Abstract
Background
Accumulating evidence suggests that sleep disturbance is associated with inflammation and related disorders including cardiovascular disease, arthritis, and diabetes mellitus. This study was undertaken to test the effects of sleep loss on activation of nuclear factor (NF) -κB, a transcription factor that serves a critical role in the inflammatory signaling cascade.
Methods
In 14 healthy adults (7 females; 7 males), peripheral blood mononuclear cell NF-κB was repeatedly assessed, along with enumeration of lymphocyte subpopulations, in the morning after baseline sleep, partial sleep deprivation (awake from 23:00 h to 03:00 h), and recovery sleep.
Results
In the morning after a night of sleep loss, mononuclear cell NF-κB activation was significantly greater compared with morning levels following uninterrupted baseline or recovery sleep, in which the response was found in females but not in males.
Conclusions
These results identify NF-κB activation as a molecular pathway by which sleep disturbance may influence leukocyte inflammatory gene expression and the risk of inflammation-related disease.
Introduction
Epidemiological data implicate poor sleep as a predictor of chronic disease and mortality in some (1), but not all studies (2). Given that risk of a wide spectrum of medical conditions including cardiovascular disease, arthritis, diabetes, certain cancers, obesity, and functional decline is associated with activation of cellular signals that initiate expression of inflammatory cytokines (3–5), it is increasingly important to consider the consequences of sleep loss on inflammatory mechanisms.
Experimental sleep deprivation has been found to alter immune responses (6) and to induce increases in circulating levels of inflammatory markers such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP)(7, 8). Whereas increases of IL-6 might be a compensatory mechanism to suppress inflammation via activation of anti-inflammatory cytokines (9), sleep loss activates cellular expression of IL-6 and TNF-α, with effects on up-stream sources of cellular inflammatory cytokine expression (10). In peripheral blood mononuclear cells (PBMC), transcription of IL-6 mRNA and TNF-α mRNA is up-regulated after sleep deprivation, and DNA microarray analyses show that increased inflammatory gene expression constitutes one element of a more general genomic response to sleep deprivation (10).
The nuclear factor (NF)-κB transcription control pathway plays a key role in controlling cellular expression of pro-inflammatory genes (11), but the relationship between sleep and NF-κB activation has not been examined in humans. In this study, we used an experimental model of partial night sleep deprivation (PSD) to determine whether NF-κB might be activated by sleep disruption patterns that are ubiquitous in the general population and often found in persons with chronic medical disorders.
Methods
Fourteen subjects (seven men and seven women) who were medically healthy, as determined by medical history, physical examination, and laboratory testing, were recruited between October 2006 and June 2007. All subjects fulfilled criteria for Never Mentally Ill as determined by Structured Clinical Interview for Diagnostic and Statistical Manual - IV (SCID) (mean age=51.8 years [SD=12.8]; 50% female; mean education level=15.8 years [SD=4.6]; mean body mass index [BMI]=26.9 [SD=3.6]; 35.7% were non-white). Females and males did not differ in mean age, education, BMI, or ethnicity (all p’s > 0.1). The sample focused on middle-aged and older adults, given the increased risk of inflammatory disorders with aging. Past or current Axis I DSM-IV psychiatric disorder, use of psychotropic medication, regular use of nonsteroidal anti-inflammatory medications, and tobacco smoking were exclusionary. Subjects regularly slept between 22:30 and 7:30 h as confirmed by 2-week sleep diaries, although use of actigraphy would have provided a more accurate assessment of sleep duration. The study was approved by the UCLA Institutional Review Board.
Subjects participated in a PSD protocol with acquisition of blood samples at 08:00, given that PSD induces an increase of cellular markers of inflammation increase at this timepoint (Supplement) (10). Nuclear extracts were prepared from isolated PBMC (12), and binding of activated NF-κB p65 to its consensus DNA sequence was measured using TransAM NF-κB p65 ELISA (Active Motif, Carlsbad, CA) (Supplement). Leukocyte cell subpopulations were enumerated in whole blood (Supplement).
Data were analyzed using SAS statistical software (version 9.13; SAS Inc, Cary, NC). To determine the effects of PSD on PBMC NF-κB activation and leukocyte subpopulations, repeated measures mixed-model analyses of variance were performed across the three conditions (baseline, PSD, recovery) covarying for age and sex. Both age and sex were covaried given a priori evidence that differences in proinflammatory cytokines are associated with these two factors. In addition, secondary analyses evaluated whether there were sex differences in the response of NF - κB. Significance level was set at p < 0.01.
Results
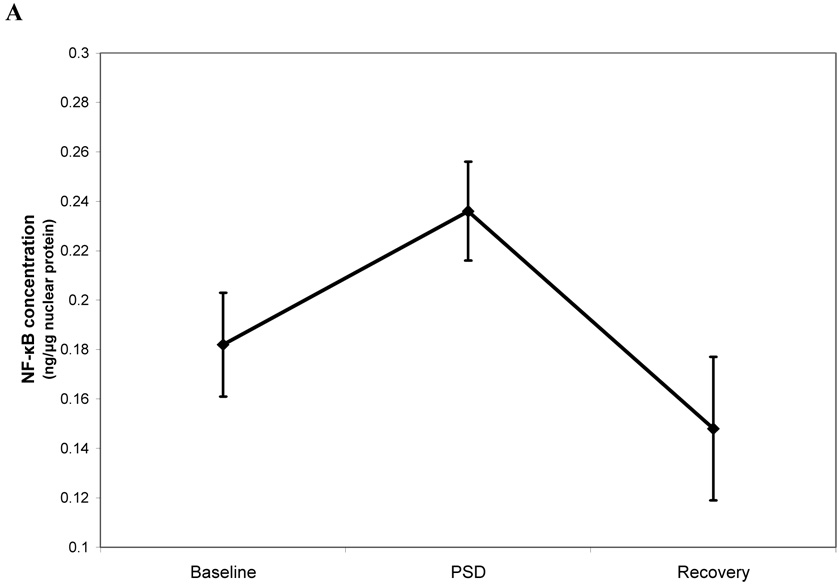
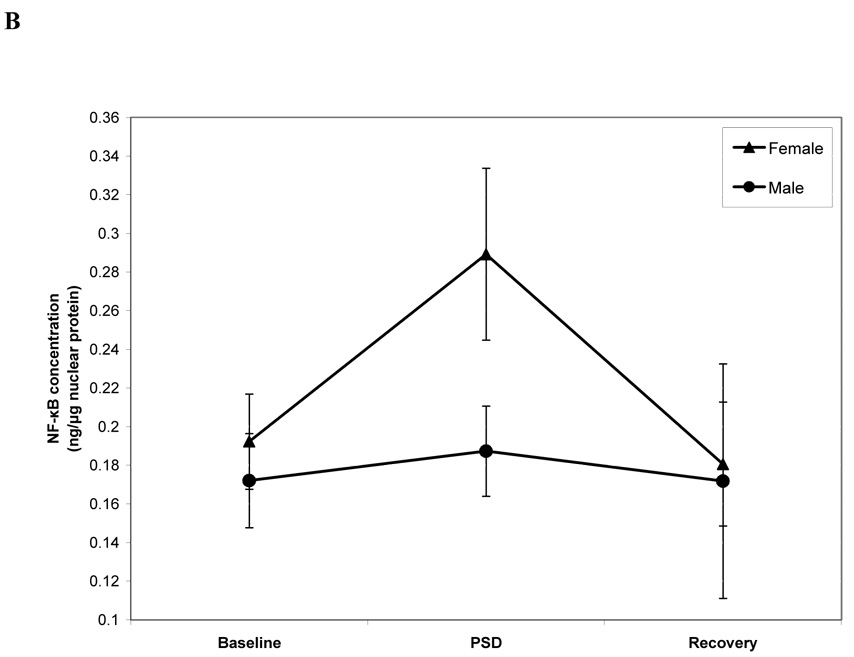
Subjects displayed an increase of PBMC NF-κB activation after PSD (condition effect: F (2, 82.0) = 7.0, p < 0.01; Figure 1A). Pairwise comparisons showed that levels of NF-κB activation were markedly increased the morning after PSD as compared to baseline- and recovery morning levels (−0.054, 95% confidence interval −0.099 to −0.009 ng NF-κB protein / µg total nuclear protein, p < 0.01; 0.057, 0.019 to 0.156, p <0.01), with a 30% increase relative to baseline levels. Levels of NF-κB activation at baseline-and recovery were similar (−0.003, −0.103 to 0.035, p = 0.68). Secondary analyses examined whether females and males differed in response of NF-κB after PSD. As shown in Figure 1B, there was a significant sex × condition interaction (F (2, 77.1) = 5.7 p < 0.01), in which females showed an increase of PBMC NF-κB activation after PSD (p < 0.001) whereas males did not (p = 0.47.) Finally, consistent with prior findings (6), PSD did not induce significant changes in leukocyte subpopulations; all p’s > 0.10 (Table 1). Moreover, levels of NF-κB were not correlated with any of the leukocyte subpopulations including NK cell number (all p’s > 0.10 by Spearman rho).
FIGURE 1.
NF-κB DNA binding in peripheral blood mononuclear cells after baseline, partial night sleep deprivation, and recovery sleep in total sample (A) and in separate groups of females and males (B).
Table 1.
Leukocyte subpopulations after baseline, PSD, and recovery sleep
| Leukocyte subpopulation | Baseline | PSD | Recovery |
|---|---|---|---|
| (% of cells) | Mean (SD) | Mean (SD) | Mean (SD) |
| CD 3 (T lymphocyte) | 67.9 (6.2) | 67.4 (9.2) | 62.2 (6.3) |
| CD 4 (T helper) | 44.7 (8.7) | 44.4 (8.5) | 39.4 (8.8) |
| CD 8 (cytotoxic T lymphocyte) | 23.3 (5.5) | 22.0 (6.0) | 23.6 (6.1) |
| CD 16,56 (natural killer) | 15.8 (6.3) | 15.3 (6.1) | 17.0 (5.3) |
| CD 19 (B lymphocyte) | 12.3 (4.8) | 11.5 (4.1) | 13.8 (3.7) |
| CD 14 (monocyte) | 19.9 (6.2) | 19.6 (5.8) | 19.8 (6.0) |
Discussion
Here we present evidence that acute sleep loss induces a rapid increase in activation of the transcription factor NF-κB in PBMC, providing a potential molecular mechanism for the effects of sleep loss on pro-inflammatory gene expression and circulating levels of inflammatory mediators (7, 8, 10). This observation that sleep loss results in nuclear translocation of NF-κB verifies previous bioinformatic indications (10) and closes an important gap in understanding the cellular mechanisms by which sleep loss enhances inflammatory biology in humans. Further studies that involve repeated measures of NF-κB are needed to evaluate the temporal dynamics of activation of this transcription factor across the diurnal period and in response to sleep loss. Nevertheless, these results are consistent with a previous study in Drosophila showing that sleep deprivation activates NF- κB (13).
Additional analyses showed that the effect of sleep loss on activation of the transcription factor NF-κB primarily occurs in females, but not in males, although these results require cautious interpretation given the small sample size and their exploratory nature. Nevertheless, if sex differences in the response of NF-κB to sleep deprivation are confirmed, these results have implications for understanding the differential risk profile for inflammatory disorders between the sexes.
The mechanisms by which sleep disruption activates NF-kB were not evaluated in this study. PSD induces marked increases in cardiovascular responses as well as in sympathoadrenal activity on awakening (14). Other data show that acute psychological stress induces adrenergic output, which facilitates in vivo release of inflammatory mediators into circulating blood, and may also contribute to NF-κB activation (15, 16). Physiological concentrations of norepinephrine are reported to be sufficient to result in a significant dose dependent increase of NF-kB-binding activity in vitro (16). Whereas enhanced ex vivo expression of NF-kB following stress is due in part to the redistribution of leucocytes, PSD did not induce any detectable change in leukocyte subset distributions. Alternatively, sleep loss can induce increases of cortisol as well as IL-6 and TNF-α. However, PSD does not alter the temporal profile of cortisol (17), and cortisol at physiological doses is known to be have potent anti-inflammatory effects by inhibiting NF-κB/Rel transcription factors and other pro-inflammatory signaling pathways (18). In contrast, sleep loss can induce expression of TNFα, which is a potent inducer of NF-kB (11).
NF-κB activation is thought to contribute to the pathophysiology of diseases such as diabetes mellitus, cardiovascular disease, and atherosclerosis (16). Given evidence that sleep disturbance is associated with each of these medical disorders (10), sleep dependent NF-κB activation may be a common mechanism in the cumulative burden that finally leads to morbidity and mortality. Indeed, difficulty falling asleep or maintaining sleep is significantly associated with risk for nonfatal myocardial infarction or cardiovascular death, even after adjustment for multiple risk factors of coronary heart disease (19). Moreover, sleep loss upregulates several pro-inflammatory cytokines and chemokines — such as TNF-α, IL-1, IL-6, and CXC-chemokine ligand 8 (CXCL8; also known as IL-8), all of which are encoded by target genes of the IKK-β̣ (inhibitor of NF-κB (IκB) kinase-κβ)-dependent NF-κB-activation pathway, and are associated with tumor development and progression in humans and mice (4). Hence, these findings may also have implications for understanding associations between insomnia, disturbances in sleep-wake activity, and cancer in humans. Alternatively, activation of inflammatory pathways and increases of circulating IL-6, for example, have been found to be associated with insomnia and with disturbances in sleep onset (20), which together raise the possibility that targeting NF-κB may have therapeutic potential for insomnia.
Loss of sleep during only part of the night is one of the most common complaints of persons who experience environmental or psychological stress, travel across time meridians, engage in shift work, or have a psychiatric disorder. The results presented herein identify a key molecular pathway by which such sleep loss may influence immune system gene expression and inflammatory biology. These data should motivate further investigations to define the effects of recurrent sleep loss, as well as insomnia, on inflammatory mechanisms that underlie risk of cardiovascular and progression of chronic inflammatory disorders such as rheumatoid arthritis in humans. Finally, given that inflammation is a biologic consequence of aging, testing of interventions that target sleep might identify new strategies to constrain inflammation in older adults.
Supplementary Material
Acknowledgments
The authors reported no biomedical financial interests or potential conflicts of interest. This work was supported in part by grants T32-MH18399, HL 079955, AG 026364, CA 10014152, CA116778, RR00827, P30-AG028748, General Clinical Research Centers Program, the UCLA Cousins Center at the Semel Institute for Neurosciences, and the UCLA Older Americans Independence Center Inflammatory Biology Core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dew MA, Hoch CC, Buysse DJ, Monk TH, Begely AE, Houck PR, et al. Healthy older adults' sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Medicine. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 2.Phillips B, Mannino DM. Does insomnia kill? Sleep. 2005;28:965–971. doi: 10.1093/sleep/28.8.965. [DOI] [PubMed] [Google Scholar]
- 3.Volpato S, Guralnik JM, Ferrucci L, Balfour J, Chaves P, Fried LP, et al. Cardiovascular disease, interleukin-6, and risk of mortality in older women: the women's health and aging study. Circulation. 2001;103:947–953. doi: 10.1161/01.cir.103.7.947. [DOI] [PubMed] [Google Scholar]
- 4.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 5.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irwin M, McClintick J, Costlow C, Fortner M, White J, Gillin JC. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J. 1996;10:643–653. doi: 10.1096/fasebj.10.5.8621064. [DOI] [PubMed] [Google Scholar]
- 7.Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, Smith EO, et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–170. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- 8.Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 9.Petersen AM, Pedersen BK. The role of IL-6 in mediating the anti-inflammatory effects of exercise. J Physiol Pharmacol. 2006;57(Suppl 10):43–51. [PubMed] [Google Scholar]
- 10.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 11.Pascual G, Glass CK. Nuclear receptors versus inflammation: mechanisms of transrepression. Trends Endocrinol Metab. 2006;17:321–327. doi: 10.1016/j.tem.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Richlin VA, Arevalo JM, Zack JA, Cole SW. Stress-induced enhancement of NF-kappaB DNA-binding in the peripheral blood leukocyte pool: effects of lymphocyte redistribution. Brain Behav Immun. 2004;18:231–237. doi: 10.1016/j.bbi.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Williams JA, Sathyanarayanan S, Hendricks JC, Sehgal A. Interaction between sleep and the immune response in Drosophila: a role for the NFkappaB relish. Sleep. 2007;30:389–400. doi: 10.1093/sleep/30.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irwin MR, Ziegler M. Sleep deprivation potentiates activation of cardiovascular and catecholamine responses in abstinent alcoholics. Hypertension. 2005;45:252–257. doi: 10.1161/01.HYP.0000153517.44295.07. [DOI] [PubMed] [Google Scholar]
- 15.Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 16.Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redwine L, Hauger RL, Gillin JC, Irwin M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab. 2000;85:3597–3603. doi: 10.1210/jcem.85.10.6871. [DOI] [PubMed] [Google Scholar]
- 18.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 19.Appels A, Bär FW, Bär J, Bruggeman C, de Baets M. Inflammation, depressive symptomtology, and coronary artery disease. Psychosomatic Medicine. 2000;62:601–605. doi: 10.1097/00006842-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Irwin M, Rinetti G, Redwine L, Motivala S, Dang J, Ehlers C. Nocturnal proinflammatory cytokine-associated sleep disturbances in abstinent African American alcoholics. Brain Behav Immun. 2004;18:349–360. doi: 10.1016/j.bbi.2004.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.