISA-based Detection of Released APs.
ELISA plates (Nunc, Naperville, IL) were coated overnight at 4°C with 50 μl of 5 μg/ml purified AP.6 antibody in 50 mM Na2CO3, pH 9.6. The plates were thoroughly washed with PBS and blocked with blocking buffer at 4°C overnight. Buffer AT (45 μl) and 5 μl of the supernatants generated in the coat release assay were plated into each well and allowed to bind overnight at 4°C. Captured APs were detected by sequential incubation with antibody 0927 diluted 1:500 in blocking buffer and HRP-conjugated species-purified donkey anti-rabbit antibody diluted 1:2500. Each antibody binding step was incubated at least 1 h at 37°C and was followed by three PBS washes. ELISA plates were developed with o-phenylenediamine and H2O2 (Carter et al., 1993).
Quantitation of Clathrin Release from CCV.
The reaction supernatant generated above (25 μl) was subjected to SDS-PAGE (Harlow and Lane, 1988), stained with Coomassie blue. The amount of clathrin heavy chain present was quantitated by laser scanning densitometry (Molecular Dynamics, Sunnyvale, CA) and comparison with a standard curve.
Electron Microscopy.
Samples for electron microscopy were bound to glow-discharged Formvar carbon-coated grids for 5 min, washed briefly with four changes of water, and stained for 10 min with 2% uranylacetate. Electron microscopic images were obtained with a Jeol (Tokyo, Japan) JEM 1200D II.
Phosphorylation and Immunoprecipitation of APs.
AP release assays were performed with 0.5 μg hsc70, 65 μg cytosol, and 25 μM ATP, in the presence of an ATP-regenerating system, and 10 μCi of γ32P-labeled ATP (Amersham) for 5 min at 25°C. To demonstrate whether phosphorylation occurred before, during, or after AP release from CCV, APs released from CCV in the cytosol-dependent reaction described above were compared with APs released from CCV with 0.5 M Tris. After centrifugation, the latter APs were incubated with 65 μg cytosol and 25 μM ATP, in the presence of an ATP-regenerating system, and 10 μCi of γ32P-labeled ATP (Amersham) for 5 min at 25°C. The reactions were stopped at 4°C and centrifuged as described above to separate released coat components. AP2 was immunoprecipitated in the presence of 1 mM sodium orthovanadate with the monoclonal antibody AP.6. Analysis was performed on dried gels after SDS-PAGE using a PhosphorImager (Molecular Dynamics).
Sucrose Gradients.
AP release assays described above were scaled up 10-fold, and the supernatants were centrifuged for 16 h into 13-ml 5–20% sucrose gradients, made in buffer AT. For comparison, Tris-released APs were sedimented in parallel gradients. A total of 20 fractions were collected and subjected to standard Western blot protocols for APs.
Gel Filtration of Bovine Brain Cytosol.
Bovine brain cytosol (200 μl; 13 mg/ml) was applied to a 24-ml Superdex 200 gel filtration column (Pharmacia) on a Pharmacia fast-performance liquid chromatography system and run in assay buffer. Fractions (1 ml) were collected, and 8 μl of each fraction were assayed for AP- and clathrin-releasing activity in the presence and absence of hsc70.
RESULTS
An ATP-dependent Cytosolic Factor Is Required for the Release of APs from CCV
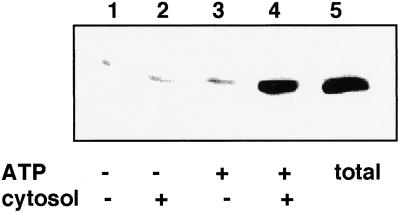
To determine the requirements for AP release from CCV, purified bovine brain CCV were incubated at 25°C for 5 min in the presence or absence of ATP and bovine brain cytosol. The release of APs was determined by Western blot of the supernatant after centrifugation of the reaction mixture at 100,000 × g for 10 min. AP2 was detected with a rabbit polyclonal antibody (0927) which predominantly recognizes the 100-kDa α subunit of the AP2 complex (Figure 1). The majority of AP2 present on the CCV was released by incubation with ATP and cytosol (Figure 1, lane 4, compared with total in lane 5), whereas very little of the AP2 complex was released by incubation in the absence of either cytosol (Figure 1, lanes 1 and 3) or ATP (Figure 1, lanes 1 and 2).
Figure 1.
AP Release from CCV is dependent on cytosol and ATP. CCV were incubated without (lanes 1 and 3) or with (lane 2 and 4) 65 μg of bovine brain cytosol; and with (lanes 3 and 4) or without (lanes 1 and 2) 800 μM ATP with a regenerating system. Hexokinase and glucose were included in samples without ATP. Released APs, present in the supernatant after centrifugation, were detected on Western blots with polyclonal antibody 0927.
CCV isolated from bovine brain are primarily plasma membrane derived; therefore, AP2 is the most abundant of the AP complexes on these vesicles (Goud et al., 1985; Robinson, 1987). Although the lower amount of AP1 in this CCV preparation made its detection difficult (especially over the cytosolic background), it appeared by Western blot using the AP1-specific antibody 100/3 (Ahle et al., 1988) that AP1 was also released in a cytosol- and ATP-dependent manner (our unpublished results). AP1 release was not further investigated.
Quantitative Detection of APs Released from CCV
To further study the release of the AP2 complex (referred to as APs throughout the remainder of this paper), we developed a quantitative ELISA-based assay for soluble APs. The linear range of this assay was established using supernatants derived from CCV treated with 0.5 M Tris, pH 7.0, which releases nearly all of the clathrin and APs from the vesicle (Keen et al., 1979). For quantitation of released APs, supernatants were applied to ELISA plates coated with AP.6, a mouse monoclonal antibody against the α subunit of AP2. Tartrate was included in the binding buffer to prevent AP aggregation (Schroder and Ungewickell, 1991). The captured APs were then detected with an α-adaptin–specific rabbit polyclonal antibody (0927). These two antibodies recognize nonoverlapping sites on the α-adaptin and do not interfere with each other’s binding. Finally, bound 0927 was detected with a species-purified and HRP-conjugated donkey anti-rabbit antibody. The amount of APs captured on the ELISA plate was quantitated using the o-phenylenediamine-based color detection system for HRP (Carter et al., 1993). This sandwich ELISA gave a linear response for APs released from 75–400 ng of CCVs (our unpublished data).
Characterization of the Cytosolic AP Release Activity
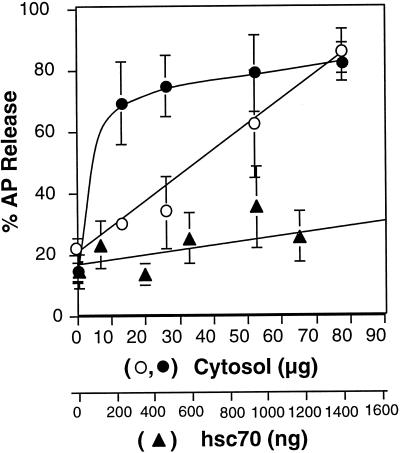
This sensitive and quantitative AP detection system was used to examine the mechanism of cytosol-mediated release of APs from CCV. Consistent with previous reports (Schlossman et al., 1984; Heuser and Keen, 1988; Greene and Eisenberg, 1990; Buxbaum and Woodman, 1995), incubation with 0.5 μg of purified hsc70 did not support AP release (total reaction volume, 50 μl) (Figure 2, solid triangles), although clathrin was quantitatively released (our unpublished results, but see Figure 4A). In contrast, APs were efficiently released from CCV incubated in the presence of crude bovine brain cytosol (Figure 2, open circles). AP release showed a direct linear dependence on cytosol concentration in the range of 10–80 μg/assay (0.2–1.6 mg/ml) (Figure 2, open circles). The cytosolic content of AP2 was not detectable on the ELISA at the concentrations applied (our unpublished data). Hsc70 is an abundant protein (20 ng hsc70/μg brain cytosol); hence, maximal clathrin release required lower concentrations of cytosol (25 μg/assay or 0.5 mg/ml, our unpublished results). Although hsc70 was not sufficient to support AP release, its presence dramatically reduced the cytosol requirement for AP release (Figure 2, solid circles). These data suggest that hsc70 and an additional cytosolic factor(s) is required for the release of APs from CCV.
Figure 2.
Cytosol titration into AP release assay. (A) CCVs (3.75 μg) were incubated in the presence of an ATP-regenerating system with increasing amounts of cytosol in the absence (open circle) or presence (closed circle) of 0.5 μg of hsc70 or with increasing amounts of hsc70 in the absence of cytosol (closed triangle) for 5 min. at 25°C. AP release from CCV was detected by ELISA after centrifugation at 4°C, as described in MATERIALS AND METHODS. Hsc70 concentration is indicated by the lower abscissa and is equivalent to the amount of hsc70 present in the corresponding amount of cytosol.
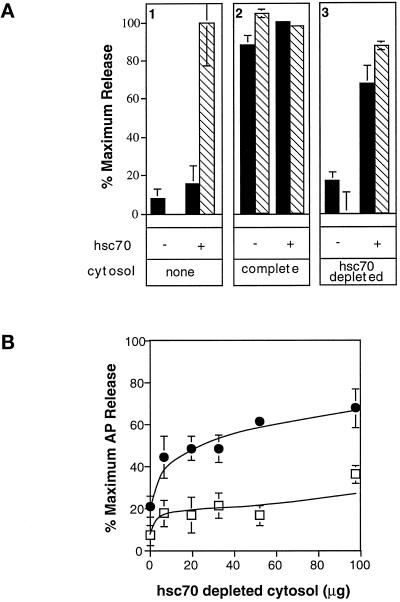
Figure 4.
AP release requires Hsc70. (A) CCV were incubated with or without hsc70 as indicated: without additional cytosol (panel 1), with 65 μg of complete cytosol (panel 2), or with 65 μg of hsc70-depleted cytosol (panel 3). AP release (solid bars) was detected by ELISA, and clathrin release (hatched bars) was detected by densitometry of Coomassie- stained SDS-PAGE gels. (B) AP release was measured by ELISA after incubation of CCV with hsc70-depleted cytosol in the absence (open squares) and presence (closed circles) of 0.5 μg of hsc70. Error bars are hidden by the symbol in some cases.
The maximum AP release achieved in our assay was approximately 80% (± 10%) of the total AP content, as compared with APs released with 0.5 M Tris, pH 7.0 (our unpublished results). It is important to note that after prolonged storage of the CCV, the maximal AP release observed could vary significantly between experiments. This may be due to deterioration of the CCV preparation. CCVs older than 2–3 wk showed increased nonspecific AP release along with reduced maximal AP release. In addition, even APs released from these vesicles with 0.5 M Tris gave aberrant results on the ELISA, suggesting that structural changes had occurred. Therefore, to ensure high reproducibility, care was taken to use fresh preparations of CCVs. AP release is represented as a percentage of maximal AP release in a standard reaction condition (5 min incubation with ATP, hsc70, and 65 μg bovine brain cytosol) throughout the remainder of this paper to enable comparison between preparations.
Rapid Cytosol-dependent Release of APs from CCV
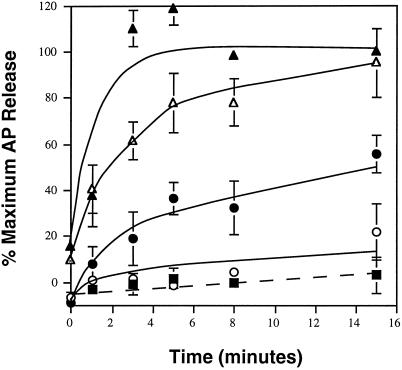
To further characterize the cytosol- and hsc70-dependent release of APs, we examined the kinetics of release under conditions of limiting as well as nonlimiting cytosol concentrations, in both the presence and absence of hsc70. Consistent with the previous results, little AP release occurred when CCV were incubated with hsc70 (Figure 3, solid squares), even when incubated for long times. Likewise, very little AP release occurred with limiting concentrations (6.5 μg) of cytosol (Figure 3, open circles). Addition of hsc70 to this low cytosol concentration increased the rate of AP release (Figure 3, solid circles). Increasing the amount of cytosol in the reaction (to 31 μg) also resulted in a dramatic increase in both the rate and extent of AP release. Eighty percent of maximal AP release was observed within the first 5 min of incubation, and maximal release was observed after 15 min (Figure 3, open triangles). Even at this higher concentration of cytosol, addition of hsc70 significantly increased the reaction rate, and maximal release was observed within 2–3 min (Figure 3, solid triangles). Thus, hsc70 appears to be one of the rate-limiting components for AP release.
Figure 3.
Kinetics of AP release at 37°C. CCV were incubated at 37°C for 0–15 min in the presence (solid symbols) or absence (open symbols) of 0.3 μg hsc70 and no additional cytosol (squares), 6.5 μg cytosol (circles), 31 μg cytosol (triangles) as described in Figure 2. Error bars are hidden by the symbol in some cases.
Hsc70 Is Required for AP release from CCV
We have demonstrated that hsc70 increases the rate of AP release from CCV; however, these experiments do not establish an absolute requirement for hsc70 in this reaction. To address this question directly, we generated hsc70- depleted bovine brain cytosol for use in the AP release assay. Quantitative Western blotting indicated that greater than 95% of hsc70 was immunodepleted from the bovine brain cytosol by incubation with the anti-hsc70 monoclonal antibody 1B5 immobilized on Gamma Bind beads (our unpublished results). To confirm the efficiency of immunodepletion we first examined clathrin release (Figure 4A, hatched bars). As expected, hsc70 (Figure 4A, panel 1) or crude cytosol alone (Figure 4A, panel 2) supported the efficient release of clathrin from CCV. In contrast, hsc70-depleted cytosol was unable to support clathrin release (Figure 4A, panel 3), confirming the near-complete removal of hsc70. Addition of hsc70 to concentrations equivalent to approximately half of the amount removed by depletion completely restored clathrin release activity (Figure 4A, panel 3). These results provide direct evidence that hsc70 is the only cytosolic component that is both necessary and sufficient for clathrin release.
Having functionally confirmed the removal of hsc70 from immunodepleted cytosol, we examined AP release (Figure 4A, solid bars). As previously shown, AP release is not supported by hsc70 alone (Figure 4A, panel 1) but is supported by crude cytosol (Figure 4A, panel 2). Importantly, hsc70-depleted cytosol did not support efficient AP release from CCV (Figure 4A, panel 3), even when added at high concentrations (Figure 4B, open squares). These results establish that hsc70 is required for AP release. Depletion of cytosol with nonspecific antibodies (i.e., mock-depleted cytosol) did not affect AP release activity (our unpublished results). Addition of hsc70 to hsc70-depleted cytosol restored AP release to 65% of control levels (Figure 4A, panel 3). The finding that neither hsc70 nor hsc70 depleted cytosol on their own supported substantial AP release, whereas together they mediated efficient AP release establishes a requirement for both hsc70 and an as-yet-unidentified cytosolic factor(s) in the uncoating reaction. Small molecules or salt effects were not responsible for this cytosolic activity, as it was not removed either by gel filtration on Sepharose G25 or by dialysis (our unpublished results, but see Figure 8).
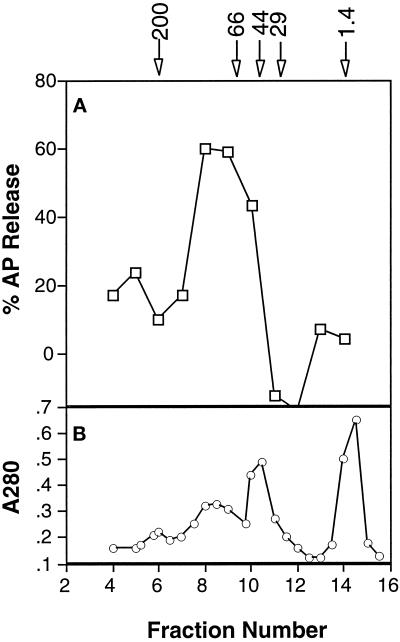
Figure 8.
Superdex 200 gel filtration of cytosol. Cytosol (200 μl of 13 mg/ml) was applied to a 24 ml Superdex 200 column and equilibrated and eluted in buffer AK. Fractions (1 ml) were collected. (A) AP release from CCV incubated with hsc70, ATP, and 8 μl of the indicated fraction was detected by ELISA. (B) Protein elution profile. The column was calibrated with β-amylase (200 kDa), BSA (66 kDa), ovalbumin (44 kDa), carbonic anhydrase (29 kDa), and vitamin B12 (1.4 kDa), which eluted as indicated in panel A.
In contrast to the complete restoration of clathrin release activity, addition of hsc70 to depleted cytosol only partially restored AP release. This difference may reflect the partial loss, along with hsc70, of the other cytosolic factor(s) required. Consistent with this, substantially higher concentrations of hsc70-depleted cytosol (∼2 mg/ml) than of complete cytosol (∼0.3 mg/ml) were required, together with hsc70, to mediate maximal release of APs from CCV (compare Figure 2, solid circles, and Figure 4B, solid circles).
Uncoupling AP Release from Clathrin Release
APs form the innermost shell of the vesicle coat, encased by the overlying lattice (Vigers et al., 1986a, 1986b). Therefore, it was reasonable to assume that the release of clathrin from the CCV would be a prerequisite for AP release. If this were true, then the observed hsc70 requirement for AP release might reflect an indirect requirement for clathrin release. Alternatively, hsc70 might also be directly required for AP release. To distinguish these two possibilities and to begin to explore the mechanisms of AP release, we sought conditions to study AP release independently of clathrin release. One approach to uncouple these two events was to establish a staged reaction, whereby clathrin-free CCV could be generated in the first stage and used to study cytosol-dependent AP release in the second-stage incubation. To generate clathrin-free vesicles, CCV were incubated with 10 mM Tris, pH 8.5, 1 mM EDTA for 10 min at 4°C, centrifuged, and resuspended. Under these conditions the majority of the APs remained associated with the vesicle, while the majority of clathrin was released (Beck et al., 1992). However, the association of APs with the clathrin-stripped vesicles was significantly destabilized by this treatment. Hence, APs present on the vesicles recovered after centrifugation were rapidly lost without any additional factors and even when incubated at 4°C (our unpublished results). Similarly, removal of clathrin from CCV with hsc70 followed by centrifugation and resuspension also resulted in destabilization of the AP–vesicle association (our unpublished results). This instability was due to processing of the vesicles after clathrin release (i.e., centrifugation and resuspension) rather than to the removal of clathrin per se. The data in Figures 2 and 3 establish that APs remain associated with clathrin-free vesicles generated during lengthy incubations with hsc70 or by the presence of high concentrations of hsc70, respectively, provided that these vesicles were not centrifuged and resuspended. Thus, we were unable to separate clathrin and AP release using staged reactions.
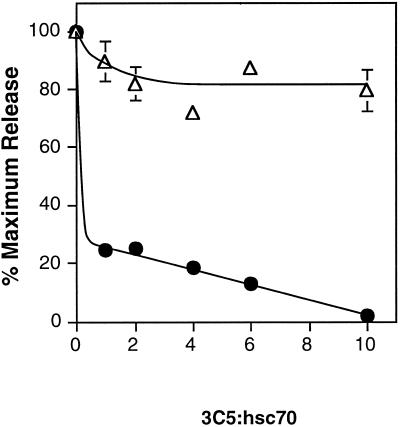
In further probing hsc70 function using immunological reagents, we discovered that the hsc70 function blocking monoclonal antibody 3C5 (Höning et al., 1994) could be used to uncouple AP release from clathrin release. This antibody recognizes the peptide-binding domain of hsc70 and is thought to compete with clathrin for binding to hsc70. 3C5 has been shown previously to be a potent inhibitor of the clathrin-uncoating reaction (Höning et al., 1994). At 10-fold molar excess of 3C5 to hsc70, the clathrin-uncoating reaction was completely inhibited (Figure 5, filled circles). Surprisingly, even when clathrin release was inhibited by >90%, 80% of APs were released from CCV (Figure 5, open triangles). A control mouse IgG1 antibody had no effect on either clathrin or AP release (our unpublished results). It was possible that clathrin remained pelletable under assay conditions in the presence of 3C5, not because it was associated with vesicles but because a pelletable immune complex was formed between the released clathrin–hsc70 complex and the anti-hsc70 antibody. To distinguish between these two possibilities, after the uncoating reaction and centrifugation, the pellets were resuspended in 10 mM Tris, pH 9.0, 1 mM EDTA. These conditions are sufficient to release greater than 90% of clathrin from the vesicle (our unpublished results), without disrupting a 3C5–hsc70–clathrin-immune aggregate. All of the clathrin present in the pellets was released under these conditions, confirming that it was vesicle associated and not in an immune aggregate (our unpublished results). The ability to uncouple these uncoating events suggests that complete release of clathrin is not a prerequisite for AP release.
Figure 5.
The anti-Hsc70 antibody 3C5 inhibits clathrin but not AP release. CCV were incubated with 65 μg of cytosol, an ATP-regenerating mixture with 800 μM ATP, and 0- to 10-fold molar excess of 3C5 to hsc70. AP release (open triangle) was measured by ELISA, and clathrin release (closed circle) was measured by densitometry of Coomassie-stained SDS-PAGE gels. Error bars are hidden by the symbol in some cases.
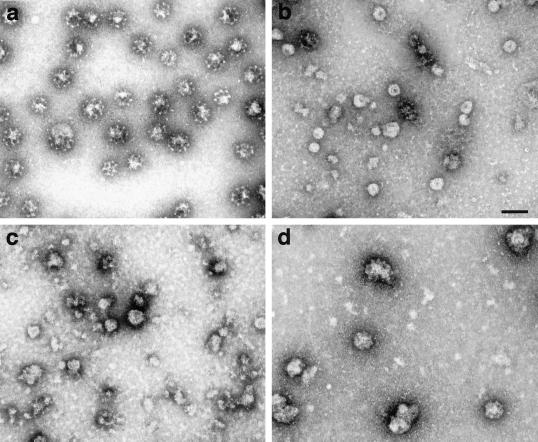
Morphological Comparison of CCV from Which APs and/or Clathrin Have Been Released
To confirm the biochemical results that demonstrate the uncoupling of AP release from clathrin release, we used negative stain electron microscopy to examine the morphology of coated vesicles depleted of either clathrin, APs, or both. CCV were incubated with and without cytosol and hsc70, and this reaction mixture was bound to Formvar carbon-coated grids without centrifugation of the samples. Images of fully coated vesicles show the characteristic polygonal lattice (Figure 6a). Vesicles uncoated with hsc70 (Figure 6b) or with cytosol and hsc70 (Figure 6c) were morphologically indistinguishable under these conditions. In neither case could we detect coat material on the vesicles. Although we were unable to detect residual APs on vesicles using these imaging techniques, others have reported APs on hsc70-uncoated vesicles using quick-freeze deep etch methodology (Heuser and Keen, 1988; Heuser and Steer, 1989). The increased density seen around the vesicles in panel C was likely due to nonspecific interactions of cytosolic proteins with the electron microscopic grid as well as with the uncoated vesicle. When uncoating was carried out in the presence of hsc70 and cytosol as well as fourfold molar excess of 3C5, clathrin remained associated with the vesicle: parallel incubations revealed that 90% of APs were released from the vesicle while 85% of the clathrin remained (see also Figure 5). However, since the structural organization of the clathrin lattice appears to be distorted, we cannot rule out the possibility that some clathrin rearrangements have occurred to facilitate AP release. Nonetheless, a coat structure is detectable on these AP-depleted vesicles (Figure 6d). In addition, we did not detect large aggregates that might represent pelletable immune complexes, consistent with our biochemical results indicating that the remaining clathrin could be released with 10 mM Tris, pH 9.0. Together, these morphological and biochemical results suggest that complete clathrin release is not a prerequisite to AP release.
Figure 6.
Morphological comparison of coated and uncoated CCV. CCV were incubated with ATP (a), 0.5 μg hsc70 (b), 0.5 μg hsc70 and 10 μg cytosol (c), or 10 μg cytosol, 0.5 μg hsc70, and fourfold molar excess of 3C5 (d) under standard conditions. The entire incubation mixtures (i.e., without centrifugation) were applied to Formvar carbon-coated grids and negatively stained with 2% uranyl acetate. Bar, 100 μm .
Nucleotide Requirements for the Release of APs and Clathrin from CCV
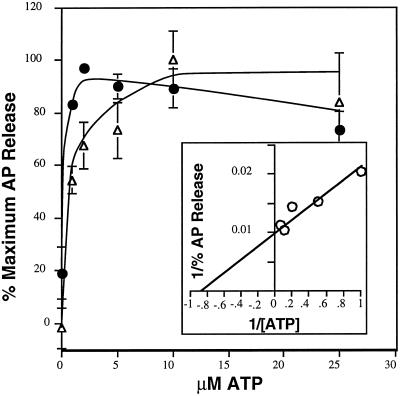
Our data suggest that hsc70, in addition to its role in clathrin release, plays a direct role in AP release. However, while necessary for AP release, hsc70 is not sufficient. Therefore, to further characterize the mechanism of AP release and to determine whether the ATP requirement for AP release reflected the involvement of hsc70, the other cytosolic factor(s), or both, we compared the nucleotide requirements for AP and clathrin release. Consistent with previous findings (Braell et al., 1984; Gao et al., 1993; Buxbaum and Woodman, 1996), we show that hsc70 is a high-affinity ATPase (Figure 7A, circles). A Lineweaver-Burk double-reciprocal plot was used to estimate the Km for ATP of hsc70 in the clathrin-uncoating reaction. We estimated this Km to be 0.5 μM (our unpublished results), which is consistent with previously published values of 0.6–0.7 μM (Braell et al., 1984; Schmid and Rothman, 1985). In contrast, higher concentrations of ATP were required to support maximal AP release (Figure 6a, triangles). Using double-reciprocal plots (Figure 7, inset), we estimated the Km for ATP of the cytosolic factor required for AP release to be 1.2 μM. Neither clathrin nor APs were released from CCV in the absence of ATP. Both AP and clathrin release was determined from the same samples, allowing us to directly compare these two Km values. This comparison indicates that the cytosolic AP-releasing activity requires greater than twofold more ATP than is required by hsc70 to support clathrin release. There was no significant difference in the ATP dependence for AP release when assayed in the presence (our unpublished results) or absence of 3C5, confirming the specificity of the observed ATP requirement for AP release.
Figure 7.
ATP dependence of AP and clathrin release. CCV were incubated with 65 μg cytosol, hsc70, an ATP-regenerating system, and the indicated amount of ATP. Clathrin release (closed circle, as determined by densitometry of Coomassie-stained gels) and AP release (open triangle, as determined by ELISA) were measured for each sample. Inset shows a double-reciprocal plot for ATP dependence of AP release. Error bars are hidden by the symbol in some cases.
Using the conditions identified in the above experiments for maximal uncoating (50 μM ATP in the presence of an ATP-regenerating system), we compared the effects of several nonhydrolyzable nucleotide analogs on clathrin and AP release. As reported previously, there was a slight inhibition of clathrin release (to ∼80% of control release) from CCV with the addition of 1 mM ATPγS or adenosine 5′-(β,γ-imido)triphosphate (AMP-PNP) (Table 1, column 1). ADPβS inhibited clathrin release by ∼30%. This was less than the previously reported inhibitory effects of ADP (Braell et al., 1984) and could reflect differences in affinity for this ADP analog. In contrast, neither of these adenosine nucleotide analogs significantly inhibited AP release (Table 1, column 2); in fact, ADPβS appeared to be slightly stimulatory. These differential nucleotide requirements further support the finding that an additional cytosolic factor(s) is required for AP release and suggest that this activity is ATP dependent.
Table 1.
Nucleotide effects in the presence of a regenerating system
| Treatment | Clathrin release | AP release |
|---|---|---|
| Control | [100] | [100] |
| +ATPγS | 81.1 ± 18.0 | 97 ± 11.1 |
| +AMP-PNP | 73.9 ± 12.9 | 90.7 ± 6.4 |
| +ADPβS | 66.6 ± 2.4 | 121.8 ± 6.2 |
| +GTP | 114.2 ± 2.6 | 97.6 ± 2.0 |
| +GTPγS | 83.9 ± 4.5 | 107.0 ± 13.4 |
| +3C5 | 6.8 ± 11.7 | 71.7 ± 15.8 |
AP and clathrin release were measured, as described in MATERIALS AND METHODS, after incubation of CCV with 65 μg cytosol, hsc70, 50 μM ATP, and ATP-regenerating system (control conditions); 1 mM of each nucleotide or nonhydrolyzable nucleotide analog was included in the reactions as indicated. Both AP and clathrin release are normalized to control conditions; results are shown ±SE.
Small GTPases of the Sar and Arf family are known to regulate coat assembly and disassembly (reviewed by Schmid and Damke, 1995; Rothman and Wieland, 1996; Schekman and Orci, 1996). We therefore examined the effects of GTP and the nonhydrolyzable GTP analog GTPγS on the uncoating reaction. As can be seen in Table 1, GTP had no effect (98% of controls) and GTPγS analogs had a slight stimulatory affect on AP release (107% of controls). In contrast, GTP slightly stimulated clathrin release, while GTPγS was slightly inhibitory.
Hsc70 cycles between the ATP- and ADP-bound forms in its clathrin-uncoating activity. In the ATP-bound form, hsc70 binds clathrin (Heuser and Steer, 1989; Prasad et al., 1994). ATP hydrolysis, ADP release, and finally, rebinding of ATP are thought to be required to release the bound clathrin so that a new clathrin molecule can be recognized by the peptide-binding site (Heuser and Steer, 1989). Although ATP hydrolysis is required for repeated rounds of clathrin uncoating, when hsc70 is present in high excess, ATPγS is sufficient to support clathrin release (Heuser and Steer, 1989; Buxbaum and Woodman, 1995). In contrast, these same studies have shown that AMP-PNP is not sufficient to support clathrin release, probably due to steric constraints on the ATP-binding site by this nonhydrolyzable nucleotide analog.
We have repeated these results and have shown that in the absence of additional ATP, 2 mM ATPγS supports ∼70% clathrin release, while AMP-PNP supports only 12% of maximal clathrin release (Table 2, column 1). In addition, we show that ATPγS, but not AMP-PNP, supports AP release. Approximately 70% maximal AP release is supported with ATPγS, but only 15% of maximal AP release is supported by AMP-PNP (Table 2, column 2). Together, these studies suggest that both clathrin and AP release are ATP-dependent reactions.
Table 2.
Nucleotide effects in the absence of a regenerating system
| Treatment | Clathrin release | AP release |
|---|---|---|
| Control (ATP regenerating) | [100] | [100] |
| 2 mM ATP | 100.8 ± 6.7 | 107.0 ± 12.7 |
| 2 mM ATPγS | 71.1 ± 4.6 | 72.0 ± 1.25 |
| 2 mM AMP-PNP | 12.2 ± 0.9 | 16.5 ± 0.24 |
The release of APs and clathrin from CCV was measured, as described in Table 1, after incubation of CCV with 50 μg cytosol, 1 μg hsc70, and 2 mM of the indicated nucleotide or nonhydrolyzable nucleotide analog, or 800 μM ATP with a regenerating system (control condition). AP and clathrin release are normalized to control conditions.
Released AP2 Complexes Appear Unmodified
Cytosolic APs have recently been shown to be phosphorylated (Wilde and Brodsky, 1996.) Therefore, we examined the possibility that APs became phosphorylated during their ATP- and cytosol-dependent release from CCV. Using γ-32P-labeled ATP we were unable to detect phosphorylation of AP2 complexes immunoprecipitated after their release under our assay conditions (our unpublished results). Clathrin is released from coated vesicles as a complex with hsc70; therefore, we also examined whether APs were released in a complex with other factors. We found that APs released from CCV by incubation with cytosol cosedimented with APs released from the vesicle by incubation with 0.5 M Tris on sucrose density gradients and we were unable to detect interacting proteins (our unpublished results). Further analysis of the mechanism of AP release awaits purification of the cytosolic factor(s) responsible.
Cytosolic AP-Releasing Activity Fractionates as a Single Species by Gel Filtration
The data presented above suggest that AP release is mediated by a novel cytosolic activity. We used gel filtration to confirm this directly. Superdex 200 is a broad range sizing matrix that allows the separation of proteins in the range of 10–200 kDa. Cytosol was subjected to gel filtration chromatography, and fractions were assayed in the presence or absence of hsc70. As expected, activity was not detected in the absence of hsc70 (our unpublished results); however, when assayed in the presence of hsc70, we found that the cytosolic AP-releasing activity eluted as a single peak from this column (Figure 8A). The apparent size of this activity was 100 kDa and was determined by comparison to the mobility of known markers on this column (indicated at the top of Figure 8A). This activity is resolved from the major protein peaks (Figure 8B) and from the clathrin-releasing activity (our unpublished results). These data establish that AP release is mediated by a unique cytosolic activity and confirm that this novel assay provides the functional basis for isolation and identification of this factor.
DISCUSSION
Receptor-mediated endocytosis via CCV is a primary mechanism for the efficient internalization of extracellular solutes. One of the reasons for the high efficiency is that the cellular components used in this process are efficiently recycled for multiple rounds of endocytosis. For example, clathrin is released from coated vesicles by the action of hsc70, and the resulting soluble clathrin is used in the formation of new coated pits. Although the mechanism for clathrin release and recycling is well characterized, very little is known about the mechanism and requirements for AP release and recycling. We developed a novel and quantitative assay to study the release of APs from CCV. Using this assay we have demonstrated that AP release requires hsc70 in addition to an unidentified cytosolic factor(s) and ATP.
AP release is directly dependent on cytosol. Here we demonstrate that hsc70 is a rate-limiting cytosolic component for AP release. Its addition to crude cytosol significantly reduces the cytosol dependence and enhances the rate of the AP release reaction. Given that hsc70 is an abundant cytosolic component, it is paradoxical that its concentration is limiting. These limitations are likely explained by the numerous functions that hsc70 plays in the cell, e.g., maintenance of the native folded structure of cytosolic proteins (Hartl et al., 1994). Importantly, as previously reported (Schlossman et al., 1984; Heuser and Keen, 1988; Greene and Eisenberg, 1990; Buxbaum and Woodman, 1995), hsc70, while necessary, is not sufficient to support the release of APs from CCV. Several lines of evidence suggest that, in addition to hsc70, other cytosolic factor(s) are required for AP release. First, neither hsc70 nor hsc70-depleted cytosol can support AP release, whereas together these distinct fractions support efficient AP release. Second, maximal AP release requires twofold more ATP than is required for hsc70- mediated clathrin release. Thus, the additional factor may directly bind ATP. Alternatively, this factor may interact with hsc70 in such a way that it alters the affinity of hsc70 for ATP. Previous studies (Gross and Hessefort, 1996) have identified a 66-kDa protein that does alter the affinity of hsc70 for ATP and is required to promote the recycling of hsc70. In addition to the differing ATP requirements, ADPβS inhibits clathrin release but not AP release. Third, cytosol-dependent AP release can be uncoupled from hsc70-dependent clathrin release. Finally, AP-releasing activity can be resolved from clathrin-releasing activity and total protein by gel filtration chromatography.
Immunodepletion of hsc70 from cytosol indicates that it is required for AP release. One function of hsc70 is to release clathrin. However, our data suggest that hsc70 plays a dual role in the release of both coat constituents from CCV. Using an antibody (3C5) that recognizes the clathrin-binding site of hsc70 as a specific inhibitor, we demonstrated that complete clathrin release was not a prerequisite to AP release. We cannot rule out that rearrangements of the clathrin lattice mediated by hsc70 in the presence of 3C5 reflect its requirement for AP release. However, our findings that AP release can occur without release of clathrin are consistent with in vitro coat assembly assays that have established that APs can be integrated into the coat after clathrin assembly (Ahle and Ungewickell, 1989; Keen et al., 1991; Prasad and Keen, 1991). Furthermore, APs can be selectively removed from coated vesicles by limited proteolysis without dissociating the clathrin coat (Matsui and Kirchhausen, 1990; Schroder and Ungewickell, 1991; Traub et al., 1995). Thus, our data suggest that, in addition to the well-documented function of hsc70 in releasing clathrin from CCV, hsc70 also plays a direct role in the release of APs.
How can the 3C5 antibody selectively inhibit hsc70-mediated clathrin release while not interfering with the putative second role in AP release? One possibility is that AP release may utilize a domain of hsc70 that is distinct from the clathrin-binding site. Several proteins that interact with hsc70 at a site distinct from the peptide/clathrin-binding site have been identified; these include DnaJ homologues, other heat shock proteins, and kinases (Perdew and Whitelaw, 1991; Cyr et al., 1994; Czar et al., 1994; Pratt and Toft, 1997). Clathrin release by hsc70, a mammalian DnaK homologue, requires the presence of the mammalian DnaJ family member auxilin (Ahle and Ungewickell, 1990; Ungewickell et al., 1995). DnaJ domain-containing proteins have been postulated to regulate the functional diversity of DnaK homologues by targeting them to different substrates (Cyr et al., 1994). It is possible that hsc70 functions to recruit or to interact with these other factors (possibly a novel DnaJ homologue) to affect AP release. Consistent with this, hsc70-depleted cytosol has a significantly reduced AP-releasing activity. This may be the result of the selective codepletion of an additional AP-releasing factor together with hsc70 due to their interaction in the cytosol.
The dual role of hsc70 in the uncoating reaction not only suggests that AP and clathrin release may be coordinated but that they may also occur by a common mechanism. The kinetics of clathrin and AP release are similar and biphasic. There is an initial and rapid release of both APs and clathrin, which is complete within 3–5 min. This initial “burst” is followed by a much slower steady-state rate of release (Greene and Eisenberg, 1990). Addition of hsc70 to cytosol enhances the rate of this initial burst of AP release. These kinetics, together with the potential association of the cytosolic AP-releasing factor(s) and hsc70, suggest that AP release may occur in concert with clathrin release.
Clathrin is released in a stable complex with hsc70, with up to three hsc70 molecules bound to the vertex of the triskelion (Schlossman et al., 1984; Heuser and Steer, 1989). Thus, it is possible that APs are also released in a stable complex with the uncoating factor(s). Our preliminary efforts to detect such complexes or proteins associating with APs have proven unsuccessful to date. This may reflect inappropriate reaction conditions for the formation of a stable complex, dissociation of the complex after AP release through the subsequent action of other cytosolic proteins, or suboptimal gradient conditions. Hence, we cannot eliminate this mechanism for the release of APs until we have examined the process using purified components.
Phosphorylation of APs is another possible mechanism for the release of APs. This is especially attractive since cytosolic APs have been shown to be preferentially phosphorylated (Wilde and Brodsky, 1996). In addition, hsc70 has been shown to associate with a kinase activity (reviewed in Pratt and Toft, 1997). This interaction would also explain the selective codepletion of the AP-releasing activity with hsc70. Consistent with the possibility that the AP-releasing factor is a kinase, ATPγS, which can support kinase activity, also supports AP release, whereas AMP-PNP does not. Efforts to detect phosphorylated APs after their cytosol-mediated release from CCV were unsuccessful. This result, however, does not eliminate the possibility that AP release is phosphorylation dependent because these reactions were performed in the presence of crude cytosol, and therefore the APs were accessible to cytosolic phosphatases. A more complete understanding of the mechanism and requirements for AP release awaits the purification of the cytosolic AP-releasing factor(s) responsible.
ACKNOWLEDGMENTS
We are grateful to Jenny Hinshaw for preliminary work in establishing the AP release assay, as well as Chandra Gullette for her help in developing conditions for ELISA detection of APs. We are also indebted to Sanja Sever and Fiona Simpson for their critical reading of this manuscript. Marius Lötscher and Michael J. McCaffery helped with electron microscopy, which was performed in the Center for Molecular Medicine-Electron Microscopy Core Facility at University of California, San Diego, supported by National Cancer Institute grant CA-58689. We are indebted to F. M. Brodsky for her advice in establishing an ELISA-based AP detection system. This work was supported by National Institutes of Health grant GM-42455 to S.L.S. L.A.H. was supported by American Cancer Society grant PF-4317, and S.L.N. was supported by American Heart Association grant 97–12. This is The Scripps Research Institute manuscript no. 11443-CB.
Abbreviations used:
- AP
adaptor protein complex
- CCV
clathrin-coated vesicles
REFERENCES
- Ahle S, Mann A, Eichelsbacher U, Ungewickell E. Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO J. 1988;7:919–929. doi: 10.1002/j.1460-2075.1988.tb02897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahle S, Ungewickell E. Identification of a clathrin binding subunit in the HA2 adaptor protein complex. J Biol Chem. 1989;264:20089–20093. [PubMed] [Google Scholar]
- Ahle S, Ungewickell E. Auxilin, a newly identified clathrin-associated protein in coated vesicles from bovine brain. J Cell Biol. 1990;111:19–29. doi: 10.1083/jcb.111.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball CL, Hunt SP, Robinson MS. Expression and localisation of alpha-adaptin isofoms. J Cell Sci. 1995;108:2865–2875. doi: 10.1242/jcs.108.8.2865. [DOI] [PubMed] [Google Scholar]
- Beck KA, Chang M, Brodsky FM, Keen JH. Clathrin assembly protein AP-2 induces membrane aggregation of membrane vesicles: a possible role for AP-2 in endosome formation. J Cell Biol. 1992;119:787–796. doi: 10.1083/jcb.119.4.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braell WA, Schlossman DM, Schmid SL, Rothman JE. Dissociation of clathrin coats coupled to the hydrolysis of ATP: role of an uncoating ATPase. J Cell Biol. 1984;99:734–741. doi: 10.1083/jcb.99.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum E, Woodman PG. Selective action of uncoating ATPase towards clathrin-coated vesicles from brain. J Cell Sci. 1995;108:1295–1306. doi: 10.1242/jcs.108.3.1295. [DOI] [PubMed] [Google Scholar]
- Buxbaum E, Woodman PG. Binding ATP and ATP analogs to the uncoating ATPase Hsc70 (70 kDa heat-shock cognate protein) Biochem J. 1996;318:923–929. doi: 10.1042/bj3180923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LL, Redelmeier TE, Woolenweber LA, Schmid SL. Multiple GTP-binding proteins participate in clathrin-coated vesicle-mediated endocytosis. J Cell Biol. 1993;120:37–45. doi: 10.1083/jcb.120.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin DJ, Straubinger RM, Acton S, Nathke I, Brodsky FM. 100-kDa polypeptides in peripheral clathrin-coated vesicles are required for receptor-mediated endocytosis. Proc Natl Acad Sci USA. 1989;86:9289–9293. doi: 10.1073/pnas.86.23.9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther RA, Pearse BM. Assembly and packing of clathrin into coats. J Cell Biol. 1981;91:790–797. doi: 10.1083/jcb.91.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr DM, Langer T, Douglas MG. DnaJ-like proteins: molecular chaperones and specific regulators of Hsp70. Trends Biochem Sci. 1994;19:176–181. doi: 10.1016/0968-0004(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Czar MJ, Owens-Grillo JK, Dittmar KD, Hutchinson KA, Zacharek AM, Leach KL, Dieble MR, Jr, Pratt WB. Characterization of the protein-protein interactions determining the heat shock protein (hsp90-hsp70-hsp56) heterocomplex. J Biol Chem. 1994;269:11155–11161. [PubMed] [Google Scholar]
- Dell’ Angelica EC, Klumperman J, Stoorvogel W, Bonifacino JS. Association of the AP-3 adaptor complex with clathrin. Science. 1998;280:431–434. doi: 10.1126/science.280.5362.431. [DOI] [PubMed] [Google Scholar]
- Gao B, Emoto Y, Greene L, Eisenberg E. Nucleotide binding properties of bovine brain uncoating ATPase. J Biol Chem. 1993;268:8507–8513. [PubMed] [Google Scholar]
- Goud B, Huet C, Louvard D. Assembled and unassembled pools of clathrin: A quantitative study using an enzyme immunoassay. J Cell Biol. 1985;100:521–527. doi: 10.1083/jcb.100.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LE, Eisenberg E. Dissociation of clathrin from coated vesicles by the uncoating ATPase. J Biol Chem. 1990;265:6682–6687. [PubMed] [Google Scholar]
- Gross M, Hessefort S. Purification and characterization of a 66-kDa protein from rabbit reticulocyte lysate which promotes recycling of hsp70. J Biol Chem. 1996;271:16833–16841. doi: 10.1074/jbc.271.28.16833. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1988. [Google Scholar]
- Hartl F-U, Hlodan R, Langer T. Molecular chaperones in protein folding: the art of avoiding sticky situations. Trends Biochem Sci. 1994;19:20–25. doi: 10.1016/0968-0004(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Heuser J, Steer CJ. Trimeric binding of the 70-kD uncoating ATPase to the vertices of clathrin triskelia: a candidate intermediate in the vesicle uncoating reaction. J Cell Biol. 1989;109:1457–1466. doi: 10.1083/jcb.109.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser JE, Keen JH. Deep-etch visualization of proteins involved in clathrin assembly. J Cell Biol. 1988;107:877–886. doi: 10.1083/jcb.107.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höning S, Kreimer G, Robenek H, Jockusch BM. Receptor-mediated endocytosis is sensitive to antibodies against the uncoating ATPase (hsc70) J Cell Sci. 1994;107:1185–1196. doi: 10.1242/jcs.107.5.1185. [DOI] [PubMed] [Google Scholar]
- Kartenbeck J. Preparation of membrane-depleted polygonal coat structures from isolated coated vesicles. Cell Biol Int Rep. 1978;2:457–464. doi: 10.1016/0309-1651(78)90097-8. [DOI] [PubMed] [Google Scholar]
- Keen JH, Beck KA, Kirchhausen T, Jarrett T. Clathrin domains involved in recognition by assembly protein AP-2. J Biol Chem. 1991;266:7950–7956. [PubMed] [Google Scholar]
- Keen JH, Willingham MC, Pastan IH. Clathrin-coated vesicles: isolation, dissociation and factor dependent reassociation of clathrin baskets. Cell. 1979;16:303–312. doi: 10.1016/0092-8674(79)90007-2. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T, Bonifacino JS, Reizman H. Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr Opin Cell Biol. 1997;9:488–495. doi: 10.1016/s0955-0674(97)80024-5. [DOI] [PubMed] [Google Scholar]
- Mahaffey DT, Peeler JS, Brodsky FM, Anderson RGW. Clathrin-coated pits contain an integral membrane protein that binds the AP-2 subunit with high affinity. J Biol Chem. 1990;265:16514–16520. [PubMed] [Google Scholar]
- Matsui W, Kirchhausen T. Stabilization of clathrin coats by the core of the clathrin-associated protein complex AP-2. Biochemistry. 1990;29:10791–10798. doi: 10.1021/bi00500a011. [DOI] [PubMed] [Google Scholar]
- Ohno H, Stewart J, M.-Fournier C, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Pearse BMF. Coated vesicles from human placenta carry ferritin, transferrin and immunoglobulin. Proc Natl Acad Sci USA. 1982;79:451–455. doi: 10.1073/pnas.79.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse BMF. Receptors compete for adaptors found in plasma membrane coated pits. EMBO J. 1988;7:3331–3336. doi: 10.1002/j.1460-2075.1988.tb03204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse BMF, Robinson MS. Purification and properties of 100 kd proteins from coated vesicles and their reconstitution with clathrin. EMBO J. 1984;3:1951–1957. doi: 10.1002/j.1460-2075.1984.tb02075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdew GH, Whitelaw ML. Evidence that the 90 kDa heat shock protein (hsc90) exists in cytosol in heteromeric complexes containing hsc70 and three other proteins with Mr of 63,000, 56,000 and 50,000. J Biol Chem. 1991;266:6708–6713. [PubMed] [Google Scholar]
- Prasad K, Heuser J, Eisenberg E, Greene L. Complex formation between clathrin and uncoating ATPase. J Biol Chem. 1994;269:6931–6939. [PubMed] [Google Scholar]
- Prasad K, Keen JH. Interaction of assembly protein AP-2 and its isolated subunits with clathrin. Biochemistry. 1991;30:5590–5597. doi: 10.1021/bi00236a036. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Steroid hormone interactions with heat shock proteins and immunophilin chaperones. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- Robinson MS. 100-kD coated vesicle proteins: molecular heterogeneity and intracellular distribution studied with monoclonal antibodies. J Cell Biol. 1987;104:887–895. doi: 10.1083/jcb.104.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS. The role of clathrin, adaptors and dynamin in endocytosis. Curr Opin Cell Biol. 1994;6:538–544. doi: 10.1016/0955-0674(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Schmid SL. Enzymatic recycling of clathrin from coated vesicles. Cell. 1986;46:5–9. doi: 10.1016/0092-8674(86)90852-4. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- Schlossman DM, Schmid SL, Braell WA, Rothman JE. An enzyme that removes clathrin coats: purification of an uncoating ATPase. J Cell Biol. 1984;99:723–733. doi: 10.1083/jcb.99.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- Schmid SL, Damke H. Coated vesicles: a diversity of form and function. FASEB J. 1995;9:1445–1453. doi: 10.1096/fasebj.9.14.7589986. [DOI] [PubMed] [Google Scholar]
- Schmid SL, Rothman JE. Two classes of binding sites for uncoating protein in clathrin triskelions. J Biol Chem. 1985;260:10050–10056. [PubMed] [Google Scholar]
- Schroder S, Ungewickell E. Subunit interaction and function of clathrin-coated vesicle adaptors from the Golgi and the plasma membrane. J Biol Chem. 1991;266:7910–7918. [PubMed] [Google Scholar]
- Simpson F, Peden AA, Christopoulon L, Robinson MS. Characterization of the adaptor-related protein complex, AP-3. J Cell Biol. 1997;137:835–845. doi: 10.1083/jcb.137.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa MA, Schmidt B, von Figura K, Hille-Rehfeld A. In vitro binding of plasma membrane-coated vesicle adaptors to the cytoplasmic domain of lysosomal acid phosphatase. J Biol Chem. 1993;268:12537–12543. [PubMed] [Google Scholar]
- Traub LM, Kornfeld S, Ungewickell E. Different domains of the AP-1 adaptor complex are required for Golgi membrane binding and clathrin recruitment. J Biol Chem. 1995;270:4933–4942. doi: 10.1074/jbc.270.9.4933. [DOI] [PubMed] [Google Scholar]
- Ungewickell E, Ungewickell H, Holstein SE, Lindner R, Prasad K, Barouch W, Martin B, Greene LE, Eisenberg E. Role of auxilin in uncoating clathrin-coated vesicles. Nature. 1995;378:632–635. doi: 10.1038/378632a0. [DOI] [PubMed] [Google Scholar]
- Vigers GPA, Crowther RA, Pearse BMF. Location of the 100kD-50 kD accessory proteins in clathrin coats. EMBO J. 1986a;5:2079–2085. doi: 10.1002/j.1460-2075.1986.tb04469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigers GPA, Crowther RA, Pearse BMF. Three-dimensional structure of clathrin cages in ice. EMBO J. 1986b;5:529–534. doi: 10.1002/j.1460-2075.1986.tb04242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde A, Brodsky FM. In vivo phosphorylation of adaptors regulates their interaction with clathrin. J Cell Biol. 1996;135:635–646. doi: 10.1083/jcb.135.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward MP, Roth TF. Coated vesicles: characterization, selective dissociation, and reassembly. Proc Natl Acad Sci USA. 1978;75:4394–4398. doi: 10.1073/pnas.75.9.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]