Abstract
The chromosomal high-mobility group A (HMGA) proteins, comprising of HMGA1a, HMGA1b and HMGA2, play important roles in the regulation of numerous processes in eukaryotic cells, such as transcriptional regulation, DNA repair, RNA processing, and chromatin remodeling. The biological activities of HMGA1 proteins are highly regulated by their post-translational modifications (PTMs), including acetylation, methylation and phosphorylation. Recently, it was found that the homeodomain-interacting protein kinase-2 (HIPK2), a newly identified serine/threonine kinase, co-immunoprecipitated with, and phosphorylated HMGA1 proteins. However, the sites and the biological significance of the phosphorylation have not been elucidated. Here, we found that HIPK2 phosphorylates HMGA1a at Ser-35, Thr-52, and Thr-77, and HMGA1b at Thr-41 and Thr-66. In addition, we demonstrated that cdc2, which is known to phosphorylate HMGA1 proteins, could induce the phosphorylation of HMGA1 proteins at the same Ser/Thr sites. The two kinases, however, exhibited different site preferences for the phosphorylation: The preference for HIPK2 phosphorylation followed the order of Thr-77 > Thr-52 > Ser-35, whereas the order for cdc2 phosphorylation was Thr-52 > Thr-77 > Ser-35. Moreover, we found that the HIPK2-phosphorylated HMGA1a reduced the binding affinity of HMGA1a to human germ line ε promoter, and the drop in binding affinity induced by HIPK2 phosphorylation was lower than that introduced by cdc2 phosphorylation, which is consistent with the notion that the second AT-hook in HMGA1a is more important for DNA binding than the third AT-hook.
Synopsis
Here we report that both HIPK2 and cdc2 phosphorylate HMGA1a at Ser-35, Thr-52 and Thr-77, but the two kinases exhibit different site preferences. Moreover, we found that HIPK2-induced phosphorylation of HMGA1a reduced the binding affinity of HMGA1a to DNA, and the drop in binding affinity was lower than that introduced by cdc2 phosphorylation, confirming that the second AT-hook in HMGA1a is more important than the third AT-hook for DNA binding.
Introduction
The chromosomal high-mobility group A (HMGA) proteins, comprising of HMGA1a, HMGA1b and HMGA2, play important roles in the regulation of numerous processes in eukaryotic cells, such as the assembly of chromatin and the regulation of gene transcription (1, 2). HMGA1 proteins are expressed abundantly in embryonic, rapidly proliferating, and tumor cells, but they are absent or expressed at very low levels in normal cells (2-6). HMGA1a and HMGA1b, formerly known as HMG-I and HMG-Y, respectively, are encoded by the same gene (HMGA1), and they are identical in sequence except for an 11-amino acid internal deletion in HMGA1b due to alternative splicing (7, 8).
The characteristic feature of HMGA1 proteins is the presence of three highly conserved DNA-binding motifs (called AT-hooks), which bind to the minor groove of AT-rich DNA (9-13). These AT-hooks are differently spaced along the protein molecules, which results in the interactions of these two protein isoforms with differently spaced AT-rich DNA regions (3). In mammals, the AT-hook motifs share the consensus sequence of P-R-G-R-P flanked by other positively charged residues (14). The first and second AT-hooks in HMGA1a are the main mediators of DNA binding in vivo (15). The second AT-hook of HMGA1a can penetrate the minor groove of AT-rich DNA which stabilizes the B-form DNA, thereby facilitating the binding of other transcription factors in the opposing major groove (16).
Other than binding to DNA, HMGA1 proteins also interact with many other proteins, and the HMGA1-interacting proteins can be grouped into three classes, which include mRNA processing proteins, chromatin remodeling factors and structural proteins (17). Recently, co-immunoprecipitation and pull-down experiments revealed the direct interaction between HMGA1a and p53 family members (p53, Tap63α and Tap73α), and the down-regulation of HMGA1 resulted in enhanced transcriptional activity of these proteins and increased apoptosis (18).
Like histones, the biological activities of the HMGA1 proteins are highly regulated by their post-translational modifications (PTMs), including acetylation, methylation and phosphorylation, which are dynamic and respond rapidly to extracellular stimuli (3). In this regard, the acetylation of Lys-64 destabilizes the enhanceosome, a high-order nucleosome complex formed in response to virus infection, whereas the acetylation of Lys-70 potentiates transcription of interferon-β gene by stabilizing the enhanceosome (19, 20). Other than Lys-64 and Lys-70, our previous studies revealed that Lys-14, Lys-66 and Lys-73 were also acetylated in PC-3 human prostate cancer cells (21, 22). In addition, HMGA1a protein undergoes methylation during apoptosis of tumor cells, and an increase in methylation is a distinct feature of apoptotic leukemic cells (23, 24). It was revealed recently that protein arginine methyltransferase 6 (PRMT6) methylated specifically HMGA1a at Arg-57 and Arg-59 (25). In PC-3 human prostate cancer cells, Arg-25 in HMGA1a, but not in HMGA1b, was both mono- and dimethylated, with the dimethylation being in either symmetric or asymmetric form (26). In addition, in-vitro methylation with several PRMTs showed that PRMT1 might be involved in the asymmetric dimethylation of Arg-25 (27). Aside from arginine methylation, Lys-30 and Lys-54 were also found to be methylated in HMGA1a purified from human breast tumor tissues (28).
HMGA1 proteins, along with histone H1, are among the most highly phosphorylated proteins in the nucleus (29, 30), and a direct link between apoptosis and the degree of phosphorylation in HMGA1a was found for leukemic cells (31). Phosphorylation of HMGA1 proteins was first detected by Lund et al. (30) in Ehrilich ascites cells. Further research revealed that HMGA1 proteins could be phosphorylated by several kinases including cdc2 kinase (32, 33), protein kinase C (PKC) (34) and protein kinase CK2 (35) at different sites. In particular, cdc2 kinase was capable of adding phosphate groups to Thr-52 and Thr-77 of human HMGA1a, whereas PKC was shown to catalyze the phosphorylation of HMGA1a at Thr-20, Ser-43, and Ser-63 (34). The phosphorylation sites induced by CK2 were on three serine residues in the acidic C-terminal tail, i.e., Ser-98, Ser-101 and Ser-102 in HMGA1a and the corresponding residues in HMGA1b (35-37). The CK2-induced phosphorylation alters the proteins’ conformation, stability as well as binding specificity (38, 39). The cdc2-mediated phosphorylation of HMGA1a reduces, by more than 20-fold, the protein’s ability to bind AT-rich DNA substrates (40, 41).
Homeodomain-interacting protein kinase-2 (HIPK2), a newly identified serine/threonine nuclear kinase belonging to the DYRK family (42), was originally identified as a corepressor of homeodomain transcription factors (43). Recently, it was revealed that HIPK2 interacts directly with, and phosphorylates p53 at Ser-46, thereby activating the p53-dependent gene transcription and apoptosis (44-47). Ionizing radiation-induced up-regulation of HIPK2 correlates strictly with the phosphorylation of p53 at Ser-46 (48). A reduction of HIPK2 in tumor cells can result in a robust decrease in the phosphorylation level of Ser-46 in p53 and reduce the apoptotic response upon cisplatin treatment or UV-irradiation (47, 49). Moreover, HIPK2 depletion abolishes the PCAF-mediated acetylation of p53 (50). HIPK2 can also induce the phosphorylation of Pax6 at Thr-281, Thr-304 and Thr-373, resulting in the transactivation of Pax6 by enhancing its interaction with p300 (51). These findings underscored the important roles of HIPK2 in the phosphorylation of nuclear proteins.
Recently, it was observed that HIPK2 co-immunoprecipitated with and phosphorylated HMGA1 proteins, and the overexpression of HIPK2 inhibited cell growth (52). Co-immunoprecipitation experiments with HMGA1a deletion mutants revealed that the region within the second AT-hook and that housing residues 54-63 are important for its binding toward HIPK2 (52). The phosphorylation sites induced by HIPK2, however, have not been determined.
Our initial goal was to locate the sites of HIPK2-induced phosphorylation of HMGA1 proteins and to assess how the phosphorylation affects the proteins’ binding toward DNA. Here we report that HIPK2 phosphorylates HMGA1a at Ser-35, Thr-52, and Thr-77 and HMGA1b at Thr-41 and Thr-66. All the phosphorylation sites precede a proline. We also demonstrated that cdc2 could induce the phosphorylation of HMGA1 proteins at the same Ser/Thr residues. However, these two kinases exhibit different site preferences for the phosphorylation of HMGA1a. Moreover, we found that the HIPK2-mediated phosphorylation decreased the binding affinity of HMGA1a to DNA. In contrast, in the absence of ATP, HIPK2 could increase the binding affinity of HMGA1a to DNA. In addition, electrophoretic mobility shift assay (EMSA) results showed that the decrease in DNA binding affinity induced by HIPK2 phosphorylation was lower than that caused by cdc2 phosphorylation.
Materials and Methods
Purification of HMGA1 proteins
Full-length recombinant human HMGA1a and HMGA1b proteins were overexpressed in E. coli BL21 DE3 pLysS cells (Invitrogen, Carlsbad, CA) followed by extraction with 5% perchloric acid as reported by Reeves (53). Recombinant HMGA1 proteins were further purified on an Agilent 1100 HPLC system (Agilent Technologies, Palo Alto, CA) by using a 4.6 × 250 mm C4 column (Grace Vydac, Hesperia, CA). The flow rate was 1.0 mL/min, and a 60-min gradient of 5-50% CH3CN in 0.1% aqueous solution of trifluoroacetic acid (TFA) was employed. The purified proteins were quantified by Bradford protein assay (Bio-Rad, Hercules, CA).
Phosphorylation of HMGA1 proteins by HIPK2 and cdc2
For the HIPK2-mediated phosphorylation, 20 μg of recombinant HMGA1a was incubated with 0.5 μg of HIPK2 (Upstate, Temecula, CA) in a 20-μL buffer containing 25 mM HEPES (pH 7.5), 10 mM MgCl2, 1 mM DTT and 20 mM ATP at 30°C for 30 min.
Cdc2-induced phosphorylation was performed by incubating 20 μg of recombinant HMGA1 proteins with 0.13 μg of cdc2 kinase (New England Biolabs, Beverly, MA) at 30°C for 30 min in a 20-μL reaction buffer, which contained 50 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 1 mM EGTA, 2 mM DTT, 0.01% Brij 35, and 20 mM ATP.
Phosphorylated HMGA1 proteins were isolated from the reaction mixtures by using HPLC on the Agilent 1100 system with a 2.0 × 250 mm C4 column (Phenomenex, Torrance, CA). The flow rate was 200 μL/min, and a 40-min gradient of 5-40% CH3CN in 0.1% aqueous solution of TFA was employed. The chromatogram was obtained by absorbance detection at 220 nm.
Enzymatic digestion
After HPLC purification, the phosphorylated proteins were dried in a Speed-vac concentrator and resuspended in 50 mM NH4HCO3. The proteins were then digested with sequencing-grade modified trypsin (Roche Applied Science, Indianapolis, IN) at an enzyme-to-substrate ratio of approximately 1:25, and the digestion was continued at 37°C overnight.
Mass spectrometry
Liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) experiments were carried out by coupling directly the effluent from a Zorbax SB-C18 capillary column (0.5×150 mm, 5 μm in particle size, Agilent Technologies) to an LTQ linear ion trap mass spectrometer (Thermo Electron Co., San Jose, CA). Sequential elution of peptides was accomplished by using a 65-min linear gradient of 2-50% acetonitrile in 0.6% acetic acid delivered by the Agilent 1100 capillary HPLC pump at a flow rate of 6 μL/min. MS/MS experiments were carried out in either the data-dependent scan mode or the pre-selected ion mode. Helium was employed as the collision gas and the normalized collision energy was 35%. The width for precursor ion isolation was set to 3.0 (m/z) with an activation Q of 0.25 and an activation time of 30 ms. The spray voltage was 4.5 kV and the temperature for the heated capillary was 275°C.
For the quantification of the relative levels of phosphorylation of the identified Ser/Thr residues, LC-MS/MS experiments were set up to monitor the sequential fragmentations of precursor ions for the phosphorylated and unphosphorylated forms of the peptides of KTTTTPGR, EPSEVPTPK and EPSEVPTPK. Relative phosphorylation level of each identified residue was quantified by calculating the ratio of the peak area found in the total-ion chromatogram for monitoring the fragmentation of the phosphorylated peptide over the sum of the corresponding peak areas observed for the unmodified and phosphorylated forms of the same peptide.
Electrophoretic mobility shift assay
EMSA was employed to assess the binding affinities of the recombinant and phosphorylated HMGA1a toward human germ line promoter oligodeoxyribonucleotides (ODNs). A 36-mer ODN d(CCA CTG CCC GGC ACA GAA ATA ACA ACC ACG GTT ACT) and its complementary strand (obtained from IDT, Coralville, IA) were annealed and labeled at the 5′-end with [γ-32P] ATP. The duplex substrate (25 nM) was then incubated with varying concentrations of recombinant or phosphorylated HMGA1a in the binding buffer, which contained 10 mM Tris-HCl (pH 7.7), 5% glycerol, 1 mM MgCl2, 0.1 mM EDTA, 80 mM NaCl, and 200 μg/mL BSA at room temperature for 30 min. The reaction products were resolved on a 6% (1:29) native polyacrylamide gel, which was run at 150 V at 4°C for 1 h. Gel band intensities for the labeled duplex DNA and the DNA-protein complex were quantified by using a Typhoon 9410 Variable Mode Imager (Amersham Biosciences, Piscataway, NJ) and ImageQuant version 5.2 (Amersham Biosciences, Piscataway, NJ). The percentages of protein-bound duplex were quantified from the radioactivity in each band.
Results
Ser-35, Thr-52, and Thr-77 in HMGA1a were phosphorylated by HIPK2
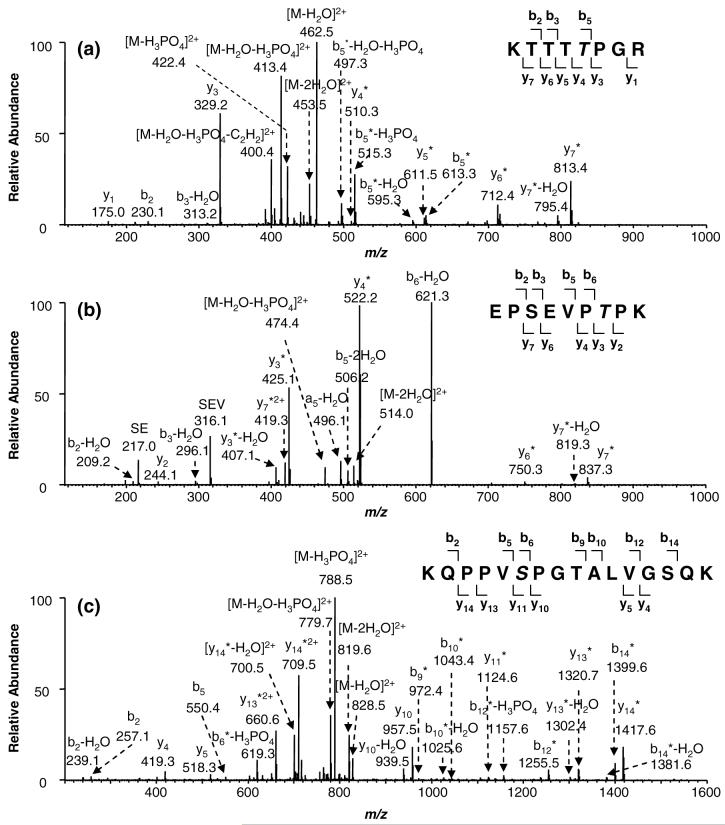
In an attempt to identify the phosphorylation sites in HMGA1 proteins induced by HIPK2, recombinant HMGA1 proteins were overexpressed, purified and subjected to in-vitro phosphorylation with HIPK2. The HMGA1 proteins were purified from the reaction mixture by using HPLC and digested with trypsin. LC-ESI-MS/MS analysis of the tryptic peptides revealed that Ser-35, Thr-52, and Thr-77 in HMGA1a were phosphorylated (Figure 1).
Figure 1.
Product-ion spectra of the ESI-produced [M + 2H]2+ ions of tryptic peptides of (a) KTTTT77PGR (m/z 472.5), (b) EPSEVPT52PK (m/z 532.4), and (c) KQPPVS35PGTALVGSQK (m/z 837.5) in HMGA1a that was phosphorylated by HIPK2. The phosphorylated residues are highlighted in gray italic fonts in the peptide sequences. An asterisk (*) indicates that a fragment ion bears a phosphate group. A scheme summarizing the observed fragment ions for each peptide is shown on the top right of each panel.
In the product-ion spectrum of the [M + 2H]2+ ion of the phosphopeptide KTTTT77PGR (m/z 472.5, Figure 1a), the most abundant fragment ion of m/z 462.5 is attributed to the loss of a water molecule, whereas the second most abundant ion of m/z 413.4 is due to the further loss of a phosphoric acid moiety (H3PO4). The observed y4*, y5*, y6*, y7* and b5* ions exhibit a mass increase of 80 Da (HPO3) with respect to the calculated masses of the corresponding fragments for the unmodified peptide. The observed masses for the y3, b2 and b3 ions are, however, the same as those for the calculated masses of the corresponding fragments for the unmodified peptide. These results demonstrate unambiguously that Thr-77 is the only phosphorylated residue in this peptide.
LC-MS/MS analysis also allowed us to identify another monophosphorylated peptide EPS48EVPT52PK (m/z 532.4, MS/MS shown in Figure 1b). The mass difference between the y2 and y3* ions is consistent with the mass of a phosphothreonine, showing that Thr-52 is phosphorylated. Meanwhile, the mass difference between the y6* and y7* ions, as well as that between [b2 - H2O] and [b3 - H2O] ions, is in keeping with the mass an unmodified serine, revealing that Ser-48 in this peptide is unphosphorylated.
The phosphorylation of Ser-35 in HMGA1a was revealed by the product-ion spectrum of the phosphopeptide KQPPVS35PGT38ALVGS43QK (spectrum shown in Figure 1c). The most abundant fragment ion, of m/z 788.5, in the spectrum is ascribed to form from the neutral loss of an H3PO4 (98 Da) molecule from the precursor ion (m/z 837.5), which is very common for phosphoserine-carrying peptides (54). The m/z values observed for the y4, y5 and y10 ions are the same as those for the corresponding ions of the unmodified peptide, ruling out the possibility of phosphorylation of Ser-43 or Thr-38. Moreover, the observation of two pairs of ions flanking Ser-35, i.e., y10 and y11*, b5 and b6*, supporting that Ser-35 is phosphorylated.
Taken together, the above results demonstrate that HIPK2 can phosphorylate HMGA1a at Ser-35, Thr-52, and Thr-77, in which Thr-52 and Thr-77 are very close to the second and third AT-hooks, respectively. Likewise, HMGA1b, a splicing variant of HMGA1a, was also phosphorylated by HIPK2 at the corresponding sites, i.e., Thr-41 and Thr-66 (Figure S1). It is also worth noting that all the phosphorylation sites identified here precede a proline residue, suggesting that HIPK2 could belong to the big family of proline-directed protein kinases.
It is worth noting that the fragmentation patterns for phosphopeptides, especially for phosphoserine (pSer)- and phosphothreonine (pThr)-containing peptides, have been well documented (54-58). The neutral loss of a phosphoric acid (H3PO4) from the molecular ions of proteolytic peptides is a characteristic marker for pSer/pThr-bearing peptides (57). However, the fragmentation patterns, especially the tendency to lose the H3PO4 moiety, may depend on the instrument configuration, charge state, amino acid sequence, etc. (56-58) In this respect, the pSer35-containing peptide (Figure 1c) showed apparent neutral loss of a phosphoric acid, whereas the pThr-containing peptides (Figure 1a & 1b) give ions arising from such kind of neutral loss in relatively low abundance. The latter observation is consistent with the notion that the pThr-containing peptides undergo complicated fragmentation on losing the H3PO4 component in an ion-trap mass spectrometer (55).
HIPK2 & cdc2 phosphorylate HMGA1 at the same sites but show different site preferences
Cdc2 kinase (also known as cdk1, p34cdc2), associated with a regulatory cyclin subunit, controls the eukaryotic cell cycle (59, 60). Previous studies demonstrated that HMGA1 proteins are in-vitro substrates for cdc2 and the kinase phosphorylates HMGA1a at Thr-52 and Thr-77 (32, 33). Since Ser-35, Thr-52 and Thr-77 can all be phosphorylated by HIPK2, we suspect that, other than the previously reported sites of Thr-52 and Thr-77, Ser-35 might also be phosphorylated by cdc2.
To explore whether cdc2 can also induce the phosphorylation of Ser-35, we treated HMGA1a with cdc2 in the presence of ATP, isolated the HMGA1a protein from the reaction mixture by HPLC, digested the protein with trypsin, and analyzed the digestion mixture by LC-MS/MS. It turned out that cdc2 could indeed phosphorylate, aside from the known phosphorylation sites (namely, Thr-52 and Thr-77), Ser-35 in HMGA1a (Figure S2). Thus, the above data support that the two kinases catalyze the phosphorylation of the same residues in HMGA1 proteins and it is important to examine whether HIPK2 and cdc2 exhibit different site preferences. To this end, we set out to quantify the relative phosphorylation level for each modified residue by setting the mass spectrometer to fragment selectively the ions corresponding to both the phosphorylated and unmodified forms of peptides of KQPPVSPGTALVGSQK, EPSEVPTPK and KTTTTPGR. The percentage of phosphorylation was calculated based on the ratio of the abundance of the ion for the phosphorylated peptide over the sum of the abundances of ions for the unmodified and phosphorylated forms of the same peptide.
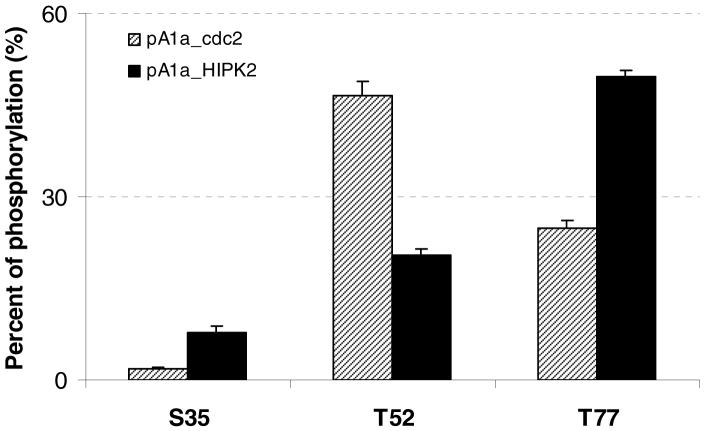
The quantification results demonstrated that HIPK2 and cdc2 indeed exhibit different substrate specificity profile for HMGA1a phosphorylation (Figure 2). The preference for HIPK2 phosphorylation follows the order of Thr-77 > Thr-52 > Ser-35, whereas the selectivity of cdc2 phosphorylation follows the sequence of Thr-52 > Thr-77 > Ser-35. In particular, the primary HIPK2-induced phosphorylation site (i.e., Thr-77) is close to the third AT-hook, while the major cdc2-catalyzed phosphorylation site (i.e., Thr-52) is located closely to the second AT-hook. In addition, the low phosphorylation level at Ser-35 may explain why this residue was not found to be phosphorylated by cdc2 in previous studies (32, 33). cdc2 is known to recognize substrates containing the consensus sequence of Ser/Thr-Pro-X-Z (where X is a polar amino acid and Z is generally a basic amino acid) (61), indicating that Ser-35 might not be a major site for cdc2 phosphorylation. Similar as what we found for HMGA1a, HIPK2 and cdc2 also exhibit different site preferences for HMGA1b phosphorylation: HIPK2 prefers the phosphorylation of Thr-66 over Thr-41, whereas cdc2 phosphorylates Thr-41 more efficiently than Thr-66 (Figure S3). In this respect, it is worth noting that phosphorylation may alter the ionization efficiency of a peptide (62). Thus, the actual percentage of phosphorylation may differ from what we estimated from the mass spectral results. However, we believe that the comparison of the levels of phosphorylation induced by the two different kinases is valid.
Figure 2.
Histograms showing that HIPK2 and cdc2 exhibit different site preferences on the in-vitro phosphorylation of HMGA1a. Phosphorylations mediated by HIPK2 (“pA1a_HIPK2”) and cdc2 (“pA1a_cdc2”) are represented by black and shaded bars, respectively. The level of phosphorylation of each modified residue was quantified by using LC-MS/MS (see Materials and Methods). The data represent the means and standard deviations of results from three independent measurements.
HIPK2 modulates HMGA1a’s DNA binding ability
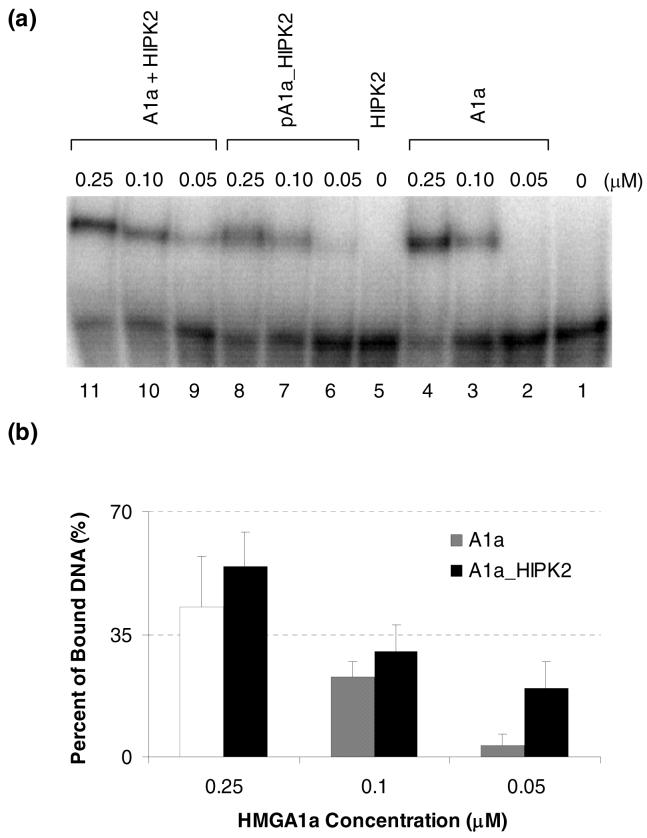
Because HMGA1 proteins are known to bind to the minor groove of AT-rich DNA thereby regulating gene transcription, it is important to assess how HIPK2 affects HMGA1a’s DNA binding activity. To this end, we performed EMSA experiments to assess the binding of recombinant HMGA1a, HIPK2-phosphorylated HMGA1a, and HMGA1a together with HIPK2 but no ATP, toward a radiolabeled fragment of the human germ line promoter, which is known to be a substrate for HMGA1a binding (37).
As shown in Figure 3a, HIPK2-phosphorylated HMGA1a (lanes 6-8) consistently exhibits lower binding affinity toward DNA than the control recombinant HMGA1a (lanes 2-4). On the other hand, although HIPK2 itself does not exhibit any binding toward the DNA probe (lane 5), HIPK2, in the absence of ATP, enhanced the DNA binding activity of HMGA1a (lanes 9-11). Along this line, it was shown previously that the presence of HIPK2 could stimulate the DNA binding ability of NK-3 homeoprotein (43). Additionally, HIPK2 can not only phosphorylate p53 (44), but also mediate the acetylation of p53 by PCAF (50). Therefore, the biological function of HIPK2 could go far beyond its capability to phosphorylate protein substrates containing Ser/Thr-Pro.
Figure 3.
HIPK2 modulates HMGA1a’s binding affinity towards human germ line promoter. (a) EMSA of HMGA1a (control, lanes 2-4), HIPK2-phosphorylated HMGA1a (lanes 6-8) and HMGA1a in the presence of HIPK2 but not ATP (lanes 9-11). “A1a” and “pA1a_HIPK2” represent recombinant and HIPK2-phosphorylated HMGA1a proteins, respectively. (b) Quantification results based on triplicate measurements of the control recombinant HMGA1a (in shaded bar) and HMGA1a in the presence of HIPK2 (in black bar) at different protein concentrations. For the EMSA results of pA1a_HIPK2 shown in lanes 9-11, HIPK2 was also present in the binding reaction mixture.
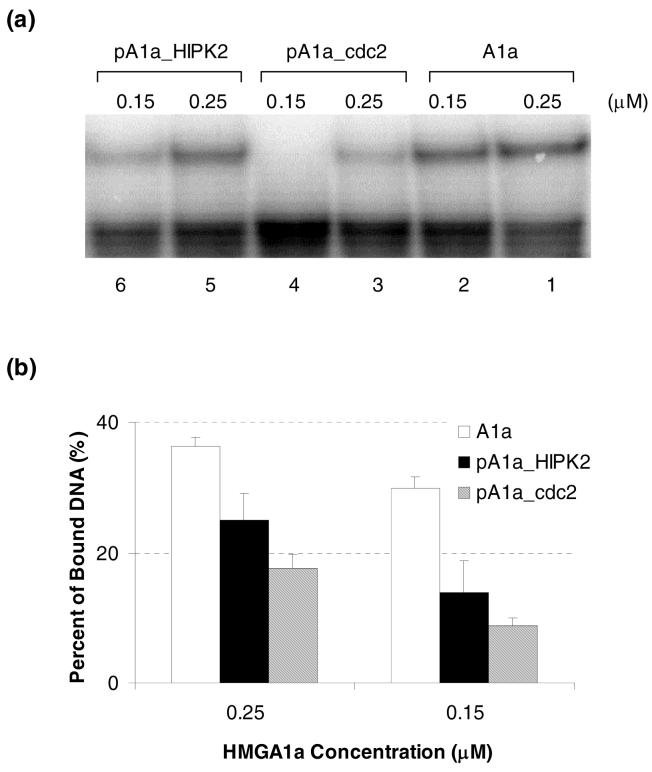
Cdc2-phosphorylated HMGA1a has lower binding affinity to DNA than HIPK2-phosphorylated HMGA1a
Since cdc2 and HIPK2 exhibit different site preferences toward HMGA1a phosphorylation, we next asked whether the HMGA1a phosphorylated by the two kinases shows different abilities toward DNA binding. To exclude any interference introduced by the presence of either HIPK2 or cdc2, we purified HMGA1a from the phosphorylation mixture by HPLC prior to carrying out the binding experiments. As shown in Figure 4a, both cdc2- and HIPK2-induced phosphorylation (lanes 3-4 & lanes 5-6, respectively) results in a decrease in HMGA1a’s DNA binding ability (lanes 1-2 illustrate the binding with recombinant HMGA1a). Quantification results from triplicate EMSA measurements revealed that the cdc2-phosphorylated HMGA1a had lower DNA binding ability than the HIPK2-phosphorylated counterpart (Figure 4b). As discussed above, Thr-52, close to the second DNA binding AT-hook motif, is the primary phosphorylation site induced by cdc2, whereas Thr-77, close to the third AT-hook, is the major phosphorylation site induced by HIPK2. Our results are consistent with the view that the second AT-hook in HMGA1a is more important than the third AT-hook for DNA binding (15, 16).
Figure 4.
(a) EMSA for the binding of recombinant HMGA1a (lanes 1-2), cdc2-phosphorylated HMGA1a (lanes 3-4) and HIPK2-phosphorylated HMGA1a (lanes 5-6) to human germ line ε promoter. (b) Quantitative results from triplicate measurements from (a) of HMGA1a (“A1a” in white bar), HIPK2-phosphorylated HMGA1a (“pA1a_HIPK2” in black bar) and cdc2-phosphorylated HMGA1a (“pA1a_cdc2” in shaded bar) at different protein concentrations. To exclude the interference introduced by the presence of either HIPK2 or cdc2, these kinases were removed prior to carrying out the EMSA experiments.
Discussion
The phosphorylation of HMGA1 proteins was originally found to be associated with cellular transformation and proliferation (30, 63). A link between HMGA1a phosphorylation and apoptosis in leukemic cells was reported and HMGA1a could bear many phosphate groups in vivo, including Thr-20, Ser-35, Ser-43, Thr-52, Thr-77, Ser-98, Ser-101, and Ser-102 (21, 31). The kinases, which could be responsible for the in-vivo phosphorylation of these sites, include cdc2, CK2, and PKC (21, 31, 33, 36, 37).
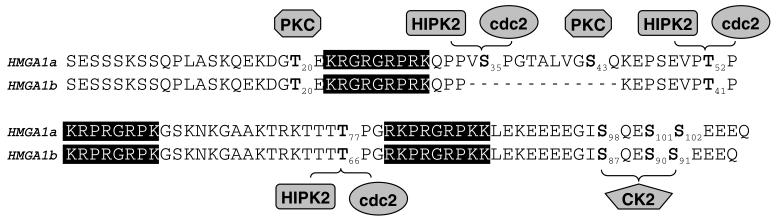
Results obtained in this study extend the knowledge of kinases involved in the phosphorylation of HMGA1 proteins. In this respect, we report here, for the first time, that HIPK2 and cdc2 can phosphorylate both HMGA1a and HMGA1b at the same sites (Figure 5), i.e., Ser-35, Thr-52 and Thr-77 in HMGA1a and Thr-41 and Thr-66 in HMGA1b. It was observed that HIPK2 is expressed at a low level in breast carcinomas (64), and consistent with this result, none of the three sites phosphorylated by cdc2 or HIPK2 were found to be phosphorylated in breast tumor tissues (28).
Figure 5.
Diagram of human HMGA1a and HMGA1b showing the phosphorylated residues (in bold) that have been detected in vivo in human cells and the corresponding kinases that could be responsible for the phosphorylation of these residues in-vivo. The three AT-hooks, functional motifs binding to DNA, are highlighted in black.
It is worth noting that all the three modified residues precede a proline residue. Cdc2 has long been known to phosphorylate the Ser/Thr residues followed by a proline (65). The recently discovered HIPK2, on the other hand, is suggested to be among the DYRK family of protein kinases (42). However, several lines of evidence suggest that HIPK2 could also belong to the big family of proline-directed protein kinases, including cyclin-dependent protein kinases (CDKs), mitogen-activated protein kinases (MAPKs), and glycogen synthase kinase-3 (GSK-3) (66, 67). Tumor suppressor protein p53 can be phosphorylated by HIPK2 at Ser-46 preceding a proline (44, 45, 68), which regulates p53’s function in apoptosis. In addition, Groucho, a corepressor for specific transcription factors, was found to be phosphorylated by HIPK2 at Ser-196, which is again followed by a proline (69). Moreover, HIPK2 phosphorylates the activation domain of Pax6, which augments Pax6 transactivation, and all the three identified phosphorylation sites, i.e., Thr-281, Thr-304, and Thr-373, precede a proline (51). Taken together, the above results suggest that HIPK2 is a proline-directed kinase.
Quantitative results revealed that cdc2 and HIPK2 exhibit different site preferences. In particular, HIPK2 prefers the phosphorylation of Thr-77 followed by Thr-52, whereas cdc2 shows higher phosphorylation level at Thr-52 than at Thr-77. p53 can also be phosphorylated by both HIPK2 and cdc2, and the major phosphorylation sites are Ser-46 and Ser-315, respectively, which are both followed by a proline. In addition, Ser-46 in p53 and Ser-413 in Pax6 can also be phosphorylated by p38 MAP kinase (70, 71). These findings raise the possibility that HIPK2 may cooperate with other proline-directed kinases, i.e., cdc2 and MAPK, to tune finely the biological functions of the corresponding protein substrates.
The reversible phosphorylation of proteins on serine or threonine residues preceding a proline (Ser/Thr-Pro) is a major cellular signaling mechanism (67). Peptidyl-prolyl cis/trans isomerase (PPIase) Pin 1 was shown to target pSer/pThr-Pro-containing proteins and induce a unique conformation switch by isomerizing the pSer/pThr-Pro bonds (72, 73). The conformational change, caused by phosphorylation with Pro-directed kinases followed by peptide bond isomerization with Pin 1, affects significantly a protein’s activity such as its interactions with other proteins and/or DNA (67).
Since the most distinct feature of HMGA1 proteins from other nuclear proteins is their DNA binding ability through the three AT-hooks (11), and the major phosphorylation sites induced by HIPK2 are very close to the N-termini of the second and third AT-hooks, we focused on the effect of HIPK2-induced phosphorylation on HMGA1a’s DNA binding ability. Our results revealed that the phosphorylation of HMGA1a by HIPK2 resulted in a reduction of its binding to DNA. It is of interest to note that HIPK2, in the absence of ATP, augments the binding affinity of HMGA1a to human germline ε promoter harboring an AT-rich region, suggesting that HIPK2 may play multiple roles in regulating HMGA1a’s activity.
We also compared the DNA binding affinity of the HMGA1a protein that is phosphorylated by either HIPK2 or cdc2. The results showed that the cdc2-phosphorylated HMGA1a exhibited lower binding affinity to DNA than the HIPK2-phosphorylated counterpart. Since the overall phosphorylation levels induced by these two kinases are comparable under our experimental conditions (Figure 2), this difference can only be attributed to the difference in the site preferences for the phosphorylation mediated by two protein kinases. The primary cdc2-induced phosphorylation site, Thr-52, is close to the N-terminus of the second AT-hook of HMGA1a (Figure 5). The major phosphorylation site induced by HIPK2 is, however, in close proximity to the N-terminus of the third AT-hook. Our results are, therefore, consistent with the argument that the second AT-hook is the most important DNA binding motif in HMGA1a (39, 40, 74, 75).
In summary, the sites of HIPK2-induced phosphorylation of HMGA1a were unambiguously located to be Ser-35, Thr-52 and Thr-77. EMSA results revealed that the binding ability of HMGA1a was modulated by HIPK2. Although cdc2 can also phosphorylate the same three sites, these two kinases exhibit different site preferences, suggesting that phosphorylation of HMGA1a may be highly regulated, which, in turn may control the expression of its target genes. Moreover, we noticed that each of the three HIPK2-phosphorylated residues was followed by a proline. The observation here, together with previous observation that HIPK2 phosphorylated the Ser/Thr residues preceding a proline in p53, pax6 and Groucho (44, 45, 51, 68, 69), suggests that HIPK2 is a member of the big family of proline-directed kinases. In addition, all the reported HIPK2 substrates can also be phosphorylated by another proline-directed kinase, though the major modification sites may be different from one another, suggesting that the proline-directed kinases might be tightly regulated to achieve the appropriate level of phosphorylation at the appropriate sites.
Supplementary Material
Acknowledgment
The authors want to thank the National Institutes of Health for supporting this research (Grant No. R01 CA116522) and Dr. Yan Zou for helpful discussion.
Abbreviations
- HMG
high-mobility group
- PTM
post-translational modification
- HIPK2
homeodomain-interacting protein kinase-2
- PRMT
protein arginine methyltransferase
- PKC
protein kinase C
- CK2
casein kinase 2 (or protein kinase CK2)
- CDK
cyclin-dependent protein kinase
- MAPK
mitogen-activated protein kinase
- GSK-3
glycogen synthase kinase-3
- PPIase
peptidyl-prolyl cis/trans isomerase
- TFA
trifluoroacetic acid
- ODN
oligodeoxyribonucleotide
- ESI
electrospray ionization
- MALDI
matrix-assisted laser desorption/ionization
- TOF
time-of-flight
- MS/MS
tandem mass spectrometry
- EMSA
electrophoretic mobility shift assay
References
- (1).Bustin M, Reeves R. High-mobility-group chromosomal proteins: architectural components that facilitate chromatin function. Prog. Nucleic Acid Res. Mol. Biol. 1996;54:35–100. doi: 10.1016/s0079-6603(08)60360-8. [DOI] [PubMed] [Google Scholar]
- (2).Sgarra R, Rustighi A, Tessari MA, Di Bernardo J, Altamura S, Fusco A, Manfioletti G, Giancotti V. Nuclear phosphoproteins HMGA and their relationship with chromatin structure and cancer. FEBS Lett. 2004;574:1–8. doi: 10.1016/j.febslet.2004.08.013. [DOI] [PubMed] [Google Scholar]
- (3).Reeves R. Molecular biology of HMGA proteins: hubs of nuclear function. Gene. 2001;277:63–81. doi: 10.1016/s0378-1119(01)00689-8. [DOI] [PubMed] [Google Scholar]
- (4).Fedele M, Bandiera A, Chiappetta G, Battista S, Viglietto G, Manfioletti G, Casamassimi A, Santoro M, Giancotti V, Fusco A. Human colorectal carcinomas express high levels of high mobility group HMGI(Y) proteins. Cancer Res. 1996;56:1896–1901. [PubMed] [Google Scholar]
- (5).Bandiera A, Bonifacio D, Manfioletti G, Mantovani F, Rustighi A, Zanconati F, Fusco A, Di Bonito L, Giancotti V. Expression of HMGI(Y) proteins in squamous intraepithelial and invasive lesions of the uterine cervix. Cancer Res. 1998;58:426–431. [PubMed] [Google Scholar]
- (6).Chiappetta G, Tallini G, De Biasio MC, Manfioletti G, Martinez-Tello FJ, Pentimalli F, de Nigris F, Mastro A, Botti G, Fedele M, Berger N, Santoro M, Giancotti V, Fusco A. Detection of high mobility group I HMGI(Y) protein in the diagnosis of thyroid tumors: HMGI(Y) expression represents a potential diagnostic indicator of carcinoma. Cancer Res. 1998;58:4193–4198. [PubMed] [Google Scholar]
- (7).Johnson KR, Lehn DA, Elton TS, Barr PJ, Reeves R. Complete murine cDNA sequence, genomic structure, and tissue expression of the high mobility group protein HMG-I(Y) J. Biol. Chem. 1988;263:18338–18342. [PubMed] [Google Scholar]
- (8).Johnson KR, Lehn DA, Reeves R. Alternative processing of mRNAs encoding mammalian chromosomal high-mobility-group proteins HMG-I and HMG-Y. Mol. Cell. Biol. 1989;9:2114–2123. doi: 10.1128/mcb.9.5.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Strauss F, Varshavsky A. A protein binds to a satellite DNA repeat at three specific sites that would be brought into mutual proximity by DNA folding in the nucleosome. Cell. 1984;37:889–901. doi: 10.1016/0092-8674(84)90424-0. [DOI] [PubMed] [Google Scholar]
- (10).Solomon MJ, Strauss F, Varshavsky A. A mammalian high mobility group protein recognizes any stretch of six A.T base pairs in duplex DNA. Proc. Natl. Acad. Sci. USA. 1986;83:1276–1280. doi: 10.1073/pnas.83.5.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Reeves R, Nissen MS. The A.T-DNA-binding domain of mammalian high mobility group I chromosomal proteins. A novel peptide motif for recognizing DNA structure. J. Biol. Chem. 1990;265:8573–8582. [PubMed] [Google Scholar]
- (12).Reeves R, Elton TS, Nissen MS, Lehn D, Johnson KR. Posttranscriptional gene regulation and specific binding of the nonhistone protein HMG-I by the 3′ untranslated region of bovine interleukin 2 cDNA. Proc. Natl. Acad. Sci. USA. 1987;84:6531–6535. doi: 10.1073/pnas.84.18.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Maher JF, Nathans D. Multivalent DNA-binding properties of the HMG-1 proteins. Proc. Natl. Acad. Sci. USA. 1996;93:6716–6720. doi: 10.1073/pnas.93.13.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Reeves R, Beckerbauer L. HMGI/Y proteins: flexible regulators of transcription and chromatin structure. Biochim. Biophys. Acta. 2001;1519:13–29. doi: 10.1016/s0167-4781(01)00215-9. [DOI] [PubMed] [Google Scholar]
- (15).Harrer M, Luhrs H, Bustin M, Scheer U, Hock R. Dynamic interaction of HMGA1a proteins with chromatin. J. Cell Sci. 2004;117:3459–3471. doi: 10.1242/jcs.01160. [DOI] [PubMed] [Google Scholar]
- (16).Huth JR, Bewley CA, Nissen MS, Evans JN, Reeves R, Gronenborn AM, Clore GM. The solution structure of an HMG-I(Y)-DNA complex defines a new architectural minor groove binding motif. Nat. Struct. Biol. 1997;4:657–665. doi: 10.1038/nsb0897-657. [DOI] [PubMed] [Google Scholar]
- (17).Sgarra R, Tessari MA, Di Bernardo J, Rustighi A, Zago P, Liberatori S, Armini A, Bini L, Giancotti V, Manfioletti G. Discovering high mobility group A molecular partners in tumour cells. Proteomics. 2005;5:1494–1506. doi: 10.1002/pmic.200401028. [DOI] [PubMed] [Google Scholar]
- (18).Frasca F, Rustighi A, Malaguarnera R, Altamura S, Vigneri P, Del Sal G, Giancotti V, Pezzino V, Vigneri R, Manfioletti G. HMGA1 inhibits the function of p53 family members in thyroid cancer cells. Cancer Res. 2006;66:2980–2989. doi: 10.1158/0008-5472.CAN-05-2637. [DOI] [PubMed] [Google Scholar]
- (19).Munshi N, Agalioti T, Lomvardas S, Merika M, Chen G, Thanos D. Coordination of a transcriptional switch by HMGI(Y) acetylation. Science. 2001;293:1133–1136. doi: 10.1126/science.293.5532.1133. [DOI] [PubMed] [Google Scholar]
- (20).Munshi N, Merika M, Yie J, Senger K, Chen G, Thanos D. Acetylation of HMG I(Y) by CBP turns off IFN beta expression by disrupting the enhanceosome. Mol. Cell. 1998;2:457–467. doi: 10.1016/s1097-2765(00)80145-8. [DOI] [PubMed] [Google Scholar]
- (21).Jiang X, Wang Y. Acetylation and phosphorylation of high-mobility group A1 proteins in PC-3 human tumor cells. Biochemistry. 2006;45:7194–7201. doi: 10.1021/bi060504v. [DOI] [PubMed] [Google Scholar]
- (22).Zhang Q, Zhang K, Zou Y, Perna A, Wang Y. A Quantitative Study on the in-vitro and in-vivo Acetylation of High Mobility Group A1 Proteins. J. Am. Chem. Soc. Mass Spectrom. 2007;18:1569–1578. doi: 10.1016/j.jasms.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Sgarra R, Diana F, Bellarosa C, Dekleva V, Rustighi A, Toller M, Manfioletti G, Giancotti V. During apoptosis of tumor cells HMGA1a protein undergoes methylation: identification of the modification site by mass spectrometry. Biochemistry. 2003;42:3575–3585. doi: 10.1021/bi027338l. [DOI] [PubMed] [Google Scholar]
- (24).Sgarra R, Diana F, Rustighi A, Manfioletti G, Giancotti V. Increase of HMGA1a protein methylation is a distinctive characteristic of leukaemic cells induced to undergo apoptosis. Cell Death Differ. 2003;10:386–389. doi: 10.1038/sj.cdd.4401184. [DOI] [PubMed] [Google Scholar]
- (25).Sgarra R, Lee J, Tessari MA, Altamura S, Spolaore B, Giancotti V, Bedford MT, Manfioletti G. The AT-hook of the chromatin architectural transcription factor high mobility group A1a is arginine-methylated by protein arginine methyltransferase 6. J. Biol. Chem. 2006;281:3764–3772. doi: 10.1074/jbc.M510231200. [DOI] [PubMed] [Google Scholar]
- (26).Zou Y, Wang Y. Tandem mass spectrometry for the examination of the posttranslational modifications of high-mobility group A1 proteins: symmetric and asymmetric dimethylation of Arg25 in HMGA1a protein. Biochemistry. 2005;44:6293–6301. doi: 10.1021/bi0475525. [DOI] [PubMed] [Google Scholar]
- (27).Zou Y, Webb K, Perna AD, Zhang Q, Clarke S, Wang Y. A mass spectrometric study on the in vitro methylation of HMGA1a and HMGA1b proteins by PRMTs: methylation specificity, the effect of binding to AT-rich duplex DNA, and the effect of C-terminal phosphorylation. Biochemistry. 2007;46:7896–7906. doi: 10.1021/bi6024897. [DOI] [PubMed] [Google Scholar]
- (28).Zou Y, Wang Y. Mass spectrometric analysis of high-mobility group proteins and their post-translational modifications in normal and cancerous human breast tissues. J. Proteome Res. 2007;6:2304–2314. doi: 10.1021/pr070072q. [DOI] [PubMed] [Google Scholar]
- (29).Elton TS, Reeves R. Purification and postsynthetic modifications of Friend erythroleukemic cell high mobility group protein HMG-I. Anal. Biochem. 1986;157:53–62. doi: 10.1016/0003-2697(86)90195-8. [DOI] [PubMed] [Google Scholar]
- (30).Lund T, Holtlund J, Laland SG. On the phosphorylation of low molecular mass HMG (high mobility group) proteins in Ehrlich ascites cells. FEBS Lett. 1985;180:275–279. doi: 10.1016/0014-5793(85)81085-1. [DOI] [PubMed] [Google Scholar]
- (31).Diana F, Sgarra R, Manfioletti G, Rustighi A, Poletto D, Sciortino MT, Mastino A, Giancotti V. A link between apoptosis and degree of phosphorylation of high mobility group A1a protein in leukemic cells. J. Biol. Chem. 2001;276:11354–11361. doi: 10.1074/jbc.M009521200. [DOI] [PubMed] [Google Scholar]
- (32).Reeves R, Langan TA, Nissen MS. Phosphorylation of the DNA-binding domain of nonhistone high-mobility group I protein by cdc2 kinase: reduction of binding affinity. Proc. Natl. Acad. Sci. USA. 1991;88:1671–1675. doi: 10.1073/pnas.88.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Lund T, Laland SG. The metaphase specific phosphorylation of HMG I. Biochem. Biophys. Res. Commun. 1990;171:342–347. doi: 10.1016/0006-291x(90)91399-d. [DOI] [PubMed] [Google Scholar]
- (34).Xiao DM, Pak JH, Wang X, Sato T, Huang FL, Chen HC, Huang KP. Phosphorylation of HMG-I by protein kinase C attenuates its binding affinity to the promoter regions of protein kinase C gamma and neurogranin/RC3 genes. J. Neurochem. 2000;74:392–399. doi: 10.1046/j.1471-4159.2000.0740392.x. [DOI] [PubMed] [Google Scholar]
- (35).Palvimo J, Linnala-Kankkunen A. Identification of sites on chromosomal protein HMG-I phosphorylated by casein kinase II. FEBS Lett. 1989;257:101–104. doi: 10.1016/0014-5793(89)81796-x. [DOI] [PubMed] [Google Scholar]
- (36).Ferranti P, Malorni A, Marino G, Pucci P, Goodwin GH, Manfioletti G, Giancotti V. Mass spectrometric analysis of the HMGY protein from Lewis lung carcinoma. Identification of phosphorylation sites. J. Biol. Chem. 1992;267:22486–22489. [PubMed] [Google Scholar]
- (37).Wang DZ, Ray P, Boothby M. Interleukin 4-inducible phosphorylation of HMG-I(Y) is inhibited by rapamycin. J. Biol. Chem. 1995;270:22924–22932. doi: 10.1074/jbc.270.39.22924. [DOI] [PubMed] [Google Scholar]
- (38).Wisniewski JR, Szewczuk Z, Petry I, Schwanbeck R, Renner U. Constitutive phosphorylation of the acidic tails of the high mobility group 1 proteins by casein kinase II alters their conformation, stability, and DNA binding specificity. J. Biol. Chem. 1999;274:20116–20122. [PubMed] [Google Scholar]
- (39).Schwanbeck R, Gymnopoulos M, Petry I, Piekielko A, Szewczuk Z, Heyduk T, Zechel K, Wisniewski JR. Consecutive steps of phosphorylation affect conformation and DNA binding of the chironomus high mobility group A protein. J. Biol. Chem. 2001;276:26012–26021. doi: 10.1074/jbc.M011053200. [DOI] [PubMed] [Google Scholar]
- (40).Piekielko A, Drung A, Rogalla P, Schwanbeck R, Heyduk T, Gerharz M, Bullerdiek J, Wisniewski JR. Distinct organization of DNA complexes of various HMGI/Y family proteins and their modulation upon mitotic phosphorylation. J. Biol. Chem. 2001;276:1984–1992. doi: 10.1074/jbc.M004065200. [DOI] [PubMed] [Google Scholar]
- (41).Nissen MS, Langan TA, Reeves R. Phosphorylation by cdc2 kinase modulates DNA binding activity of high mobility group I nonhistone chromatin protein. J. Biol. Chem. 1991;266:19945–19952. [PubMed] [Google Scholar]
- (42).Hofmann TG, Mincheva A, Lichter P, Droge W, Schmitz ML. Human homeodomain-interacting protein kinase-2 (HIPK2) is a member of the DYRK family of protein kinases and maps to chromosome 7q32-q34. Biochimie. 2000;82:1123–1127. doi: 10.1016/s0300-9084(00)01196-2. [DOI] [PubMed] [Google Scholar]
- (43).Kim YH, Choi CY, Lee SJ, Conti MA, Kim Y. Homeodomain-interacting protein kinases, a novel family of co-repressors for homeodomain transcription factors. J. Biol. Chem. 1998;273:25875–25879. doi: 10.1074/jbc.273.40.25875. [DOI] [PubMed] [Google Scholar]
- (44).D’Orazi G, Cecchinelli B, Bruno T, Manni I, Higashimoto Y, Saito S, Gostissa M, Coen S, Marchetti A, Del Sal G, Piaggio G, Fanciulli M, Appella E, Soddu S. Homeodomain-interacting protein kinase-2 phosphorylates p53 at Ser 46 and mediates apoptosis. Nat. Cell Biol. 2002;4:11–19. doi: 10.1038/ncb714. [DOI] [PubMed] [Google Scholar]
- (45).Hofmann TG, Moller A, Sirma H, Zentgraf H, Taya Y, Droge W, Will H, Schmitz ML. Regulation of p53 activity by its interaction with homeodomain-interacting protein kinase-2. Nat. Cell Biol. 2002;4:1–10. doi: 10.1038/ncb715. [DOI] [PubMed] [Google Scholar]
- (46).Di Stefano V, Blandino G, Sacchi A, Soddu S, D’Orazi G. HIPK2 neutralizes MDM2 inhibition rescuing p53 transcriptional activity and apoptotic function. Oncogene. 2004;23:5185–5192. doi: 10.1038/sj.onc.1207656. [DOI] [PubMed] [Google Scholar]
- (47).Di Stefano V, Rinaldo C, Sacchi A, Soddu S, D’Orazi G. Homeodomain-interacting protein kinase-2 activity and p53 phosphorylation are critical events for cisplatin-mediated apoptosis. Exp. Cell Res. 2004;293:311–320. doi: 10.1016/j.yexcr.2003.09.032. [DOI] [PubMed] [Google Scholar]
- (48).Dauth I, Kruger J, Hofmann TG. Homeodomain-interacting protein kinase 2 is the ionizing radiation-activated p53 serine 46 kinase and is regulated by ATM. Cancer Res. 2007;67:2274–2279. doi: 10.1158/0008-5472.CAN-06-2884. [DOI] [PubMed] [Google Scholar]
- (49).Cecchinelli B, Porrello A, Lazzari C, Gradi A, Bossi G, D’Angelo M, Sacchi A, Soddu S. Ser58 of mouse p53 is the homologue of human Ser46 and is phosphorylated by HIPK2 in apoptosis. Cell Death Differ. 2006;13:1994–1997. doi: 10.1038/sj.cdd.4401933. [DOI] [PubMed] [Google Scholar]
- (50).Di Stefano V, Soddu S, Sacchi A, D’Orazi G. HIPK2 contributes to PCAF-mediated p53 acetylation and selective transactivation of p21Waf1 after nonapoptotic DNA damage. Oncogene. 2005;24:5431–5442. doi: 10.1038/sj.onc.1208717. [DOI] [PubMed] [Google Scholar]
- (51).Kim EA, Noh YT, Ryu MJ, Kim HT, Lee SE, Kim CH, Lee C, Kim YH, Choi CY. Phosphorylation and transactivation of Pax6 by homeodomain-interacting protein kinase 2. J. Biol. Chem. 2006;281:7489–7497. doi: 10.1074/jbc.M507227200. [DOI] [PubMed] [Google Scholar]
- (52).Pierantoni GM, Fedele M, Pentimalli F, Benvenuto G, Pero R, Viglietto G, Santoro M, Chiariotti L, Fusco A. High mobility group I (Y) proteins bind HIPK2, a serine-threonine kinase protein which inhibits cell growth. Oncogene. 2001;20:6132–6141. doi: 10.1038/sj.onc.1204635. [DOI] [PubMed] [Google Scholar]
- (53).Reeves R. HMGA proteins: isolation, biochemical modifications, and nucleosome interactions. Methods Enzymol. 2004;375:297–322. doi: 10.1016/s0076-6879(03)75020-4. [DOI] [PubMed] [Google Scholar]
- (54).Tholey A, Reed J, Lehmann WD. Electrospray tandem mass spectrometric studies of phosphopeptides and phosphopeptide analogues. J. Mass Spectrom. 1999;34:117–123. doi: 10.1002/(SICI)1096-9888(199902)34:2<117::AID-JMS769>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- (55).DeGnore JP, Qin J. Fragmentation of phosphopeptides in an ion trap mass spectrometer. J. Am. Soc. Mass Spectrom. 1998;9:1175–1188. doi: 10.1016/S1044-0305(98)00088-9. [DOI] [PubMed] [Google Scholar]
- (56).Moyer SC, Cotter RJ, Woods AS. Fragmentation of phosphopeptides by atmospheric pressure MALDI and ESI/Ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2002;13:274–283. doi: 10.1016/S1044-0305(01)00361-0. [DOI] [PubMed] [Google Scholar]
- (57).Lehmann WD, Kruger R, Salek M, Hung CW, Wolschin F, Weckwerth W. Neutral loss-based phosphopeptide recognition: a collection of caveats. J. Proteome Res. 2007;6:2866–2873. doi: 10.1021/pr060573w. [DOI] [PubMed] [Google Scholar]
- (58).Leitner A, Foettinger A, Lindner W. Improving fragmentation of poorly fragmenting peptides and phosphopeptides during collision-induced dissociation by malondialdehyde modification of arginine residues. J. Mass Spectrom. 2007;42:950–959. doi: 10.1002/jms.1233. [DOI] [PubMed] [Google Scholar]
- (59).Bashir T, Pagano M. Cdk1: the dominant sibling of Cdk2. Nat. Cell Biol. 2005;7:779–781. doi: 10.1038/ncb0805-779. [DOI] [PubMed] [Google Scholar]
- (60).Atherton-Fessler S, Parker LL, Geahlen RL, Piwnica-Worms H. Mechanisms of p34cdc2 regulation. Mol. Cell. Biol. 1993;13:1675–1685. doi: 10.1128/mcb.13.3.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Moreno S, Nurse P. Substrates for p34cdc2: in vivo veritas? Cell. 1990;61:549–551. doi: 10.1016/0092-8674(90)90463-o. [DOI] [PubMed] [Google Scholar]
- (62).Gao Y, Wang Y. A method to determine the ionization efficiency change of peptides caused by phosphorylation. J. Am. Soc. Mass Spectrom. 2007 doi: 10.1016/j.jasms.2007.08.010. doi:10.1016/j.jasms.2007.1008.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Giancotti V, Pani B, D’Andrea P, Berlingieri MT, Di Fiore PP, Fusco A, Vecchio G, Philp R, Crane-Robinson C, Nicolas RH, et al. Elevated levels of a specific class of nuclear phosphoproteins in cells transformed with v-ras and v-mos oncogenes and by cotransfection with c-myc and polyoma middle T genes. EMBO J. 1987;6:1981–1987. doi: 10.1002/j.1460-2075.1987.tb02461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Pierantoni GM, Bulfone A, Pentimalli F, Fedele M, Iuliano R, Santoro M, Chiariotti L, Ballabio A, Fusco A. The homeodomain-interacting protein kinase 2 gene is expressed late in embryogenesis and preferentially in retina, muscle, and neural tissues. Biochem. Biophys. Res. Commun. 2002;290:942–947. doi: 10.1006/bbrc.2001.6310. [DOI] [PubMed] [Google Scholar]
- (65).Pearson RB, Kemp BE. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- (66).Hall FL, Vulliet PR. Proline-directed protein phosphorylation and cell cycle regulation. Curr. Opin. Cell Biol. 1991;3:176–184. doi: 10.1016/0955-0674(91)90136-m. [DOI] [PubMed] [Google Scholar]
- (67).Lu KP, Liou YC, Zhou XZ. Pinning down proline-directed phosphorylation signaling. Trends Cell Biol. 2002;12:164–172. doi: 10.1016/s0962-8924(02)02253-5. [DOI] [PubMed] [Google Scholar]
- (68).Wesierska-Gadek J, Schmitz ML, Ranftler C. Roscovitine-activated HIP2 kinase induces phosphorylation of wt p53 at Ser-46 in human MCF-7 breast cancer cells. J. Cell. Biochem. 2007;100:865–874. doi: 10.1002/jcb.21211. [DOI] [PubMed] [Google Scholar]
- (69).Choi CY, Kim YH, Kim YO, Park SJ, Kim EA, Riemenschneider W, Gajewski K, Schulz RA, Kim Y. Phosphorylation by the DHIPK2 protein kinase modulates the corepressor activity of Groucho. J. Biol. Chem. 2005;280:21427–21436. doi: 10.1074/jbc.M500496200. [DOI] [PubMed] [Google Scholar]
- (70).Mikkola I, Bruun JA, Bjorkoy G, Holm T, Johansen T. Phosphorylation of the transactivation domain of Pax6 by extracellular signal-regulated kinase and p38 mitogen-activated protein kinase. J. Biol. Chem. 1999;274:15115–15126. doi: 10.1074/jbc.274.21.15115. [DOI] [PubMed] [Google Scholar]
- (71).Bulavin DV, Saito S, Hollander MC, Sakaguchi K, Anderson CW, Appella E, Fornace AJ., Jr. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 1999;18:6845–6854. doi: 10.1093/emboj/18.23.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Yaffe MB, Schutkowski M, Shen M, Zhou XZ, Stukenberg PT, Rahfeld JU, Xu J, Kuang J, Kirschner MW, Fischer G, Cantley LC, Lu KP. Sequence-specific and phosphorylation-dependent proline isomerization: a potential mitotic regulatory mechanism. Science. 1997;278:1957–1960. doi: 10.1126/science.278.5345.1957. [DOI] [PubMed] [Google Scholar]
- (73).Ranganathan R, Lu KP, Hunter T, Noel JP. Structural and functional analysis of the mitotic rotamase Pin1 suggests substrate recognition is phosphorylation dependent. Cell. 1997;89:875–886. doi: 10.1016/s0092-8674(00)80273-1. [DOI] [PubMed] [Google Scholar]
- (74).Yie J, Liang S, Merika M, Thanos D. Intra- and intermolecular cooperative binding of high-mobility-group protein I(Y) to the beta-interferon promoter. Mol. Cell. Biol. 1997;17:3649–3662. doi: 10.1128/mcb.17.7.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Frank O, Schwanbeck R, Wisniewski JR. Protein footprinting reveals specific binding modes of a high mobility group protein I to DNAs of different conformation. J. Biol. Chem. 1998;273:20015–20020. doi: 10.1074/jbc.273.32.20015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.