Abstract
A quantitative proteomic analysis of changes in protein expression accompanying the differentiation of P19 mouse embryonal carcinoma cells into neuron-like cells using isobaric tag technology coupled with LC-MS/MS revealed protein changes reflecting withdrawal from the cell cycle accompanied by a dynamic reorganization of the cytoskeleton and an up-regulation of mitochondrial biogenesis. Further study of quantitative changes in abundance of individual proteins in a purified mitochondrial fraction showed that most mitochondrial proteins increased significantly in abundance. A set of chaperone proteins did not participate in this increase, suggesting that neuron-like cells are relatively deficient in mitochondrial chaperones. We developed a procedure to account for differences in recovery of mitochondrial proteins during purification of organelles from distinct cell or tissue sources. Proteomic data supported by RT-PCR analysis suggests that enhanced mitochondrial biogenesis during neuronal differentiation may reflect a large increase in expression of PGC-1α combined with down-regulation of its negative regulator, p160 Mybbp1a.
Keywords: embryonal carcinoma, mitochondrial biogenesis, quantitative proteomics, cell cycle, neurogenesis
Introduction
The P19 embryonal carcinoma cell model has been adopted by many laboratories studying neuronal differentiation 1, 2. P19 cells are undifferentiated, divide rapidly, and, like stem cells, are multi-potential. In the presence of varied inducers, P19 cells can differentiate in vitro into derivatives of all three germ layers, endoderm, mesoderm, and ectoderm 2. When these cells are injected into mouse blastocysts, they differentiate into a broad range of cell types 3. P19 cells can be induced to withdraw from the cell cycle and to differentiate along the neuronal pathway with the addition of a vitamin A derivative, all-trans-retinoic acid (ATRA), combined with altered culture conditions. The cells differentiate into neurons, glia and fibroblast-like cells in much the same way as neural stem cells are thought to develop through radial glia as common progenitors of both neurons and glia 1, 4, 5. P19 neuron-like cells (NLCs) in culture have small cell bodies with long processes similar to the axons and dendrites of cultured brain cells.
Previous studies in other labs have investigated the global proteomic changes of neuronal differentiation. A study of the in vitro differentiation of adult rat hippocampal neural stem cells by two-dimensional electrophoresis (2DE), resulted in the identification of 367 regulated spots, of which 128 could be identified, although many of these proteins were isoforms 6. A similar 2DE analysis of protein abundance in P19 cells undergoing neuronal development revealed 500 abundant polypeptides, 17 of which were regulated 7. However, this early study did not identify many of these proteins. A subsequent study of P19 cell neural differentiation identified only 28 differentially expressed proteins 8. A distinct set of proteomic changes is observed when P19 cells are induced to differentiate along the cardiomyocyte lineage 9. Approximately 1200 proteins were studied via 2DE analysis of E14 cells during differentiation into dopaminergic neurons, but only 23 spots were expressed differentially in a consistent pattern 10. A recent 2DE-proteome analysis of proliferating and differentiating human neuronal ReNcell VM stem cells identified 402 spots with differential expression of 49 protein spots 11.
We compared the protein profile of dividing P19 cells with that of NLCs using a relatively new isobaric tagged proteomic approach that incorporates multidimensional chromatography coupled with MS/MS, as an alternative to conventional 2DE. The use of this isobaric tag method, which has been stimulated by the commercialization of iTRAQ® reagents (Applied Biosystems), enables complex sets of proteins to be compared quantitatively using mass spectrometry without 2D gels 12.
The pattern of protein expression in our study shows dynamic changes that accompany differentiation into NLCs. We identified multiple polypeptides for over 500 proteins, 182 of which were observed consistently in replicate experiments using total cell lysates. Statistical analysis of the data revealed 119 (~65%) of these 182 proteins significantly increased or decreased in abundance during neuronal differentiation. Mitochondrial purification allowed for a deeper coverage of mitochondrial proteins in response to NLC differentiation. A total of 59 mitochondrial proteins were observed consistently in replicate experiments. This work provides the most extensive quantitative analysis to date of proteomic changes accompanying neuronal differentiation along with a novel approach to monitor changes in protein abundance in a subcellular fraction.
Experimental Section
Cell culture
P19 cells were maintained in complete medium (alpha modified MEM, 2.5% fetal bovine serum (FBS), 7.5% bovine calf serum (BCS), and 1% penicillin-streptomycin) at 37°C in a 5% CO2 atmosphere. The cultures were passaged every 2–3 days at a 1:20 to 1:30 dilution. P19 cells in exponential growth were trypsinized and transferred to siliconized glass dishes at a density of 105 cells per ml of ATRA induction medium (alpha modified MEM, 2.5% fetal bovine serum (FBS) and penicillin-streptomycin containing 500 nM ATRA), where the cells aggregated for 4 days with a medium change on day 2. On day 4, the aggregates were trypsinized and cells were plated at a density of 2×107 per 10 cm poly-L-lysine treated tissue culture dish in complete medium (alpha modified MEM, 2.5% fetal bovine serum (FBS), 7.5% bovine calf serum (BCS), and penicillin-streptomycin). From day 5 to 7, the complete medium was supplemented with cytosine arabinoside (AraC) to kill fibroblast-like cells that did not respond to ATRA induction. Cells were maintained in NB/B27 (Neurobasal-A medium with the B27 supplement) from days 7 to 11 when neuron-like cells were harvested.
Total cell protein preparation
P19 and ATRA-induced neuron-like cells (NLC) were lysed in an extraction buffer containing 9 M urea, 2.5 M thiourea, 5% CHAPS, 0.2% ampholytes, 2 mM DTT. The proteins samples were diluted 1 to 5 in distilled water before protein was determined by Bradford assay, with diluted lysis buffer serving as the blank and BSA as the quantitative protein standard.
Mitochondrial purification
All handling was at 4°C, except for the nuclease treatment step. All buffers contained the protease inhibitors PMSF, Pepstatin A, Leupeptin and E64. 30 plates of P19 day 0 or 60 plates of NLC day 11 cells were washed off dishes and pelleted at 900 × g for 5 min, resuspended in TD buffer (135 mM NaCl, 5 mM KCl, 25 mM Tris-HCl, pH 7.6, 0.7 mM Na2HPO4), and repelleted by spinning at 900 × g for 5 min. The cells were resuspended in 9 ml of hypotonic buffer (20 mM Hepes pH 8, 5 mM KCl, 1.5 mM MgCl2, 2 mM DTT) and homogenized with 10–15 strokes of a glass dounce homogenizer. 6 ml of 2.5X MSH (525 mM mannitol, 175 mM sucrose, 50 mM Hepes, pH 8, 5 mM EDTA), were added per 9 ml. Nuclei were removed by two successive centrifugation steps for 5 min at 1500 g. The post nuclear supernatant (PNS) containing the mitochondria was centrifuged for 15 min at 13,000 rpm to pellet the crude mitochondria (CM).
Mitochondria were resuspended in 3 ml of MSH buffer (210 mM mannitol, 70 mM sucrose, 20 mM Hepes, pH 8, 2 mM EDTA) containing 60 mM KCl, 1 mM K2HPO4, 10 mM MgCl2, 1 mM ADP, 5 mM Na-glutamate, 5 mM Na-malate, 60 μl of 2 mg/ml RNase-free DNAse I (Sigma Type II; 2000 U/mg) and of 0.05 U/ml Benzonase (adapted from 13). The crude mitochondria were incubated at 37 °C for 15 min. EDTA was added after the incubation to chelate Mg. The crude mitochondria were then layered above 10 ml of 0.8 M sucrose, 20 mM Hepes, pH 8, 1 mM EDTA pelleted at 4 °C at 13,000 rpm for 15 min, and resuspended in 0.5 ml 1X MSH containing 50 μg/ml BSA.
Crude mitochondria were resuspended in 0.5 ml 1X MSH and layered on a 0.8 M/1.5 M preformed sucrose step gradient in a Beckman SW41 tube. Gradients were centrifuged at 23,000 rpm at 4 °C for 30 min. Mitochondria, which sediment to the 0.8 M/1.5 M sucrose interface, were collected with a sterile Pasteur pipette. The mitochondria were diluted with 4 volumes of 1X MSH containing 50 μg/ml BSA, repelleted at 13000 rpm and resuspended in 0.5 ml 1X MSH. Protein content was determined using the Bradford dye binding assay as above. 50 to 100 μg aliquots of mitochondria were pelleted and frozen at −80°C.
Proteolysis and iTRAQ® reagent labeling
50 μg samples of protein from undifferentiated P19 cells and day 11 NLC were precipitated with acetone for 1 h at −20 °C and collected by centrifugation at 14,000 rpm. The pellet of protein from each sample was then resuspended in 30 μl of dissolution buffer (iTRAQ® reagent, Applied Biosystems) containing 0.05% SDS final concentration. Each 50 μg sample of protein was reduced, alkylated, digested with trypsin and labeled with the isobaric reagents according to the manufacturer’s protocol. Differentially labeled peptide samples were pooled and subjected to strong cation exchange chromatography fractionation on a Waters 625 HPLC, equipped with a 200 × 4.6 mm, 5 μm PolyLC Polysulfoethyl A column (The Nest Group, Inc.). Peptides were eluted with a gradient of 0 to 0.4 M KCl in 5 mM KH2PO4, 25% acetonitrile, pH 3.0. The resulting peptide fractions were lyophilized under vacuum to 50 μl volume.
Protein mass spectrometric analysis
The HPLC-separated peptides were run on a nano-LC/ESI/MSMS API Qstar Pulsar i quadrupole-time of flight (Q-TOF) tandem mass spectrometer (Applied Biosystems/MDS Sciex, Foster City, CA) interfaced with an LC Packings Nano LC System which included an Ultimate micro pump, a FAMOS micro auto-sampler and a Switchos micro column switch module (LC Packings, Sunnyvale, CA). The peptide separation was carried out on a Waters NanoEase Symmetry column (3.5 μm C18, 75 μm ID × 15 cm) with a 60 min linear gradient from 2% to 30% solvent B. Solvent A was 2% acetonitrile (ACN) with 0.1% formic acid and solvent B was 88% ACN, 10% isopropanol with 0.1% formic acid. The flow rate was 300 nL/min. A 300 μm id × 5 mm μ-Pre-column Cartridge (LC Packings) was used for sample desalting and pre-concentration.
Immunoblot Analysis
Total proteins (3 μg) were separated by SDS-PAGE and electrophoretically transferred to PVDF membrane. The membranes were blocked with 5% non-fat dried milk in Tween-PBS (1x PBS containing 0.5% Tween 20) for 1 hr at room temperature. Following blocking, membranes were incubated with the primary antibodies as follows (source and dilution factor in parentheses): monoclonal antibodies Oct 4 (Santa Cruz, 1:1000), β3 tubulin (R&D Systems, 1:1000), GAPDH (RDI, 1:5000), complex I, II and III (MitoSciences MSC101 vs NDUFA9 1:1000, MSC 204 vs SDHA 1:10,000 and MSC303 vs UQCRC1 1:2000, respectively), Complex IV (Calbiochem, Cox I 1:1000), Porin (Calbiochem, 1:1000), AIF (Santa Cruz, 1:200), prohibitin (Neomarkers, 1:500); polyclonal antibodies, goat anti-NRF1 (gift from R. Scarpulla, 1:1000) or rabbit anti-HSP60 (Santa Cruz, 1:500), MnSOD (RDI, 1:1000), PGC-1α (Chemicon, 1:500) and GFAP (DAKO, 1:1000). Primary antibodies were incubated overnight at room temperature. Secondary anti-mouse or anti-rabbit antibodies were either fluorescein-modified (Amersham Life Science, 1:2500) or phosphatase-labeled (KPL, 1:5000) and were incubated for 1 hr at room temperature. Between the primary and secondary antibodies or before detection, membranes were washed three times for 5 min each in Tween-PBS.
Flow Cytometry
β3-tubulin and glial fibrillary acidic protein (GFAP) expression in P19 and NLCs were measured using a Becton Dickinson FACSCAN analyzer. 1 × 106 cells in suspension were dispersed and centrifuged at 200 g for 5 min. The supernatant was removed and the cells were washed with PBS twice then centrifuged again. The PBS was removed and the cells were fixed in 4% PFA in PBS for 30 min at room temperature. The cells were centrifuged at 200 g for 5 min to remove excess PFA and washed twice with PBS. Permeabilization buffer (0.25 % Triton X-100 in PBS) was added and the cells incubated at room temperature for 30 min. After washing twice with PBS, the cells were blocked for 30 min at room temperature in 5% normal swine serum, 0.25 % Triton X-100 in PBS. The cells were washed with PBS twice then centrifuged again and incubated in primary antibody (β3-tubulin (R&D Systems, 1:1000) or GFAP (DAKO, 1:500)) at room temperature for 2h. The cells were washed with PBS twice then centrifuged again and incubated in secondary antibody (Alexa fluor 488 goat anti-mouse IgG or Alexa fluor 488 goat anti-rabbit IgG; both Molecular Probes reagents) at 1:10,000 dilution at room temperature for 1h. The cells were washed with PBS twice then centrifuged again and resuspended in 500 μl of PBS for analysis.
Nucleic Acid Extraction and Analysis
Total DNA was purified from cells at days 0, 4 and 11 of the differentiation program using a standard Proteinase K/phenol extraction method 14. DNA was quantified by UV absorbance and digested with EcoRI. Fragments were separated by agarose gel electrophoresis and Southern blotted to Hybond N+ membrane (Waters). Specific fragments were detected by simultaneous hybridization with random-primer labeled 32P probes for mouse mtDNA residues 1751–3804 and mouse rDNA (BK000964) residues 10198–10743, which hybridize to fragments of 2053 and 6606 base pairs, respectively. Total RNA from P19 day 0 and day 11 NLCs was extracted by homogenization in TRIzol® reagent (Invitrogen). After mixing with chloroform the lysate was centrifuged at 12,000 × g for 15 min at 4° C. RNA was precipitated from the aqueous phase with isopropyl alcohol and dissolved in ribonuclease-free water. RNA was quantified and first strand cDNA was generated from 5 μg RNA template using Superscript™ III Reverse Transcriptase (Invitrogen). The cDNA was stored at −20C.
Quantitative RTPCR
Quantitative PCR was carried out using the Qiagen QuantiTect SyBr Green PCR Kit and a MJ Research DNA Engine Opticon 2 PCR machine. The gene expression of 3-phosphoglycerate dehydrogenase (Phgdh) was used as an internal control to normalize the results obtained for genes of interest with respect to mitochondrial biogenesis at neuronal differentiation time points days 0, 4 and 11. Equal amounts of starting cDNA template for each time point were added to PCR reactions with the gene of interest in neuronal differentiation as follows:
Phgdh (CCTCACCAGTGCCTTCTCTC and TCTCTCAGAAGGCCGACAAT),
NRF1 (GCACCTTTGGAGAATGTGGT and CTGAGCCTGGGTCATTTTGT),
PGC-1α (AAGGTCCCCAGGCAGTAGAT and GCGGTATTCATCCCTCTTGA),
PGC1β (TTCCCAGAACTGGATGAAGG and TCTGGAACTGAGGCTGGTCT),
PRC-1 (TTCAGTGGTCAGATGCTTGC and ACCAGCTCACTCAAGGAGGA),
Mybbp1a (GGAGATCTTGCTGTCCTTGC and TCATCAGTGACCACCACGTT), and
TFAM (CTTCAACCACCACACCACTG and TCTCTCCTCCATGCGTTCTT). The cDNA was heated at 95 °C for 15 min before PCR consisting of 40 cycles of 30 s at 94 °C, 30s at 60 °C and 1 minute at 72. Quantitative PCR products were analyzed using Opticon Monitor Analysis Software Version 2.02.
Protein identification and quantification
Four experiments were performed comparing undifferentiated P19 and day 11 ATRA-treated NLC. Mass spectrometer data files in wiff format were processed with Pro-Quant software (Applied Biosystems) for quantification and identification of iTRAQ® labeled peptides by searching against an interrogator database constructed from GenBank NR release 19. Carboxamidomethylcysteine was selected as a fixed modification and methionine oxidation was permitted. One missed trypsin cleavage was allowed. Mass tolerance was set to 0.15 Da in MS mode and 0.1 Da in MS/MS analysis. To attempt to assess the frequency of false positives, we repeated data analysis with a mock database containing reversed-sequences. We saw occasional apparent peptide hits, as might be expected by chance, but never observed a reversed protein with more than one peptide hit. Pro-Group software (Applied Biosystems) was utilized to search results from Pro-Quant to determine protein ID confidence, description of expression differences and detection of protein isoforms. This software combines peptide hits from several fractions that together comprise a complete experiment to determine the number of peptides identified for a particular protein. Results were then imported into an Excel file and truncated to a list of peptides identified with confidence > 90%. Each individual peptide was linked to an independent mass tag ratio. The resulting peptides that identified a single protein were used to calculate a new averaged mass tag ratio for that protein. These averages characteristically had a standard deviation of less than +/− 17.6 %. Complete data sets from independent experiments were compared to one another and a final average ratio for each protein identified in the data set was calculated. Only those proteins identified in at least two independent experiments by peptides with > 90% confidence were interpreted in this analysis. All of the identified proteins are then submitted to the MiGenes database (http://www.pharm.stonybrook.edu/migenes/) using the batch search mode. MiGenes is a relational database of mitochondrially-related gene products that collates information on all proteins with GO annotations identifying them as mitochondrial 15.
Statistical analysis
Each protein was characterized by a ratio of mass tag signal intensity reflecting the relative abundance of the protein in neuronal-like cells relative to undifferentiated cells. These abundance ratios were converted to a log10 scale to overcome the lack of symmetry in raw protein abundance ratios and to calculate a mean, μ, and standard deviation, σ, for the log protein abundance. The observed mean of the relative protein abundance was μ = 1.10 and the log of this value was 0.04. The standard deviation of the log relative protein abundance was σ = 0.29. In order to determine the biological significance of relative protein expression data, we formulated the null hypothesis H0 that all protein abundance data fit a normal distribution characterized by the population mean = μ. The hypothesis of interest is whether the relative log10 abundance ratio of a protein X = μ, or if the alternative hypothesis is correct, that X ≠ μ. Using a two-tailed t test, we defined criteria to determine whether the abundance of a protein was significantly distinct from the normal distribution characterized by the population mean, μ. We consider a protein to be significantly up or down-regulated if the p-value of its abundance ratio relative to the mean was ≤ 0.05.
Results
Homogeneous differentiation of P19 cells to NLC
We chose the P19 cell line for our studies as a system expected to be capable of homogeneous differentiation. The non-induced, replicating P19 cells express the pluripotent stem cell marker Oct4, which is quickly lost as cells differentiate (Figure 1A) 16. Upon ATRA induction, cell aggregation, and a total of 11 days of culture, including growth in supplemented neurobasal medium from days 7–11, P19 cells differentiate to NLC that express the mature neuronal marker β3-tubulin (Figure 1A) 17. Since neurons and glia share a common neuroectodermal progenitor, we sought to investigate whether our differentiated cultures might contain a mix of neurons and glia. Parallel immunoblot experiments failed to detect glial fibrillary acidic protein (GFAP) as a glial marker under conditions that readily detected this protein in a mouse brain homogenate (Figure 1B) 18. Similarly, flow cytometry of NLC detected homogeneous staining with β3-tubulin and no detectable sub-population expressing GFAP (Figure 1C). We conclude that the ATRA induction protocol results in a fairly uniform population of cells.
Figure 1.
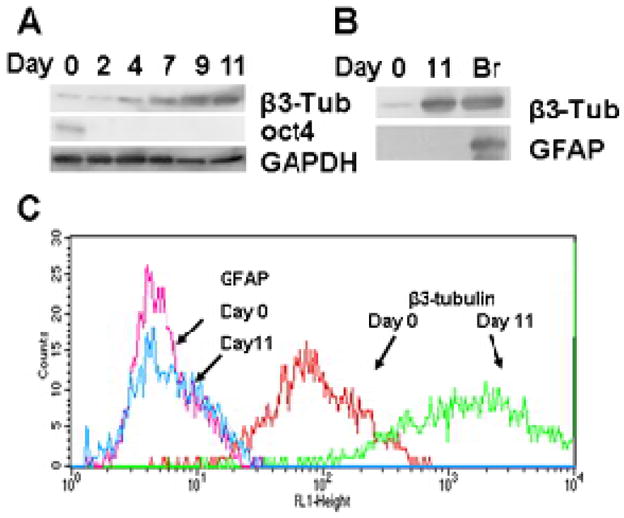
Differentiation of P19 embryonal carcinoma cells to neuron-like cells. A. In vitro differentiated P19 cells express markers of mature neurons. 3 μg samples of total protein from days 0, 2, 4, 7, 9 and 11 of the developmental program were fractionated by SDS-PAGE, transferred to a PVDF membrane and probed with various antisera: β3-tubulin, marker for mature neurons; Oct4, a marker for pluripotent stem cells; GAPDH, a loading control. B. 3 μg samples of total P19 cell protein from days 0 or 11 or from a mouse brain homogenate (Br) were fractionated by SDS-PAGE and probed by immunoblotting with antibodies directed against β3 tubulin and GFAP (glial fibrillary acidic protein). C. Undifferentiated P19 cells (day 0) and day 11 NLCs were subjected to flow cytometry to analyze the distribution of cells responding to a glial marker GFAP, and a neuronal marker β3-tubulin as indicated.
Proteomic analysis of total cell lysates
Total cell protein was prepared from undifferentiated P19 cells and differentiated NLC for quantitative multidimensional LC-MS/MS analysis of isobaric tagged peptides as described in Materials and Methods. In four replicate experiments, we obtained quantitative data on multiple peptides for over 500 proteins. 182 proteins were identified in at least two experiments by peptides with >90% confidence (Supplementary Table 1). In over 90% of the cases, multiple qualifying peptides were observed in each experiment. Since the isobaric tag approach to quantitative proteomics using iTRAQ® reagents is a new technology, we examined the data to determine their reliability. First, we observed that multiple peptides in replicate experiments converged to an average characterized by a standard deviation of less than +/− 17%. Second, in several instances, we obtained quantitative data on proteins that are known to be components of larger complexes. These proteins generally increased or decreased in parallel with their binding partners. For example, two subunits of Na+/K+ ATPase were increased 3.26 and 2.64-fold while two subunits of mitochondrial F1 ATPase were increased 1.86 and 1.77-fold (Supplementary Table 1). Finally, in total cell lysates, 19 ribosomal proteins all decreased to 70+/−14% of control abundance during differentiation (Supplementary Table 1). These results provide additional confidence in the precision of protein abundance measurements using the isobaric tag method.
119 of the 182 proteins identified, approximately 65%, were differentially expressed at the p=0.05 confidence level, with 53 of these up-regulated and 66 down-regulated (Figure 2). This result indicates that neuronal differentiation entails a major re-organization of cell structure and function. We grouped the proteins listed in Supplementary Table 1 according to gene ontology classifications to reveal a number of categories that showed significant disparities in the numbers of up- and down-regulated proteins, as shown in Table 1. The most consistent patterns of alteration in protein abundance reflect withdrawal of cells from the cell cycle, reorganization of the cytoskeleton to adopt a neuronal morphology and a shift in metabolism involving enhanced mitochondrial biogenesis.
Figure 2.
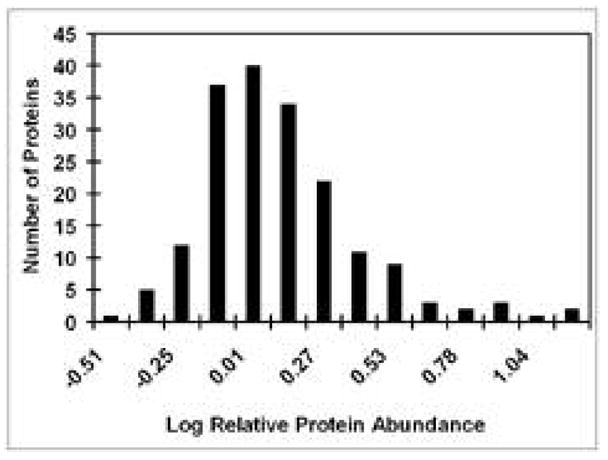
Changes in abundance of total cell proteins during neuronal differentiation. The distribution of the number of proteins with log iTRAQ® ratios in the indicated ranges is shown.
Table 1. Analysis of quantitative changes in abundance of total cell proteins by GO category.
Proteins identified by LC-MS/MS were classified according to their GO annotations. The numbers of proteins in each class that were increased, decreased or unchanged (NC) in abundance are indicated along with the average fold-change for proteins in each class.
| GO Category | Up | NC | Down | Avg |
|---|---|---|---|---|
| Cytoskeleton | 16 | 6 | 3 | 3.10 |
| Transcription | 0 | 7 | 4 | 0.86 |
| Translation/Protein Biosynthesis | 1 | 3 | 27 | 0.68 |
| Metabolism | 1 | 6 | 3 | 1.03 |
| Cell Cycle/DNA metabolism | 0 | 1 | 7 | 0.54 |
| mRNA Processing | 2 | 12 | 6 | 0.98 |
| Protein Modification | 0 | 2 | 0 | 1.03 |
| Protein Catabolism | 0 | 2 | 1 | 0.83 |
| Neurogenesis | 10 | 0 | 0 | 3.77 |
| Nucleotide Biosynthesis | 0 | 3 | 0 | 0.95 |
| Protein Folding/Chaperone | 4 | 8 | 5 | 0.98 |
| Signal Transduction | 5 | 3 | 0 | 1.92 |
| Hydrolase Activity | 1 | 0 | 2 | 1.00 |
| Transport | 3 | 7 | 1 | 1.42 |
| Response to Stress/Oxidative stress | 0 | 0 | 2 | 0.69 |
| Mitochondrial categories: | ||||
| TCA cycle | 3 | 0 | 0 | 2.00 |
| Transport | 4 | 1 | 0 | 1.66 |
| ATP Synthetase | 2 | 0 | 0 | 1.82 |
| Electron Transport | 1 | 2 | 0 | 1.45 |
| Oxidative Stress | 0 | 1 | 0 | 1.22 |
| Protein Folding/Chaperones | 0 | 4 | 0 | 0.97 |
The withdrawal of P19 cells from the cell cycle may be expected to entail remarkable changes, since the undifferentiated cells grow very rapidly with a generation time of approximately 13 hours. However, we observed relatively few proteins with GO annotations involved in roles in cell cycle regulation, no doubt reflecting the fact that most regulatory proteins are of low abundance and difficult to capture in a quantitative proteomic screen of total cell proteins. The two cell cycle-related proteins we observed were among the most significantly down-regulated proteins in the data set: SET translocation (SET), an oncoprotein involved in cell cycle regulation and chromatin remodeling 19–23, and prothymosin alpha, a highly abundant oncoprotein necessary for cell cycle progression and proliferation 24.
The non-proliferative state is characterized by a decrease in protein synthesis 8. While neuronal differentiation requires new synthesis of specific proteins, as discussed below, the overall quantity of new protein required is evidently less than that required for the continued rapid doubling of the undifferentiated cell population. Thus, Table 1 shows a significant decrease in abundance of several translation initiation factors as well as ribosomal proteins. Overall, 27 of 31 proteins annotated as having a role in protein biosynthesis showed a significant decrease in abundance, while only one, ribophorin I, increased. Ribophorin I is not a core component of the protein synthesis machinery, but is an endoplasmic reticulum protein involved in protein glycosylation. The modest up-regulation of ribophorin I may reflect the increased synthesis and ER processing of membrane proteins in NLCs.
We quantified significant increases in abundance of ten proteins involved in neurogenesis during the differentiation of P19 cells to NLC including Basp1, brain abundant membrane attached signal protein 1 (also known as NAP22 and CAP23), a downstream effector of neuregulins 25 and a functionally related protein, GAP-43 26. These proteins function with MARCKS, another protein we found to be significantly up-regulated, in the remodeling of the actin cytoskeleton 27. Several members of a family of dihydropyrimidinase-like proteins were also up-regulated during neuronal differentiation. These proteins, which are also known as collapsing response mediator proteins, are thought to be involved in axonal growth, neuronal differentiation, and one of them, dihydropyrimidinase-like 5, is reported to co-localize with mitochondria 28, 29. These examples illustrate the intimate co-regulation of markers of neurogenesis with the remodeling of the cytoskeleton in NLC (Table 1). We documented significant increases in the abundance of 16 other cytoskeletal proteins in this study. These proteins play clear functional and/or regulatory roles in axonal outgrowth in developing neurons, extending other recent work by Inberg et al. 30.
Enhanced mitochondrial biogenesis in NLC
The results in Table 1 and Supplementary Table 1 also document increased levels of a number of proteins involved in mitochondrial energy metabolism, including TCA cycle proteins and components of the mitochondrial respiratory complexes. To obtain a deeper coverage of mitochondrial proteins, we repeated the iTRAQ® analysis with mitochondria purified from undifferentiated cells and NLC. Western blot analyses to detect representative markers for mitochondrial and cytosolic fractions confirmed that the mitochondria were highly enriched (Figure 3). We performed three experiments using isobaric tags to compare proteins in independent preparations of mitochondria from undifferentiated P19 cells and NLC obtained using the 11-day in vitro differentiation protocol. These experiments provided quantitative data on 129 mitochondrial proteins, but our analysis was confined to 59 proteins for which we obtained multiple peptides in repeat experiments (Supplementary Table 2).
Figure 3.
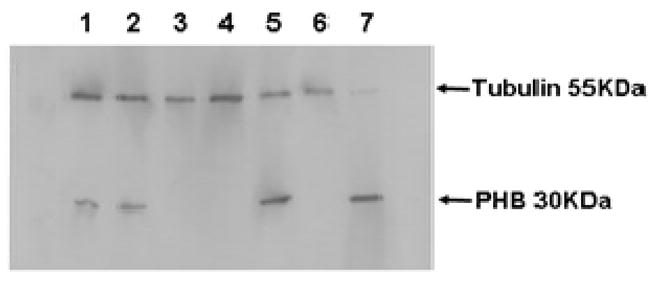
Purity of the mitochondrial preparation. 3 μg of protein from each fraction during purification was subjected to electrophoresis. Proteins were transferred to PVDF membrane and probing with antibodies directed against tubulin as a cytoskeletal marker and prohibitin as a mitochondrial marker. Lanes contained the following fractions: 1. Total homogenate; 2. Post-nuclear supernatant; 3. Nuclear pellet; 4. Post-mitochondrial supernatant; 5. Crude mitochondrial pellet; 6. Microsomal fraction; and 7. Purified mitochondria.
The raw data on peptide ratios obtained using purified mitochondrial fractions consistently showed a lower degree of up-regulation than we observed in total cell extracts prepared by homogenizing the cell pellets directly in CHAPS/urea buffer. This discrepancy is illustrated by analyzing the behavior of 14 proteins that we observed in both total cell homogenates and purified mitochondrial preparations. These 14 proteins showed a range of 33 to 89% greater relative abundance in total homogenates than in purified mitochondria (Table 2). What is the source of this disparity? Since cell fractionation involves increased handling, we consider that the total cell data most likely provides the best estimate of the absolute change in relative abundance of these proteins. Apparently, there is either a greater loss of mitochondria during purification from NLCs or that there may be a greater representation of non-mitochondrial proteins in the mitochondrial fraction from NLC than from undifferentiated P19 cells. To evaluate the latter possibility, we analyzed the iTRAQ® abundance data on all non-mitochondrial proteins identified in the mitochondrial fraction that were also observed in the total cell lysates. 38 proteins fitting these criteria are listed in Supplementary Table 3. These were increased in abundance in the mitochondrial fraction by an average of 2.06-fold. This trend is led by a set of 15 proteins annotated as cytoskeletal or neurogenesis proteins that were increased in abundance in the mitochondrial fraction by an average factor of 3.3-fold. These proteins could simply represent contaminants, but their selective nature is consistent with a remodeling of the contacts of the outer mitochondrial membrane with other cellular components during neurogenesis. For example, it appears that neurogenesis involves increased association of mitochondria with such proteins as tubulin, MARCKS, GAP43 and collapsing response mediator (dihydropyrimidinase-like) protein family members (Supplementary Table 3) possibly to aid in transport of mitochondria to neuronal processes. As a second example, we note that although the abundance of proteins in the glycolysis pathway is decreased during neurogenesis, this trend is reversed for two proteins, aldolase 1 and pyruvate kinase 3, that showed a slightly increased abundance in the mitochondrial fraction in NLCs. The docking of proteins on the outer mitochondrial membrane is of increasing interest in cell signaling and will be an important subject for future research.
Table 2. Comparison of abundance changes for 14 mitochondrial proteins observed in both the total cell protein and the mitochondrial fraction.
The relative abundance of 14 mitochondrial proteins for NLC relative to undifferentiated cells is shown for total cell lysates and for the purified mitochondrial fraction. The right-hand column shows the ratio of total cell to mitochondrial protein data.
| Accession | Protein Name | Gene | iTRAQ® Cell | Abundance Mito | Data Cell/Mito |
|---|---|---|---|---|---|
| NP_031531 | F1 ATPase alpha | Atp5a1 | 1.861 | 1.137 | 1.637 |
| NP_058054 | F1 ATPase beta | Atp5b | 1.770 | 0.951 | 1.861 |
| NP_035824 | VDAC 1 | Vdac1 | 2.034 | 1.193 | 1.705 |
| NP_079843 | cytochrome c-1 | Cyc1 | 1.313 | 0.695 | 1.888 |
| NP_079986 | Complex III, Rieske Fe-S 1 | Uqcrfs1 | 1.786 | 1.050 | 1.701 |
| NP_034607 | HSP 60 | Hsp60 | 0.845 | 0.636 | 1.328 |
| NP_034611 | HSP 70 | GRP75 | 1.016 | 0.654 | 1.554 |
| NP_032329 | HSP 10 | Hspe1 | 0.846 | 0.506 | 1.673 |
| NP_031557 | Prohibitin 2 | BAP | 1.170 | 0.686 | 1.706 |
| NP_032643 | MDH 2 | MDH | 1.751 | 1.154 | 1.518 |
| NP_080720 | Citrate synthase | Cis | 1.911 | 1.275 | 1.499 |
| NP_542364 | aconitase 2 | Aco2 | 2.332 | 1.533 | 1.521 |
| NP_031477 | Ant 2 | Slc25a5 | 1.242 | 0.844 | 1.472 |
| NP_598429 | Phosphate carrier | Phc | 1.523 | 0.986 | 1.543 |
| Average | 1.615 |
For our present purpose of understanding changes in the abundance of known mitochondrial proteins, we sought to develop a strategy to normalize the data in Supplementary Figure 2 to account for the discrepancy in relative protein abundance in total lysates and purified mitochondria. We considered that one way to accomplish this would be to develop an internal standard. However, as an alternative to a single standard, we decided to use a set of internal standards, the group of 14 mitochondrial proteins observed in both total cell lysates and the mitochondrial fraction. The data on relative abundance of these 14 proteins in both the total cell lysate and the mitochondrial fraction is shown in Table 2. These are all relatively abundant proteins representing chaperones, membrane carriers, TCA cycle enzymes and members respiratory complexes. They are contained in outer membrane, inner membrane and matrix fractions. It is evident that the total cell data show a greater increase in abundance with differentiation than the corresponding data from the mitochondrial fraction, with an average increase of 61+/− 15%. Thus, we normalized the raw data on relative abundance of each of the 59 mitochondrial proteins by multiplying by a factor of 1.61 to reflect the apparent selective loss or dilution (with other proteins) of these 14 internal standard proteins (Supplementary Table 2). This normalization procedure brings the relative abundance of the internal standard proteins into line with their abundance in the total cell lysate and provides a basis to consider the behavior of the less abundant mitochondrial proteins that were not observed in the total cell lysate. This is a generally applicable method to deal with systematic changes in recovery of subcellular organelles in proteomic research.
Our detailed analysis of the normalized data on abundance of mitochondrial proteins is shown in Figure 4, which illustrates the relative abundance of most of the mitochondrial proteins listed in Supplementary Table 2, organized according to the functional classification of proteins as components of the electron transport chain, ATP synthetase, the TCA cycle, transporters and chaperones. This analysis shows that neuronal differentiation is accompanied by a broadly-based up-regulation of mitochondrial proteins. However, some individual classes, such as the chaperone proteins and components of the mitochondrial protein import machinery did not increase in parallel with other mitochondrial proteins. In order to validate the quantitative changes in mitochondrial proteins observed using LC-MS/MS, immunoblots were performed to monitor individual proteins in respiratory complexes as well as transporters, chaperones and a scavenger of reactive oxygen species (Figure 5). Components of the electron transport chain and cytochrome C were all up-regulated, although only a relatively minor increase was observed for complex II, SDHA. This is consistent with the increased reliance of neurons on glycolysis, which provides reducing equivalents to the electron transport chain at the level of NADH through complex I. Relatively little change was observed for ETFB (Supplementary Table 2), which participates in the delivery of electrons at the level of FADH2 to respiratory complex II, and for the SDHA subunit of complex II itself (Figure 5). We did not observe peptides derived from complex II in our proteomic analysis. The marked increase observed for the superoxide scavenger MnSOD in Figure 5 may reflect the participation of PGC-1α in the neuronal development program, as discussed below.
Figure 4.

Quantitative changes in abundance of mitochondrial proteins during differentiation. The changes in abundance during neuronal differentiation are shown for several classes of mitochondrial proteins. Panel A includes proteins belonging to respiratory complexes I, III and IV. Panel B shows subunits of F1, F0 ATPase (Complex V). Panel C shows changes in abundance of proteins in the TCA cycle. Panels D and E depict data for membrane transporters and chaperones, respectively. Note the differences in scale between different panels.
Figure 5.
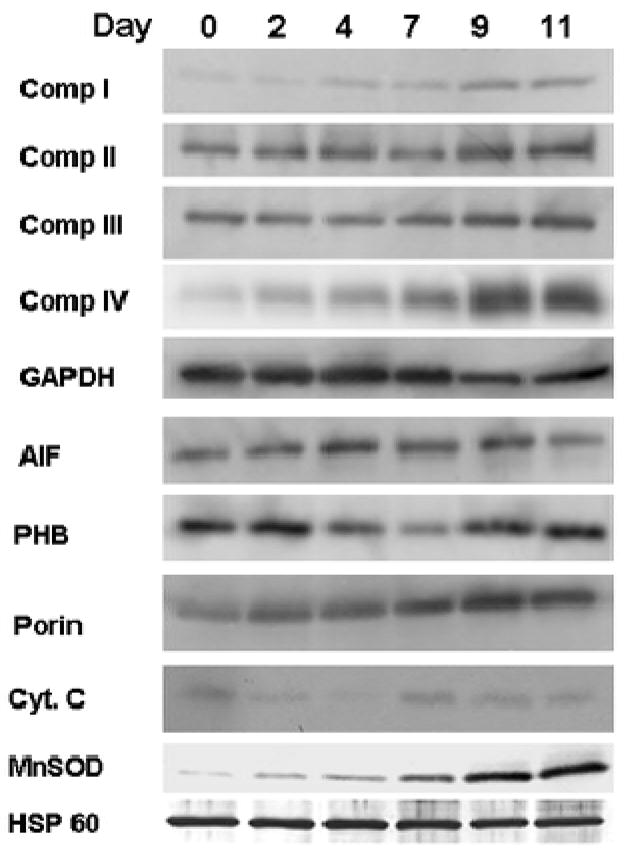
Immunoblots validate changes in expression of mitochondrial proteins during differentiation. 3 μg samples of total cell proteins from cultures at different stages in the induction program from day 0 through 11 were subjected to SDS-PAGE, blotted and probed for a variety of mitochondrial proteins. Antibodies used were specific for individual polypeptides of the respiratory complexes I–IV, GAPDH (loading control), Porin (VDAC), Cyt. C (cytochrome C), AIF (apoptosis inducing factor), PHB (prohibitin 1), MnSOD (manganese superoxide dismutase), and HSP 60 (heat shock protein 60).
Two results obtained in this study suggested a mechanism for the enhanced mitochondrial biogenesis that we observed. First, differentiation resulted in increased abundance of a diverse collection of mitochondrial proteins, suggesting a broadly-based regulatory switch. This is also indicated by a 2.1-fold increase in mtDNA copy number measured relative to nuclear ribosomal DNA (rDNA) during the 11-day developmental program (Figure 6). Second, one of the most significantly down-regulated cellular proteins we identified was the protein Mybbp1a, also known as p160 myb binding protein 1. Fan et al. 31 showed that binding of Mybbp1a to PGC-1α can down-regulate mitochondrial respiration. We reasoned that down-regulation of Mybbp1a during neuronal differentiation might relieve repression of PGC-1α. Since PGC-1α stimulates NRF-1, which up-regulates expression of numerous nuclear genes encoding mitochondrial proteins 32, decreased Mybbp1a results in enhanced mitochondrial biogenesis. Unfortunately, protein sequencing did not identify PGC-1α or NRF-1. Therefore, we monitored expression of these factors at either the RNA level using quantitative RT-PCR or at the protein level using immunoblots (Table 3 and Figure 7). In addition to studying the mRNA abundance of PGC-1α we monitored two other potential regulators of mitochondrial respiration, PGC-1β and PRC-1 from the same family. Undifferentiated cells contain an abundance of PRC-1 mRNA, along with a lower quantity of PGC-1β, while PGC-1α is, rather surprisingly, very rare. In contrast, in NLCs the abundance of PGC-1α mRNA is increased approximately 150-fold while PGC-1β mRNA increases slightly and PRC1 mRNA levels are similar to those of undifferentiated cells. The increased PGC-1α mRNA level is also reflected at the protein level (Figure 7). Interestingly, these changes in levels of mRNAs encoding PGC1-family co-activators do not result in a marked increase in mRNA for either NRF-1 or TFAM (data not shown). We suggest that this increase in PGC-1α, coupled with relief of inhibition by Mybbp1a, promotes overall mitochondrial biogenesis, with selectively greater effects on some proteins, such as MnSOD, than on others.
Figure 6.
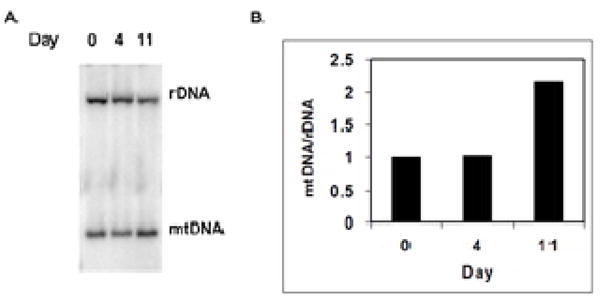
Increase of mtDNA during neuronal differentiation. A. Total cellular DNA was prepared from undifferentiated cells and from cells at days 4 and 11 of the differentiation program. 10 μg samples of DNA were digested with EcoRI, fractionated by agarose gel electrophoresis, transferred to a Hybond N+ membrane and probed with a mixture of 32P-labeled probes to detect both nuclear rDNA and mtDNA. Hybridization of probes to DNA was detected and quantified with a phosphorimager. B. Quantification of mitochondrial DNA to nuclear ribosomal DNA.
Table 3. Differential expression of mRNAs regulated upon NLC differentiation.
Quantitative mRNA analysis of cycle threshold (Ct) changes of Phgdh (internal control), PGC1α, PRC1, and mybbp1a for undifferentiated P19 cells day 0, 4, and 11. (S.D. = standard deviation).
| Relative Ct Values
|
Relative to Day 0
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Day 0 | S.D.
+/− |
Day 4 | S.D
+/− |
Day 11 | S.D
+/− |
Δ Ct
Day 4 |
Δ Ct
Day 11 |
Fold Δ
Day 4 |
Fold Δ
Day 11 |
|
| ||||||||||
| PHGDH | 19.83 | 0.30 | 18.67 | 0.27 | 22.54 | 0.10 | −1.16 | 2.71 | 2.24 | 0.15 |
| PGC1α | 30.21 | 0.33 | 30.95 | 0.23 | 22.93 | 0.09 | 0.74 | −7.28 | 0.60 | 155.88 |
| PGC1β | 30.07 | 0.24 | 28.06 | 0.20 | 26.31 | 0.11 | −2.01 | −3.75 | 4.03 | 13.49 |
| PRC1 | 23.77 | 0.32 | 22.26 | 0.51 | 23.52 | 0.22 | −1.51 | −0.25 | 2.85 | 1.19 |
| Mybbp1a | 20.10 | 0.19 | 20.78 | 0.41 | 21.99 | 0.49 | 0.68 | 1.89 | 0.63 | 0.27 |
Figure 7.
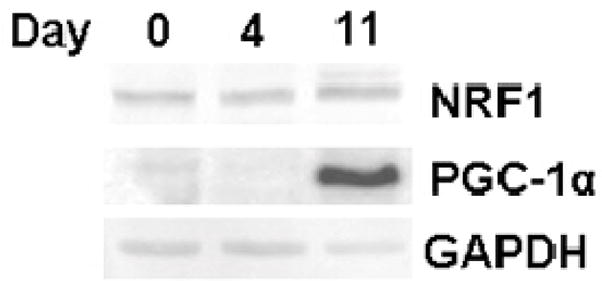
Differential expression of protein regulated upon NLC differentiation. Immunoblot analysis of NRF1, PGC-1α and GAPDH at days 0, 4 and 11 of the differentiation time course.
Discussion
Quantitative proteomic analysis of a neuronal differentiation pathway
The P19 embryonal carcinoma cell system has proven to be a very valuable model for studying neuronal differentiation 1, 33, 34. This process provides a clear illustration of the concept that proliferation and differentiation are mutually incompatible cell states. Upon exposure to ATRA and altered cell culture conditions, P19 cells engage in two to three rounds of cell division, then withdraw from the cell cycle and undergo a fairly uniform transition to NLCs through a differentiation program characterized by decreased expression of Oct4 and other markers of proliferative cells coincident with the acquisition of neuronal markers, such as β3-tubulin (Figure 1). The P19-derived NLCs express an array of other neuronal markers and exhibit synaptic structures and electrophysiological characteristics of neurons 1.
The P19 cell neuronal program has been studied previously at both the RNA 35, 36 and protein levels 7. The previous 2DE study by Ray and Gottlieb 7 did not reveal any down-regulated proteins, nor did they indicate the identities of the 17 up-regulated proteins observed. Advances in 2DE technology and peptide mass spectrometry made after the publication of this early study, including the use of DIGE as an internally standardized quantitative method 37, would presumably permit a more complete analysis of proteomic changes. However, we elected to use an isobaric tag proteomic method to quantify changes in protein abundance. Our study documenting the relative abundance of over 200 proteins represents a major advance in understanding this neuronal differentiation pathway, although this still represents only a sampling of the full proteome. We found that a substantial fraction of cellular proteins vary in abundance during neuronal differentiation. Significant decreases were observed for proteins involved in the cell cycle, nuclear DNA synthesis and translation, while marked increases were observed for proteins involved in remodeling the cytoskeleton and related activities required for neurogenesis, as noted above.
Modulation of bioenergetic pathways and mitochondrial function during neuronal differentiation
It is well known that neurons use glucose as their major energy source. The enhanced mitochondrial oxidative phosphorylation in NLCs involves induction of the aspartate-glutamate carrier aralar (Slc25a12; Supplementary Table 2) to assist in transfer of NADH reducing equivalents to the mitochondrial matrix through the malate-aspartate shuttle 38. The primary structure of aralar differs from that of the majority of mitochondrial carriers in that it contains a large EF-hand calcium binding domain that presumably functions to couple calcium signaling to neuronal metabolism 39, 40. We were surprised to find that differentiation of P19 cells to NLCs entailed a modest but consistent depression in the levels of enzymes involved in glycolysis, including aldolase, triosephosphate isomerase, glyceraldehyde 3-phosphate dehydrogenase, enolase and pyruvate kinase (Supplementary Table 1). A simultaneous decrease in lactate dehydrogenase coupled with a broad up-regulation of proteins involved in the citric acid cycle and oxidative phosphorylation indicate that the differentiating cells produce less lactic acid, but transfer acetate more efficiently to mitochondrial metabolism. This transition makes more efficient use of carbohydrate energy, providing more ATP to satisfy the demands of the developing neuron. The mitochondrially-generated ATP is made available to the cell through up-regulation of the adenine nucleotide transporter (principally ANT1; Supplementary Table 2) and creatine kinase (Supplementary Table 1). It would be of great interest to conduct a comparative study of these shifts in metabolic proteins using normal stem cells induced to undergo neuronal differentiation. P19 cells are highly transformed tumor-derived cells that may be even more dependent on glycolysis than neurons.
The changes in mitochondrial protein composition indicated in Figure 4 and Supplementary Table 2 reflect a generalized increase in mitochondrial biogenesis. Singh and Veltri 41 previously documented an increase in the fraction of cytoplasm occupied by mitochondria in P19-derived NLC. However, their study also reported the paradoxical result that the quantity of mtDNA appeared to decrease during differentiation. This result would be difficult to reconcile with the requirement of mtDNA transcription and translation to provide the 13 mtDNA-encoded subunits of mitochondrial respiratory complexes. We reinvestigated the quantity of mtDNA in P19 and NLC cells using an internally-controlled Southern blotting procedure and found that the mtDNA content relative to nuclear rDNA increased 2.1-fold (Figure 6), in parallel with the increases observed for most components of the electron transport chain. While most components of the mitochondrial respiratory complexes observed in our work increased in abundance approximately 1.6 to 2-fold, Western blotting suggests that the COX1 subunit of cytochrome oxidase increased more dramatically (Figure 5). It has long been known that neurons are distinguished by an unusually high content of cytochrome oxidase 42, and that this content responds to neuronal activity. However, the basis for the disproportionate increase in cytochrome oxidase subunit 1 remains uncertain.
Neuronal differentiation does not involve a coordinated increase in abundance of all mitochondrial proteins. Some proteins, such as chaperones and three components of the protein import apparatus, showed little change (Supplementary Table 2). It is interesting to note that the accumulation of proteins involved in importation and refolding of mitochondrial proteins does not keep pace with the general increase in abundance of cargo proteins. This is illustrated by the behavior of HSP60, which does not increase perceptibly during neuronal differentiation according to our mass spectrometry (Figure 4) and immunoblot results (Figure 5). HSP60 is a known disease gene that is mutated in some patients with spastic hereditary paraplegia 43. It will be interesting to determine whether a reduced relative level of this or other chaperones is observed in neurons in other settings.
Overall control of the differentiation program
Much of the developmental transition during neurogenesis is likely to result from changes at the level of transcription. Since the developmental program is primed by exposure of cells to all-trans retinoic acid, early transcriptional modulation may involve RAR and RXR factors 44, or may reflect the down-regulation of cyclin D1 as cells exit the cell cycle. However, much of the increase in mitochondrial proteins documented in Figure 5 occurs later in the differentiation program as primitive NLCs begin to extend axonal processes.
The list of nuclear transcription factors most commonly implicated in control of mitochondrial proliferation includes nuclear respiratory factors (NRF1 and 2), and peroxisome proliferator-activated receptor α/γ(PPARα/γ), all of which can employ PGC-1α as a coactivator. PGC-1α also collaborates with estrogen related receptor-α (ERRα) 45 to influence expression of nuclear genes encoding mitochondrial proteins. While expression of PGC-1α has been shown previously to respond to RXR receptor activation, our data in Table 3 and Figure 7 provide the first evidence, to our knowledge, that PGC-1α is significantly up-regulated during neuronal differentiation. Additionally these data show that there is a consistently high abundance of PRC-1 mRNA from day 0 to 11, with a dramatic increase in abundance of PGC-1α. PGC-1α is known to induce mitochondrial biogenesis and oxidative metabolism in muscle cells, adipocytes and cardiomyocytes 46, 47. Recently, St. Pierre et al. 48 showed that PGC-1α stimulates MnSOD expression, as we observed (Figure 5). The phenotype of PGC-1α knockout mice provided the first clue to the importance of this factor in neuronal function 49. These KO mice contain spongiform lesions in the striatum leading to hyperactivity that is phenocopied to some extent by the action of mutant Huntingtin protein which has recently been shown to antagonize expression of PGC-1α50. These results will stimulate additional study of the complex role of PGC-1α in neurogenesis.
Our proteomic data suggest that down-regulation of Mybbp1a may also impact the regulation of mitochondrial biogenesis by PGC-1α during neuronal differentiation. Mybbp1a can inhibit the ability of PGC-1α to stimulate transcription 31. Thus, enhanced mitochondrial biogenesis during neuronal differentiation may reflect a major effect of increased PGC-1α and secondary effect of decreased levels of its antagonist, Mybbp1a. The potential interaction of these mechanisms is illustrated in Figure 8, which depicts the manner in which these two mechanisms together may contribute to increased mitochondrial biogenesis. Despite this apparent complexity, Figure 8 clearly oversimplifies the situation since it does not account for the possible contribution of either PGC-1β or PRC1 to this pathway. Another regulatory pathway that might exert an influence during neuronal differentiation is the anticipated decrease in cyclin D1, which is reported to control expression of a cohort of genes involved in mitochondrial biogenesis 51. We suggest that the P19 neuronal differentiation pathway provides a fertile opportunity to explore these regulatory circuits in greater detail.
Figure 8.
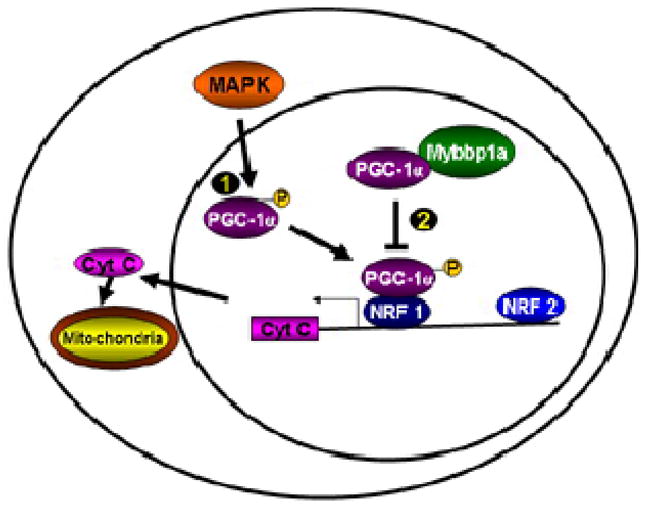
PGC-1α and NRF1 modulate expression of nuclear genes encoding mitochondrial proteins. Neuronal differentiation involves a dramatic up-regulation of expression of PGC-1α. Upon phosphorylation it can actively bind its regulatory partner NRF1 to activate expression of nuclear genes encoding mitochondrial proteins such as cytochrome C (Cyt C)52. A second regulatory mechanism is illustrated when PGC-1α is bound by Mybbp1a (p160), cannot be phosphorylated and cannot activate NRF131.
Supplementary Material
The iTRAQ® ratio of protein abundance in NLC vs undifferentiated cells is shown with the standard deviation, S.D. of peptide abundance. Columns on the right show the number of peptides observed by mass spectrometry analysis in repetitive experiments and the number of experiments in which the protein was detected.
The mass tag ratio of protein abundance in the mitochondrial fraction of NLC vs undifferentiated cells is shown. The ratios of protein abundance were normalized using the common set of 14 proteins observed in both the total cell and mitochondrial experiments as described in the text. Column headings are as given in Supplementary Table 1.
38 of the proteins reported in the total cell analysis in Supplementary Figure 1 were also observed as apparent contaminants in the mitochondrial fraction. The accession numbers and names of these proteins are shown along with the relative abundance measured using iTRAQ® ratio analysis of both the mitochondrial fraction and total cell lysate.
Acknowledgments
We thank Margaret Romaine and Stephanie Burke for technical assistance and Dr. Weiping Xie of the USB Proteomics Facility for LC-MS/MS services. We thank Chun Zhou and Erich Bremer for bioinformatics assistance, Dr. John Chen for statistical advice and Dr. Stella Tsirka for critical review of the manuscript. This research was supported by NIEHS grant R01-ES012039.
References
- 1.Bain G, Ray WJ, Yao M, Gottlieb DI. From Embryonal Carcinoma Cells to Neurons: The P19 Pathway. BioEssays. 1994;16:343–348. doi: 10.1002/bies.950160509. [DOI] [PubMed] [Google Scholar]
- 2.McBurney MW, Rogers BJ. Isolation of male embryonic carcinoma cells and their chromosome replication patterns. Dev Biol. 1982;89:503–508. doi: 10.1016/0012-1606(82)90338-4. [DOI] [PubMed] [Google Scholar]
- 3.Rossant J, McBurney MW. The developmental potential of a euploid male teratocarcinoma cell line after blastocyst injection. J Embryol Exp Morph. 1982;70:99–112. [PubMed] [Google Scholar]
- 4.Meeks JP, Mennerick S. Feeding hungry neurons: astrocytes deliver food for thought. Neuron. 2003;37(2):187–9. doi: 10.1016/s0896-6273(03)00027-8. [DOI] [PubMed] [Google Scholar]
- 5.Voutsinos-Porche B, Bonvento G, Tanaka K, Steiner P, Welker E, Chatton JY, Magistretti PJ, Pellerin L. Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron. 2003;37(2):275–86. doi: 10.1016/s0896-6273(02)01170-4. [DOI] [PubMed] [Google Scholar]
- 6.Maurer MH, Feldmann RE, Jr, Futterer CD, Butlin J, Kuschinsky W. Comprehensive Proteome Expression Profiling of Undifferentiated versus Differentiated Neural Stem Cells from Adult Rat Hippocampus. Neurochemical Research. 2004;29(6):1129–1144. doi: 10.1023/b:nere.0000023600.25994.11. [DOI] [PubMed] [Google Scholar]
- 7.Ray WJ, Gottlieb DI. Regulation of Protein Abundance in Pluripotent Cells Undergoing Commitment to the Neural Lineage. J Cell Physiology. 1996;168:264–275. doi: 10.1002/(SICI)1097-4652(199608)168:2<264::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 8.An J, Yuan Q, Wang C, Liu L, Tang K, Tian HY, Jing NH, Zhao FK. Differential display of proteins involved in the neural differentiation of mouse embryonic carcinoma P19 cells by comparative proteomic analysis. Proteomics. 2005;5(6):1656–68. doi: 10.1002/pmic.200401049. [DOI] [PubMed] [Google Scholar]
- 9.Wen J, Xia Q, Lu C, Yin L, Hu J, Gong Y, Yin B, Monzen K, Yuan J, Qiang B, Zhang X, Peng X. Proteomic analysis of cardiomyocytes differentiation in mouse embryonic carcinoma P19CL6 cells. J Cell Biochem. 2007;102:149–160. doi: 10.1002/jcb.21285. [DOI] [PubMed] [Google Scholar]
- 10.Wang D, Gao L. Proteomic analysis of neural differentiation of mouse embryonic stem cells. Proteomics. 2005;5(17):4414–26. doi: 10.1002/pmic.200401304. [DOI] [PubMed] [Google Scholar]
- 11.Hoffrogge R, Mikkat S, Scharf C, Beyer S, Christoph H, Pahnke J, Mix E, Berth M, Uhrmacher A, Zubrzycki IZ, Miljan E, Volker U, Rolfs A. 2-DE proteome analysis of a proliferating and differentiating human neuronal stem cell line (ReNcell VM) Proteomics. 2006;6(6):1833–47. doi: 10.1002/pmic.200500556. [DOI] [PubMed] [Google Scholar]
- 12.Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ. Multiplexed Protein Quantitation in Saccharomyces cerevisiae Using Amine-reactive Isobaric Tagging Reagents. Mol Cell Proteomics. 2004;3(12):1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 13.Enriquez J, Fernandez-Silva P, Perez-Martos A, Lopez-Perez M, Montoya J. The synthesis of mRNA in isolated mitochondria can be maintained for several hours and is inhibited by high levels of ATP. Eur J Biochem. 1996;237:601–610. doi: 10.1111/j.1432-1033.1996.0601p.x. [DOI] [PubMed] [Google Scholar]
- 14.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 15.Basu S, Bremer E, Zhou C, Bogenhagen DF. MiGenes: a searchable interspecies database of mitochondrial proteins curated using gene ontology annotation. Bioinformatics. 2006;22(4):485–492. doi: 10.1093/bioinformatics/btk009. [DOI] [PubMed] [Google Scholar]
- 16.Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005;26(2):495–502. doi: 10.1093/carcin/bgh321. [DOI] [PubMed] [Google Scholar]
- 17.Braun H, Schafer K, Hollt V. BetaIII tubulin-expressing neurons reveal enhanced neurogenesis in hippocampal and cortical structures after a contusion trauma in rats. J Neurotrauma. 2002;19(8):975–83. doi: 10.1089/089771502320317122. [DOI] [PubMed] [Google Scholar]
- 18.Lu QR, Park JK, Noll E, Chan JA, Alberta J, Yuk D, Alzamora MG, Louis DN, Stiles CD, Rowitch DH, Black PM. Oligodendrocyte lineage genes (OLIG) as molecular markers for human glial brain tumors. Proc Natl Acad Sci U S A. 2001;98(19):10851–6. doi: 10.1073/pnas.181340798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M, Makkinje A, Damuni Z. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J Biol Chem. 1996;271:11059–11062. doi: 10.1074/jbc.271.19.11059. [DOI] [PubMed] [Google Scholar]
- 20.Karetsou Z, Martic G, Sflomos G, Papamarcaki T. The histone chaperone SET/TAF-I beta interacts functionally with the CREB-binding protein. Biochem Biophys Res Comm. 2005;335:322–327. doi: 10.1016/j.bbrc.2005.06.210. [DOI] [PubMed] [Google Scholar]
- 21.Adler HT, Nallaseth FS, Walter G, Tkachuk DC. HRX leukemic fusion proteins form a heterocomplex with the leukemia-associated protein SET and protein phosphatase 2A. J Biol Chem. 1997;272:28407–28414. doi: 10.1074/jbc.272.45.28407. [DOI] [PubMed] [Google Scholar]
- 22.Estanyol JM, Jaumot M, Casanovas O, Rodriguez-Vilarrupla A, Agell N, Bachs O. The protein SET regulates the inhibitory effect of p21(Cip1) on cyclin E-cyclin-dependent kinase 2 activity. J Biol Chem. 1999;274:33161–33165. doi: 10.1074/jbc.274.46.33161. [DOI] [PubMed] [Google Scholar]
- 23.Canela N, Rodriguez-Vilarrupla A, Estanyol JM, Diaz C, Pujol MJ, Agell N, Bachs O. The SET protein regulates G2/M transition by modulating cyclin B-cyclin-dependent kinase 1 activity. J Biol Chem. 2003;278:1158–1164. doi: 10.1074/jbc.M207497200. [DOI] [PubMed] [Google Scholar]
- 24.Letsas KP, Frangou-Lazaridis M. Surfing on prothymosin alpha proliferation and anti-apoptotic properties. Neoplasma. 2006;53(2):92–96. [PubMed] [Google Scholar]
- 25.Mattar P, Britz O, Johannes C, Nieto M, Ma L, Rebeyka A, Klenin N, Polleux F, Guillemot F, Schuurmans C. A screen for downstream effectors of Neurogenin2 in the embryonic neocortex. Dev Biol. 2004;273(2):373–389. doi: 10.1016/j.ydbio.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Frey T, Mannella C. The internal structure of mitochondria. Trends Biochem Sci. 2000;25:319–325. doi: 10.1016/s0968-0004(00)01609-1. [DOI] [PubMed] [Google Scholar]
- 27.Laux T, Fukami K, Thelen M, Golub T, Frey D, Caroni P. GAP43, MARCKS, and CAP23 Modulate PI(4,5)P2 at Plasmalemmal Rafts, and Regulate Cell Cortex Actin Dynamics through a Common Mechanism. J Cell Biol. 2000;149(7):1455–1472. doi: 10.1083/jcb.149.7.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukada M, Watakabe I, Yuasa-Kawada J, Kawachi H, Kuroiwa A, Matsuda Y, Noda M. Molecular Characterization of CRMP5, a Novel Member of the Collapsin Response Mediator Protein Family. J Biol Chem. 2000;275(48):37957–37965. doi: 10.1074/jbc.M003277200. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi S, Inatome R, Yamamura H, Yanagi S. Isolation and expression of a novel mitochondrial septin that interacts with CRMP/CRAM in the developing neurones. Genes Cells. 2003;8(2):81–93. doi: 10.1046/j.1365-2443.2003.00617.x. [DOI] [PubMed] [Google Scholar]
- 30.Inberg A, Bogoch Y, Bledi Y, Linial M. Cellular processes underlying maturation of P19 neurons: Changes in protein folding regimen and cytoskeleton organization. Proteomics. 2007;7(6):910–920. doi: 10.1002/pmic.200600547. [DOI] [PubMed] [Google Scholar]
- 31.Fan M, Rhee J, St-Pierre J, Handschin C, Puigserver P, Lin J, Jaeger S, Erdjument-Bromage H, Tempst P, Spiegelman BM. Suppression of mitochondrial respiration through recruitment of p160 myb binding protein to PGC-1{alpha}: modulation by p38 MAPK. Genes Dev. 2004;18(3):278–289. doi: 10.1101/gad.1152204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cam H, Balciunaite E, Blais A, Spektor A, Scarpulla RC, Young R, Kluger Y, Dynlacht BD. A Common Set of Gene Regulatory Networks Links Metabolism and Growth Inhibition. Mol Cell. 2004;16(3):399–411. doi: 10.1016/j.molcel.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 33.Jones-Villeneuve EM, McBurney MW, Rogers KA, Kalnins VI. Retinoic Acid Induces Embryonal Carcinoma Cells to Differentiate into Neurons and Glial Cells. J Cell Biol. 1982;94:253–262. doi: 10.1083/jcb.94.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones-Villeneuve E, Rudnicki M, Harris J, McBurney M. Retinoic acid-induced neural differentiation of embryonal carcinoma cells. Mol Cell Bio. 1983;3:2271–2279. doi: 10.1128/mcb.3.12.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bain G, Mansergh F, Wride M, Hance J, Isogawa A, Rancourt S, Ray W, Yoshimura Y, Tsuzuki T, Gottlieb D, Rancourt D. ES cell Neural Differentiation Reveals a Substantial Number of Novel ESTs. Funct Integr Genomics. 2000;1:127–139. doi: 10.1007/s101420000014. [DOI] [PubMed] [Google Scholar]
- 36.Wei Y, Harris T, Childs G. Global gene expression patterns during neural differentiation of P19 embryonic carcinoma cells. Differentiation. 2002;70:204–219. doi: 10.1046/j.1432-0436.2002.700409.x. [DOI] [PubMed] [Google Scholar]
- 37.Unlu M, Morgan M, Minden J. Difference gel electrophoresis: A single gel method for detecting changes in protein extracts. Electrophoresis. 1997;18:2071–2077. doi: 10.1002/elps.1150181133. [DOI] [PubMed] [Google Scholar]
- 38.Nicholls D, Ferguson S. Bioenergetics. Vol. 3. Academic Press; Amsterdam: 2002. [Google Scholar]
- 39.Ramos M, del Arco A, Pardo B, Martinez-Serrano A, Martinez-Morales JR, Kobayashi K, Yasuda T, Bogonez E, Bovolenta P, Saheki T, Satrustegui J. Developmental changes in the Ca2+-regulated mitochondrial aspartate-glutamate carrier aralar1 in brain and prominent expression in the spinal cord. Developmental Brain Research. 2003;143(1):33–46. doi: 10.1016/s0165-3806(03)00097-x. [DOI] [PubMed] [Google Scholar]
- 40.Pardo B, Contreras L, Serrano A, Ramos M, Kobayashi K, Iijima M, Saheki T, Satrustegui J. Essential role of aralar in the transduction of small Ca2+ signals to neuronal mitochondria. J Biol Chem. 2006;281(2):1039–47. doi: 10.1074/jbc.M507270200. [DOI] [PubMed] [Google Scholar]
- 41.Singh G, Veltri K. Effects of Differentiation of Embryonal Carcinoma cells (P19) on Mitochondrial DNA Content in vitro. In Vitro Cell Dev Biol. 1991;27A:557–561. doi: 10.1007/BF02631286. [DOI] [PubMed] [Google Scholar]
- 42.Wong-Riley MTT. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends in Neurosciences. 1989;12(3):94–101. doi: 10.1016/0166-2236(89)90165-3. [DOI] [PubMed] [Google Scholar]
- 43.Hansen JJ, Durr A, Cournu-Rebeix I, Georgopoulos C, Ang D, Nielsen MN, Davoine CS, Brice A, Fontaine B, Gregersen N, Bross P. Hereditary spastic paraplegia SPG13 is associated with a mutation in the gene encoding the mitochondrial chaperonin Hsp60. Am J Hum Genet. 2002;70:1328–1332. doi: 10.1086/339935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leid M, Kastner P, Chambon P. Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem Sci. 1992;17:427–433. doi: 10.1016/0968-0004(92)90014-z. [DOI] [PubMed] [Google Scholar]
- 45.Mootha VK, Handschin C, Arlow D, Xie X, StPierre J, Sihag S, Yang W, Altshuler D, Puigserver P, Patterson N, Willy PJ, Schulman IG, Heyman RA, Lander ES, Spiegelman BM. Err{alpha} and Gabpa/b specify PGC-1{alpha}-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc Natl Acad Sci U S A. 2004;101(17):6570–6575. doi: 10.1073/pnas.0401401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schreiber S, Emter R, Hock MB, Knutti D, Cardenas J, Podvinec M, Oakeley EJ, Kralli A. The estrogen-related receptor a (ERRa) functions in PPARg coactivator 1a (PGC-1a)-induced mitochondrial biogenesis. Proc Natl Acad Sci U S A. 2004;101(17):6472–6477. doi: 10.1073/pnas.0308686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scarpulla R. Transcriptional activators and coactivators in the nuclear control of mitochondrial function in mammalian cells. Gene. 2002;286:81–89. doi: 10.1016/s0378-1119(01)00809-5. [DOI] [PubMed] [Google Scholar]
- 48.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W. Suppression of Reactive Oxygen Species and Neurodegeneration by the PGC-1 Transcriptional Coactivators. Cell. 2006;127(2):397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 49.Lin J, Wu P-H, Tarr PT, Lindenberg KS, St-Pierre J, Zhang C-Y, Mootha VK, Jager S, Vianna CR, Reznick RM. Defects in Adaptive Energy Metabolism with CNS-Linked Hyperactivity in PGC-1[alpha] Null Mice. Cell. 2004;119(1):121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 50.Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional Repression of PGC-1[alpha] by Mutant Huntingtin Leads to Mitochondrial Dysfunction and Neurodegeneration. Cell. 2006;127(1):59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 51.Sakamaki T, Casimiro MC, Ju X, Quong AA, Katiyar S, Liu M, Jiao X, Li A, Zhang X, Lu Y, Wang C, Byers S, Nicholson R, Link T, Shemluck M, Yang J, Fricke ST, Novikoff PM, Papanikolaou A, Arnold A, Albanese C, Pestell R. Cyclin D1 Determines Mitochondrial Function In Vivo. Mol Cell Biol. 2006;26(14):5449–5469. doi: 10.1128/MCB.02074-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metabolism. 2005;1(6):361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The iTRAQ® ratio of protein abundance in NLC vs undifferentiated cells is shown with the standard deviation, S.D. of peptide abundance. Columns on the right show the number of peptides observed by mass spectrometry analysis in repetitive experiments and the number of experiments in which the protein was detected.
The mass tag ratio of protein abundance in the mitochondrial fraction of NLC vs undifferentiated cells is shown. The ratios of protein abundance were normalized using the common set of 14 proteins observed in both the total cell and mitochondrial experiments as described in the text. Column headings are as given in Supplementary Table 1.
38 of the proteins reported in the total cell analysis in Supplementary Figure 1 were also observed as apparent contaminants in the mitochondrial fraction. The accession numbers and names of these proteins are shown along with the relative abundance measured using iTRAQ® ratio analysis of both the mitochondrial fraction and total cell lysate.


