Abstract
Background
The effects of intestinal inflammation on the central neurons projecting to the enteric nervous system are unknown. The dorsal motor nucleus of the vagus signals to the gastrointestinal system. Ghrelin is elevated in patients with inflammatory bowel disease and has been implicated as an inflammatory mediator. The purpose of this study was to investigate the effects of gastrointestinal inflammation on the dorsal motor nucleus of the vagus in rats, as well as the effects of pro-inflammatory cytokines and ghrelin on neurons from the dorsal motor nucleus of the vagus in vitro.
Methods
DiI was injected into the stomach wall of rats to retrogradely label neurons of the dorsal motor nucleus of the vagus. Intestinal inflammation was induced with indomethacin injection. Serial serum ghrelin measurements were performed. Tissue was examined under fluorescent microscopy. In vitro studies used primary culture of neurons from the dorsal motor nucleus of the vagus. RT-PCR for cytokine transcripts and immunohistochemistry for cytokine receptors were performed. Cell proliferation and apoptosis were measured by ELISA.
Results
A significant decrease of DiI labeling was demonstrated in the dorsal motor nucleus of the vagus of animals injected with indomethacin. Serum levels of ghrelin were significantly elevated two days after induction of inflammation. In vitro, apoptosis and cell proliferation were measured after 24 hour exposure to experimental conditions. Ghrelin alone had no effect on apoptosis. Exposure to IL-1β or TNF-α increased apoptosis. The addition of ghrelin to cytokine resulted in significant decreases in apoptosis compared to cytokine alone. Ghrelin significantly increased neuronal proliferation. Exposure to IL-1β, IL-6, or TNF-α significantly decreased proliferation. The addition of ghrelin to TNF-α or IL-6 significantly increased cellular proliferation compared to cytokine alone.
Conclusions
Neurons from the dorsal motor nucleus of the vagus which project to the stomach are reduced in number after induction of colitis in rats. In vitro, pro-inflammatory cytokines increase apoptosis and decrease cell proliferation of neurons from the dorsal motor nucleus of the vagus. These effects are attenuated by ghrelin.
Introduction
Inflammatory bowel diseases are common, but poorly understood clinical problems. The presumed etiology of some forms of inflammatory bowel disease involves an autoimmune reaction resulting in intestinal inflammation. The inflammatory response is characterized by alterations in circulating cytokines. The pro-inflammatory cytokine tumor necrosis factor alpha (TNF-α) may have a role in intestinal inflammation, showing increased circulating levels along with other pro-inflammatory cytokines such as interleukin-1 (IL-1) and interleukin-6 (IL-6).1
A commonly studied animal model for inflammatory bowel disease (IBD) uses mucosal application of the hapten trinitrobenzenesulfonic acid solution (TNBS) to modify self antigens and induce colonic inflammation.2 Systemically administered indomethacin can also induce gastrointestinal inflammation with features of human diseases.3 Animal studies in models of acute intestinal inflammation have demonstrated structural and functional changes in the enteric nervous system.4 However, the effects of intestinal inflammation on neurons in the central nervous system projecting to the enteric nervous system are largely unknown.
The dorsal vagal complex is the primary central structure which signals to the enteric nervous system. The dorsal vagal complex is located in the dorsal medulla and consists of three components: the area postrema, the nucleus of the solitary tract, and the dorsal motor nucleus of the vagus (DMV). The area postrema abuts the fourth ventricle and detects chemical changes in the blood and cerebrospinal fluid. Through primary vagal afferents, gastrointestinal stimuli activate the nucleus of the solitary tract. The DMV integrates peripheral and central signals and provides the parasympathetic efferent outflow to the gastrointestinal system through the vagus nerve.
Ghrelin, a 28 amino acid peptide first described in 1999, is secreted by gastric oxyntic glands and circulates in blood.5 In rats, ghrelin has been found to stimulate food intake, to induce adiposity, and to increase body weight. In the gastrointestinal system, ghrelin regulates secretion of gastric acid, gastric motility, and pancreatic protein output by a vagus nerve-dependent mechanism.6 More recently, ghrelin has been implicated as an inflammatory mediator with levels elevated in the plasma of rats and humans with sepsis.7
Previous studies have demonstrated that the DMV responds to systemic endotoxin, and TNBS-induced colitis increases permeability through the blood-brain barrier in both rats and rabbits.8 Thus, it is possible that the DMV is exposed to pro-inflammatory cytokines and ghrelin circulating in blood in the setting of acute intestinal inflammation. In this study, we investigate the effects of gastrointestinal inflammation on the DMV in rats, as well as the effects of pro-inflammatory cytokines and ghrelin on DMV cells in vitro.
Materials and Methods
Chemicals and solutions
Neurobasal medium A, phosphate buffer solution (PBS), B27 supplement, L-glutamine, penicillin and streptomycin were from Gibco (Grand Island, NY, USA). β fibroblast growth factor (βFGF) and anti-fade reagent were from Invitrogen (Carlsbad, CA, USA). Poly-d-lysine, trypsin type I, protease type XIV, 2,4,6-trinitrobenzenesulfonic acid solution (TNBS), Triton X-100, indomethacin, fluorescein isothiocyanate (FITC) conjugated goat anti-mouse IgG, biotin-conjugated goat anti-rabbit IgG, and goat serum were purchased from Sigma-Aldrich (St. Louis, MO). Recombinant interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) were obtained from Calbiochem (Darmstadt, Germany). Dihydrochloride (DAPI) and DiI were from Molecular Probes (Eugene, OR, USA). Ghrelin was from Phoenix Pharmaceuticals (Burlingame, CA, USA). Cell Death Detection ELISA and Cell Proliferation ELISA kits were purchased from Roche Diagnostics (Basel, Switzerland). Rabbit anti-IL-1 receptor antibody, rabbit anti-IL-6 receptor antibody, rabbit anti-TNF-α receptor antibody, and mouse serum were purchased from Santa Cruz Biotechnology (Santa Cruz, California). Mouse anti-Thy-1 monoclonal antibody was from Chemicon (Temecula, CA, USA). Streptavidin-FITC was from Biolegend (San Diego, CA, USA).
Retrograde tracing of DMV neurons
Animal studies were approved by the University of Michigan Committee on Use and Care of Animals. Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 200–220 grams were kept in individual cages with 12 hour light/dark cycles and were given chow food and water ad libitum. Rats were anesthetized with intramuscular injection of a mixture of urethane and ketamine (10 and 90 mg/kg body weight, respectively). Laparotomy was performed and the fluorescent carbocyanine dye, DiI, was injected into the stomach wall. DiI is taken up by nerve terminals in the periphery and moves retrogradely up the axon to the cell body to specifically stain the neurons in the DMV projecting to the stomach.9
Two models of intestinal inflammation were utilized. In the first, animals in which midbrain neurons were labeled underwent induction of acute intestinal inflammation by injection of indomethacin (7.5 mg/kg).3 In the second model, intestinal inflammation was induced by 0.5 cc enemas of trinitrobenzenesulfonic acid solution (TNBS) at a dose of 30 mg in 50% ethanol.2 Control animals were given 0.5 cc enemas of 50% ethanol. Animals were given chow food and water ad libitum and sacrificed after one week. At necropsy, the colon was inspected and its weight measured. The brainstem was removed and post-fixed for 2 days at 4°C, then kept in 0.1 M phosphate buffer containing 20% sucrose for 24 h. The medulla oblongata containing the DMV was sectioned into 60 µm slices at the interaural level of −4.24 to −5.08 mm according to the atlas of Paxinos and Watson using the Leica CM 1850 cryostat (Wetzlar, Germany). Nuclei were counterstained with DAPI and fluorescent microscopy was used to assess DiI staining. Total positive cells at the levels of the ventricle, area postrema and caudal area were counted and data were expressed as mean ± SEM.
Measurement of octanoyl ghrelin and desacyl-ghrelin
Male Sprague-Dawley rats were divided into groups receiving vehicle injection or subcutaneously injected indomethacin (7.5 mg/kg, x2 at 24 hr interval). Animals were sacrificed at days 2, 5 and 10 after induction of intestinal inflammation. After an overnight fast, blood was obtained from rats and placed in tubes with 1 mg/ml EDTA-2Na and 500 U/ml aprotinin, immediately centrifuged at 4 °C, acidified with 1 M HCl and stored at −80 C° until assay. Plasma levels of octanoyl (active) ghrelin and desacyl ghrelin were measured using a two-site sandwich enzyme-linked immunosorbent assay (ELISA). Standard curves were fitted and results calculated with 4-parameter logistic function.
In vitro culture of DMV cells
DMV neurons were isolated from 2–5 day old Sprague–Dawley rats in a manner previously described.10 Rats were euthanized. The brainstem was rapidly removed, and chilled at 0 °C in a dissection solution containing: NaCl 138 mM, KCl 4 mM, MgCl2 1 mM, CaCl2 2 mM, glucose 20 mM, HEPES 10 mM. Tissue blocks were prepared and sectioned transversely into 400 µm slices at the level of the obex using a Vibratome 3000 (Redding, CA). The DMV area was identified under a dissecting microscope as the area immediately ventral to the nuclei of the solitary tract and dorsal to the XII nuclei. DMV tissue was excised and then digested in an enzyme solution containing protease type XIV (0.6 mg/ml) and trypsin type I (0.4 mg/ml) at 32 °C for 30–60 minutes. The tissue was then dissociated by gentle trituration with pipettes. Cells were plated onto poly-d-lysine coated 25 mm slides in 35mm culture dishes. Neurons were maintained at 37 °C in an atmosphere of 5% CO2 in serum-free culture media containing Neurobasal medium A containing 2% B27 supplement, 2 mM glutamine, 1% penicillin and streptomycin, and 5 ng/ml βFGF. After 4 days, one-half of the medium was replaced and experiments were conducted at 7 days.
Immunohistochemistry
After one week in culture, culture media was removed and cells were fixed with 4% para-formaldehyde for 30 minutes at room temperature. Cells were washed with PBS for three five-minute intervals. Non-specific staining was suppressed by blocking with 10% goat serum in 0.1% Triton X-100 for 60 minutes. Anti-IL-1 receptor polyclonal antibody (1:50), anti-IL-6 receptor polyclonal antibody (1:50), anti-TNF receptor polyclonal antibody (1:50), and anti-Thy-1 monoclonal antibody (1:200) were diluted in 10% goat serum and applied to DMV cells overnight at 4°C. Cells were exposed to three five-minute washes with PBS. Biotinylated goat anti-rabbit IgG was used as secondary antibody for IL-1 receptor, IL-6 receptor, and TNF receptor. FITC-conjugated goat anti-mouse IgG was used as secondary antibody for Thy-1. Secondary antibodies were diluted 1:200 in 10% goat serum and added to cells for 1 h at room temperature. Cells were exposed to three five-minute washes with PBS. Streptavidin-conjugated FITC was diluted 1:500 in 10% goat serum and added to cells exposed to biotinylated secondary antibody for 45 min at room temperature. Cells were treated with three five-minute washes with PBS. DAPI was diluted 1:5000 in PBS and added to cells for 5 min at room temperature for nuclear counterstaining. Cells were washed and coverslips were mounted on glass microscope slides using anti-fade reagent. An Olympus BX51 fluorescent microscope was used to examine DMV cells, and images were captured with a Spot 2 digital camera (Diagnostic Instruments, Inc, Sterling Heights, MI).
RT-PCR
Total RNA was isolated from rat DMV tissues. Single-strand cDNA synthesis was performed as follows: 30 µl of reverse transcription mixture contained 1 µg of DNase I pre-treated total RNA, 0.75 µg of oligo d(T) primer, 6 µl of 5×RT buffer, 10 mM dithiothreitol, 0.5 mM deoxynucleotides, 50 units of RNase inhibitor, and 240 units of reverse transcriptase (Invitrogen, Carlsbad, CA). The RT reaction was carried out at 40°C for 70 min followed by heat inactivation at 95°C for 3 min. Forty cycles were run. PCR primers were deduced from published rat sequences. PCR was performed in a total 25 µl volume, containing 2.5µl cDNA, 5 mM MgCl2, 0.2 mM dNTPs, 0.25 µM each primer, 1.25 U Ampli Taq Polymerase (Roche Diagnostics, Indianapolis, IN), and 1µl of a 800× diluted SYBR Green I stock (Roche). Melt curve analysis was from 600 C to 950 C at 0.20 C/second with Optics Ch1 On. PCR product was also visualized by 1.5% agarose gel electrophoresis. For negative controls, PCR reactions were performed for the primer pairs in the absence of transcript.
Apoptosis and cellular proliferation measurements
Apoptosis and cellular proliferation were measured in primary DMV neuronal cell culture. 5000 cells were plated onto each well of a 96-well plate. Cells were maintained for 1 week at which time they were exposed to experimental conditions or control for 24 hours. Experimental conditions consisted of fresh media in addition to cytokine and/or ghrelin, while the control condition was fresh media. Apoptosis was measured using a commercially available colorimetric enzyme sandwich-linked immunosorbent assay (ELISA) following cell lysis and immunochemical determination of histone-complexed DNA fragments. Similarly, after 24 hours of experimental conditions or control, cellular proliferation was measured using a commercially available colorimetric ELISA after 3 hours of BrdU incorporation. Experimental conditions consisted of fresh media without βFGF, a strong proliferative factor in vitro, with the addition of cytokine and/or ghrelin. The control condition was fresh media without βFGF. At least 6 repetitions were performed for each experimental condition.
Data analysis
The software used was GraphPad Prism® from GraphPad Software Inc. (San Diego, CA). Results are expressed as mean ± SEM. Data were analyzed using ANOVA and a Student’s t-test as appropriate. Significance was accepted as P < 0.05.
Results
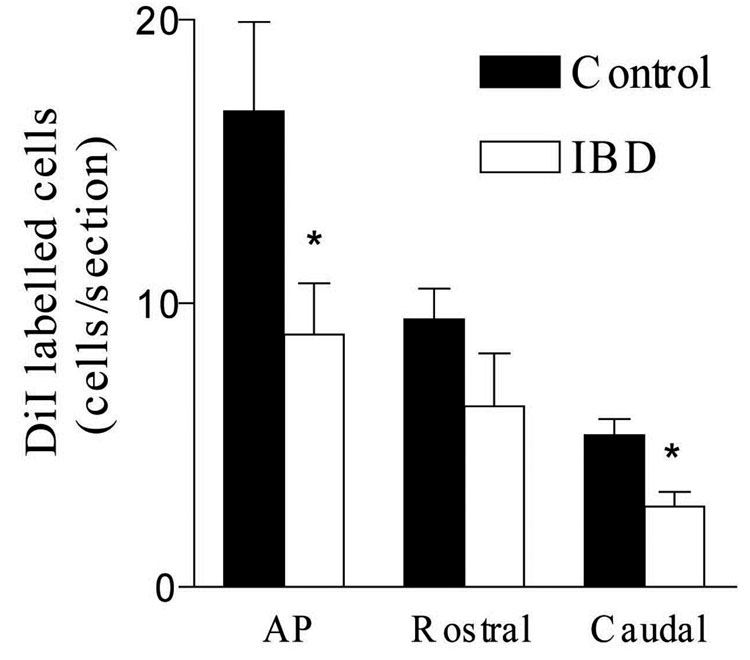
Retrograde tracing of efferent gastrointestinal neurons demonstrated their origin in the nucleus of the solitary tract, area postrema and the dorsal motor nucleus of the vagal nerve. In control animals, both right and left regions of the medulla contributed equally to gastric innervation. In the presence of intestinal inflammation induced by injection of indomethacin, significant reductions in labeled neurons projecting to the gastrointestinal tract were observed. As shown in Figure 1, 40–50% reductions in labeled neurons were observed in all three areas of the DMV. Similarly significant reductions in labeled DMV neurons, approximately 70%, were observed in animals in which intestinal inflammation was induced by TNBS. Reduction of visualized DMV neurons in animals in which labeling occurred prior to the induction of intestinal inflammation strongly suggests that inflammatory signals were transmitted from the intestine to the CNS.
Figure 1.
DiI labelled neurons in the DMV. 40–50% reductions in labeled neurons were observed with TNBS-induced colitis in all three areas of the DMV. Labeled neurons per brain section when comparing controls to animals with TNBS-induced IBD were: adjacent to the area postrema (AP), 16.8 ± 3.2 vs. 8.9 ± 1.8; rostral DMV 9.5 ± 1.1 vs. 6.4 ± 1.8; caudal DMV, 5.4 ± 0.6 vs. 2.9 ± 0.5. *P < 0.05.
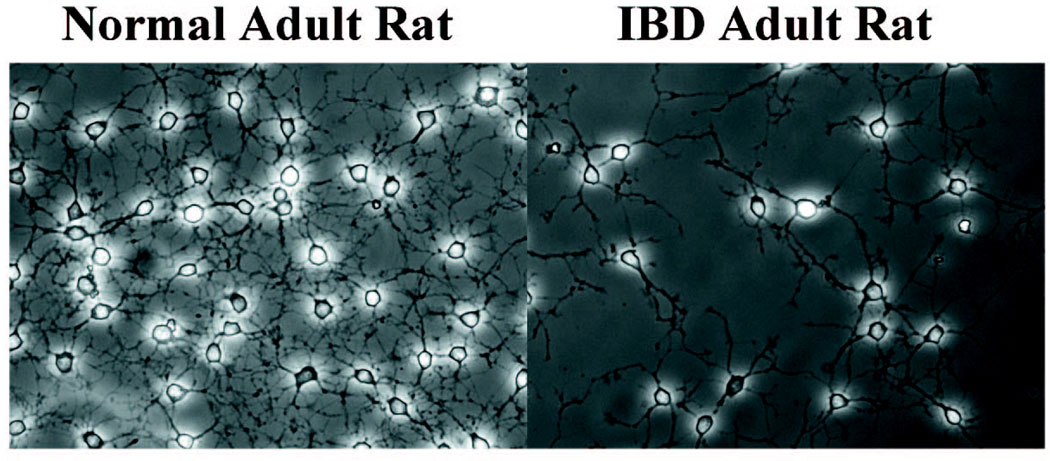
Using microdissection, dorsal motor nucleus neurons were obtained and then maintained in culture for up to 10 days. They expressed a neuronal phenotype, with a dense, phase-bright cell body and branching dendritic processes (Figure 2). Similarly, midbrain neurons from rats in which intestinal inflammation had been induced by indomethacin were maintained in primary culture with similar neuronal phenotypes (Figure 2).
Figure 2.
Cell culture of DMV cells from control animals and animals with indomethacin-induced intestinal inflammation. Cells expressed a neuronal phenotype, with a dense, phase-bright cell body and branching dendritic processes. Similarly, medullary neurons from rats in which intestinal inflammation had been induced by indomethacin were maintained in primary culture demonstrating similar phenotypes.
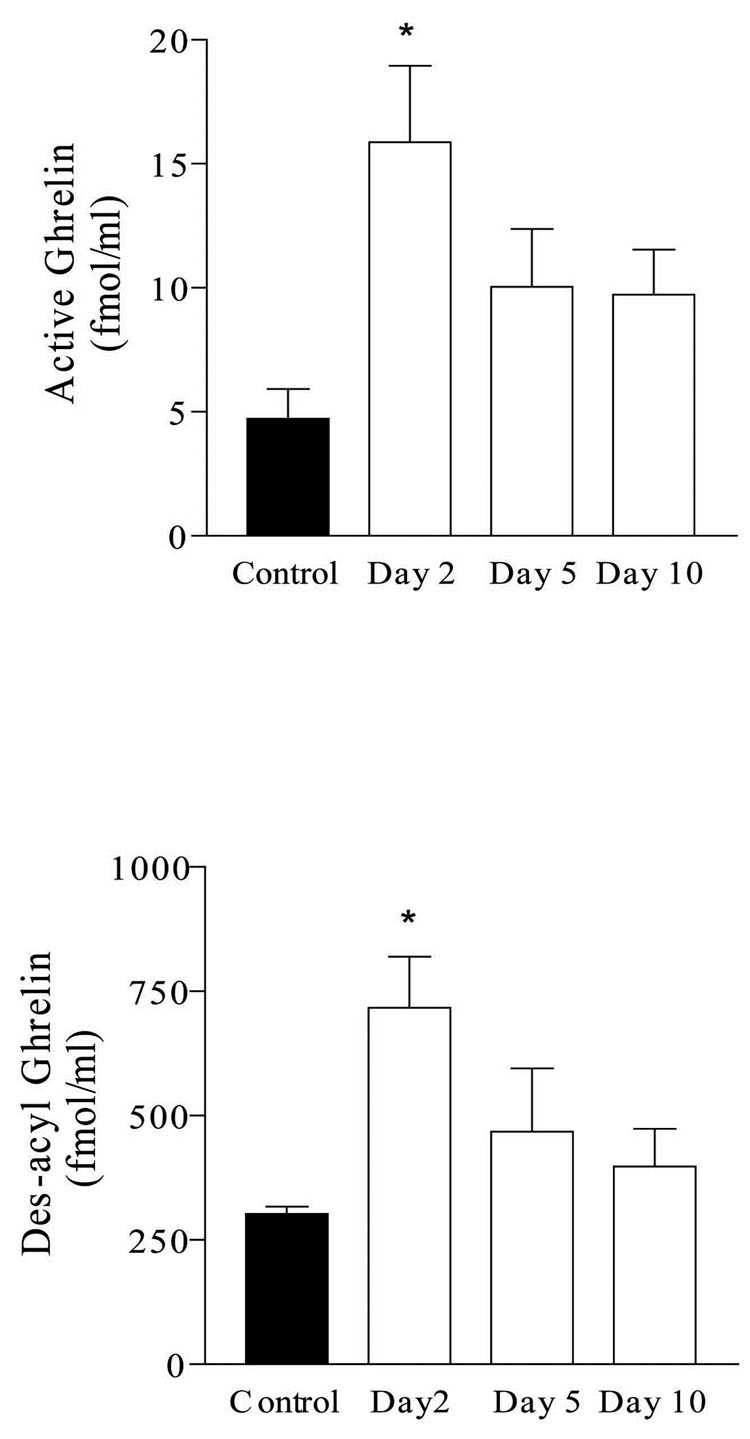
Serum levels of octanoyl ghrelin and desacyl-ghrelin were both significantly elevated within 2 days of induction of intestinal inflammation (Figure 3). Octanoyl ghrelin levels were 4.7 ± 1.2 fmol/ml prior to IBD induction, 15.9 ± 3.1 fmol/ml two days later, 10.0 ± 2.3 fmol/ml on day 5, and 9.7 ± 1.8 fmol/ml on day 10. Des-acyl ghrelin levels were 302.8 ± 14.3 fmol/ml prior to IBD induction, 717.4 ± 102.1 fmol/ml two days later, 468.3 ± 127.0 fmol/ml on day 5, and 398.0 ± 76.1 fmol/ml on day 10. In a previous study, we have demonstrated expression of ghrelin receptor in the DMV by immunoreactivity to a specific antibody as well as mRNA transcription.11
Figure 3.
Serum ghrelin levels. Octanoyl (active) ghrelin and des-acyl ghrelin levels were measured prior to indomethacin injection, and 2, 5, and 10 days after IBD induction. *P < 0.01.
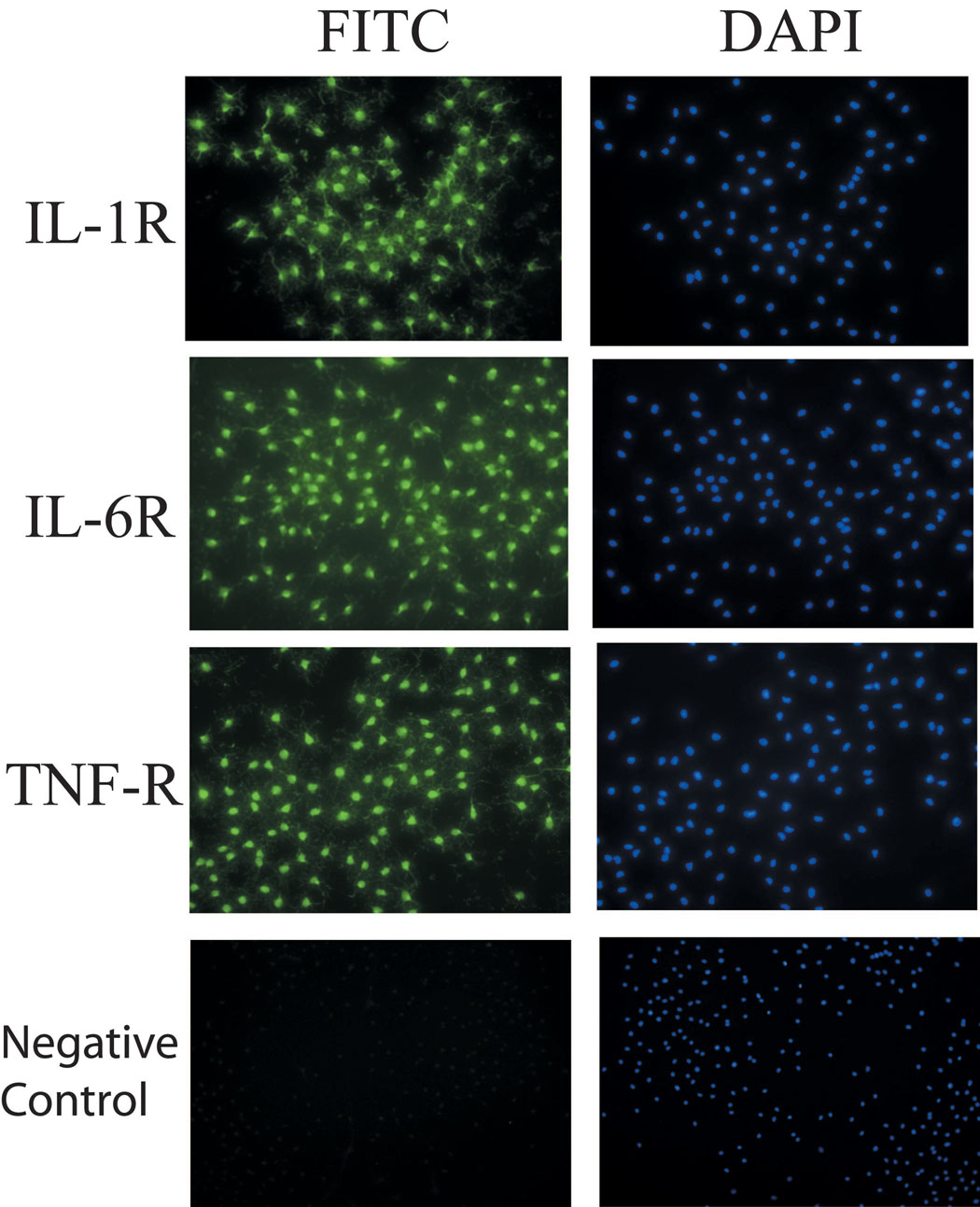
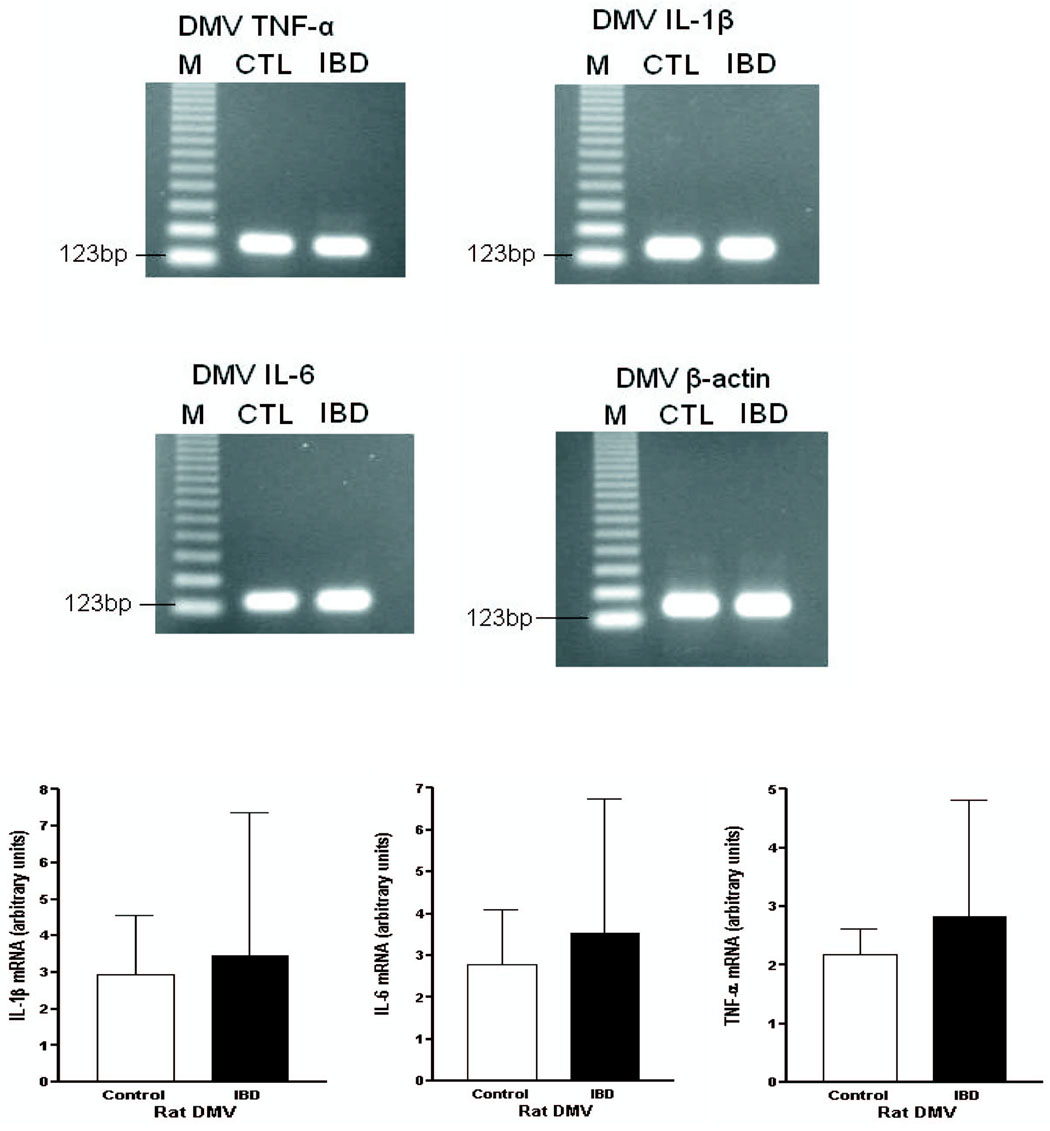
Transcripts for receptors for IL-1β, IL-6 and TNF-α were expressed in rat vagal nuclei, and receptor expression was also demonstrated by immunocytochemical staining for these proteins (Figure 4). mRNA levels of these cytokines within the DMV were not increased by the induction of intestinal inflammation (Figure 5).
Figure 4.
Immunostaining of cytokine receptors in primary cell culture. FITC staining of cytokine receptors for IL-1β, IL-6, and TNF-α in the left panel. Negative control used IgG as primary antibody. Dapi staining of nuclei in the right panel.
Figure 5.
Cytokine expression in DMV. Northern blot analysis of mRNA PCR products demonstrates that IL-1β, IL-6 and TNF-α mRNAs are expressed in rat DMV. Transcription of these cytokines is not increased by indomethacin-induced intestinal inflammation (IBD). Densitometry of the blots was performed, and these results are also shown in the lower panel. M = Nucleic acid marker; CTL = Control.
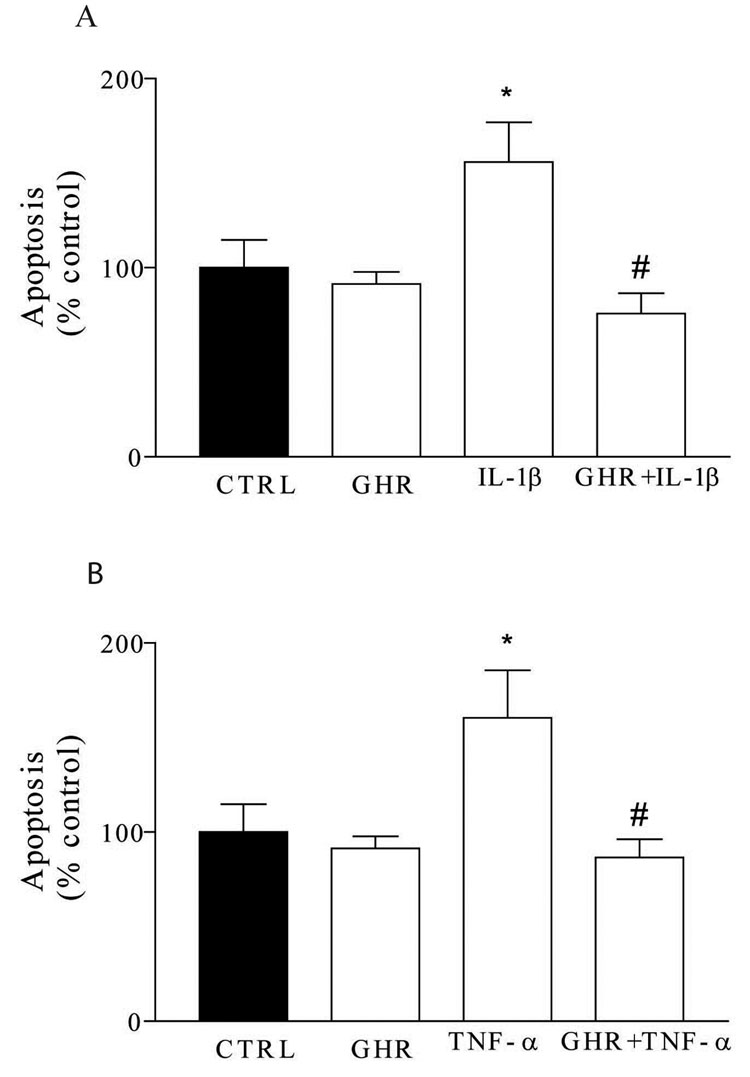
Using in vitro primary DMV neuronal cell culture, the effects of pro-inflammatory cytokines on apoptosis was measured. Cells were individually exposed to three different cytokines, IL-1β, TNF-α, and IL-6, in the presence or absence of ghrelin, for 24 hours. This was followed by cell lysis and colorimetric ELISA measurement of apoptosis. All apoptosis measurements were normalized to control experiments in which cells were incubated in fresh media devoid of cytokine. IL-1β, IL-6, and TNF-α, each at 10ng/ml, significantly increased apoptosis (Figure 6A). There was no significant change in neuronal apoptosis after 24 hours exposure to ghrelin alone. When DMV neurons were exposed to both IL-1β and ghrelin 10−8M apoptotic rates returned to control levels (Figure 6A). Similarly, TNF-α 10 ng/ml significantly increased apoptosis while TNF-α and ghrelin 10−8M together returned apoptosis to control levels (Figure 6B). IL-6 did not significantly alter DMV apoptotic rates.
Figure 6.
Ghrelin alters the effect of cytokines on apoptosis. A. Ghrelin (GHR) alone did not change apoptotic rates compared to control (CTRL). Exposure to IL-1β alone increased apoptotic rates (155 ± 21% of control). Exposure to IL-1β in the presence of ghrelin resulted in apoptotic rates significantly lower than cytokine alone, and similar to control experiments. B. Exposure to TNF-α alone increased apoptotic rates to 160 ± 25% of control. Exposure to TNF-α in the presence of ghrelin resulted in apoptotic rates of 87 ± 10% of control, significantly lower than cytokine alone, and similar to control experiments. *P < 0.05 compared to control. #P < 0.05 compared to cytokine exposure alone.
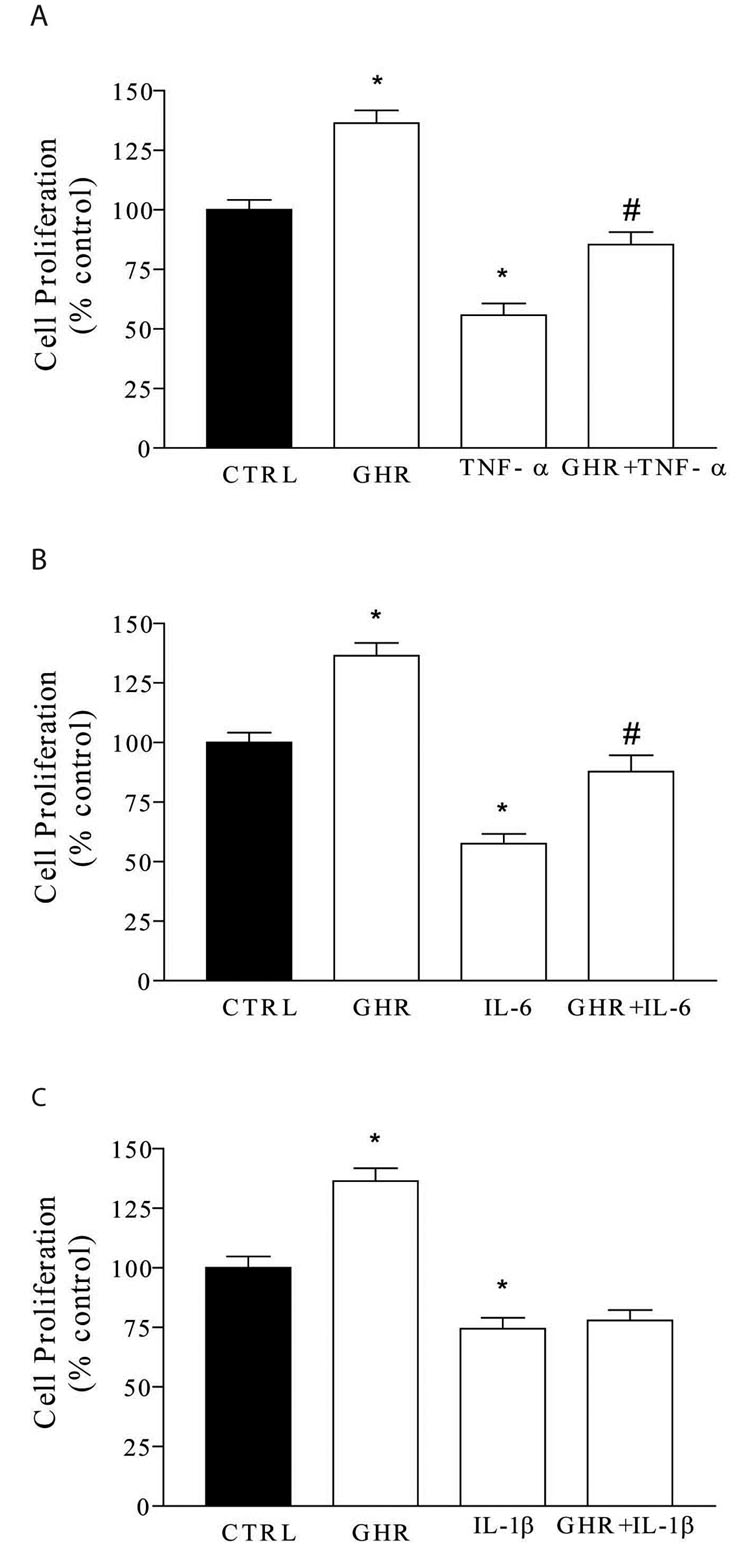
Cell proliferation was measured using a colorimetric ELISA measuring BrdU incorporation. Cells were individually exposed to IL-1β, TNF-α, and IL-6, in the presence or absence of ghrelin, for 24 hours followed by 3 hours of BrdU incorporation. All BrdU incorporation measurements were normalized to control experiments. In a previous publication, we have reported that ghrelin stimulates neurogenesis in the DMV 11. In the current study, ghrelin 10−7M caused significantly increased neuronal proliferation relative to control (Figure 7). Although TNF-α (1 ng/ml) significantly decreased cell proliferation, when cells were exposed to both TNF-α and ghrelin 10−7M, BrdU incorporation was similar to control levels (Figure 7A). Similarly, IL-6 1 ng/ml alone decreased cell proliferation, while cells exposed to both IL-6 and ghrelin 10−7M had indices of cellular proliferation similar to control levels (Figure 7B). IL-1β decreased neuronal proliferation, but the addition of ghrelin did not alter the effects of IL-1β (Figure 7C).
Figure 7.
Ghrelin alters the effect of cytokines on proliferation. A. Ghrelin (GHR) alone stimulates cellular proliferation in vitro (136 ± 5% of control (CTRL)). Exposure to TNF-α alone decreased cellular proliferation rates to 56 ± 5% of control. Exposure to TNF-α in the presence of ghrelin resulted in proliferation rates of 85 ± 5% of control, significantly higher than cytokine alone, and similar to control experiments. B. Exposure to IL-6 alone decreased cellular proliferation. IL-6 in the presence of ghrelin resulted in proliferation rates significantly higher than cytokine alone, and similar to control experiments. C. IL-1β alone decreased cellular proliferation. Cellular proliferation of IL-1β in the presence of ghrelin was similar to IL-1β alone. *P < 0.01 compared to control. #P < 0.01 compared to cytokine alone.
Discussion
These results indicate that intestinal inflammation adversely affects neuronal survival in the DMV. In addition, ghrelin attenuates the effects of inflammatory cytokines on the DMV. Several findings support these conclusions. Two differing experimental models of IBD cause loss of labeled neurons from the DMV. Neurons can be cultured from the DMV from both control animals and from animals with IBD so that functions may be investigated in vitro. In animals with IBD, circulating ghrelin is elevated, and ghrelin receptor protein is present in the DMV. IL-1β, IL-6 and TNF-α receptor expression are observed in the DMV. Transcription of the pro-inflammatory cytokines IL-1β, IL-6 and TNF-α is not increased locally by intestinal inflammation, suggesting DMV exposure to cytokines comes from circulating sources. In vitro, cytokine exposure increases apoptosis and decreases cellular proliferation. Both of these effects are reversed by ghrelin.
The hapten-induced model of colonic inflammation displays both pathologic and clinical similarities to human IBD.2 In this model, TNBS is delivered intrarectally and binds to intestinal proteins to stimulate a delayed-type hypersensitivity reaction and exaggerated T cell activation. 24 to 48 hours after administration of TNBS, animals develop diarrhea as well as mucosal ulceration and bowel wall edema. Indomethacin administration also results in small bowel inflammation in experimental animal models.12 Depending on the number of doses of indomethacin administered, this model results in both acute and chronic inflammatory changes, sharing clinical, histological, and pathophysiological characteristics with Crohn’s disease.3
Intestinal inflammation causes several changes in the enteric nervous system. Nearly half of all neurons are lost within 48 hours after the induction of TNBS colitis.13 There are functional changes in enteric neurons from inflamed areas characterized by hyperexcitability.14 Further alterations occur in neuromediator content and neurotransmitter release. For example, there is reduced expression and function of the serotonin reuptake transporter.15 These numerous changes potentially contribute to altered sensory information during intestinal inflammation. Investigative studies have expanded knowledge about the effects of IBD in the enteric nervous system. In contradistinction, there is a lack of understanding of the actions of IBD on the central nervous system.
The DMV is an important central nervous structure with projections to the gastrointestinal system. The DMV possesses a leaky blood–brain barrier whose permeability is increased with TNBS-induced colitis.8 There are also transport systems for entry of the pro-inflammatory cytokines TNF-α and IL-1β into the CNS.16 Systemic elevation of TNF-α is seen in humans and animal models of IBD, contributing to symptoms of anorexia, nausea, and vomiting.17 IL-1β has also been shown to directly act on the DMV to inhibit gut motility.18 It is plausible that acute intestinal inflammation elevates levels of circulating cytokines and ghrelin which have access to the DMV.
Ghrelin is a novel 28 amino acid peptide which was initially purified from rat stomach and localized to gastric oxyntic glands.5 Ghrelin receptor has been localized in the DMV.19 In the dorsal vagal complex, direct application of ghrelin stimulates food intake.20 Ghrelin has also been described to act on the dorsal vagal complex to stimulate pancreatic protein secretion.21
There is mounting evidence that ghrelin also possesses inflammatory modulating effects. Ghrelin inhibits activation-induced cytokine expression in human monocytes, lymphocytes, and endothelial cells.22 Exposure of peritoneal macrophages to ghrelin prevented endotoxin-induced IL-6 release.23 Ghrelin levels were increased in arthritic rats, and administration of ghrelin decreased circulating levels of IL-6, ameliorated the external symptoms of arthritis and decreased the arthritis score.23 Ghrelin secretion is enhanced and provides protection in animal models of peptic ulcer disease.24 Rats with sepsis by the cecal ligation and perforation model had plasma ghrelin levels 50% higher than control animals.25 Administration of ghrelin inhibited lipopolysaccharide-induced pro-inflammatory cytokine production, ameliorated hypotension, and significantly decreased mortality in rats with sepsis.7 Ghrelin attenuated pancreatic damage in a rat model of acute pancreatitis.26 Ghrelin administration in rats who underwent ligation of the common pancreaticobiliary duct decreased pancreatic and hepatic destruction which was accompanied by reduced injury to kidneys and lungs.27 Specific to intestinal inflammation, humans with active IBD have increased serum levels of ghrelin.28 In mice with TNBS-induced colitis, ghrelin administration abrogated body weigh loss, diarrhea, colonic inflammation, and increased survival.29
Though using DMV neurons in cell culture is useful for investigative studies, there are also inherent limitations. Cells may be damaged or altered by the isolation process. Furthermore, normal nervous connections found in vivo are not maintained in culture. Despite these limitations, studies using cell culture can help discern cellular responses to stimuli. Another potential limitation is the possibility that the increased serum ghrelin levels in rats with TNBS-colitis were due to decreased food intake. However, no changes in ghrelin levels were demonstrated in healthy patients with normal feeding versus after two days of fasting.30
Intestinal inflammation causes numerous physiologic alterations. Clinically, diarrhea characterizes inflammatory bowel disease. Injury to the bowel wall includes damage to the enteric neurons. Elevated levels of cytokines contribute to anorexia and fever. Serum ghrelin levels are also elevated, and attenuate the effects of cytokines at the cellular level. This study suggests that intestinal inflammation may affect the structure of the DMV, and ghrelin acts as an inflammatory modulator.
Acknowledgments
NIH Grants T32CA009672 and RO1DK054032.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sun FF, Lai PS, Yue G, et al. Pattern of cytokine and adhesion molecule mRNA in hapten-induced relapsing colon inflammation in the rat. Inflammation. 2001;25(1):33–45. doi: 10.1023/a:1007023611478. [DOI] [PubMed] [Google Scholar]
- 2.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96(3):795–803. [PubMed] [Google Scholar]
- 3.Yamada T, Deitch E, Specian RD, Perry MA, Sartor RB, Grisham MB. Mechanisms of acute and chronic intestinal inflammation induced by indomethacin. Inflammation. 1993;17(6):641–662. doi: 10.1007/BF00920471. [DOI] [PubMed] [Google Scholar]
- 4.Lomax AE, Linden DR, Mawe GM, Sharkey KA. Effects of gastrointestinal inflammation on enteroendocrine cells and enteric neural reflex circuits. Auton Neurosci. 2006:126–127. 250–257. doi: 10.1016/j.autneu.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 6.Date Y, Nakazato M, Murakami N, Kojima M, Kangawa K, Matsukura S. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochemical and biophysical research communications. 2001;280(3):904–907. doi: 10.1006/bbrc.2000.4212. [DOI] [PubMed] [Google Scholar]
- 7.Chang L, Zhao J, Yang J, Zhang Z, Du J, Tang C. Therapeutic effects of ghrelin on endotoxic shock in rats. European journal of pharmacology. 2003;473(2–3):171–176. doi: 10.1016/s0014-2999(03)01972-1. [DOI] [PubMed] [Google Scholar]
- 8.Hathaway CA, Appleyard CB, Percy WH, Williams JL. Experimental colitis increases blood-brain barrier permeability in rabbits. The American journal of physiology. 1999;276(5 Pt 1):G1174–G1180. doi: 10.1152/ajpgi.1999.276.5.G1174. [DOI] [PubMed] [Google Scholar]
- 9.Browning KN, Renehan WE, Travagli RA. Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. The Journal of physiology. 1999;517(Pt 2):521–532. doi: 10.1111/j.1469-7793.1999.0521t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, Hu Y, Newman EA, Mulholland MW. Serum-free culture of rat postnatal neurons derived from the dorsal motor nucleus of the vagus. Journal of neuroscience methods. 2006;150(1):1–7. doi: 10.1016/j.jneumeth.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, Lin TR, Hu Y, et al. Ghrelin stimulates neurogenesis in the dorsal motor nucleus of the vagus. The Journal of physiology. 2004;559(Pt 3):729–737. doi: 10.1113/jphysiol.2004.064121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kent TH, Cardelli RM, Stamler FW. Small intestinal ulcers and intestinal flora in rats given indomethacin. The American journal of pathology. 1969;54(2):237–249. [PMC free article] [PubMed] [Google Scholar]
- 13.Sanovic S, Lamb DP, Blennerhassett MG. Damage to the enteric nervous system in experimental colitis. The American journal of pathology. 1999;155(4):1051–1057. doi: 10.1016/S0002-9440(10)65207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linden DR, Sharkey KA, Mawe GM. Enhanced excitability of myenteric AH neurones in the inflamed guinea-pig distal colon. The Journal of physiology. 2003;547(Pt 2):589–601. doi: 10.1113/jphysiol.2002.035147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linden DR, Foley KF, McQuoid C, Simpson J, Sharkey KA, Mawe GM. Serotonin transporter function and expression are reduced in mice with TNBS-induced colitis. Neurogastroenterol Motil. 2005;17(4):565–574. doi: 10.1111/j.1365-2982.2005.00673.x. [DOI] [PubMed] [Google Scholar]
- 16.Banks WA, Kastin AJ, Gutierrez EG. Interleukin-1 alpha in blood has direct access to cortical brain cells. Neuroscience letters. 1993;163(1):41–44. doi: 10.1016/0304-3940(93)90224-9. [DOI] [PubMed] [Google Scholar]
- 17.Kapas L, Hong L, Cady AB, et al. Somnogenic, pyrogenic, and anorectic activities of tumor necrosis factor-alpha and TNF-alpha fragments. The American journal of physiology. 1992;263(3 Pt 2):R708–R715. doi: 10.1152/ajpregu.1992.263.3.R708. [DOI] [PubMed] [Google Scholar]
- 18.Morrow NS, Quinonez G, Weiner H, Tache Y, Garrick T. Interleukin-1 beta in the dorsal vagal complex inhibits TRH analogue-induced stimulation of gastric contractility. The American journal of physiology. 1995;269(2 Pt 1):G196–G202. doi: 10.1152/ajpgi.1995.269.2.G196. [DOI] [PubMed] [Google Scholar]
- 19.Hou Z, Miao Y, Gao L, Pan H, Zhu S. Ghrelin-containing neuron in cerebral cortex and hypothalamus linked with the DVC of brainstem in rat. Regulatory peptides. 2006;134(2–3):126–131. doi: 10.1016/j.regpep.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes. 2003;52(9):2260–2265. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Wu X, Zhao Y, Chen S, Owyang C. Ghrelin acts on the dorsal vagal complex to stimulate pancreatic protein secretion. American journal of physiology. 2006;290(6):G1350–G1358. doi: 10.1152/ajpgi.00493.2005. [DOI] [PubMed] [Google Scholar]
- 22.Dixit VD, Schaffer EM, Pyle RS, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. The Journal of clinical investigation. 2004;114(1):57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Granado M, Priego T, Martin AI, Villanua MA, Lopez-Calderon A. Ghrelin receptor agonist GHRP-2 prevents arthritis-induced increase in E3 ubiquitin-ligating enzymes MuRF1 and MAFbx gene expression in skeletal muscle. Am J Physiol Endocrinol Metab. 2005;289(6):E1007–E1014. doi: 10.1152/ajpendo.00109.2005. [DOI] [PubMed] [Google Scholar]
- 24.Fukuhara S, Suzuki H, Masaoka T, et al. Enhanced ghrelin secretion in rats with cysteamine-induced duodenal ulcers. American journal of physiology. 2005;289(1):G138–G145. doi: 10.1152/ajpgi.00298.2004. [DOI] [PubMed] [Google Scholar]
- 25.Chang L, Du JB, Gao LR, Pang YZ, Tang CS. Effect of ghrelin on septic shock in rats. Acta pharmacologica Sinica. 2003;24(1):45–49. [PubMed] [Google Scholar]
- 26.Dembinski A, Warzecha Z, Ceranowicz P, et al. Ghrelin attenuates the development of acute pancreatitis in rat. J Physiol Pharmacol. 2003;54(4):561–573. [PubMed] [Google Scholar]
- 27.Kasimay O, Iseri SO, Barlas A, et al. Ghrelin ameliorates pancreaticobiliary inflammation and associated remote organ injury in rats. Hepatol Res. 2006;36(1):11–19. doi: 10.1016/j.hepres.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Peracchi M, Bardella MT, Caprioli F, et al. Circulating ghrelin levels in patients with inflammatory bowel disease. Gut. 2006;55(3):432–433. doi: 10.1136/gut.2005.079483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Rey E, Chorny A, Delgado M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology. 2006;130(6):1707–1720. doi: 10.1053/j.gastro.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 30.Avram AM, Jaffe CA, Symons KV, Barkan AL. Endogenous circulating ghrelin does not mediate growth hormone rhythmicity or response to fasting. The Journal of clinical endocrinology and metabolism. 2005;90(5):2982–2987. doi: 10.1210/jc.2004-1785. [DOI] [PubMed] [Google Scholar]